Abstract
Introduction
Intraperitoneal dissemination is a major problem resulting in very poor prognosis and a rapid marked deterioration in the quality of life of patients. Pressurised intraperitoneal aerosol chemotherapy (PIPAC) is an emergent laparoscopic procedure aiming to maximise local efficacy and to reduce systemic side effects.
Methods and analysis
Nab-PIPAC, a bicentre open-label phase IB, aims to evaluate safety of nab-paclitaxel and cisplatin association using in patients with peritoneal carcinomatosis (PC) of gastric, pancreatic or ovarian origin as ≥1 prior line of systemic therapy. Using a 3+3 design, sequential intraperitoneal laparoscopic application of nab-paclitaxel (7.5, 15, 25, 37.5, 52.5 and 70 mg/m2) and cisplatin (10.5 mg/m2) through a nebuliser to a high-pressure injector at ambient temperature with a maximal upstream pressure of 300 psi. Treatment maintained for 30 min at a pressure of 12 mm Hg and repeated4–6 weeks intervals for three courses total.
A total of 6–36 patients are expected, accrual is ongoing. Results are expected in 2024.
The primary objective of Nab-PIPAC trial is to assess tolerability and safety of nab-paclitaxel and cisplatin combination administered intraperitoneally by PIPAC in patients with PC of gastric, pancreatic or ovarian origin. This study will determine maximum tolerated dose and provide pharmacokinetic data.
Ethic and dissemination
Ethical approval was obtained from the ethical committees of Geneva and Vaud (CCER-2018-01327). The study findings will be published in an open-access, peer-reviewed journal and presented at relevant conferences and research meetings.
Trial registration number
Keywords: Gynaecological oncology, Adult oncology, Gastrointestinal tumours
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first study that evaluate pressurised intraperitoneal aerosol of nab-paclitaxel and cisplatin intraperitoneal administration; it will determine maximum tolerated dose and provide pharmacokinetic data.
This study includes quality-of-life assessments to investigate the clinical benefit of pressurised intraperitoneal aerosol chemotherapy (PIPAC).
Within this population, the dose-limiting toxicity assessment is challenged by peritoneal carcinomatosis (PC) symptoms.
The efficacy assessment will be limited by small sample size and heterogeneity in tumour’s organ origin of participants.
The study includes a translational research programme to characterise longitudinal changes induced by PIPAC on tumour immune microenvironment in patients with PC.
Introduction
Intraperitoneal (IP) dissemination of malignant tumours is a major problem in the management of digestive and gynaecological cancers resulting in very poor prognosis and a rapid marked deterioration in the quality of life of these patients. Malignancies most likely to spread to the peritoneum include ovarian (60%–70%), gastric (15%–43%), colorectal (8%–25%), pancreatic, peritoneal mesothelioma and pseudomyxoma peritonei.1
Maintaining the quality of life of patients in palliative oncology is of great importance. Surgical and/or systemic treatments (intravenous chemotherapy) have limited efficacy in the palliation of symptoms related to peritoneal carcinomatosis (PC) at the cost of systemic toxicities, which are usually significant. Intravenous chemotherapy efficacy is usually short lived due to poor penetration into the peritoneal cavity. The role of IP chemotherapy is to maximise tumour penetration and optimise cell death while minimising systemic toxicity.2 IP chemotherapy is a recommended treatment for epithelial ovarian cancer in combination with maximal cytoreductive surgery and is the standard treatment of pseudomyxoma peritonei.3–5 It has also been studied in several cancers of digestive origin.6
Pressurised intraperitoneal aerosol chemotherapy (PIPAC) is a laparoscopic procedure used for the IP application of a pressurised aerolisation of chemotherapy, hence optimising therapeutic ratio of the substance administered between local and systemic concentrations, resulting in an improvement of local efficacy and reduction of systemic toxicity. Its application could be repeated at a 4–6 weeks’ interval.7 A phase I study aiming to report the maximal tolerated dose (MTD) of cisplatin and doxorubicin administered intraperitoneally by PIPAC has found that dose level (DL) of 10.5 mg/m2 and 2.1 mg/m2 of cisplatin and doxorubicin, respectively.8 A second phase 1 conducted in patients suffering from PC of gastrointestinal origin evaluated the safety and efficacy of oxaliplatin administered by PIPAC and found recommended phase II dose is 120 mg/m2; of note, in this study, 12.5% (3/24) patients developed acute pancreatitis as dose-limiting toxicity (DLT).8 9 Overall, PIPAC was shown to be feasible and safe in patients with refractory carcinomatosis of various origins, with a low incidence of reported serious adverse events (SAEs) (2%–15%) and,10 surgery-related complications (12%)11–13 and meaningful clinical benefit and histological response rate.11–13 Laparoscopic access and repeatability were 83%–100% and 38%–82%, respectively.14 PIPAC was followed by a modest and transitory inflammatory response, no haematological, renal or hepatic toxicity were observed even after repetitive administration.15 Quality of life and symptoms were not impacted by PIPAC therapy.16 The available evidence on PIPAC was summarised by a systematic review confirming its feasibility and tolerance profile.17 With a standardised surgical approach and dedicated safety checklist, PIPAC could be safely introduced in clinical routine with minimal learning curve.18 19 The overall tumour response ranged between 40% and 75% in PC of ovarian and gastric origin with three successive PIPAC cycles with cisplatin and doxorubicin.11–13 Additionally, practice of this new drug administration method was studied within an international expert panel showing excellent standardisation of PIPAC among expert centres opening the door for registries and multi-centre studies.20
