Abstract
The enhanced gastric epithelial cell apoptosis observed during infection with Helicobacter pylori has been suggested to be of significance in the etiology of gastritis, peptic ulcers, and neoplasia. To investigate the cell death signaling induced by H. pylori infection, human gastric epithelial cells were incubated with H. pylori for up to 72 h. H. pylori infection induced the activation of caspase -8, -9, and -3 and the expression of the proapoptotic Bcl-2 family proteins Bad and Bid. The peak of the activity of the caspases occurred at 24 h. At this time, the inhibition of caspase-8 or -9 almost completely suppressed H. pylori-induced apoptosis. Inhibition of caspase-8 suppressed the expression of Bad and Bid and the subsequent activation of caspase-9 and -3. These observations indicate that H. pylori induces apoptosis through a pathway involving the sequential induction of apical caspase-8 activity, the proapoptotic proteins Bad and Bid, caspase-9 activity, and effector caspase-3 activity. Activation of the pathway was independent of CagA or vacuolating toxin. A membrane fraction of H. pylori was sufficient to activate this pathway, and treatment with proteinase K eliminated the activity. Apoptotic activity of the membrane fraction was significantly increased by incubating the bacteria under serum-starved conditions for 24 h. These observations suggest that environmental conditions in the human stomach could induce H. pylori-mediated pathogenesis, leading to a variety of clinical outcomes.
Helicobacter pylori is a human-specific gastric pathogen that colonizes the stomachs of at least half the world's population (7, 19, 21). Infection with H. pylori is strongly associated with gastric atrophy, peptic ulceration, and gastric cancer (8, 15, 18, 49, 59). H. pylori attaches to the gastric epithelium and exerts its pathogenic actions on the defense system responsible for the maintenance of mucosa homeostasis (37).
Apoptosis, programmed cell death, plays an important role in the regulation of epithelial cell numbers in the gastrointestinal tract (30). Deregulation of the apoptotic pathway is implicated in a number of disease processes in the gastrointestine (60). In H. pylori-induced chronic gastritis, cell loss by apoptosis is excessive compared with proliferation (54). In vivo studies demonstrate that infection with H. pylori triggers apoptosis of gastric epithelial cells (53). It is suggested that acceleration of apoptosis plays an important role in H. pylori-mediated pathogenesis (14, 28, 36, 42, 43).
To date, a number of studies have investigated the pathogenicity of H. pylori in relation to cytotoxic products, including urease, Cag, and vacuolating toxin (VacA). Recently potential apoptosis-inducing activity was reported in VacA (26) and urease (24). However, this viewpoint has often failed to elucidate the diverse pathomorphisms in H. pylori infections. Thus, it has been suggested that it is important to investigate the host factors which might affect cellular responses that would be involved in the development of gastric mucosal disorders. El-Omar et al. accordingly reported that interleukin 1 gene cluster polymorphisms suspected of enhancing the production of interleukin 1β are associated with an increased risk of both hypochlorhydria induced by H. pylori and gastric cancer (22). Apoptosis in H. pylori-associated gastritis accompanies the activation of Fas and the Fas ligand system (34, 61, 73) in epithelial cells. Fas is a member of the tumor necrosis factor receptor family, which, when bound by its ligand, activates caspase-8, an initiator of the downstream apoptotic process that includes the cleavage of other death substrates, cellular and nuclear morphological changes and, ultimately, cell death (56, 62, 70). The inflammatory mediators gamma interferon (IFN-γ) and tumor necrosis factor alpha augment apoptosis induced by H. pylori (72). Variations in host responses including these inflammatory mediators might cause the H. pylori-mediated pathogenesis to result in a variety of clinical outcomes.
Recent studies indicate that many apoptotic responses are initiated by activation of the apical caspase-8 or caspase-9: the former by the tumor necrosis factor receptor family (11, 55) and the latter by the release of cytochrome c following mitochondrial damage (66, 78). Activation of either of these two initiator caspases can lead to activation of the effector caspase-3 (67, 70).
In this study we explored the involvement of these signal pathways in H. pylori-induced apoptosis and the influence of growth conditions on the apoptosis-inducing activity of H. pylori.
MATERIALS AND METHODS
Bacterial strains, cell lines, and media.
H. pylori strains NGY 273, isolated from a 61-year-old female with atrophic gastritis, NGY 621, from a 64-year-old male with gastritis, NGY 1268, from a 56-year-old male with early gastric cancer (type IIc), and NGY 1281, from a 53-year-old male with a gastric ulcer, were used. These strains were freshly isolated from biopsy specimens and stored at −80°C in brain-heart infusion (BHI) broth containing 10% fetal calf serum (FCS) and 15% glycerol. The strain NGY 273 was cagA negative and vacuolating toxin negative, while the other strains were cagA positive and vacuolating toxin positive. The presence of cagA was confirmed by PCR with the primer pair 5′-GGCAATGGTGGTCCTGGAGCTAGGC-3′ (nucleotides 1495 to 1519 in cagA) and 5′-GGAAATCTTTAATCTCAGTTCGG-3′ (nucleotides 1797 to 1819 in cagA). The presence of VacA was determined by the method of Cover et al. (16). Concentrated broth supernatants were incubated with HeLa cells (CRL-JCRB9004; Health Science Research Resource Bank, Osaka, Japan) for 24 h at 37°C, and vacuolation was assessed by bright-field microscopy.
