Abstract
The wealth of epidemiological evidence in the scientific world underscores the possibility that a plant-based diet can reduce the prevalence of common diseases such as diabetes, cardiovascular disease, cancer, and stroke. The therapeutic effects of plant sources are partly explained by phenolic secondary metabolites or polyphenolic compounds. Therefore, polyphenolic compounds, which are widely distributed in plants, are of great interest for the development of effective specific drugs with antioxidant and anti-inflammatory effects. Moreover, polyphenol compounds have no harmful effects due to their natural biocompatibility and safety. Numerous studies have highlighted the potential of some industrial food wastes from plant material processing, including apple peels and mashed potatoes, grape skins, tomato and carrot peels, pomegranate peels and seeds, and many others. These byproducts are considered low-cost sources of natural biological compounds, including antioxidants, which have beneficial effects on human health.
The polyphenol complex of pomegranate peel (Punica granatum L.), which makes up half of the pomegranate fruit, has more pronounced antioxidant and anti-inflammatory properties than other parts. And the most important active components of pomegranate peel, which are found only in this plant, are punicalagin, followed by ellagic acid and gallic acid. It is known that these polyphenolic compounds of pomegranate peel have the most pronounced therapeutic effect. Several studies have shown the protective effect of ellagic acid, punicalagin, against oxidative stress damage caused by free radicals. The potential of pomegranate peel as an antioxidant and therapeutic component in various biological systems is high, according to scientific sources.
However, despite extensive research in recent years, a review of sources has shown that there is insufficient evidence to support the therapeutic effects of polyphenolic compounds from pomegranate peels. The role of pomegranate peel polyphenolic compounds, including flavonoids, as antioxidants in various biological systems also requires further research. Of particular importance are the mechanisms by which antioxidants influence the cellular response against oxidative stress. The purpose of this review was to report our current knowledge of plant polyphenolic compounds and their classification, and to evaluate the potential of phenolic compounds from pomegranate peels with significant antioxidant and therapeutic effects.
Keywords: Polyphenols, Flavonoids, By-products, Pomegranate peel, Radical reactions, Antioxidants, Therapeutic
1. Introduction
The concept of plant-based nutrition has become a fundamental issue worldwide due to its universal health benefits (Lavecchia et al., 2013, Kumar and Pandey, 2013). Various scientific studies have described plant raw materials as a reliable source of food, aromatic, medicinal and cosmetic ingredients and other special materials (Marchev et al., 2020, Andrea and Verpoortea, 2011, Luchian et al., 2019).
Evidence that fruits and vegetables, along with other plant foods, are important components of a healthy diet is based on a large body of scientific research showing that their consumption helps prevent chronic diseases. Fruits and vegetables contain a whole range of chemical products, especially secondary metabolites of polyphenolic nature. These chemical products have beneficial biological and antioxidant activity important to humans, including ascorbic acid, carotenoids and polyphenols such as flavonoids and phenolic acids (Ruiz-Torralba et al., 2018, Hasan et al., 2017). Additionally, these substances interrupt rapidly occurring oxidative processes and form weak radicals that can be easily excreted.
On the other hand, the processing of fruits and vegetables generates significant amounts of biological residues or by-products in the form of peels, seeds, and pulp. To manage food waste and prevent its loss, the Food Waste Management Hierarchy - Principle 3R: Reduce, Reuse, and Recycle - has been introduced in several countries. The environmental outlook and impact of discarded food waste is changing according to this approach (Dri et al., 2018). For fruits and vegetables, losses can be as high as 50 % throughout the supply chain. According to FAO (Fao, 2018), the FAO's goal is to reduce food waste by about 50 % by 2050 and to use processed waste as raw material. The FAO database shows that the average percentage of waste from fruit and vegetable processing is less than 10 %, but in some cases up to 40 % (https://www.fao.org/platform-food-loss-waste/flw-data/en/ Database, 2022). It is worth mentioning that these by-products contain many valuable nutrients (Gulsunoglu et al., 2019).
This review presents recently published results on the use of promising by-products from pomegranate peels in various biological systems, focusing on the antioxidant activity of phenolic compounds from pomegranate peels.
2. Methodology
Information was not selected via the Yandex or Google search engines, but via the Web of Science, Scopus, PubMed, and Google Scholar bibliographic databases. In order to simplify the further search for suitable literature, a pre-selection of criteria for scientific papers was made (phrases for certain keywords, impact factors of publications, etc.). In selecting sources, research articles, review articles, experimental articles, literature reviews, and book chapters, the number of times they were cited in the past year was considered. Only relevant information was considered, so 125 sources were covered in this work.
3. Polyphenolic compounds from plant sources and their classification
Polyphenols are widely distributed in the plant world as secondary metabolites, especially in fruits and vegetables. Polyphenols are a well-known group of phenolic systems characterized by at least two phenyl rings and one or more hydroxyl groups. There are about 10,000 different phenolic structures known, which are widely distributed in the plant world and are also found in foods (Coppo and Marchese, 2014, Crozier et al., 2009).
The classification of polyphenols is based on the analysis of the structure of the phenolic part of the molecule. But their diversity is also largely determined by molecules of carbohydrates, organic acids, and other substances attached to scaffold (Singla et al., ,2019).
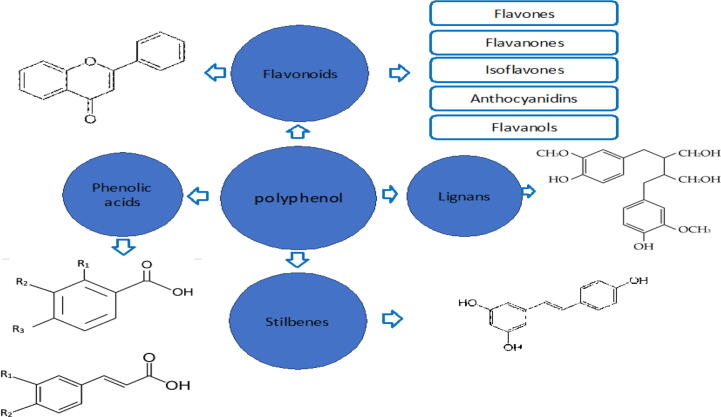
Consequently, polyphenols are simply classified into flavonoids and non-flavonoids. Alternatively, polyphenols are divided into many subclasses depending on the number of phenolic rings in their molecular structure, the substituent groups and the structural elements that bind these rings together (Pandey and Rizvi, 2009). Polyphenols are thus classified according to their origin, polyphenol function, and chemical structure, and major groups include flavonoids, phenolic acids, stilbenoids, and lignans with thousands of compounds in each group (Fig. 1) (Ofosu et al., 2020).
Fig. 1.
The major groups of polyphenols include flavonoids, phenolic acids, stilbenoids and lignans.
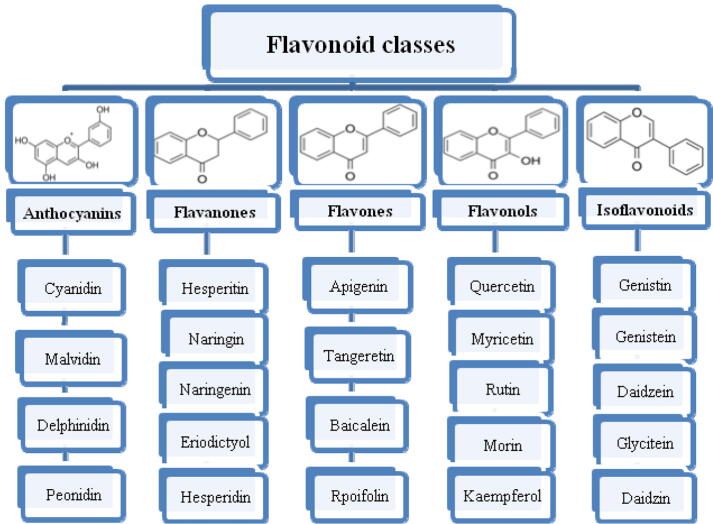
One of the most studied classes of polyphenolic compounds is flavonoids. The phenolic backbone of flavonoid molecules contains 15 carbon atoms and consists of two aromatic rings connected through three carbon atoms. Classification of flavonoids is based on differences in the structure of the three carbon atoms that connect the rings. Different ways of closing this ring, related to differences in the oxidation degree of the first ring, give different classes of flavonoids (Es-Safi et al., 2007). Thus, due to differences in the structure of flavonoid compounds, flavonoids are classified as flavanols, flavanones, flavonols, isoflavones, flavones, and anthocyanins with their corresponding subclasses (Fig. 2).
