Abstract
A histologic study was performed on the livers of wild-type (WT), severe combined immunodeficient (SCID), hydrocortisone acetate (HC)-treated WT, and HC-treated SCID mice infected intravenously with 105 CFU of Mycobacterium bovis BCG. It was found that infection progressed faster in SCID mice than in WT mice and that HC treatment caused exacerbation of infection in both types of mice. In all cases infection in the liver was confined to granulomas that were populated predominantly by macrophages. Higher levels of infection in HC-treated SCID mice, but not HC-treated WT mice, were associated with extensive infection and destruction of parenchymal cells at the margins of granulomas. The results indicate that in the absence of T-cell-mediated immunity and of HC-sensitive T-cell-independent defense mechanisms, macrophages are incapable of restricting BCG growth and of confining infection to their cytoplasm. Consequently, BCG bacilli are released into the extracellular environment, where they are ingested by neighboring parenchymal cells.
It is generally believed that Mycobacterium tuberculosis, Mycobacterium bovis, and other members of the Mycobacterium tuberculosis complex are intracellular pathogens that reside in their hosts almost exclusively in macrophages. Therefore, these pathogens remain confined to the cytoplasm of the very host cells that are equipped to express innate and acquired antimicrobial defense mechanisms against them. The apparent absence of evidence showing that M. tuberculosis and M. bovis can also infect parenchymal cells means either that parenchymal cells are not capable of phagocytosing these pathogens or that parenchymal cells are not provided the opportunity to ingest M. tuberculosis or M. bovis bacilli during the normal course of infection. The second possibility seems more likely given the evidence (9–12, 18) that a variety of nonphagocytic cells are capable of ingesting and supporting the growth of M. tuberculosis in vitro. There is no reason to postulate, moreover, that parenchymal cells would not be capable of ingesting M. tuberculosis and M. bovis in the in vivo setting if given the opportunity to do so. Presumably, parenchymal cells do not become infected because of the ability of the host to rapidly mobilize enough macrophages to sites of M. tuberculosis or M. bovis multiplication to ensure that the pathogens are always confined to the cytoplasm of these phagocytic cells. The relatively slow doubling times of M. tuberculosis or M. bovis would help to prevent M. tuberculosis or M. bovis from reaching overwhelming numbers before specific, T-cell-mediated immunity is acquired. The upregulation of macrophage antimycobacterial defenses subsequent to the acquisition of specific immunity would further ensure that infection is confined to macrophage cytoplasm. If this line of reasoning is correct, one would expect to see infection of parenchymal cells in a host in which macrophages are prevented from expressing innate and acquired antibacterial defenses. It was shown by a previous study (13), in this connection, that whereas immunocompetent mice are capable of slowly resolving BCG infection in major organs, BCG infection is progressive in severe combined immunodeficient (SCID) mice and is even more progressive in SCID mice that are treated with hydrocortisone (HC). Because it was also shown that BCG infection in SCID mice is confined to macrophages in granulomas, it was suggested (13) that HC treatment causes exacerbation of infection in SCID mice by virtue of its ability to suppress the expression of macrophage-based, innate defense mechanisms capable of slowing the intracellular growth of mycobacteria. It is known (3, 20), in support of this interpretation, that glucocorticoids, by way of inhibiting activation of NF-κB, can prevent macrophages from synthesizing and secreting tumor necrosis factor alpha and other proinflammatory cytokines considered essential for the expression of innate and acquired defenses at sites of infection. It seemed reasonable to suspect that if BCG possessed the potential to infect parenchymal cells, this potential would be realized in SCID mice treated with HC. The purpose of this study is to show that this is the case in the liver.
MATERIALS AND METHODS
Mice and BCG infection.
Wild-type (WT) CB17 and CB17 SCID mice 8 to 10 weeks of age were obtained from the Trudeau Institute Animal Breeding Facility (Saranac Lake, N.Y.). BCG Pasteur (TMC 1101) was grown as a dispersed culture in Proskauer and Beck medium containing 0.01% Tween 80, harvested in log phase, dispensed in 1-ml vials, and stored at −70°C. To infect mice a vial was thawed, and the culture was subjected to 5 s of ultrasound to break up clumps and diluted appropriately in saline containing 0.01% Tween. The mice were inoculated intravenously with 105 BCG CFU in a volume of 0.2 ml. BCG CFU were enumerated in the lungs, liver, and spleen on day 30 of infection by plating 10-fold serial dilutions of whole-organ homogenates on enriched Middlebrook 7H11 agar and counting colonies after 3 weeks of incubation at 37°C. Differences between means of CFU per organ were determined by Student's t test.