H. pylori strains were grown on 7% horse blood agar plates at 37°C under microaerophilic conditions. Fresh plates were started from the glycerol stocks each week and passaged after 48 h. Liquid cultures of H. pylori were grown in BHI broth supplemented with 10% FCS under the same conditions for 24 h with agitation. The human gastric adenocarcinoma cell line AGS was obtained from American Type Culture Collections (CRL-1739) and maintained in Ham's F12 medium with 10% FCS. Cells were serum starved for 16 h and incubated with H. pylori at a bacterium/cell ratio of 100:1 for up to 72 h in the medium without serum.
Preparation of cellular membrane fraction from H. pylori.
The bacteria grown in BHI broth with 10% FCS were harvested, washed with phosphate-buffered saline (PBS), and resuspended in serum-free RPMI 1640 medium. This bacterial suspension was incubated for 24 h unless otherwise stated. Then cells were harvested, washed, and resuspended in precooled PBS (4 ml per 100 ml of original culture). Cells were disrupted by one passage through a French pressure cell at 120 MPa. After low-speed centrifugation (5000 × g; 30 min) to remove cellular debris and unbroken cells, cellular membrane was sedimented by centrifuging at 100,000 × g for 1 h at 4°C. The cellular membrane was resuspended in PBS to a protein concentration of 1 mg/ml. The supernatant was used as the cytosolic fraction. Where indicated, the membrane preparation was digested with proteinase K (Wako) at a concentration of 1 μg/ml at 50°C for 3 h. The membrane fraction was then sedimented by centrifuging at 100,000 × g for 1 h at 4°C to remove the proteinase K.
Reagents.
Caspase-8 inhibitor, Z-IETD-FMK, and caspase-9 inhibitor, Z-LEHD-FMK, were purchased from Calbiochem (San Diego, Calif.). Antibodies to Bad and Bid were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Antibodies to phosphorylated Bad at serine-112 or -136 were from New England BioLabs, Inc. (Beverly, Mass.).
Assessment of apoptosis.
Cells in suspension and trypsinized cells were pelleted and resuspended in PBS. Then cells were incubated with 100 nM Hoechst 33342 (Molecular Probes, Eugene, Oreg.) for 5 min at room temperature. A drop of the suspension was applied to a microscope slide, and apoptotic cells were assessed by fluorescence microscopy. Nuclei with highly condensed and fragmented chromatin were considered apoptotic. Apoptotic cells were enumerated by counting 500 cells in multiple randomly selected fields. The apoptotic index was expressed as the percentage of apoptotic cells per 500 cells enumerated.
Caspase activity assay.
The activies of the apical caspase-8 and -9 and effector caspase-3 were determined using Caspase Colorimetric Protease Assay kits (Medical & Biological Laboratories, Nagoya, Japan) according to the manufacturer's instructions.
Western blotting.
Cells were lysed in a buffer containing 1% Triton X-100, 10 mM Tris-HCl, pH 7.4, and protease inhibitors, and the resulting insoluble material was removed by centrifugation. For the analysis of cytosolic proteins, a digitonin permeabilization technique (33) was used to release cytosol from cells. Fifty-microgram protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, Calif.), and immunoblotted overnight with antibodies at a concentration of 1:1,000 (vol/vol). Immunocomplexes were visualized by enhanced chemiluminescence detection (ECL; Amersham Pharmacia Biotech, Uppsala, Sweden) using horseradish peroxidase-conjugated secondary antibodies following the protocol provided by the manufacturer.
Statistical analyses.
Results are expressed as the means ± the standard deviations (SD). Induction of apoptosis and activities of caspases were compared using a two-tailed Student t test and considered significant if the P values were <0.01.
RESULTS
Evaluation of H. pylori-induced apoptosis.
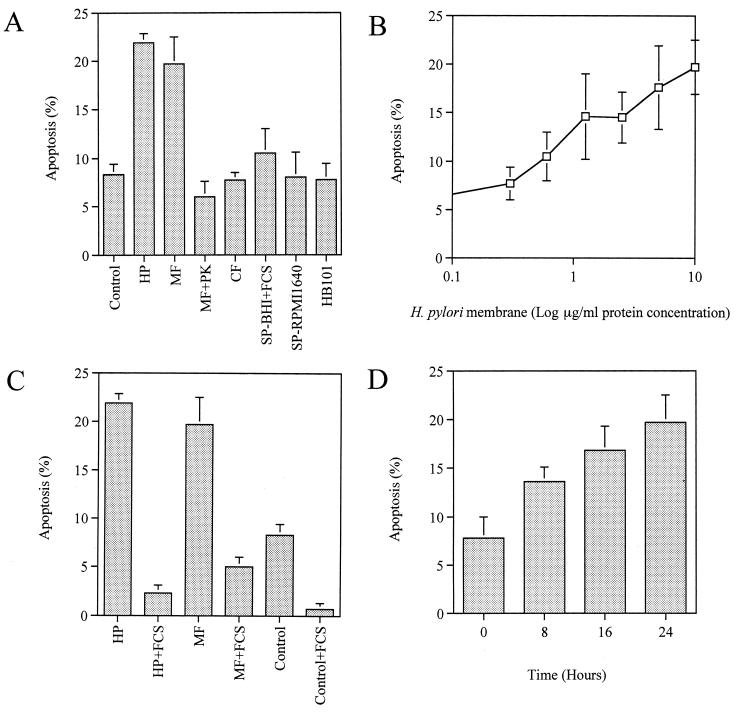
