Abstract
Non-alcoholic fatty liver disease (NAFLD) is an increasingly prevalent ailment worldwide. Moreover, de novo lipogenesis (DNL) is considered a critical factor in the development of NAFLD; hence, its inhibition is a promising target for the prevention of fatty liver disease. There is evidence to indicate that AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) may play a crucial role in DNL and are the regulatory proteins in type 2 diabetes mellitus, obesity and cardiovascular disease. Therefore, AMPK and SIRT1 may be promising targets for the treatment of NAFLD. The present review article thus aimed to summarize the findings of clinical studies published during the past decade that suggested the beneficial effects of AMPK and SIRT1, using their specific activators and their combined effects on fatty liver disease.
Keywords: AMP-activated protein kinase, sirtuin 1, mechanism, non-alcoholic fatty liver disease, randomized control trial
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a condition where the accumulation of lipids exceeds 5% of hepatocytes and is not generated by alcohol, drug consumption or does not damage hepatocytes (1). The global prevalence of NAFLD is increasing, with ~20–30% of patients presenting with early-stage disease (2,3). This disease is currently of great concern as it may increase the risk of developing other subsequent anomalies, as for example type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) (4).
It has been revealed that de novo lipogenesis (DNL) may be crucial for the development of NAFLD (5). It occurs primarily in hepatocytes and is triggered mainly by a high intake of glucose or fructose. DNL turns excessive glucose or fructose into fatty acid and triglycerides (6). DNL is a normal process for the maintenance of homeostasis in the body, and its increased activation may potentially cause hepatic steatosis (7). Therefore, the inhibition of DNL is highly pursued as a therapeutic target for lipid metabolism-related disease.
The sterol regulatory element-binding protein 1c (SREBP1c) and carbohydrate response element-binding protein (ChREBP) are key transcription factors that play a crucial role in DNL (8). Several studies have revealed that SREBP1c and ChREBP increase the expression of lipogenic enzymes related to DNL (9–11). The simultaneous activity of SREBP1c and ChREBP is a normal process for the maintenance of cell homeostasis; however, at excessive levels, the cell has a specific mechanism to terminate the signalling activation. Several proteins are responsible for reducing DNL, including AMP-activated protein kinase (AMPK) (12).
AMPK regulates DNL through several mechanisms, phosphorylating and inactivating acetyl-CoA carboxylase (ACC), thus inhibiting fatty acid biosynthesis (13). Furthermore, AMPK also inhibits transcriptional regulators, including SREBP1c and ChREBP. The activation of AMPK has been reported to be blocked the nuclear translocation of SREBP1c and attenuates aberrant lipogenesis in diabetic mice (14). In another study on 3T3-L1 cells, AMPK was revealed to phosphorylate the precursor of SREBP1c and prevented the conversion of SREBP1c into its mature form (15). It also regulates the activity of ChREBP, as demonstrated in an ethanol-induced fatty liver experiment, where AMPK was inhibited by ethanol, while ChREBP activity increased significantly (16). Therefore, AMPK is considered one of the proteins that can maintain cell balance, specifically concerning lipid metabolism.
Sirtuin 1 (SIRT1) is also well-known as a regulatory protein (17). Several studies have reported the activation of SIRT1 in lowering the expression of DNL enzymes (18,19). Furthermore, the increased activity of SIRT1 decreases the expression of SREBP1c, while the knockout SIRT1 has been reported to elevated the expression of ChREBP in HepG2 cells (19). This demonstrates the importance of SIRT1 in the regulation of lipid metabolism, specifically in DNL.
The effects of AMPK and SIRT1 activation on lipid metabolism are well known; however, there are still concerns as to whether the combination of their activators is beneficial for pathological lipid metabolism-related diseases, including fatty liver disease. Therefore, the present review article aimed to summarize the role of AMPK and SIRT1 in NAFLD, based on evidence obtained from randomized control studies.
2. Data collection methods
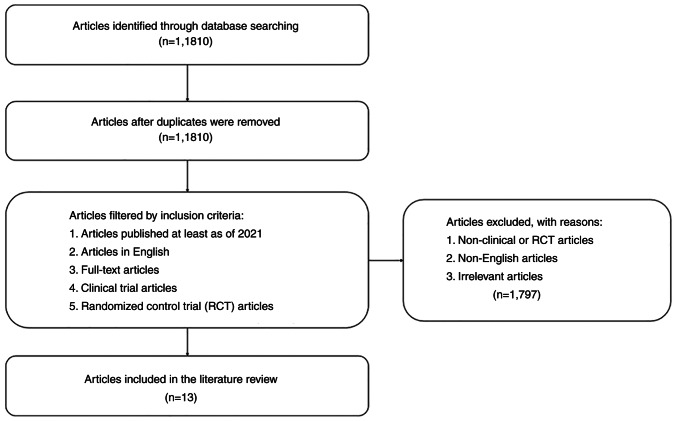
The present review summarizes the result of randomized control studies related to the effect of AMPK and SIRT1 activators on NAFLD. Articles were obtained from the PubMed database identified using the key words ‘SIRT1 activator AND NAFLD’, ‘Resveratrol AND fatty liver’, ‘AMPK activator AND NAFLD’, as well as ‘Metformin AND fatty liver’. Only clinical or randomized control trial articles published over the last 10 years were included. By contrast, articles that did not include SIRT1 and AMPK activators in patients with NAFLD were excluded. The method used for data collection is summarized in Fig. 1. In total, 13 articles were collected, and the data are presented in Table I, arranged by the protein, its activator name, subject, treatment, duration, type of study, outcome and references, and the results of these studies were then discussed.
Figure 1.
Flowchart for the literature search.
Table I.