Fig. 2.
Flavanoid classes and subclasses.
Other flavonoid compounds may also include compounds such as bioflavonoids (e.g., ginkgetin), prenylflavonoids, flavonolignans (e.g., silybin), and esters of flavonoid glycoside, chalcones, and proanthocyanins (Singla et al., 2019).
The classifications presented confirm the great variety of polyphenolic compounds of plant origin. Thus, to date, several thousand polyphenolic compounds have been characterized and grouped into different classes in plants. Table 1 presents some of the most studied plant sources with high content of polyphenolic compounds and their antioxidant activity, which has a positive effect on human health.
Table 1.
Plant sources with high content of polyphenolic compounds and their health effects.
| Plant raw materials | Representatives of polyphenolic compounds | Health effects | Source of literature |
|---|---|---|---|
| Bersama abyssinica (Meliathaceae) | Isoquercitin, syringaldehyde and other phenolic substances |
Antimicrobial, antiviral, antioxidant and hypoglycemic activities |
(Zekeya et al., 2020) |
| Tea leaves | Catechins (epigallocatechin-3-gallate (EGCG) and epicatechin − 3-gallate (ECG)) | Antibacterial, inhibit LtxA-mediated cytotoxicity in human white blood cells | (Hyung et al., 2019, Yang-Hee et al., 2013) |
| Origanum vulgare (Lamiaceae) | Phenolic acids, luteolin | Anticancer, anti-inflammatory, antioxidant and antimicrobial activities | (Parra et al., 2021) |
| Mallotus philippinensis | Quercetin, rottlerin | Anti-inflammatory, antiasthmatic, analgesic, and antioxidant activity | (Mayank et al., 2014) |
| Chokeberry (Aronia melanocarpa) fruits and leaves | Proanthocyanidins Total anthocyaninFlavanols (Quercetin derivatives (sum) )Total flavonoids (phenolic acids) |
Antioxidant effect | (Lee et al., 2014, Jurendić, 2021) |
| Lingonberry (Vaccinium vitis-idaea L.) | Anthocyanins | Cardiovascular, metabolic, brain, and gastrointestinal health and cancer prevention | (Ross and David, 2015) |
| Hawthorn Fruits (Crataegus) | Proanthocyanidins, total polyphenols, flavonoids cyanidin |
Antioxidant, anticarcinogenic, and anti-inflammatory effects | (Farouk et al., 2013) |
| Citrullus colocynthis Schrad (Cucurbitaceae) | Flavonoids and flavone glycosides |
Antibacterial and anticandidal properties | (Marzouk et al., 2009, Kapoor et al., 2020) |
| Pre-ripe and ripe mango | Gallic acid, chlorogenic acid, carotenoids | Antioxidant, immuno-stimulating, and antiviral properties | (Bhatt Anjali &, 2016) |
| Cranberry extract | Proanthocyanidins | Prebiotic effect promote the presence of Akkermansia in the gut microbiota |
(Anhê et al., 2016) |
| Pomegranate (Punica granatum) juice |
Punicalagin and ellagic acid |
anti-carcinogenic and anti-inflammatory properties | (Sari et al., 2018) |
| Red pomegranate (Punica granatum L.) juice | Flavonoids and tannins |
Probiotic action | (Riani et al., 2019) |
| Pomegranate (Punica granatum) fruits |
Ellagitannins, punicalagin, anthocyanins (3-glucosides and 3,5- glucosides of delphinidin, cyanidin, and pelargonidin) and flavonols | as future anticancer agents, antiproliferative, anti-invasive, and antimetastatic effects, | (Eleonora et al., 2015) |
| Pomegranate (Punica granatum) peel |
Gallic acid and flavonoids (quercetin, kaempferol and luteolin glycosides) | Anti-S. aureus and anti-S. enterica activities | (Aboulgasem and Azab, 2015) |
| Carrots | Anthocyanins,chlorogenic acid, carotenoids | Antioxidant activity | (Sun et al., 2009) |
| Mustard (Brassica juncea) | Sinapic acid, gallic acid, vanillic acid, caffeic acid, p-Coumaric acid, ferulic acid, p-Hydroxybenzaldehyde, vanillin, Epigallocatechin gallat, epicatechin gallat, rutin, naringin, proanthocyanidins, protocatechuic acid, p-hydroxybenzoic acid, catechin, chlorogenic acid | Anti-oxidation, anti-inflammation, and bacteriostatic and antiviral activity | (Tian and Deng, 2020) |
| Soy seeds | Isoflavones (daidzein, daidzin, genistein and genistin) | Antioxidant and anti-inflammatory activity | (Maciej et al., 2020) |
| Kale (Brassica oleracea L. var. acephala) | Total flavonoid, quercetin, kamempferol, total phenol, hydroxycinnamic acids, carotenoids |
Protective role in coronary artery disease, anti-inflammatory activity, antigenotoxic ability, anti-microbial against specific microorganisms | (Neela Satheesh & Solomon Workneh Fanta, 2020) |
| Onion (Allium cepa L.) | Quercetin derivates, cyanidin | Antioxidant, inhibitory activities | (Ouyang et al., 2018, Rita et al., 2020) |
Table 1 confirms the diversity of polyphenolic compounds and their plant sources, which include pomegranate fruit and byproducts.
It is also evident that a variety of polyphenolic compounds are formed by methylation, glycosylation, or esterification of hydroxyl groups, as well as by dimerization and polymerization. Such formation of new compounds results in the inclusion of different substituent groups in the molecule (Plaza et al., 2014). For example, as demonstrated in (Metsämuuronen and Sirén, 2019) methylation of polyphenolic compounds hydroxyl groups intensifies biological activity of stilbenes and O-methylation of hydroxyl groups in flavonoids decreases their reactivity and improves their antimicrobial properties. Therefore, each class of polyphenolic compounds has a wide range of effects on different biological systems.
4. The role of polyphenolic compounds of pomegranate peel, including flavonoids, as antioxidants in various biological systems
Biological wastes, which are produced in significant quantities each year in the agri-food industry, pose a serious problem for disposal. In recent years, much attention has been paid to the extraction of phenols and other nutrients from agricultural wastes. They can be a cheap and safe source of natural food supplements and ingredients (Nag and Sit, 2018, Tandokazi et al., 2020). Therefore, the use of biological residues or by-products, including antioxidants, meets the current consumer desire for health-promoting nutrient compounds (Carpentieri et al., 2021). Some of these food wastes have been studied and are considered acceptable sources of phenolic antioxidants. In one paper (Balasundram et al., 2006), the peels of fruits such as oranges, apples, peaches, and others were found to contain a higher amount of phenols than the edible fleshy parts.
As reported in (Singha et al., 2018), pomegranate peel is a valuable source of bioactive compounds such as phenolic acids (hydroxycinnamic acid and hydrobenzoic acid), hydrolyzable tannins (ellagitannins, gallotannins, and gallagylesters), and flavonoids compared to the other parts of the pomegranate. Numerous studies have shown that the peel of the pomegranate is the component with the higher polyphenol content among the seeds, peel and juice (Fourati et al., 2019, Zaki et al., 2015).
To that, in the processing of pomegranate (Punica granatum) approximately 500 g/kg of pomegranate fruit falls on the inedible part - the peel. The remaining half contains about 400 g/kg of juice and 100 g/kg of seeds (Al-Rawahi et al., 2013).
According to a study by Tunisian scientists, the total polyphenols in pomegranate peel amounted to 85.60 ± 4.87 mg equivalents of gallic acid (GAE) per g of dry weight (mg GAE/g DW), flavonoids (51.52 ± 8,14 mg equivalents of rutin per g of dry weight (mg RE/g DW), anthocyanins (102.2 ± 16.4 mg equivalents of cyanidin-3-glucoside per g DW (mg CGE/g DW) and hydrolyzable tannins (139.63 ± 4.25 mg tannic acid equivalents per g dry weight (mg TAE/g DW) (Walid et al., 2012).
Other US pomegranate peel studies showed the highest total phenols (20.24 % or 202.4 mg GAE/g DW), proanthocyanidins (2.65 % or 26.5 mg TAE/g DW), and flavonoids (3.92 % or 39.2 mg RE/g DW) in an extract prepared by ethyl acetate extraction (Wang et al., 2011).
The authors from Spain reported total polyphenols content of 54.84 mg per acid equivalents/g sample, the flavonoids content of 42.36 mg per rutin equivalent /g sample, and the hydrolyzed tannins content of 21.25 mg per catechin equivalent/g of pomegranate peel sample (Viuda-Martos et al., 2013).