Quantitation of apoptotic AGS cells by fluorescence microscopy demonstrated that incubation with H. pylori induced a significant increase in apoptosis compared to the apoptosis of untreated cells at 24 h (21.9% ± 1.0% versus 8.3% ± 1.1%; P < 0.01). No significant difference in the level of apoptosis was observed among the strains examined, suggesting that apoptosis was independent of the secretory proteins CagA and VacA. A membrane fraction of H. pylori at a protein concentration of 10 μg/ml elicited a comparable response (Fig. 1A). Apoptosis was completely blocked by pretreating the membrane fraction preparation with proteinase K, indicating the involvement of an H. pylori membrane protein(s) in the initiation of apoptotic signaling. Induction of apoptosis by the membrane fraction preparation was dose dependent (Fig. 1B). Neither the cytosolic fraction nor the concentrated culture supernatant from BHI broth or RPMI 1640 medium induced apoptosis, nor did Escherichia coli HB101 induce apoptosis of AGS cells (Fig. 1A). The apoptotic effect of H. pylori was significantly suppressed by the addition of serum (21.9% ± 1.0% versus 2.3% ± 0.8% by live bacteria; 19.7% ± 2.8% versus 5.0% ± 1.0% by membrane fraction; P < 0.01) (Fig. 1C). Moreover, transfer of the bacteria grown in BHI broth with FCS to serum-free RPMI 1640 medium induced a time-dependent increase in the apoptotic activity of the membrane fraction (Fig. 1D). At 0 h, apoptosis-inducing activity was no more than the control level (7.8% ± 2.2% versus 8.3% ± 1.1%), while at 24 h it had increased to a level comparable to that elicited by the live bacteria under the serum-deprived conditions (19.7% ± 2.8% versus 21.9% ± 1.0%). These results indicate an antiapoptotic effect of serum on both AGS cells and the bacteria. Hence, the following analyses of H. pylori-induced apoptosis were performed using serum-deprived AGS cells as described in Materials and Methods unless otherwise stated.
FIG. 1.
Quantitation of H. pylori-induced apoptosis by fluorescence microscopy. Since there was no significant difference among the strains examined, data from NGY 1281 are shown as representative. Results are expressed as the mean percentages of apoptotic cells per 500 cells enumerated. Bars represent mean values ± SD from three experiments. (A) Induction of apoptosis by live H. pylori (HP), H. pylori membrane fraction (MF), membrane fraction pretreated with proteinase K (MF+PK), H. pylori cytosol (CF), culture supernatant from BHI broth with FCS (SP-BHI+FCS), culture supernatant from RPMI 1640 medium (SP-RPMI 1640), and E. coli HB101 (HB101). (B) Dose-dependent induction of apoptosis by the membrane fraction preparation. (C) Effect of serum on H. pylori-mediated apoptosis. (D) Time-dependent increase in apoptotic activity of the membrane fraction during incubation in serum-free RPMI 1640 medium. Membrane fraction preparations were added at a protein concentration of 10 μg/ml.
Caspase activation and effect of caspase inhibitors on H. pylori-induced apoptosis.
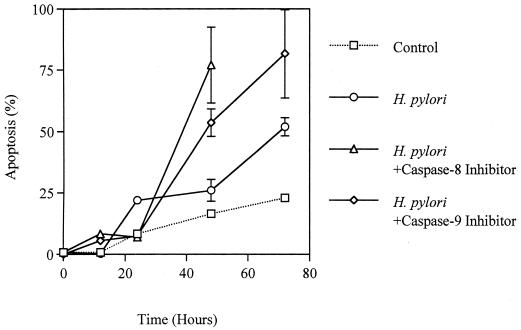
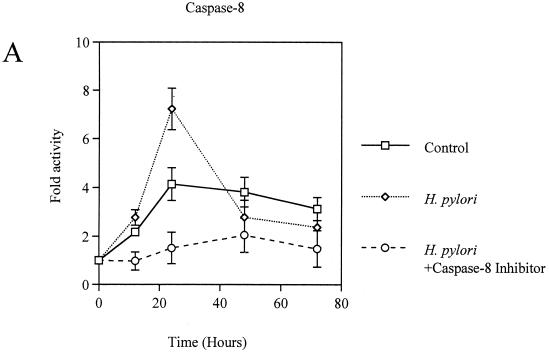
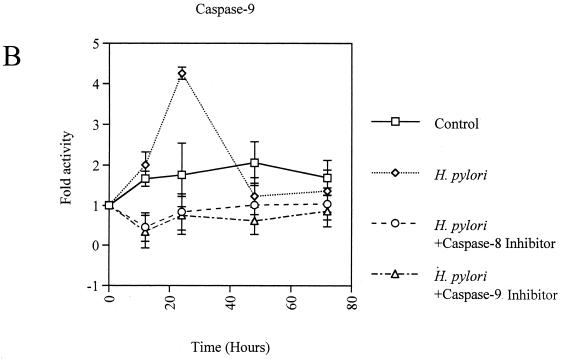
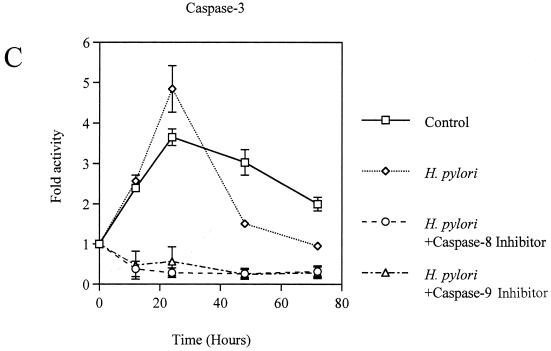
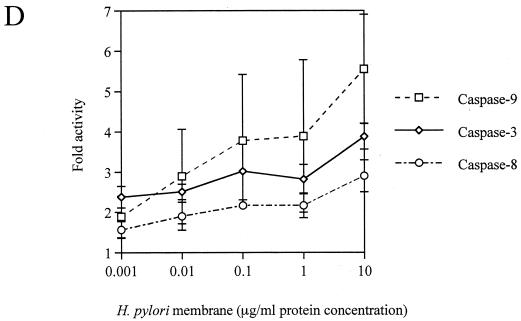
Coculture of AGS cells with H. pylori induced apoptosis in a time-dependent manner (Fig. 2). The activities of caspase-8, -9, and -3 were significantly elevated in H. pylori-infected AGS cells, with the peak occurring at 24 h, compared with control AGS cells (fold activation, for caspase-8, 7.2 ± 0.9 versus 4.1 ± 0.7; for caspase-9, 4.3 ± 0.1 versus 1.8 ± 0.8; for caspase-3, 4.8 ± 0.6 versus 3.6 ± 0.2; P < 0.01) (Fig. 3A, B, and C). These responses were also elicited by the membrane fraction preparations of H. pylori. Activation of the caspases by the membrane fractions was dose dependent (Fig. 3D). Treatment of the preparations with proteinase K eliminated the activity. To determine the contribution of caspase-8 and -9 to the activation of caspase-3, AGS cells were incubated with H. pylori in a medium containing 40 μM caspase-8 inhibitor Z-IETD-FMK or 20 μM caspase-9 inhibitor Z-LEHD-FMK. As Fig. 3C shows, caspase-3 activation was completely inhibited by both of these caspase inhibitors during the entire time course examined. In addition, caspase-8 