Abstract
Carnitine is a medically needful nutrient that contributes in the production of energy and the metabolism of fatty acids. Bioavailability is higher in vegetarians than in people who eat meat. Deficits in carnitine transporters occur as a result of genetic mutations or in combination with other illnesses such like hepatic or renal disease. Carnitine deficit can arise in diseases such endocrine maladies, cardiomyopathy, diabetes, malnutrition, aging, sepsis, and cirrhosis due to abnormalities in carnitine regulation. The exogenously provided molecule is obviously useful in people with primary carnitine deficits, which can be life-threatening, and also some secondary deficiencies, including such organic acidurias: by eradicating hypotonia, muscle weakness, motor skills, and wasting are all improved l-carnitine (LC) have reported to improve myocardial functionality and metabolism in ischemic heart disease patients, as well as athletic performance in individuals with angina pectoris. Furthermore, although some intriguing data indicates that LC could be useful in a variety of conditions, including carnitine deficiency caused by long-term total parenteral supplementation or chronic hemodialysis, hyperlipidemias, and the prevention of anthracyclines and valproate-induced toxicity, such findings must be viewed with caution.
Keywords: l-carnitine, Nutrition, Pathology, Anti-wasting effect, Health benefits
Abbreviations: LC, l-carnitine; PCD, Primary carnitine deficiency; SCD, Secondary carnitine deficiency; HFD, High-Fat Diet; NF-kB, Nuclear factor-kB; ROS, Reactive oxygen species; COPD, Chronic obstructive pulmonary disease; AIF, Apoptosis-inducing factor; AD, Alzheimer's disease; VD, Vascular dementia; BBB, Blood–brain barrier; TLE, Temporal lobe epilepsy; GOT, Glutamic oxaloacetic transaminase; CHF, Chronic heart failure; MI, myocardial infarction; ESRD, End-stage renal disease; MTX, Methotrexate; HCC, Hepatocellular carcinoma; CC, Cancer cachexia; OSL, Observed safe level; HOI, Highest observed intake
1. Introduction
Carnitine (3-hydroxy-4-N-trimethylammoniobutanoate) is being researched extensively since its development 100 years ago. Research to date has expanded the knowledge of carnitine's function in metabolism, and increase in research interest in the use of carnitine in therapeutics is noticed. This is partly due to the discovery of mechanisms for both primary and secondary carnitine deficit, as well as carnitine’s use as a therapeutics and supplement. Furthermore, characterizing the biological processes of carnitine production has resurrected this characteristic of carnitine homeostasis (Almannai et al., 2019).
Human carnitine status fluctuates depending on body composition, gender, and food. Carnitine consumption in the diet is favorably correlated with plasma carnitine levels. The approach used to determine carnitine content of the food is outdated and ineffective. Nonetheless, carnitine in the diet is vital. The carnitine biosynthesis enzymes' molecular biology has been completed. Carnitine synthesis is a very effective system that requires pathways in several organs. The abundance of trimethyllysine from tissue proteins determines overall biosynthesis. There has yet to be a case of carnitine depletion caused by a biosynthetic error (J.L. et al., 2010, Ringseis et al., 2018, Steiber et al., 2004).
Carnitine is produced endogenously from two essential amino acids, methionine and lysine, when it is not received from diet. This can occur in the brain, liver, and kidneys (Cave et al., 2008). Because skeletal and cardiac muscles have the greatest quantities, they are unable to synthesis carnitine and must obtain it from plasma. Microbes in the intestinal tract mostly destroy unabsorbed LC (Rebouche, 2004). Carnitine is almost entirely intracellular (99 %) (Cave et al., 2008). Carbohydrate metabolism is influenced by carnitine. Carnitine regulatory abnormalities have been attributed to diabetic complications, trauma, hemodialysis, starvation, obesity, cardiomyopathy, fasting, endocrine imbalances, medication interactions, and other conditions (Guerra et al., 2021).
Dietary LC administration, the pharmacologically active form, is beneficial to patients with uremia and can positively affect neuropathic pain, nerve conduction, and immunological role in diabetic individuals, as well as saving lives in patients with primary carnitine deficiency (Bonomini et al., 2011, Cha, 2008, Ribas et al., 2014). Carnitine has shown potential for treating of a number of neurological illnesses, including Alzheimer's disease, Parkinson disease, hepatic encephalopathy, autism spectrum disorder, and other painful neuropathies (Malaguarnera, 2013, Pettegrew et al., 1995). In dry eyes, topical treatment provides osmoprotection while also modulating immunological and proinflammatory responses (Jin et al., 2019). Carnitine is acknowledged as a dietary supplementary material in the treatment of cardiovascular maladies (Ferrari et al., 2004) and there is growing evidence that it may be effective in the treatment of obesity (Gao et al., 2020), decreasing intolerance of glucose, and raising expenditure of total energy (Van Weyenberg et al., 2009).
The goal of this study is to outline LC 's role in human nutrition and disease, as well as to discuss the main research topics in this subject. This review discussed in detail the pathological states for LC deficiencies, as well as toxicological profile and safety concerns too.
2. Methodology
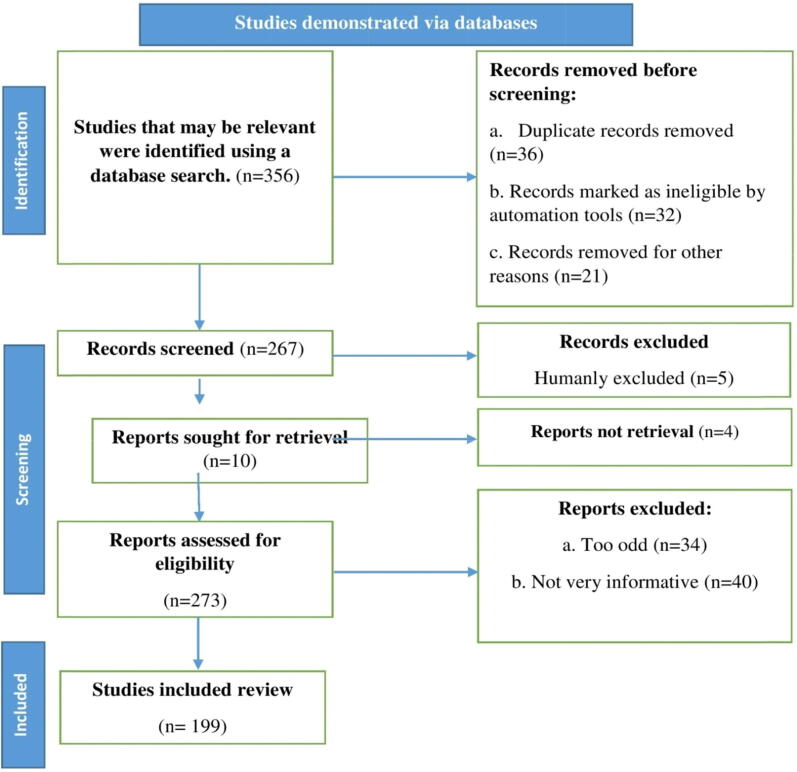
Using resources such as Elsevier, Science Direct, Scopus, PubMed, and Web of Science, we performed a literature search and identified recent related literatures. In our searches, we used the keywords l-carnitine, nutrition, pathology, and health perspective. We chose and assessed research reports, literature reviews, and original research publications in English. We also checked over the citations and added them where necessary. Page et al. (Page et al., 2021) suggested employing an algorithm that incorporated all of the procedures required in determining the study's relevant content, as shown in the flow chart in Fig. 1.
Fig. 1.
Diagram showing the search strategy, the number of records identified and the exclusion and inclusion criteria; n = number of literatures.
3. l-carnitine deficiencies and pathological states
Two separate carnitine deficiency conditions have been recorded, albeit establishing a precise difference between “primary” and “secondary” carnitine deficit can be problematic in certain circumstances (Angelini et al., 1987). Primary carnitine deficiency (PCD) is an autosomal recessive disorder characterized by a lack of plasma membrane carnitine transport owing to a shortcoming in the OCTN2 carnitine transporter. This shortage inhibits tissue uptake, resulting in reduced accumulation in the skeletal muscle and heart, as well as potentiating increased renal carnitine loss (Burwinkel et al., 1999, Y et al.., 1999), resulting in systemic carnitine depletion (Reuter et al., 2008). Patients with primary deficiency excrete (free) carnitine through the urine due to impaired renal absorption (Cederbaum et al., 2002, Spiekerkoetter et al., 2003). The only known instances of primary carnitine insufficiency are genetic abnormalities of transporter activity (Pons and De Vivo, 1995).
PCD affects 1–5 people in 10,000 and most usually presents between the ages of 1 and 7 (Magoulas and El-Hattab, 2012). Hypoketotic hypoglycemia encephalopathy is the most prevalent symptom of PCD. There has also been evidence of cardiomyopathy (Erguven et al., 2007). SLC22A5 is the gene that causes PCD. A number of mutations have been identified (Mayatepek et al., 2000). PCD affects three tissues/organs: the skeletal muscle, which develops myopathy, the central nervous system, which develops encephalopathy due to hypoketotic hypoglycemia; and the cardiac muscle, which develops progressive cardiomyopathy. LC supplementation is a life-saving therapeutic for these individuals (Mayatepek et al., 2000).
Secondary deficiency can be characterized by substantial excretion of carnitine via the urine in acyl-carnitine form. This is owing to the some organic acid accumulation (Rinaldo et al., 1998, Thangavelu, 2010). Secondary carnitine deficiency (SCD) is triggered by excessive carnitine loss, a couple of metabolic disorders, poor carnitine content in the diet or malabsorption, Fanconi syndrome (free carnitine loss through renal tubule), peritoneal dialysis or elevated excretion of acyl-carnitine (Winter SC, 2003). As a minimum, 15 syndromes have been identified in which carnitine deficit is thought to be caused by genetic abnormalities in intermediate metabolism or other circumstances. Patients with SCD accumulate organic acids, resulting in increased excretion of acyl-carnitine in the urine (Thangavelu, 2010).
SCD is substantially more prevalent and has a less severe short-term clinical impact (Reuter et al., 2008). SCD, unlike PCD, is caused by or occurs in combination with other conditions such as liver or renal disease, fatty acid metabolic abnormalities, or the use of therapeutic drugs like valproic acid or pivampicillin. SCD is found in individuals with renal tubular diseases, where carnitine excretion may be high, as well as hemodialysis patients. Inadequate synthesis of carnitine produces carnitine depletion in dialysis patients, resulting in carnitine depletion and a simultaneous relative rise in esterified carnitine in certain individuals (Matera et al., 2003). Through normalizing the lowered carnitine palmitoyl transferase function in red cells, LC can help uremic patients with a variety of complications, including muscle symptoms, impaired exercise and functional capacities, cardiac complications, and erythropoietin-resistant anemia (Argani et al., 2005).
4. Nutritional profile
The area of dietary supplements is surely diverse and expanding these days: every year, a large number of new goods are introduced to the market. This is mirrored in a new therapeutic restructuring that has resulted in dietary supplement regulations being changed (Giammarioli et al., 2013, Lordan, 2021, Santini and Novellino, 2014). LC is a major nutritional substance found in animal food because endogenic synthesis is inadequate to meet metabolic demands. PCD is uncommon, while SCD is more common, associated with many inborn metabolic abnormalities and acquired medical or iatrogenic diseases, such as zidovudine and valproate medication. Other chronic disorders, including Alzheimer's disease, diabetes, and heart failure, when combined with diseases that induce improved catabolism, could result in SCD (Kepka et al., 2020).
There are currently no documented suggested carnitine reference values. In most circumstances, the predicted average carnitine necessities for an adult are 20–200 mg/day, which are fulfilled by food and endogenous production. Fish, Meat, and dairy sources supply at least 80 % of the desired LC (Rigault et al., 2008). It is necessary to incorporate carnitine-rich foods into a healthy diet based on rational dietary norms (Rospond and Chlopicka, 2013), including carnitine supplementation. However, it should be noted that the bioavailability of LC obtained through meals is approximately-four times higher than that obtained from dietary supplements. Furthermore, a High-Fat Diet (HFD) is capable of increasing carnitine and its metabolites production (Kelly, 1998, Kepka et al., 2020).
5. l-carnitine: Health benefits
5.1. Anti-wasting effect
Muscle wasting or atrophy, often known as reduction in skeletal muscle mass, is now a frequent hallmark of various chronic disorders, including infectious diseases and cancer (Lenk et al., 2010, Powers et al., 2016, Remels et al., 2013). Muscle wasting is also known as cachexia in chronic conditions, and it is commonly attended with adipose tissue loss (Evans et al., 2008). Because mass and integrity of skeletal muscle are essential for whole-body metabolism and health, increasing muscle wasting is a crucial indicator that leads to treatment intolerance, a poor prognosis, and, as a result, a higher mortality rate in inpatients. Furthermore, muscle wasting reduces a patient's quality of life by causing muscular wasting and chronic weariness (fatigue) (Gramignano et al., 2006, Sakurauchi et al., 1998). Since muscle loss has such a detrimental impact on a patient's prognosis and quality of life, developing effective treatment options to combat muscle wasting is a crucial goal of research (Furrer and Handschin, 2019, Sawicka et al., 2018). An experimental investigation précises the results of clinical and animal studies demonstrating the role of LC supplementation or its derivatives on pivotal molecular mechanisms attributed to the reduction of muscle mass under pathological circumstances to assess the applicability of LC as an anti-wasting agent for medical application, such as reduced synthesis of protein, increased proteolysis, myonuclear apoptosis, mitochondrial dysfunction, OS, and inflammation. According to findings both from animal study and clinical trials, LC supplementation improves nitrogen balance either by enhanced synthesis of protein or decreased protein breakdown, apoptosis prevention, and revocation of inflammatory responses under pathological conditions. Furthermore, supplementation with LC has a positive impact on numerous essential pathways involved in pathological skeletal muscle loss, which might explain at least some of the anti-catabolic benefits and improvements in fatigue-related metrics seen in chronic illness people who received it (Montesano et al., 2015, Ringseis et al., 2013).