The pomegranate peel contains a large number of phenols including flavonoids (anthocyanins, catechins, and other complexed flavonoids) and hydrolyzable tannins (punicalin, pedunculagin, punicalagin, gallic acid and ellagic acid). Other phytochemicals identified from the pomegranate are organic and phenolic acids. The following article provides a comprehensive review of the scientific literature on the major active phenolic compounds in the peel of pomegranate cultivars grown in different countries, which have been identified and quantified by advances in separation and spectrometry (Smaoui et al., 2019).
As indicated in Table 2, a number of other studies have shown that the major antioxidant compounds of pomegranate peel are unique to this plant.
Table 2.
Main antioxidant compounds of pomegranate peel, unique for this plant.
| Punicalagin *Punicalin |
Gallic acid | Ellagic acid | p‐coumaric acid | Caffeic acid | Catechin *Quercetin |
Refe-rence |
|---|---|---|---|---|---|---|
| 12.43 % *18.5 % |
not defined | 2.75 % | not defined | not defined | not defined | (Vishal et al., 2011) |
| not defined | not defined | 11–12 mg/100 g | not defined | not defined | 27.7–50 mg/100 g | (Zaki et al., 2015) |
| 1082.97 m/z *780.99 m/z |
not defined | 300.80 m/z | not defined | not defined | not defined | (Sepúlveda et al., 2018) |
| 85 % (w/w) of total tannins | not defined | 1.3 % (w/w) of total tannins | not defined | not defined | not defined | (Seeram et al., 2005) |
| 86 mg/g | 11.1 mg/g | 240 mg/g | 5.8 mg/g | not defined | 12 mg/g *3 mg/g |
(Marra et al., 2022) |
| 138.8–143.64 mg/g dry matter | not defined | not defined | not defined | not defined | not defined | (Kaderides et al., 2019) |
| 16.67 mg/g | not defined | 0.15 mg/g | not defined | not defined | not defined | (Beatriz et al., 2016) |
| (α + β) varied 128.02–146.61 mg/g | 0.051 mg/g | 10.12–22.53 mg/g | not defined | not defined | not defined | (Kazemi et al., 2016) |
| 541 m/z | 169 m/z | 301 m/z | 163 m/z | 197 m/z | 289 m/z | (Ambigaipalan et al., 2016) |
| 28,03–104,14 mg/g *203–840 µg/g |
10–73 µg/g | 1580–4514 µg/g | not defined | not defined | 115–613 µg/g *8.81–86.11 µg/g |
(Man et al., 2022) |
| not defined | 30.4 mg/g | 148.9 mg/g | 5.6 mg/g | 21.4 mg/g | not defined | (Dikmen et al., 2011) |
| 4.05 ± 0.26 % w/w | not defined | 0.63 ± 0.04 % w/w | not defined | not defined | not defined | (Laosirisathian et al., 2020) |
| 4792.3–6894.8 mg/100 g (FW) - (HT) Hydrolyzable tannins (HT) include gallotannins, ellagic acid derivatives, and gallagyl tannins, which comprise mainly punicalagin isomers and punicalin. |
3.8–5.2 mg/100 g (FW) | 18.9–21.4 mg/100 g (FW) | 110.7–125.6 *92.7–91.1 mg/ 100 g FW |
(Pande and Akoh, 2009 Oct 28) | ||
| not defined | not defined | 7.06 % −13.63 % w/w | not defined | not defined | not defined | (Pharkphoom et al., 2010) |
| 56.78 *200.02 mg/g DW |
5.52 mg/g DW | 20.93 mg/g DW | not defined | not defined | not defined | (Stojanović et al., 2017) |
| not defined |
124.1 ± 16.5 mg/100 g DW | 34.7 ± 2.9 mg/100 g DW |
3.9 ± 1,7 mg/100 g DW |
14.7 ± 10.8 mg/100 g DW |
*1.9 ± 0.5 mg/100 g DW |
(Mansour et al., 2013) |
| 98.020 mg/g | 2.5 mg/g | 12.561 mg/g | 0.086 mg/g | 0.458 mg/g | 3.275 *0,215 mg/g |
(El-hadary and Hassanien, 2019) |
| (α + β) varied 40.09 ± 2.70–43.18 ± 4.62 mg/l | 32.62 ± 1.36 mg/l | 98.13 ± 5.45 mg/l | not defined | not defined | not defined | (Qu et al., 2012 Jun 1) |
| 65.38 mg/100 mg |
2.53 mg/100 mg | 2.93 mg/100 mg | not defined | 0.03 mg/100 mg | 12.66 mg/100 g | (Song et al., 2016 Jul) |
| (α + β) varied 5.4 ± 0.8–231.18 ± 20 mg/g | not defined | 3.7 ± 0.2–14.7 ± 0.1 mg/g | not defined | not defined | not defined | (Rosas-Burgos et al., 2017 Feb) |
| not defined | 52.3 ± 0.1 mg/100 g | not defined | not defined | 22.2 ± 0.3 mg/100 g | 76.5 ± 0.4 mg/100 g | (Singh et al., 2016 Nov) |
As shown by the authors of the paper (Lampakis et al., 2021), the main phytochemical component classes identified so far in pomegranate peels are phenolic acids (ellagic acid and gallic acid), flavonoids (quercetin, cyanide and complex substances) and hydrolyzable tannins (punicalin and other complex substances). Other authors point out that the main phenolic compounds of pomegranate fruit with high antioxidant activity reported in the literature are flavonoids (anthocyanins such as pelargonidin, delfinidin, cyanidin as well as their derivatives and anthoxanthins such as catechin, epicatechin and quercetin), tannins (ellagitannins and ellagic acid derivatives such as punicalagin, punicalin and pedunculagin) and phenolic acids (such as chlorogenic, caffeic, syringic, synaptic, p-coumaric, ferulic, ellagic, gallic and cinnamic acids) (Shaygannia et al., 2016, Singh et al., 2018, Wu and Tian, 2017, Poyrazoğlu et al., 2002). The data in Table 2 confirm the authors' data.
Many studies have reported the role and importance of pomegranate peel polyphenolic compounds, including flavonoids, as a rich source of natural antioxidants in various biological systems. The use of components of pomegranate fruit, including the peel, as natural supplements to fortify source products (yoghurt, meat products) has been reported. Synthetic antioxidants should be increasingly restricted because of their undesirable effects (Viuda-Martos et al., 2010, Kakkar et al., 2021, El-Said Marwa et al., 2014, Hassan et al., 2017, Das et al., 2021).
For example, one study suggests that functional ice cream fortified with punicalagins from pomegranate peels may provide antioxidant and thus health benefits to consumers (Çam et al., 2013). In this context, some studies support the benefits of breads enriched with fiber and antioxidant substances from pomegranate peels for a healthy and nutritious diet (Sayed-Ahmed, 2014, Sulieman Abdel Moneim et al., 2016). Greek authors describe examples of possible applications of bioactive compounds from pomegranate fruit byproducts, including the peels, and for obtaining various types of food products with satisfactory results (dairy products, films and packaging coatings, meat and fish products, cereals and nuts (Kandylis and Evangelos, 2020).
Research into the possibility of enriching fruit juices with pomegranate peel extracts (PPE) occupies a special place. PPEs are a rich source of antioxidant compounds for the preparation of functional fruit juice- based beverages with antioxidant and anti-inflammatory activity. Thus, the authors note that commercial guava juices fortified with pomegranate peel extracts exhibited increased levels of antioxidant activity and did not degrade sensory performance (Barros et al., 2014). However, another work shows that the added amount must be limited to 0.5 % due to the astringent odour of pomegranate peels. Although the addition of pomegranate peel powder increases the antioxidant activity of fortified tomato and orange juices (Mastrodi et al., 2012).
Enrichment of carrot juice with PPE leads to stable color of the product and improvement of its bioactive properties. Moreover, all physicochemical parameters of the fortified juice were stable during 28 days of storage (Trigo et al., 2020). The authors emphasize the importance of balancing pomegranate peel extract (0.5 and 1.0 g PPE per 100 mL) in apple juice fortification to avoid toxicity and sensory quality deterioration (Altunkaya et al., 2013). Other authors point out that pomegranate peels have a bactericidal effect against some pathogenic bacteria and can be used as preservatives and stabilizers for proper food storage (Qin et al., 2013, Akhtar et al., 2015, Al-Zoreky, 2009).
