Abstract
Background:
Eastern Europe and Central Asia have intertwined HIV and incarceration epidemics, concentrated in people who inject drugs. Moldova is one of the few countries in this region that offers methadone within prisons, but uptake and post-release retention remains suboptimal. Screening, brief intervention, and referral to treatment (SBIRT) procedures are a potential implementation strategy to address this problem.
Methods:
From June 1, 2017 to March 3, 2018, we conducted a 2-stage SBIRT strategy in nine prisons and four pre-trial detention facilities in Moldova among incarcerated persons with opioid use disorder (OUD; N = 121) and within 90 days of release. Survey results were analyzed to evaluate the effect of the SBIRT strategy on the uptake of and post-release retention on methadone maintenance treatment (MMT).
Results:
Among the 121 screened with OUD, 27 were on MMT at baseline within the prison and this number increased to 41 after the two-step SBIRT intervention, reflecting a 51.9% increase over baseline. Eleven (78.6%) of the 14 participants that newly started MMT did so only after completing both SBIRT sessions. The brief intervention did not significantly improve knowledge about methadone but did improve attitudes towards it. Among the 41 participants who received methadone during this trial, 40 (97.6%) were retained 6 months after release; the one participant not retained was on methadone at the time of the intervention and had planned to taper off.
Conclusion:
The SBIRT strategy significantly improved participant attitudes, but treatment initiation mostly occurred after completing both sessions, including soon after release, but remained low overall. Work within the Moldovan prison subculture to dispel negative myths and misinformation is needed to further scale-up OAT in Moldova.
Keywords: HIV, Incarceration, Moldova, Opioid agonist therapy, SBIRT, Methadone, Implementation science
Introduction
By year-end 2018, over 10.7 million people were incarcerated globally. The countries of Eastern Europe and Central Asia (EECA) have some of the highest incarceration rates in the world, with over 850,000 individuals incarcerated and a median prison population rate of 180 per 100,000 population across 16 countries (Walmsley, 2018). Incarceration in EECA is influenced by laws that favor incarceration over treatment, especially for people who inject drugs (PWID). Among incarcerated people in the region, over one-third have injected drugs at some point prior to their arrest, with heroin accounting for over 80% of drugs injected (LaMonaca et al., 2019). In some EECA settings, prisons become a source for initiation of drug use (Azbel et al., 2018; Izenberg et al., 2014). Criminalizing drug use, with police often engaging in punitive practices toward PWID, results in a concentration of PWID and people with HIV (PWH) in prisons (Altice et al., 2016; Csete et al., 2016; Dolan et al., 2016; Kamarulzaman et al., 2016). Targeting of PWID by police in the region reduces the likelihood that PWID will engage in effective HIV prevention programs like syringe services programs and opioid agonist therapies (OAT) like maintenance on methadone (MMT) or buprenorphine (BMT), which in turn increases injection-related risks (LaMonaca et al., 2019).
Unlike global trends, HIV incidence and mortality continue to increase in EECA where the HIV epidemic is concentrated in PWID with opioid use disorder (OUD) (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2020) and syndemic with incarceration (Bromberg et al., 2020). In 2019, nearly half of all new HIV infections in the region occurred among PWID (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2020). Consequently, these intertwined opioid and incarceration epidemics result in HIV prevalence among incarcerated people in the region being several-fold higher than in the surrounding community (Altice et al., 2016).
OAT is internationally recognized as the most effective form of treatment for OUD (Altice et al., 2010; Degenhardt et al., 2019; Lawrinson et al., 2008; Mattick et al., 2014; Stone et al., 2021). It reduces drug injection levels, resulting in decreased transmission of HIV and HCV, decreased mortality (Sordo et al., 2017), and decreased criminal activity, while increasing engagement in HIV and HCV treatment (Degenhardt et al., 2019; Low et al., 2016; Stone et al., 2021). Until 2020, OAT was only available in prisons in three countries in EECA: Armenia, Kyrgyzstan, and Moldova (LaMonaca et al., 2019). Modeling studies from the region show that OAT scale-up is one of the most effective and MMT is the most cost-effective HIV prevention strategy (Alistar et al., 2011; Degenhardt et al., 2019; Stone et al., 2021; Tan et al., 2020), with scale-up in prisons and community transition after release being the most effective strategy to reduce HIV transmission (Altice et al., 2016; Stone et al., 2016) and death (Degenhardt et al., 2019; Stone et al., 2021).
Implementation strategies to scale-up OAT have been limited by both individual and structural challenges, but in the EECA region in particular, scale-up has been hindered by negative attitudes and myths surrounding OAT in both community and prison contexts (Polonsky et al., 2016a). OAT scale-up in the criminal justice system (CJS) has been controversial even in high-income countries, and negative attitudes and moral biases remain a significant barrier to OAT enrollment globally (Peterson et al., 2010; Torrens et al., 2013). In the presence of negative attitudes toward OAT in prisons, many prisoners do not access OAT, as they are embedded within a stigmatizing culture and are afraid to be ostracized and bullied (Polonsky et al., 2016a). Studies of incarcerated persons in Ukraine suggest that while incarcerated, PWID remain optimistic about being “cured” of their drug addiction, resulting in low interest in MMT. Their optimism, however, wanes soon after release when relapse occurs in the setting of elevated stigma about being a PWID (Polonsky et al., 2016b). Similar findings have been observed in Kyrgyzstan, with tapering off MMT before release being common and associated with poor post-release outcomes (Bachireddy et al., 2022). Low willingness to initiate methadone and tapering off before release is often influenced by the informal prison leadership who often oppose MMT (Azbel et al., 2022; Liberman et al., 2021). Implementation strategies to scale-up OAT in prisons and effectively transition individuals to treatment in the community post-release are urgently needed (Altice et al., 2016). We therefore pilot-tested a screening, two-step brief intervention, and linkage to treatment (SBIRT) strategy in Moldovan prisons (only MMT is available) to address the country’s HIV prevention goals by increasing uptake of OAT before release and retaining them on treatment after release.
Methods
Given epidemiological and mathematical findings from EECA that scaling up OAT in prisons and transitioning them to treatment after release reduces HIV transmission (Altice et al., 2016), we conducted a SBIRT strategy in 9 prisons and 4 pre-trial detention facilities in Moldova to increase uptake of and post-release retention on OAT. This study received approval from Institutional Review Boards at Yale University and the Ukrainian Institute on Public Health Policy.
Context
Moldova is a land-locked country in the EECA region with a population of 3.5 million. There are an estimated 27,500 PWID in Moldova (Costin-Codreanu & Cotelnic-Harea, 2020; LaMonaca et al., 2019), and an average daily census of 7,510 incarcerated individuals (incarceration rate: 212 per 100,000) (Walmsley, 2018). The prevalence of HIV in Moldova was 0.7% in 2019 (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2020) and is higher among PWID and prisoners. Nearly half (40% to 48%) of PWID in Moldova have been imprisoned, and about half of them have injected drugs while in prison (Cotelnic-Harea & Costin-Codreanu, 2020). Recently, the Moldovan penitentiary system was estimated to contain approximately 1,600 PWID (Costin-Codreanu et al., 2016). Methadone was introduced in Moldovan prisons as a pilot study in 2005 (LaMonaca et al., 2019), one of the earliest OAT programs in EECA prisons; BMT is not available. Before this intervention, incarcerated persons often transitioned off MMT before release due to suboptimal transition to a community program (Hoover & Jurgens, 2009), making post-release retention a priority. MMT has since been implemented in 13 prisons, and patients may continue MMT after release though most do not. Nevertheless, MMT coverage in Moldova remains low, with only 2.7% of PWID being treated in 2019 (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2020), including treatment in 9 prisons, 4 pre-trial detention centers, and 10 community MMT programs.