Histology.
A small piece of the left dorsal lobe of each of the livers used for enumerating bacteria was removed and fixed in 10% neutral buffered fomaldehyde for 24 h and then washed in running tap water. The pieces were dehydrated in graded ethanol solutions and embedded in paraffin by standard methods or in glycol methacrylate (JB-4 embedding kit; Polysciences, Inc., Warrington, Pa.) according to the manufacturer's instructions. Paraffin sections were cut on a rotary microtome and after dewaxing were stained for acid-fast bacteria with a modified acid-fast stain (4) and counterstained with methylene blue. Sections of glycol methacrylate-embedded liver were cut with glass knives and stained with 2% crystal violet. Photomicrography was performed with a Nikon Microphot-Fx microscope.
For electron microscopy two infected mice were killed by CO2 asphyxiation and quickly perfused via the left ventricle with 5 ml of heparinized phosphate-buffered saline (PBS) and then with PBS containing 2% glutaraldehyde. Pieces of perfused liver were removed, diced into 1-mm2 pieces, and fixed for 2 h in the glutaraldehyde solution. The pieces were then washed in PBS and postfixed for 1 h in 1% osmium tetroxide and then for 1 h in 1% uranyl acetate. Dehydration was in 70 and 100% ethanol, and embedding was in Epon. Thin sections were stained with lead acetate and viewed and photographed with a JEOL 1200EX electron microscope.
HC.
HC was obtained in emulsion form from United Research Laboratories (Philadelphia, P.). It was given subcutaneously in a dose of 1.25 mg on days 15, 17, 20, and 23 of infection.
RESULTS
Growth of BCG in WT and SCID mice.
Table 1 shows that by day 30 of infection BCG had grown to much higher numbers in the organs of SCID mice than in the organs of WT mice and that infection in both types of mice was exacerbated by treatment with HC given between days 15 and 23. Differences in the mean CFU per organ between any two groups of mice shown in Table 1 were highly significant (P < 0.001) according to Student's t test. The results are in agreement with those of more detailed previously published studies (13, 14), which showed that while BCG infection is slowly resolved in WT mice, it is progressive in SCID mice and is much more progressive in HC-treated SCID mice. The results indicate that mice posses a glucocorticoid-sensitive defense mechanism capable of retarding the growth of BCG in the lungs, liver, and spleen in the absence of T-cell-mediated immunity. They also indicate that this mechanism probably operates in WT mice in the presence of specific immunity.
TABLE 1.
Growth of BCG in WT and SCID mice
| Mouse group | Log10 CFU of BCGa in:
|
||
|---|---|---|---|
| Liver | Spleen | Lung | |
| WT | 5.533 ± 0.11 | 5.45 ± 0.09 | 4.88 ± 0.19 |
| SCID | 6.61 ± 0.36 | 6.82 ± 0.24 | 6.29 ± 0.23 |
| WT + HC2 | 7.31 ± 0.27 | 6.72 ± 0.06 | 5.55 ± 0.02 |
| SCID + HC | 8.66 ± 0.58 | 8.39 ± 0.24 | 7.03 ± 0.27 |
Mean ± standard deviation of results for five mice per group on day 30 of infection.
WT and SCID mice were given 1.25 mg of HC acetate on days 15, 17, 20, and 23 of infection.
Differences among means per given organ were highly significant (P < 0.001) by Students's t test.
Histologic consequences of higher levels of BCG infection.
The liver was chosen as the organ in which to look for infection of parenchymal cells because of the ease of distinguishing parenchymal cells (hepatocytes) from other cells on the basis of size, morphology, and staining characteristics. The intravenous route of inoculation was chosen because it ensured that the liver would contain an adequate number of sites of infection to examine histologically. Day 30 was chosen as the time to study the liver because at this stage of infection bacterial numbers were considered high enough in HC-treated SCID mice to result in infection of parenchymal cells, if, in fact, parenchymal cells were capable of becoming infected.
