Abstract
Keratin pearls (KP) is an important indicator of the degree of tumor cell differentiation of esophageal squamous cell carcinomas (ESCC). However, the independent prognostic value of KP in ESCC patients remains unclear. The hematoxylin-eosin (H&E) stained tissue microarrays (TMAs) or whole slides of the patients were prepared to identify the existence of KP. Kaplan-Meier (KM) survival analysis as well as univariate and multivariate Cox regression analyses were used to evaluate the prognostic value of KP. A nomogram based on KP and other clinicopathologic characteristics was constructed. The C-index, calibration curve, Receiver Operating Characteristic (ROC) curve, and Decision Curve Analysis (DCA) were used to evaluate the nomogram. The results indicated KP is a protective factor against lymph node metastasis and is closely associated with the differentiation degree in ESCC patients. KM survival analysis showed that the overall survival (OS) of patients with KP was significantly better than for patients without KP. In addition, multivariate Cox regression analysis revealed that KP was an independent predictor of OS. Furthermore, ROC curve demonstrated that KP combined with differentiation degree could more accurately predict the 5-year survival rate than differentiation degree alone. Importantly, the nomogram showed good discrimination and calibration abilities in both training and validation groups, which could more accurately predict the 3-, 5-, and 10-year survival rates of ESCC patients and adds to the predictive value of TNM stage alone. In conclusion, KP is an independent predictor of prognosis in patients with ESCC and provides incremental prognostic value to degree of differentiation.
Keywords: Keratin pearls, prognostic value, esophageal squamous cell carcinoma, nomogram
Introduction
The prognosis of patients with esophageal squamous cell carcinoma (ESCC) is extremely poor, with a 5-year survival rate of less than 30% [1]. Accurate prediction of a patient’s prognosis is essential for the initial clinical management and the subsequent treatment decision-making [2]; therefore, it is imperative to identify factors with significant prognostic value. Pathological studies have provided many important prognostic factors, such as pathological stage, lymph node metastasis, and differentiation degree [3].
For squamous cell carcinoma such as ESCC, keratin pearls (KP) is one of the most distinctive pathological structures. KP are formed by local squamous epithelium accumulation [4], which is easily recognized under a microscope [5] and is considered a marker of well-differentiated squamous cell carcinoma [6]. Since the degree of differentiation has been proven to be an important factor affecting the prognosis of patients with ESCC [7], we speculated that KP status could also serve as an independent predictive marker for the prognosis of ESCC patients. In addition, according to our long-term experience in the study of ESCC, Chinese pathologists rarely record the presence or absence of KP in ESCC in their pathologic diagnosis. Hence, if the addition of KP status may improve the prediction of the prognosis of ESCC patients. Therefore, this study aimed not only to evaluate the prognostic value of KP but also clarify the association between KP and other clinicopathologic characteristics in patients with ESCC.
Materials and methods
Patients and follow-up
All patient information in this study was obtained from esophageal and gastric cardia carcinoma clinical diagnosis, pathology, and follow-up databases (1973-2022) which were established by the State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University [8].
The patient inclusion criteria for this study were: (1) treated by esophagectomy surgery and confirmed as ESCC by pathologic diagnosis after surgery; (2) did not receive radiotherapy or chemotherapy before surgery; and (3) hematoxylin-eosin (H&E) stained tissue microarrays (TMAs) and whole slides of cancer tissue were available in our database so that we could identify the presence of KP.
The TNM stage of the patients was determined according to the 6th edition of the TNM staging system which was established by Union for International Cancer Control (UICC) in 2002 [9].
The follow-up with patients was through telephone interview, mail (mainly in the 1970s and 1990s), and home visits from village doctors to directly contact the patients or their families. In addition, we also sought help from public service systems, such as the new cooperative medical database, the medical security bureau database, or the citizen death information registration management system. Patients were followed up every 3 months during the first year after surgery, followed by annual follow-up until their death [8]. The last follow-up was completed in January 2021. This study was conducted after approval from the Ethics Committee of Zhengzhou University and was performed in accordance with the Helsinki Declaration.
Construction of TMAs
All of our samples were histologically screened by H&E stained slides first; representative regions with high tumor purity were selected and marked in the paraffin block. Then we’ll take two cores (a cylinder with 1.5 mm diameter and 3-5 mm height) from one paraffin block, and each TMA contains 150 cores (TMAs were constructed by Wuhan Servicebio Technology Co., Wuhan, China). To ensure reproducibility and homogeneity, we stained TMA slides and reviewed the histologic diagnosis.
Identification of KP and grouping of patients
We used a combination of H&E-stained TMAs and whole slides to identify KP (Figure 1). We first examined the TMAs of all patients, and patients with positive KP staining in the TMA were classified into the group of ESCC with KP. For each patient with negative TMA KP staining, an additional H&E stained whole slide of cancer tissue was performed to redetermine whether KP existed, and patients with positive KP staining were also classified into the group of ESCC with KP. Patients with KP negative staining in both TMA and whole slide were classified into the group of ESCC without KP. All the TMAs and whole slides were examined independently by two experienced pathologists, and a third pathologist was called in if there was a disagreement.
Figure 1.