Developing technologies to extract high-value compounds, including polyphenols, from pomegranate peels and other agroindustrial wastes will enable the expansion of food and health applications into other areas: Development of edible films, biomaterials, carbon dots, microbial media, biochar and biosorbents, hydrogen production, water treatment, and boiler fuel (Lucarini et al., 2021). Liu Hui-Min et al (Liu et al., 2021) have recently shown that fermentation of pomegranate peels with lactobacilli releases polyphenols such as ellagic acid more efficiently than extraction with water. The outstanding antioxidant abilities and protective effect against H2O2 -induced oxidative damage of fermented plant sources could promote their use as a functional raw material for cosmetics.
Antioxidants of fruit extracts including pomegranates peels tannic acids is reported to be effective against S. Typhimurium, S. Enteritidis, E. coli and S. aureus, and have biomedical and biotechnological applications including medicine, food, animal feed, cosmetic substances, and pharmaceuticals (Suriyaprom et al., 2022).
In conclusion, due to the versatility, high nutritional value and antioxidant activity of pomegranate peels, many industries are interested in the special benefits and new opportunities of using pomegranate peels as a promising raw material for the development of various products with antioxidant function.
5. Antioxidant and therapeutic effects of pomegranate peel extract
Obviously, the body receives exogenous antioxidants from food that absorb free radicals such as peroxide, hydroperoxide or lipid peroxide and inhibit oxidative mechanisms. In other words, the above bioactive compounds have various biological properties, such as anti-cancer, anti-inflammatory, antioxidant, antibacterial, antimicrobial, antiviral, anti-obesity, anti-depression, prevention and treatment of diabetes and cataracts, and also have effects on the immune system (Shaygannia et al., 2015, Sayago-Ayerdi et al., 2021, Abigail et al., 2016).
Antioxidants are compounds that inhibit or suppress free radical reactions and delay or suppress cellular damage. Although the mechanism of antioxidant protection varies from compound to compound, the existence of antioxidant protection itself is universal. The body and living cells have evolved several mechanisms for protection against reactive oxygen species, including an enzymatic one involving enzymes and non-enzymatic forms using antioxidant compounds (Irina and Mohamed, 2012, Sharma, 2014). Free radicals are constantly generated by living cells and reactions involving them. They are the main cause of many human diseases as well as aging in general. Adverse effects occur with an excess of free radicals when a phenomenon called oxidative stress occurs in the body (Hidekazu et al., 2012).
Therefore, plant phenolic compounds as antioxidants are becoming increasingly popular practices to maintain optimal body function and can be called “the seventh major nutrient” (Kurutas, 2016, Lv et al., 2021, Mo et al., 2022 Jun). Some authors claim that the metabolites of ellagitannin geranium, chlorogenic acid, and epigallocatechin gallate have stronger antioxidant activity than their respective parent compounds. Therefore, these metabolites are believed to play an important role in the prevention of oxidative stress-related diseases by acting as biological antioxidants after the consumption of functional polyphenols (Adwas et al., 2019).
According to the authors, the mechanisms of antioxidant effects on redox processes in the human body are
-
1)
blocking the formation of free radicals.
-
2)
elimination of oxidants.
-
3)
transformation of toxic free radicals into less toxic substances.
-
4)
blocking the production of moderately toxic metabolites and inflammatory mediators.
-
5)
blocking the growth of secondary oxidant chains.
-
6)
repair of damaged molecules.
-
7)
Initiation and enhancement of the body's antioxidant defense system.
All of these defense mechanisms work together to protect the body from oxidative stress (Nimse and Dilipkumar, 2015).
Thus, the protective effect of flavonoids is due to several mechanisms, such as free radical scavenging, enzyme inhibition, and metal ion chelation.
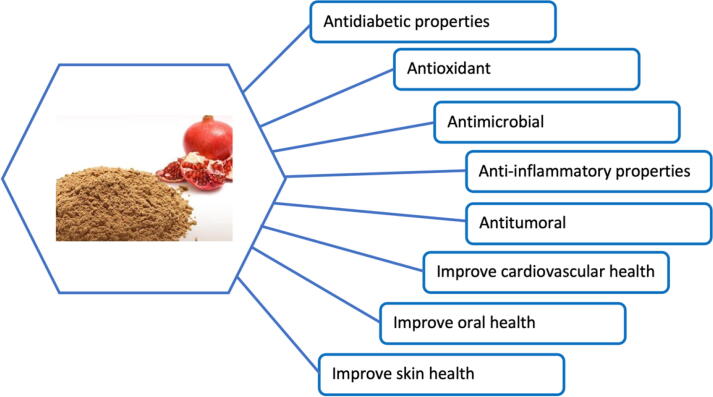
In this context, several works show antioxidant and therapeutic effects of pomegranate peel extract (Fig. 3), which may be due to the high content of polyphenols in this product. Thus, the data of the work indicate that the ethanol extract of pomegranate peel is a potent nephropreventive agent. And the mechanism of action of PPE, in their opinion, is the induction of various antioxidant and phase-forming enzymes II and the purification of reactive oxygen species (Ahmed and Ali, 2010).
Fig. 3.
Antioxidant and therapeutic effects of pomegranate peel extract.
The following work shows that PPE protects the liver and kidneys by inhibiting oxidative stress induced by toxic aluminum, stimulating antioxidant activity, and increasing the level of anti-apoptotic protein (Moneim et al., 2013). According to (Wu et al., 2019), pomegranate peel extract can be used as a therapeutic and immunoregulatory agent because it has high antioxidant activity in vivo.
Researchers from China studied the antioxidant activity of key pomegranate peel extracts such as ellagic acid, punicalin, and punicalagin in vitro and investigated their ability to scavenge free radicals, prevent fat oxidation, and their antioxidant activity in vivo under oxidative stress. According to the authors' report, ellagic acid is more efficient in protecting the body from oxidative stress in vivo compared to punicalin and punicalagin (Sun et al., 2017).
The authors from Tunisia point out that pomegranate peel added to the diet provides strong protection against barium-induced oxidative stress in red blood cells. Pomegranate peel extract reduces toxicity by scavenging free radicals and preventing DNA damage (Elwej et al., 2016).
Authors from Egypt emphasise the inhibitory effect of green tea and pomegranate peel extracts on the bacterium Streptococcus mutans and the reduction of its adherence to the tooth surface of orthodontic patients (Abd-El-Aziz and Sallam, 2020). Other articles (Eliana Harue Endo, 2012, Noory et al., 2020, Seeram et al., 2005) present published results on various aspects of of pomegranate peel polyphenols focusing on its medicinal properties. Pomegranate peel polyphenols help to treat oral infections caused by C. albicans, reduce profen oxidative stress and improvement liver and kidney functions, as well as exhibit antiproliferative, apoptotic and antioxidant activities.
Several studies have been performed to evaluate a beneficial effect of and selected medicinal plants polyphenols against bacterial agents associated with bovine mastitis or Eimeria infection in chickens (Raheema, 2016, Amber et al., 2017, Freitas et al., 2019, Khorrami et al., 2022, Yang et al., 2022, Matsumoto et al., 2015). Pomegranate peel represents an important therapeutic potential, not only for human health but also for animals.
Thus, the results of the reviewed works allow us to consider the mechanisms of influence of the plant-based antioxidants, including polyphenolic compounds of pomegranate peel, on the redox processes in the human body, for the prevention and treatment of various diseases.
6. Conclusions and future perspectives
The search for new sources of polyphenolic compounds from plant raw materials capable of maintaining the balance of redox processes in the human body, the so-called antioxidant status of the body, is an urgent direction of scientific research in the field of biotechnology. Particular attention is being paid to secondary foods as inexpensive and natural sources of antioxidants, which include the byproducts of pomegranate fruit, such as the peel.
Compared to other parts of the fruit, pomegranate peel contains significant amounts of polyphenolic compounds such as hydrolyzable tannins (punicalin, punicalagin, ellagic acid, and gallic acid), flavonoids (anthocyanins and catechins), and other substances that contribute to the high antioxidant activity of pomegranate peel.
However, important aspects such as bioavailability, absorption, metabolism, biotransformation, and pharmacokinetics of pomegranate peel polyphenolic compounds are not well understood. Also, extensive clinical experiments are needed to prove the preventive and therapeutic potential of pomegranate peel.