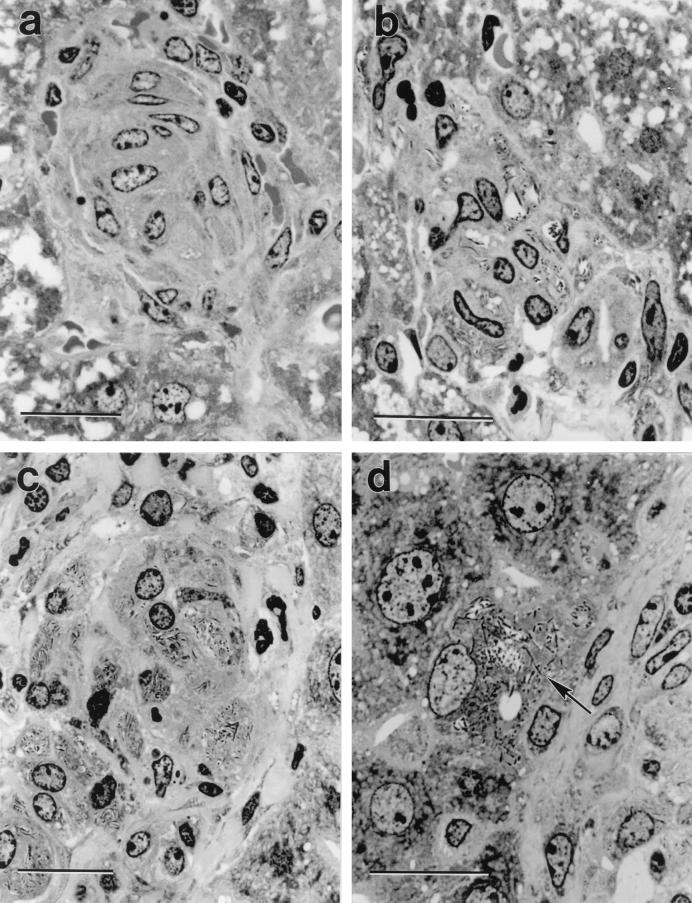
A microscopic examination of sections of wax-embedded livers (not shown) from the same mice used to obtain the results in Table 1 revealed that acid-fast bacilli were confined to granulomas scattered throughout the liver. Granulomas in the livers of HC-treated SCID mice were much larger, more numerous, and more heavily infected than those in the livers of untreated SCID mice. Again, liver granulomas in untreated SCID mice were larger and more numerous and contained more acid-fast bacilli than those in HC-treated WT and untreated WT mice. Sections of plastic-embedded livers revealed much more detail about the types of cells infected with BCG. In agreement with the results of previous histology studies (13, 14) it was found (Fig. 1) that BCG bacilli in liver granulomas of WT and SCID mice were confined to the cytoplasm of macrophages. However, the macrophages in granulomas of SCID mice were more numerous and contained more acid-fast bacilli than those in WT mice. Closer examination showed, moreover, that in livers of SCID mice occasional hepatocytes were infected with BCG at the margins of granulomas (Fig. 1). Infected cells at the margins of granulomas were easily identified as hepatocytes on the basis of being the same large size and morphology as the cells that made up the bulk of liver parenchyma.
FIG. 1.
Crystal violet-stained, 2-μm-thick plastic section of a liver granuloma. The granuloma in the WT mouse (a) is compact and comprised predominantly of concentrically arranged macrophages, whereas the granuloma in the HC-treated WT mouse (b) is comprised of a loose collection of macrophages containing large numbers of BCG bacilli. The granuloma in the SCID mouse (c) is also comprised predominantly of macrophages that are heavily infected with BCG. The higher power micrograph of the edge of a granuloma in a SCID mouse (d) shows a hepatocyte at the margin of the granuloma (arrow) heavily infected with BCG. Bars represent 20 μm. Magnifications: ×1,060 (a), ×1,280 (b), ×1020 (c), and ×1,280 (d).