inhibition also blocked the activation of caspase-9 (Fig. 3B). These results indicate a crucial role of caspase-8 and -9 in the activation of effector caspase-3 and a cascade process of their sequential activation in H. pylori-infected AGS cells. No significant difference was observed among the strains examined. At 24 h, the apoptosis was almost completely blocked by inhibition of either caspase-8 or -9 (21.9% ± 0.95% versus 6.9% ± 1.1% and 7.4% ± 1.3%, respectively; P < 0.01) (Fig. 2), indicating that these caspases play a critical role at this time point. However, these caspase inhibitors did not suppress the apoptosis beyond that time (Fig. 2), suggesting an involvement of caspase-independent pathways in the late phase, when a relatively high degree of apoptosis is induced. Similar phenomena were elicited by the membrane fraction preparations from H. pylori (data not shown). These observations indicate that caspase-8, -9, and -3 exert their apoptotic function at an early phase, when activities of these caspases attain relatively high levels.
FIG. 2.
Time course of H. pylori NGY 1281-induced apoptosis in AGS cells in the absence or presence of caspase inhibitors. Results are expressed as the mean percentages of apoptotic cells per 500 cells enumerated. Bars represent mean values ± SD from three experiments.
FIG. 3.
(A to C) Activation of caspase-8 (A), -9 (B), and -3 (C) in H. pylori NGY1281-infected AGS cells. Effects of the inhibitors of caspase-8 and -9 were also examined. (D) Dose-dependent activation of caspase-8, -9, and -3 by H. pylori membrane fraction at 24 h. AGS cells were exposed to the H. pylori membrane preparation at the indicated protein concentration for 24 h. Bars represent mean values ± SD from three experiments.
Expression of Bad and Bid during H. pylori infection.
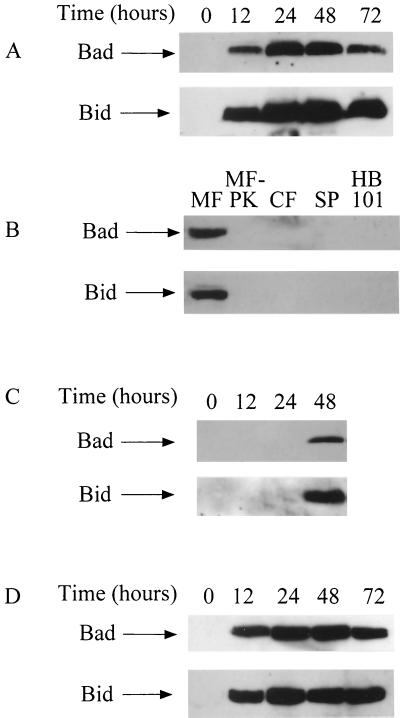
Since the activation of caspase-9 takes place by the release of cytochrome c following mitochondrial damage (66, 78), we investigated the expression of the proapoptotic Bcl-2 family member proteins Bad and Bid, which potentially exert their proapoptotic activity in mitochondria to cause a disruption of the mitochondrial membrane (74, 75). Bad and Bid were barely detectable in control AGS cells, but after the incubation with H. pylori, there was a significant increase in the expression of these proteins (Fig. 4A). No apparent difference was observed among the strains examined. As expected, the membrane preparations of H. pylori elicited the same response (Fig. 4B). The proteinase K-treated membrane preparations, cytosolic fraction, and concentrated supernatant showed no activity in inducing the expression of these proteins, nor did E. coli HB101 induce these proteins (Fig. 4B). Bad phosphorylated at either Ser-112 or Ser-136, whose proapoptotic activity was lost by an alteration in the subcellular location from mitochondria to cytosol (76), was not detected at any time between 12 and 72 h (not shown) during H. pylori infection. The Bid protein detected was a 13-kDa protein which was reported to possess mitochondrial damage-inducing activity (45). Moreover, Bad and Bid were not detected in the cytosolic extracts of AGS cells (data not shown). These observations suggest that Bad and Bid exert their death-promoting effects in the mitochondria of H. pylori-infected AGS cells. Inhibition of caspase-8 suppressed the expression of Bad and Bid between 12 and 24 h (Fig. 4C). This suggests that the induction of the proapoptotic proteins Bad and Bid at the early phase requires caspase-8 activity. In the late phase, alternative factors seem to induce the expression of Bad and Bid, which does not lead to the activation of caspase-9. These findings are consistent with the results obtained by a caspase activity assay showing the inhibitory effect of the caspase-8 inhibitor on the activation of caspase-9. In contrast, the inhibition of caspase-9 did not notably affect the expression of these proteins (Fig. 4D). This is in agreement with previous studies implicating this caspase activity in the mitochondrial pathway.