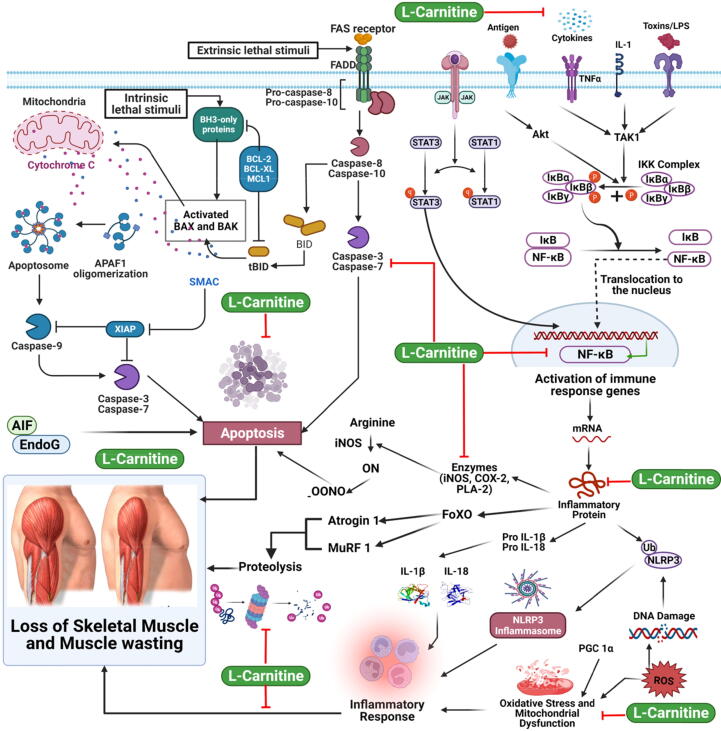
A transcriptional regulator of atrogin-1 and MuRF1 has been shown to be nuclear factor-kB (NF-kB), an induced transcription factor. Atrogin-1 and MuRF1 expression in skeletal muscle is increased by cytokines or reactive oxygen species (ROS) and constitutive activation of NF-kB in animal models, leading to muscle loss and atrophy (Ringseis et al., 2013). In contrast, blocking muscle NF-kB activation prevents inflammatory and atrophy signaling in skeletal muscle, resulting in significant muscle mass preservation in response to systemic inflammation (Langen et al., 2012). A recent research found that administering LC to tumor-bearing rats at doses of 1 g/kg b.w. causes downregulation of MuRF1 and atrogin-1 (Table 1) (Busquets et al., 2012). In contrast, caspase-3 within skeletal muscle treated with LC appeared to be reduced. Furthermore, these biological effects were accompanied with LC administration by a reduction in muscle wasting induced by tumor and a betterment in physical performance as measured by locomotor movements, stereotyped movements, and total physical activity during 21 h, mean velocity, and total distance travelled (Busquets et al., 2012). Accordingly, the findings strongly showed that LC administration can reduce muscle wasting in cancer cachexia animals. In CHF model of rat treated by monocrotaline, reduced caspase-3 expression in skeletal muscle were similarly reported in response to LC administration at a dose of 50 g/kg b.w. (Vescovo et al., 2002), whereas this study did not look into the influence of LC on the ubiquitin–proteasome system (UPS). This suggests that LC administration prevents muscle loss not just by lowering UPS activity, but also by preventing actomyosin complex disassociation, which is essential for the UPS to break down the monomeric contractile proteins (Vescovo et al., 2002). Considering that this implication of LC supplementation was followed by increased physical activity and decreased muscle loss, these studies and investigations that LC's suppression of skeletal muscle apoptosis leads at least largely to LC's anti-wasting activity. In another experiment, rats were given a combination of LC and lipoic acid to see how it affected age-dependent apoptosis in skeletal muscle fibers (Table 1) (Tamilselvan et al., 2007). Numerous lines of evidence from animal and human investigations have shown that when there are pathological disorders linked with muscle wasting such cancer and Chronic Obstructive Pulmonary Disease (COPD), apoptosis (myonuclear apoptosis) is elevated (Pirestani et al., 2011). Multinucleated myofiber apoptosis is distinct from mononucleated cell apoptosis, which results in the complete loss of the cell's nucleus, but does not always lead to cell death. This process is known as apoptotic nuclear death. Receptor-mediated or mitochondrial apoptosis are two ways in which myonuclear apoptosis can be triggered. Radiation and ROS, both of which are systemically enhanced under a number of pathological situations associated with muscle atrophy, are important “death-inducing” stimuli for both the receptor-mediated and mitochondrial apoptotic pathways. Caspases, which are cysteine-dependent aspartate-directed proteases regulated by Bcl-2 family proteins, heat shock proteins, and apoptosis inhibitors, become active in the cytosol upon activation and then cleave a wide range of proteins, are common features of the diverse routes leading to apoptosis (Fig. 2) (Genazzani et al., 2011). Caspase-3 activation is considered a critical stage in apoptosis, especially in skeletal muscle, due to its function in filamentous actin breakdown (Fig. 2) (Dokmeci, 2005). The mitochondrial release of apoptosis-inducing factor (AIF) and of endonuclease G (EndoG), which is extremely specific for myonuclei, occur, which are responsible for DNA fragmentation and chromatin condensation, as well as the collapse of the nuclear envelope (Fig. 2) (Genazzani et al., 2011).
Table 1.
Preclinical findings on the use of l-carnitine in disease management.
| Diseases | Compounds | Dose/concentration | Study model | Findings | References |
|---|---|---|---|---|---|
| Anti-Wasting effect | l-carnitine | 0, 50, 100, 500 and 1000 mM | In vitro (HepG2 cells) | ↓transcript levels of several genes involved in the UPS in skeletal muscle and liver of piglets | (Keller et al., 2012) |
| l-carnitine | 500 mg | In vivo (crossbred pigs) | Differentially expressed genes involved cytoskeletal protein binding, IGF binding, transcription factor activity, and insulin receptor binding. Identified genes with the molecular function Transcription factor activity encoded primarily transcription factors | (Keller et al., 2011) | |
| l-carnitine | 50 mg-1kg−1 | In vivo (Sprague–Dawley male Rats) |
Inhibit caspases and to decrease the levels of TNF-α and sphingosine | (Vescovo et al., 2002) | |
| l-carnitine | 2, 5, 10 mM | In vitro (skeletal muscle cells) | Reduction in apoptosis | (Vescovo et al., 2002) | |
| Acetyl-l-carnitine (ALCAR) | 0.4 g/ kg of body weight | In vivo (Male Wistar rats) | ↓glycolytic enzyme expression ↓capacity of fat oxidation |
(Moriggi et al., 2008) | |
| Renal disease | Glycine propionyl l-carnitine (GPLC) | 35 mg/kg BW/day | In vivo (Male Wistar rats) | ↓lipid peroxidation ↑level of GSH Inhibiting the increase in serum TNF-a, ATM-Kinase, MAP-Kinase expression, and Caspase-3 and Bax m-RNA |
(Ganai et al., 2014) |
| l-carnitine | 500 mg/kg followed by 250 mg/kg for 5 days | In vivo (Sprague–Dawley male Rats) |
Inhibiting cisplatin-induced injury. Preventing doxorubicin induced cardiac metabolic damage Modulating the relationship between MMP-9 and TIMP-3 |
(Martinez et al., 2009) |
|
| l-carnitine | 300 mg/Kg body weight/ week | In vivo (Male albino rats) | ↓serum ALT, AST, urea, creatinine, uric acids and MDA levels ↑GSH, catalase |
(Tousson et al., 2014) | |
| l-carnitine | 400 mg/kg body weight/day | In vivo (Male Wistar rats) | ↑expression of PPAR-γ ↓pro-oxidative and proinflammatory status |
(Zambrano et al., 2014) | |
| l-carnitine | (200 mg · kg − 1 ·d − 1, ip | In vivo (Sprague–Dawley male Rats) |
Anti-inflammatory and antioxidant effects attenuating programmed cell death via PI3K/AKT/PTEN signaling | (Zheng et al., 2021) | |
| l-carnitine | 250 mg/kg body weight | In vivo (Male Wistar rats) | Improving kidney functioning, cognitive functioning histological damage | (Abu Ahmad et al., 2016) | |
| l-carnitine | 200 mg/kg, i.p. | In vivo (Sprague–Dawley male Rats) |
Protection against functional, biochemical and morphological damage and iron accumulation in glycerol-induced myoglobinuric ARF | (Aydogdu et al., 2006) | |
| l-carnitine | 300 mg/kg | In vivo (Sprague–Dawley male Rats) |
Improving Renal urinary concentrating function. ↑AQP2 accumulation at the apical plasma membranes of the renal-collecting ducts ↑GSα protein, adenylyl cyclase, and serum AVP |
(Gao et al., 2017) | |
| l-carnitine | 40 mg/kg or 200 mg/kg | In vivo (Sprague–Dawley male Rats) |
Less severe proximal tubular necrosis. Greater mild proximal tubular necrosis |
(Kopple et al., 2002) | |
| l-carnitine | 1.5 mmol/l | In vivo (Balb/c mice) | Preventing the effect of maternal SE on renal underdevelopment involves global epigenetic alterations from birth | (Stangenberg et al., 2019) | |
| l-carnitine | 250 mg/ml | In vivo (Sprague–Dawley male Rats) |
↓Lys phospholipids, free fatty acids, and nitro tyrosine, amelioration in MDA, SOD, and phospholipase A2 activity |
(Liu et al., 2012) | |
| l-carnitine | 1, 10, 50, 100,200, 500, 1000 μM | In vitro (Human proximal tubule epithelial cell) | ↓H2O2-induced cell viability loss, intracellular ROS generation, and lipid peroxidation, ↓total antioxidative capacity of GPX, catalase |
(Ye et al., 2010) | |
|
Liver disease |
l-carnitine | 200 mg/kg/day | In vivo (Wistar albino rats) | Protecting the liver cells against IRI damage Improving the oxidative stress and inflammatory states ↓the expression of vascular adhesion molecules ↑NO level |
(Soc et al., 2022) |
| l-carnitine | 40, 200 mg/kg and 1 g/kg | In vitro (MDCK, Mpcpt, MDCK-hOCTN2 cells) | ↓clozapine-induced liver TG and TCHO, abnormal lipid metabolism |
(Wang et al., 2019) | |
| l-carnitine | 125–250 mg/kg BW | In vivo (Kungming mice) | fatty acid oxidation ↓LC/ALC ratio in the liver |
(Xia et al., 2011) | |
| l-carnitine | 100 mg/kg | In vivo (Male Wistar rats) | No significant changes |
(Yonezawa et al., 2005) | |
| l-carnitine | 200 mg/kg | In vivo (Male Wistar rats) | ↓lipid peroxidation and significant ↑antioxidant activity |
(Canbaz et al., 2007) | |
| l-carnitine | 200 mg/kg | In vivo (Wistar albino mice) | ↓MDA, GSH, serum levels for AST, ALT and LDH, histopathological studies | (C̈ekin et al., 2013) | |
| Alzheimer disease | Acetyl-l-carnitine (ALCAR) | 500 mg | In vivo (C57B/6 Mice) |
↓ROS in normal mice Delaying progression of age-related cognitive decline |
(Suchy et al., 2009) |
| Acetyl-l-carnitine (ALC) | 50 mg/day per rat | In vivo (Male Sprague–Dawley rat) | Improving the HCV-induced memory deficit Abolishing the HCV- induced tau hyperphosphorylation Suppressing the phosphorylation of b-amyloid precursor proteins (APP) |
(Zhou et al., 2011) | |
| Acetyl-l-carnitine (ALC) | 100 mgkgy1 b.wt. | In vivo (Male Fischer rats Charles) | Modulating cerebral glucose utilization Reducing total 14CO2 release from [U-14C] glucose. |
(Aureli et al., 1998) | |
| l-acetyl carnitine | 30 and 60 mg.kg–1 | In vivo (Sprague-Dawley rats) | ↓citrate synthase and glutamate dehydrogenase activities ↑cytochrome oxidase and a-ketoglutarate dehydrogenase activities |
(Gorini et al., 1998) | |
| Acetyl-l-carnitine (ALC) | 0.2 % [wt/vol] | In vivo (rats) | ↑proliferation of intact mitochondria and reducing the density of mitochondria ↓number of severely damaged mitochondria ↑number of intact mitochondria |
(Aliev et al., 2009) | |
| l-carnitine | 300 mg/kg body wt/day | In vivo (male Wistar rats) | ↑levels of dopamine, epinephrine, and serotonin | (Juliet et al., 2003) | |
| Depression | Acetyl-l-carnitine (ALC) | 100 mg.kg − 1 | In vivo (Swiss albino mice) | ↑ARTN levels in spinal cord, hippocampus, and prefrontal cortex | (Di Cesare Mannelli et al., 2011) |
| Acetyl-l-carnitine (ALCAR) | 0.5 g/kg | In vivo (mice) | ↓ [3-13C] lactate, GABA,glucose metabolism ↑ myo-inositol |
(Smeland et al., 2012) | |
| Epilepsy | l-carnitine | 100 mg/kg/day | In vivo (Sprague Dawley rats) | ↓ Seizure score ↓Oxidative stress marker malondialdehyde (MDA) ↓PTZ-induced elevation in protein expression of caspase-3 |
(Hussein et al., 2018) |
| l-carnitine | (5, 10 or 20 mmol/kg | ↓Frequency of clonic as well as tonic seizures |
(Yu et al., 1997) |
||
| Parkinson Disease | Acetyl-l-carnitine (ALC) | 100 or 200 mg/kg/day | In vivo (male Wistar rats) | Abrogating neuroinflammation, apoptosis, astrogliosis, and oxidative stress | (Afshin-Majd et al., 2017) |
| Acetyl-l-carnitine (ALC) | 100 mg/kg | In vivo (male C57BL6 Mice) |
↓Microglial activation and astrocytic reactivity Protection against MPTP-induced damage to endothelial cells. |
(Burks et al., 2019) | |
| Acetyl-l-carnitine (ALC) | 10 or 100 mg/kg | In vivo (Sprague-Dawley rats) | Preventing loss of TH and a decline of DAT level, activation of both microglia and astroglia | (Acosta et al., 2020) | |
| Acetyl-l-carnitine (ALC) | 1 mM | In vitro (PC12 cell) | ↓MPP + and METH-evoked toxicity Partial n restoration of mitochondrial function |
(Virmani et al., 2004) | |
| Acetyl-l-carnitine (ALC) | 0–10 µM | In vitro (SK-N-MC human neuroblastoma cell) | ↑Mitochondrial biogenesis ↓ROS Upregulating peroxisome proliferator-activated receptor-γ coactivator 1-α |
(Zhang et al., 2010) | |
| Sexual function | l-carnitine | (1.5 mM) | In vitro (Bovine embryos) | ↑Blastocyst rate at day 8 ↑Blastocyst total number of cells Positive effect on embryo development rate and quality |
(Ghanem, 2015) |
| l-carnitine | 100 ml i.p injection (25 mg/ml) | In vivo (female BALB/c mice) | ↑Macrophages, T cells, IFN-γ and TNF-α |
(Dionyssopoulou et al., 2005) | |
| l-carnitine | 0, 0.25, 0.5, or 1 mg/mL | In vitro (Porcine oocyte) | ↓ROS, apoptosis in activated blastocysts. Accelerating nuclear maturation, and preventing oxidative damage |
(Wu et al., 2011) | |
| l-carnitine | 0, 0.3, 0.6, 1.2, 2.5, 5.0, and 10 mg/mL | In vitro (mouse embryos) | Improvement in %BDR Reducing blocking effect of AD, H2O2, and TNF-a ↓level of DNA damage |
(Abdelrazik et al., 2009) | |
| l-carnitine | 10 mM | In vitro (pig (Sus scrofa) oocytes) | ↑Expression levels of DNMT1, PCNA, FGFR2, and POU5F1 mRNA, BAX and p-Bcl-xl mRNA. ↑intracellular GSH synthesis ROS |
(You et al., 2012) | |
| Acetyl-l-carnitine (ALC) | 2 mM | In vitro (lamb oocyte) | Improving blastocyst rate. ↑Mitochondrial mass ↑Expression of genes involved in mitochondrial biogenesis |
(Reader et al., 2015) | |
| l-carnitine | 3.72 mM | In vitro (GV–oocytes) | ↓Proportion of oocytes with mitochondrial aggregates ↑Activity of mitochondria ↑Proportions of oocytes |
(Moawad et al., 2014) | |
| l-carnitine | 125 ppm | In vivo (White Leghorns) | ↓Hatchling yolk sac weights ↑egg yolk Improving sperm concentration |
(Zhai et al., 2008) | |
| l-carnitine | 100 mg/kg | In vivo (Sprague Dawley rats) | Attenuation in histological changes and other indices increase by ischemia | (Usta et al., 2008) | |
| l-carnitine | 500 mg/kg | In vivo (Sprague Dawley rats) | ↑HSP70, amelioration in histological changes | (Guan et al., 2009) | |
| Cardiovascular risk | Propionyl l-carnitine | 60 mg. kg −1/ day −1 | In vivo (Wistar-Kyoto rats) | ↑Cardiac work in hypertrophied hearts |
(Schönekess et al., 1995) |
| l-carnitine | 1.2 % | In vivo (Wistar rats) | ↓Left ventricular interstitial collagen content ↓Blood pressure ↓Diastolic stiffness constant ↑Cardiac remodeling and improved cardiac function |
(O’Brien et al., 2010) | |
| Carnitine along with lipoic acid | 300 mg/kg body weight/day | In vivo (Albino rats) | Effective supplement against free radical induced damage to the cardiac tissue | (Savitha et al., 2007) | |
| Carnitine | 0.15 % w/v | In vivo (Wistar Albino rats) | ↓Lymphocytic infiltration of myocardium ↓Serum BNP concentration |
(Strilakou et al., 2013) | |
| Anticancer activity | l-carnitine | 100 mg/kg | In vivo (Male LEC rats) | Showed potential inhibitory effects on the initiation of hepatocarcinogenesis | (Chang et al., 2005) |
| l-carnitine | 1 g/kg | In vivo (Albino rats) |
l-carnitine preserves hepatic lipid metabolism in tumor-bearing animals |
(Silvério et al., 2012) | |
| l-carnitine | 9 mg/kg/day | In vivo (Mice) |
l-carnitine exerts its ameliorative effects in cancer cachexia in association with the PPAR-γ signaling pathway |
(Jiang et al., 2015) | |
| Antioxidant activity | l-carnitine | 400 or 880 mg | Fish | Reduces hepatic and renal oxidative stress and may be effective when used for the prophylaxis and treatment of CYN-related intoxication in fish | (Guzmán-Guillén et al., 2013) |
| Antidiabetic activity | l-carnitine | 30 mmol | Mouse | Improved glucose tolerance ability | (Yoshikawa et al., 2003) |
|
Obesity |
l-carnitine | 250 mg/kg | In vivo (Wistar rats) | ↓Final body weight, serum AI, and serum ALT Normalizing lipid profile |
(Esmail et al., 2021) |
| l-carnitine | 1 g | In vivo (ICR mice) | Inhibiting body weight increase ↓GPT, GOT and TG |
(Tao et al., 2015) |
Fig. 2.