Classical chemotherapy components used intravenously for ovarian, gastric and pancreatic neoplasias belong to the taxane and platin cytostatic families. Nab-paclitaxel is a nanoparticle albumin-bound formulation of paclitaxel specifically designed to overcome the limitations of conventional paclitaxel formulations, including the barriers to effective drug delivery of highly lipophilic agents.21 Nab-paclitaxel has fewer side effects, shows increased tumour cell cytotoxicity, and patients have higher overall response rates, compared with equal doses of solvent-based paclitaxel in many solid malignancies.22 It has been studied intravenously in many solid tumours, including four phases II clinical trials for recurrent ovarian cancer23–26 and in two phases II and a phase III trial for recurrent gastric adenocarcinoma.27–30 IP administration of paclitaxel is a standard therapy for advanced epithelial ovarian carcinoma in North America,31 but the IP administration of nab-paclitaxel has been little studied. IP administration of nab-paclitaxel has been evaluated in phase I trial in 27 patients with gynaecological and digestive PC, with an IP MTD of 140 mg/m2. IP administration of nab-paclitaxel showed higher (~150 fold) peritoneal exposure to the drug compared with the plasma exposure with a low interpatient and intrapatient variability.32
Preclinical reports have shown that nab-paclitaxel has an enhanced antitumoural activity due to its internalisation through macropinocytosis by the macrophages of the tumour environment (tumour-associated macrophages, TAMs) leading to antitumoural immunomodulatory effect.33 Macropinocytosis is a form of endocytosis in which a large fluid-filled vesicle is pinched off from the cell membrane and brought into the interior of the cell. This is particulary relevant as PC from tumours highly infiltrated by TAMs have an especially poor prognosis, this hold true for pancreatic, gastric and ovarian carcinoma.34–36 TAMs may be polarised in two phenotypes: type M1 or type M2. TAMs of the M2 phenotype are known to promote tumour proliferation by suppressing antitumour immune reactions and inducing angiogenesis and are associated with a poor prognosis in numerous cancers.34 On the contrary, a ratio favouring type M1 TAMs confers a better prognosis.37 A mechanism of action of nab-paclitaxel recently identified in preclinical models of pancreatic cancers is its capacity to polarise TAMs towards M1 activation state. Nab-paclitaxel-mediated M1 induction might result in a positive feedback signalling, further promoting uptake of drug and enhancing its M1-activating effects in autocrine and paracrine fashions.22
We hypothesised that nab-paclitaxel could be a good candidate for IP administration by PIPAC in patients with PC, as this route allows reduced systemic toxicity and increased intratumoural drug concentration. We expect that this local intervention might rebalance favourably PC immune environment, leading to a prolonged local control and potentially a survival benefit. As PIPAC procedure is commonly repeated three times, collecting PC samples before each procedure is a unique opportunity for longitudinal studies of changes in peritoneal tumour immune microenvironment on exposure to in situ therapy.
Methods and analysis
Trial design
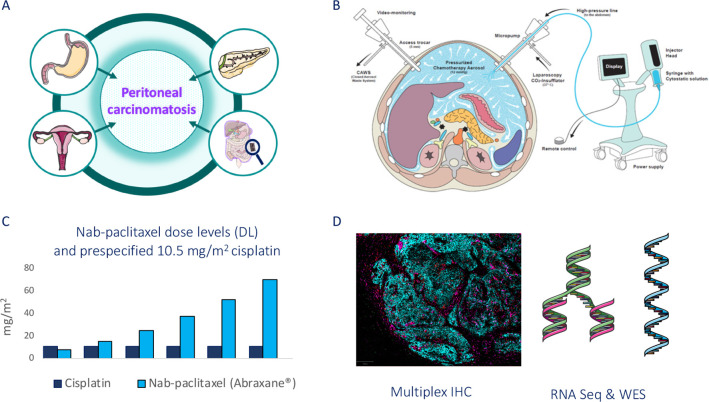
It is a prospective sequential open-label non-randomised multicentric conventional phase IB with a single dose escalation of the investigational drug (nab-paclitaxel, Abraxane) performed in association with a prespecified cisplatin dose administered intraperitoneally by PIPAC (figure 1).38
Figure 1.
Nab-PIPAC study design. (A) Peritoneal carcinomatosis from oesogastric, pancreatic, ovarian origin and primitive peritoneal mesothelioma; (B) PIPAC procedure; (C) 3+3 escalating dose level (DL) design; (D) translational research using multiplex immunohistochemistry (IHC), RNA sequnecing and whole exome sequencing. This figure was partly generated using PIPAC schema published in Hübner, M., et al.47 and Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license. PIPAC, pressurised intraperitoneal aerosol chemotherapy.
Study population and recruitment
This study is intended for patients with peritoneal carcinomas from neoplasias known to be sensitive to platin and/or taxane chemotherapy, who are in a palliative situation, due to peritoneal metastatic spreading, but still in good shape and would offer them an additional therapy that might improve their quality of life and potentially their survival. According to inclusion and exclusion criteria (table 1), the study population will include all voluntary patients aged >18 years, psychologically and physically able to follow the trial procedures and to give a written informed consent, suffering from PC, with limited extraperitoneal metastases from pancreatic, oesogastric, ovarian cancers or primitive peritoneal mesothelioma, for whom standard therapies have been exhausted, or not feasible, or having residual disease following first line of therapy.