Enrollment Procedures
From June 1, 2017 to March 3, 2018, prison personnel referred only incarceration persons who met the following inclusion criteria: were at least 18 years old, met ICD-10 diagnostic criteria for opioid dependence, were within 90 days of release, and had a post-release address within 40km of a community MMT site. Prisons and pre-trial detention centers were chosen based on access to MMT within the prison and their location relative to community MMT programs to ensure that participants could continue to access MMT after release. There were four prisons that failed to meet these inclusion criteria. We focused on the 90 days before release for two reasons: 1) to allow incarcerated individuals interested in OAT sufficient time to achieve a stable dose prior to transition to the community, a factor that has been associated with post-release retention (Wickersham et al., 2013a,b); and 2) to provide the option of starting MMT soon after release given potential concerns about bullying and ostracism by other incarcerated persons who are part of the prison hierarchy (O’Hara et al., 2021; Polonsky et al., 2016a).
After referral, a trained researcher met each participant in a private room where screening procedures and informed consent were conducted, including an explanation of the study aims and the risks and benefits of participation. Participants were also informed during this process that participation or non-participation in the study was not linked to any rewards or punishments. Enrolled participants were re-consented prior to their release from prison.
Baseline assessment
After informed consent, the researcher supervised a 30-minute survey administered using a computer-assisted survey instrument (CASI) to avoid written documentation, followed by testing for HIV, HCV, HBV, and syphilis. The survey included sections on interest in OAT (10-point Likert Scale), drug use prior to incarceration, the validated 19-item Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) (Miller & Tonigan, 1997), and standardized questions about knowledge and attitudes toward OAT (Polonsky et al., 2015, Polonsky et al., 2016a). Prison authorities could not access participant survey responses as they were housed on a computer and linked to an anonymous identification number. HIV testing was conducted in accordance with national guidelines, including pre- and post-test counseling. After the survey, participants completed SBIRT procedures.
Modified SBIRT activities
SBIRT activities were modified for the prison context where drug use assessments focused on the 12 months before incarceration and those who were interested in MMT were linked immediately to treatment even before completing any brief intervention. Opioid dependence was assessed using the Rapid Opioid Dependence Screen (RODS) (Wickersham et al., 2015). The first brief intervention, based on motivational interviewing, focused on initiating MMT for those on treatment and for remaining on it for those already on MMT. The second, more intensive intervention was conducted just before release to provide opportunities for starting MMT, but more importantly, to promote post-release retention, emphasize the risks of opioid use immediately after release, and to provide resources to seek or continue treatment in the community. Participants were told that they could start MMT at any time before release or could do so immediately upon release if they sought to avoid ostracism and bullying within prison (Polonsky et al., 2016a).
The brief intervention included evidence-based information on the risks and benefits of MMT during their time in prison, as well as after release. Instructions about how to access MMT services following release in the community were addressed. Participants were asked to weigh the risks and benefits personally to improve engagement. The brief intervention lasted approximately 20 minutes, with time made available for any questions from participants. After the intervention, participants were asked again about their interest in OAT using the 10-point Likert Scale.
Anyone interested in MMT after the brief intervention was referred directly to the prison medical staff for MMT initiation. Anyone already on methadone was urged to maintain dosages adequate to support post-release retention. Participants who initiated methadone did so using a standardized national protocol approved by the Ministry of Health (MoH) (Ministry of Health, 2018; Wickersham et al., 2013a). All participants, irrespective of their interest in MMT or current enrollment status, were provided details on how to access methadone after release. This was done in recognition that some incarcerated participants may have perceived initiating methadone while incarcerated as putting them at risk for bullying or ostracism (Polonsky et al., 2016a).
One week before release, a second more intensive intervention was conducted to expand upon information from the brief intervention and to focus on retention on treatment and related risks during the transitional process. This second 30-minute intervention was intended to promote the retention of individuals on MMT following release to the community, but also to provide opportunity to start MMT immediately after release for those not on it. Participants were provided a document with contact information, clinic hours, and directions to the MMT location nearest to their post-release residence.
Follow-Up
Participants who did not return to prison were surveyed at months 1, 3, and 6 in the community after release. As in the baseline surveys, they were assessed for their interest in OAT, drug use, and standardized assessments of readiness for MMT (SOCRATES), and knowledge and attitudes toward OAT. A national registry of all patients who have ever received methadone, whether in prison or in the community is maintained by the Ministry of Health and used to verify being on methadone.
Analyses
All statistical analyses were conducted using Statistical Analysis Software (SAS). Participants were assessed for their pre- and post-intervention interest in OAT using a 10-point Likert scale, ranging from 0 (not interested at all) to 10 (extremely interested). Despite a median score of 2, values were dichotomized using a value of 5 or more as interested in OAT. Similarly, there were three attitude and six knowledge statements assessed using a 5-point Likert scale, ranging from 1 (strongly disagree) to 5 (strongly agree). Composite attitude and knowledge scores were calculated by summing the three attitude and six knowledge statements, respectively. These scores were then analyzed as a continuous variable. Mean differences between scores at baseline and month 1 were also assessed.
For the SOCRATES, composite subscales of recognition (7 items), ambivalence (4 items, and taking steps (8 items) scores were created by summing the respective 5-point Likert scale items. Means scores were calculated for each subscale and compared using paired t-tests. Additionally, low and high score designations for each of these groups were assigned using predetermined rankings (Miller & Tonigan, 1996). Mean differences between composite scores at baseline and month 1 were also assessed.
For those not on MMT at the time of screening, factors contributing to interest in and initiation of MMT were assessed. These factors included sex, age, marital status, education, housing, overdose experience prior to incarceration, injection practices prior to incarceration, HIV status, major depression, composite attitude and knowledge scores, and categorical SOCRATES scores. Unadjusted associations between these factors and the outcomes of interest were calculated using various statistical tests. Paired t-tests were used for the comparison between mean scores from baseline and month one for attitudes, knowledge, and SOCRATES scores. Independent samples t-tests were used to compare means for SOCRATES scores, age, and attitude and knowledge scores for those who were/were not interested in or did/did not initiate MMT. Chi-square was used for the categorical variables. If 25% or more of the cells had counts less than five, Fisher’s Exact tests were used. A parsimonious multivariable model was used to assess correlates of initiation of MMT by including variables significant in the bivariate model at p<0.20. Two of these variables, composite measures of attitudes toward and knowledge about MMT were collinear and the final model incorporated the composite attitudes variable based on goodness-of-fit using AIC. The percent increase in the number of individuals on MMT after the SBIRT strategy was reported, reflected as the percentage increase over the number on MMT at baseline. An OUD cascade was created, which included retention on MMT six months after release.
Results
The distribution of participants over time is depicted in Fig. 1. Of the 181 individuals referred, 140 (77.3%) met screening criteria for opioid dependence. Of these, 121 (86.4%) enrolled in the study. By the end of the study, six months after release, there were 110 (90.9%) participants retained.
Fig. 1.