Anti-wasting effect of l-Carnitine.
On the other hand, Oxidative stress has been linked to muscle atrophy, according to new research (Di Liberato et al., 2014). Occurs when the antioxidant capacity of cells is depleted, resulting in the generation of oxidants such peroxynitrite and superoxide radicals (ROS-like). This leads to damage to proteins, lipids, and even DNA. Increased levels of ROS in the blood and/or impaired antioxidant ability in chronically unwell patients with muscle loss have both been related to muscle wasting and ROS as powerful inducers of the UPS pathway, respectively. Oxidative stress is also a contributor to muscle wasting, as ROS are well-known stimulators of apoptotic pathways, showing that oxidative stress is a factor in muscle wasting (Fig. 2) (Ringseis et al., 2013).
5.2. Neuropharmacological effects
5.2.1. Alzheimer’s disease
Alzheimer's disease (AD) is defined by the degeneration of synapses and neurons in the cerebral cortex and some subcortical sites, resulting in atrophy and degeneration of the affected regions in the temporal and parietal lobes, as well as portions of the callosal gyrus and the frontal lobe (Das et al., 2021, Kabir et al., 2021).
The American FDA has authorized LC as powder, fluid, tablet, or capsules for the treatment of primary and secondary carnitine insufficiency. Experimental results from in vitro and in vivo studies found no evidence of LC toxicity (Rebouche, 2004). There have been no reports of LC intoxication. Healthy people were given LC supplementation at various dosages ranging from 250 mg to 2.0 g (maximum safe dose) per day (Chen et al., 2015). A meta-analysis containing 21 randomized, double-blind, and placebo-controlled studies in duration from 3 months to 1 year revealed that ALC either restored cognitive dysfunction or slowed cognitive deterioration. Continued to improve cognitive performance and a slower rate of cognitive impairment were clinically and statistically significant, with the size of the effects growing with time. The majority of the trials employed daily dosages of 1.5–2.0 g of LC, which were well tolerated (Wollen, 2010). In patients with moderate (initial) dementia caused by AD and vascular dementia (VD), administration with ALC at dosages of 2.25–3.0 g/day resulted in a substantial treatment response in individuals with AD relative to placebo-treated and VD patients (Gavrilova et al., 2011). In a separate trial, 11 patients with Alzheimer's disease were administered intravenously with ALC at 30 mg/kg for ten days. Multiple dosages of ALC administered intravenously and orally increased ALC CSF and plasma concentrations in Alzheimer's disease patients, suggesting that ALC efficiently passes the blood–brain barrier (BBB) (Parnetti et al., 1992).
The hydrophobicity of LC is increased by acetylation, allowing ALC to pass the blood–brain barrier. ALC has a neuroprotective effect on brain cells, promotes energy metabolism and nerve cell structure repair, and reduces mitochondrial malfunction and apoptosis (Zhang et al., 2015), boosting memory and creative thinking. Several research have revealed that ALC has a positive effect on the brain and behavioral in aging and Alzheimer's disease patients. ALC improves clinical and cognitive function in Alzheimer's patients in the short and medium term (3 and 6 months) at various dosages (1.5–3.0 g/day). ALC also delays the decline of cognitive function in Alzheimer's patients with a 1-year administration at a dosage of 2.0 g/day (CALVANI et al., 1992).
5.2.2. Parkinson disease (PD)
PD is the second leading neuronal condition among the elderly, and its prevalence is predicted to rise as the aging population. Gradual loss of dopaminergic neurons in the substantia nigra pars compacta of the midbrain is a hallmark of Parkinson's disease (Bahbah et al., 2021, Muangpaisan et al., 2011, Wirdefeldt et al., 2011). Natural antioxidants and nutraceuticals could help to slow the development of neurological conditions like Parkinson's disease, according to new study. These molecules can be used as adjuvant treatment to reduce the amount of prescription medication needed (Chattopadhyaya et al., 2015, Koppula et al., 2012, Sedaghat et al., 2014). ALC is thought to be a naturally produced metabolic intermediate implicated in the transportation of acetyl units across mitochondrial membranes in anabolic and catabolic processes. An investigation was carried out to assess the neurotherapeutic properties of ALC in contrast to 6-hydroxydopamine-induced PD model and to investigate certain associated processes. In this work, rats with intrastriatal 6-OHDA lesions were given ALC at dosages of 100 or 200 mg/kg/day for one week. ALC (200 mg/kg) reduced the rotational asymmetry induced by apomorphine and decreased the latency to initiate and total time in the narrow beam test, decreased MDA, increased catalase activity and GSH level, deterred reduction of nigral tyrosine hydroxylase (TH)-positive neurons and striatal TH-immunoreactivity, and decreased striatal GFAP. However, ALC at both dosages reduced nigral DNA fragmentation, which is a good biomarker of apoptosis. The findings of this work clearly indicate that ALC has a neurotherapeutic impact in a 6-OHDA-mediated model of PD by inhibiting apoptosis, neuroinflammation, astrogliosis, and OS, and that it might be considered as a potential supplementary treatment option for PD management (Afshin-Majd et al., 2017).
5.2.3. Autism spectrum disorder
LC is essential for the proper functioning of the CNS, particularly in the fatty acid metabolism. It has been shown that persons with ASD have altered carnitine metabolism and aberrant fatty acid metabolism. ASD is a multifaceted neurodevelopmental disorder that is often diagnosed in infancy. People with ASD need to be classified carefully since this clinical group might include patients with cognitive disabilities or high functioning, seizures, linguistic problems, or linked Mendelian genetic disorders. LC contributes in the oxidation of long-chain fatty acid in the brain, boosts acetylcholine production (acyl group donor), increases protein-43 expression, inhibits cell damage and neuronal damage, and boosts neurotransmission. The analysis of acylcarnitines in a dried blood spot and the determination of LC in serum/plasma may be relevant in the diagnosis and treatment of ASD. Modifications in acylcarnitine levels in ASD children could suggest mitochondrial malfunction and aberrant fatty acid metabolism. In ASD, LC shortage or dysregulation of LC metabolism is associated with disruptions in other metabolic pathways, such as the Krebs cycle and respiratory chain complex activity, indicating mitochondrial malfunction. LC supplementation may help ASD patients with their behavioral and cognitive problems (Kępka et al., 2021). In the following randomized trial, the effect of a larger LC administration (100 mg/kg) for 6 months in 35 patients with ASD was studied, and a lot of progress in symptoms of ASD was noted (Fahmy, S.F.; El-hamamsy, M.H.; Zaki, O.K.; Badary, 2013). Goin-Kochel et al. conducted an 8-week study in which 10 boys with ASD (including a patient with hereditary ASD with a TMLHE gene deficit) were given carnitine in three separate dosages starting at 200 mg/kg and escalating to 400 mg/kg/day, up to a maximum of 6 g daily. They demonstrated that large dosages of LC, up to 400 mg per day, are safe, despite moderate diarrhea and unusual body odor (fishy odor) being the main side effects. The efficacy of LC treatment was shown after four and eight weeks of careful and extensive psychological monitoring of the boys, which demonstrated strong positive correlations between serum raised free and total LC levels and improvements in cognitive functioning (Goin-Kochel et al., 2019).
5.2.4. Depression
Despite the availability of several antidepressants, many individuals with depression do not get an acceptable response, necessitating the emergence of new antidepressants with diverse mechanisms of action. For its numerous roles relating to neuroplasticity, ALC has the potential to be a new antidepressant with a novel mechanism of action. The neuroplasticity influence, transmembrane modulation, and neurotransmitter control of ALC may all have a role in the therapy of depression, according to animal models. In four RCTs, ALC was found to be more effective than placebo (PBO) in individuals with depression. Two RCTs found that it is more effective than PBO in the management of dysthymia, and two more RCTs found it is as useful as amisulpride and fluoxetine to cure dysthymic disorder (Wang et al., 2014). ALC performs a variety of crucial functions, such as promoting acetyl CoA absorption into mitochondria during oxidation of fatty acid, increasing acetylcholine synthesis, accelerating protein and transmembrane phospholipid formation, and preventing undue cellular injury in the neuron (Di Cesare Mannelli et al., 2011).
5.2.5. Epilepsy
Epilepsy is a serious neurological condition associated with abnormal brain electrical activity. Disturbance in the metabolism and homeostasis of main inhibitory and excitatory neurotransmitters, glutamate and GABA, are important pathogenic pathways. The majority of current pharmacological therapies are targeted at lowering neural excitability and thereby avoiding seizures. Many individuals, however, are resistant to therapy and adverse effects are common. The most frequent kind of drug-resistant epilepsy in adults is temporal lobe epilepsy (TLE). The pilocarpine-status epilepticus model in rats resembles the pathophysiology and chronic spontaneous seizures of TLE, whereas the pentylenetetrazole kindling paradigm demonstrates chronic caused limbic seizures. A growing body of data from TLE research indicates to abnormalities in neurons and astrocytes as significant metabolic modifications. A review reported treatments that improve astrocyte–neuronal connections by boosting mitochondrial metabolism. The substances under consideration are the natural transport molecule ALC and the heptanoate triglyceride triheptanoin. Both offer acetyl moieties for tricarboxylic acid cycle oxidation, however heptanoate additionally gives propionyl-CoA, which following carboxylation can create succinyl-CoA, leading in anaplerosis (M.G. et al., 2015).
5.3. Obesity
Supplementing with LC is shown to benefit with obesity caused by a HFD, as well as decreasing hepatic and blood lipid levels and alleviating fatty liver. However, it is still unclear if LC can help with inconsistent obesity induced by feeding and lipids metabolism disorders (Xia et al., 2011). An investigation looked into the role of LC on the obesity caused by irregular feeding in mice. After 8-week trial with LC administration, body weight growth and epididymal fat excess weight caused by delayed feeding were considerably reduced. Furthermore, LC supplementation reduced blood triglyceride (TG) levels and glutamic oxaloacetic transaminase (GOT) levels, which had been considerably raised by irregular eating. Furthermore, animals treated with LC did not show the hallmarks of glucose intolerance that were seen in mice with obesity induced by irregular feeding (Tao et al., 2015). In one study, a combination of nicotinamide riboside and LC enhanced metabolism in the liver and reduced obesity and liver steatosis (Salic et al., 2019). This experiment assessed the potentiality of combination therapy with both nicotinamide riboside and LC in preventing obesogenic liver damage, which are components that can improve transfer of fatty acid through the inner mitochondrial membrane and increase NAD + levels, both of which are required for TCA cycle and β-oxidation. For 21 weeks, Leiden mice were fed a high-fat diet enriched with LC, NR, or both (COMBI). HFD lowered LC plasma levels, which were then normalized by LC. Supplementing with NR increased plasma metabolite levels, indicating efficient delivery. Despite the fact that both groups had similar food intake and ambulatory exercise, COMBI therapy significantly reduced body weight increase induced by HFD, fat mass growth, and hepatic steatosis (Salic et al., 2019). A study looked at the nutritional effects of trimethylamine N-oxide and LC on reducing obesity in mice caused by a HFD. After adjusting for several confounding variables, the researchers found a substantial relationship between increased blood LC levels and obesity in women but not in males. Serum TMAO level was linked with age but not obesity. Dietary TMAO showed no effect on fat formation in mice fed a HFD. LC supplementation, on the other hand, reduced obesity caused by a HFD in both female and male mice by increasing lipolysis and decreasing lipogenesis in adipose tissues. The current experiment extends to the evidence regarding the links among LC, TMAO, and obesity (Gao et al., 2020).
LC treatment improved the lipid profile and reduced the ultimate body weight, serum ALT, and serum AI levels. Histopathological studies of the liver of HFD-fed rats revealed steatosis, which was alleviated by LC administration, while atorvastatin had no effect on the treatment of hepatic lesions. Overall, the outcomes of this research revealed that LC reduced metabolic and histological alterations in the hepatic tissues of rats administered an HFD (Esmail et al., 2021).
5.4. Cardiovascular risk
Worldwide, approximately 25–30 million individuals are affected by cardiac disorders, notably chronic heart failure (CHF) (Song et al., 2017). Cardiac failure is characterized by myocardial infarction (MI), ventricular systolic dysfunction, and reduced cardiac muscle contraction along with the flow of blood (Roan et al., 1982). Angiotensin and corticosteroid receptor antagonists, ACE inhibitors, β-blockers, and Ca-channel antagonists are common therapies for cardiac arrest (Kolkhof et al., 2015, Lipworth et al., 2016). These pharmacological medications induce vasodilation, lower blood pressure and vascular resistance, and enhance blood flow and oxygen delivery to cardiac muscles. They can be taken alone or in combination, based on the patient's condition and demands. However, long-term administration of the medications has been related to unfavorable hepatotoxic/hematologic consequences, as well as renal failure and hyperkalemia (Sidorenkov and Navis, 2014).
The two most widely investigated forms of LC, propionyl and acetyl-LC, contribute to decrease the formation of toxic metabolites produced in coronary thrombosis and embolism. Thus, LC, particularly propionyl-LC, has been recommended as a therapy for a variety of cardiac issues, including reperfusion injury, cardiopulmonary arrest, coronary infarction, accidental blood circulation abnormalities, hypercholesterolemia, toxic myocardial injury, and diabetes (M.A.,1997). According to meta-analysis findings, LC has a substantial effect in preventing cardiovascular disease. The data revealed that LC supplementation reduced arrhythmia, ventricular dysfunction, and angina pectoris pain, leading in a lower risk of heart attack and death (DiNicolantonio et al., 2013). Carnitine also helped with hypertension induced by stress, hyperosmolar hyperglycemia, diabetic ketoacidosis, insulin resistance, insulin-dependent DM, obesity, and other physiological conditions (Wang et al., 2018).
Many investigations on the potential health benefits of carnitine on heart disease were undertaken in the last two decades. The identification of carnitine disorder, which was linked to skeletal and cardiac myopathies among other symptoms, sparked initial attention (Atar et al., 1997). The success of carnitine therapy in ischemic heart disease was first attributed to the higher in fat oxidation in cardiac myocytes, which resulted in an increased energy supply. However, new research suggests that LC may have other roles in protecting cardiac cells from hypoxia, ischemia, and OS. Thus, carnitine is thought to be cardioprotective due to its indirect influence on lowering harmful acylCoA derivative levels and modulating glucose metabolism (Ferrari et al., 2004, Lango et al., 2001). The intake of LC lowers the intramitochondrial acetyl-CoA to free CoA ratio, which promotes pyruvate dehydrogenase activity and glucose oxidation. Aside from its effects on ischemic heart disease, LC has been shown to be useful in arrhythmia, endothelial function, circulatory failure, and peripheral blood disorders (Shankar et al., 2004).