Table 1.
Inclusion/exclusion criteria for all participants
| Inclusion | Exclusion |
| Informed consent as documented by signature | Predominant extraperitoneal metastases at the discretion of the study team after discussion at the multidisciplinary tumour board |
| Age ≥18 years | Bowel obstruction, active gastroduodenal ulcer or ongoing abdominal infection (bacterial, viral or fungal) |
| Who are psychologically able to follow the trial procedures | Chemotherapy or surgery within the last 2 weeks prior to enrolment |
| With peritoneal carcinomatosis from pancreatic, oesogastric, epithelial ovarian cancers or primitive peritoneal mesothelioma | General or local (abdominal) contra-indications for laparoscopic surgery |
| Not candidate for surgical cytoreduction and IP/HIPEC based on expert multidisciplinary board | Known allergy to cisplatin or other platinum-containing compounds or to compounds of similar chemical or biological composition of nab-paclitaxel |
| Who received at least one line of chemotherapy and for whom standard therapies have been exhausted or not feasible. Patients with residual disease following the first line of therapy or following secondary debulking are eligible. | Severe organ dysfunction including: renal impairment (calculated GFR <60 mL/min/1.73 m2); myelosuppression (platelet count <100 x10∧9/L, haemoglobin <90 g/L, neutrophil granulocytes <1.500 /mL); INR ≥2; hepatic impairment (serum total bilirubin ≥1.5 mg/dL, AST/ALT >1.5 x ULN); severe respiratory or neurological impairment (grade 3); severe myocardial insufficiency (NYHA class >2), recent myocardial infarction, severe arrhythmias |
| ECOG 0, 1 or 2 | Pregnancy or breastfeeding, women who can become pregnant must ensure effective contraception |
| Life expectancy >3 months | Known or suspected non-compliance, inability to follow the procedures of the study, for example, due to language problems, psychological disorders, dementia, etc of the participant |
| History or current evidence of any condition, therapy, or laboratory abnormality that might confound the results of the trial, interfere with the subject’s participation for the full duration of the trial, or is not in the best interest of the subject to participate, in the opinion of the treating investigator. |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECOG, Eastern Cooperative Oncology Group performance status scale; GFR, glomerular filtration rate; HIPEC, hyperthermic intraperitoneal chemotherapy; INR, international normalised ratio; IP, intraperintoaneal; NYHA, New York Heart Association functional classification; ULN, upper limit of normal.
Recruitment of voluntary participants will be done during the oncological multidisciplinary tumour board (gynaeco-oncology and gastrointestinal oncology), the oncological clinic and referral from private practice and other hospitals. Enrolment started in 2021 with 6 patients enrolled in the two first DL, expected trial completion year is 2023.
Study location
This study will be conducted at the Hôpitaux Universitaires de Genève (HUG) and Centre Hospitalier Universitaire Vaudois, Switzerland.
Determination of sample size
The study design consists in a modified Fibonacci sequence (nab-paclitaxel dose increase by 100%, 67%, 50%, 40% and 33% for all the rest): 7.5 mg/m2, 15 mg/m2, 25 mg/m2, 37.5 mg/m2, 52.5 mg/m2 and 70 mg/m2. Three patients are treated at each DL until the first DLT in the first cycle of treatment (defined as grade 3 or 4, CTCAE version 5.039) occurred.
If one patient among the three of the first cohort experiences a DLT within 4 weeks from the first cycle of PIPAC, then an additional cohort of three patients is treated at the same DL. If no patient out of three or one patient out of six experiences a DLT within 4 weeks from the first cycle of PIPAC, the dose is escalated. The MTD is (MDT) defined as the lowest DL at which ≥33% (≥ 2/6) subjects experienced a DLT during the first cycle of treatment.40
Within each cohort, a time frame after the first PIPAC procedure will be respected before starting the treatment of the next patient.
In the first cohort of a DL:
The first two patients can be enrolled simultaneously in cycle 1, while the third patient could be included only after at least one of the previous patients has completed the DLT monitoring period without experiencing any DLT.
In case only one patient was initially enrolled: The next two patients of the same cohort can be enrolled simultaneously only after the first patient has completed the DLT reporting period without experiencing a DLT. The next two patients of the same cohort should be enrolled sequentially, if the first patient experienced a DLT.
In the second cohort of a DL:
If no DLT was experienced in the first cohort of three patients: two patients can be enrolled simultaneously, while the third patient should be included only when at least one of the previous patients has completed the DLT monitoring period without experiencing any DLT.
If one DLT was experienced in the first cohort of three patients: the three planned patients of the second cohort should be enrolled sequentially.
According to the occurrence of a DLT, we are expecting enrollment of 6–36 patients.
Study outcomes
Primary
The study seeks primarily to assess short-term safety and tolerability of the IP association of cisplatin and nab-paclitaxel administration by PIPAC and to determine the MTD of nab-paclitaxel administered IP by PIPAC in concomitance with cisplatin. MTD defined as the lowest DL at which ≥33% (≥ 2/6) of patients experience DLT in the first cycle of treatment in accordance to CTCAE criteria version 5.0.39 DLT is defined as any CTCAE grade 3 or 4 AE determined to be possibly, probably or definitely related to nab-paclitaxel and cisplatin IP administration.