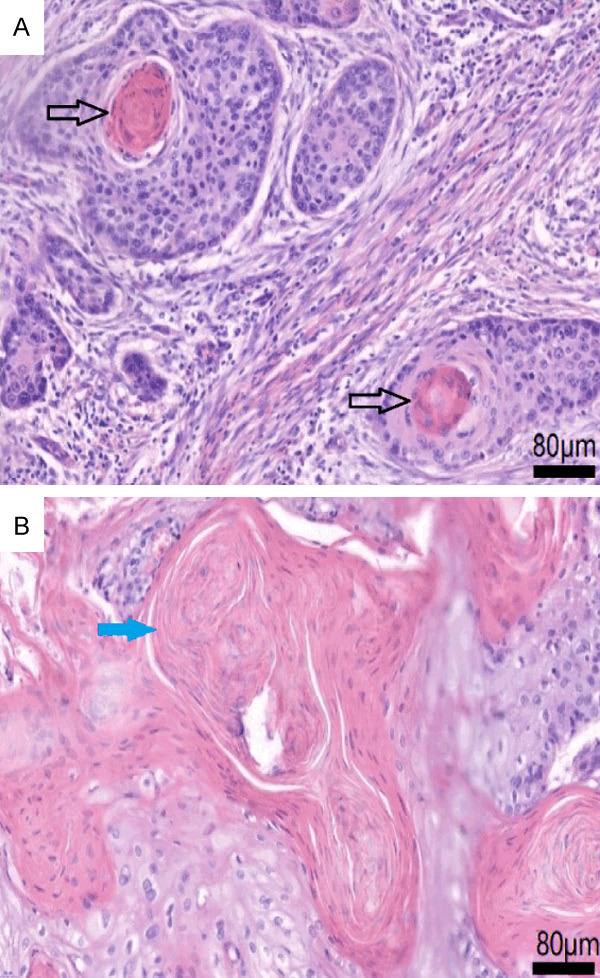
Hematoxylin-eosin (H&E) stained pictures of keratin pearls (KP). KP areeosinophilic whorled shaped aggregates of flattened keratinocytes. KP can separate from each other (A, magnification × 400, labeled by black arrow) or fuse with each other (B, magnification × 400, labeled by blue arrow).
Statistical analysis
Categorical variables were given as frequency and percentage. Pearson’s chi-square test was used to assess the association between KP and other clinicopathologic characteristics. Overall survival (OS) were compared using Kaplan-Meier (KM) method with log-rank test. Univariate and multivariate Cox regression analyses were carried out to estimate the hazard ratios (HR) and 95% confidence intervals (CI). Collinearity diagnosis was conducted to evaluate the collinearity among the covariates in the multivariate Cox regression model. A nomogram was used to predict the prognosis of patients more intuitively; the C-index and calibration curve were used to evaluate the discrimination and calibration abilities of the nomogram. Receiver Operating Characteristic (ROC) curve and Decision Curve Analysis (DCA) were used to compare the discrimination abilities and clinical benefit between different models, and Delong’s test was used to compare different ROC curves. A value of P<0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistical software for Windows, version 21.0 (SPSS, Chicago, IL, USA) and R (version 3.6.3).
Results
Analysis of the association between KP and other clinicopathologic characteristics
There were 3069 (30.0%) cases of ESCC with KP and 7144 (70.0%) cases of ESCC without KP in our cohort. There were significant differences in the distribution of regions (high incidence area and low incidence area), family history, degree of tumor differentiation, and pathologic stage between ESCC patients with KP and without KP. Compared to patients without KP, patients with KP had slightly lower high incidence area and family history (64% vs 66%, P = 0.006; 47% vs 49%, P = 0.049). However, the proportion of tumor with high or moderate differentiation was significantly higher in patients with KP (18% vs 8% or 68% vs 57%, respectively, P<0.001), and the proportion of patients with stage II was significantly higher in ESCC with KP (67% vs 59%, P<0.001) (Table 1). The formation of KP varied greatly among patients with different degrees of tumor differentiation. Among cases that were well-differentiated, moderately differentiated, and poorly differentiated, the frequencies were (49.4%, 33.8%, and 15.2%, respectively, P<0.001) (Table 1). There was a lower risk of lymph node metastasis in patients with KP than in patients without KP [34.1% vs 43.0%, P<0.001, relative risk (RR) = 0.688, 95% CI: 0.630-0.751] (Table 3).
Table 1.