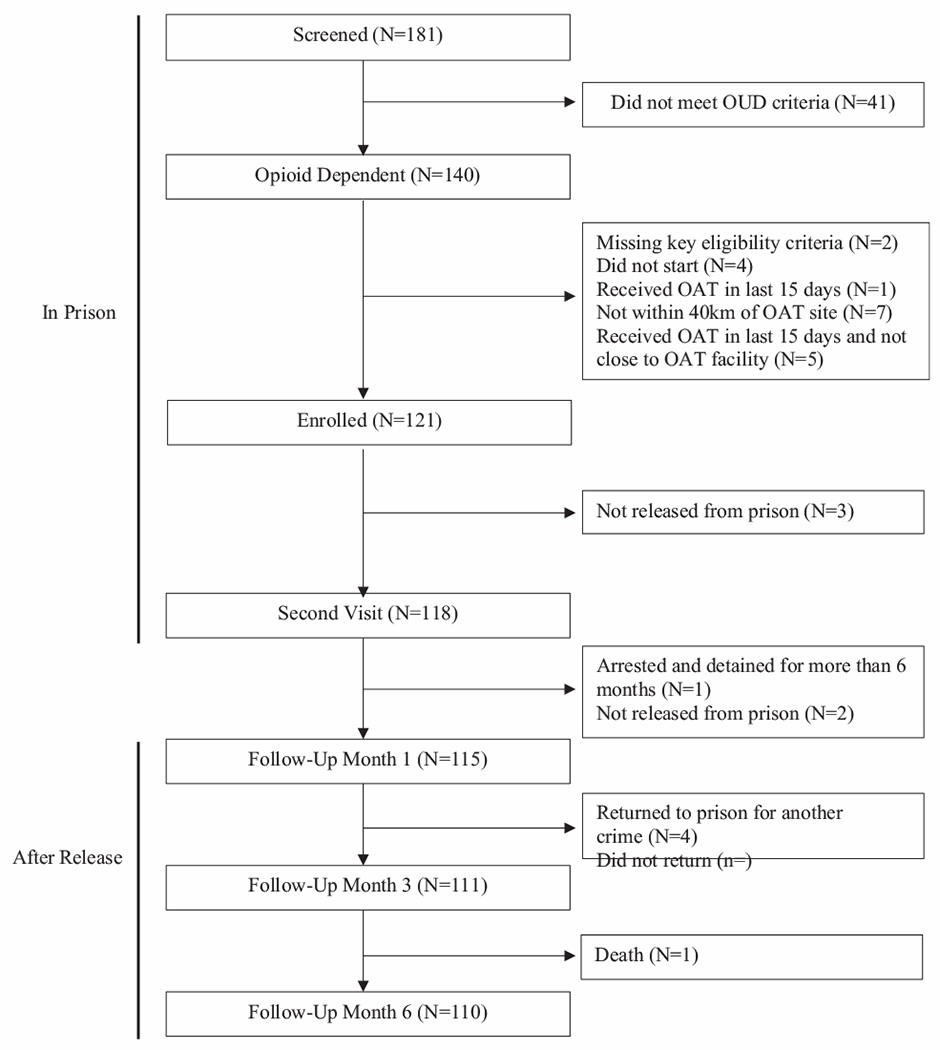
Distribution of study participants over time.
Fig. 2 depicts the timeline of MMT initiation for participants. Of the 121 enrolled participants, 27 (22.3%) were already on MMT at the time of enrollment. None of these had continued methadone from the community and all of these had started methadone after entry to the prison. An additional 14 of the remaining 94 participants (14.9%) subsequently initiated MMT, with 3 doing so between the two brief interventions, 10 before the 1-month post-release interview, and 2 more between 1 and 3 months after release. Of the 10 individuals who initiated MMT before the 1-month post-release interview, 2 did so prior to release from prison, with the remaining 8 individuals initiating following release from prison. One participant initiated and stopped MMT between the intensive intervention and 1 month follow-up. This individual then initiated MMT again before the month 3 follow-up. No participant initiated MMT between the month 3 and month 6 follow-up visit. One individual who was on MMT at enrollment stopped MMT before the month 3 follow-up due to a planned completion of their treatment. Fig. 3 provides the retention on MMT after release, stratified by those on and not on methadone at the time of release to reflect that MMT initiation occurred both within prison and after release. Retention at 6 months was 97.6% overall. It was 100% for the 14 who initiated methadone after the SBIRT intervention and 96.3% for the 27 who were on methadone before the intervention, mostly due to planned discontinuation from treatment. MMT uptake occurred in 6 of the 13 sites in this study. Of the 14 individuals who newly initiated MMT during the course of the study, five came from the same prison site.
Fig. 2.
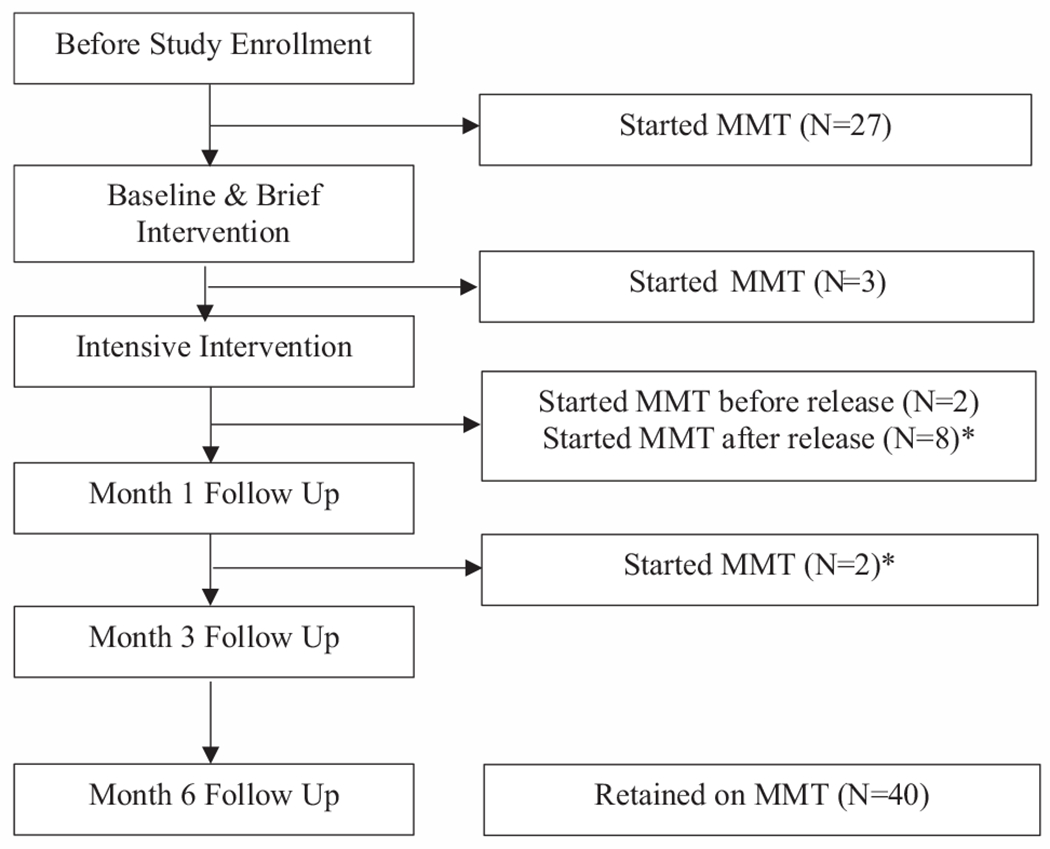
Timeline for methadone maintenance treatment with 27 being on treatment upon screening and 14 new patient enrolling. *One individual initiated and ended OAT between the intensive intervention and month 1 follow up, and initiated OAT again before the month 3 follow up.
Fig. 3.