DLTs define as:
Haematologic
Febrile neutropenia grade >3 for more than 7 days.
Platelet count decreased grade 3 or 4 for more than 7 days
Thrombocytopenia requiring transfusion.
Non-haematologic
Any grade ≥3 non-haematological trial treatment-emergent AE (TEAE). Exception: non-clinically significant non-haematological laboratory findings.
Any treatment-related AE that leads to a delay of treatment in the start of cycle 2 of >14 days.
Abdominal pain grade ≥3 during more than 7 days and requiring opioides treatment. Pain will be estimated with Visual Analogue scale for pain (VAS). The highest VAS value of the day taken in bed will be recorded in the electronic case report form (eCRF).
AE related to the primary tumour, such as progression of the disease, will not be considered as DLTs.
Documentation of AE and SAE with predefined toxicity criteria will be applied using CTCAE V.5.0 criteria,38 documented before and after the first, second and third course of treatment (D0/D10 of each cycle). Surgical complications will be assessed according to Clavien classification and Comprehensive Complication Index (CCI).41
Secondary
To report pharmacokinetic analysis of free plasmatic concentrations of nab-paclitaxel at predose, end of infusion, H1, H4 and 24 hours after the first PIPAC treatment for the two first patients treated for each new DL.
To evaluate histological regression and objective tumour response rate assessed according to peritoneal regression grade score system (PRGS)42 at D1 of second and third PIPAC cycles. Histological regression will be assessed by pathological review of repeated peritoneal biopsies proceeded during laparoscopy before each PIPAC cycle, according to the new regression system for peritoneal cancer.
To assess the objective response rate and the clinical benefit rate, defined by revised RECIST v.1.1 criteria.43
To evaluate any benefit in QoL assessed by EORTC QLQ-C30 V.3.0 (European Organisation for Research and Treatment of Cancer, quality of life C30 questionnaire) and VAS for pain questionnaires filled by the patient itself before (D0) and after (D10) each cycle of PIPAC application.
Correlatives
To assess predictive relevance and reproducibility of radiological assessment of Peritoneal Carcinomatosis Index (PCI) by abdominal CT enterography at screening and EOT visit, when available.
To evaluate of the impact of locally administered nab-paclitaxel and cisplatin on intratumoural immune response (spatial distribution of immune cell subsets) assessed by multispectral IHC; Assessment of the presence of TAMs (CD68+, CD163+, Tie2+), regulatory T cells (Foxp3+), TILs (CD8+), plasmacytoid dendritic cells (BDCA2+), resident T cells (CD103+), PD-L1/PD1 and other immune cells to be defined.
To quantify gene expression by RNAseq, performed on formalin-fixed paraffin embedded (FFPE) tumour samples. Bioinformatic processing will be based on a standardised pipeline (bwa, edgeR); downstream analysis will include unsupervised hierarchical clustering for the discovery of underlying subgroups, differential expression analysis of matched samples before and during treatment and differential expression analysis between responders and non-responders. Gene expression information can also be used to deconvolute immune infiltrates (for example, CIBERSORT, TIMER), supplementing the immunohistochemical estimates.
To evaluate potential predictive biomarkers using whole-exome sequencing performed on blood and FFPE tumour samples obtained before and during treatment and processed on a standardised pipeline (bcbio) for the identification of pathogenic variants (mutations) and copy number alterations (CNVkit). Mutational signatures can also be derived from exome data and have been associated with distinct biological processes, such as deficient DNA repair. We plan to compare the patterns of responders with non-responders in the hope of identifying candidate biomarkers. In addition, we will examine the changes that might have resulted from exposure to treatment, such as the expansion of potentially resistant clones.
Outcomes of interest include (A) the distribution of gene expression profiles, (B) the gene expression changes during treatment, (C) the gene expression differences between responders and non-responders, (D) mutational patterns (pathogenic variants, mutational signatures) predicting response, (E) subclonal changes in response to treatment, for example expansion of resistant clones and resistance mutations.
Study intervention
Dose rationale
The choice of 10.5 mg/m2 cisplatin dose has been based on the result of the recent phase I escalation dose of cisplatin and doxorubicin association administered IP by PIPAC.8 The MTD dose in the phase I of IP nab-paclitaxel administration by catheterism was 140 mg/m2,32 we are expecting MTD at a lower dose for this phase I as PIPAC has an enhanced activity and penetration than conventional IP treatment. Cisplatin 10.5 mg/m2 body surface in 150 mL NaCl 0,9% (concentration of 1 mg/mL) and nab-paclitaxel (Abraxane) in 150 mL NaCl 0,9% at escalating doses will be applied sequentially through a nebuliser (Capnopen, Capnomed, Villigendorf, Germany) to a high-pressure injector (Accutron HP-D Injecteur, Medtron AG) with a flow rate of 0.6–0.7 mL/s and a median droplet size of 11 (3-15) μm at ambient temperature and at a maximal upstream pressure of 300 psi intraperitoneally. Treatment will be maintained for 30 min after administration at a pressure of 12 mm Hg.
PIPAC will be performed only by gynaecological or gastrointestinal surgeon who has already completed a special training and will be repeated q4–6 week’s intervals for a total of three courses procedure. The length of stay in hospital for the PIPAC procedure is about 4 days. PIPAC procedure will be performed as follow.18
PIPAC procedure
Intervention under general anaesthesia.