Clinical trials of AMPK and SIRT1 activators on patients with NAFLD.
| Protein | Activator | Subjects | Treatment and duration of study | Type of study | Outcome | (Refs.) |
|---|---|---|---|---|---|---|
| AMPK | Metformin | 173 children with NAFLD | 500 mg twice a day for 96 weeks | Randomized, placebo-controlled, double blind |
|
(57) |
| Metformin combined with N-acetylcysteine | 53 patients with NAFLD | 850-1,500 mg/day for 48 weeks | Open-label multicenter randomized trial |
|
(58) | |
| PXL770 | 12 patients with NAFLD |
|
Randomized, double-blind, placebo |
|
(65) | |
| Metformin | 63 patients with NAFLD | 500 mg metformin once a day for 4–12 month | Randomized, placebo-controlled |
|
(68) | |
| Metformin | 10 patients at a risk of developing NAFLD | 500 mg once a day for 12 weeks | Single center, open label trial |
|
(69) | |
| Metformin | 35 patients with NAFLD | 850 mg daily for 24 weeks | Prospective controlled trial |
|
(70) | |
| Metformin | 29 patients with type 2 diabetes and NAFLD |
|
Single center, open-label, prospective, randomized trial |
|
(71) | |
| SIRT1 | Resveratrol | 50 patients with NAFLD | 500 mg once a day for 12 weeks | Randomized, placebo-controlled, double blind |
|
(75) |
| Resveratrol | 60 patients with NAFLD | 150 mg Twice a day for 3 months | Randomized, placebo-controlled, double blind |
|
(76) | |
| Resveratrol | 25 patients with NAFLD | 500 mg once a day for 12 weeks | Randomized, placebo-controlled, double blind |
|
(48) | |
| Resveratrol | 28 patients with NAFLD | 1,5 g daily for 6 months | Randomized, placebo-controlled, double blind |
|
(49) | |
| Resveratrol | 44 patients with NAFLD | 50 mg and 200 mg once a day for 6 months | Randomized |
|
(86) | |
| AMPK and SIRT1 | Metformin + leucine + sildenafil (NS-0200) | 91 patients with NAFLD |
|
Randomized, placebo-controlled, double blind |
|
(103) |
AMPK, AMP-activated protein kinase; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD Activity Score; DNL, de novo lipogenesis; HOMA-IR, homeostatic model assessment for insulin resistance; ALT, alanine aminotransferase; AST, aspartate aminotransferase; VLDL, very low-density lipoprotein; LDL, low-density lipoprotein; HDL-c, high-density lipoprotein cholesterol.
3. De novo lipogenesis
DNL is considered the primary factor in the development of fatty liver disease (7). In a pathological condition, such as NAFLD, DNL activation increases, generating excessive fat and culminating in intrahepatic lipid accumulation (5,20). Furthermore, DNL is a biosynthetic pathway for the productions of fatty acids and triglycerides from a non-lipid source, triggered by a high presence of carbohydrates or by insulin receptor-mediated signalling. The pathway is highly regulated by two significant factors, namely transcriptional regulation of DNL enzyme and allosteric regulation of ACC (21).
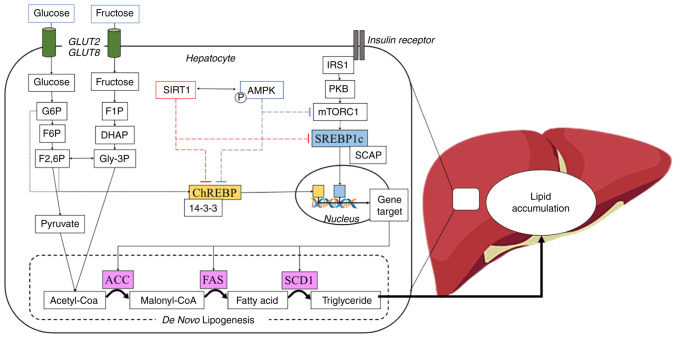
The transcriptional regulation of the DNL enzyme includes two transcription factor proteins, namely SREBP1c and ChREBP (Fig. 2). The influx of glucose and the signalling from insulin induce the activation of ChREBP and SREBP1c, respectively. Under basal conditions, the binding of SREBP1c to SREBP cleavage-activating protein (SCAP) and insulin-induced gene 1 (INSIG1) protein on the endoplasmic reticulum, prevents its translocation to the nucleus (8). Subsequently, INSIG1 is dissociated via the phosphorylation of SREBP1c and SCAP is cleaved by S1 and S2 proteases in the Golgi apparatus, and eventually, SREBP1c expresssion is released (8). Additionally, ChREBP is anchored by 14-3-3 protein and the phosphorylation of this complex permits the free ChREBP entry the nucleus (22). Furthermore, SREBP1c and ChREBP bind to the promoter gene target in the nucleus and start the transcription of lipogenic genes, including fatty acid synthase (FAS), stearoyl-CoA desaturase 1 (SCD1) and ACC (23).
Figure 2.
Schematic overview of DNL and its transcriptional regulation. Carbohydrates, including glucose or fructose enter hepatocyte cells and become a sensor for DNL activation. Glucose is converted to G6P followed by isomerization to F6P and F2,6P through the glycolysis process. By contrast, fructose also converts to Gly-3P through fructolysis and further converts to F2,6P. G6P and F2,6P induce dephosphorylation of ChREBP, and it detaches from 14-3-3 protein into an active form. Moreover, the activation of insulin receptor leads to the phosphorylation of IRS1, further activating the mTORC pathway and induces the nuclear translocation of SREBP1c. In the feedback response, SIRT1 and AMPK prevent the nuclear translocation of ChREBP and SREBP1c, resulting in the inhibition of DNL transcriptional regulation. DNL, de novo lipogenesis; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; Gly-3P, glycerol 3-phosphate; F2,6P, fructose 2,6-bisphosphate; ChREBP, carbohydrate response element-binding protein; IRS1, insulin receptor substrate 1; mTORC, mammalian target of rapamycin complex; SREBP1c, sterol regulatory element-binding protein 1c; SIRT1, Sirtuin 1; AMPK, AMP-activated protein kinase.
The inhibition of SREBP1c and ChREBP reduces the production of lipogenic genes as well as lipogenesis (24,25). Several proteins such as AMPK have been reported to inhibit the activity of SREBP1c and ChREBP. Another possible inhibitory mechanism of AMPK is predicted through SIRT1 which reportedly blocked both SREBP1c and ChREBP (19,23,26).
4. AMPK
The body has a system to maintain energy balance, in the form of adenosine triphosphate (ATP). When cellular ATP levels are reduced, the AMPK pathway is activated, phosphorylating the growth-regulating enzymes along with proteins, in order to generate ATP and decrease ATP consumption (27). AMPK is considered the master regulator of numerous proteins responsible for aging, inflammation, redox and the metabolism of lipids and glucose (28).