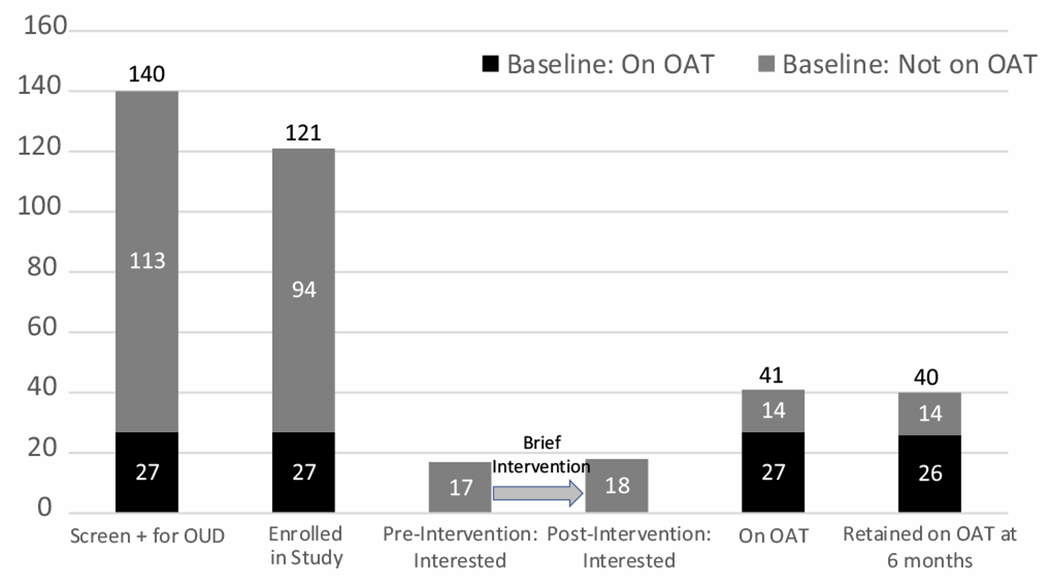
The opioid agonist treatment cascade for incarcerated people with opioid dependence in Moldovan prisons. Interest in OAT did not markedly increase following the brief intervention. Of the 14 participants who newly initiated OAT after the brief intervention, 11 were interested and 3 were not interested in OAT.
Table 1 presents the participant characteristics (N = 121), stratified by MMT enrollment at the start of the study period. Age, screening positive for HCV and injecting nearly daily were significantly associated with MMT status at the time of study enrollment (p=0.021, p = 0.004, and p=0.004, respectively). No other participant characteristics were significantly associated with enrollment in MMT at the start of the study period.
Table 1.
Baseline participant characteristics (N = 121).
| Variable | Not on MMT N = 94 (%) |
On MMT N = 27 (%) |
p-valuea |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 86 (91.5) | 25 (92.6) | |
| Female | 8 (8.5) | 2 (7.4) | |
| Age, mean ± SD (range) | 34.4 ± 7.4 (21, 57) | 38.2 ± 8.4 (25, 64) | 0.021* |
| Marital Status | 0.592 | ||
| Partnered | 33 (35.1) | 11 (40.7) | |
| Not partnered | 61 (64.9) | 16 (59.3) | |
| Education | 0.691 | ||
| Secondary or less | 87 (92.6) | 24 (88.9) | |
| Beyond secondary | 7 (7.4) | 3 (11.1) | |
| Pre-incarceration Housing | 0.702 | ||
| Own residence (rent/own) | 17 (18.1) | 5 (18.5) | |
| Friend or Relative’s home | 71 (75.5) | 19 (70.4) | |
| Other | 6 (6.4) | 3 (11.1) | |
| Overdose in the 6 months before incarceration | 0.782 | ||
| Yes | 17 (18.1) | 4 (14.8) | |
| No | 77 (81.2) | 23 (85.2) | |
| Injected nearly daily in last 30 days before incarceration | 0.004* | ||
| Yes | 19 (20.2) | 13 (48.2) | |
| No | 75 (79.8) | 14 (51.8) | |
| HIV Screening | 0.730 | ||
| Positive | 11 (11.7) | 2 (7.4) | |
| Negative | 83 (88.3) | 25 (92.6) | |
| Hepatitis B Surface Antigen | 0.782 | ||
| Positive | 17 (18.1) | 4 (14.8) | |
| Negative | 77 (81.2) | 23 (85.2) | |
| Hepatitis C Screening | 0.004* | ||
| Positive | 40 (42.6) | 7 (25.9) | |
| Negative | 54 (57.4) | 20 (74.1) | |
| Syphilis (RPR) | 0.682 | ||
| Positive | 7 (7.4) | 1 (3.7) | |
| Negative | 87 (92.6) | 26 (96.3) | |
| Major Depression (CESD-10) | 0.544 | ||
| Yes | 46 (48.9) | 15 (55.6) | |
| No | 48 (51.1) | 12 (44.4) |
p-value from t-test for continuous variables and Fischer’s Exact or χ2 test for categorical variables.
The opioid treatment cascade for the Moldovan prison population is shown in Fig. 3. At the time of enrollment, 27 participants were on MMT and interest in MMT for those not on MMT did not markedly increase (from 17 to 18 participants) after the brief intervention. Ultimately, 41 participants were on MMT by the end of the study (including the 27 on MMT at time of enrollment). Of the 14 participants who newly initiated MMT during the course of the study, 11 were interested and 3 were not interested in MMT immediately after the brief intervention.
The self-reported pre- and post-intervention median interest in MMT remained unchanged at 2.0 (IQR: 0 – 4.0). The pre- and post-intervention median difficulty of starting treatment scores (4.0 (IQR: 1.0 – 9.0) and 4.00 (IQR: 1.0 – 8.5), respectively) and median importance of receiving MMT scores (2.0 (IQR: 0 – 3.5) and 2.0 (0 – 4.0), respectively) also remained unchanged.
Table 2 presents findings from the attitudes and knowledge statements at baseline and one month after release. Attitudes consisted of three statements and knowledge consisted of six. All three of the attitudes statements significantly improved, while only one of the knowledge statements improved significantly. The only knowledge statement that improved significantly was that MMT reduces an opioid dependent individuals’ consumption of illicit opioids. Though statistically significant, the effect size was relatively small, with the greatest difference being 0.37 for the measure of buprenorphine versus methadone for the treatment of opioid addiction. When creating the composite attitudes and knowledge scores, only the mean composite attitudes score significantly improved (10.20 (SD±3.11) vs 10.97 (SD±2.7); p<0.001).
Table 2.
Comparison of mean scores regarding attitudes toward and knowledge about opioid agonist therapies at baseline within prison and one month after release (N = 115).
| Statementsa | Baseline Mean (SD) | Month 1 Follow-Up Mean (SD) | p-value | Effect size Cohen’s d |
|---|---|---|---|---|
| Attitude Statements | ||||
| 1. Opioid agonist therapy services should be available in the community so that all people who suffer from opioid addiction and want opioid agonist therapy can receive it. | 3.83 (1.22) | 4.01 (1.08) | 0.021* | 0.22 |
| 2. Opioid agonist therapy services should be introduced into prisons so that all inmates who suffer from opioid addiction and want opioid substitution therapy can receive it. | 3.84 (1.23) | 4.03 (1.06) | 0.004* | 0.28 |
| 3. For people who have opioid addiction, it would be much better to treat them with buprenorphine rather than methadone. | 2.53 (1.34) | 2.93 (1.39) | <0.001* | 0.37 |
| Composite Attitude Score | 10.20 (3.11) | 10.97 (2.7) | <0.001* | 0.43 |
|
| ||||
| Knowledge Statements | ||||
| 4. Opioid agonist therapies reduces opioid dependent individuals’ consumption of illicit opioids. | 3.57 (1.20) | 3.76 (1.15) | 0.023* | 0.22 |
| 5. Opioid agonist therapies reduces opioid dependent individuals’ risk of acquiring or transmitting HIV. | 4.04 (1.08) | 4.06 (1.07) | 0.905 | |
| 6. Opioid agonist therapies improves adherence to HIV medications in HIV-infected opioid dependent individuals. | 3.81 (1.24) | 3.82 (1.24) | 0.199 | |
| 7. Opioid agonist therapies increases opioid dependent patients’ adherence to tuberculosis medication | 3.96 (1.06) | 3.95 (1.07) | 0.602 | |
| 8. Opioid agonist therapies decreases opioid dependent individuals’ risk of dying from overdose. | 3.95 (1.10) | 3.97 (0.98) | 0.731 | |
| 9. Opioid agonist therapies reduces addicts’ criminal activities. | 3.65 (1.24) | 3.76 (1.14) | 0.315 | |
| Composite Knowledge Score | 23.07 (5.62) | 23.30 (5.45) | 0.508 | |
Range of responses is 1-5 (1=strongly disagree, 5 = strongly agree).