Antibiotic prophylaxis with commercial cephalosporine administered during anaesthetic induction.
Introduction of a 5 mm optic after insufflation with Hasson technique.
Insufflation capnoperitoneum at 12 mm Hg and insertion of 1 trocar of 10/12 for the nebuliser, 1 trocar for the camera and one working trocar (ascites removal, biopsies, peritonectomy).
Removal of ascites and documentation of the volume and cytology.
Documentation of the PCI (surgical PCI score and Fagotti score for ovarian carcinoma).44 45
Five punch peritoneal biopsies in the 4 quadrants of the abdomen and 1 peritonectomy of several cm2.
Connection of the nebuliser (Capnopen, Capnomed) to a high-pressure injector (Accutron HP-D Injecteur, Medtron G) and insertion into the abdomen.
Pressurised dose of cisplatin and nab-paclitaxel at escalating doses will be applied via the nebuliser and injector, with a flow rate of 0.6–0.7 mL/s.
Maximal upstream pressure of 300 psi.
Treatment to be maintained for up to 30 min after administration at ambient temperature and a pressure of 12 mm Hg.
Study assessment and schedule
The study schedule is summarised in table 2. Safety will be assessed by the surgeon at each medical visit before (D0) and after (D10) each PIPAC course and at the EOT visit by a dedicated oncology physician, with predefined toxicity criteria which will be documented according to the CTCAE V.5.0 criteria39 and consigned in the patient file and eCRF. Surgical complications will be assessed according to Clavien classification and CCI.41
Table 2.
Study schedule
| Visit no | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Visit name | Screening | C1D0 | C1D1 | C1D2 | C1D3 | C1D10 | C2D0 | C2D1 | C2D2 | C2D3 | C2D10 | C3D0 | C3D1 | C3D2 | C3D3 | C3D10 | CXD56* | Q3M |
| Scheduling window | −28 | −3 | ±3 | −3 | ±3 | ±3 | −3 | ±3 | ±3 | ±7 | ±14 | |||||||
| Patient information and consent | x | |||||||||||||||||
| Demographics | x | |||||||||||||||||
| Medical history | x | |||||||||||||||||
| Inclusion/exclusion criteria | x | |||||||||||||||||
| Physical examination† | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| ECOG score | x | x | x | x | x | x | x | x | ||||||||||
| AE/SAE | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| DLT evaluation‡ | x | |||||||||||||||||
| Concomitant medication | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| Vital signs | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| Haematology§ | x§ | x | x | x | x | x | x | x | ||||||||||
| Serum chemistry§ | x§ | x | x | x | x | x | x | x | ||||||||||
| Coagulation§ | x§ | |||||||||||||||||
| Serum tumour biomarker§ | x§ | x | x | x | ||||||||||||||
| Urine analysis§ | x§ | |||||||||||||||||
| Pregnancy test | (x)¶ | (x) | (x) | (x) | ||||||||||||||
| Chest CT | x | x | ||||||||||||||||
| CT enterography** (CT-PCI score) |
x | X†† | x | |||||||||||||||
| ECG | x | |||||||||||||||||
| Ascite volume | x | x | x | |||||||||||||||
| PIPAC | x | x | x | |||||||||||||||
| Surgical PCI score | x | x | x | |||||||||||||||
| Biopsies (standard and translational) | x | x | x | |||||||||||||||
| Blood sampling (translational research) | x | |||||||||||||||||
| PK (free plasmatic nab-paclitaxel)‡‡ | x | x | ||||||||||||||||
| QoL | x | x | x | x | x | x | x | |||||||||||
| VAS§§ | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| Survival status | x | |||||||||||||||||
| Pathology and Molecular analysis | x | |||||||||||||||||
| Disease status | x |
*To be performed 56 days after the last PIPAC administration.
†Neurological examination with pallesthesia and abdominal circumference only requested at screening visit.
‡For dose-limiting toxicities, refer to study outcomes section.
§To be performed within 10 days prior to registration.
¶To be performed within 7 days prior to registration.
**When available.
††3 days before in order to assess the PCI score before the next administration.
‡‡Blood samples for pharmacokinetic analysis will be performed at the following time points: Predose 30 min (±5 min), end of infusion (±15 min), 1 hour (±15 min), 4 hours (±30 min), 24 hours (±4 hours). Samples collected at the first cycle of PIPAC for the two first patients treated for each dose level escalation.
§§Highest value of the day taken in bed.
AE, adverse event; DLT, dose-limiting toxicity; ECOG, Eastern Cooperative Oncology Group; PCI, Peritoneal Carcinomatosis Index; PIPAC, pressurised intraperitoneal aerosol chemotherapy; QoL, quality of life; SAE, serious AE; VAS, Visual Analogue Scale.
Sequence and duration of all study periods:
Screening phase (S, D-28): will consist in checking that every candidate meets the inclusion criteria and does not have any exclusion criteria. The physician will explain the purpose, the design, the risk and benefit balance of the study and the necessity of a good compliance. All the procedures and tests for the screening phase mentioned in the flow chart must have been completed during the defined interval timeline of 28 following days.