5.5. Renal disease
LC has piqued the interest of patients with end-stage renal disease (ESRD) since reduced renal synthesis and dialysis losses have been proposed as reasons of LC deficit in hemodialysis patients (Guarnieri et al., 2001). Dialysis-associated carnitine deficit, in turn, has been linked to a number of symptoms seen in uremic patients, including fatigability, cardiomyopathy, anemia, and skeletal muscle weakness (Eknoyan et al., 2003). Carnitine administration is not indicated for uremic patients on a regular basis (Schröder et al., 2003), although symptomatic dialysis patients who do not react to normal treatment can benefits from it (Schreiber, 2006).
Inadequate food intake, intestinal malabsorption, deposition of metabolic intermediates, diminished renal synthesis, and dialysis age are all possible causes of carnitine metabolism abnormalities in ESRD patients on PD (Di Liberato et al., 2014). Carnitine abnormalities may also be caused by daily losses in dialysate during dialysis dwell time (E et al., 2007, Evans, 2003). Carnitine metabolism in Parkinson's disease patients may be influenced by the function of peritoneal membrane and dialysis method. Patients with a higher peritoneal transport rates had an increased carnitine metabolic state than those with low peritoneal transport rates. Furthermore, individuals treated with APD had lower levels of free and acetyl-carnitine than those treated with CAPD. This observation may be due to the distinct nature of the PD depurative treatments, since APD is characterized by shorter and bigger volume stays, which may promote the elimination of carnitine molecules (Di Liberato et al., 2014).
Several observational studies indicated a relationship between carnitine system and lipid metabolic problems in Parkinson's disease patients (Marín VB, Azocar M, Molina M, Guerrero JL, Ratner R, 2006). Waradi et al. conducted the only randomized study to date, looking at the effects of oral LC (100 mg/kg/day) given to six young patients with CAPD for two months. The mean plasma carnitine content rose considerably, while serum triglyceride levels did not alter (Table 2) (Bonomini et al., 2019). The benefits of LC (50 mg/kg/day) administered orally to 20 participants for 30 days on PD were studied by Kosan et al. (Koşan et al., 2003). There was a substantial reduction in blood apolipoprotein B levels at the conclusion of the research, but no alteration in the other lipid levels tested. Because high apolipoprotein B levels is an additional risk factor for developing coronary artery disease and atherosclerosis, the authors recommend that children with PD take carnitine supplements to prevent cardiovascular problems (Koşan et al., 2003).
Table 2.
Clinical findings on the use of l-carnitine in disease management.
| Study design | Participants | Dose | Outcomes | References |
|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled study | 645 cancer patients | 250 mg/day, with doses increased in increments of 500 mg to a maximum target dose of 3000 mg/day | Having beneficial effects on symptoms of fatigue, depression, and quality of sleep | (Cruciani et al., 2004) |
| Randomized trial | One hundred seven patients | 500 mg oral l-carnitine each morning on non-dialysis days or after dialysis treatment for 12 weeks | Improvement in muscular symptoms, dialysis-associated muscle symptoms | (Sakurauchi et al., 1998) |
| Double-blind Pilot Study | Forty-two subjects | 1500 mg l-carnitine-l-tartrate for 24 weeks | Did not affect either the skeletal muscle strength or circulating markers | (Sawicka et al., 2018) |
| Randomized trial | 162 women with CC– resistant PCOS | 3 g oral L-carnitine | Improvements in menstrual pattern, follicle- stimulating hormone, luteinizing hormone, free testosterone, and insulin resistance, lipid profile. | (El Sharkwy and Abd El Aziz, 2019) |
| Double-Blind Randomized Clinical Trial | 147 women with PCOS | ALC (500 mg, 15 mg, and 1500 mg, respectively) twice daily for 12 weeks | ↑Adiponectin ↓HOMA-IR Testosterone, FSH, and LH significantly improved |
(Tauqir et al., 2021) |
| Double-blind, randomized controlled clinical trial | Sixty-two overweight/obese women with PCOS | 1000 mg/d l-carnitine capsule | No beneficial effect on liver fat content and cardiometabolic outcomes | (Sangouni et al., 2021) |
| Randomized controlled trial Alshimaa |
100 children with type 1 diabetes mellitus | l-carnitine (50 mg/kg/day) | ↓Total cholesterol and low- density lipoprotein ↑High-density lipoprotein |
(Badreldeen et al., 2021) |
| Pilot trial of | 40 patients with severe TBI | 2 g/day for one week | Neurocognitive function and NSE significantly improved | (Mahmoodpoor et al., 2018) |
| Randomized trial | 47 patients with coronary artery disease | LC supplementation (1000 mg/d) for 12 weeks | HDL-C and Apo-A1 levels ↓TG levels |
(Lee et al., 2016) |
| Randomized trial | 62 patients with peripheral artery disease | PLC (2 g daily, n = 32) 6 months | ↓PWT g improving walking performance in both treatment arms |
(Hiatt et al., 2011) |
| Multicenter, Randomized, Double-blinded, Controlled Trial | 232 patients with Diabetic Peripheral Neuropathy | ALC 500 mg | ↓NSS and NDS Improved Neurophysiological parameters |
(Li et al., 2016) |
| Randomized, double-blind, multicenter trial | Four-hundred nine subjects with Taxane-Induced Neuropathy | ALC (1000 mg three times a day) | Significantly worse CIPN over two years Reduction in NTX scores |
(Hershman et al., 2018) |
| Pilot, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial | 56 patients with kidney transplantation | 3 g of oral l-carnitine administered in 3 divided doses each day for 4 consecutive days | No protective effects of oral l-carnitine supplementation against DGF occurrence recipients. 3-month graft loss was lower in the l-carnitine supplemented group. |
(Jafari et al., 2017) |
| Randomized, clinical trial | 54 patients in the carnitine and 62 patients in the placebo group with antituberculosis drug-induced hepatotoxicity (ATDH) were | 1000 mg oral carnitine solution twice daily for 4 weeks | Significantly decreased the rate of ATDH | (Hatamkhani et al., 2014) |
| Randomized, triple-blind, placebo-controlled clinical trial | 30 patients in the l-carnitine group and 25 patients in placebo group with nonalcoholic fatty liver disease (NAFLD) | 50 mg/kg/day l-carnitine twice a day or identical placebo per day for three months | Did not have significant effect on improving biochemical and sonographic markers of NAFLD in children and adolescents | (Saneian et al., 2021) |
| Pilot study | 2 groups with GDR ≥ 7.9 (n = 16) or > 7.9 (n = 16) | 24-week oral acetyl-l-carnitine (1 g twice daily) | ↑GDR from 4.89-1.47 to 6.72-3.12 mg/kg per minute. Improving glucose tolerance |
(Ruggenenti et al., 2009) |
| Randomized, Placebo-Controlled Trial | 28 patients (26 [93.0 %] males) with a mean age ± SD of 58.1 ± 10.5 years | 50 mg/kg/day oral dose of l-carnitine | ↑left ventricle ejection fraction (p = 0.002) and 14.3 % (p = 0.006) and 3.3 % (p > 0.05). Did not demonstrate any additional benefit in reverse remodeling |
(Da Silva Guimarães et al., 2017) |
| Multi-institutional, randomized, exploratory trial | Fifty-nine breast cancer patients | IP (125 g) once daily for 21 days contains l-carnitine (50 mg) | Changes in the worst level of fatigue, GFS, and current feeling of fatigue HADS, EORTC QLQ-C30, and EORTC QLQ-BR23 scores were not significantly different |
(Iwase et al., 2016) |
An experiment was carried out to look into the involvement of LC in renal and hepatic damage induced by methotrexate (MTX). When compared to total proteins in the MTX and self-healing groups, MTX treated with LC showed a substantial drop in blood AST, ALT, urea, uric acids, creatinine, and MDA levels and a significant rise in catalase, GSH, etc. Histopathological findings confirmed the biochemical findings and l-ability carnitine's to reduce kidney and liver toxicity. Throughout co– and post-treatment, LC demonstrated a variety of protective mechanisms against MTX-induced kidney and liver damage. It may infer that LC medication during MTX chemotherapy has favorable qualities and can minimize MTX-induced liver and kidney damage (Tousson et al., 2014).
Table 2. Clinical findings on the use of l-carnitine in disease management.
5.6. Liver disease
LC serves an important role in sustaining liver function because of its influence on lipid metabolism. The findings that individuals with PCD might appear with fatty liver disease, which could be owing to decreased intrahepatic and serum LC levels, supports the role of LC in liver health. Additionally, research suggests that LC administration can lower hepatic fat and the hepatic enzymes AST and ALT in people with NAFLD. LC has been reported to promote insulin sensitivity and increase PDH flux. Studies demonstrating lower intrahepatic fat and improved liver enzymes following LC intake indicate that LC may be a potential supplement for improving or delaying the course of NAFLD (Savic et al., 2020).
An inverse correlation between acyl-carnitine length and disease severity was found in a study of 241 individuals with biopsy-proven NAFLD and 23 patients having hepatocellular carcinoma (HCC). HCC, fibrosis, and inflammation were all attributed to plasma long-chain acyl-carnitine, but not free l-carnitine. Medium-chain acyl-carnitines were shown to be lower as the severity of NAFLD worsened (Enooku et al., 2019).
Plasma LC levels were shown to be lower in obese rats fed a HFD for a year, and this was concomitant to lower expression of hepatic regulatory LC genes. The effects of LC therapy were reversible. Streptozotocin-induced diabetic rats given LC (3 g/kg/day for five weeks) had better liver enzymes and choline levels in the liver, whereas TG levels in the plasma were reduced (Noland et al., 2009).
There are significant differences in the data, and it is unclear whether LC raises and when LC lowers free fatty acids (Sakai et al., 2016). In obese individuals, for instance, LC decreases all free fatty acids by delivering them to the mitochondria (Isaeva and Gapparova, 2018). An experiment looked at 13 patients with chronic liver disease before and after four weeks of LC treatment (1800 mg/day) and discovered that after LC treatment, free fatty acid levels and whole-body carbohydrate oxidation increased, but whole-body fatty acid and protein oxidation dropped dramatically (Sakai et al., 2016). Others here have found no alterations in energy metabolism when individuals with cirrhosis of the liver were given LC, but they did find increases in exercise tolerance, which suggests good energy metabolism. Evidence from experimental diabetic animals suggest that LC has an influence on energy metabolism, with one research finding that LC administration increases oxidation of fatty acid (Xia et al., 2011).
5.7. Antidiabetic activity
Carnitine administration reduced insulin resistance in mice fed a HFD devoid of affecting their consumption or weight. Carnitine enhanced insulin-induced glucose metabolism in diabetic and obese mice, as well as circulating levels of acyl-carnitine and urine excretion. Carnitine infusions increased glucose metabolism in healthy adults when assessed using a hyperinsulinaemic euglycaemic clamp, owing to a non-oxidative process that leads in glycogen accumulation (Stephens et al., 2006, Stephens et al., 2007). Carnitine administration enhanced glycogen storage and glucose oxidation in T2DM patients (Vidal-Casariego et al., 2013). Plasma levels of LC are 25 % lower in diabetic patients with problems than in those without difficulties, and it has been hypothesized that carnitine might be a beneficial therapeutic in T2DM (Poorabbas et al., 2007, Ringseis et al., 2012).
Severe hypoglycemia was shown to cause mitochondrial swelling in the brain, followed by neuronal cell death. Because LC efficiently protects mitochondrial function in various cells in vivo and in vitro, one investigation in male Wistar rats looked into its effects on brain damage caused by hypoglycemia shock. Animals were fed 0.1 % LC-containing water for one week before being given insulin to produce hypoglycemia. Though LC had no effect on the death of rats suffering from hypoglycemia shock, the Morris water-maze test revealed that it increased the cognitive performance of those that survived. LC markedly protected neuronal damage by inhibiting the rise in oxidized glutathione and mitochondrial dysfunctions in the hippocampus. In hippocampal neural cells grown in glucose-depleted media, LC also prevented the reduction in mitochondrial membrane potential and the formation of ROS. These findings show that LC protects the hippocampus from hypoglycemia-induced neuronal injury by sustaining mitochondrial activity. As a result, LC could have therapeutic benefits in individuals suffering from hypoglycemia caused by an excess of insulin (Hino et al., 2005).
5.8. Antioxidant activity
The impact of LC on renal epithelial cells of rat subjected to leptin to induce the OS hallmark of obesity was investigated. Leptin stimulated the production of superoxide anion (O2•) via NADPH oxidase (through the PI3 K/Akt pathway), the expression of NOX2, and the levels of nitrotyrosine. NOX4 expression and H2O2 levels, on the other hand, decreased following leptin administration. Additionally, leptin affected the expression of antioxidative enzymes, catalase and SOD, and increased the mRNA expression of pro-inflammatory factors. LC reversed all leptin-induced alterations to their pre-treatment levels. Finally, leptin administration caused oxidative damage and inflammation in NRK-52E cells, which could be alleviated by preincubation with LC. Remarkably, LC increased NOX4 expression and reinstated the release of its product, hydrogen peroxide, indicating that NOX4 may defend against leptin-induced kidney injury (Blanca et al., 2016). Another research analyzed the effect of LC pre-treatment on OS induced by A. ovalisporum in cells. Various OS markers, such as lipid peroxidation, DNA oxidation, protein oxidation, and the ratio of reduced to oxidized glutathione, as well as the activities of catalase, SOD, NADPH oxidase, and gamma-glutamyl-cysteine synthetase, were assessed in the liver. The study's findings indicated for the first time that LC dietary supplementation had favorable antioxidant effects on OS state in fish. There were no pro-oxidant effects identified at any of the LC dosages tested, indicating that LC is a chemoprotectant that decreases hepatic and renal OS and may be beneficial in the prevention and treatment of CYN-related intoxication in fish (Guzmán-Guillén et al., 2013).
5.9. Anticancer activity
Cancer cachexia (CC), characterized by a gradual loss of weight, is linked to reduced energy production. Because individuals with CC have aberrantly low amounts of LC in muscle tissue, mitochondrial β-oxidation of long-chain fatty acids does not proceed proficiently (Szefel et al., 2012). LC improves CC in mice, in part through the carnitine palmitoyltransferase-associated PPAR-signaling pathway. LC has been shown to alleviate cachectic symptoms. In cancer cachectic mice, oral treatment of LC enriched cachexia parameters and metabolic parameters at 9 mg/kg/day. LC reduced increased blood levels of TNF-α and IL-6 (Jiang et al., 2015).
The benefits of LC administration on expression of genes and hepatic fat metabolism-associated proteins were examined in cachectic tumor-bearing rats. Wistar rats were given either LC or saline at a dose of 1 g/kg. In tumor-bearing rats, LC protects hepatic lipid metabolism, indicating that supplementation might be beneficial in cachexia (Silvério et al., 2012). The carnitine has been examined in a variety of cancer types, both clinical and experimental. Chang et al. postulated that the antioxidant properties of LC may be used to inhibit hepatocarcinogenesis in Long-Evans Cinnamon rats (Chang et al., 2005). LC has been proven in vitro to slow the development of colon cancer cells by reversing the obstruction of mitochondrial fatty acid import in cancerous cells (Hoang et al., 2007). Researchers have hypothesized that acetyl-LC may have a role to play in preventing and alleviating chemotherapy-induced neuropathy, such as that seen in patients taking ifosfamide (Hockenberry et al., 2009). Furthermore, several investigations have indicated that the advantages of acetyl-l-CAR occur without reducing the medicines' anticancer effects (Li et al., 2011, Rebecca et al., 2007).
5.10. Sexual function
LC has been shown to help with infertility management (Abdelrazik, 2008, Binienda and Virmani, 2003, Dunning, 2012). The effect of LC on infertility of male has been well established. It was shown to be associated with spermatozoa epididymal maturation (Aliabadi et al., 2012, Cheng and Chen, 2008). It was shown to limit the impact of OS in sperm induced by free radical and ROS, as well as pathological sperm abnormalities such as ATP depletion, lipid peroxidation, inadequate axonemal phosphorylation, and loss of motility and viability (Dokmeci, 2005). Researchers and scientists contemplating using LC as a therapy for female infertility because it has been shown to be a strong antioxidant with few negative effects (Ismail et al., 2014, Samimi et al., 2016). OS has been shown to have a variety of effects on female reproductive system (Wu et al., 2011).