Clinicopathologic characteristics of patients with esophageal squamous cell carcinoma (ESCC) with keratin pearls (KP) and ESCC without KP, n (%)
| Characteristic | No of Patients Examined | ESCC with KP | ESCC without KP | P value |
|---|---|---|---|---|
|
| ||||
| 3069 (30.0) | 7144 (70.0) | |||
| Age, y, at diagnosis | ||||
| Male | ||||
| <40 | 50 (0.76) | 14 (0.7) | 36 (0.8) | 0.706 |
| 40- | 578 (8.82) | 173 (8.7) | 405 (8.9) | |
| 50- | 2323 (35.45) | 711 (35.6) | 1612 (35.4) | |
| 60- | 2658 (40.57) | 828 (41.5) | 1830 (40.2) | |
| ≥70 | 943 (14.39) | 271 (13.6) | 672 (14.8) | |
| Female | ||||
| <40 | 26 (0.71) | 7 (0.7) | 19 (0.7) | 0.817 |
| 40- | 226 (6.17) | 74 (6.9) | 152 (5.9) | |
| 50- | 1220 (33.32) | 351 (32.7) | 869 (33.6) | |
| 60- | 1555 (42.47) | 456 (42.5) | 1099 (42.4) | |
| ≥70 | 634 (17.32) | 184 (17.2) | 450 (17.4) | |
| Area | ||||
| High incidence area | 6699 (65.59) | 1952 (63.6) | 4747 (66.4) | 0.006 |
| Low incidence area | 3514 (34.41) | 1117 (36.4) | 2397 (33.6) | |
| Smoking history | ||||
| No | 5457 (53.43) | 1634 (53.2) | 3823 (53.5) | 0.801 |
| Yes | 4756 (46.57) | 1435 (46.8) | 3321 (46.5) | |
| History of alcohol intake | ||||
| No | 7168 (70.19) | 2175 (70.9) | 4993 (69.9) | 0.321 |
| Yes | 3045 (29.81) | 894 (29.1) | 2151 (30.1) | |
| Family history | ||||
| Negative | 5239 (51.30) | 1620 (52.8) | 3619 (50.7) | 0.049 |
| Positive | 4974 (48.70) | 1449 (47.2) | 3525 (49.3) | |
| Tumor location | ||||
| Cervical | 45 (0.44) | 11 (0.4) | 34 (0.5) | 0.102 |
| Upper | 1720 (16.84) | 542 (17.7) | 1178 (16.5) | |
| Median | 6636 (64.98) | 1944 (63.3) | 4692 (65.7) | |
| Lower | 1812 (17.74) | 572 (18.6) | 1240 (17.4) | |
| Differentiation degree of tumor | ||||
| Low | 2974 (29.12) | 452 (14.7) | 2522 (35.3) | |
| Moderate | 6150 (60.22) | 2079 (67.7) | 4071 (57.0) | <0.001 |
| Well | 1089 (10.66) | 538 (17.5) | 551 (7.7) | |
| Stage | ||||
| Stage I | 673 (6.59) | 141 (4.6) | 532 (7.4) | <0.001 |
| Stage II | 6300 (61.69) | 2056 (67.0) | 4244 (59.4) | |
| Stage III | 3159 (30.93) | 856 (27.9) | 2303 (32.2) | |
| Stage IV | 81 (0.79) | 16 (0.5) | 65 (0.9) | |
Table 3.
Relationship between keratin pearls (KP) and risk of lymph node metastasis in esophageal squamous cell carcinoma (ESCC) patients, n (%)
| N1 | N0 | P value | RR and 95% CI | |
|---|---|---|---|---|
| ESCC with KP | 1048 (34.1) | 2021 (65.9) | <0.001 | 0.688 (0.630-0.751) |
| ESCC without KP | 3071 (43.0) | 4073 (57.0) | - | - |
RR: risk ratio, CI: confidence interval.
The prognostic value of KP in patients with ESCC
Kaplan-Meier curves illustrated that unadjusted overall survival (OS) was significantly different between patients without KP and with different KP subtypes (Figure 2), as the OS of patients with KP was significantly better than for patients without KP (P<0.001) (Figure 2).
Figure 2.
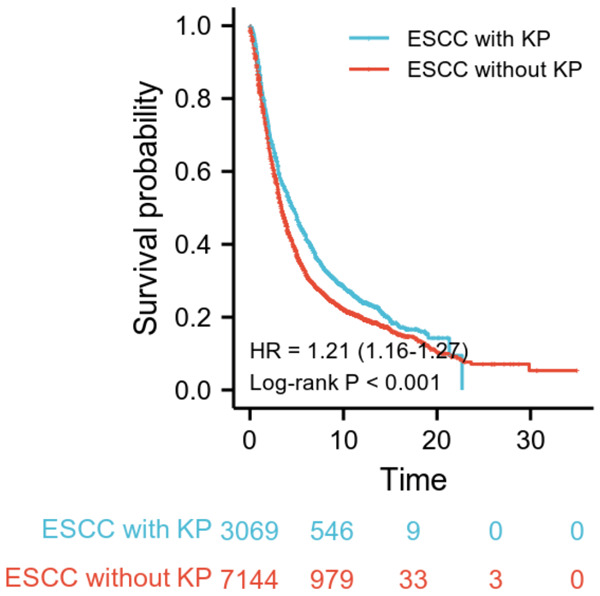
Kaplan-Meier (KM) survival analysis based onkeratin pearls (KP) in esophageal squamous cell carcinoma (ESCC) patients. KM survival curves of ESCC with KP and ESCC without KP. The curves showed that the overall survival (OS) of ESCC patients with KP was significantly better than for ESCC patients without KP.
Furthermore, we used X-tile software to select the best cut-off value for age, and the result showed that 65 years old was the best cut-off; hence, we used this cut-off in the univariate and multivariate Cox regression analyses. Univariate Cox regression analysis showed that KP, sex, age, area, smoking history, history of alcohol intake, family history, differentiation degree and tumor stage were associated with OS in patients with ESCC (P<0.05) (Table 2).
Table 2.