Treatment readiness using the three subscales of the SOCRATES were compared in Fig. 4 at baseline (N = 89) and at the one-month follow-up (N = 83). Neither the mean score or the composition of low, moderate, and high levels of the Recognition and Ambivalence subscales improved after the intervention. The mean scores for Taking Steps significantly increased (p=0.007) from 27.21 (SD±7.59) to 29.04 (SD±7.50) (data not shown), but importantly the proportion of individuals with moderate to high taking steps scores increased (11.6% to 14.9% and 14.0% to 14.9%, respectively), with a resulting decrease in the proportion with low scores from baseline to follow-up (74.4% to 70.2%).
Fig. 4.
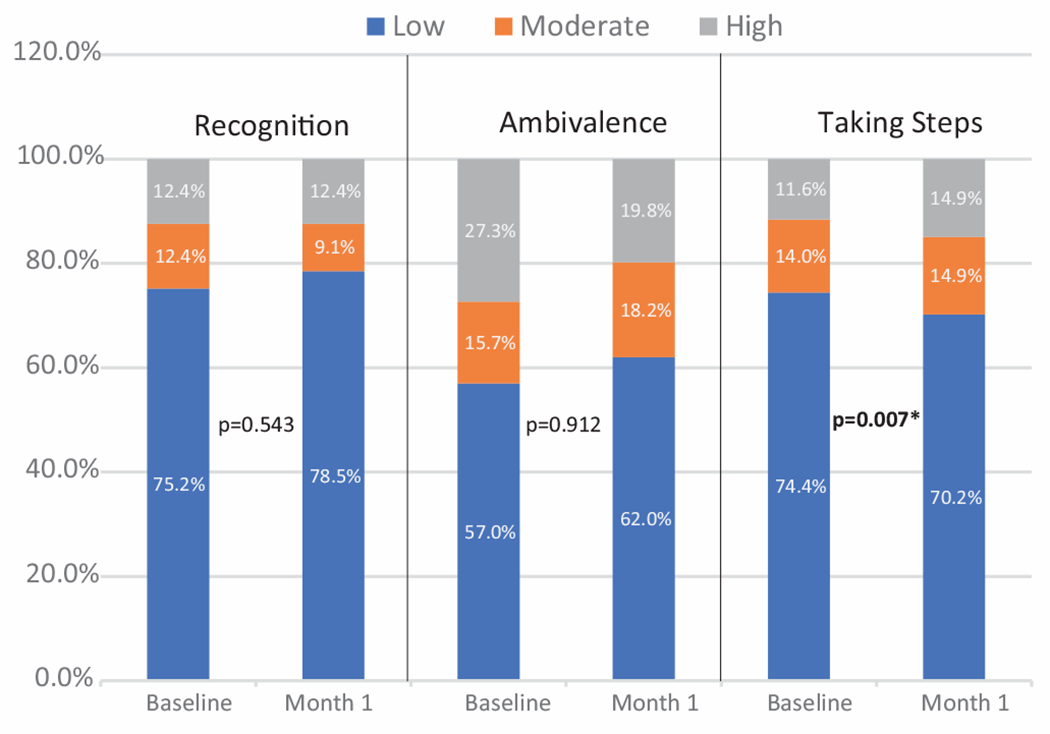
Treatment readiness using the SOCRATES subscales. N = 89 at baseline and N = 83 at Month 1.
Table 3 provides the bivariate associations between a number of variables and interest in MMT and initiation of MMT. Both the composite variables for attitudes and knowledge toward MMT were significantly correlated with interest in and initiation of MMT. Two components of the stages of change, however, were significant for interest in methadone (recognition and ambivalence), but were not significantly different for initiation of methadone. For initiation of MMT, however, having experienced an overdose in the 6 months before incarceration was significantly associated with initiating MMT (p=0.037). Though not presented, participants being interested in MMT was significantly correlated with initiation of MMT (p=0.002). In the multivariable model examining predictors of initiating MMT for variables significant at p<0.20, after excluding the composite score for knowledge about MMT due to collinearity with the composite attitude score, only three variables remained in the model. The composite attitude score remained significant (AOR=1.6; 95% CI=1.1-2.2; p=0.0068), while overdose in the 6 months before incarceration was no longer significant (AOR=0.4; 95% CI=0.1-1.7; p=0.20).
Table 3.
Bivariate associations between variables of interest and participant interest in opioid agonist therapies (N = 88) and initiation of opioid agonist therapies (N = 90).
| Variables | Interested in Methadone Maintenance Treatment |
Initiated Methadone Maintenance Treatment |
||||
|---|---|---|---|---|---|---|
| Yes n (%) | No n (%) | p-value* | Yes n (%) | No n (%) | p-value* | |
| Sex | 0.180 | 0.348 | ||||
| Male | 14 (17.5) | 66 (82.5) | 14 (17.1) | 68 (82.9) | ||
| Female | 3 (37.5) | 5 (62.5) | 0 (0.0) | 8 (100.0) | ||
| Age (mean ± SD) | 36.24±6.39 | 34.03±7.29 | 0.255 | 34.43±5.23 | 34.54±7.36 | 0.957 |
| Marital Status | 0.813 | 0.219 | ||||
| Partnered | 5 (17.9) | 23 (82.1) | 7 (21.9) | 25 (78.1) | ||
| Not Partnered | 12 (20.0) | 48 (80.0) | 7 (12.1) | 51 (87.9) | ||
| Education | 0.616 | 1.000 | ||||
| Secondary or less | 15 (18.5) | 66 (81.5) | 13 (15.7) | 70 (84.3) | ||
| Beyond secondary | 2 (28.6) | 5 (71.4) | 1 (14.3) | 6 (85.7) | ||
| Pre-incarceration Housing | 0.307 | 0.892 | ||||
| Own residence (rent/own) | 5 (29.4) | 12 (70.6) | 2 (11.8) | 15 (88.2) | ||
| Friend or relative’s residence | 12 (18.2) | 54 (81.8) | 11 (16.4) | 56 (83.6) | ||
| Other | 0 (0.0) | 5 (100.0) | 1 (16.7) | 5 (83.3) | ||
| Overdose in the 6 months before incarceration | 0.090 | 0.037* | ||||
| Yes | 5 (35.7) | 9 (64.3) | 5 (33.3) | 10 (66.7) | ||
| No | 12 (16.2) | 62 (83.8) | 9 (12.0) | 66 (88.0) | ||
| Injected nearly daily in the last 30 days before incarceration | 0.743 | 0.726 | ||||
| Yes | 4 (22.2) | 14 (77.8) | 2 (10.5) | 17 (89.5) | ||
| No | 13 (18.6) | 57 (81.4) | 12 (16.9) | 59 (83.1) | ||
| HIV Screening | 0.679 | 1.000 | ||||
| Positive | 1 (10.0) | 16 (20.5) | 1 (10.0) | 13 (16.2) | ||
| Negative | 9 (90.0) | 62 (79.5) | 9 (90.0) | 67 (83.8) | ||
| Moderate to severe depression (CESD-10) | 0.632 | 0.623 | ||||
| Yes | 9 (21.4) | 33 (78.6) | 6 (13.6) | 38 (86.4) | ||
| No | 8 (17.4) | 38 (82.6) | 8 (17.4) | 38 (82.6) | ||
| Composite Attitude Score (mean ± SD) | 12.12±2.29 | 10.15±2.69 | 0.007* | 12.71±1.82 | 10.28±2.69 | 0.002* |
| Composite Knowledge Score (mean ± SD) | 25.25±4.40 | 21.40±5.34 | 0.006* | 26.07±4.76 | 21.76±5.24 | 0.005* |
| Stages of Change Scores (mean ± SD) | ||||||
| Recognition Score | 30.47±5.64 | 24.66±7.49 | 0.004 | 25.78±10.24 | 25.95±7.21 | 0.950 |
| Ambivalence Score | 17.71±2.28 | 13.87±4.14 | <0.001 | 14.58±4.11 | 16.11±3.79 | 0.290 |
| Taking Steps Score | 30.29±6.12 | 26.54±7.80 | 0.680 | 28.44±5.64 | 27.38±7.78 | 0.690 |
p-value from t-test for continuous variables and Fischer’s Exact or χ2 test for categorical variable.