Based on the crystal structure of the protein, AMPK is a trimeric complex, consisting of a catalytic α subunit and two regulatory subunits, namely β and γ. The α subunit contains a kinase domain and an important residue (Thr172), which is phosphorylated by upstream kinases. The β subunit contains a binding site for carbohydrates that causes AMPK to associate with glycogen. Additionally, the γ subunit acts as a sensor for changes in the AMP/ADP ratio (29). When AMP increases and ADP decreases, AMP binds to the γ subunit, activating AMPK through three mechanisms, namely: i) The phosphorylation of Thr172 by stimulating the upstream proteins or stabilizing AMPK into a substrate more susceptible to phosphorylation; ii) AMP prevents the dephosphorylation by the phosphatase on Thr172; and iii) AMP causes allosteric activation of Thr172 in the α sub-unit (30,31). The major upstream kinase of AMPK is liver kinase B1 (LKB1) and Ca2+/calmodulin-dependent protein (CaMKK) which phosphorylates AMPK in Thr172 (32,33). LKB1 is the main upstream activator of AMPK. It is activated by the stress signal or by the presence of activators, including aminoimidazole-4-carboxamide ribonucleoside and metformin (34). In addition, CaMKK is highly distributed in neural tissue to respond to neuronal depolarization (35).
AMPK is known to play an essential role in various metabolic-related diseases, such as NAFLD. Its activity causes the inhibition of DNL through the suppression of SREBP1c and ChREBP. AMPK inhibits the activation of SREBP1c through the phosphorylation at Ser372 residue and prevents the cleavage process by protease (14). Furthermore, a recent study demonstrated that it suppresses SREBP1c expression through the mTOR and LXRa proteins (36). ChREBP is also phosphorylated at the Ser568 residue by AMPK, causing re-binding to 14-3-3 protein and the subsequent conversion into an inactive form as well as preventing lipid synthesis (22,36).
5. SIRT1
SIRT1 is a class III family of histone deacetylases, and their reactions require nicotinamide adenine (NAD+) to concurrently deacetylate histones and non-histone from proteins involved in metabolic processes and stress responses (17,37). It is widely expressed in mammalian cells in a number of organs, including the brain, adipose tissue, kidneys, pancreas, endothelium, spleen, skeletal muscle and liver. Furthermore, its expression is known to be involved in several diseases, including metabolic diseases and age-related diseases, as well as CVD (38). SIRT1 is a protein that regulates metabolism, including fat cell accumulation and maturation, lipid metabolism in the liver, systemic inflammation, nutrition sensing and circadian rhythms (39). Previous studies have demonstrated that SIRT1 inhibits DNL enzymes, as well as their key regulator proteins, SREBP1c and ChREBP, culminating in abolishing perturbation of hepatic lipid metabolism (19,40).
The primary function of SIRT1 is to deacetylate the acetyl-lysine residue of histone substrate or non-histone proteins, including transcription factors, co-regulators and enzymes (41,42). Therefore, SIRT1 has multiple physiological functions, particularly in metabolism. It has been characterized as the ‘master of metabolic regulators’, due to its pivotal role in maintaining the homeostasis of lipid metabolism by affecting several proteins involved. SREBP1c is a critical transcription factor that initiates several lipogenic genes, inducing lipogenesis within the cell. SIRT1 inhibits SREBP1c activity and decreases lipogenesis in mouse liver (19). Another lipogenesis inducer aside SREBP1c and ChREBP is SIRT1 (43). Furthermore, AMPK, which is the natural regulator of ChREBP and SREBP1c, is also affected by SIRT1 activity through an indirect mechanism by deacetylating the upstream kinase of AMPK, LKB1 (18,44). This demonstrates that SIRT1 plays a prominent role in the development of lipid-related diseases, including non-alcoholic liver disease. This is in line with several studies demonstrating that SIRT1 activator alleviates fatty liver in rodent models and NAFLD patients (45–49).
A previous in silico study revealed that the crystal structure of SIRT1 is composed of the following three major domains: the catalytic, N-terminal, and C-terminal (50). The catalytic region consists of the binding site of substrate and NAD+ that promotes the deacetylation of lysine, whereas the N- and C-terminals bind to several compounds such as resveratrol, suramin, or EX-527 and regulate SIRT1 deacetylase activity (51). Inside the cell, SIRT1 is localized in the cytoplasm and affects other proteins, including NF-κB, peroxisome proliferator-activated receptor γ, peroxisome proliferator-activated receptor-γ coactivator, AMPK and p-53 (52,53), while in the nucleus, it affects the translocation of proteins, including FOXO3a and several antioxidant genes such as SOD2/3, HO-1, and NQO-1 (54).
6. AMPK activators in NAFLD clinical studies
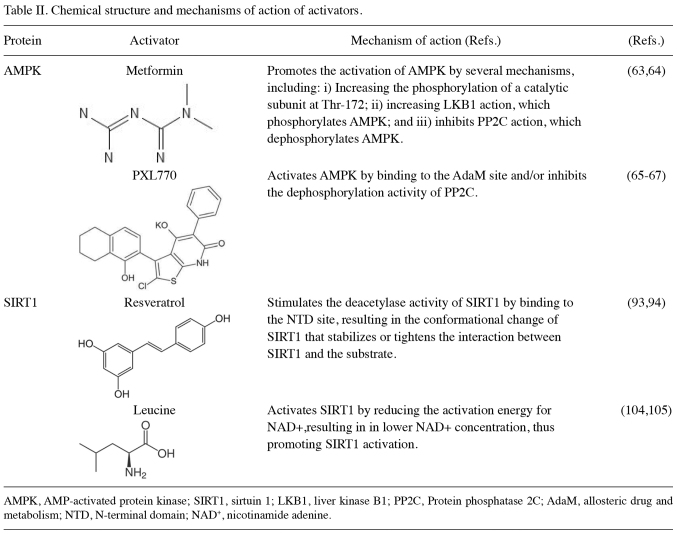
Evidence supports the role of AMPK in metabolism-related diseases, such as NALFD (55). AMPK regulates other proteins and provides homeostasis within the cell through several mechanisms involved in lipid metabolism, glucose metabolism, protein metabolism, autopaghy, and mitochondrial biogenesis (27,55,56). It has been well-established that AMPK is involved in the prevention of hepatic steatosis. Metformin, an indirect activator of AMPK, has been widely studied for its effects on NAFLD. Several clinical trials have reported the beneficial effects of metformin on certain features of NAFLD. A previous randomized control trial on children diagnosed with NAFLD and treated with metformin at 500 mg twice per day for 24 months, reported an improvement in steatosis grade and lipid profiles (57). Moreover, an open-label, multi-centred, randomized trial, reported that metformin in combination with acetylcysteine administered for 12 months led to the significant improvement in the NAFLD Activity Score measured by liver biopsies of adult patients with NAFLD (58). Acetylcysteine provides a potent antioxidant effect on the liver, thereby protecting the liver from oxidative stress (59,60). AMPK activity also affects the antioxidant defense system in cells (61,62). A combination of AMPK activator and antioxidant such as acetylcysteine yielded a positive impact against hepatic steatosis (58). AMPK activation through metformin exerts a beneficial effect by reducing hepatic steatosis in patients with NAFLD (57,58). Another study with a direct AMPK activator, PXL770, supports this statement. The mechanisms of action of metformin and PXL770 as activators of AMPK are summarized in Table II (58–67). A randomized, double-blind, placebo-controlled trial reported that treatment using PXL770 for 12 weeks decreased DNL percentage and improved glucose metabolism. Lipid profiles concerning triglycerides and very-low-density lipoprotein (VLDL) decreased in the PXL770 group compared to the placebo group (65). Furthermore, AMPK activation, direct or indirect, has a beneficial effect by reducing steatosis in patients with NAFLD.