At the same time, the lack of scientific information should not hinder further research in this direction. As pomegranate peels have demonstrated their potential as promising bioactive antioxidants, they may be a potential source of functional food ingredients and nutraceuticals that can be used in various areas of biotechnology and other industries.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by grant funding for scientific, and (or) scientific, and technical projects for 2021-2023 with a period of implementation of 12 months (MES RK) (Grant No. AP09563292).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd-El-Aziz A., Sallam R.A. Antibacterial effect of green tea and pomegranate peel extracts on Streptococcus mutans of orthodontic treated patients. J. Radiat. Res. Appl. Sci. 2020;13(1):132–143. doi: 10.1080/16878507.2019.1693733. [DOI] [Google Scholar]
- Abigail R.-M., Luisa C.-I., Candy C.-Á., Pimentel-González D.J., Brenda A.-S. Antioxidant activity, antimicrobial and effects in the immune system of plants and fruits extracts. Fron. Life Sci. 2016;9(2):90–98. doi: 10.1080/21553769.2015.1104388. [DOI] [Google Scholar]
- Aboulgasem G.J., Azab A.E. The potential protective effects of pomegranate juice against (S)-(-)-1-methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt induced serum biochemical changes in rabbits. Int. J. Sci. Res. 2015;4(11):360–371. [Google Scholar]
- Adwas A.A., Elsayed A.S.I., Azab A.E., et al. Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng. 2019;6(1):43–47. doi: 10.15406/jabb.2019.06.00173. [DOI] [Google Scholar]
- Ahmed Mahgoub, M., Ali Safaa, E. 2010. Protective effect of pomegranate peel ethanol extract against ferric nitrilotriacetate induced renal oxidative damage in rats, Journal of Cell and Molecular Biology 7(2) & 8(1):35-43, http://jcmb.halic.edu.tr.
- Akhtar S., Ismail T., Fraternale D., Sestili P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015;174:417–425. doi: 10.1016/j.foodchem.2014.11.035. ISSN 0308–8146, [DOI] [PubMed] [Google Scholar]
- Al-Rawahi A.S., Rahman M.S., Guizani N., Essa M.M. Chemical composition, water sorption isotherm, and phenolic contents in fresh and dried pomegranate peels. Dry. Technol. 2013;31(3):257–263. [Google Scholar]
- Altunkaya A., Hedegaard R.V., Harholt J., Brimer L., Gökmen V., Skibsted L.H. Palatability and chemical safety of apple juice fortified with pomegranate peel extract. Food Funct. 2013;4(10):1468–1473. doi: 10.1039/C3FO60150A. [DOI] [PubMed] [Google Scholar]
- Al-Zoreky N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International journal of food microbiology. 2009;134(3):244–248. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Amber R., Adnan M., Tariq A., Khan S.h., Niaz M.S., et al. Antibacterial activity of selected medicinal plants of northwest Pakistan traditionally used against Mastitis in livestock. Saudi Journal of Biological Sciences. 2017 doi: 10.1016/j.sjbs.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambigaipalan P., Camargo A., Shahidi F. Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J. Agric. Food Chem. 2016;64(34):6584–6604. doi: 10.1021/acs.jafc.6b02950. [DOI] [PubMed] [Google Scholar]
- Andrea A., Verpoortea R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011;34(1):785–801. doi: 10.1016/j.indcrop.2011.01.019. [DOI] [Google Scholar]
- Anhê F.F., Geneviève P., Denis R., Yves D., Emile L., André M. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes. 2016;7(2):146–153. doi: 10.1080/19490976.2016.1142036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Barros Z., Salgado J., Melo P., Biazotto F. Enrichment of Commercially-Prepared Juice With Pomegranate (Punica granatum L.) Peel Extract as a Source of Antioxidants. J Food Res. 2014;Vol. 3: 6:179–187. doi: 10.5539/jfr.v3n6p179. [DOI] [Google Scholar]
- Gullon Beatriz, Pintado Manuela E., Pérez-Álvarez José A., Viuda-Martos Manuel. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction, Food Control, 2016; ISSN: 0956-7135, Vol: 59, Page: 94-98, https://doi.org/10.1016/j.foodcont.2015.05.025.
- Bhatt Anjali & Patel Vinayak.A Comparative Study Between Antioxidant Potential of Ripe and Pre-ripe Mango Using Conventional and Physiological Extraction. Int.l J. Fruit Sci. 2016;16(1):57–68. doi: 10.1080/15538362.2015.1044693. [DOI] [Google Scholar]
- Çam M., Erdoğan F., Aslan D., Dinç M. Enrichment of functional properties of ice cream with pomegranate by-products. J. Food Sci. 2013;78(10):1543–1550. doi: 10.1111/1750-3841.12258. PMID: 24102443. [DOI] [PubMed] [Google Scholar]
- Carpentieri S., Soltanipour F., Ferrari G., Pataro G., Donsì F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants. 2021;10:1417. doi: 10.3390/antiox10091417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppo E., Marchese A. Antibacterial Activity of Polyphenols. Curr. Pharm. Biotechnol. 2014;15:380–390. doi: 10.2174/138920101504140825121142. [DOI] [PubMed] [Google Scholar]
- Crozier A., Jaganath I.B., Clifford M.N. Dietary phenolics: chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009;26:1001–1043. doi: 10.1039/B802662A. [DOI] [PubMed] [Google Scholar]
- Das A.K., Nanda P.K., Chowdhury N.R., Dandapat P., Gagaoua M., Chauhan P., Pateiro M., Lorenzo J.M. Application of Pomegranate by-Products in Muscle Foods: Oxidative Indices, Colour Stability, Shelf Life and Health Benefits. Molecules. 2021;26(2):467. doi: 10.3390/molecules26020467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen, M., Ozturk, N., Ozturk, Y. 2011. The antioxidant potency of Punica granatum L. Fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J Med Food. 2011 Dec;14(12):1638-46. doi: 10.1089/jmf.2011.0062. Epub 2011 Aug 23. PMID: 21861726. [DOI] [PubMed]
- Dri M., Canfora P., Antonopoulos I.S., Gaudillat P. Publications Office of the European Union; Luxembourg: 2018. Best Environmental Management Practice for the Waste Management Sector, JRC Science for Policy Report, EUR 29136 EN. [Google Scholar]
- Turrini Eleonora, Ferruzzi Lorenzo, Fimognari Carmela. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid. Med.Cell. Longev., 2015; vol. 2015, Article ID 938475, 19 pages. https://doi.org/10.1155/2015/938475. [DOI] [PMC free article] [PubMed]
- El-hadary A., Hassanien M. Phenolic profiles, antihyperglycemic, antihyperlipidemic, and antioxidant properties of pomegranate (Punica granatum) peel extract. J. Food Biochem. 2019;43:1–9. doi: 10.1111/jfbc.12803. [DOI] [PubMed] [Google Scholar]
- Eliana Harue Endo, Tânia Ueda-Nakamura, Celso Vataru Nakamura, Benedito Prado Dias Filho. Activity of Spray-dried Microparticles Containing Pomegranate Peel Extract against Candida albicans. Molecules. 2012; 17:10094-10107. [DOI] [PMC free article] [PubMed]
- El-Said Marwa M., Haggag H.F., Fakhr El-Din Hala M., Gad A.S., Farahat A.M. Antioxidant activities and physical properties of stirred yoghurt fortified with pomegranate peel extracts. Ann. Agric. Sci. 2014;Vol. 59: 2:207–212. doi: 10.1016/j.aoas.2014.11.007. [DOI] [Google Scholar]
- Elwej A., Salah G., Ben K.C., Fakhfakh F., Zeghal N., Ben A.I. Protective effects of pomegranate peel against hematotoxicity, chromosomal aberrations, and genotoxicity induced by barium chloride in adult rats. Pharmac. Biol. 2016;54(6):964–974. doi: 10.3109/13880209.2015.1087035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Es-Safi N.E., Ghidouche S., Ducrot P.H. Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules (Basel, Switzerland) 2007;12(9):2228–2258. doi: 10.3390/12092228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fao The future of food and agriculture – Alternative pathways to 2050. Summary version. Rome. 2018;60:pp. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Farouk M., Mohamed J., Kalthoum C.J., Munevver S., Atalay S., Malika T.-A. Phenolic Contents and Antioxidant Potential of Crataegus Fruits Grown in Tunisia as Determined by DPPH, FRAP, and β-Carotene/Linoleic Acid Assay. J. Chem. 2013;2013vol:6 pages. doi: 10.1155/2013/378264. [DOI] [Google Scholar]
- Fourati M., Smaoui S., Ennouri K., Hlima H., Ben E.K., Chakchouk-Mtibaa A., Sellem I., Mellouli L. Multiresponse Optimization of Pomegranate Peel Extraction by Statistical versus Artificial Intelligence: Predictive Approach for Foodborne Bacterial Pathogen Inactivation. Evid.-based Complement. Altern. Med. 2019;2019:18 p. doi: 10.1155/2019/1542615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas T., Marques F., Kuster R., et al. Punica granatum L. Inhibits the Growth of Microorganisms Associated with Bovine Mastitis. The Natural Products Journal. 2019 doi: 10.2174/2210315509666191111105143. [DOI] [Google Scholar]
- Gulsunoglu Z., Karbancioglu-Guler F., Raes K., Kilic-Akyilmaz M. Soluble and insoluble-bound phenolics and antioxidant activity of various industrial plant wastes. Int. J. Food Prop. 2019;22(1):1501–1510. doi: 10.1080/10942912.2019.1656233. [DOI] [Google Scholar]
- Hasan M.M., Bashir T., Bae H. Use of Ultrasonication Technology for the Increased Production of Plant Secondary Metabolites. Molecules. 2017;22, 7:1046. doi: 10.3390/molecules22071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S., Abolghasem G., Hassan K., Taher M.M. Effects of dietary α-tocopherol acetate, pomegranate peel, and pomegranate peel extract on phenolic content, fatty acid composition, and meat quality of broiler chickens. J. Appl. Anim. Res. 2017;45(1):629–636. doi: 10.1080/09712119.2016.1248841. [DOI] [Google Scholar]
- Ishimoto Hidekazu, Tai Akihiro, Yoshimura Morio, Amakura Yoshiaki, Yoshida Takashi, Hatano Tsutomu, Ito Hideyuki. Antioxidative Properties of Functional Polyphenols and Their Metabolites., Biosci., Biotechnol., Biochem., 2012; 76:2, 395-399, DOI: 10.1271/bbb.110717. [DOI] [PubMed]
- https://www.fao.org/platform-food-loss-waste/flw-data/en/ Database. 2022. Available online: (accessed on 20 September 2022).