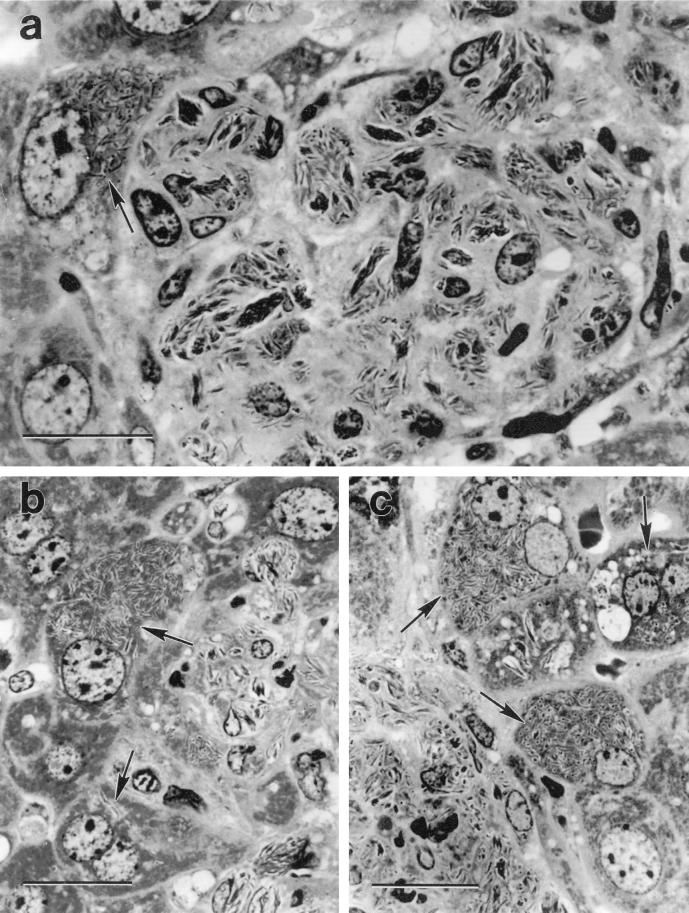
Infection of hepatocytes was much more extensive at the margins of liver granulomas in HC-treated SCID mice. This is illustrated in Fig. 2, where it can be seen that liver granulomas in these mice were larger and the number of bacilli per granuloma was much higher than in untreated SCID mice. Indeed, in granulomas in HC-treated SCID mice BCG bacilli formed large compact aggregates in the cytoplasm of macrophages. The cytoplasm of hepatocytes at the margins of these granulomas was also replete with BCG bacilli, and in some cases hepatocytes beyond the margins of granulomas were heavily infected with BCG. The overall histological interpretations given are based on an examination of six or more sections cut at intervals along a block of embedded liver from each of the five mice in the groups shown in Table 1. All sections of granulomas from HC-treated SCID mice displayed at least two heavily infected hepatocytes at their margins. In contrast, infected hepatocytes were only occasionally seen at the edge of granulomas in liver sections of untreated SCID mice.
FIG. 2.
Crystal violet-stained, 2-μm-thick plastic sections of liver granulomas in SCID mice treated with HC. The micrograph of a whole granuloma (a) shows very heavily infected macrophages containing dense rafts of BCG and a heavily infected hepatocyte (arrow) at the margin of the granuloma. Note that the infected hepatocyte is the same large size as the uninfected hepatocyte below it and is much lager than macrophages in the granuloma. Each of the other two micrographs (b and c) was selected to show heavily infected hepatocytes (arrows) at the margins of granulomas. Bars represent 20 μm. Magnifications: ×1,360 (a) and ×1,190 (b and c).