Discussion
This study provides evidence that a modified screening, brief intervention, and linkage to MMT strategy with increased attention to transitioning prisoners to treatment in the community can complement existing OAT scale-up strategies and help Moldova meet its HIV prevention goals. Implementation of SBIRT not only increased the number of people on methadone who might benefit from it, but also resulted in high levels of retention on treatment 6 months after release. Of the 121 individuals in this sample who might have benefited from OAT, the proportion who ultimately received OAT increased from 22.3% to 33.9% (27 to 41 persons), a 51.9% increase of 14 over the baseline 27 patients on OAT after the SBIRT strategy. Of interest is that the level of interest in OAT generally did not improve using the measure we selected, but new patients enrolled in OAT which would typically indicate an improvement in interest. One additional insight is that those who expressed interest in methadone were significantly more likely to recognize the need for it and where ambivalent about treatment, but this relationship did not persist for those who initated methadone. The finding that interest in methadone did not necessarily translate to initiation may be explained by the lack of taking steps in those that were interested. One potential strategy to consider integrating into the SBIRT intervention would be to more actively assist the person with treatment, either through patient or peer navigation.
The observed increase in enrollment after SBIRT may have been driven by significant improvements in attitudes toward OAT, rather than knowledge improvements. Not only did the brief intervention play a role in OAT initiation, but may have also contributed to retention as post-release treatment benefits were incorporated into the content. Though not measured here, improved attitudes toward OAT may serve as a longterm strategy to treat a chronic relapsing disease, potentially contributing to both uptake and retention (Polonsky et al., 2016a, Polonsky et al., 2016b). Future studies should consider the elements of the intervention content that were changed among participants.
A systematic review of brief interventions questioned the benefits of SBIRT (Young et al., 2014), which is a recommended implementation strategy to address drug and alcohol use disorders (Substance Abuse and Mental Health Services Administration (SAMHSA), 2014). Though the data for SBIRT is more compelling with alcohol than for other drugs (Platt et al., 2016), there are several factors here that may help explain the level of OAT uptake in the present study. First, the linkage to treatment only included methadone, an evidence-based treatment that was readily accessible to participants. In many settings, individuals eligible for treatment with OAT may be willing to take a medication but have a stated preference for buprenorphine (Uebelacker et al., 2016). Had buprenorphine been an option in this setting, scale-up may have increased. This is especially important since OAT, inclusive of both methadone and buprenorphine, is the most effective treatment in large studies of people relative to residential treatment, abstinence-based treatments like behavioral counseling, or even an opioid antagonist like extended-release naltrexone (Wakeman et al., 2020). In these studies, using any treatment strategy besides OAT had similar overdose rates, death, and hospitalization for a serious condition relative to no treatment at all (Wakeman et al., 2020). Second, SBIRT activities were conducted in a controlled setting where access to methadone could be guaranteed. In other studies of SBIRT, any drug use identified during screening, including those drugs for which there is no evidence-based treatment, could be included and treatment resources may be limited or inaccessible. These modifications may, in part, have contributed to increased OAT uptake in this setting.
Despite the observed success in increasing OAT uptake, one might question why more participants did not enroll on OAT. Of importance is that the brief interventions did not substantially improve knowledge about OAT, aside from participants learning that OAT substantially reduces the consumption of illicit opioids. It did, however, significantly improve attitudes toward OAT and result in high retention levels after release. Attitudes toward OAT have been problematic in the EECA region, including observations from Moldova (Polonsky et al., 2016a). Though not studied here, the presence of informal prison hierarchies, a caste system that governs prisoner interactions, is common to the EECA region. Depending on the influence of the informal hierarchy, the leadership may impose social ostracism on those who access OAT within prison and place them in the lowest caste, which limits their ability to gain benefits while within the prison. Concern about public humiliation may markedly reduce the likelihood that many incarcerated persons will initiate OAT (Liberman et al., 2021; Slade & Azbel, 2022). In prisons in Kyrgyzstan, an incarcerated person’s standing within the hierarchy greatly influences the consequences an individual faces for entering a methadone program (Liberman et al., 2021), with those already in lower castes being more likely to enroll in OAT than those in higher castes. Findings from additional studies suggest that a subset of incarcerated persons in Moldova experienced social ostracism while they were on methadone in prison (Council of Europe, 2018; Polonsky et al., 2016a). A lack of support for OAT by prison personnel has also been noted as a barrier to access (Polonsky et al., 2015). The role of the prison subculture in Moldova may explain why OAT scale-up was not more than observed and in part may explain the increased uptake as people were leaving incarceration.
Two factors related to the brief intervention itself may have influenced OAT uptake. Typically, brief interventions improve knowledge, which was observed only for methadone’s role in reducing opioid use (but not for other factors). Even though the fidelity checklist suggested that information delivery was complete, the understanding by participants may have been limited. Alternatively, the deliverer of the brief intervention may have successfully improved attitudes about OAT, as evidenced by our findings, but not sufficiently enough to move individuals far enough along the Stages of Change continuum to take action and start methadone. While there was a statistically significant increase in the taking steps subscale, the clinical significance may have been minimal with the proportion in the “low” levels only decreasing from 74.4% to 70.2%, which translates to only a few participants. Though not measured, the extent to which the participant perceived stigma during the intervention could influence engagement or even willingness to seek treatment.
To address potential concerns surrounding stigma in the prison setting or completeness of content delivered during the brief intervention, informed decision aids may be considered to overcome the inherent limitations in person-delivered interventions. Informed decision aids, when properly developed and tested, help improve patient decision-making, effectively engage them in the decision process, and generally result in higher satisfaction and improved patient outcomes (Bekker et al., 2003; Lewis & Pignone, 2009). Such decision aids for OAT in this setting, however, should at a minimum incorporate elements about attitudes and myths of OAT that are a major narrative in prisons (Polonsky et al., 2016a) and limit OAT uptake among this key population. Such decision aids should address OUD as a chronic, relapsing condition, incorporate post-release consequences like heightened overdose (Merrall et al., 2010) and HIV risk-taking (Altice et al., 2016; Stone et al., 2021; Stone et al., 2018), and other health benefits after release. The extent to which these decision aids might also assist with attitudes toward OAT in prison by the prison hierarchy is worth future investigations. Without addressing the general attitudes and knowledge concerning methadone, OAT scale-up in Moldovan prisons will not optimize its role in HIV prevention.
The post-release retention on MMT in this study remained high, and higher than reported elsewhere. In one pilot study of incarcerated persons with HIV in Malaysia, approximately half were retained on methadone, with higher retention for those on higher dosages (Wickersham et al., 2013a). A subsequent and larger trial in the same Malaysian prison found retention on MMT to be just under 50% (48.4%) at six months (Chandra et al., 2019). An observational study from Kyrgyzstan found just over a third (35.4%) remained on methadone for six months after release (Bachireddy et al., 2022), which is similar to studies in the U.S. (Gordon et al., 2008). Unlike our study, however, none of these intervened to keep participants in treatment post-release.
