Abstract
Objectives
To assess whether frailty can be assessed using a smartphone and whether daily walking speed (DWS) is associated with frailty.
Design
Cross-sectional study.
Setting
Three prefectures (Kanagawa, Saitama and Tokyo) in Japan.
Participants
The study enrolled 163 participants (65 in the robust group, 69 in the prefrailty group and 29 in the frailty group) by sending letters to house owners aged≥55 years.
Primary and secondary outcome measures
The participants downloaded the DWS measurement application on their smartphones, which measured the daily walking (DW) parameters (DWS, step length and cadence) and the Kihon checklist for frailty assessment. The differences in the DW parameters between the robust, prefrailty and frailty groups were examined using one-way analysis of variance. We conducted logistic regression analysis for the Crude model (each DW parameter), model 1 (adjusted for the number of steps) and model 2 (model 1+age, sex and the number of chronic diseases).
Results
DWS was marginally significantly slower in the frailty group than in the prefrailty and robust group (robust 1.26 m/s vs prefrailty 1.25 m/s vs frailty 1.19 m/s, p=0.060). Step length was significantly smaller in the frailty group than in the robust group (robust 66.1 cm vs prefrailty 65.9 vs frailty 62.3 cm, p<0.01). Logistic regression analysis for the three models revealed that DWS was significantly associated with frailty.
Conclusions
DWS measured using the smartphone application was associated with frailty. This was probably due to the shorter step length and body height seen in frail individuals.
Keywords: GERIATRIC MEDICINE, SPORTS MEDICINE, PREVENTIVE MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The participants accessed the website for the smartphone application using the QR code printed on the invitation letter and downloaded the daily walking speed (DWS) measurement application on their smartphones.
Unlike previous studies, frailty in this study was assessed using a web-based smartphone application, and frail participants were included.
The participants did not have to go to a designated place and could answer the Kihon checklist through the application.
However, the participants were not randomly selected but were those with access to a smartphone and those interested in frailty prevention and health promotion.
In addition, DWS was limited to outdoor measurements as the application was based on GPS.
Introduction
Walking speed is closely associated with many health outcomes and predicts dependency and death in older individuals.1 2 A meta-analysis showed that decreased walking speed is associated with the incidence of cardiovascular diseases and associated mortality.3 Based on these studies, walking speed is recognised as the sixth vital sign, following blood pressure, pulse, respiration, temperature and pain.4 Usual walking speed has been often measured by recording the time required to walk a certain distance using a stopwatch in the previous studies. This method can measure the walking speed easily and accurately and has been used in several studies; however, concerns have been raised since the participants are required to come to a specific measurement site, and they can intentionally change their walking speed.
Recent studies have measured daily walking speed (DWS) using wearable accelerometers and smartphone applications.5–9 If DWS can be used for health assessment in a manner similar to the conventional ‘laboratory walking speed (LWS)’, such daily measurements can be used for the early detection of health risks, continuous health assessment and health promotion. However, the measurement of DWS is not well-established, and its definition differs depending on the study, with variations in factors such as differences in sensor type used for measurement (accelerometer vs GPS), range of days for measurement (14 days vs 1 week), and representative value (average vs percentile). In addition, previous studies on DWS have only shown the relationship between average6 10 or percentile values of DWS8 9 and LWS, minimal detectable change in 95% (MDC95) of average of DWS,7 and age-sex reference values for average DWS,11 and only a few studies have investigated the association between DWS and health outcomes.
Recent studies have reported an association between DWS and prefrailty.12 13 Frailty is a state in which vulnerability increases owing to ageing, and the risk of dependency and death increases.14 The prevention of frailty is extremely important for maintaining the health of older individuals. However, few studies on DWS have examined the association between DWS and frailty. Kawai et al12 used the Japanese version of the Cardiovascular Health Study (CHS) criteria, which comprises five domains (weight loss, weakness, slowness, exhaustion and low activity) to assess frailty15; however, participants corresponding to frailty were not included in the study. Takayanagi et al13 used the Kihon checklist (KCL),16 which comprises 25 questions to assess frailty; however, participants with frailty were excluded from the study. These studies, which recruited participants from a cohort study involving community-dwelling older adults and measured DWS using a smartphone application or an accelerometer, could not include frail participants because the participants were required to go to the survey venue or designated location for collecting and uploading the data, which may be difficult for frail participants. Soltani et al17 recently reported the discriminability of DWS for frailty; however, the frailty definition included only the body mass index and handgrip strength and was limited to weight loss and weakness.