Similarly, antioxidant properties of ALC, the major acetyl ester of LC, have been demonstrated to have favorable effects on reproductive functioning (Aliabadi et al., 2012). ALC is required for intermediate metabolism in mammals, serving as an acetyl group donor and aiding the transport of fatty acids. ALC was shown to have cholinomimetic properties as well as modulating the GABA system. It can modulate neuronal function and hence the hypothalamo-pituitary gonadal axis to impose its influence on female reproduction since it is widespread in the hypothalamus (Liu et al., 2004).
Many research investigations employing carnitines to cure female infertility and increase reproductive function have previously been conducted in humans (Genazzani et al., 2011, Latifian et al., 2015) and animal models. It has also been utilized in ART to address comparable issues (Pirestani et al., 2011).
The majority of human studies used LC as a supplementation to relieve or treat the condition of female infertility. Supplementing with both LC and ALC relieves illnesses such as endometriosis, polycystic ovarian syndrome, and amenorrhea, according to several researches. Carnitines have been shown to promote oocyte health and boost sex hormone and gonadotropins levels (Genazzani et al., 2011).
6. Toxicological profile and safety concerns
A couple of clinical investigations have been focused on the role of LC in energy balance and weight loss. But regarding the safety issues, only a few are in consideration (Rubin et al., 2001). According to the OSL risk assessment approach, intakes up to 2000 mg/day of LC equivalents for chronic supplementation provide high evidence of safety, and this amount is designated as the observed safe level (OSL). The evidence for consumption above 2000 mg/day is insufficient for a confident assessment of long-term safety, despite the fact that far greater amounts have been tested without adverse effects and may be safe. (J.N. and A., 2006).
In a subchronic toxicity test including two genotoxicity assays, the safety of LC was evaluated. Rats were fed diets containing upto 50,000 ppm LC for 90 days, followed by a 4 weeks recovery phase, in a 90-day subchronic investigation. There were no treatment-related changes in mortality, gross pathology, hematology, ophthalmology, or histopathology. Significant increase in water and food intake, variations in the absolute and relative weights of seminal vesicles, and effects noted in urinalysis evaluations were all deemed to be of no toxicological significance because they either represented a physiological response, were temporary and disappeared at the end of the recovery phase, or weren't associated with any microscopic alteration. In both the presence and absence of metabolic activation, l-carnitine did not exhibit any carcinogenic activity in different bacterial strains at doses up to 5,000 g/plate, and it also did not cause chromosomal abnormalities in human cells. These trials' findings confirm that using l-carnitine and l-tartrate as a dietary source of l-carnitine is safe (Bellamine and Durkee, 2021).
7. Concluding remarks
Since the 1960 s, carnitine as a dietary supplement has already been marketed as therapeutic in a variety of illnesses involving carnitine deficiency and poor oxidation of fatty acid, implying that pharmacologic or nutritional carnitine supplements may be advantageous in some conditions. However, as per Stanley, there have only been two unambiguous cases of illnesses directly caused by carnitine deficit that have offered definitive proof of benefits from carnitine supplementation during the previous 40 years.
Carnitine is produced and obtained in adequate amounts by most healthy persons, even vegetarians. Because individuals' needs can exceed food intake during particular illness conditions, carnitine is classified as a “conditionally essential” nutrient. Because LC seems to have a low bioavailability and absorption, a rapid renal clearance, and active uptake into tissues, the amount of LC that may be increased in plasma with oral treatment, even up to and surpassing 2 mg, is restricted. Intravenous LC supplementation may be more efficacious if renal function is not affected, as above 95 % of LC filtered via glomeruli is stored, and excessive exogenous LC is rapidly eliminated.
Notwithstanding this, extensive research has been conducted on the benefits of prophylactic doses of carnitine in a variety of illness situations, while there is some dispute and misunderstanding about its usage in normal nutrition. Carnitine is a naturally occurring chemical that is non-toxic in oral levels up to several grams; therefore, supplements are frequently advised for primary and secondary deficits. Supplemental consumption of carnitine is generally tolerated since it is easily eliminated. When employed as a medicine, data from both rat and human research suggests health benefits.
Institutional review board statement
Not applicable.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelrazik H. L-carnitine and assisted reproduction. Arch Med Sci. 2008;5(1A):s43–s47. [Google Scholar]
- Abdelrazik H., Sharma R., Mahfouz R., Agarwal A. L-Carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil. Steril. 2009;91:589–596. doi: 10.1016/j.fertnstert.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Abu Ahmad N., Armaly Z., Berman S., Jabour A., Aga-Mizrachi S., Mosenego-Ornan E., Avital A. L-Carnitine improves cognitive and renal functions in a rat model of chronic kidney disease. Physiol. Behav. 2016;164:182–188. doi: 10.1016/j.physbeh.2016.05.036. [DOI] [PubMed] [Google Scholar]
- Acosta M.F., Muralidhran P., Abrahamson M.D., Grijalva C.L., Carver M., Tang H., Klinger C., Fineman J.R., Black S.M., Mansour H.M. Comparison of L-Carnitine and L-Carnitine HCL salt for targeted lung treatment of pulmonary hypertension (PH) as inhalation aerosols: Design, comprehensive characterization, in vitro 2D/3D cell cultures, and in vivo MCT-Rat model of PH. Pulm. Pharmacol. Ther. 2020;65 doi: 10.1016/j.pupt.2021.101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshin-Majd S., Bashiri K., Kiasalari Z., Baluchnejadmojarad T., Sedaghat R., Roghani M. Acetyl-L-carnitine protects dopaminergic nigrostriatal pathway in 6-hydroxydopamine-induced model of Parkinson’s disease in the rat. Biomed. Pharmacother. 2017;89:1–9. doi: 10.1016/j.biopha.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Aliabadi E., Mehranjani M.S., Borzoei Z., Talaei-Khozani T., Mirkhani H., Tabesh H. Effects of L-carnitine and L-acetyl-carnitine on testicular sperm motility and chromatin quality. Iran. J. Reprod. Med. 2012;10:77–82. [PMC free article] [PubMed] [Google Scholar]
- Aliev G., Liu J., Shenk J.C., Fischbach K., Pacheco G.J., Chen S.G., Obrenovich M.E., Ward W.F., Richardson A.G., Smith M.A., Gasimov E., Perry G., Ames B.N. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J. Cell. Mol. Med. 2009;13:320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almannai M., Alfadhel M., El-Hattab A.W. Carnitine inborn errors of metabolism. Molecules. 2019;24 doi: 10.3390/molecules24183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini C., Trevisan C., Isaya G., Pegolo G., Vergani L. Clinical varieties of carnitine and carnitine palmitoyltransferase deficiency. Clin. Biochem. 1987;20:1–7. doi: 10.1016/S0009-9120(87)80090-5. [DOI] [PubMed] [Google Scholar]
- Argani H., Rahbaninoubar M., Ghorbanihagjo A., Golmohammadi Z., Rashtchizadeh N. Effect of L-carnitine on the serum lipoproteins and HDL-C subclasses in hemodialysis patients. Nephron. Clin. Pract. 2005;101 doi: 10.1159/000087411. [DOI] [PubMed] [Google Scholar]
- Atar D., Spiess M., Mandinova A., Cierpka H., Noll G., Lüscher T.F. Carnitine - From cellular mechanisms to potential clinical applications in heart disease. Eur. J. Clin. Invest. 1997;27:973–976. doi: 10.1046/j.1365-2362.1997.2360783.x. [DOI] [PubMed] [Google Scholar]
- Aureli T., Di Cocco M.E., Puccetti C., Ricciolini R., Scalibastri M., Miccheli A., Manetti C., Conti F. Acetyl-L-carnitine modulates glucose metabolism and stimulates glycogen synthesis in rat brain. Brain Res. 1998;796:75–81. doi: 10.1016/S0006-8993(98)00319-9. [DOI] [PubMed] [Google Scholar]
- Aydogdu N., Atmaca G., Yalcin O., Taskiran R., Tastekin E., Kaymak K. Protective effects of L-carnitine on myoglobinuric acute renal failure in rats. Clin. Exp. Pharmacol. Physiol. 2006;33:119–124. doi: 10.1111/j.1440-1681.2006.04336.x. [DOI] [PubMed] [Google Scholar]
- Badreldeen A., El Razaky O., Erfan A., El-Bendary A., El Amrousy D. Comparative study of the efficacy of captopril, simvastatin, and L-carnitine as cardioprotective drugs in children with type 1 diabetes mellitus: A randomised controlled trial. Cardiol. Young. 2021;31:1315–1322. doi: 10.1017/S1047951121000226. [DOI] [PubMed] [Google Scholar]
- Bahbah E.I., Ghozy S., Attia M.S., Negida A., Emran T.B., Mitra S., Albadrani G.M., Abdel-Daim M.M., Uddin M.S., Simal-Gandara J. Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Mar. Drugs. 2021;19 doi: 10.3390/md19040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamine A., Durkee S. Genotoxicity and subchronic oral toxicity studies of L-carnitine and L-carnitine L-tartrate. J. Drug Metab. Toxicol. 2021;12:1–12. [Google Scholar]
- Binienda Z., Virmani A. The Mitochondriotropic Effects of L-carnitine and its Esters in the Central Nervous System. Curr. Med. Chem. - Cent. Nerv. Syst. Agents. 2003;3:275–282. doi: 10.2174/1568015033477659. [DOI] [Google Scholar]
- Blanca A.J., Ruiz-Armenta M.V., Zambrano S., Salsoso R., Miguel-Carrasco J.L., Fortuño A., Revilla E., Mate A., Vázquez C.M. Leptin Induces Oxidative Stress Through Activation of NADPH Oxidase in Renal Tubular Cells: Antioxidant Effect of L-Carnitine. J. Cell. Biochem. 2016;2281–2288 doi: 10.1002/jcb.25526. [DOI] [PubMed] [Google Scholar]
- Bonomini M., Zammit V., Pusey C.D., De Vecchi A., Arduini A. Pharmacological use of l-carnitine in uremic anemia: Has its full potential been exploited? Pharmacol. Res. 2011;63:157–164. doi: 10.1016/j.phrs.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Bonomini M., Di Liberato L., Zammit V., Arduini A. Current opinion on usage of L-carnitine in end-stage renal disease patients on peritoneal dialysis. Molecules. 2019;24 doi: 10.3390/molecules24193449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks S., Raymick J., Robinson B., Hanig J., Sarkar S. Neuroprotective effects of acetyl-L-carnitine (ALC) in a chronic MPTP-induced Parkinson’s disease mouse model: Endothelial and microglial effects. Neurosci. Lett. 2019;703:86–95. doi: 10.1016/j.neulet.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Burwinkel B., Kreuder J., Schweitzer S., Vorgerd M., Gempel K., Gerbitz K.D., Kilimann M.W. Carnitine transporter OCTN2 mutations in systemic primary carnitine deficiency: A novel Arg169Gln mutation and a recurrent arg282ter mutation associated with an unconventional splicing abnormality. Biochem. Biophys. Res. Commun. 1999;261:484–487. doi: 10.1006/bbrc.1999.1060. [DOI] [PubMed] [Google Scholar]
- Busquets S., Serpe R., Toledo M., Betancourt A., Marmonti E., Orpí M., Pin F., Capdevila E., Madeddu C., López-Soriano F.J., Mantovani G., Macciò A., Argilés J.M. L-Carnitine: An adequate supplement for a multi-targeted anti-wasting therapy in cancer. Clin. Nutr. 2012;31:889–895. doi: 10.1016/j.clnu.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Calvani M., Carta A., Benedetti N., Iannuccelli M., Caruso G. Action of Acetyl-L-Carnitine in Neurodegeneration and Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 1992;663:483–486. doi: 10.1111/j.1749-6632.1992.tb38710.x. [DOI] [PubMed] [Google Scholar]
- Canbaz H., Akca T., Tataroglu C., Caglikulekci M., Dirlik M., Ayaz L., Ustunsoy A.B., Tasdelen B., Aydin S. The effects of exogenous l-carnitine on lipid peroxidation and tissue damage in an experimental warm hepatic ischemia-reperfusion injury model. Curr. Ther. Res. - Clin. Exp. 2007;68:32–46. doi: 10.1016/j.curtheres.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M.C., Hurt R.T., Frazier T.H., Matheson P.J., Garrison R.N., McClain C.J., McClave S.A. Obesity, inflammation, and the potential application of pharmaconutrition. Nutr. Clin. Pract. 2008;23:16–34. doi: 10.1177/011542650802300116. [DOI] [PubMed] [Google Scholar]
- Cederbaum S.D., Koo-McCoy S., Tein I., Hsu B.Y.L., Ganguly A., Vilain E., Dipple K., Cvitanovic-Sojat L., Stanley C. Carnitine membrane transporter deficiency: A long-term follow up and OCTN2 mutation in the first documented case of primary carnitine deficiency. Mol. Genet. Metab. 2002;77:195–201. doi: 10.1016/S1096-7192(02)00169-5. [DOI] [PubMed] [Google Scholar]
- C̈ekin, A.H., GüR, G., Türkoǧlu, S., Aldemir, D., Uǧur Yilmaz, Gürsoy, M., Taşkoparan, M., Boyacioǧlu, S., 2013. The protective effect of L-carnitine on hepatic ischemia-reperfusion injury in rats. Turkish J. Gastroenterol. 24, 51–56. https://doi.org/10.4318/tjg.2013.0645. [PubMed]
- Cha Y.S. Effects of L-carnitine on obesity, diabetes, and as an ergogenic aid. Asia Pac. J. Clin. Nutr. 2008;17:306–308. [PubMed] [Google Scholar]
- Chang B.J., Nishikawa M., Nishiguchi S., Inoue M. L-carnitine inhibits hepatocarcinogenesis via protection of mitochondria. Int. J. Cancer. 2005;113:719–729. doi: 10.1002/ijc.20636. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya I., Gupta S., Mohammed A., Mushtaq N., Chauhan S., Ghosh S. Neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced Parkinsonism in rats. BMC Complement. Altern. Med. 2015;15 doi: 10.1186/s12906-015-0815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Yang M., Zhou M., Xiao J., Guo J., He L., Xing R. L-carnitine for cognitive enhancement in people without cognitive impairment. Cochrane Database Syst. Rev. 2015;2015 doi: 10.1002/14651858.CD009374.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.J., Chen T. Clinical efficacy of combined L-carnitine and acetyl-L-carnitine on idiopathic asthenospermia. Zhonghua Nan Ke Xue. 2008;14:149–151. [PubMed] [Google Scholar]
- Cruciani R.A., Dvorkin E., Homel P., Culliney B., Malamud S., Shaiova L., Fleishman S., Lapin J., Klein E., Lesage P., Portenoy R., Esteban-Cruciani N. L-carnitine supplementation for the treatment of fatigue and depressed mood in cancer patients with carnitine deficiency: A preliminary analysis. Ann. N. Y. Acad. Sci. 2004;1033:168–176. doi: 10.1196/annals.1320.016. [DOI] [PubMed] [Google Scholar]
- Da Silva Guimarães S., De Souza Cruz W., Da Silva L., MacIel G., Huguenin A.B., De Carvalho M., Costa B., Da Silva G., Da Costa C., D’Ippolito J.A., Colafranceschi A., Scalco F., Boaventura G. Effect of L-Carnitine Supplementation on Reverse Remodeling in Patients with Ischemic Heart Disease Undergoing Coronary Artery Bypass Grafting: A Randomized, Placebo-Controlled Trial. Ann. Nutr. Metab. 2017;70:106–110. doi: 10.1159/000465531. [DOI] [PubMed] [Google Scholar]
- Das R., Rauf A., Akhter S., Islam M.N., Emran T.B., Mitra S., Khan I.N., Mubarak M.S. Role of Withaferin A and Its Derivatives in the Management of Alzheimer’s Disease: Recent Trends and Future Perspectives. Molecules. 2021;26 doi: 10.3390/molecules26123696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare Mannelli L., Vivoli E., Salvicchi A., Schiavone N., Koverech A., Messano M., Nicolai R., Benatti P., Bartolini A., Ghelardini C. Antidepressant-like effect of artemin in mice: A mechanism for acetyl-l-carnitine activity on depression. Psychopharmacology (Berl). 2011;218:347–356. doi: 10.1007/s00213-011-2326-0. [DOI] [PubMed] [Google Scholar]
- Di Liberato L., Arduini A., Rossi C., Di Castelnuovo A., Posari C., Sacchetta P., Urbani A., Bonomini M. l-Carnitine status in end-stage renal disease patients on automated peritoneal dialysis. J. Nephrol. 2014;27:699–706. doi: 10.1007/s40620-014-0076-x. [DOI] [PubMed] [Google Scholar]