FIG. 4.
Western blot analysis for Bad and Bid. Data from NGY 1281 are shown. (A) Changes in expression of Bad and Bid after incubation with H. pylori. (B) Induction of Bad and Bid by membrane fraction (MF), membrane fraction pretreated with proteinase K (MF-PK), cytosolic fraction (CF), concentrated supernatant (SP), or E. coli HB101 (HB101). Each fraction was added to AGS cells at a protein concentration of 10 μg/ml. E. coli HB101 was added at a bacterium/cell ratio of 10:1. Resuts at 24 h are shown as representative. (C) Effect of the caspase-8 inhibitor on expression of Bad and Bid after incubation with H. pylori. (D) Effect of the caspase-9 inhibitor on expression of Bad and Bid after incubation with H. pylori.
Addition of serum to the medium, by which induction of apoptosis was significantly suppressed, as shown in Fig. 1C, had no apparent inhibitory effect on the expression of Bad and Bid (data not shown), indicating that the antiapoptotic effect of serum works on the downstream steps or on other signaling pathways.
DISCUSSION
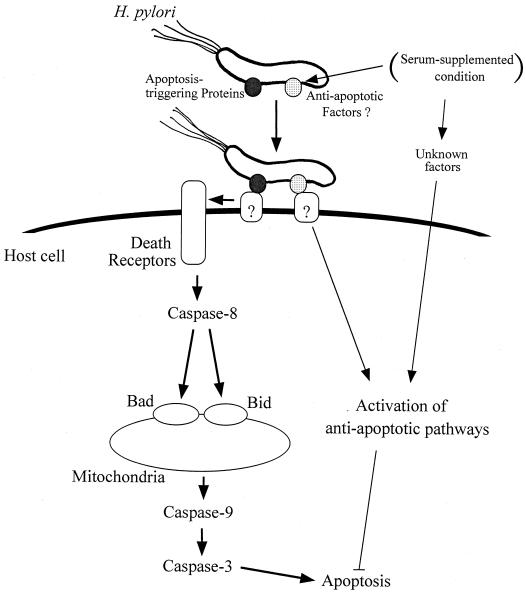
In this study we demonstrate that H. pylori induces apoptosis through a pathway involving the sequential induction of caspase-8 activity, the proapoptotic proteins Bad and Bid, caspase-9 activity, and effector caspase-3 activity (Fig. 5). Although the mechanism by which caspase-8 induces Bad and Bid has been unclear until now, our results indicate that the activation of caspase-8 is an initiator for the downstream signaling cascade. This pathway appears to play a critical role in the early phase and induces a rather low degree of apoptosis. In the late phase, when a relatively high degree of apoptosis is induced, alternative pathways would likely mediate H. pylori-induced apoptosis. In both phases, contact of the bacterial membrane protein(s) with the host cell appears to trigger initiation of the apoptotic pathways, and apoptosis was significantly inhibited by the addition of serum. Serum appears to exert an antiapoptotic effect on both the host cell and the bacteria. These results indicate that the pathogenic activities of H. pylori would be largely affected by environmental conditions. We used AGS cells for these studies, since the AGS cell line serves as a suitable model for investigating these apoptotic pathways compared with other available gastric cell lines (13, 34). Since AGS cells undergo apoptosis rather than necrosis in response to the infection with H. pylori, it has been suggested that the response of AGS cells to infection with the bacterium mimics an in vivo setting (13, 34).
FIG. 5.
Possible mechanism for the H. pylori-induced apoptotic signaling pathway. The apoptosis-triggering protein(s) attaches to the host cell surface and then stimulates the death receptors through unknown systems. Stimulation of the death receptors induces apoptosis through sequential induction of caspase-8 activity, proapoptotic proteins Bad and Bid, caspase-9 activity, and caspase-3 activity. Under the serum-supplemented condition, expression of antiapoptotic factors of H. pylori would presumably increase, leading to activation of antiapoptotic pathways of the host cell. Antiapoptotic substances, such as growth factors, would also be responsible for inhibition of H. pylori-mediated apoptosis.