Table II.
Chemical structure and mechanisms of action of activators.
Metformin also has a beneficial effect on the lipid profiles of patients with NAFLD, according to a previous trial, where 500 mg metformin administered for 4 months significantly decreased liver enzyme and triglyceride levels, and increased high-density lipoprotein (HDL)-cholesterol levels in patients (68). This is in line with another study which revealed that 500 mg metformin administered for 3 months decreased VLDL and triglyceride levels in 10 patients who were at a risk of developing NAFLD (69). Another study similarly reported that the daily administration of 850 mg metformin for 6 months reduced liver enzyme, total cholesterol and triglyceride levels, and increased HDL-cholesterol levels (70). Furthermore, in children diagnosed with NAFLD, treatment with metformin 500 mg twice per day, for 24 months, led to a beneficial effect in the form of improvement in lipid profiles (57). Different doses of metformin, including 250 mg three times per day, 500 mg three times per day, and 1,000 mg twice per day, administered for 6 months, have been shown to produce similar results, namely an improvement in liver enzyme levels and lipid profiles in patients with T2DM and NAFLD (71). Lipid profiles are greatly influenced by metformin at various doses in children and adult patients.
AMPK activation is involved in several mechanisms in lipid metabolism. A previous study revealed that the activation of AMPK decreased SREBP1c activity in mice fed a high-fat diet, thereby attenuating hepatic steatosis (14). SREB1c regulates the protein that is crucial for lipid and glucose metabolism. AMPK activation has been reported to inhibit fat-forming enzymes, including ACC, FAS and SCD1 through SREBP1 inhibition, leading to decreased intracellular fat accumulation (15). Another possible mechanism is through the inhibition of 6-phosphogluconate dehydrogenase (6PGD), which is an enzyme involved in glycolysis. A previous in vitro study demonstrated that the inhibition of 6PGD activated the AMPK pathway and reduced ACC1 activity, thereby inhibiting lipid biosynthesis (72). 6PGD is the third enzyme in the pentose phosphate pathway (PPP) which is responsible for converting the 6-phosphogluconate into ribulose 5-phosphate (R-5-P). The upregulation of R-5-P frequently antagonizes the LKB1 complex, resulting in the decrease of AMPK activity. Another protein involved in this mechanism is mammalian target of rapamycin complex 1 (mTORC), which is the upstream protein target of SREBP1c. In the cancer cell, activation of mTORC may upregulate the PPP through SREBP1c (73). It is well known that AMPK activity inhibits mTORC; therefore, it may also alter the PPP, resulting in the reduction of the lipogenesis. PPP may be a critical pathway in lipogenesis. In a recent study on cancer cells, metformin was reported to interfere with several enzymes related to PPP and decreased the effect of PPP via modulation of mTORC (74). However, the information about the association between metformin and 6PGD remains unclear (74). Briefly, in vitro, in vivo, or clinical trials have provided evidence that AMPK activation may be a critical step in improving lipid metabolism.
7. The SIRT1 activator, resveratrol, in NAFLD clinical studies
In recent years, the use of resveratrol as a therapy for certain diseases has attracted increasing attention, due to its beneficial effects in reducing insulin resistance, the risk of CVD, hyperlipidemia, obesity and fatty liver-related diseases, such as NAFLD. Several clinical studies have demonstrated the beneficial effects of resveratrol in patients with NAFLD (Table I). A previous randomized, placebo-controlled, double-blinded study on 50 patients with NAFLD treated with 500 mg resveratrol daily for 3 months indicated an improvement in anthropometric measurements (weight, body mass index, waist circumference), liver enzyme levels, inflammatory marker levels and liver steatosis compared to the placebo group. It was proven that liver steatosis and inflammatory cytokines, including TNF-α, IL-6 and NF-κB were reduced by resveratrol for the activation of SIRT1 (75). Another randomized trial also reported that a lower dose of resveratrol (150 mg/day) for 3 months reduced the TNF-α level in patients (76). SIRT1 activation in hepatocytes in steatosis is associated with the inflammation system, preventing further hepatocyte damage (77).
Inflammation and oxidative stress have been widely reported in hepatic steatosis, due to elevated lipid peroxidation and free radical production, eventually leading to cell damage or dysfunction (78–80). A previous study on mice revealed that resveratrol inhibited the activity of NF-κB and TNF-α (81). The inhibition of SIRT1 expression can lead to an increase in inflammatory cytokine levels. Moreover, SIRT1 activation induces nuclear factor erythroid 2-related factor 2 activity, thereby providing a protective effect through the antioxidant defense system of the cell (82,83). Other pre-clinical studies have reported that resveratrol ameliorates high-fat diet induced fatty liver disease, culminating in decreased triglyceride levels (84,85). These studies generally confirm that SIRT1 activation may inhibit fatty liver and improve the inflammation condition in hepatic steatosis both in animals and humans.