We customised the DWS application for examining certain health indicators by using a chatbot to measure frailty without going to a designated location. This study aimed to examine whether frailty can be assessed using this application and elucidate the association between DWS and frailty.
Methods
Participants
This cross-sectional study was conducted in three prefectures, Kanagawa, Saitama and Tokyo, in Japan. These are neighbouring prefectures, and the environmental characteristics of the regions are similar. The participants were recruited by sending letters to house owners aged 55 years or older who lived in a house provided by a housemaker. The housemakers solicited their participation in the research, which aimed at promoting frailty prevention using the smartphone application. The letters were sent twice to recruit as many participants as possible. The participants accessed the download site using the QR code printed on the invitation letter and downloaded the DWS measurement application on their smartphones after reading the study documentation displayed on the site and consenting to participate in the study. The application was limited to Android smartphones. Individuals were included in this study if they habitually used a smartphone, could walk independently, and were not recommended restricted physical exercises by a doctor. We did not examine whether participants received help downloading or operating the application. The sample size was planned to be n=34 for frailty group and n=100 for prefrailty and robust group, assuming that the ratio of frailty to prefrailty and robust is 1: 3 with effect size d=0.5 and a power of 0.8.
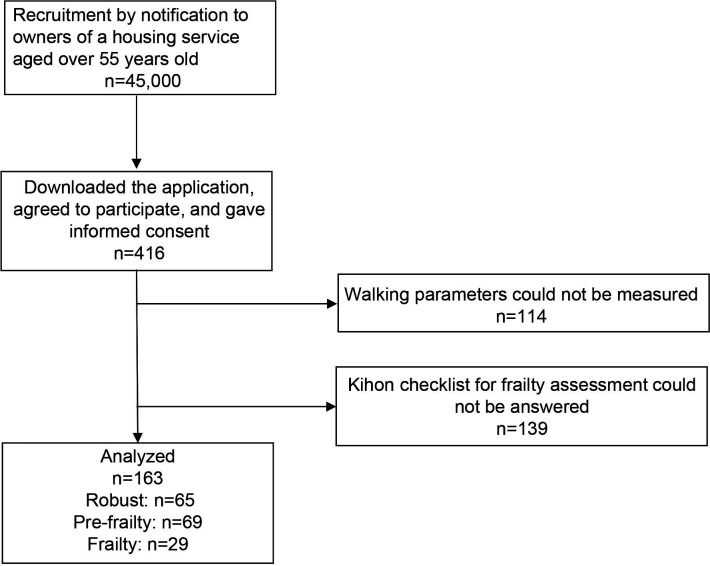
Between August 2020 and January 2021, 416 participants downloaded the application. Among them, 163 participants who could measure DWS and frailty were included in the analysis (figure 1).
Figure 1.
Flowchart of the study participation.
Patient and public involvement
No patient involved.
Measurement of daily walking parameters
Daily walking (DW) parameters, such as walking speed, step length and cadence during daily life, were measured using a smartphone application (Chami, InfoDeliver, Tokyo, Japan). The application automatically measured DWS in a manner imperceptible to the participants. The walking start time was determined by the pedometer application programming interface (API) response in the smartphone operating system and geomagnetic sensors installed in the smartphone. A stable walking trajectory was detected from position information acquired by the smartphone GPS during walking using the linear least squares method (patent number: WO2016043081).7 When the pedometer API and GPS detected a stable walking trajectory ≥20 m, the walking speed was measured until interrupted. The use of GPS implied that measurements were limited to outdoor walking. GPS measurements may be difficult to obtain because of buildings and terrain types, including outdoors. However, this problem was overcome using all positions measured by the GPS during walking and using the average value of walking speed measured multiple times a day, rather than using only the beginning and ending positions.