Univariate and multivariate Cox regression analyses of keratin pearls (KP) and other clinicopathologic characteristics
| Characteristic | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| ESCC with KP/ESCC without KP | 0.815 (0.774, 0.858) | <0.001 | 0.872 (0.828, 918) | <0.001 |
| Female/Male | 0.877 (0.836, 0.921) | <0.001 | 0.940 (0.848, 0.943) | <0.001 |
| Age >65/Age ≤65 | 1.383 (1.316, 1.454) | <0.001 | 1.385 (1.318, 1.456) | <0.001 |
| LIA/HIA | 0.824 (0.784, 0.866) | <0.001 | 0.874 (0.831, 0.919) | <0.001 |
| Smoking history Positive/Negative | 1.119 (1.069, 1.172) | <0.001 | - | 0.429 |
| Alcohol intake Yes/No | 1.139 (1.083, 1.198) | <0.001 | 1.068 (1.011, 1.128) | 0.018 |
| Family history Positive/Negative | 1.276 (1.218, 1.336) | <0.001 | 1.231 (1.174, 1.290) | <0.001 |
| Differentiation degree Moderate/Low | 0.812 (0.771, 0.854) | <0.001 | 0.805 (0.765, 0.847) | <0.001 |
| Differentiation degree Well/Low | 0.635 (0.583, 0.693) | <0.001 | 0.661 (0.606, 0.722) | <0.001 |
| Stage II/Stage I | 2.405 (2.135, 2.708) | <0.001 | 2.521 (2.238, 2.841) | <0.001 |
| Stage III/Stage I | 3.915 (3.467, 4.421) | <0.001 | 3.915 (3.466, 4.423) | <0.001 |
| Stage IV/Stage I | 5.950 (4.613, 7.674) | <0.001 | 5.669 (4.394, 7.314) | <0.001 |
ESCC: esophageal squamous cell carcinoma, LIA: low incidence area, HIA: high incidence area.
We then included all the above variables in a multivariate Cox regression analysis and found that KP was an independent predictor of prognosis in patients with ESCC. The overall mortality was significantly lower in patients with KP (P<0.001, adjusted HR = 0.872, 95% CI: 0.828-0.918) than in patients without KP (Table 2). In addition, other independent predictors of prognosis in ESCC patients were gender (female vs male, P<0.001, adjusted HR = 0.940, 95% CI: 0.848-0.943), age at diagnosis (>65 vs ≤65, P<0.001, adjusted HR = 1.385, 95% CI: 1.318-1.456), area (LIA vs HIA, P<0.001, adjusted HR = 0.874, 95% CI: 0.831-0.919), alcohol intake (yes vs no, P = 0.018, adjusted HR = 0.874, 95% CI: 0.831-0.919), family history (positive vs negative, P<0.001, adjusted HR = 1.231, 95% CI: 1.174-1.290), differentiation degree (moderate vs low, P<0.001, adjusted HR = 0.805, 95% CI: 0.765-0.847; well vs low, P<0.001, adjusted HR = 0.661, 95% CI: 0.606-0.722) and TNM stage (II vs I, P<0.001, adjusted HR = 2.521, 95% CI: 2.238-2.841; III vs I, P<0.001, adjusted HR = 3.915, 95% CI: 3.466-4.423; IV vs I, P<0.001, adjusted HR = 5.669, 95% CI: 4.394-7.314) (Table 2).