Intervention phase (C1D1, C2D1±3, C3D1±3): During the intervention phase, each participant intends to have three cycles of PIPAC repeated at q4–6 week’s intervals. Before each PIPAC cycle (D0), a preoperative medical visit by the surgeon and laboratory tests will be performed. The estimated duration of the hospitalisation is 4 days. After each cycle of PIPAC, a medical visit will be done at day 10 (D10) to evaluate the toxicity and report any TEAE assessed by using CTCAE criteria V.5.0,38 monitoring of vital signs and laboratory parameters and filling the QoL questionnaires (QLQ-C30 V.3.0 and VAS scale). The highest VAS value of the day taken in bed will be recorded in the eCRF.
End of treatment visit (EOT, CXD56+/−7): will consist of a medical visit done 2 months after the last PIPAC cycle, with assessment of clinical and biological parameters, chest and CT enterography when available. TEAEs and QoL evaluation will be consigned by the physician in patient’s file.
Expected duration of participant’s participation: from the screening phase till the EOT visit, the total participation time will be 6–8 months.
Data handling and monitoring
Data generation, transmission, archiving and analysis of personal data within this study, strictly follows Swiss legal requirements according to the federal law on data security, as well as the regulation on professional secrecy in clinical research. Prerequisite is the voluntary approval of the participant given by signing the informed consent prior start of participation of the clinical trial. Informed consent will be obtained by dedicated treating physicians or physician from the DFDL unit (Department of Oncology, HUG). Health related personal data captured during this project are strictly confidential and accessible only by investigators and authorised personnel. Coding will safeguard participants’ confidentiality. Data management is performed by DFDL unit (Department of Oncology, HUG). Data monitoring is performed by UIC (Unité d’Investigation Clinique), a unit which is part of CRC (Centre de Recherche Clinique/CTU) at HUG and the Faculty of Medicine of Geneva University (UNIGE). UIC is certified ISO 9001/2008, and the unit guarantees best practices in the field of clinical data management. Data are physically stored in a relational database management system, using a deidentified dedicated clinical database management system software (secuTrial). All study documents will be archived on site for the minimum of at least 10 years after study termination. A risk-based monitoring will be conducted by the UIC and the frequency of monitoring visits will be determined by factors such as the frequency of subject visits and the site enrolment rate. On study completion, the sponsor representative will visit the site to conduct a study termination visit. The source data/documents will be accessible to monitors and questions will be answered during monitoring.
Statistical analysis plan
Participant characteristics
Patient characteristics will be tabulated for visual comparison. For quantitative variables, the following descriptive statistics will be given: N, Mean and 95% CI, SD, median andIQR (for non-normally distributed); for qualitative variables, the frequency and percentage of patients within each category will be provided.
Adverse events
TEAEs and SAEs will be summarised by presenting the number and percentage of patients having any AE, having any event by body system and having each individual AE (incidence, relationship to Nab-PIPAC, severity according CTCAE vV.5.0).39 AEs that result in death (other than disease progression), discontinuation or SAEs will be presented separately. Any other information, for example, time of onset, duration, resolution, action to be taken, assessment of intensity, relationship with study treatment will be listed for all participants.
Laboratory parameters
All laboratory results mentioned in the eCRF monitored at each planned visit which are not in line with the laboratory normal ranges and/or the CTCAE V.5.0 criteria39 will be summarised by presenting shift tables using normal ranges, by presenting summary statistics of raw data and changes from baseline values (mean, median, SD, range) and by flagging of notable values in data listings.
Vital signs
Vital signs at baseline and change from baseline will be summarised by changes from baseline values (mean, median, SD, range) and by flagging of notable values in data listings.
The trial will end in case of more than one grade 5 event related to the investigational product or to the study procedure (CTCAE V.5.0).39 Deaths due to progressive disease are not considered as grade 5 events. Patients who will prematurely withdraw from the study will be displayed and summarised by primary reason and treatment. No deviation(s) from the planned analyses will be justified.
A safety report will be performed 16 weeks after the last eligible patient has completed the last third cycle of PIPAC. Intention-to-treat analysis will be performed on all patients who receive at least two cycles of PIPAC. The final efficacy analysis will be performed 1 year after the last eligible patient has completed the last follow-up visit. Survivals will be reported using Kaplan-Meier curves. A final report will be issued at the end of the trial. The statistical analysis will be conducted by a dedicated biostatistician.
Patient and public involvement
No patient involved.
Discussion
Recently, Ceelen et al reported results of their phase 1 evaluating the safety of nab-paclitaxel administration by PIPAC in patients with PC from ovarian, breast, gastric, hepatobiliary or pancreatic origin. In this study, PIPAC was associated to concomitant systemic treatment in 65% of the 21 enrolled patients. Safety results were encouraging, with no major surgical complications or mortality and manageable haematological toxicity. Unless patients have known hepatobiliary functional impairment, the MTD and recommended phase II dose was defined as 140 mg/m2. Overall response rate according to PRGS was 35% (7/21) with stable disease present in 35% (7/21).46
In comparison to the phase I reported by Ceelen et al, our study varies in its inclusion criteria, definition of DLT and design. For instance, they excluded fatigue and abdominal symptoms (nausea and abdominal pain) from their definition of DLT, while we consider any grade ≥3 non-haematological TEAEs, including abdominal pain as a DLT. Further, they allowed systemic chemotherapy prior to and in between two PIPAC cycles, which is not the case in our study. Finally, their study investigated nab-paclitaxel monotherapy while we combine it with cisplatin. Such differences in the design are expected to lead to differences in the DLT between both studies.