Although walking speed in daily life can change depending on the environment and situation of walking; however, in our previous study,11 we showed that walking speed measured using this application multiple times in daily life has a single-peaked normal distribution. Therefore, we defined the average of the walking speed measured in daily life as DWS and reported on the excellent test–retest reliability of DWS.7 This application was used in a study on the changes in walking behaviour due to the COVID-19 pandemic18 and a study on seasonal changes in DWS.19
The application can measure the DW step length and cadence from the number of steps on the step counter in addition to walking speed. We defined the average values during the measurement period as DWS, DW step length and DW cadence. The DW step length modified by body height was also calculated. The MDC95 for DWS, DW step length and DW cadence in our previous study7 was 0.101 m/s, 5.662 step/min and 3.498 cm, respectively.
Frailty assessment
Frailty was assessed using KCL, which consists of 25 questions and has been validated using the Japanese version of the CHS criteria for frailty assessment.16 The KCL is a simple yes/no questionnaire that assesses multiple aspects of physical, oral, cognitive and psychosocial functions. Total KCL score was significantly associated with prefrailty and frailty based on the CHS criteria in the previous study. Further, this study showed that prefrailty and frailty by KCL can predict the incidence of 3-year dependency and mortality in older adults.16 According to the study, scores of ≥8, 4–7 and 0–3 were evaluated as frail, prefrailty and robust, respectively.
In this study, the text of each question in KCL was displayed in the chatbot programme of the application, and the participants responded by pressing the ‘Yes’ or ‘No’ buttons.
Other measurements
The participants self-reported their height, weight, history of chronic disease (high blood pressure, diabetes, stroke, cancer and heart disease), hip and knee pain complaints, self-rated health (very healthy, healthy enough, not very healthy and not healthy), psychological well-being (WHO-5 Well-Being Index (WHO-5)),20 dietary variety score (DVS)21 and Tokyo Metropolitan Institute of Gerontology Index of Competence (TMIG-IC).22 These questions were also displayed in the chatbot programme, and the participants answered them through the programme.
The WHO-5 is a five-question psychological well-being index; the participants selected one of the five options: 0, no time; 1, some of the time; 2, less than half the time; 3, more than half the time; 4, most of the time and 5, all of the time (total score range: 0–25 points). A higher score reflected better psychological well-being. DVS covers 10 food groups (fish and shellfish, meat, eggs, milk, soybean/soybean products, green and yellow vegetables, potatoes, seaweeds, fruits, and fats and oils). One point was added for consuming items from the food groups almost every day (total score range: 0–10 points). A higher score reflected a more diverse food intake. TMIG-IC is an index of higher functional capacity, consisting of 13 items (0–13 points). A higher score reflected higher functional capacity.
Statistical analysis
The differences between the robust, prefrailty and frailty groups for all variables were examined using one-way analysis of variance for continuous variables and the χ2 test for categorical variables. We conducted logistic regression analysis using the prefrailty and robust versus frailty as the dependent variable. We examined the Crude model (each DW parameter), model 1 (adjusted for the number of steps) and model 2 (model 1+age, sex and the number of chronic diseases) to assess the associations between each DW parameter and frailty. SPSS V.27.0 J (IBM Japan) was used for all statistical analyses, and the significance level was set at 5%.
Results
The mean age (SD, range) of the participants was 72.1 (6.85, 57–93) years. There were 163 participants in the study, with 65 participants in the robust group, 69 in the prefrailty group and 29 in the frailty group (table 1). Height, weight, history of stroke, knee pain complaints, self-rated health, KCL, WHO-5 and DW step length had a significant main effect between the robust, prefrailty, and frailty groups.
Table 1.