Since there was a closely association between KP and the degree of differentiation, a collinearity diagnosis was conducted on the multivariate Cox regression model, and no strong collinearity was found among the covariates in the model [all variance inflation factor (VIF) <1.5].
Subgroup analysis of KP
To further evaluate the prognostic value of KP in ESCC patients, a subgroup analysis stratified by patients’ characteristics was conducted. The results showed that patients with KP had better survival than patients without KP in most subsets, including male, female, high incidence area (HIA), low incidence area (LIA), moderately differentiated, low-differentiation degree, stage II, and stage III or IV. However, there was no significant difference between the survival of patients with KP and patients without KP in patients with high differentiation degrees and in patients in stage I (Figure 3).
Figure 3.
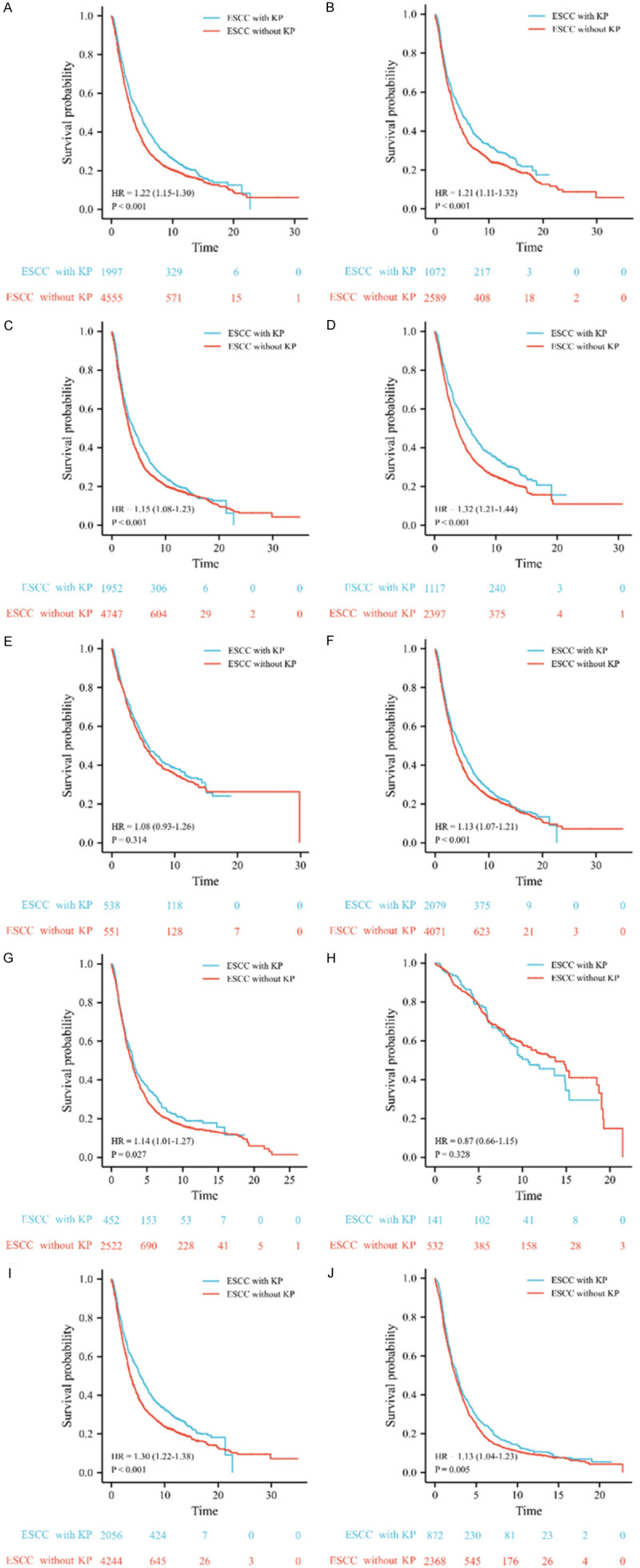
Kaplan-Meier (KM) survival curves of esophageal squamous cell carcinoma (ESCC) with keratin pearls (KP) and ESCC without KP. ESCC with KP has better survival than ESCC without KP in males (A), females (B), patients from high incidence area (HIA) (C), patients from low incidence area (LIA) (D), patients with moderately differentiated tumor (F), and patients with poorly differentiated tumor (G), patients in stage II (I), and patients in stage III or IV (J). However, there is no significant difference between the survival of ESCC with KP and ESCC without KP in patients with high differentiation degree (E) and patients in stage I (H).
Incremental prognostic value of KP in predicting the survival of ESCC patients
Moreover, we used an ROC curve to further investigate whether KP could provide incremental prognostic value to differentiation degree. We used differentiation degree as a predictor, and differentiation degree combined with KP as another way to predict the 5- and 10-year OS of ESCC patients. The ROC curves showed that differentiation degree combined with KP could more accurately predict the 5-year OS (P<0.001) and 10-year OS (P = 0.003) than differentiation degree alone (Figure 4).
Figure 4.
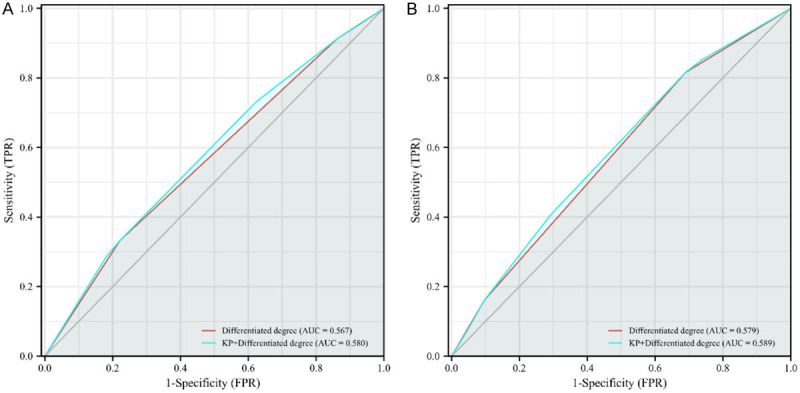
Receiver operator characteristic (ROC) curves of different indicators for predicting 5-year (A) and 10-year (B) survival rates in patients with esophageal squamous cell carcinoma (ESCC). The red line represents differentiation degree, the blue line represents keratin pearls (KP) combined with differentiation degree, and the gray line is the guide line. AUC: areas under the receiver operating characteristic curve.