Registration and categorisation of study
The study is registered at www.clinicaltrials.gov (NCT04000906). Swiss National Clinical trial Portal (SNCTP000003129 via BASEC).
The clinical trial comes under category B (clinical trials of medicinal products).
Data sharing will follow ICMJE statements. All individual deidentified participant data collected during the trial (including data dictionary) will be shared following publication, no end date. Data will be shared for meta-analysis or any academic purpose. Related documents will be available (study protocol, ICF).
Supplementary Material
Footnotes
Contributors: NL conceptualised the original study and drafted the manuscript. MH, IL-G, MU, MDM and FR contributed to refining the study design. MH, IL-G, AD, FR, PP, CT, NM and MU critically revised the manuscript. NL was the principal investigator (2018-2019), IL-G and AD are the current principal investigators (2019-2022). IL-G is the lead researcher. All authors have approved the final draft of the manuscript.
Funding: This study was supported by Hôpitaux Universitaires de Genève 'Young investigator research and development project' grant (PRD 9-2019-I), the division of visceral surgery (HUG), the division of gynaecology (HUG), the clinical research unit (DFDL) of the department of oncology (HUG), the department of oncology (CHUV), the division of visceral surgery (CHUV), la fondation pour l’innovation sur le cancer et la biologie and the 'Fond’action contre le cancer' foundation.
Competing interests: None declared for IL-G, FR, MU, AD, PP, CT, MDM and NM. MH declares the following competing interests: ENCARE Consultant fee (institution); Nestlé; Research funding Capnomed Sponsoring of scientific meetings MSD; Fresenius Speaker honorary (institution); ERAS society Board member, chair education; ISSPP Board member, chair education.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Cortés-Guiral D, Hübner M, Alyami M, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers 2021;7:91. 10.1038/s41572-021-00326-6 [DOI] [PubMed] [Google Scholar]
- 2.Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978;62:1–11. [PubMed] [Google Scholar]
- 3.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the gynecologic oncology group, southwestern oncology group, and eastern cooperative oncology group. J Clin Oncol 2001;19:1001–7. 10.1200/JCO.2001.19.4.1001 [DOI] [PubMed] [Google Scholar]
- 4.van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 2018;378:230–40. 10.1056/NEJMoa1708618 [DOI] [PubMed] [Google Scholar]
- 5.Govaerts K, Lurvink RJ, De Hingh IHJT, et al. Appendiceal tumours and pseudomyxoma peritonei: literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur J Surg Oncol 2021;47:11–35. 10.1016/j.ejso.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 6.Alyami M, Hübner M, Grass F, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol 2019;20:e368–77. 10.1016/S1470-2045(19)30318-3 [DOI] [PubMed] [Google Scholar]
- 7.Solass W, Kerb R, Mürdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553–9. 10.1245/s10434-013-3213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong LAW, van Erp NP, Bijelic L. Pressurized intraperitoneal aerosol chemotherapy: the road from promise to proof. Clin Cancer Res 2021;27:1830–2. 10.1158/1078-0432.CCR-20-4342 [DOI] [PubMed] [Google Scholar]
- 9.Kim G, Tan HL, Sundar R, et al. PIPAC-OX: a phase I study of oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy in patients with peritoneal metastases. Clin Cancer Res 2021;27:1875–81. 10.1158/1078-0432.CCR-20-2152 [DOI] [PubMed] [Google Scholar]
- 10.Blanco A, Giger-Pabst U, Solass W, et al. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol 2013;20:2311–6. 10.1245/s10434-012-2840-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tempfer CB, Giger-Pabst U, Seebacher V, et al. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol 2018;150:23–30. 10.1016/j.ygyno.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 12.Tempfer CB, Winnekendonk G, Solass W, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol 2015;137:223–8. 10.1016/j.ygyno.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 13.De Simone M, Vaira M, Argenziano M, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with oxaliplatin, cisplatin, and doxorubicin in patients with peritoneal carcinomatosis: an open-label, single-arm, phase II clinical trial. Biomedicines 2020;8:102. 10.3390/biomedicines8050102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hübner M, Teixeira Farinha H, Grass F, et al. Feasibility and safety of pressurized intraperitoneal aerosol chemotherapy for peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract 2017;2017:1–7. 10.1155/2017/6852749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teixeira Farinha H, Grass F, Labgaa I, et al. Inflammatory response and toxicity after pressurized intraperitoneal aerosol chemotherapy. J Cancer 2018;9:13–20. 10.7150/jca.21460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teixeira Farinha H, Grass F, Kefleyesus A, et al. Impact of pressurized intraperitoneal aerosol chemotherapy on quality of life and symptoms in patients with peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract 2017;2017:1–10. 10.1155/2017/4596176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grass F, Vuagniaux A, Teixeira-Farinha H, et al. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg 2017;104:669–78. 10.1002/bjs.10521 [DOI] [PubMed] [Google Scholar]