- DiNicolantonio J.J., Lavie C.J., Fares H., Menezes A.R., O’Keefe J.H. L-carnitine in the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Mayo Clin. Proc. 2013;88:544–551. doi: 10.1016/j.mayocp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Dionyssopoulou E., Vassiliadis S., Evangeliou A., Koumantakis E.E., Athanassakis I. Constitutive or induced elevated levels of L-carnitine correlate with the cytokine and cellular profile of endometriosis. J. Reprod. Immunol. 2005;65:159–170. doi: 10.1016/j.jri.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Dokmeci D. Oxidative stress, male infertility and the role of carnitines. Folia Med. (Plovdiv) 2005;47:26–30. [PubMed] [Google Scholar]
- Dunning K.R.R.R. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim. Reprod. Sci. 2012;134:69–75. doi: 10.1016/j.anireprosci.2012.08.013. [DOI] [PubMed] [Google Scholar]
- ., V., U., C., M.G., C., F., E., P., S., T., D.P., G., L., L.T., D., M., C., R., C., F., P., 2007. Effect of carnitine supplementation on lipid profile and anemia in children on chronic dialysis. Pediatr. Nephrol. 22, 727–733. [DOI] [PubMed]
- Eknoyan G., Latos D.L., Lindberg J. Practice recommendations for the use of L-Carnitine in dialysis-related carnitine disorder National Kidney Foundation Carnitine Consensus Conference. Am. J. Kidney Dis. 2003;41:868–876. doi: 10.1016/s0272-6386(03)00110-0. [DOI] [PubMed] [Google Scholar]
- El Sharkwy I.A., Abd El Aziz W.M. Randomized controlled trial of N-acetylcysteine versus l-carnitine among women with clomiphene-citrate-resistant polycystic ovary syndrome. Int. J. Gynecol. Obstet. 2019;147:59–64. doi: 10.1002/ijgo.12902. [DOI] [PubMed] [Google Scholar]
- Enooku K., Nakagawa H., Fujiwara N., Kondo M., Minami T., Hoshida Y., Shibahara J., Tateishi R., Koike K. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-47216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erguven M., Yilmaz O., Koc S., Caki S., Ayhan Y., Donmez M., Dolunay G. A case of early diagnosed carnitine deficiency presenting with respiratory symptoms. Ann. Nutr. Metab. 2007;51:331–334. doi: 10.1159/000107675. [DOI] [PubMed] [Google Scholar]
- Esmail M., Anwar S., Kandeil M., El-Zanaty A.M., Abdel-Gabbar M. Effect of Nigella sativa, atorvastatin, or L-Carnitine on high fat diet-induced obesity in adult male Albino rats. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111818. [DOI] [PubMed] [Google Scholar]
- Evans A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am. J. Kidney Dis. 2003;41 doi: 10.1016/S0272-6386(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Evans W.J., Morley J.E., Argilés J., Bales C., Baracos V., Guttridge D., Jatoi A., Kalantar-Zadeh K., Lochs H., Mantovani G., Marks D., Mitch W.E., Muscaritoli M., Najand A., Ponikowski P., Rossi Fanelli F., Schambelan M., Schols A., Schuster M., Thomas D., Wolfe R., Anker S.D. Cachexia: A new definition. Clin. Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Fahmy S.F., El-hamamsy M.H., Zaki O.K., Badary O. L-Carnitine supplementation improves the behavioral symptoms in autistic children. Res. Autism Spectr. Disord. 2013;7:159–166. [Google Scholar]
- Ferrari R., Merli E., Cicchitelli G., Mele D., Fucili A., Ceconi C. Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: A review. Ann. N. Y. Acad. Sci. 2004;1033:79–91. doi: 10.1196/annals.1320.007. [DOI] [PubMed] [Google Scholar]
- Furrer R., Handschin C. Muscle wasting diseases: Novel targets and treatments. Annu. Rev. Pharmacol. Toxicol. 2019;59:315–339. doi: 10.1146/annurev-pharmtox-010818-021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganai A.A., Jahan S., Ahad A., Abdin M.Z., Farooqi H. Glycine propionyl l-carnitine attenuates d-Galactosamine induced fulminant hepatic failure in wistar rats. Chem. Biol. Interact. 2014;214:33–40. doi: 10.1016/j.cbi.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Gao J., Gu Z., Li M., Xu Y., Gao Y., Wei J., Liang B., Na Y. L-Carnitine Ameliorates the Decrease of Aquaporin 2 Levels in Rats with Cisplatin-Induced Kidney Injury. Nephron. 2017;135:315–325. doi: 10.1159/000455052. [DOI] [PubMed] [Google Scholar]
- Gao X., Sun G., Randell E., Tian Y., Zhou H. Systematic investigation of the relationships of trimethylamine: N -oxide and l-carnitine with obesity in both humans and rodents. Food Funct. 2020;11:7707–7716. doi: 10.1039/d0fo01743d. [DOI] [PubMed] [Google Scholar]
- Gavrilova S.I., Kalyn Y.B., Kolykhalov I.V., Roshchina I.F., Selezneva N.D. Acetyl-L-carnitine (carnicetine) in the treatment of early stages of Alzheimer’s disease and vascular dementia. Zhurnal Nevrol. i Psihiatr. Im. S.S. Korsakova. 2011;111:16–22. [PubMed] [Google Scholar]
- Genazzani A.D., Lanzoni C., Ricchieri F., Santagni S., Rattighieri E., Chierchia E., Monteleone P., Jasonni V.M. Acetyl-L-carnitine (ALC) administration positively affects reproductive axis in hypogonadotropic women with functional hypothalamic amenorrhea. J. Endocrinol. Invest. 2011;34:287–291. doi: 10.3275/6997. [DOI] [PubMed] [Google Scholar]
- Ghanem N. L-carnitine improved bovine blastocyst rate and quality when supplemented at different preimplantation stages. Egypt. J. Anim. Prod. 2015;52:89–99. doi: 10.21608/ejap.2015.93625. [DOI] [Google Scholar]
- Giammarioli S., Boniglia C., Carratù B., Ciarrocchi M., Chiarotti F., Mosca M., Sanzini E. Use of food supplements and determinants of usage in a sample Italian adult population. Public Health Nutr. 2013;16:1768–1781. doi: 10.1017/S1368980012004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goin-Kochel R.P., Scaglia F., Schaaf C.P., Berry L.N., Dang D., Nowel K.P., Laakman A.L., Dowell L.R., Minard C.G., Loh A., Beaudet A.L. Side Effects and Behavioral Outcomes Following High-Dose Carnitine Supplementation Among Young Males With Autism Spectrum Disorder: A Pilot Study. Glob. Pediatr. Heal. 2019;6 doi: 10.1177/2333794X19830696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini A., D’Angelo A., Villa R.F. Action of L-acetylcarnitine on different cerebral mitochondrial populations from cerebral cortex. Neurochem. Res. 1998;23:1485–1491. doi: 10.1023/A:1020907400905. [DOI] [PubMed] [Google Scholar]
- Gramignano G., Lusso M.R., Madeddu C., Massa E., Serpe R., Deiana L., Lamonica G., Dessì M., Spiga C., Astara G., MacCiò A., Mantovani G. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition. 2006;22:136–145. doi: 10.1016/j.nut.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Guan Y., Zheng X.M., Yang Z.W., Li S.W. Protective effects of L-carnitine upon testicular ischemia-reperfusion damage in rats. Natl. Med. J. China. 2009;89:1858–1861. doi: 10.3760/cma.j.issn.0376-2491.2009.26.019. [DOI] [PubMed] [Google Scholar]
- Guarnieri G., Situlin R., Biolo G. Carnitine metabolism in uremia. Am. J. Kidney Dis. 2001;38 doi: 10.1053/ajkd.2001.27408. [DOI] [PubMed] [Google Scholar]
- Guerra J.V.S., Dias M.M.G., Brilhante A.J.V.C., Terra M.F., García-Arévalo M., Figueira A.C.M. Multifactorial basis and therapeutic strategies in metabolism-related diseases. Nutrients. 2021;13 doi: 10.3390/nu13082830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Guillén R., Prieto A.I., Vázquez C.M., Vasconcelos V., Cameán A.M. The protective role of l-carnitine against cylindrospermopsin-induced oxidative stress in tilapia (Oreochromis niloticus) Aquat. Toxicol. 2013;132–133:141–150. doi: 10.1016/j.aquatox.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Hatamkhani S., Khalili H., Karimzadeh I., Dashti-Khavidaki S., Abdollahi A., Jafari S. Carnitine for prevention of antituberculosis drug-induced hepatotoxicity: A randomized, clinical trial. J. Gastroenterol. Hepatol. 2014;29:997–1004. doi: 10.1111/jgh.12474. [DOI] [PubMed] [Google Scholar]
- Hershman D.L., Unger J.M., Crew K.D., Till C., Greenlee H., Minasian L.M., Moinpour C.M., Lew D.L., Fehrenbacher L., Wade J.L., Wong S.F., Fisch M.J., Henry N.L., Albain K.S. Two-year trends of taxane-induced neuropathy in women enrolled in a randomized trial of acetyl-L-carnitine (SWOG S0715) J. Natl. Cancer Inst. 2018;110:669–676. doi: 10.1093/jnci/djx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt W.R., Creager M.A., Amato A., Brass E.P. Effect of propionyl-l-carnitine on a background of monitored exercise in patients with claudication secondary to peripheral artery disease. J. Cardiopulm. Rehabil. Prev. 2011;31:125–132. doi: 10.1097/HCR.0b013e3181f1fd65. [DOI] [PubMed] [Google Scholar]
- Hino K., Nishikawa M., Sato E., Inoue M. L-Carnitine inhibits hypoglycemia-induced brain damage in the rat. Brain Res. 2005;1053:77–87. doi: 10.1016/j.brainres.2005.06.062. [DOI] [PubMed] [Google Scholar]
- Hoang B.X., Graeme Shaw D., Pham P., Levine S.A. Neuro-bioenergetic concepts in cancer prevention and treatment. Med. Hypotheses. 2007;68:832–843. doi: 10.1016/j.mehy.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Hockenberry M.J., Hooke M.C., Gregurich M., McCarthy K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J. Pediatr. Hematol. Oncol. 2009;31:664–669. doi: 10.1097/MPH.0b013e3181b259a7. [DOI] [PubMed] [Google Scholar]
- Hussein A.M., Adel M., El-Mesery M., Abbas K.M., Ali A.N., Abulseoud O.A. L-carnitine modulates epileptic seizures in pentylenetetrazole-kindled rats via suppression of apoptosis and Autophagy and Upregulation of Hsp70. Brain Sci. 2018;8:1–18. doi: 10.3390/brainsci8030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva A.P., Gapparova K.M. The effect of L-carnitine on lipid metabolism in patients with obesity. Clin. Nutr. 2018;37:S38–S39. doi: 10.1016/j.clnu.2018.06.1187. [DOI] [Google Scholar]
- Ismail A.M., Hamed A.H., Saso S., Thabet H.H. Adding L-carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;180:148–152. doi: 10.1016/j.ejogrb.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Iwase S., Kawaguchi T., Yotsumoto D., Doi T., Miyara K., Odagiri H., Kitamura K., Ariyoshi K., Miyaji T., Ishiki H., Inoue K., Tsutsumi C., Sagara Y., Yamaguchi T. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and l-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial (JORTC-CAM01) Support. Care Cancer. 2016;24:637–646. doi: 10.1007/s00520-015-2824-4. [DOI] [PubMed] [Google Scholar]
- J.L., F., P.A., S., J., V., M.D., W., Q., G., 2010. Role of carnitine in disease. Nutr. Metab. 7, 1–14. [DOI] [PMC free article] [PubMed]
- J.N., H., A., S., 2006. Risk assessment for carnitine. Regul. Toxicol. Pharmacol. 46, 23–28. [DOI] [PubMed]
- Jafari A., Khatami M.R., Dashti-Khavidaki S., Lessan-Pezeshki M., Abdollahi A., Moghaddas A. Protective Effects of L-Carnitine Against Delayed Graft Function in Kidney Transplant Recipients: A Pilot, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. J. Ren. Nutr. 2017;27:113–126. doi: 10.1053/j.jrn.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Jiang F., Zhang Z., Zhang Y., Pan X., Yu L., Liu S. L-Carnitine Ameliorates Cancer Cachexia in Mice Partly via the Carnitine Palmitoyltransferase-Associated PPAR-γ Signaling Pathway. Oncol. Res. Treat. 2015;38:511–516. doi: 10.1159/000439550. [DOI] [PubMed] [Google Scholar]
- Jin M., Pan T., Cheng X., Zhu T.T., Sun P., Zhou F., Ding X., Zhou Q.C. Effects of supplemental dietary L-carnitine and bile acids on growth performance, antioxidant and immune ability, histopathological changes and inflammatory response in juvenile black seabream (Acanthopagrus schlegelii) fed high-fat diet. Aquaculture. 2019;504:199–209. doi: 10.1016/j.aquaculture.2019.01.063. [DOI] [Google Scholar]
- Juliet P.A.R., Balasubramaniam D., Balasubramaniam N., Panneerselvam C. Carnitine: A Neuromodulator in Aged Rats. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2003;58:970–974. doi: 10.1093/gerona/58.11.b970. [DOI] [PubMed] [Google Scholar]
- Kabir M.T., Uddin M.S., Jeandet P., Emran T.B., Mitra S., Albadrani G.M., Sayed A.A., Abdel-Daim M.M., Simal-Gandara J. Anti-Alzheimer’s molecules derived from marine life: Understanding molecular mechanisms and therapeutic potential. Mar. Drugs. 2021;19 doi: 10.3390/md19050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J., Ringseis R., Priebe S., Guthke R., Kluge H., Eder K. Dietary L-carnitine alters gene expression in skeletal muscle of piglets. Mol. Nutr. Food Res. 2011;55:419–429. doi: 10.1002/mnfr.201000293. [DOI] [PubMed] [Google Scholar]
- Keller J., Ringseis R., Koc A., Lukas I., Kluge H., Eder K. Supplementation with L-carnitine downregulates genes of the ubiquitin proteasome system in the skeletal muscle and liver of piglets. Animal. 2012;6:70–78. doi: 10.1017/S1751731111001327. [DOI] [PubMed] [Google Scholar]
- Kelly G.S. L-Carnitine: therapeutic applications of a conditionally-essential amino acid. Altern. Med. Rev. 1998;3:345–360. [PubMed] [Google Scholar]
- Kepka A., Ochocinska A., Borzym-kluczyk M., Skorupa E., Stasiewicz-jarocka B., Chojnowska S., Waszkiewicz N. Preventive role of l-carnitine and balanced diet in Alzheimer’s disease. Nutrients. 2020;12:1–21. doi: 10.3390/nu12071987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kępka A., Ochocińska A., Chojnowska S., Borzym-Kluczyk M., Skorupa E., Knaś M., Waszkiewicz N. Potential role of l-carnitine in autism spectrum disorder. J. Clin. Med. 2021;10:1–26. doi: 10.3390/jcm10061202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolkhof P., Nowack C., Eitner F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr. Opin. Nephrol. Hypertens. 2015;24:417–424. doi: 10.1097/MNH.0000000000000147. [DOI] [PubMed] [Google Scholar]
- Kopple J.D., Ding H., Letoha A., Ivanyi B., Qing D.P.Y., Dux L., Wang H.Y., Sonkodi S. L-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol. Dial. Transplant. 2002;17:2122–2131. doi: 10.1093/ndt/17.12.2122. [DOI] [PubMed] [Google Scholar]
- Koppula S., Kumar H., More S.V., Kim B.W., Kim I.S., Choi D.K. Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. Int. J. Mol. Sci. 2012;13:10608–10629. doi: 10.3390/ijms130810608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koşan C., Sever L., Arisoy N., Çalisşkan S., Kasapçopur Ö. Carnitine supplementation improves apolipoprotein B levels in pediatric peritoneal dialysis patients. Pediatr. Nephrol. 2003;18:1184–1188. doi: 10.1007/s00467-003-1302-2. [DOI] [PubMed] [Google Scholar]
- Langen R.C.J., Haegens A., Vernooy J.H.J., Wouters E.F.M., De Winther M.P.J., Carlsen H., Steele C., Shoelson S.E., Schols A.M.W.J. NF-κB activation is required for the transition of pulmonary inflammation to muscle atrophy. Am. J. Respir. Cell Mol. Biol. 2012;47:288–297. doi: 10.1165/rcmb.2011-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lango R., Smolenski R.T., Narkiewicz M., Suchorzewska J., Lysiak-Szydlowska W. Influence of L-carnitine and its derivatives on myocardial metabolism and function in ischemic heart disease and during cardiopulmonary bypass. Cardiovasc. Res. 2001;51:21–29. doi: 10.1016/S0008-6363(01)00313-3. [DOI] [PubMed] [Google Scholar]
- Latifian S., Hamdi K., Totakneh R. Effect of addition of l-carnitine in polycystic ovary syndrome (PCOS) patients with clomiphene citrate and gonadotropin resistant. Int J Curr Res Acad Rev. 2015;3:469–476. [Google Scholar]