Characteristics of the participants
| Robust (n=65) | Prefrailty (n=69) | Frailty (n=29) | P value* |
|||||||
| n | (%) | n | (%) | n | (%) | |||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| Sex (female) | 24 | (36.9) | 21 | (30.4) | 14 | (48.3) | 0.242 | |||
| Age (years) | 65 | 70.5 | 5.67 | 69 | 73.2 | 6.87 | 29 | 72.6 | 8.62 | 0.070 |
| Height (cm) | 59 | 162.7 | 6.93 | 67 | 163.5 | 7.28 | 27 | 158.3 | 8.72 | 0.009 |
| Weight (kg) | 58 | 59.8 | 8.96 | 68 | 59.8 | 8.11 | 26 | 54.8 | 10.13 | 0.031 |
| Chronic disease | ||||||||||
| Hypertension | 23 | (35.9) | 23 | (33.3) | 13 | (44.8) | 0.556 | |||
| Diabetes | 11 | (17.2) | 8 | (11.6) | 3 | (10.3) | 0.549 | |||
| Stroke | 0 | 0 | 0 | 0 | 2 | (6.9) | 0.01 | |||
| Cancer | 7 | (10.9) | 5 | (7.2) | 3 | (10.3) | 0.745 | |||
| Heart disease | 7 | (10.9) | 6 | (8.7) | 2 | (6.9) | 0.805 | |||
| Hip pain | 23 | (35.9) | 22 | (31.9) | 10 | (34.5) | 0.883 | |||
| Knee pain | 9 | (14.1) | 16 | (23.2) | 11 | (37.9) | 0.036 | |||
| Self-rated health | 0.001 | |||||||||
| Very healthy | 8 | (12.5) | 7 | (10.1) | 1 | (3.4) | ||||
| Healthy enough | 52 | (81.3) | 53 | (76.8) | 16 | (55.2) | ||||
| Not very healthy | 3 | (4.7) | 8 | (11.6) | 8 | (27.6) | ||||
| Not healthy | 1 | (1.6) | 1 | (1.4) | 4 | (13.8) | ||||
| Health assessment | ||||||||||
| KCL | 65 | 1.9 | 1.01 | 69 | 5.3 | 1.04 | 29 | 10.6 | 2.4 | <0.001 |
| WHO-5 | 54 | 18.2 | 3.65 | 56 | 15.6 | 4.73 | 20 | 11.7 | 4.01 | <0.001 |
| DVS | 32 | 5.5 | 1.95 | 23 | 4.9 | 1.93 | 11 | 4.8 | 1.72 | 0.388 |
| TMIG-IC | 35 | 12.3 | 1.05 | 29 | 11.8 | 0.83 | 11 | 11.7 | 1.01 | 0.062 |
| Daily walking parameters | ||||||||||
| DWS (m/s) | 65 | 1.27 | 0.12 | 69 | 1.25 | 0.14 | 29 | 1.19 | 0.21 | 0.06 |
| DW step length (cm) | 65 | 66.4 | 5.26 | 69 | 65.9 | 5.74 | 29 | 62.3 | 8.35 | 0.009 |
| DW step length/height (%) | 59 | 40.8 | 2.58 | 67 | 40.4 | 3.42 | 27 | 39.8 | 4.13 | 0.397 |
| DW cadence (step/min) | 65 | 115.1 | 7.34 | 69 | 114.5 | 7.61 | 29 | 114.6 | 8.69 | 0.913 |
| Number of steps (steps/day) | 65 | 2427.1 | 1815.08 | 69 | 2699.5 | 2799.45 | 29 | 2810.6 | 2416.51 | 0.71 |
| Number of measurements | 65 | 1468.7 | 1361.74 | 69 | 1478.8 | 1683.49 | 29 | 1290.7 | 1170.59 | 0.832 |
*One-way analysis of variance or χ2 test. Number in bold indicate statistically significance (p<0.05).
DVS, Dietary Variety Score; DW, daily life walking; DWS, daily life walking speed; KCL, Kihon checklist; TMIG-IC, Tokyo Metropolitan Institute of Gerontology Index of Competence.