Recently, several bacterial pathogens have been found to trigger apoptosis in host cells in vitro or in vivo, and several types of mechanisms have been elucidated (27). Introduction of bacterial proteins into the host cell via the type III secretion pathway has been demonstrated to be involved in triggering apoptosis by enteropathogenic E. coli (17), Salmonella (52), Shigella (79, 80), and Yersinia (51). In enterohemorrhagic E. coli-mediated apoptosis, secreted Shiga toxins trigger apoptotic signals by binding to the receptor on the host cell (4, 35, 40, 41). However, H. pylori-induced triggering of cell death appears to differ from that resulting from the enteric bacteria, since exposure of the host cell to an H. pylori membrane protein(s) was sufficient to trigger the apoptotic pathways. In addition, compared to the enteric bacterium-mediated apoptosis, H. pylori is much weaker in its ability to cause cell death. The enteric bacteria cause more than 50% of cell death within a few hours. In contrast, H. pylori induced only up to 25% of host cell death in 24 h. In Pseudomonas aeruginosa (12) and Campylobacter jejuni (77), apoptotic activities of the outer membrane porins have been identified. Incubation of the porins purified from these organisms with the host cell causes a rather small degree of apoptosis (12, 77), which is comparable to that of H. pylori. In this mechanism, by interacting with the plasma membranes of the host cells, porins become embedded as hydrophilic pores in the phospholipid bilayer, damaging the structure and function of this part of the host cell architecture and leading to the activation of apoptotic signaling pathways (12). Since the H. pylori membrane fraction prepared as described in Materials and Methods contains a variety of membrane proteins, including outer membrane porins, involvement of this type of mechanism could be possible. Although H. pylori porins possess immunological activities, including the release of a series of inflammatory mediators (71), their apoptotic activities remain to be elucidated. Recently apoptosis-inducing activity of H. pylori urease was reported (24). H. pylori urease is suggested to be present on the surface of the bacterium (20). However, in our preliminary examination, the membrane fraction preparation that had no apoptosis-inducing activity did have apparent urease activity (data not shown), suggesting an involvement of another membrane protein(s) in triggering apoptosis.
Our results showed a potent antiapoptotic effect of serum on both AGS cells and the bacteria. Antiapoptotic substances, such as growth factors, would be responsible for the inhibition of apoptosis of the host cells. The mechanism by which serum eliminates the apoptotic activity of H. pylori is unclear. However, an antiapoptotic substance(s) expressed by H. pylori in response to serum would likely be involved, since H. pylori infection could stimulate potential antiapoptotic signals, including tyrosine kinases (2, 46, 58, 63–65, 68), protein kinase C (5, 6, 69), and the transcription factor NF-κB (25, 29, 32, 39, 48, 57). The ability of H. pylori to stimulate antiapoptotic pathways is not surprising, since there appears to be no obvious benefit for H. pylori from rapid host cell killing, which would result in the loss of colonization sites. The variability in the apoptosis-inducing activity of H. pylori might be a result of the adaptation of the bacterium to environmental conditions.
In the human stomach, the degree of apoptosis induced is affected by the associated inflammatory response. H. pylori infection induces a number of inflammatory mediators, including cytokines and chemokines (10). In vitro studies demonstrated that IFN-γ and tumor necrosis factor alpha, which are increased in the gastric mucosa during H. pylori infection (10, 38), augment the apoptosis induced by H. pylori (61, 72). IFN-γ is postulated to upregulate the expression of the Fas receptor on gastric epithelial cells (61, 73), and these tumor necrosis factor receptors activate caspase-8 (11, 50, 55). Taken together, our results support and extend recent evidence indicating the functional role of the tumor necrosis factor receptor family in H. pylori-induced apoptosis (31, 34, 61, 72, 73). The pathway initiated by the activation of caspase-8 that causes a rather small degree of apoptosis may be involved in the latent pathomorphisms in vivo. Activation of alternative pathways that induce a high degree of apoptosis may lead to severe cell loss, which is characteristic of ulceration. The environmental conditions in the stomach would exert a significant influence on the stimulation of these pathways. To further delineate the role of the pathways in the pathogenesis of H. pylori-mediated disease, an examination of the long-term time course of apoptosis and proliferation will be required (23), and in vivo studies, including those using animal models of human disease (44), should be undertaken.
To date, strain-specific genetic diversity has been proposed to be involved in the organism's ability to cause different diseases or even to be beneficial to the infected host and participate in the lifelong chronicity of infection (1, 3, 9, 47). However, our results suggest the need for further studies on host factors for a better understanding of the pathogenicity of H. pylori.