- Lee B.J., Lin J.S., Lin Y.C., Lin P.T. Effects of L-carnitine supplementation on lipid profiles in patients with coronary artery disease. Lipids Health Dis. 2016;15:1–8. doi: 10.1186/s12944-016-0277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk K., Schuler G., Adams V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia. Sarcopenia Muscle. 2010;1:9–21. doi: 10.1007/s13539-010-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Chen X., Li Q., Du J., Liu Z., Peng Y., Xu M., Li Q., Lei M., Wang C., Zheng S., Zhang X., Yu H., Shi J., Tao S., Feng P., Tian H. Effects of acetyl-L-carnitine and methylcobalamin for diabetic peripheral neuropathy: A multicenter, randomized, double-blind, controlled trial. J. Diabetes Investig. 2016;7:777–785. doi: 10.1111/jdi.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li Q.D., Wang P.Z., Wang M.S., Cui J., Diao T.Y., Li Q.H. The effect of oxidized low-density lipoprotein combined with adriamycin on the proliferation of Eca-109 cell line. Lipids Health Dis. 2011;10 doi: 10.1186/1476-511X-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipworth B., Skinner D., Devereux G., Thomas V., Jie J.L.Z., Martin J., Carter V., Price D.B. Underuse of β-blockers in heart failure and chronic obstructive pulmonary disease. Heart. 2016;102:1909–1914. doi: 10.1136/heartjnl-2016-309458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Head E., Kuratsune H., Cotman C.W., Ames B.N. Comparison of the effects of L-carnitine and acetyl-L-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann. N. Y. Acad. Sci. 2004;1033:117–131. doi: 10.1196/annals.1320.011. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yan S., Ji C., Dai W., Hu W., Zhang W., Mei C. Metabolomic changes and protective effect of L-carnitine in rat kidney ischemia/reperfusion injury. Kidney Blood Press. Res. 2012;35:373–381. doi: 10.1159/000336171. [DOI] [PubMed] [Google Scholar]
- Lordan R. Dietary supplements and nutraceuticals market growth during the coronavirus pandemic – Implications for consumers and regulatory oversight. PharmaNutrition. 2021;18 doi: 10.1016/j.phanu.2021.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M.A., A., 1997. Carnitine and its derivatives in cardiovascular disease. Prog. Cardiovasc. Dis. 40, 265–286. [DOI] [PubMed]
- M.G., H., T., M., O.B., S., T.W., M., H., E., S., J., K., B., U., S., 2015. Modification of Astrocyte Metabolism as an Approach to the Treatment of Epilepsy: Triheptanoin and Acetyl-l-Carnitine. Neurochem. Res. [DOI] [PubMed]
- Magoulas P.L., El-Hattab A.W. Systemic primary carnitine deficiency: An overview of clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 2012;7:1. doi: 10.1186/1750-1172-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodpoor A., Shokouhi G., Hamishehkar H., Soleimanpour H., Sanaie S., Porhomayon J., Rasouli F., Nader N.D. A pilot trial of L-carnitine in patients with traumatic brain injury: Effects on biomarkers of injury. J. Crit. Care. 2018;45:128–132. doi: 10.1016/j.jcrc.2018.01.029. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M. Acetyl-L-carnitine in hepatic encephalopathy. Metab. Brain Dis. 2013;28:193–199. doi: 10.1007/s11011-013-9376-4. [DOI] [PubMed] [Google Scholar]
- Marín, V.B., Azocar, M., Molina, M., Guerrero, J.L., Ratner, R., C.F. 2006. Total carnitine and acylated carnitine ratio: relationship of free carnitine with lipid parameters in pediatric dialysis patients. Adv. Perit. Dial. Conf. Perit. Dial. 22, 130–135. [PubMed]
- Martinez G., Costantino G., Clementi A., Puglia M., Clementi S., Cantarella G., De Meo L., Matera M. Cisplatin-induced kidney injury in the rat: l-carnitine modulates the relationship between MMP-9 and TIMP-3. Exp. Toxicol. Pathol. 2009;61:183–188. doi: 10.1016/j.etp.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Matera M., Bellinghieri G., Costantino G., Santoro D., Calvani M., Savica V. History of L-carnitine: Implications for renal disease. J. Ren. Nutr. 2003;13:2–14. doi: 10.1053/jren.2003.50010. [DOI] [PubMed] [Google Scholar]
- Mayatepek E., Nezu J., Tamai I., Oku A., Katsura M., Shimane M., Tsuji A. Two novel missense mutations of the OCTN2 gene (W283R and V446F) in a patient with primary systemic carnitine deficiency. Hum. Mutat. 2000;15:118. doi: 10.1002/(SICI)1098-1004(200001)15:1<118::AID-HUMU28>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Moawad A.R., Xu B., Tan S.L., Taketo T. L-Carnitine supplementation during vitrification of mouse germinal vesicle stage-oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes. Hum. Reprod. 2014;29:2256–2268. doi: 10.1093/humrep/deu201. [DOI] [PubMed] [Google Scholar]
- Montesano A., Senesi P., Luzi L., Benedini S., Terruzzi I. Potential therapeutic role of L-carnitine in skeletal muscle oxidative stress and atrophy conditions. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/646171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggi M., Cassano P., Vasso M., Capitanio D., Fania C., Musicco C., Pesce V., Gadaleta M.N., Gelfi C. A DIGE approach for the assessment of rat soleus muscle changes during unloading: Effect of acetyl-L-carnitine supplementation. Proteomics. 2008;8:3588–3604. doi: 10.1002/pmic.200701176. [DOI] [PubMed] [Google Scholar]
- Muangpaisan W., Mathews A., Hori H., Seidel D. A systematic review of the worldwide prevalence and incidence of Parkinson’s disease. J. Med. Assoc. Thail. 2011;94:749–755. [PubMed] [Google Scholar]
- Noland R.C., Koves T.R., Seiler S.E., Lum H., Lust R.M., Ilkayeva O., Stevens R.D., Hegardt F.G., Muoio D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien D., Chunduri P., Iyer A., Brown L. L-carnitine attenuates cardiac remodelling rather than vascular remodelling in deoxycorticosterone acetate-salt hypertensive rats. Basic Clin. Pharmacol. Toxicol. 2010;106:296–301. doi: 10.1111/j.1742-7843.2009.00480.x. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021;10 doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnetti L., Gaiti A., Mecocci P., Cadini D., Senin U. Pharmacokinetics of IV and oral acetyl-L-carnitine in a multiple dose regimen in patients with senile dementia of Alzheimer type. Eur. J. Clin. Pharmacol. 1992;42:89–93. doi: 10.1007/BF00314926. [DOI] [PubMed] [Google Scholar]
- Pettegrew J.W., Klunk W.E., Panchalingam K., Kanfer J.N., McClure R.J. Clinical and neurochemical effects of acetyl-L-carnitine in Alzheimer’s disease. Neurobiol. Aging. 1995;16:1–4. doi: 10.1016/0197-4580(95)80001-8. [DOI] [PubMed] [Google Scholar]
- Pirestani A., Aghakhani M., Tabatabaei S.N., Ghalamkari G., Baharlo F. Effects of dietary L-Carnitine and Choline Chloride Compound on Reproduction Indices and Udder Immune System in Holstein Dairy Cattle. Life Sci. Technol. 2011;3:59–61. [Google Scholar]
- Pons R., De Vivo D.C. Primary and secondary carnitine deficiency syndromes. J. Child Neurol. 1995;10 doi: 10.1177/0883073895010002s03. [DOI] [PubMed] [Google Scholar]
- Poorabbas A., Fallah F., Bagdadchi J., Mahdavi R., Aliasgarzadeh A., Asadi Y., Koushavar H., Vahed Jabbari M. Determination of free L-carnitine levels in type II diabetic women with and without complications. Eur. J. Clin. Nutr. 2007;61:892–895. doi: 10.1038/sj.ejcn.1602594. [DOI] [PubMed] [Google Scholar]
- Powers S.K., Lynch G.S., Murphy K.T., Reid M.B., Zijdewind I. Disease-induced skeletal muscle atrophy and fatigue. Med. Sci. Sports Exerc. 2016;48:2307–2319. doi: 10.1249/MSS.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader K.L., Cox N.R., Stanton J.A.L., Juengel J.L. Effects of acetyl-L-carnitine on lamb oocyte blastocyst rate, ultrastructure, and mitochondrial DNA copy number. Theriogenology. 2015;83:1484–1492. doi: 10.1016/j.theriogenology.2015.01.028. [DOI] [PubMed] [Google Scholar]
- Rebecca, J.T., Andrew, J.M., Mark, H., Luc, J.C. van L., David, C.-S., 2007. Reduced plasma free fatty acid availability during exercise: effect on gene expression. Eur. J. Appl. Physiol. V99, 485. [DOI] [PubMed]
- Rebouche C.J. Kinetics, pharmacokinetics, and regulation of L-Carnitine and acetyl-L-carnitine metabolism. Ann. N. Y. Acad. Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- Remels A.H.V., Gosker H.R., Langen R.C.J., Schols A.M.W.J. The mechanisms of cachexia underlying muscle dysfunction in COPD. J. Appl. Physiol. 2013;114:1253–1262. doi: 10.1152/japplphysiol.00790.2012. [DOI] [PubMed] [Google Scholar]
- Reuter S.E., Faull R.J., Evans A.M. L-carnitine supplementation in the dialysis population: Are Australian patients missing out? Nephrology. 2008;13:3–16. doi: 10.1111/j.1440-1797.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- Ribas G.S., Vargas C.R., Wajner M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene. 2014;533:469–476. doi: 10.1016/j.gene.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Rigault C., Mazué F., Bernard A., Demarquoy J., Le Borgne F. Changes in l-carnitine content of fish and meat during domestic cooking. Meat Sci. 2008;78:331–335. doi: 10.1016/j.meatsci.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Rinaldo P., Raymond K., Al-Odaib A., Bennett M.J. Clinical and biochemical features of fatty acid oxidation disorders. Curr. Opin. Pediatr. 1998;10:615–621. doi: 10.1097/00008480-199810060-00014. [DOI] [PubMed] [Google Scholar]
- Ringseis R., Keller J., Eder K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: Evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur. J. Nutr. 2012;51:1–18. doi: 10.1007/s00394-011-0284-2. [DOI] [PubMed] [Google Scholar]
- Ringseis R., Keller J., Eder K. Mechanisms underlying the anti-wasting effect of l-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013;52:1421–1442. doi: 10.1007/s00394-013-0511-0. [DOI] [PubMed] [Google Scholar]
- Ringseis R., Keller J., Eder K. Basic mechanisms of the regulation of L-carnitine status in monogastrics and efficacy of L-carnitine as a feed additive in pigs and poultry. J. Anim. Physiol. Anim. Nutr. (Berl) 2018;102:1686–1719. doi: 10.1111/jpn.12959. [DOI] [PubMed] [Google Scholar]
- Roan P.G., Buja M., Saffer S., Izquierdo C., Hagler H., Duke B., Hillis L.D., Willerson J.T. Effects of systemic hypertension on ischemic and nonischemic regional left ventricular function in awake, unsedated dogs after experimental coronary occlusion. Circulation. 1982;65:115–125. doi: 10.1161/01.CIR.65.1.115. [DOI] [PubMed] [Google Scholar]
- Rospond B., Chlopicka J. The biological function of L-carnitine and its content in the particular food examples. Przeglad Lek. 2013;70:85–91. [PubMed] [Google Scholar]
- Rubin M.R., Volek J.S., Gómez A.L., Ratamess N.A., French D.N., Sharman M.J., Kraemer W.J. Safety Measures of L-Carnitine L-Tartrate Supplementation in Healthy Men. J. Strength Cond. Res. 2001;15:486–490. doi: 10.1519/1533-4287(2001)015<0486:SMOLCL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P., Cattaneo D., Loriga G., Ledda F., Motterlini N., Gherardi G., Orisio S., Remuzzi G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: Effects of acetyl-l-carnitine therapy. Hypertension. 2009;54:567–574. doi: 10.1161/HYPERTENSIONAHA.109.132522. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Nishikawa H., Enomoto H., Yoh K., Iwata Y., Hasegawa K., Nakano C., Kishino K., Shimono Y., Takata R., Nishimura T., Aizawa N., Ikeda N., Takashima T., Ishii A., Iijima H., Nishiguchi S. Effect of L-Carnitine in Patients With Liver Cirrhosis on Energy Metabolism Using Indirect Calorimetry: A Pilot Study. J. Clin. Med. Res. 2016;8:863–869. doi: 10.14740/jocmr2734w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurauchi Y., Matsumoto Y., Shinzato T., Takai I., Nakamura Y., Sato M., Nakai S., Miwa M., Morita H., Miwa T., Amano I., Maeda K. Effects of L-carnitine supplementation on muscular symptoms in hemodialyzed patients. Am. J. Kidney Dis. 1998;32:258–264. doi: 10.1053/ajkd.1998.v32.pm9708610. [DOI] [PubMed] [Google Scholar]
- Salic K., Gart E., Seidel F., Verschuren L., Caspers M., van Duyvenvoorde W., Wong K.E., Keijer J., Bobeldijk-Pastorova I., Wielinga P.Y., Kleemann R. Combined treatment with L-carnitine and nicotinamide riboside improves hepatic metabolism and attenuates obesity and liver steatosis. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi M., Jamilian M., Ebrahimi F.A., Rahimi M., Tajbakhsh B., Asemi Z. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. (Oxf) 2016;84:851–857. doi: 10.1111/cen.13003. [DOI] [PubMed] [Google Scholar]
- Saneian H., Khalilian L., Heidari-Beni M., Khademian M., Famouri F., Nasri P., Hassanzadeh A., Kelishadi R. Effect of l -carnitine supplementation on children and adolescents with nonalcoholic fatty liver disease (NAFLD): A randomized, triple-blind, placebo-controlled clinical trial. J. Pediatr. Endocrinol. Metab. 2021;34:897–904. doi: 10.1515/jpem-2020-0642. [DOI] [PubMed] [Google Scholar]
- Sangouni A.A., Sasanfar B., Ghadiri-Anari A., Hosseinzadeh M. Effect of L-carnitine supplementation on liver fat content and cardiometabolic indices in overweight/obese women with polycystic ovary syndrome: A randomized controlled trial. Clin. Nutr. ESPEN. 2021;46:54–59. doi: 10.1016/j.clnesp.2021.08.005. [DOI] [PubMed] [Google Scholar]
- Santini A., Novellino E. Nutraceuticals: Beyond the Diet Before the Drugs. Curr. Bioact. Compd. 2014;10:1–12. doi: 10.2174/157340721001140724145924. [DOI] [Google Scholar]
- Savic D., Hodson L., Neubauer S., Pavlides M. The importance of the fatty acid transporter l-carnitine in non-alcoholic fatty liver disease (Nafld) Nutrients. 2020;12:1–17. doi: 10.3390/nu12082178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitha S., Naveen B., Panneerselvam C. Carnitine and lipoate ameliorates lipofuscin accumulation and monoamine oxidase activity in aged rat heart. Eur. J. Pharmacol. 2007;574:61–65. doi: 10.1016/j.ejphar.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Sawicka A.K., Hartmane D., Lipinska P., Wojtowicz E., Lysiak-Szydlowska W., Olek R.A. L-carnitine supplementation in older women. A pilot study on aging skeletal muscle mass and function. Nutrients. 2018;10 doi: 10.3390/nu10020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönekess B.O., Allard M.F., Lopaschuk G.D. Propionyl l-carnitine improvement of hypertrophied rat heart function is associated with an increase in cardiac efficiency. Eur. J. Pharmacol. 1995;286:155–166. doi: 10.1016/0014-2999(95)00442-N. [DOI] [PubMed] [Google Scholar]
- Schreiber B.D. Debate forum: Levocarnitine therapy is rational and justified in selected dialysis patients. Blood Purif. 2006;24:128–139. doi: 10.1159/000089449. [DOI] [PubMed] [Google Scholar]
- Schröder C.H., Edefonti A., Fischbach M., Klaus G., Rönnholm K., Schaefer F., Simkova E., Stefanidis D., Strazdins V., Vande Walle J., Watson A., Zurowska A. The management of anemia in pediatric peritoneal dialysis patients. Guidelines by an ad hoc European committee. Pediatr. Nephrol. 2003;18:805–809. doi: 10.1007/s00467-003-1126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat R., Roghani M., Khalili M. Neuroprotective effect of thymoquinone, the Nigella sativa bioactive compound, in 6-hydroxydopamine-induced hemi-parkinsonian rat model. Iran. J. Pharm. Res. 2014;13:227–234. [PMC free article] [PubMed] [Google Scholar]
- Shankar S.S., Mirzamohammadi B., Walsh J.P., Steinberg H.O. L-carnitine may attenuate free fatty acid-induced endothelial dysfunction. Ann. N. Y. Acad. Sci. 2004;1033:189–197. doi: 10.1196/annals.1320.018. [DOI] [PubMed] [Google Scholar]
- Sidorenkov G., Navis G. Safety of ACE inhibitor therapies in patients with chronic kidney disease. Expert Opin. Drug Saf. 2014;13:1383–1395. doi: 10.1517/14740338.2014.951328. [DOI] [PubMed] [Google Scholar]
- Silvério R., Laviano A., Fanelli F.R., Seelaender M. L-Carnitine induces recovery of liver lipid metabolism in cancer cachexia. Amino Acids. 2012;42:1783–1792. doi: 10.1007/s00726-011-0898-y. [DOI] [PubMed] [Google Scholar]
- Smeland O.B., Meisingset T.W., Borges K., Sonnewald U. Chronic acetyl-l-carnitine alters brain energy metabolism and increases noradrenaline and serotonin content in healthy mice. Neurochem. Int. 2012;61:100–107. doi: 10.1016/j.neuint.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Soc, E., Sci, P., El-damarawi, M.A., Nassef, N.A., 2022. Protective Effect of the Combined use of L-carnitine and L-arginine against Hepatic Ischemia-Reperfusion Injury in Rats 213–230.