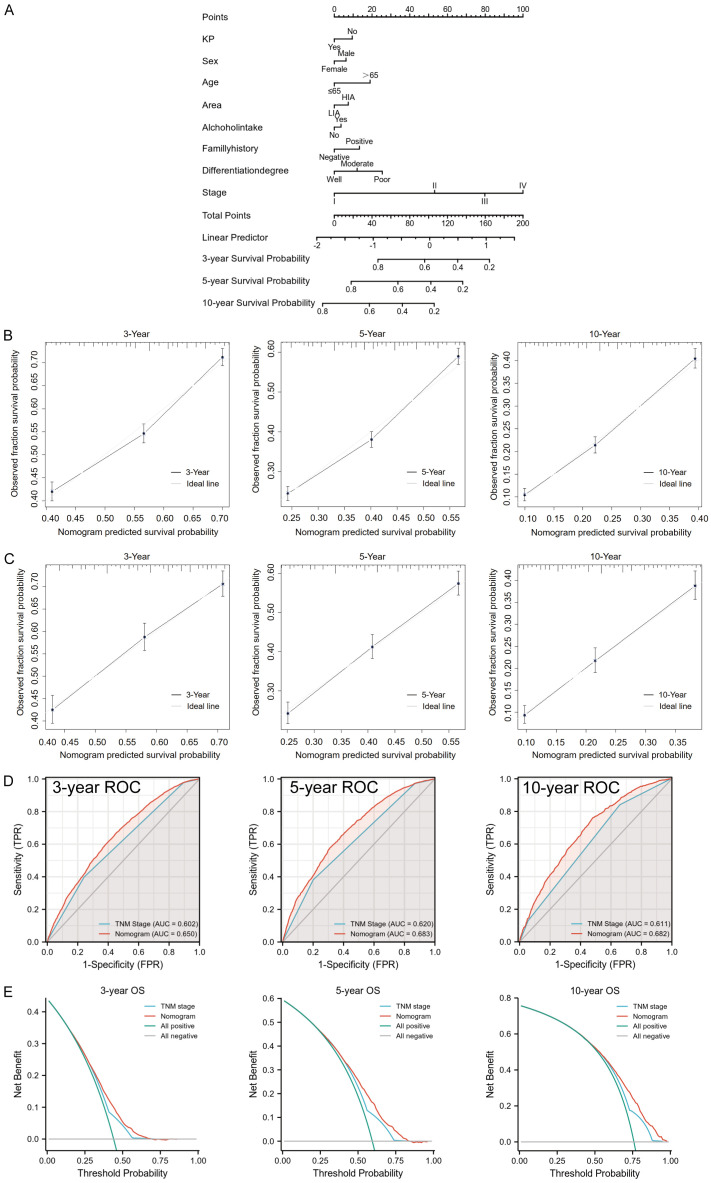
Construction and validation of prognostic nomogram based on KP and other clinicopathologic characteristics
Patients were randomly divided into a training group (n = 7149, 70%) and validation group (n = 3064, 30%). In order to predict the prognosis of patients more intuitively, we used the data of the training group to construct a nomogram based on KP and other clinicopathologic characteristics (Figure 5A). In the training group, the nomogram showed good discrimination ability, with a C-index of 0.624. In addition, the calibration curve demonstrated high consistency between the prediction and the actual observation about the probability of 3-, 5-, and 10-year survival in the training group (Figure 5B), indicating that the model had strong calibration ability. We further validated the discriminative and calibration abilities of this nomogram model in the validation group and determined that the C-index of the nomogram was 0.617. Importantly, the calibration curve also showed a good relation between the nomogram prediction and actual observations for the probability of 3-, 5-, and 10-year survival (Figure 5C).
Figure 5.
A nomogram based on keratin pearls (KP) and other clinicopathologic characteristics was constructed to predict the prognosis of esophageal squamous cell carcinoma (ESCC) patients in the training group. A. The nomogram for predicting the overall survival (OS) of ESCC patients at 3, 5, and 10 years. B. The nomogram calibration curves of 3-, 5-, and 10-year survival probabilities in the training group. C. The nomogram calibration curves of 3-, 5-, and 10-year survival probabilities in the validation group. D. The receiver operator characteristic (ROC) curves of the nomogram and TNM stage for the survival prediction of ESCC patients at 3, 5, and 10 years in the training group. E. Decision curve analysis (DCA) of the nomogram and TNM stage for the 3-, 5-, and 10-year survival prediction of ESCC patients in the training group. AUC: areas under the receiver operating characteristic curve.
We also compared the nomogram model with TNM stage in the training group using ROC curve and decision curve analysis (DCA). The ROC curve showed that the nomogram model could more accurately predict the 3-, 5-, and 10-year survival rates of ESCC patients than TNM stage did (all the P<0.001) (Figure 5D). The DCA curves illustrated that the nomogram could better predict the 3-, 5- and 10-year OS in the training group, as it added more net benefit compared to the TNM stage for almost all threshold probabilities, with both the treat-all-patients scheme and the treat-none scheme (Figure 5E).
Discussion
In this study, we determined that: (1) KP is an independent predictor of prognosis in patients with ESCC; (2) KP provides incremental prognostic value to differentiation degree; (3) KP is a protective factor against lymph node metastasis; and (4) KP is closely associated with the differentiation degree in ESCC patients. However, there was no strong collinearity between the two covariates in predicting the OS of ESCC patients.
To our knowledge, this study was the first and the largest cohort to investigate the independent prognostic value of KP in ESCC. Because of the close association between KP and the degree of differentiation, pathologists and clinicians are accustomed to using the degree of differentiation as a proxy for the presence of KP, which may be the main reason why the independent prognostic value of KP has been overlooked. However, a previous study has found that the Aldehyde dehydrogenase-1 (ALDH1) protein in vulvar squamous carcinoma tissue was always positively expressed in the KP, and the positive expression of ALDH1 was significantly associated with good prognosis in those patients [10], suggesting that vulvar squamous cell carcinomas patients with KP may have better survival; nevertheless, this remains to be further evaluated. In addition, a previous study showed that DNA was absent, and that the cell membrane fluidity was reduced in the center of KP [11]. Therefore, compared to squamous cell carcinoma without KP, squamous cell carcinoma with KP may be less invasive, which may explain the better prognosis of ESCC patients with KP. Furthermore, Yasuzumi et al. found that after bleomycin treatment in tongue cancer patients, the cancer cells in the lesions would gather to form cancer cell nests and then gradually transform into cancer pearls (also known as KP), followed by degeneration to necrosis [12]. Thus, KP may represent a stage in the necrosis process of squamous cell carcinoma, and chemotherapy may promote the formation of KP. For ESCC patients with KP, their tumor tissues have undergone changes similar to those seen after chemotherapy, thereby contributing to a better prognosis. Consistently, our results suggest that ESCC patients without KP have a higher risk of lymph node metastasis, which may also attribute to the poorer prognosis of patients without KP.