The participants in the frailty group were significantly shorter in height and lighter in weight than those in the prefrailty and robust groups (p<0.01). Further, the participants in the frailty group had a significantly higher frequency of stroke history (p<0.01) and knee pain complaints (p<0.05) than those in the prefrailty and robust groups. Compared with the prefrailty and robust groups, the proportion of those who were not very healthy and not healthy per the self-rated health was significantly higher in the frailty group (p<0.001). WHO-5 score was significantly lower in the frailty group than in the prefrailty and robust groups (p<0.001). The DW step length was significantly smaller in the frailty group than in the robust group (p<0.01). DWS tended to be slower in the frailty group than in the prefrailty and robust groups (p=0.060). No significant differences in DW step length modified by body height were observed between the frailty and robust groups. Logistic regression analysis for all three models (Crude model, model 1 and model 2) revealed the same tendencies: DWS and DW step length were significantly associated with frailty (table 2).
Table 2.
Logistic regression analysis with frailty as the dependent variable and each DW parameter as independent variables
| Crude model | Model 1 | Model 2 | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| DWS (m/s) | 0.024 | 0.001 to 0.571 | 0.012 | 0.000 to 0.368 | 0.022 | 0.001 to 0.784 |
| DW step length (cm) | 0.902 | 0.842 to 0.967 | 0.895 | 0.832 to 0.962 | 0.907 | 0.841 to 0.978 |
| DW cadence (step/min) | 0.997 | 0.946 to 1.051 | 0.994 | 0.941 to 1.050 | 0.999 | 0.941 to 1.061 |
Model 1: adjusted for the number of steps; Model 2: model 1+adjusted for age, sex and the number of chronic diseases.
Numbers in bold font are statistically significant (p<0.05).
DW, daily life walking; DWS, daily life walking speed.
Discussion
This study examined whether DWS is associated with frailty using a smartphone application. Since some previous studies on DWS could not include a sufficient number of participants with frailty, only the association with prefrailty was reported. However, in this study, frailty was assessed using a web-based smartphone application, and frail participants were included. The results showed that the DW step length was smaller, and DWS tended to be lesser in the frailty group compared with the robust group.
The study participants were house owners residing in houses provided by a private housemaker. This housing service provides an urban detached house, suggesting that the residents may be those whose socioeconomic statuses were higher than those of community-dwelling older adults. Additionally, the participants were those who could use a smartphone since they could access the QR code. In our previous study, we found that smartphone-based study participants were younger, had a higher physical function, and were healthier than non-participants.10 Only 0.9% of the 45 000 participants who downloaded the application received an invitation letter. The number of individuals who read the study document in the letter may have been even fewer. The participants should be interested in health information.
However, the mean age of the participants in this study, which included 29 (17.8%) frail participants, was not significantly different from that of the participants in the community cohort. To recruit more participants, this study invited people aged ≥55 years. Although participants under 60 years were included in the analysis, there were only two participants aged 57 and 59. Therefore, almost all participants were older individuals. A previous study in which frailty was assessed using KCL, similar to that in this study, from a large cohort of more than 5000 community-dwelling older individuals, reported that the prevalence of frailty was 17.2%, which was also similar to that in this study.16 In addition, the DWS (1.25 m/s) in this study was not significantly different from that reported in the previous study (1.28 m/s), measured using a smartphone application in the community cohort.10 Therefore, the participants of this study probably had good socioeconomic status and could use a smartphone; however, these participants were considered similar to those recruited from the community from the frailty and DWS perspectives.
KCL is usually examined using a self-administered questionnaire. In this study, the participants entered KCL using a smartphone application, and frailty was assessed from the recorded data. As described above, since the prevalence of frailty in this study was similar to that of a previous study in which frailty was assessed using a self-administered questionnaire in the community cohort, we believe that frailty can be assessed using a smartphone application.
Additionally, there was a significant difference between the robust, prefrailty and frailty groups in height, weight, history of stroke, knee pain complaint, self-rated health, KCL and WHO-5, indicating a reasonable result that reflects frailty. However, statistical differences in DVS and TMIG-IC reported to be associated with frailty21 and decline with age23 were not found between the groups. KCL consists of 25 items. Although there were more items than in other questionnaires, KCL items were asked in the first half of the conversation with the chatbot, and priority was given to assessing frailty. However, there were many questions, such as the 10 items for DVS and 13 items for TMIG-IC, which were asked in the latter half of the conversation. Consequently, the number of participants who responded to the questionnaire was lower than those who provided KCL information. This lack of statistical power might explain why no statistical differences were observed in DVS and TMIG-IC between the robust and frailty groups. Thus, future research with a larger number of participants is required. Overall, we believe that the frailty assessment in this study using the application would be appropriate.