- Song X., Qu H., Yang Z., Rong J., Cai W., Zhou H. Efficacy and Safety of L-Carnitine Treatment for Chronic Heart Failure: A Meta-Analysis of Randomized Controlled Trials. Biomed Res. Int. 2017;2017 doi: 10.1155/2017/6274854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter U., Huener G., Baykal T., Demirkol M., Duran M., Wanders R., Nezu J., Mayatepek E. Silent and symptomatic primary carnitine deficiency within the same family due to identical mutations in the organic cation/carnitine transporter OCTN2. J. Inherit. Metab. Dis. 2003;26:613–615. doi: 10.1023/A:1025968502527. [DOI] [PubMed] [Google Scholar]
- Stangenberg S., Nguyen L.T., Chan Y.L., Zaky A., Pollock C.A., Chen H., Saad S. Maternal L-carnitine supplementation ameliorates renal underdevelopment and epigenetic changes in male mice offspring due to maternal smoking. Clin. Exp. Pharmacol. Physiol. 2019;46:183–193. doi: 10.1111/1440-1681.13038. [DOI] [PubMed] [Google Scholar]
- Steiber A., Kerner J., Hoppel C.L. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 2004;25:455–473. doi: 10.1016/j.mam.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Stephens F.B., Constantin-Teodosiu D., Laithwaite D., Simpson E.J., Greenhaff P.L. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J. Clin. Endocrinol. Metab. 2006;91:5013–5018. doi: 10.1210/jc.2006-1584. [DOI] [PubMed] [Google Scholar]
- Stephens F.B., Constantin-teodosiu D., Greenhaff P.L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007;581:431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilakou A.A., Lazaris A.C., Perelas A.I., Mourouzis I.S., Douzis I.C., Karkalousos P.L., Stylianaki A.T., Pantos C.I., Liapi C.A. Heart dysfunction induced by choline-deficiency in adult rats: The protective role of l-carnitine. Eur. J. Pharmacol. 2013;709:20–27. doi: 10.1016/j.ejphar.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Suchy J., Chan A., Shea T.B. Dietary supplementation with a combination of α-lipoic acid, acetyl-l-carnitine, glycerophosphocoline, docosahexaenoic acid, and phosphatidylserine reduces oxidative damage to murine brain and improves cognitive performance. Nutr. Res. 2009;29:70–74. doi: 10.1016/j.nutres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Szefel J., Kruszewski W.J., Ciesielski M., Szajewski M., Kawecki K., Aleksandrowicz-Wrona E., Jankun J., Łysiak-SzydŁowska W. L-carnitine and cancer cachexia. I. L-carnitine distribution and metabolic disorders in cancer cachexia. Oncol. Rep. 2012;28:319–323. doi: 10.3892/or.2012.1804. [DOI] [PubMed] [Google Scholar]
- Tamilselvan J., Jayaraman G., Sivarajan K., Panneerselvam C. Age-dependent upregulation of p53 and cytochrome c release and susceptibility to apoptosis in skeletal muscle fiber of aged rats: Role of carnitine and lipoic acid. Free Radic. Biol. Med. 2007;43:1656–1669. doi: 10.1016/j.freeradbiomed.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Tao W., Anqi G., Qingyu S., Yangjian Q., Ying K., Zhiping S., Shumin S., Zhengwei F. L-Carnitine intake prevents irregular feeding-induced obesity and lipid metabolism disorder. Gene. 2015;554:148–154. doi: 10.1016/j.gene.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Tauqir S., Israr M., Rauf B., Malik M.O., Habib S.H., Shah F.A., Usman M., Raza M.A., Shah I., Badshah H., Ehtesham E., Shah M. Acetyl-l-Carnitine Ameliorates Metabolic and Endocrine Alterations in Women with PCOS: A Double-Blind Randomized Clinical Trial. Adv. Ther. 2021;38:3842–3856. doi: 10.1007/s12325-021-01789-5. [DOI] [PubMed] [Google Scholar]
- Thangavelu S. Fatty Acid Oxidation disorders. Indian J. Pract. Pediatr. 2010;12:181–183. doi: 10.21037/atm.2018.10.57. [DOI] [Google Scholar]
- Tousson E., Tawfeek Zaki Z., Ali Abu-Shaeir W., Hassan H. Methotrexate-induced Hepatic and Renal Toxicity: Role of L-carnitine in Treatment “Methotrexate-induced Hepatic and Renal Toxicity: Role of L-carnitine in Treatment. Biomed. Biotechnol. 2014;2:85–92. [Google Scholar]
- Usta U., Inan M., Erbas H., Aydogdu N., Oz Puyan F., Altaner S. Tissue damage in rat ovaries subjected to torsion and detorsion: Effects of l-carnitine and N-acetyl cysteine. Pediatr. Surg. Int. 2008;24:567–573. doi: 10.1007/s00383-008-2123-y. [DOI] [PubMed] [Google Scholar]
- Van Weyenberg S., Buyse J., Janssens G.P.J. Increased plasma leptin through l-carnitine supplementation is associated with an enhanced glucose tolerance in healthy ponies. J. Anim. Physiol. Anim. Nutr. (Berl) 2009;93:203–208. doi: 10.1111/j.1439-0396.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Vescovo G., Ravara B., Gobbo V., Sandri M., Angelini A., Barbera M.D., Dona M., Peluso G., Calvani M., Mosconi L., Libera L.D. L-carnitine: A potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am. J. Physiol. - Cell Physiol. 2002;283 doi: 10.1152/ajpcell.00046.2002. [DOI] [PubMed] [Google Scholar]
- Vidal-Casariego A., Burgos-Peláez R., Martínez-Faedo C., Calvo-Gracia F., Valero-Zanuy M.Á., Luengo-Pérez L.M., Cuerda-Compés C. Metabolic effects of L-carnitine on type 2 diabetes mellitus: Systematic review and meta-analysis. Exp. Clin. Endocrinol. Diabetes. 2013;121:234–238. doi: 10.1055/s-0033-1333688. [DOI] [PubMed] [Google Scholar]
- Virmani A., Gaetani F., Binienda Z., Xu A., Duhart H., Ali S.F. Role of mitochondrial dysfunction in neurotoxicity of MPP+: Partial protection of PC12 cells by acetyl-L-carnitine. Ann. N. Y. Acad. Sci. 2004;1025:267–273. doi: 10.1196/annals.1316.033. [DOI] [PubMed] [Google Scholar]
- Wang W., Bai M., Jiang T., Li C., Li P., Zhou H., Wang Z., Li L., Jiang H. Clozapine-induced reduction of L-carnitine reabsorption via inhibition/down-regulation of renal carnitine/organic cation transporter 2 contributes to liver lipid metabolic disorder in mice. Toxicol. Appl. Pharmacol. 2019;363:47–56. doi: 10.1016/j.taap.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Wang S.M., Han C., Lee S.J., Patkar A.A., Masand P.S., Pae C.U. A review of current evidence for acetyl-l-carnitine in the treatment of depression. J. Psychiatr. Res. 2014;53:30–37. doi: 10.1016/j.jpsychires.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Wang Z.Y., Liu Y.Y., Liu G.H., Lu H.B., Mao C.Y. L-Carnitine and heart disease. Life Sci. 2018;194:88–97. doi: 10.1016/j.lfs.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Winter S.C. Treatment of carnitine deficiency. J. Inherit. Metab. Dis. 2003;26(2–3):171–180. doi: 10.1023/a:1024433100257. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K., Adami H.O., Cole P., Trichopoulos D., Mandel J. Epidemiology and etiology of Parkinson’s disease: A review of the evidence. Eur. J. Epidemiol. 2011;26 doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- Wollen K.A. Alzheimer’s disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern. Med. Rev. 2010;15:223–244. [PubMed] [Google Scholar]
- Wu G.Q., Jia B.Y., Li J.J., Fu X.W., Zhou G.B., Hou Y.P., Zhu S.E. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology. 2011;76:785–793. doi: 10.1016/j.theriogenology.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Xia Y., Li Q., Zhong W., Dong J., Wang Z., Wang C. L-carnitine ameliorated fatty liver in high-calorie diet/STZ-induced type 2 diabetic mice by improving mitochondrial function. Diabetol. Metab. Syndr. 2011;3 doi: 10.1186/1758-5996-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Y., W., J., Y., V., G., N., L., 1999. Mutations in the organic cation/carnitine transporter OCTN2 in primary carnitine deficiency. Proc. Natl. Acad. Sci. U. S. A. 96, 2356–2360. [DOI] [PMC free article] [PubMed]
- Ye J., Li J., Yu Y., Wei Q., Deng W., Yu L. L-carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul. Pept. 2010;161:58–66. doi: 10.1016/j.regpep.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Yonezawa K., Tolba R.H., Wetter A., Yamamoto Y., Yamaoka Y., Minor T. L-carnitine could not improve hepatic warm ischemia-reperfusion injury despite ameliorated blood flow. J. Surg. Res. 2005;125:16–22. doi: 10.1016/j.jss.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y., Ueda E., Sakurai H., Kojima Y. Anti-diabetes effect of Zn(II)/carnitine complex by oral administration. Chem. Pharm. Bull. 2003;51:230–231. doi: 10.1248/cpb.51.230. [DOI] [PubMed] [Google Scholar]
- You J., Lee J., Hyun S.H., Lee E. L-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology. 2012;78:235–243. doi: 10.1016/j.theriogenology.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Yu Z., Iryo Y., Matsuoka M., Igisu H., Ikeda M. Suppression of pentylenetetrazol-induced seizures by carnitine in mice. Naunyn. Schmiedebergs. Arch. Pharmacol. 1997;355:545–549. doi: 10.1007/PL00004981. [DOI] [PubMed] [Google Scholar]
- Zambrano S., Blanca A.J., Ruiz-Armenta M.V., Miguel-Carrasco J.L., Arévalo M., Mate A., Vázquez C.M. L-carnitine attenuates the development of kidney fibrosis in hypertensive rats by upregulating PPAR-γ. Am. J. Hypertens. 2014;27:460–470. doi: 10.1093/ajh/hpt268. [DOI] [PubMed] [Google Scholar]
- Zhai W., Neuman S.L., Latour M.A., Hester P.Y. The effect of male and female supplementation of L-carnitine on reproductive traits of white leghorns. Poult. Sci. 2008;87:1171–1181. doi: 10.3382/ps.2007-00325. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. yu, Fan, Z. kai, Cao, Y., Jia, Z. qiang, Li, G., Zhi, X. dong, Yu, D. shui, Lv, G., 2015. Acetyl-l-carnitineamelioratesmitochondrial damage and apoptosis following spinal cord injury in rats. Neurosci. Lett. 604, 18–23. https://doi.org/10.1016/j.neulet.2015.06.021. [DOI] [PubMed]
- Zhang H., Jia H., Liu J., Ao N., Yan B., Shen W., Wang X., Li X., Luo C., Liu J. Combined R-α-lipoic acid and acetyl-L-carnitine exerts efficient preventative effects in a cellular model of Parkinson’s disease. J. Cell. Mol. Med. 2010;14:215–225. doi: 10.1111/j.1582-4934.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H. lan, Zhang, H. yue, Zhu, C. lian, Li, H. ying, Cui, S., Jin, Jian, Piao, S. guo, Jiang, Y. ji, Xuan, M. ying, Jin, Ji zhe, Jin, Y. shun, Lee, J. pyo, Chung, B. ha, Choi, B. soon, Yang, C. woo, Li, C., 2021. L -Carnitine protects against tacrolimus-induced renal injury by attenuating programmed cell death via PI3K/AKT/PTEN signaling. Acta Pharmacol. Sin. 42, 77–87. https://doi.org/10.1038/s41401-020-0449-8. [DOI] [PMC free article] [PubMed]
- Zhou P., Chen Z., Zhao N., Liu D., Guo Z.Y., Tan L., Hu J., Wang Q., Wang J.Z., Zhu L.Q. Acetyl-l-carnitine attenuates homocysteine-induced alzheimer-like histopathological and behavioral abnormalities. Rejuvenation Res. 2011;14:669–679. doi: 10.1089/rej.2011.1195. [DOI] [PubMed] [Google Scholar]