In several clinical trials, SIRT1 activation by resveratrol at different doses has been shown to lead to a decrease in lipid content. A double-blind, randomized, placebo-controlled trial with 60 participants with NAFLD treated with 150 mg resveratrol, twice per day, for 3 months, reported a significant decrease in liver enzyme, total cholesterol and low density lipoprotein (LDL)-cholesterol levels, and homeostatic model assessment for insulin resistance (HOMA-IR) compared to the placebo group (76). According to a previous study, lower doses of resveratrol, such as 150 mg reduced the intrahepatic lipid content (47). By contrast, a randomized control trial failed to show the beneficial role of resveratrol in glucose metabolism and lipid profile in higher doses, but not in the steatosis level (48). The lipid profile comprising triglycerides, LDL-cholesterol, total cholesterol and HDL, as well as HOMA-IR did not differ not significantly between the cohort treated with 500 mg resveratrol for 3 months and the placebo group. However, this trial demonstrated a significant reduction in hepatic steatosis grade and also liver enzyme, indicating the beneficial effect of resveratrol for steatosis patients (48). Another randomized control trial reported a 3.8% lipid content reduction in patients with NAFLD treated with high doses of resveratrol 1.5 g daily, for 6 months (49). Concerning lower daily doses of 50 and 200 mg for 6 months, a lower triglyceride and LDL level in patients with NAFLD has also been observed (86).
In a previous animal study, resveratrol demonstrated an undoubtedly beneficial effect on lipid metabolism (87). Lipid levels, including triglycerides, LDL-cholesterol and total cholesterol are significantly depleted in mice with hepatic steatosis treated with resveratrol (86–88). Additionally, it may also improve glucose metabolism (81,84,89) and reduce the hepatic steatosis score in high-fat/carbohydrate-induced NAFLD rats (90,91). Moreover, a previous study revealed that the overexpression of SIRT1 culminated in the alleviation of high-fat diet-induced hepatic steatosis and glucose intolerance in mice (42). Another pre-clinical study reported that mice lacking SIRT1 expression in the liver had hepatic steatosis accompanied by elevated AST levels (92). These studies prove that SIRT1 activity improves lipid and glucose metabolism in NAFLD animal models and in vitro study.
Several mechanisms have been proposed for resveratrol in the treatment of NAFLD. The proposed mechanisms of SIRT1 activators are summarized in Table II (93,94). As a direct SIRT1 activator, resveratrol is crucial for lipid metabolism (95,96). The activation of SIRT1 inhibits SREBP1c activity, thereby preventing lipogenesis (19). SIRT1 also inhibits the activity of lipogenesis enzymes, including ACC and FAS (81). In an indirect mechanism, it activates the AMPK pathway to amplify the effect of AMPK on maintaining the homeostasis of lipid metabolism (97,98). In general, SIRT1 has been proven, in clinical investigations except from in vitro and in vivo studies, to possess a crucial role in improving fatty liver conditions.
8. The combination of AMPK and SIRT1 activation
AMPK and SIRT1 interact with each other, affecting lipid metabolism. It has been previously reviewed that AMPK and SIRT1 simultaneously function, in order to regulate other proteins (99). A combination of resveratrol and metformin decreased glucose and triglyceride levels as well as improved liver function in diabetic mice (100). A previous study also reported that a similar combination reduced liver weight and visceral fat in mice (100). Furthermore, the concurrent activation of AMPK and SIRT1 pathways contributes to decreasing lipogenesis, thereby alleviating hepatic steatosis in mice with NAFLD (101,102).
A previous randomized control trial of 91 participants with NAFLD reported that a combination of leucine and metformin given daily for 16 weeks culminated in decreased hepatic fat and a significantly increased fatty acid oxidation compared to the placebo group (103). L-leucine directly activates SIRT1 through allosteric interaction in an in vitro study. Its mechanism of action as an activator of SIRT1 is also summarized in Table II (104,105). Furthermore, leucine also affects AMPK activity (106,107). The combination of leucine and metformin produced a beneficial effect related to NAFLD features. Several studies have also demonstrated that the activation of AMPK and SIRT1 plays a principal role to improve NAFLD features (15,46–48,57,67). However, clinical trials that adopt the combination of AMPK and SIRT1 activators are still lacking; hence, further research on the combinatory use of these activators is required, in order to elucidate a strong correlation between AMPK and SIRT1 in lipid metabolism.
9. Conclusions
The existing data indicated that SIRT1 and AMPK might have a pivotal role in the pathogenesis of NAFLD. Both of Activators of SIRT1 and activators of AMPK, produce a benefit in preventing lipogenesis, thus reduce the impact of fatty liver. Several randomized control trials have proven that treatment using SIRT1 and AMPK activators in patients with NAFLD can improve hepatic steatosis, prevent inflammation, and inhibit lipogenesis. However, further studies are warranted for the confirmation of the effects of SIRT1 and AMPK activator alone or in combination for the treatment of fatty liver-related diseases. The present review demonstrates that SIRT1 and AMPK activators are promising therapeutics for treating NAFLD.
Acknowledgements
Not applicable.
Funding Statement
The present study was funded by the Doctoral Research Grant (PDD) 2022 provided by the Indonesian Ministry of Education and Culture (Grant nos. 094/E5/PG.02.00.PT/2022 and 044/E5/RA.02.00.PM/2022).
Availability of data and materials
Not applicable.