- 18.Hübner M, Grass F, Teixeira-Farinha H, et al. Pressurized IntraPeritoneal aerosol chemotherapy - practical aspects. Eur J Surg Oncol 2017;43:1102–9. 10.1016/j.ejso.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 19.Solass W, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol 2013;20:3504–11. 10.1245/s10434-013-3039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowacki M, Alyami M, Villeneuve L, et al. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: an international survey study. Eur J Surg Oncol 2018;44:991–6. 10.1016/j.ejso.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 21.Schettini F, Giuliano M, De Placido S, et al. Nab-paclitaxel for the treatment of triple-negative breast cancer: rationale, clinical data and future perspectives. Cancer Treat Rev 2016;50:129–41. 10.1016/j.ctrv.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 22.Cullis J, Siolas D, Avanzi A, et al. Macropinocytosis of nab-paclitaxel drives macrophage activation in pancreatic cancer. Cancer Immunol Res 2017;5:182–90. 10.1158/2326-6066.CIR-16-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman RL, Brady WE, McMeekin DS, et al. A phase II evaluation of nanoparticle, albumin-bound (NAB) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a gynecologic oncology group study. Gynecol Oncol 2011;122:111–5. 10.1016/j.ygyno.2011.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao JB, Swensen RE, Ovenell KJ, et al. Phase II trial of albumin-bound paclitaxel and granulocyte macrophage colony-stimulating factor as an immune modulator in recurrent platinum resistant ovarian cancer. Gynecol Oncol 2017;144:480–5. 10.1016/j.ygyno.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 25.Tillmanns TD, Lowe MP, Walker MS, et al. Phase II clinical trial of bevacizumab with albumin-bound paclitaxel in patients with recurrent, platinum-resistant primary epithelial ovarian or primary peritoneal carcinoma. Gynecol Oncol 2013;128:221–8. 10.1016/j.ygyno.2012.08.039 [DOI] [PubMed] [Google Scholar]
- 26.Al Housseini A, Daugherty P, Sofidiya M, et al. A phase II, non-randomized study of abraxane plus carboplatin in patients with recurrent platinum-sensitive ovarian or primary peritoneal cancer: evaluation of side effects and tolerability. JCO 2008;26:16556. 10.1200/jco.2008.26.15_suppl.16556 [DOI] [Google Scholar]
- 27.He M-M, Wang F, Jin Y, et al. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci 2018;109:3575–82. 10.1111/cas.13813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki Y, Nishina T, Yasui H, et al. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci 2014;105:812–7. 10.1111/cas.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katsaounis P, Kotsakis A, Kentepozidis N, et al. Nab-paclitaxel as second-line treatment in advanced gastric cancer: a multicenter phase II study of the hellenic oncology research group. Ann Gastroenterol. 2018;31:65-70. 10.20524/aog.2017.0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (absolute): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:277–87. 10.1016/S2468-1253(16)30219-9 [DOI] [PubMed] [Google Scholar]
- 31.NNCN guzidelines . Available: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf [Accessed July 2022].
- 32.Cristea MC, Frankel P, Synold T, et al. A phase I trial of intraperitoneal nab-paclitaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Cancer Chemother Pharmacol 2019;83:589–98. 10.1007/s00280-019-03767-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006;12:1317–24. 10.1158/1078-0432.CCR-05-1634 [DOI] [PubMed] [Google Scholar]
- 34.Takaishi K, Komohara Y, Tashiro H, et al. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via STAT3 activation. Cancer Sci 2010;101:2128–36. 10.1111/j.1349-7006.2010.01652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi T, Fushida S, Yamamoto Y, et al. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer 2016;19:1052–65. 10.1007/s10120-015-0579-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mielgo A, Schmid MC. Impact of tumour associated macrophages in pancreatic cancer. BMB Rep 2013;46:131–8. 10.5483/BMBRep.2013.46.3.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, He Y, Sun X, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 2014;7:19. 10.1186/1757-2215-7-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hübner M, Teixeira H, Demartines N. PIPAC – Chimiothérapieintrapéritonéale vaporisée. In: Un traitement innovateur de la carcinose péritonéale, Rev Med Suisse, 479, 2015: 1325–30. [PubMed] [Google Scholar]
- 39.Common terminology Criteriafor adverse events (CTCAE). Available: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf [Accessed July 2022].
- 40.Le Tourneau C, Gan HK, Razak ARA, et al. Efficiency of new dose escalation designs in dose-finding phase I trials of molecularly targeted agents. PLoS One 2012;7:e51039. 10.1371/journal.pone.0051039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solass W, Sempoux C, Detlefsen S, et al. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the peritoneal regression grading score (PRGS). Pleura Peritoneum 2016;1:99–107. 10.1515/pp-2016-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 44.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In: Sugarbaker PH, ed. Peritoneal carcinomatosis: principles of management, 82. Springer US: Cancer Treatment and Research, 1996: 359–74. [DOI] [PubMed] [Google Scholar]
- 45.Fagotti A, Ferrandina G, Fanfani F, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol 2006;13:1156–61. 10.1245/ASO.2006.08.021 [DOI] [PubMed] [Google Scholar]
- 46.Ceelen W, Sandra L, de Sande LV, et al. Phase I study of intraperitoneal aerosolized nanoparticle albumin based paclitaxel (NAB-PTX) for unresectable peritoneal metastases. EBioMedicine 2022;82:104151. 10.1016/j.ebiom.2022.104151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hübner M. PIPAC – Chimiothérapie intrapéritonéale vaporisée. un traitement innovateur de la carcinose péritonéale. Rev Med Suisse 2015:1325–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.