DWS tended to be slower in the frailty group than those in the prefrailty and robust groups; however, the difference was not statistically significant. In contrast, the DW step length was significantly smaller in the frailty group than those in the prefrailty and robust groups. The significantly smaller DW step length in the frailty group could decrease the DWS in the frailty group; however, the difference in DWS between the robust and frailty groups was 0.08 m/s, which was smaller than 0.101 m/s for MDC95 of DWS.7 Therefore, the statistical power in this study may be slightly insufficient to detect this difference. Additionally, since the DW step length modified by body height was similar between the groups, the difference in the DW step length between the groups must be caused by the difference in body height. However, body size is one of the unmodifiable features of frailty in older adults. Thus, we did not adjust for body height in the logistic regression analysis. The logistic regression analysis of the three models revealed that DWS was significantly associated with frailty. Therefore, we believe that DWS is an important factor associated with frailty.
Limitations
This study has some limitations. The participants were not randomly selected but were those who could use a smartphone and were interested in frailty prevention and health promotion. The participation rate has been low since the application was limited to Android smartphones. Participation in this study might have been biased toward healthy individuals rather than being representative of community-dwelling older adults. However, since the participants did not have to go to the designated place and could answer the KCL through the application, this study included participants with frailty with a prevalence similar to that in the community.
DWS varies according to sex and age.11 However, subgroup analysis could not be conducted in this study because of the small number of participants with frailty. An analysis stratified for sex and age will be necessary in the future. Since DWS was measured using an application based on GPS, it was limited to outdoor measurements. Further studies are needed on the association between DWS measured indoors and frailty. It may be necessary to maintain cognitive function to measure DWS and assess frailty using a smartphone application; however, cognitive function was not measured in this study. We also did not examine other possible covariates that may affect DWS, such as visual impairment, fear of falling and walking aids. Since this study had a cross-sectional design, the predictability of DWS for future frailty occurrence is unclear. Future studies, including more representative large samples, are needed.
Supplementary Material
Acknowledgments
We are grateful to the individuals who participated in this study. We also thank Tomoketsu Senri from InfoDeliver Co., Ltd., and Kaori Ito and Kyoji Yamada from Asahi Kasei Homes Corporation for their cooperation in this study.
Footnotes
Contributors: HK contributed to the conceptualisation, methodology, visualisation, investigation, writing - original draft. SO contributed to the conceptualisation, writing - review and editing, supervision, project administration. ME contributed to the data curation, writing - review and editing. KI contributed to the visualisation, investigation, writing - review and editing. HK is the guarantor of this study.
Funding: This work was supported by the Japanese Standards Association (N/A), JSPS KAKENHI (grant number: 20 K12751) and the joint research fund with Asahi Kasei Homes Corporation (N/A).
Competing interests: This study was funded by Asahi Kasei Homes Corporation. There are no other conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The datasets analysed in this study are not publicly available due to intellectual property rights, but are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the ethics committee of the Tokyo Metropolitan Institute of Gerontology (approval number: 2020-5). Participants gave informed consent to participate in the study before taking part.