- Chang En Hyung, Huang Joanne, Lin Zixiang, Brown Angela C. Catechin-mediated restructuring of a bacterial toxin inhibits activity. Biochimica et Biophysica Acta (BBA) - General Subjects, 2019; Volume 1863, Issue 1, Pages 191-198, ISSN 0304-4165,https://doi.org/10.1016/j.bbagen.2018.10.011. [DOI] [PMC free article] [PubMed]
- Irina I., Mohamed G. Biological Activities and Effects of Food Processing on Flavonoids as Phenolic Antioxidants. Adv. Appl. Biotechnol. 2012 doi: 10.5772/30690. Available from: https://www.intechopen.com/chapters/26397. [DOI] [Google Scholar]
- Jurendić T. Ščetar Mario Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants. 2021;10(7):1052. doi: 10.3390/antiox10071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaderides K., Papaoikonomou L., Serafim M., Goula A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. 2019;137:1–11. doi: 10.1016/j.cep.2019.01.006. ISSN 0255–2701, [DOI] [Google Scholar]
- Kakkar S., Tandon R., Tandon N. Utilizing fruits and vegetables waste as functional food: a review. Plant cell Biotechnol. Mol. Boil. 2021;22(17–18):41–58. https://www.ikppress.org/index.php/PCBMB/article/view/6070 Retrieved from. [Google Scholar]
- Kandylis P., Evangelos K. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods. 2020;9(2):122. doi: 10.3390/foods9020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M., Kaur N., Sharma C., Kaur G., Kaur R., Batra K., Rani J. Citrullus colocynthis an Important Plant in Indian Traditional System of Medicine. Pharmacog Rev. 2020;14(27):22–27. doi: 10.5530/phrev.2020.14.4. [DOI] [Google Scholar]
- Kazemi M., Karim R., Mirhosseini H., Hamid A.A. Optimization of Pulsed Ultrasound Assisted Technique for Extraction of Phenolics from Pomegranate Peel of Malas Variety: Punicalagin and Hydroxybenzoic Acids. Food Chem. 2016 doi: 10.1016/j.foodchem.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Khorrami P., Gholami-Ahangaran M., Moghtadaei-Khorasgani E. The efficacy of pomegranate peel extract on Eimeria shedding and growth indices in experimental coccidiosis in broiler chickens. Vet Med Sci. 2022 Mar;8(2):635–641. doi: 10.1002/vms3.714. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kumar, S., Pandey, A. 2013. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J.., Vol. 2013, ID 162750, 16 p. DOI:10.1155/2013/162750. [DOI] [PMC free article] [PubMed]
- Kurutas E. Belge. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state, Nutrition Journal (2016) 15:71 DOI 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed]
- Lampakis D., Skenderidis P., Leontopoulos S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes. 2021;9:236. doi: 10.3390/pr9020236. [DOI] [Google Scholar]
- Laosirisathian N., Saenjum C., Sirithunyalug J., Eitssayeam S., Sirithunyalug B., Chaiyana W. The Chemical Composition, Antioxidant and Anti Tyrosinase Activities, and Irritation Properties of Sripanya Punica granatum Peel Extract. Cosmetics. 2020;7:7. doi: 10.3390/cosmetics7010007. [DOI] [Google Scholar]
- Lavecchia T., Rea G., Antonacci A., Maria G.T. Healthy and Adverse Effects of Plant-Derived Functional Metabolites: The Need of Revealing their Content and Bioactivity in a Complex Food Matrix. Crit Rev Food Sci Nutr. 2013;53(2):198–213. doi: 10.1080/10408398.2010.52082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.E., Kim G.S., Park S. Determination of chokeberry (Aronia melanocarpa) polyphenol components using liquid chromatography-tandem mass spectrometry: Overall contribution to antioxidant activity. Food Chem. 2014;146:1–5. doi: 10.1016/j.foodchem.2013.09.029. [DOI] [PubMed] [Google Scholar]
- Liu H.-M., Xu P.-F., Cheng M.-Y., Lei S.-N., Liu Q.-L., Wang W. Optimization of Fermentation Process of Pomegranate Peel and Schisandra Chinensis and the Biological Activities of Fermentation Broth: Antioxidant Activity and Protective Effect Against H2O2-induced Oxidative Damage in HaCaT Cells. Molecules. 2021;26:3432. doi: 10.3390/molecules26113432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarini M., Durazzo A., Bernini R., Campo M., Vita C., Souto E.B., Lombardi-Boccia G., Ramadan M.F., Santini A., Romani A. Fruit Wastes as a Valuable Source of Value-Added Compounds: A Collaborative Perspective. Molecules. 2021;26:6338. doi: 10.3390/molecules26216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchian V., Georgescu M.I., Savulescu E., Popa V. Some aspects of morpho-anatomical features of the invasive species Eleusine indica (L.) Gaertn. Sci. Papers. Ser. A. Agron. 2019;62(1) http://agronomyjournal.usamv.ro/pdf/2019/issue_1/Art75.pdf. [Google Scholar]
- Lv Q., Long J., Gong Z., et al. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Natural Product Communications. 2021;16(7) doi: 10.1177/1934578X211027745. [DOI] [Google Scholar]
- Maciej K., Katarzyna P., Iwona J.-K., Jacek N. Isoflavone composition, polyphenols content and antioxidative activity of soybean seeds during tempeh fermentation. 2020;15:27–33. [Google Scholar]
- Man G., Xu L., Wang Y., Liao X., Xu Z. Profiling Phenolic Composition in Pomegranate Peel From Nine Selected Cultivars Using UHPLC-QTOF-MS and UPLC-QQQ-MS. Front. Nutr. 2022;8 doi: 10.3389/fnut.2021.807447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour E., Ben K.A., Lachiheb B., Abid M., Bachar K., Ferchichi A. Phenolic Compounds, Antioxidant, and Antibacterial Activities of Peel Extract from Tunisian Pomegranate. JAST. 2013;15(7):1393–1403. http://jast.modares.ac.ir/article-23-671-en.html URL. [Google Scholar]
- Marchev A.S., Yordanova Z.P., Georgiev M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020;40(4):443–458. doi: 10.1080/07388551.2020.1731414. [DOI] [PubMed] [Google Scholar]
- Marra F., Petrovicova B., Canino F., Maffia A., Mallamaci C., Muscolo A. Pomegranate Wastes Are Rich in Bioactive Compounds with Potential Benefit on Human Health. Molecules. 2022;27:5555. doi: 10.3390/molecules27175555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzouk, B., Marzouk, Z., Décor, R., Edziri, H., Haloui, E., Fenina, N., Aouni, M. 2009. Antibacterial and anticandidal screening of Tunisian Citrullus colocynthis Schrad from Medenine. J.Ethnopharmcol.,2009,Volume 125, Issue 2,Pages 344-349,ISSN 0378-8741, https://doi.org/10.1016/j.jep.2009.04.025. [DOI] [PubMed]
- Mastrodi S.J., Baroni Ferreira T.R., de Oliveira B.F., et al. Increased Antioxidant Content in Juice Enriched with Dried Extract of Pomegranate (Punica granatum) Peel. Plant Foods Hum Nutr. 2012;67:39–43. doi: 10.1007/s11130-011-0264-y. [DOI] [PubMed] [Google Scholar]
- Matsumoto L., Moreira G., Silva M., et al. In vitro antibacterial activities of pomegranate extract against standard microorganisms of bovine mastitis. Journal of medicinal plant research. 2015;9(36):950–953. doi: 10.5897/JMPR2015.5844. [DOI] [Google Scholar]
- Mayank G., Kumar G.M., Kumar S.A., Tripathi Y.B., Goel R.K. Nath Gopal Antioxidant Capacity and Radical Scavenging Effect of Polyphenol Rich Mallotus philippenensis Fruit Extract on Human Erythrocytes: An In Vitro Study. Sci. World J. 2014;2014:12 p. doi: 10.1155/2014/279451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsämuuronen S., Sirén H. Bioactive phenolic compounds, metabolism and properties: a review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem Rev. 2019;18:623–664. doi: 10.1007/s11101-019-09630-2. [DOI] [Google Scholar]
- Mo Y., Ma J., Gao W., Zhang L., Li J., Zang J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front Nutr. 2022 Jun;9(9) doi: 10.3389/fnut.2022.887113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel Moneim, Ahmed E., Othman, Mohamed S., Mohmoud, Sahar M., El-Deib, Kamal M. Pomegranate peel attenuates aluminum-induced hepatorenal toxicity. Toxicol. Mech.Methods, 2013; Vol. 23:8,624-633, DOI:10.3109/15376516.2013.8234 [DOI] [PubMed]
- Nag S., Sit N. Optimization of ultrasound assisted enzymatic extraction of polyphenols from pomegranate peels based on phytochemical content and antioxidant property. Food Measure. 2018;12:1734–1743. doi: 10.1007/s11694-018-9788-2. [DOI] [Google Scholar]
- Neela Satheesh & Solomon Workneh Fanta. Kale: Review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food & Agric., 2020; 6:1, DOI: 10.1080/23311932.2020.1811048.