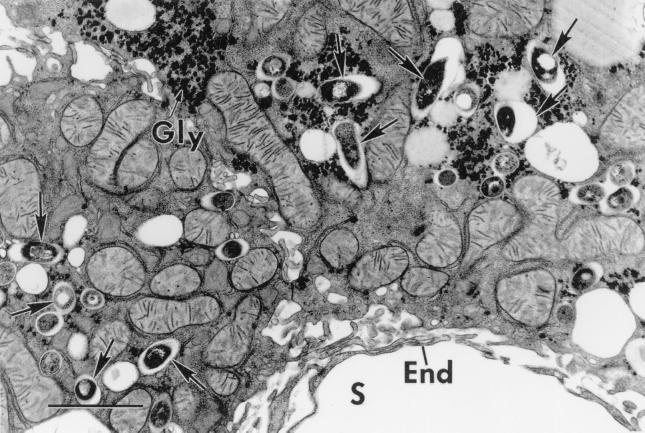
Electron microscopy of HC-treated SCID livers provided additional evidence that BCG caused extensive infection of hepatocytes. This is illustrated in Fig. 3, which shows part of a heavily infected hepatocyte that was situated at the edge of a granuloma on one side, and can be seen to be adjacent to a liver sinusoid on the other. BCG bacilli can be seen to be numerous in its cytoplasm and to be confined to phagocytic vesicles. There was no evidence that BCG bacilli were present free in the cytosol. Given that the cell shown in Fig. 3 is large, is situated external to a sinusoid on the parenchymal side of sinusoid endothelium, and has the same ultrastructure as parenchymal cells that were seen elsewhere in the section, there can be no doubt that it is a hepatocyte. Also, the fact that it contains glycogen deposits as well as mitochondria rich in cristae and with an electron-dense matrix typical of those described for hepatocytes (2) makes it difficult to identify it as any cell other than a hepatocyte. Macrophages in granulomas viewed by electron microscopy (not shown) contained smaller mitochondria with fewer cristae and were devoid of glycogen granules.
FIG. 3.
Electron micrograph of an infected hepatocyte in an HC-treated SCID mouse. The hepatocyte was situated at the edge of a granuloma (out of view at the top) and next to a sinusoid (S) marginated by typical endothelium (E). The hepatocyte is heavily infected with BCG bacilli (arrows) that are enclosed in phagosomes. Mitochondria and glycogen deposits (G) typical of hepatocytes are clearly visible. Bars, 2 μm.
In contrast to its effect on SCID mice, HC treatment did not result in infection of hepatocytes in WT mice but resulted, instead, in atypical granuloma development (Fig. 1). Thus, whereas liver infection in WT mice was confined to compact granulomas consisting of concentrically arranged macrophages, liver infection in HC-treated WT mice was confined to loose collections of heavily infected macrophages.
DISCUSSION
It was shown in previous publications (13, 14) that whereas WT mice acquire the capacity to slowly resolve BCG infection, SCID mice allow progressive BCG growth. It was also shown (13) that treatment with HC causes exacerbation of infection in both types of mice but that infection in SCID mice reaches much higher levels. The results of the day 30 CFU counts presented here confirm these previously published findings. They serve to show that SCID mice posses an HC-sensitive, T-cell-independent mechanism(s) that functions to retard the growth of BCG at sites of infection. Since the control of BCG growth occurs in the cytoplasm of macrophages, it seems likely that the HC-sensitive defense mechanism that is ultimately responsible for retarding BCG growth is located in the cytoplasm of these cells. In this connection, it has been shown in the in vitro setting (16) that human and mouse macrophages posses innate, HC-sensitive antimicrobial mechanisms that are independent of those activated by gamma interferon and therefore by T-cell-mediated immunity. It has also been shown (17) that glucocorticoids can suppress the baseline antimicrobial action of macrophages against microbial pathogens. Presumably, HC is able to inhibit the expression of innate antimycobacterial mechanisms of macrophages in vitro partly by virtue of its ability to inhibit NF-κB-dependent transcriptional activation of macrophage genes involved in upregulation of antimycobacterial defense (1, 3, 20).
Because HC treatment also caused exacerbation of infection in WT mice, albeit to a lesser extent, it is apparent that the same innate defense mechanism might contribute to the control of BCG growth in mice with acquired specific immunity. However, interpreting the suppressive effect of HC on BCG infection in immunocompetent mice is much more difficult because of the contribution that T cells make to immunity in these mice by secreting gamma interferon and other cytokines capable of activating the antimycobacterial functions of macrophages. It is known (13) that glucocorticoids have a suppressive effect on numerous components of host immunity, including T cells and macrophages, as well as on the inflammatory response that results in the extravasation of these cells from blood into sites of infection. It is also known (13) that glucocorticoids can suppress the in vivo function of lymphocytes and phagocytic cells by inhibiting their ability to synthesize the numerous cytokines and chemokines necessary for the mediation and expression of acquired T-cell-mediated immunity. Moreover, there is evidence (6, 15) that these compounds are more suppressive of Th1 than Th2 responses. Even so, the way that they suppress resistance to infection in vivo is not well understood. The possibility that HC caused exacerbation of BCG infection in the present study because of a growth-enhancing effect on BCG is unlikely, given published evidence (7) that, if anything, glucocorticoids over a range of concentrations have a modest inhibitory effect on mycobacterial growth in culture.