Authors' contributions
SAS, HK and JL were involved in the conception and design of the study. PA principally collected previously published studies and wrote the original draft of the manuscript. HK and SAS were responsible for the acquisition of the collected articles. JL critically revised the article for intellectual content. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tiniakos DG, Anstee QM, Burt AD, editors. MacSween's Pathol Liver. 7th edition. Elsevier; Philadephia, PA: 2018. Fatty liver disease; pp. 308–371. [DOI] [Google Scholar]
- 2.Iqbal U, Perumpail B, Akhtar D, Kim D, Ahmed A. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines (Basel) 2019;6:41. doi: 10.3390/medicines6010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20:205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Byrne CD, Targher G. NAFLD: A multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Ter Horst KW, Serlie MJ. Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients. 2017;9:981. doi: 10.3390/nu9090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knebel B, Fahlbusch P, Dille M, Wahlers N, Hartwig S, Jacob S, Kettel U, Schiller M, Herebian D, Koellmer C, et al. Fatty liver due to increased de novo lipogenesis: Alterations in the hepatic peroxisomal proteome. Front Cell Dev Biol. 2019;7:248. doi: 10.3389/fcell.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferré P, Foufelle F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12:83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 9.Witte N, Muenzner M, Rietscher J, Knauer M, Heidenreich S, Nuotio-Antar AM, Graef FA, Fedders R, Tolkachov A, Goehring I, Schupp M. The glucose sensor ChREBP links de novo lipogenesis to PPARγactivity and adipocyte differentiation. Endocrinol. 2015;156:4008–4019. doi: 10.1210/EN.2015-1209. [DOI] [PubMed] [Google Scholar]
- 10.Vijayakumar A, Aryal P, Wen J, Syed I, Vazirani RP, Moraes-Vieira PM, Camporez JP, Gallop MR, Perry RJ, Peroni OD, et al. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport. Cell Rep. 2017;21:1021–1035. doi: 10.1016/j.celrep.2017.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoeckman AK, Towle HC. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J Biol Chem. 2002;277:27029–27035. doi: 10.1074/jbc.M202638200. [DOI] [PubMed] [Google Scholar]
- 12.von Loeffelholz C, Coldewey SM, Birkenfeld AL. A narrative review on the role of ampk on de novo lipogenesis in non-alcoholic fatty liver disease: Evidence from human studies. Cells. 2021;10:1822. doi: 10.3390/cells10071822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J Physiol. 2006;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JYJ, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha JH, Jang J, Chung SI, Yoon Y. AMPK and SREBP-1c mediate the anti-adipogenic effect of β-hydroxyisovalerylshikonin. Int J Mol Med. 2016;37:816–824. doi: 10.3892/ijmm.2016.2484. [DOI] [PubMed] [Google Scholar]
- 16.Liangpunsakul S, Ross RA, Crabb DW. Activation of carbohydrate response element binding protein by ethanol. J Investig Med. 2013;61:270–277. doi: 10.2310/JIM.0b013e31827c2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantó C, Auwerx J. Targeting sirtuin 1 to improve metabolism: All you need is NAD +? Pharmacol Rev. 2012;64:166–187. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paglialunga S, Dehn CA. Clinical assessment of hepatic de novo lipogenesis in non-alcoholic fatty liver disease. Lipids Health Dis. 2016;15:159. doi: 10.1186/s12944-016-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders FWB, Griffin JL. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol Rev. 2016;91:452–468. doi: 10.1111/brv.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato S, Jung H, Nakagawa T, Pawlosky R, Takeshima T, Lee WR, Sakiyama H, Laxman S, Wynn RM, Tu BP, et al. Metabolite regulation of nuclear localization of carbohydrate-response element-binding protein (ChREBP): Role of amp as an allosteric inhibitor. J Biol Chem. 2016;291:10515–10527. doi: 10.1074/jbc.M115.708982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JRB, Girard J, Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Xiaoli, Zong H, Abdulla A, Yang EST, Wang Q, Ji JY, Pessin JE, Das BC, Yang F. Inhibition of SREBP transcriptional activity by a boron-containing compound improves lipid homeostasis in diet-induced obesity. Diabetes. 2014;63:2464–2473. doi: 10.2337/db13-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen LT, Mak CH, Chen H, Zaky AA, Wong MG, Pollock CA, Saad S. SIRT1 attenuates kidney disorders in male offspring due to maternal high-fat diet. Nutrients. 2019;11:146. doi: 10.3390/nu11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzig S, Shaw RJ. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, et al. Structural basis of AMPK regulation by small molecule activators. Nat Commun. 2013;4:3017. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 31.Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 32.Gormand A, Henriksson E, Ström K, Jensen TE, Sakamoto K, Göransson O. Regulation of AMP-activated protein kinase by LKB1 and CaMKK in adipocytes. J Cell Biochem. 2011;112:1364–1375. doi: 10.1002/jcb.23053. [DOI] [PubMed] [Google Scholar]
- 33.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Lee GH, Peng C, Jeong SY, Park SA, Lee HY, Hoang TH, Kim J, Chae HJ. Ginger extract controls mTOR-SREBP1-ER stress-mitochondria dysfunction through AMPK activation in obesity model. J Funct Foods. 2021;87:104628. doi: 10.1016/j.jff.2021.104628. [DOI] [Google Scholar]
- 37.Rahman S, Islam R. Mammalian Sirt1: Insights on its biological functions. Cell Commun Signal. 2011;9:11. doi: 10.1186/1478-811X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elibol B, Kilic U. High levels of SIRT1 expression as a protective mechanism against disease-related conditions. Front Endocrinol (Lausanne) 2018;9:614. doi: 10.3389/fendo.2018.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang RH, Li C, Deng CX. Liver steatosis and increased ChREBP expression in mice carrying a liver specific SIRT1 null mutation under a normal feeding condition. Int J Biol Sci. 2010;6:682–690. doi: 10.7150/ijbs.6.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12:1069–1076. doi: 10.1038/embor.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao M, Liu D. Resveratrol suppresses T0901317-induced hepatic fat accumulation in mice. AAPS J. 2013;15:744–752. doi: 10.1208/s12248-013-9473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faghihzadeh F, Adibi P, Hekmatdoost A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. Br J Nutr. 2015;114:796–803. doi: 10.1017/S0007114515002433. [DOI] [PubMed] [Google Scholar]