- Nimse S.B., Dilipkumar P. Free radicals, natural antioxidants, and their reaction mechanisms (Review Article). RSC. Advances. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- Noory F.A., Alumeri J.K., Al-hamadawi H.A. Protective and therapeutic role of pomegranate peel extract to reduce profen side effects. Eurasia J Biosci. 2020;14:1775–1778. [Google Scholar]
- Ofosu F.K., Daliri E.-B.-M., Elahi F., Chelliah R., Lee B.-H., Oh D.-H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020;4 doi: 10.3389/fsufs.2020.525810. [DOI] [Google Scholar]
- Hui Ouyang, Kun Hou, Wanxi Peng, Zhenling Liu, Heping Deng Antioxidant and xanthine oxidase inhibitory activities of total polyphenols from onion, Saudi J.Biol. Sci., 2018;25, 7, 1509-1513, ISSN 1319-562X, https://doi.org/10.1016/j.sjbs.2017.08.005. [DOI] [PMC free article] [PubMed]
- Pande G., Akoh C.C. Antioxidant capacity and lipid characterization of six Georgia-grown pomegranate cultivars. J Agric Food Chem. 2009 Oct 28;57(20):9427–9436. doi: 10.1021/jf901880p. PMID: 19743855. [DOI] [PubMed] [Google Scholar]
- Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidat. Med. Cell. Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra C., Muñoz P., Bustos L., Parra F., Simirgiotis M.J., Escobar H. UHPLC-DAD Characterization of Origanum vulgare L. from Atacama Desert Andean Region and Antioxidant, Antibacterial and Enzyme Inhibition Activities. Molecules. 2021;26:2100. doi: 10.3390/molecules26072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharkphoom P., Atcharaporn I., Anusak S. Preparation method and stability of ellagic acid-rich pomegranate fruit peel extract. Pharm. Biol. 2010;48(2):201–205. doi: 10.3109/13880200903078503. [DOI] [PubMed] [Google Scholar]
- Plaza M., Pozzo T., Liu J., Ara K. Gulshan, Turner C., Karlsson E. Nordberg. Substituent Effects on in Vitro Antioxidizing Properties, Stability, and Solubility in Flavonoids, J.Agric. Food Chem., 2014; 62, 15: 3321–3333, DOI: 10.1021/jf405570u · Source: PubMed. [DOI] [PubMed]
- Poyrazoğlu E., Gökmen W., Artik N. Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J Food Compos Anal. 2002;15:567–575. [Google Scholar]
- Qin Y.Y., Zhang Z.H., Li L., et al. Antioxidant effect of pomegranate rind powder extract, pomegranate juice, and pomegranate seed powder extract as antioxidants in raw ground pork meat. Food Sci Biotechnol. 2013;22:1063–1069. doi: 10.1007/s10068-013-0184-8. [DOI] [Google Scholar]
- Qu W., Breksa Iii A.P., Pan Z., Ma H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012 Jun 1;132(3):1585–1591. doi: 10.1016/j.foodchem.2011.11.106. Epub 2011 Dec 1 PMID: 29243653. [DOI] [PubMed] [Google Scholar]
- Raheema R. Effect of Pomegranate Peel Extract on some Biochemical and Histopathological Parameters in Experimental Induced Mice with Staphylococcus aureus. Journal of Animal Health and Production. 2016:42–49. doi: 10.14737/journal.jahp/2016/4.2.42.49. [DOI] [Google Scholar]
- Setiadhi Riani, Sufiawati Irna, Hidayat Wahyu. Identification of active component in red pomegranate (Punica granatum L.) seeds ethanolic extract. J. Dentomaxillofac Sci., 2019;Volume 4, Number 2: 83-86 P-ISSN.2503-0817, E-ISSN.2503-0825.