Regardless of how HC exacerbates infection, the fact that it reduces the ability of macrophages at sites of infection to restrict the intracellular growth of BCG allowed it to be used as a tool to determine whether liver parenchymal cells can become infected with BCG if the ability of macrophages to contain infection is sufficiently compromised. In HC-treated SCID mice unrestricted BCG growth apparently resulted in the death of infected macrophages and the liberation of BCG bacilli into the extracellular space, where they were available for ingestion by neighboring parenchymal cells. Given that infection of cells by BCG can only occur as a result of active engulfment of BCG bacilli via plasma membrane invagination, it was not surprising to find that BCG bacilli were enclosed in phagocytic vacuoles of liver parenchymal cells. There is no reason to believe at this time that BCG and other mycobacteria are equipped with mechanisms that enable them to escape the phagocytic vacuole and enter the cytosol.
It is not suggested here, however, that BCG infection of hepatocytes can only occur in HC-treated SCID mice. On the contrary, it was evident that infection of parenchymal cells was occurring in SCID mice without the influence of HC, albeit at a much lower level. Whether more extensive hepatocyte infection would have occurred if infection had been allowed to proceed in SCID mice beyond 30 days is not known. This presumably would depend on the bacterial load in macrophages of SCID mice becoming large enough to cause these cells to die and liberate BCG bacilli into the extracellular environment. Even if liberation of BCG bacilli from individual macrophages were to occur, significant infection of hepatocytes would not be expected to take place, unless SCID mice were unable to generate enough monocytes to replace macrophages lost to infection at infectious foci. This may well have been the case in SCID mice, but not in HC-treated SCID mice, given that glucocorticoids have been shown to reduce the rate at which recently formed monocytes are released from bone marrow into the circulation (21).
Additional evidence that infection of hepatocytes was much more extensive in HC-treated SCID mice than in untreated SCID mice is seen in the demonstration that the granulomas in HC-treated SCID mice were much larger. Since the space occupied by liver granulomas in all groups of mice represents space originally occupied by parenchymal cells, it follows that the larger the granulomas are the greater the loss of parenchymal cells to infection is at these sites. Indeed, it is obvious that since the granulomas in WT mice extend beyond the boundry of sinusoids, these granulomas also occupy space originally occupied by parenchymal cells. This indicates that infection of hepatocytes might have occurred to a small extent in WT mice, presumably before specific immunity was acquired.
As for the possibility that BCG is capable of parasitizing parenchymal cells in other organs in SCID and HC-treated SCID mice, it was not investigated in this study, although it will be the subject of a study soon to be initiated in this laboratory. In light of knowledge (9–12, 18) that a variety of cell types are capable of ingesting and supporting the growth of M. tuberculosis in vitro, it seems reasonable to suspect that parenchymal cell infection in other organs would occur under the conditions described here. This is an important possibility to investigate given the significant contribution made by CD8 T cells to the expression of anti-M. tuberculosis immunity in mice and humans (5, 8, 19). Indeed, it is possible that a key role of CD8 T cells in the expression of anti-M. tuberculosis immunity might be the recognition and lysis of infected parenchymal cells rather than macrophages. The results shown here with BCG serve to draw attention to this possibility. Moreover, since BCG is an attenuated organism with a limited ability to cause disease, it may not be necessary to render mice as severely immunodeficient as done in this study to show that virulent strains of M. tuberculosis and M. bovis are capable of infecting parenchymal cells.
ACKNOWLEDGMENTS
Grant AI-37844 from the National Institute of Allergy and Infectious Diseases and grant HL-64565 from the National Heart Lung Blood Institute supported this work.