- 49.Heebøll S, Kreuzfeldt M, Hamilton-Dutoit S, Poulsen MK, Stødkilde-Jørgensen H, Møller HJ, Jessen N, Thorsen K, Hellberg YK, Pedersen SB, Grønbæk H. Placebo-controlled, randomised clinical trial: High-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand J Gastroenterol. 2016;51:456–463. doi: 10.3109/00365521.2015.1107620. [DOI] [PubMed] [Google Scholar]
- 50.Davenport AM, Huber FM, Hoelz A. Structural and functional analysis of human SIRT1. J Mol Biol. 2014;426:526–541. doi: 10.1016/j.jmb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan M, Yuan H, Brent M, Ding EC, Marmorsteins R. SIRT1 contains N- and C-terminal regions that potentiate deacetylase activity. J Biol Chem. 2012;287:2468–2476. doi: 10.1074/jbc.M111.285031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McBurney MW, Clark-Knowles KV, Caron AZ, Gray DA. SIRT1 is a highly networked protein that mediates the adaptation to chronic physiological stress. Genes Cancer. 2013;4:125–134. doi: 10.1177/1947601912474893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olmos Y, Brosens JJ, Lam EWF. Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resist Updat. 2011;14:35–44. doi: 10.1016/j.drup.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Yanagisawa S, Baker JR, Vuppusetty C, Koga T, Colley T, Fenwick P, Donnelly LE, Barnes PJ, Ito K. The dynamic shuttling of SIRT1 between cytoplasm and nuclei in bronchial epithelial cells by single and repeated cigarette smoke exposure. PLoS One. 2018;13:e0193921. doi: 10.1371/journal.pone.0193921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730–E740. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- 56.Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corey KE, Vuppalanchi R, Vos M, Kohli R, Molleston JP, Wilson L, Unalp-Arida A, Cummings OW, Lavine JE, Chalasani N, et al. Improvement in liver histology is associated with reduction in dyslipidemia in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2015;60:360–367. doi: 10.1097/MPG.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Oliveira CP, Cotrim HP, Stefano JT, Siqueira ACG, Salgado ALA, Parise ER. N-acetylcysteine and/or ursodeoxycholic acid associated with metformin in non-alcoholic steatohepatitis: An open-label multicenter randomized controlled trial. Arq Gastroenterol. 2019;56:184–190. doi: 10.1590/s0004-2803.201900000-36. [DOI] [PubMed] [Google Scholar]
- 59.Cai Z, Lou Q, Wang F, Li E, Sun J, Fang H, Xi J, Ju L. N-acetylcysteine protects against liver injure induced by carbon tetrachloride via activation of the Nrf2/HO-1 pathway. Int J Clin Exp Pathol. 2015;8:8655–8662. [PMC free article] [PubMed] [Google Scholar]
- 60.Bauerlein DK, Akbar HN, von Rosenvinge EC, Loughry ND, John PR. Benefit of N-acetylcysteine in postoperative hepatic dysfunction: Case report and review of literature. Case Reports Hepatol. 2019;2019:4730381. doi: 10.1155/2019/4730381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jansen T, Kvandová M, Daiber A, Stamm P, Frenis K, Schulz E, Münzel T, Kröller-Schön S. The AMP-activated protein kinase plays a role in antioxidant defense and regulation of vascular inflammation. Antioxidants. 2020;9:525. doi: 10.3390/antiox9060525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Yang D, Gong X, Ge P, Dai J, Lin L, Zhang L. Protective benefits of AMP-activated protein kinase in hepatic ischemia-reperfusion injury. Am J Transl Res. 2017;9:823–829. [PMC free article] [PubMed] [Google Scholar]
- 63.Meng S, Cao J, He Q, Xiong L, Chang E, Radovick S, Wondisford FE, He L. Metformin activates AMP-activated protein kinase by promoting formation of the αβγheterotrimeric complex. J Biol Chem. 2015;290:3393–3802. doi: 10.1074/jbc.M114.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem. 2011;286:1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fouqueray P, Bolze S, Dubourg J, Hallakou-Bozec S, Theurey P, Grouin JM, Chevalier C, Gluais-Dagorn P, Moller DE, Cusi K. Pharmacodynamic effects of direct AMP kinase activation in humans with insulin resistance and non-alcoholic fatty liver disease: A phase 1b study. Cell Reports Med. 2021;2:100474. doi: 10.1016/j.xcrm.2021.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gluais-Dagorn P, Foretz M, Steinberg GR, Batchuluun B, Zawistowska-Deniziak A, Lambooij JM, Guigas B, Carling D, Monternier PA, Moller DE, et al. Direct AMPK activation corrects NASH in rodents through metabolic effects and direct action on inflammation and fibrogenesis. Hepatol Commun. 2022;6:101–119. doi: 10.1002/hep4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monternier PA, Parasar P, Theurey P, Dagorn PG, Kaur N, Nagaraja TN, Fouqueray P, Bolze S, Moller DE, Singh J, Hallakou-Bozec S. Beneficial effects of the direct AMP-kinase activator PXL770 in in vitro and in vivo models of X-linked adrenoleukodystrophy. J Pharmacol Exp Ther. 2022;382:208–222. doi: 10.1124/jpet.122.001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shargorodsky M, Omelchenko E, Matas Z, Boaz M, Gavish D. Relation between augmentation index and adiponectin during one-year metformin treatment for nonalcoholic steatohepatosis: Effects beyond glucose lowering? Cardiovasc Diabetol. 2012;11:61. doi: 10.1186/1475-2840-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Green CJ, Marjot T, Walsby-Tickle J, Charlton C, Cornfield T, Westcott F, Pinnick KE, Moolla A, Hazlehurst JM, McCullagh J, et al. Metformin maintains intrahepatic triglyceride content through increased hepatic de novo lipogenesis. Eur J Endocrinol. 2022;186:367–377. doi: 10.1530/EJE-21-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Resuli B, Demiraj V, Babameto A, Sema K, Malaj V. Metformin superior to low-fat diet for the treatment of patients with nonalcoholic fatty liver disease and/or steatohepatitis. Pol Arch Med Wewn. 2012;122:68–71. [PubMed] [Google Scholar]
- 71.Feng WH, Bi Y, Li P, Yin TT, Gao CX, Shen SM, Gao LJ, Yang DH, Zhu DL. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: A randomized trial. J Diabetes Investig. 2019;10:399–407. doi: 10.1111/jdi.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang X, Peng X, Huang J. Inhibiting 6-phosphogluconate dehydrogenase selectively targets breast cancer through AMPK activation. Clin Transl Oncol. 2018;20:1145–1152. doi: 10.1007/s12094-018-1833-4. [DOI] [PubMed] [Google Scholar]
- 73.Sarfraz I, Rasul A, Hussain G, Shah MA, Zahoor AF, Asrar M, Selamoglu Z, Ji XY, Adem S, Sarker SD. 6-Phosphogluconate dehydrogenase fuels multiple aspects of cancer cells: From cancer initiation to metastasis and chemoresistance. Biofactors. 2020;46:550–562. doi: 10.1002/biof.1624. [DOI] [PubMed] [Google Scholar]