Despite the many important findings, this study has some limitations. A larger sample may have allowed us to discern differences in changes in knowledge and attitudes about OAT, conduct a more robust multivariable analyses, and allow us to ascertain the path from screening, brief intervention and eventual treatment. The pilot nature of this study, however, should be followed up with more comprehensive screening of all incarcerated people and not limited to a subset that was pre-selected to meet pre-release criteria for OAT. Screening at intake would more holistically screen and treat patients for a chronic relapsing condition. It may also have allowed incarcerated persons to make a decision about treatment before the prison hierarchy could have influenced individuals. A limited number of OAT sites available in the community following release also reduced the number of eligible participants in this study. Another limitation of this study is the lack of a reference group of prisoners not exposed to the SBIRT. It is possible that other factors outside of the intervention described here influenced the observed increase in OAT uptake.
Future studies should focus on additional strategies to promote uptake including satisfaction with treatment that can be accomplished with optimal OAT dosing, which in turn improves retention in treatment in both community (Farnum et al., 2021) and transitional care from prisons (Wickersham et al., 2013a). Moreover, implementation strategies like the NIATx treatment improvement model that focuses on quality improvement of treatment could help expand treatment as well (McCarty et al., 2007).
Conclusions
SBIRT strategies that are modified for the setting can substantially increase the number of individuals who initiate and remain on OAT. Multiple modeling studies, including for the EECA region, suggest that OAT scale-up is effective to reduce HIV transmission and death in HIV epidemics concentrated in PWID (Alistar et al., 2011; Tan et al., 2020) and the role of scaling up OAT in prisons (Degenhardt et al., 2019; Stone et al., 2016, 2021) is especially crucial to achieve this goal. It may benefit any future SBIRT strategies to reserve interventions for only those individuals who are ambivalent about OAT, while immediately initiating OAT for individuals who express an interest in it. Future studies should incorporate factors that include the prison environment itself and the informal hierarchies that exist there.
Funding statement
This work was supported by the National Institute of Health and National Institute of Drug Abuse (R01 DA029910 and R21 DA047902, Altice and F31 DA054861), the National Institute for Mental Health (T32 MH020031, Bromberg), and the Global Health Equity Scholars Program from the Fogarty International Center (D43 TW010540, Bromberg).
Footnotes
Ethics
This study received approval from the Yale University Institutional Review Board.
Declarations of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alistar SS, Owens DK, & Brandeau ML (2011). Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. Plos Medicine, 8(3), Article e1000423. 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Azbel L, Stone J, Brooks-Pollock E, Smyrnov P, Dvoriak S, … Vickerman P (2016). The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. The Lancet, 388(10050), 1228–1248. 10.1016/S0140-6736(16)30856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, & Friedland GH (2010). Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. The Lancet, 376(9738), 367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azbel L, Bromberg DJ, Dvoryak S, & Altice FL (2022). Addiction treatment as prison governance: A critical discourse analysis of methadone delivery in Kyrgyz prisons. Contemporary Drug Problems, 49(1), 106–120. [Google Scholar]
- Azbel L, Wegman MP, Polonsky M, Bachireddy C, Meyer J, Shumskaya N, … Altice FL (2018). Drug injection within prison in Kyrgyzstan: elevated HIV risk and implications for scaling up opioid agonist treatments. International Journal of Prisoner Health, 14(3), 175–187. 10.1108/IJPH-03-2017-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachireddy C, Shrestha R, Bromberg DJ, Azbel L, Kurmanalieva A, Wegman MP, … Altice FL (2022). Methadone within prison and linkage to and retention in treatment upon community release for people with opioid use disorder in Kyrgyzstan: Evaluation of a National Program. International Journal of Drug Policy, 101, 103558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker HL, Hewison J, & Thornton JG (2003). Understanding why decision aids work: linking process with outcome. Patient Education and Counseling, 50(3), 323–329. [DOI] [PubMed] [Google Scholar]
- Bromberg DJ, Mayer KH, & Altice FL (2020). Identifying and managing infectious disease syndemics in patients with HIV. Current Opinion in HIV and AIDS, 15(4), 232–242. 10.1097/COH.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra DK, Bazazi AR, Nahaboo Solim MA, Kamarulzaman A, Altice FL, & Culbert GJ (2019). Retention in clinical trials after prison release: results from a clinical trial with incarcerated men with HIV and opioid dependence in Malaysia. HIV Research & Clinical Practice, 20(1), 12–23. 10.1080/15284336.2019.1603433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costin-Codreanu T, Barbîroş I, Cotelnic-Harea T, & Caraulan L (2016). Estimarea Mărimii Grupului Deţinuţi Consumatori de Droguri Injectabile http://www.ccm.md/sites/default/files/2018-02/Raport_estimare_CDI_penitenciare_2016.pdf. [Google Scholar]
- Costin-Codreanu T, & Cotelnic-Harea T (2020). Estimating the Size of People Who Inject Drugs, Female Sex Workers, and Men Who Have Sex with Men in the Republic of Moldova, 2020. https://sdmc.md/wp-content/uploads/2021/01/National_size_estimation_RM_report_22_01_2021-ENGL.pdf. [Google Scholar]
- Cotelnic-Harea T, & Costin-Codreanu T (2020). Integrated Biological-Behavioral Surveillance Survey Among Female Sex Workers, People Who Inject Drugs and Men Who Have Sex with Men in the Republic of Moldova. https://sdmc.md/wp-content/uploads/2020/12/IBBS_REPORT_MD_2020_FINAL_eng.pdf. [Google Scholar]
- Council of Europe. (2018). Baseline Study into Criminal Subculture in Prisons in the Republic of Moldova. https://rm.coe.int/criminal-subculture-md-en-/1680796111. [Google Scholar]
- Csete J, Kamarulzaman A, Kazatchkine M, Altice F, Balicki M, Buxton J, Beyrer C, Kerr T, Lajous AM, Lewis S, Martin N, Mejia D, Camacho A, Mathieson D, Obot I, … Beyrer C (2016). Public health and international drug policy. The Lancet, 387(10026), 1427–1480. 10.1016/S0140-6736(16)00619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BD, Bruneau J, Altice FL, Henderson G, & Rahimi-Movaghar A (2019). Global patterns of opioid use and dependence: harms to populations, interventions, and future action. The Lancet, 394(10208), 1560–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan K, Wirtz AL, Moazen B, Ndeffo-Mbah M, Galvani A, Kinner SA, … Altice FL (2016). Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. The Lancet, 388(10049), 1089–1102. 10.1016/S0140-6736(16)30466-4. [DOI] [PubMed] [Google Scholar]
- Farnum SO, Makarenko I, Madden L, Mazhnaya A, Marcus R, Prokhorova T, … Altice FL (2021). The real-world impact of dosing of methadone and buprenorphine in retention on opioid agonist therapies in Ukraine. Addiction, 116 (1), 83–93. 10.1111/add.15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Schwartz RP, & O’Grady KE (2008). A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addiction, 103(8), 1333–1342. 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover J, & Jurgens R (2009). Harm Reduction in Prison: The Moldova Model(New York, NY: International Harm Reduction Development Program, Open Society Institute, Public Health Program. Accessed on July 16, 2016 at: https://www.opensocietyfoundations.org/publications/harm-reduction-prison-moldova-model. [Google Scholar]
- Izenberg JM, Bachireddy C, Wickersham JA, Soule M, Kiriazova T, Dyoriak S, & Altice FL (2014). Within-prison drug injection among HIV-infected Ukrainian prisoners: Prevalence and correlates of an extremely high-risk behaviour. International Journal of Drug Policy, 25(5), 845–852. 10.1016/j.drugpo.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). (2020). Global AIDS Update: 2020 (Seizing the Moment Tackling Entrenched Inequalities to End Epidemics Issue [PubMed] [Google Scholar]