REFERENCES
- 1.Ashwell J D, Lu F W M, Vacchio M S. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 2.Cross C C, Mercer K L. Cell and tissue ultrastructure. A functional perspective. W. H. Oxford, England: Freeman and Company; 1993. [Google Scholar]
- 3.Drouet C, Shakhov A N, Jongeneel C V. Enhancers and transcription factors controlling the inducibility of tumor necrosis factor-alpha promoter in primary macrophages. J Immunol. 1991;147:1694–1670. [PubMed] [Google Scholar]
- 4.Ellis R S, Zabrowany L A. Safer staining method for acid fast bacilli. J Clin Pathol. 1993;46:559–560. doi: 10.1136/jcp.46.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn J L, Goldstein M M, Treibold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchimont D, Galon D, Gadina M, Visconti R, Zhou Y-J, Aringer M, Frucht D M, Chrousos G P, O'Shea J J. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol. 2000;164:1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- 7.Hennes A R, Muchmore H G, McClure H G, Hammersten J F. In vitro inhibition of growth of M. tuberculosis by certain 11-oxygenated steroids. Proc Soc Exp Biol. 1959;101:145–147. doi: 10.3181/00379727-101-24861. [DOI] [PubMed] [Google Scholar]
- 8.Lewinsohn D M, Zhu L, Madison V J, Dillon D C, Fling S P, Reed S G, Grabstein K H, Alderson M R. Classically restricted human CD8 T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigen specificity. J Immunol. 2001;166:439–446. doi: 10.4049/jimmunol.166.1.439. [DOI] [PubMed] [Google Scholar]
- 9.Mapother U, Sanger J G. In vitro interaction of Mycobacterium avium with intestinal epithelial cells. Infect Immun. 1984;45:67–73. doi: 10.1128/iai.45.1.67-73.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonough K A, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P K, King C H, White E H, Murtagh J J, Quinn F D. Comparison of in vitro models of Mycobacterium tuberculosis invasion and intracellular replication. Infect Immun. 1996;64:2673–2679. doi: 10.1128/iai.64.7.2673-2679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mermudez L E, Goodman J. Mycobacterium tuberculosis invades and replicates in type II epithelial cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North R J, Izzo A. Mycobacterial virulence. Virulent strains of Mycobacterium tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177:1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North R J, Izzo A A. Granuloma formation in severe combined immunodeficient (SCID) mice in response to progressive BCG infection. Am J Pathol. 1993;142:1959–1966. [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez F. Glucocorticoids induce a Th2 response in vitro. Dev Immunol. 1998;6:233–243. doi: 10.1155/1998/73401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rook G A, Steele J, Ainsworth M, Leveton C. A direct effect of glucocorticoid hormones on the ability of human and mouse macrophages to control growth of M. tuberculosis. Eur J Respir Dis. 1987;71:286–291. [PubMed] [Google Scholar]
- 17.Schaffner A, Schaffner T. Glucocorticoid-induced impairment of macrophage antimcrobial activity: mechanisms and dependence on the state of activation. Rev Infect Dis. 1987;9(Suppl. 5):620–629. doi: 10.1093/clinids/9.supplement_5.s620. [DOI] [PubMed] [Google Scholar]
- 18.Shepard C C. A comparison of selected mycobacteria in HeLa, monkey kindey, and human amnion cells in tissue culture. J Exp Med. 1958;107:237–245. doi: 10.1084/jem.107.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith S M, Dockrell H M. Role of CD8 T cells in mycobacterial infections. Immunol Cell Biol. 2000;78:325–333. doi: 10.1046/j.1440-1711.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- 20.Steer J H, Kroeger K M, Abraham L J, Joyce D A. Glucocorticoids suppress tumor necrosis factor-alpha by human monocytic THP-1 cells by suppressing transactivation through adjacent NF-kappa B and c-Jun-activating transcription factor-2 binding sites in the promoter. J Biol Chem. 2000;16:18432–18440. doi: 10.1074/jbc.M906304199. [DOI] [PubMed] [Google Scholar]
- 21.Thompson J, van Furth R. The effect of glucocorticosteroids on the proliferation and kinetics of promonocyes and monocytes of the bone marrow. J Exp Med. 1973;137:10–21. doi: 10.1084/jem.137.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]