ACKNOWLEDGMENT
This work was supported by a grant from the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 2.Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M, Suzuki T, Sasakawa C. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 4.Barnett Foster D, Abul-Milh M, Huesca M, Lingwood C A. Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect Immun. 2000;68:3108–3115. doi: 10.1128/iai.68.6.3108-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beil W, Birkholz C, Wagner S, Sewing K F. Helicobacter pylori fatty acid cis 9,10-methyleneoctadecanoic acid increases [Ca2+]i, activates protein kinase C and stimulates acid secretion in parietal cells. Prostaglandins Leukot Essent Fatty Acids. 1998;59:119–125. doi: 10.1016/s0952-3278(98)90090-4. [DOI] [PubMed] [Google Scholar]
- 6.Beil W, Obst B, Wagner S, Sewing K F. The Helicobacter pylori fatty acid cis-9,10-methyleneoctadecanoic acid stimulates protein kinase C and increases DNA synthesis of gastric HM02 cells. Br J Cancer. 1998;77:1852–1856. doi: 10.1038/bjc.1998.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser M J. Ecology of Helicobacter pylori in the human stomach. J Clin Investig. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser M J. Helicobacter pylori and gastric diseases. Br Med J. 1998;316:1507–1510. doi: 10.1136/bmj.316.7143.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaser M J. Not all Helicobacter pylori strains are created equal: should all be eliminated? Lancet. 1997;349:1020–1022. doi: 10.1016/S0140-6736(96)09133-7. [DOI] [PubMed] [Google Scholar]
- 10.Bodger K, Crabtree J E. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998;54:139–150. doi: 10.1093/oxfordjournals.bmb.a011664. [DOI] [PubMed] [Google Scholar]
- 11.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 12.Buommino E, Morelli F, Metafora S, Rossano F, Perfetto B, Baroni A, Tufano M A. Porin from Pseudomonas aeruginosa induces apoptosis in an epithelial cell line derived from rat seminal vesicles. Infect Immun. 1999;67:4794–4800. doi: 10.1128/iai.67.9.4794-4800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Sordillo E M, Ramey W G, Reidy J, Holt P R, Krajewski S, Reed J C, Blaser M J, Moss S F. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem Biophys Res Commun. 1997;239:626–632. doi: 10.1006/bbrc.1997.7485. [DOI] [PubMed] [Google Scholar]
- 14.Correa P, Miller M J. Carcinogenesis, apoptosis and cell proliferation. Br Med Bull. 1998;54:151–162. doi: 10.1093/oxfordjournals.bmb.a011665. [DOI] [PubMed] [Google Scholar]
- 15.Cover T L, Blaser M J. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992;43:135–145. doi: 10.1146/annurev.me.43.020192.001031. [DOI] [PubMed] [Google Scholar]
- 16.Cover T L, Halter S A, Blaser M J. Characterization of HeLa cell vacuoles induced by Helicobacter pylori broth culture supernatant. Hum Pathol. 1992;23:1004–1010. doi: 10.1016/0046-8177(92)90261-z. [DOI] [PubMed] [Google Scholar]
- 17.Crane J K, Majumdar S, Pickhardt D F., III Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;675:2575–2584. doi: 10.1128/iai.67.5.2575-2584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dooley C P, Cohen H, Fitzgibbons P L, Bauer M, Appleman M D, Perez-Perez G I, Blaser M J. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 19.Dorrell N, Crabtree J E, Wren B W. Host-bacterial interactions and the pathogenesis of Helicobacter pylori infection. Trends Microbiol. 1998;6:379–382. doi: 10.1016/s0966-842x(98)01367-5. [DOI] [PubMed] [Google Scholar]
- 20.Dunn B E, Campbell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 21.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Omar E M, Carrington M, Chow W H, McColl K E, Bream J H, Young H A, Herrera J, Lissowska J, Yuan C C, Rothman N, Lanyon G, Martin M, Fraumeni J F, Jr, Rabkin C S. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 23.Falk P G, Syder A J, Guruge J L, Kirschner D, Blaser M J, Gordon J I. Theoretical and experimental approaches for studying factors defining the Helicobacter pylori-host relationship. Trends Microbiol. 2000;8:321–329. doi: 10.1016/s0966-842x(00)01780-7. [DOI] [PubMed] [Google Scholar]
- 24.Fan X, Gunasena H, Cheng Z, Espejo R, Crowe S E, Ernst P B, Reyes V E. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol. 2000;165:1918–1924. doi: 10.4049/jimmunol.165.4.1918. [DOI] [PubMed] [Google Scholar]
- 25.Foryst-Ludwig A, Naumann M. p21-activated kinase 1 activates the nuclear factor κB (NF-κ-B)-inducing kinase-iκB kinases NF-κ-B pathway and proinflammatory cytokines in H. pylori infection. J Biol Chem. 2000;275:39779–39785. doi: 10.1074/jbc.M007617200. [DOI] [PubMed] [Google Scholar]
- 26.Galmiche A, Rassow J, Doye A, Cagnol S, Chambard J C, Contamin S, de Thillot V, Just I, Ricci V, Solcia E, Van Obberghen E, Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao L Y, Kwaik Y A. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 2000;8:306–313. doi: 10.1016/s0966-842x(00)01784-4. [DOI] [PubMed] [Google Scholar]
- 28.Genta R M. Helicobacter pylori, inflammation, mucosal damage, and apoptosis: pathogenesis and definition of gastric atrophy. Gastroenterology. 1997;113(6 Suppl.):S51–S55. doi: 10.1016/s0016-5085(97)80012-1. [DOI] [PubMed] [Google Scholar]
- 29.Glocker E, Lange C, Covacci A, Bereswill S, Kist M, Pahl H L. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect Immun. 1998;66:2346–2348. doi: 10.1128/iai.66.5.2346-2348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall P A, Coates P J, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 31.Houghton J, Macera-Bloch L S, Harrison L, Kim K H, Korah R M. Tumor necrosis factor alpha and interleukin 1β up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect Immun. 2000;68:1189–1195. doi: 10.1128/iai.68.3.1189-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768–2776. doi: 10.1111/j.1572-0241.2000.02304.x. [DOI] [PubMed] [Google Scholar]