- 74.Marini C, Cossu V, Bauckneht M, Lanfranchi F, Raffa S, Orengo AM, Ravera S, Bruno S, Sambuceti G. Metformin and cancer glucose metabolism: At the bench or at the bedside? Biomolecules. 2021;11:1231. doi: 10.3390/biom11081231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, Shu F, Gao Y, Yuan L, Zhang Q, Mi M. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig Liver Dis. 2015;47:226–232. doi: 10.1016/j.dld.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 77.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rezzani R, Franco C. Liver, oxidative stress and metabolic syndromes. Nutrients. 2021;13:301. doi: 10.3390/nu13020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cichoz-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andrade JMO, Paraíso AF, de Oliveira MVM, Martins AME, Neto JF, Guimarães ALS, de Paula AM, Qureshi M, Santos SHS. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915–919. doi: 10.1016/j.nut.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 82.Chai D, Zhang L, Xi S, Cheng Y, Jiang H, Hu R. Nrf2 activation induced by Sirt1 ameliorates acute lung injury after intestinal ischemia/reperfusion through NOX4-mediated gene regulation. Cell Physiol Biochem. 2018;46:781–792. doi: 10.1159/000488736. [DOI] [PubMed] [Google Scholar]
- 83.Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett. 2019;24:36. doi: 10.1186/s11658-019-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Du F, Huang R, Lin D, Wang Y, Yang X, Huang X, Zheng B, Chen Z, Huang Y, Wang X, Chen F. Resveratrol improves liver steatosis and insulin resistance in non-alcoholic fatty liver disease in association with the gut microbiota. Front Microbiol. 2021;12:611323. doi: 10.3389/fmicb.2021.611323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wardani HA, Rahmadi M, Ardianto C, Balan SS, Kamaruddin NS, Khotib J. Development of nonalcoholic fatty liver disease model by high-fat diet in rats. J Basic Clin Physiol Pharmacol. 2020;30:1–7. doi: 10.1515/jbcpp-2019-0258. [DOI] [PubMed] [Google Scholar]
- 86.Theodotou M, Fokianos K, Moniatis D, Kadlenic R, Chrysikou A, Aristotelous A, Mouzouridou A, Diakides J, Stavrou E. Effect of resveratrol on non-alcoholic fatty liver disease. Exp Ther Med. 2019:559–565. doi: 10.3892/etm.2019.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Q, Wang Y, Han X, Fu S, Zhu C, Chen Q. Efficacy of resveratrol supplementation on glucose and lipid metabolism: A meta-analysis and systematic review. Front Physiol. 2022;13:795980. doi: 10.3389/fphys.2022.918751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao H, Zhang Y, Shu L, Song G, Ma H. Resveratrol reduces liver endoplasmic reticulum stress and improves insulin sensitivity in vivo and in vitro. Drug Des Devel Ther. 2019;13:1473–1485. doi: 10.2147/DDDT.S203833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.León D, Uribe E, Zambrano A, Salas M. Implications of resveratrol on glucose uptake and metabolism. Molecules. 2017;22:398. doi: 10.3390/molecules22030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abd El-Haleim EA, Bahgat AK, Saleh S. Resveratrol and fenofibrate ameliorate fructose-induced nonalcoholic steatohepatitis by modulation of genes expression. World J Gastroenterol. 2016;22:2931–2948. doi: 10.3748/wjg.v22.i10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ding S, Jiang J, Zhang G, Bu Y, Zhang G, Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS One. 2017;12:e0183541. doi: 10.1371/journal.pone.0183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang H, Liu Y, Wang Y, Xu S, Su D. Knockdown of Sirt1 gene in mice results in lipid accumulation in the liver mediated via PGC-1α-induced mitochondrial dysfunction and oxidative stress. Bull Exp Biol Med. 2021;172:180–186. doi: 10.47056/0365-9615-2021-172-8-212-218. [DOI] [PubMed] [Google Scholar]
- 93.Hou X, Rooklin D, Fang H, Zhang Y. Resveratrol serves as a protein-substrate interaction stabilizer in human SIRT1 activation. Sci Rep. 2016;6:38186. doi: 10.1038/srep38186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao D, Wang M, Qiu X, Liu D, Jiang H, Yang N, Xu RM. Structural basis for allosteric, substratedependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015;29:1316–1325. doi: 10.1101/gad.265462.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gertz M, Nguyen GTT, Fischer F, Suenkel B, Schlicker C, Fränzel B, Tomaschewski J, Aladini F, Becker C, Wolters D, Steegborn C. A molecular mechanism for direct sirtuin activation by resveratrol. PLoS One. 2012;7:e49761. doi: 10.1371/journal.pone.0049761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ford RJ, Desjardins EM, Steinberg GR. Are SIRT1 activators another indirect method to increase AMPK for beneficial effects on aging and the metabolic syndrome? EBioMedicine. 2017;19:16–17. doi: 10.1016/j.ebiom.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: A long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duarte-Vázquez MA, Gómez-Solis A, Gómez-Cansino R, Reyes-Esparza J, Luis Rosado J, Rodriguez-Fragoso L. Effect of combined resveratrol plus metformin therapy in db/db diabetic mice. FASEB J. 2017;31 1001.8. [Google Scholar]
- 101.Li S, Qian Q, Ying N, Lai J, Feng L, Zheng S, Jiang F, Song Q, Chai H, Dou X. Activation of the AMPK-SIRT1 pathway contributes to protective effects of Salvianolic acid A against lipotoxicity in hepatocytes and NAFLD in mice. Front Pharmacol. 2020;11:560905. doi: 10.3389/fphar.2020.560905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen XY, Cai CZ, Yu ML, Feng ZM, Zhang YW, Liu PH, Zeng H, Yu CH. LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway. World J Gastroenterol. 2019;25:6607–6618. doi: 10.3748/wjg.v25.i45.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chalasani N, Vuppalanchi R, Rinella M, Middleton MS, Siddiqui MS, Barritt AS, IV, Kolterman O, Flores O, Alonso C, Iruarrizaga-Lejarreta M, et al. Randomised clinical trial: A leucine-metformin-sildenafil combination (NS-0200) vs placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:1639–1651. doi: 10.1111/apt.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Banerjee J, Bruckbauer A, Zemel MB. Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism. 2016;65:1679–1691. doi: 10.1016/j.metabol.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 105.Bruckbauer A, Zemel MB. Synergistic effects of polyphenols and methylxanthines with leucine on AMPK/Sirtuin-mediated metabolism in muscle cells and adipocytes. PLoS One. 2014;9:e89166. doi: 10.1371/journal.pone.0089166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang C, Curry BJ, Brown PL, Zemel MB. Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. J Nutr Metab. 2014;2014:239750. doi: 10.1155/2014/239750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruckbauer A, Zemel MB. Effects of dairy consumption on SIRT1 and mitochondrial biogenesis in adipocytes and muscle cells. Nutr Metab (Lond) 2011;8:91. doi: 10.1186/1743-7075-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.