- Kamarulzaman A, Reid SE, Schwitters A, Wiessing L, El-Bassel N, Dolan K, … Altice FL (2016). Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. The Lancet, 388(10049), 1115–1126. 10.1016/S0140-6736(16)30769-3. [DOI] [PubMed] [Google Scholar]
- LaMonaca K, Dumchev K, Dvoriak S, Azbel L, Morozova O, & Altice FL (2019). HIV, drug injection, and harm reduction trends in Eastern Europe and Central Asia: Implications for international and domestic policy. Current Psychiauy Reports, 21 (7), 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrinson P, Ali R, Buavirat A, Chiamwongpaet S, Dvoryak S, Habrat B, … Moskalewicz J (2008). Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction, 103(9), 1484–1492 . [DOI] [PubMed] [Google Scholar]
- Lewis CL, & Pignone MP (2009). Promoting informed decision-making in a primary care practice by implementing decision aids. North Carolina Medical Journal 70(2), 136 . [PMC free article] [PubMed] [Google Scholar]
- Liberman AR, Bromberg DJ, Azbel L, Rozanova J, Madden L, Meyer JP, & Altice FL (2021). Decisional considerations for methadone uptake in Kyrgyz prisons: The importance of understanding context and providing accurate information. International Journal of Drug Policy, 94, Article 103209. 10.1016/j.drugpo.2021.103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, Turner KM, Looker KJ, Christensen H, & McLean S (2016). Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clinical Infectious Diseases, 63 (8), 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, & Davoli M (2014). Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews, (2). [Google Scholar]
- McCarty D, Gustafson DH, Wisdom JP, Ford J, Choi D, Molfenter T, … Cotter F (2007). The Network for the Improvement of Addiction Treatment (NIATx): enhancing access and retention. Drug and Alcohol Dependence, 88(2–3), 138–145. 10.1016/j.drugalcdep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrall EL, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, … Bird SM (2010). Meta-analysis of drug-related deaths soon after release from prison. Addiction, 105(9), 1545–1554. 10.1111/j.1360-0443.2010.02990.x ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, & Tonigan JS (1997). Assessing drinkers’ motivation for change: the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). American Psychological Association. [Google Scholar]
- Ministry of Health. (2018). Pharmacological Treatment of Opioid Dependence: National Clinical Protocol http://89.32.227.76/_files/15650-PCN%2520-225%2520Tratamentul%2520farmacologic%2520al%2520dependentei%2520de%2520opiacee.pdf.
- O’Hara GL, Liberman AR, Polonsky M, Azbel L, Marcus R, Doltu S, … Altice FL (2021). Multi-level implementation factors that influence scale-up of methadone maintenance treatment in Moldovan prisons: a qualitative study. Journal of Substance Abuse Treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Schwartz RP, Mitchell SG, Reisinger HS, Kelly SM, O’Grady KE, … Agar MH (2010). Why don’t out-of-treatment individuals enter methadone treatment programmes? International Journal of Drug Policy, 21(1), 36–42. 10.1016/j.drugpo.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt L, Melendez-Torres G, O’Donnell A, Bradley J, Newbury-Birch D, Kaner E, & Ashton C (2016). How effective are brief interventions in reducing alcohol consumption: do the setting, practitioner group and content matter? Findings from a systematic review and metaregression analysis. BMJ Open, 6(8), Article e011473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky M, Azbel L, Wickersham JA, Marcus R, Doltu S, Grishaev E, … Altice FL (2016a). Accessing methadone within Moldovan prisons: Prejudice and myths amplified by peers. International Journal of Drug Policy, 29, 91–95. 10.1016/j.drugpo.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky M, Azbel L, Wickersham JA, Taxman FS, Grishaev E, Dvoryak S, & Altice FL (2015). Challenges to implementing opioid substitution therapy in Ukrainian prisons: Personnel attitudes toward addiction, treatment, and people with HIV/AIDS. Drug and Alcohol Dependence, 148, 47–55( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky M, Rozanova J, Azbel L, Bachireddy C, Izenberg J, Kiriazova T, … Altice FL (2016b). Attitudes toward addiction, methadone treatment, and recovery among HIV-infected Ukrainian prisoners who inject drugs: Incarceration effects and exploration of mediators. AIDS and Behavior, 20(12), 2950–2960. 10.1007/s10461-016-1375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade G, & Azbel L (2022). Managing drugs in the prisoner society: heroin and social order in Kyrgyzstan’s prisons. Punishment & Society, 24 (1), 26–45. 10.1177/1462474520956280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, … Pastor-Barriuso R (2017). Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Brooks-Pollock E, Altice FL, Azbel L, Smyrnov P, Martin NK, & Vickerman P (2016). Incarceration and people who inject drugs in Ukraine: modelling its role in HIV transmission and the impact of introducing OST in prisons. Journal of the International AIDS Society, 19 https://doi.org/<Go_to_ISI>://WOS:000395394300098 [Google Scholar]
- Stone J, Degenhardt L, Grebely J, Larney S, Altice FL, Smyrnov P, … Havens JR (2021). Modelling the intervention effect of opioid agonist treatment on multiple mortality outcomes in people who inject drugs: a three-setting analysis. The Lancet Psychiatry, 8(4), 301–309. 10.1016/S2215-0366(20)30538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Fraser H, Lim AG, Walker JG, Ward Z, MacGregor L, … Vickerman P (2018). Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. The Lancet Infectious Diseases, 18(12), 1397–1409. 10.1016/S1473-3099(18)30469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2014). Screening, Brief Intervention, and Referral to Treatment (SBIRT). Taylor & Francis Online. https://www.samhsa.gov/sbirt. (Accessed 16 July 2016).
- Tan J, Altice FL, Madden LM, & Zelenev A (2020). Effect of expanding opioid agonist therapies on the HIV epidemic and mortality in Ukraine: a modelling study. The Lancet HIV, 7(2), e121–e128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens M, Fonseca F, Castillo C, & Domingo-Salvany A (2013). Methadone maintenance treatment in Spain: the success of a harm reduction approach. Bulletin of the World Health Organization, 91(2), 136–141. 10.2471/BLT.12.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelacker LA, Bailey G, Herman D, Anderson B, & Stein M (2016). Patients’ beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. Journal of Substance Abuse Treatment, 66, 48–53. 10.1016/j.jsat.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, Azocar F, & Sanghavi DM (2020). Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Network Open, 3(2), Article e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley R (2018). World prison population list (12th edition). London, UK: Institute for Criminal Policy Research. https://www.prisonstudies.org/sites/default/files/resources/downloads/wppl_12.pdf. [Google Scholar]
- Wickersham JA, Azar MM, Cannon CM, Altice FL, & Springer SA (2015). Validation of a brief measure of opioid dependence: the Rapid Opioid Dependence Screen (RODS). Journal of Correctional Health Care, 21(1), 12–26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Marcus R, Kamarulzaman A, Zahari MM, & Altice FL (2013a). Implementing methadone maintenance treatment in prisons in Malaysia. Bulletin of the World Health Organization, 91 , 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Zahari MM, Azar MM, Kamarulzaman A, & Altice FL (2013b). Methadone dose at the time of release from prison significantly influences retention in treatment: implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug and Alcohol Dependence, 132(1–2), 378–382. 10.1016/j.drugalcdep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MM, Stevens A, Galipeau J, Pirie T, Garritty C, Singh K, Yazdi F, Golfam M, Pratt M, & Turner L (2014). Effectiveness of brief interventions as part of the Screening, Brief Intervention and Referral to Treatment (SBIRT) model for reducing the nonmedical use of psychoactive substances: a systematic review. Systematic Reviews, 3(1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