Furthermore, our results showed that the proportion of ESCC with KP gradually decreased between high differentiation to low differentiation. Although this result could be predicted by the criterion of differentiation degree, our results demonstrated the specific extent of the association between KP and the degree of differentiation. In addition, we found that there was no strong collinearity between KP and differentiation degree when predicting the OS of patients, suggesting that the presence of KP and the degree of differentiation were not interchangeable. Thus it is importance to obtain KP information when examine pathologic slides of ESCC patients. Notably, our results showed that KP combined with differentiation degree was more accurate than differentiation degree alone in predicting 5- and 10-year survival rates, which also better illustrated the important prognostic value of KP for patients with ESCC.
This study not only clarified the important prognostic value of KP in ESCC, but also reported the occurrence frequency of KP, as well as analyzed the association between KP and clinicopathologic characteristics of ESCC patients. In our study cohort, patients without KP accounted for 70.0% of all patients, while those with KP accounted for 30.0%. Until now, there was no report on the specific occurrence frequency of KP in ESCC, but Sarode et al. retrospectively analyzed the histological sections of 147 patients with oral squamous cell carcinoma and found that 99 (67.3%) of them had KP [13]. In this study, the frequency of KP was 30.0%, which may be related to the difference in tumor types. Additionally, some other studies were similar to ours but used different pathological material. Cooper used TMAs to evaluate the presence of keratinization or basaloid differentiation in oropharyngeal squamous cell carcinoma [14], Courtiol used one whole slide from each patient to do deep learning-based classification of mesothelioma [15], Vuong used TMAs and whole slides to identify Epstein-Barr Virus Status in patients with gastric cancer [16]. Due to the large sample size of our study, we initially used TMAs to identify KP, which greatly improved our efficiency. Importantly, in a previous study, Dekker found that the concordance between core needle biopsy and surgical specimens was high for estrogen receptor testing in patients with breast cancer. However, they still recommend retesting estrogen receptor-negative core needle biopsy results on the surgical specimen [17], since the results may be affected by tumor heterogeneity. Our current procedure is similar to their recommendation. Caution should be taken when KP status is negative in TMAs, since the tissuesize on TMAs is small, possibly causing a false negative. So we only used the TMAs for preliminary identification of KP. As expected, after using the current procedure, the positive rate of KP in our cohort increased from the initial 27.5% to 30.0% eventually. However, if our classification method is to be used in clinical work in the future, the procedure should be adjusted. In this study, compared to patients without KP, the proportion of patients from a high incidence area and patients with family history was slightly lower in patients with KP, suggesting that ESCC with a hereditary inclination was less likely to form KP. In contrast, the proportion of patients with stage II was significantly higher in ESCC with KP, suggesting that patients with KP had a lower degree of malignancy, e.g. a lower N stage and TNM stage.
However, a limitation of this study was that treatment method wasn’t included in the multivariate Cox regression analysis since we had not been able to obtain sufficient information about it due to the long time span of our cohort, wide geographical origin of patients, and the variable hospitals in which patients were treated after surgery. Therefore in the future, we will focus further on the effect of treatment method on patients’ survival and its relationship with KP.
In conclusion, this study revealed the important prognostic value of KP in patients with ESCC and clarified the close association among KP, lymph node metastasis, and differentiation degree. Although the independent prognostic value of KP is limited, the combination of KP and differentiation degree can more accurately predict the prognosis of ESCC patients, which is important to the treatment selection. Furthermore, since recent studies have shown that KP can be observed in the magnifying endoscope of superficial ESCC [18], for those patients who cannot be treated surgically, KP status in their tumor tissues will provide valuable prognostic information. Although the specific mechanism by which KP affects lymph node metastasis and the prognosis of patients is still unclear, our findings of the association between KP and lymph node metastasis risk might provide a novel approach for the improvement of prognosis by promoting the formation of KP in ESCC tissue. Interestingly, this idea is similar to the idea of inducing differentiation, which is the major mechanism by which several of the novel therapeutic agents function in myeloid malignancies [19]. Moreover, the molecular mechanism of KP formation may be easier to elucidate than that of tumor differentiation, as the differentiation of tumors is a very complicated process. Hence, promoting the formation of KP may represent a new avenue in the treatment of ESCC and other squamous cell carcinomas in the future.
Acknowledgements
We thank all the patients and their family members whose contributions made this work possible; thousands of medical students from Xinxiang Medical University for sample collections, data collections and follow-up. Supported by the National Natural Science Foundations (81872032).
Disclosure of conflict of interest
None.