References
- 1.Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci 2016;71:63–71. 10.1093/gerona/glv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;305:50–8. 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veronese N, Stubbs B, Volpato S, et al. Association between gait speed with mortality, cardiovascular disease and cancer: a systematic review and meta-analysis of prospective cohort studies. J Am Med Dir Assoc 2018;19:981–8. 10.1016/j.jamda.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 4.Fritz S, Lusardi M. White Paper: “Walking Speed: the Sixth Vital Sign”. Journal of Geriatric Physical Therapy 2009;32:2–5. 10.1519/00139143-200932020-00002 [DOI] [PubMed] [Google Scholar]
- 5.Schimpl M, Lederer C, Daumer M. Development and validation of a new method to measure walking speed in free-living environments using the actibelt® platform. PLoS One 2011;6:e23080. 10.1371/journal.pone.0023080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takayanagi N, Sudo M, Yamashiro Y, et al. Relationship between daily and in-laboratory gait speed among healthy community-dwelling older adults. Sci Rep 2019;9:3496. 10.1038/s41598-019-39695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obuchi SP, Tsuchiya S, Kawai H. Test-Retest reliability of daily life gait speed as measured by smartphone global positioning system. Gait Posture 2018;61:282–6. 10.1016/j.gaitpost.2018.01.029 [DOI] [PubMed] [Google Scholar]
- 8.Rojer AGM, Coni A, Mellone S, et al. Robustness of in-laboratory and daily-life gait speed measures over one year in high functioning 61- to 70-year-old adults. Gerontology 2021;67:650–9. 10.1159/000514150 [DOI] [PubMed] [Google Scholar]
- 9.Van Ancum JM, van Schooten KS, Jonkman NH, et al. Gait speed assessed by a 4-m walk test is not representative of daily-life gait speed in community-dwelling adults. Maturitas 2019;121:28–34. 10.1016/j.maturitas.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 10.Kawai H, Obuchi S, Watanabe Y, et al. Association between daily living walking speed and walking speed in laboratory settings in healthy older adults. Int J Environ Res Public Health 2020;17:2707. 10.3390/ijerph17082707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obuchi SP, Kawai H, Murakawa K. Reference value on daily living walking parameters among Japanese adults. Geriatr Gerontol Int 2020;20:664–9. 10.1111/ggi.13931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai H, Obuchi S, Hirayama R, et al. Intra-day variation in daily outdoor walking speed among community-dwelling older adults. BMC Geriatr 2021;21:417. 10.1186/s12877-021-02349-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayanagi N, Sudo M, Yamashiro Y, et al. Screening prefrailty in Japanese community-dwelling older adults with daily gait speed and number of steps via tri-axial accelerometers. Sci Rep 2021;11:18673. 10.1038/s41598-021-98286-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–7. 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 16.Satake S, Shimokata H, Senda K, et al. Validity of total Kihon checklist score for predicting the incidence of 3-year dependency and mortality in a community-dwelling older population. J Am Med Dir Assoc 2017;18:552.e1–552.e6. 10.1016/j.jamda.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 17.Soltani A, Abolhassani N, Marques-Vidal P, et al. Real-World gait speed estimation, frailty and handgrip strength: a cohort-based study. Sci Rep 2021;11:18966. 10.1038/s41598-021-98359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obuchi SP, Kawai H, Ejiri M, et al. Change in outdoor walking behavior during the coronavirus disease pandemic in Japan: a longitudinal study. Gait Posture 2021;88:42–6. 10.1016/j.gaitpost.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obuchi SP, Kawai H, Garbalosa JC, et al. Walking is regulated by environmental temperature. Sci Rep 2021;11:12136. 10.1038/s41598-021-91633-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topp CW, Østergaard SD, Søndergaard S, et al. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom 2015;84:167–76. 10.1159/000376585 [DOI] [PubMed] [Google Scholar]
- 21.Motokawa K, Watanabe Y, Edahiro A, et al. Frailty severity and dietary variety in Japanese older persons: a cross-sectional study. J Nutr Health Aging 2018;22:451–6. 10.1007/s12603-018-1000-1 [DOI] [PubMed] [Google Scholar]
- 22.Koyano W, Shibata H, Nakazato K, et al. Measurement of competence: reliability and validity of the TMIG index of competence. Arch Gerontol Geriatr 1991;13:103–16. 10.1016/0167-4943(91)90053-S [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi Y, Kitamura A, Nofuji Y, et al. Association of trajectories of higher-level functional capacity with mortality and medical and long-term care costs among community-dwelling older Japanese. J Gerontol A Biol Sci Med Sci 2019;74:211–8. 10.1093/gerona/gly024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The datasets analysed in this study are not publicly available due to intellectual property rights, but are available upon reasonable request.