- Metrani Rita, Jashbir Singh, Pratibha Acharya, Guddadarangavvanahally K. Jayaprakasha, and Bhimanagouda S. Patil. Comparative Metabolomics Profiling of Polyphenols, Nutrients and Antioxidant Activities of Two Red Onion (Allium cepa L.) Cultivars. Plants 9, 2020; no. 9: 1077. https://doi.org/10.3390/plants9091077. [DOI] [PMC free article] [PubMed]
- Rosas-Burgos E.C., Burgos-Hernández A., Noguera-Artiaga L., Kačániová M., Hernández-García F., Cárdenas-López J.L., Carbonell-Barrachina Á.A. Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J Sci Food Agric. 2017 Feb;97(3):802–810. doi: 10.1002/jsfa.7799. [DOI] [PubMed] [Google Scholar]
- Ross K.A., David G. Fukumoto Lana The chemical composition, antioxidant activity and α-glucosidase inhibitory activity of water-extractable polysaccharide conjugates from northern Manitoba lingonberry. Cogent Food Agric. 2015;1: 1 , 1109781 doi: 10.1080/23311932.2015.1109781. [DOI] [Google Scholar]
- Ruiz-Torralba A., Guerra-Hernández E., García-Villanova B. Antioxidant capacity, polyphenol content and contribution to dietary intake of 52 fruits sold in Spain, CyTA –. J Food. 2018;16(1):1131–1138. doi: 10.1080/19476337.2018.1517828. [DOI] [Google Scholar]
- Sari W.A., Puspita S.O., Dhara N., Fragrantia T.C. Pomegranate Juice Inhibits Periodontal Pathogens Biofilm In Vitro. J. Dent. Sci. 2018;2(3):101–108. doi: 10.26912/sdj.v2i3.2572. DOI: [DOI] [Google Scholar]
- Sayago-Ayerdi S., García-Martínez D.L., Ramírez-Castillo A.C., Ramírez-Concepción H.R., Viuda-Martos M. Tropical Fruits and Their Co-Products as Bioactive Compounds and Their Health Effects: A Review. Foods. 2021;10:1952. doi: 10.3390/foods10081952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed-Ahmed E.F. Evalution of pomegranate peel fortified pan bread on body weight loss. Int. J. Nutr. Food Sc. 2014;3(5):411–420. doi: 10.11648/j.ijnfs.20140305.18. [DOI] [Google Scholar]
- Seeram N.P., Adams L.S., Henning S.M., Yantao N., Yanjun Z., Nair M.G., David H. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005;16(6):360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Seeram N., Lee R., Hardy M., Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005;41:49–145. doi: 10.1016/j.seppur.2004.04.003. [DOI] [Google Scholar]
- Sepúlveda L., Wong-Paz J.E., Buenrostro-Figueroa J., Ascacio-Valdés J.A., Aguilera-Carbó A., Aguilar C.N. Solid state fermentation of pomegranate husk: Recovery of ellagic acid by SEC and identification of ellagitannins by HPLC/ESI/MS. Food Biosci. 2018;22:99–104. doi: 10.1016/j.fbio.2018.01.006. [DOI] [Google Scholar]
- Sharma N. Free radicals, antioxidants and disease. Biol. Med. 2014;6(3) doi: 10.4172/0974-8369.1000214. [DOI] [Google Scholar]
- Shaygannia E., Bahmani M., Zamanzad B., Rafieian-Kopaei M. A Review Study on Punica granatum L. Journal of Evidence-Based Complementary & Alternative Medicine. 2015;21(3):221–227. doi: 10.1177/2156587215598039. [DOI] [PubMed] [Google Scholar]
- Shaygannia E., Bahmani M., Zamanzad B., Rafieian-Kopaei M. A Review Study on Punica granatum L. Journal of Evidence-Based Complementary & Alternative Medicine. 2016;221–227 doi: 10.1177/2156587215598039. [DOI] [PubMed] [Google Scholar]
- Singh J.P., Kaur A., Shevkani K., Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016 Nov;53(11):4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Singh J.P., Kaur A., Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018;261:75–86. doi: 10.1016/j.foodchem.2018.04.039. [DOI] [PubMed] [Google Scholar]
- Singha B., Singhb J. Pal, Kaurb A.l, Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review Food Chemistry, Vol.261, 2018, P.75-86, https://doi.org/10.1016/j.foodchem.2018.04.039. [DOI] [PubMed]
- Rajeev K Singla, Ashok K Dubey, Arun Garg, Ramesh K Sharma, Marco Fiorino, Sara M Ameen, Moawiya A Haddad, Masnat Al-Hiary Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures, J. AOAC Int., 2019; 102, 5, 1. 1397–1400. https://doi.org/10.1093/jaoac/102.5.1397. [DOI] [PubMed]
- Singla, R.K., Dubey, A.K., Garg, A., Sharma, R.K., Fiorino, M., Ameen, S.M., Haddad, M.A., Al-Hiary, M. 2019. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures, J. AOAC Int.,2019; Vol., 102, (5):1397-1400, DOI: 10.5740/jaoacint.19-0133. [DOI] [PubMed]
- Smaoui S., Hlima H. Ben, Mtibaa A. Ch., Fourati M., Sellem I., Elhadef K., Ennouri K., Mellouli L. Pomegranate peel as phenolic compounds source: Advanced analytical strategies and practical use in meat products, Meat Science, Vol. 158, 2019, 107914, https://doi.org/10.1016/j.meatsci.2019.107914. [DOI] [PubMed]
- Song B., Li J., Li J. Pomegranate peel extract polyphenols induced apoptosis in human hepatoma cells by mitochondrial pathway. Food Chem Toxicol. 2016 Jul;93:158–166. doi: 10.1016/j.fct.2016.04.020. Epub 2016 Apr 24 PMID: 27120393. [DOI] [PubMed] [Google Scholar]
- Stojanović I., Šavikin K., Đedović N., et al. Pomegranate peel extract ameliorates autoimmunity in animal models of multiple sclerosis and type 1 diabetes. Journal of functional foods. 2017;35:522–530. doi: 10.1016/j.jff.2017.06.021. [DOI] [Google Scholar]
- Sulieman Abdel Moneim E., Babiker Wisal A.M., Elhardallou S.B., Elkhalifa E.A., Veettil V.N. Influence of Enrichment of Wheat Bread with Pomegranate (Punica granatum L) Peels by-Products. Int. J. Food Sci. Nutr. Eng. 2016;6(1):9–13. doi: 10.5923/j.food.20160601.02. [DOI] [Google Scholar]
- Sun T., Simon P.W., Tanumihardjo S.A. Antioxidant Phytochemicals and Antioxidant Capacity of Biofortified Carrots (Daucus carota L.) of Various Colors. J. Agric. Food Chem. 2009;57(10):4142–4147. doi: 10.1021/jf9001044. [DOI] [PubMed] [Google Scholar]
- Sun Y.u., Tao X., Men Xia X.Z., Wang T. In vitro and in vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. J. Integr. Agric. 2017;16(8):1808–1818. doi: 10.1016/S2095-3119(16)61560-5. [DOI] [Google Scholar]
- Suriyaprom S., Mosoni P., Leroy S., Kaewkod T., Desvaux M., Tragoolpua Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants. 2022;11:602. doi: 10.3390/antiox11030602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandokazi M.P., Pearl M.N., Amos F.O., Linus O.U. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules. 2020;25:4690. doi: 10.3390/molecules25204690. DOI:molecules25204690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Deng F. Phytochemistry and biological activity of mustard (Brassicajuncea): a review. CyTA–J.Food. 2020;18(1):704–718. doi: 10.1080/19476337.2020.1833988. [DOI] [Google Scholar]
- Trigo João, P., Alexandre Elisabete, M. C., Silva, S., Costa, E., Saraiva Jorge, A., Pintado, M. 2020. Study of viability of high pressure extract from pomegranate peel to improve carrot juice characteristics, Food Funct., 2020; Vol.11:4, 3410- 3419, DOI - 10.1039/C9FO02922B. [DOI] [PubMed]
- Vishal J., Murugananthan G., Deepak M., Viswanatha G.L, Manohar D. Isolation and Standardization of Various Phytochemical Constituents from Methanolic Extracts of Fruit Rinds of Punica granatum, Chin. J. Nat. Med., 2011; Vol. 9:6,414-420 , DOI:10.3724/SP.J.1009.2011.00414.
- Viuda-Martos M., Fernández-López J., Pérez-Álvarez J. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010;9:635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M., Pérez-Álvarez J.A., Sendra E., Fernández-López J. In vitro antioxidant properties of pomegranate (Punica granatum) peel powder extract obtained as coproduct in the juice extraction process. J. Food Process. Preserv. 2013;37(5):772–776. doi: 10.1111/j.1745-4549.2012.00715.x. [DOI] [Google Scholar]
- Walid E., Hédia H., Nizar T., Yassine Y., Nizar N., Ali F. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plant Res. 2012;Vol. 6: 32:4724–4730. doi: 10.5897/JMPR11.995. [DOI] [Google Scholar]
- Wang Z., Pan Zh., Ma H., Atungulu G. Extract of Phenolics From Pomegranate Peels. The Open Food Science Journal, 2011; 5: 17-25, Electronic publication date 19/4/2011, DOI: 10.2174/1874256401105010017.
- Wu S., Tian L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum) Molecules. 2017;22(10):1606. doi: 10.3390/molecules22101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhu C., Zhang Y., Li Y., Sun J. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int. J.Biol.Macromol., 2019; Vol.137,504-511,ISSN01418130, https://doi.org/10.1016/j.ijbiomac.2019.06.139. [DOI] [PubMed]
- Yang Y., Ding X., Memon F.U., Zhang G., Jiang M., Hu D., Si H. Punicalagin: a monomer with anti-Eimeria tenella effect from fruit peel of Punica granatum L. Poult Sci. 2022 Oct;101(10) doi: 10.1016/j.psj.2022.102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Hee H., Young J.E., Yooheon P., Kwang-Soon S., Young K.T., Kwang-Won Y.u., Jae C.U. Suh Hyung Joo Enzymatic Improvement in the Polyphenol Extractability and Antioxidant Activity of Green Tea Extracts. Biosci. Biotechnol. Biochem. 2013;77(1):22–29. doi: 10.1271/bbb.120373. [DOI] [PubMed] [Google Scholar]
- Zaki S.A., Abdelatif S.H., Abdelmohsen N.R., Ismail F.A. Phenolic Compounds and Antioxidant Activities of Pomegranate Peels. Int. J. Food Eng. 2015;1(2):73–79. doi: 10.18178/ijfe.1.2.73-76. [DOI] [Google Scholar]
- Zekeya N., Ibrahim M., Mamiro B., Ndossi H., Kilonzo M., Mkangara M., Chacha M., Chilongola J., Kideghesho J. Potential of natural phenolic compounds as antioxidant and treatment of chronic diseases from Africa medicinal plant; Bersama abyssinica (Meliathaceae) Saudi J. Biol. Sci. 2020 doi: 10.1016/j.sjbs.2022.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]