References
- 1.Xi Y, Lin Y, Guo W, Wang X, Zhao H, Miao C, Liu W, Liu Y, Liu T, Luo Y, Fan W, Lin A, Chen Y, Sun Y, Ma Y, Niu X, Zhong C, Tan W, Zhou M, Su J, Wu C, Lin D. Multi-omic characterization of genome-wide abnormal DNA methylation reveals diagnostic and prognostic markers for esophageal squamous-cell carcinoma. Signal Transduct Target Ther. 2022;7:53. doi: 10.1038/s41392-022-00873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saner FAM, Herschtal A, Nelson BH, deFazio A, Goode EL, Ramus SJ, Pandey A, Beach JA, Fereday S, Berchuck A, Lheureux S, Pearce CL, Pharoah PD, Pike MC, Garsed DW, Bowtell DDL. Going to extremes: determinants of extraordinary response and survival in patients with cancer. Nat Rev Cancer. 2019;19:339–348. doi: 10.1038/s41568-019-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolgar JA, Triantafyllou A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol. 2009;45:361–385. doi: 10.1016/j.oraloncology.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Huang T, Chen S, Han H, Li H, Huang Z, Zhang J, Yin Q, Wang X, Ma X, Dai P, Duan D, Zou F, Chen X. Expression of Hsp90α and cyclin B1 were related to prognosis of esophageal squamous cell carcinoma and keratin pearls formation. Int J Clin Exp Pathol. 2014;7:1544–1552. [PMC free article] [PubMed] [Google Scholar]
- 5.Woolgar JA. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2006;42:229–239. doi: 10.1016/j.oraloncology.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Okawa T, Michaylira CZ, Kalabis J, Stairs DB, Nakagawa H, Andl CD, Johnstone CN, Klein-Szanto AJ, El-Deiry WS, Cukierman E, Herlyn M, Rustgi AK. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21:2788–2803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou X, Gu YK, Liu XW, Fu JH, Wang X, Zhang LJ, Luo RZ, Lin P, Yang HX. The impact of tumor cell differentiation on survival of patients with resectable esophageal squamous cell carcinomas. Ann Surg Oncol. 2015;22:1008–1014. doi: 10.1245/s10434-014-4067-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Zhao XK, Xu RH, Song X, Yang MM, Zhou FY, Lei LL, Fan ZM, Han XN, Gao SG, Wang XZ, Liu ZC, Li Li A, Gao WJ, Hu JF, Zhang LG, Wei JC, Jiao FL, Zhong K, Wang WP, Li LY, Ji JJ, Li XM, Wang LD. Transthoracic, thoracoabdominal, and transabdominal surgical approaches for gastric cardia adenocarcinomas: a survival evaluation based on a cohort of 7103 patients. World J Surg Oncol. 2022;20:217. doi: 10.1186/s12957-022-02680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual [M] 6th ed. Chicago: Springer; 2002. p. 91. [Google Scholar]
- 10.Wu Q, Shi H, Holm R, Li X, Trope C, Nesland JM, Suo Z. Aldehyde dehydrogenase-1 predicts favorable prognosis in patients with vulvar squamous cell carcinoma. Anticancer Res. 2014;34:859–865. [PubMed] [Google Scholar]
- 11.Schultz CP, Mantsch HH. Biochemical imaging and 2D classification of keratin pearls structures in oral squamous cell carcinoma. Cell Mol Biol (Noisy-le-grand) 1998;44:203–210. [PubMed] [Google Scholar]
- 12.Yasuzumi G, Hyo Y, Hoshiya T, Yasuzumi F. Effects of bleomycin on human tongue carcinoma cells as revealed by electron microscopy. Cancer Res. 1976;36:3574–3583. [PubMed] [Google Scholar]
- 13.Sarode SC, Sarode GS, Sengupta N, Sharma NK, Patil S. Calcified keratin pearlss in oral squamous cell carcinoma. Oral Oncol. 2020;109:104681. doi: 10.1016/j.oraloncology.2020.104681. [DOI] [PubMed] [Google Scholar]
- 14.Cooper T, Biron VL, Adam B, Klimowicz AC, Puttagunta L, Seikaly H. Association of keratinization with 5-year disease-specific survival in oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141:250–256. doi: 10.1001/jamaoto.2014.3335. [DOI] [PubMed] [Google Scholar]
- 15.Courtiol P, Maussion C, Moarii M, Pronier E, Pilcer S, Sefta M, Manceron P, Toldo S, Zaslavskiy M, Le Stang N, Girard N, Elemento O, Nicholson AG, Blay JY, Galateau-Sallé F, Wainrib G, Clozel T. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat Med. 2019;25:1519–1525. doi: 10.1038/s41591-019-0583-3. [DOI] [PubMed] [Google Scholar]
- 16.Vuong TTL, Song B, Kwak JT, Kim K. Prediction of Epstein-Barr virus status in gastric cancer biopsy specimens using a deep learning algorithm. JAMA Netw Open. 2022;5:e2236408. doi: 10.1001/jamanetworkopen.2022.36408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekker TJ, Smit VT, Hooijer GK, Van de Vijver MJ, Mesker WE, Tollenaar RA, Nortier JW, Kroep JR. Reliability of core needle biopsy for determining ER and HER2 status in breast cancer. Ann Oncol. 2013;24:931–937. doi: 10.1093/annonc/mds599. [DOI] [PubMed] [Google Scholar]
- 18.Baba ER, Uedo N, Rodrigues AL, da Costa Martins B, Maluf-Filho F. Keratin pearlss in magnifying endoscopy of superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2021;94:424–425. doi: 10.1016/j.gie.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Stubbins RJ, Karsan A. Differentiation therapy for myeloid malignancies: beyond cytotoxicity. Blood Cancer J. 2021;11:193. doi: 10.1038/s41408-021-00584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]