- 33.Jiang S, Cai J, Wallace D C, Jones D P. Cytochrome c-mediated apoptosis in cells lacking mitochondrial DNA. Signaling pathway involving release and caspase 3 activation is conserved. J Biol Chem. 1999;274:29905–29911. doi: 10.1074/jbc.274.42.29905. [DOI] [PubMed] [Google Scholar]
- 34.Jones N L, Day A S, Jennings H A, Sherman P M. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun. 1999;67:4237–4242. doi: 10.1128/iai.67.8.4237-4242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones N L, Islur A, Haq R, Mascarenhas M, Karmali M A, Perdue M H, Zanke B W, Sherman P M. Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am J Physiol Gastrointest Liver Physiol. 2000;278:G811–G819. doi: 10.1152/ajpgi.2000.278.5.G811. [DOI] [PubMed] [Google Scholar]
- 36.Jones N L, Shannon P T, Cutz E, Yeger H, Sherman P M. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 37.Jones N L, Sherman P M. Helicobacter pylori-epithelial cell interactions: from adhesion to apoptosis. Can J Gastroenterol. 1999;13:563–566. doi: 10.1155/1999/848346. [DOI] [PubMed] [Google Scholar]
- 38.Karttunen R, Karttunen T, Ekre H P, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keates S, Hitti Y S, Upton M, Kelly C P. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 40.Kiyokawa N, Taguchi T, Mori T, Uchida H, Sato N, Takeda T, Fujimoto J. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga toxins 1 and 2. J Infect Dis. 1998;178:178–184. doi: 10.1086/515592. [DOI] [PubMed] [Google Scholar]
- 41.Kodama T, Nagayama K, Yamada K, Ohba Y, Akeda Y, Honda T. Induction of apoptosis in human renal proximal tubular epithelial cells by Escherichia coli verocytotoxin 1 in vitro. Med Microbiol Immunol (Berlin) 1999;188:73–78. doi: 10.1007/s004300050107. [DOI] [PubMed] [Google Scholar]
- 42.Kohda K, Tanaka K, Aiba Y, Yasuda M, Miwa T, Koga Y. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut. 1999;44:456–462. doi: 10.1136/gut.44.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konturek P C, Pierzchalski P, Konturek S J, Meixner H, Faller G, Kirchner T, Hahn E G. Helicobacter pylori induces apoptosis in gastric mucosa through an upregulation of Bax expression in humans. Scand J Gastroenterol. 1999;34:375–383. doi: 10.1080/003655299750026380. [DOI] [PubMed] [Google Scholar]
- 44.Lee A, O'Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. . (Erratum, 113:732.) [DOI] [PubMed] [Google Scholar]
- 45.Li H, Zhu H, Xu C J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 46.Li S D, Kersulyte D, Lindley I J D, Neelam B, Berg D E, Crabtree J E. Multiple genes in the left half of the cag pathogenicity island of Helicobacter pylori are required for tyrosine kinase-dependent transcription of interleukin-8 in gastric epithelial cells. Infect Immun. 1999;67:3893–3899. doi: 10.1128/iai.67.8.3893-3899.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Logan R P, Berg D E. Genetic diversity of Helicobacter pylori. Lancet. 1996;348:1462–1463. doi: 10.1016/s0140-6736(05)65885-0. [DOI] [PubMed] [Google Scholar]
- 48.Maeda S, Yoshida H, Ogura K, Mitsuno Y, Hirata Y, Yamaji Y, Akanuma M, Shiratori Y, Omata M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119:97–108. doi: 10.1053/gast.2000.8540. [DOI] [PubMed] [Google Scholar]
- 49.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 50.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moss S F. Helicobacter pylori and apoptosis. Yale J Biol Med. 1998;71:53–61. [PMC free article] [PubMed] [Google Scholar]
- 54.Moss S F, Calam J, Agarwal B, Wang S, Holt P R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) deathinducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 56.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 57.Naumann M, Wessler S, Bartsch C, Wieland B, Covacci A, Haas R, Meyer T F. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J Biol Chem. 1999;274:31655–31662. doi: 10.1074/jbc.274.44.31655. [DOI] [PubMed] [Google Scholar]
- 58.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 59.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 60.Que F G, Gores G J. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996;110:1238–1243. doi: 10.1053/gast.1996.v110.pm8613014. [DOI] [PubMed] [Google Scholar]
- 61.Rudi J, Kuck D, Strand S, von Herbay A, Mariani S M, Krammer P H, Galle P R, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Investig. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 63.Segal E D, Falkow S, Tompkins L S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Segal E D, Lange C, Covacci A, Tompkins L S, Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stennicke H R, Deveraux Q L, Humke E W, Reed J C, Dixit V M, Salvesen G S. Caspase-9 can be activated without proteolytic processing. J Biol Chem. 1999;274:8359–8362. doi: 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- 67.Stennicke H R, Salvesen G S. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 68.Su B, Johansson S, Fallman M, Patarroyo M, Granstrom M, Normark S. Signal transduction-mediated adherence and entry of Helicobacter pylori into cultured cells. Gastroenterology. 1999;117:595–604. doi: 10.1016/s0016-5085(99)70452-x. [DOI] [PubMed] [Google Scholar]
- 69.Terres A M, Pajares J M, Hopkins A M, Murphy A, Moran A, Baird A W, Kelleher D. Helicobacter pylori disrupts epithelial barrier function in a process inhibited by protein kinase C activators. Infect Immun. 1998;66:2943–2950. doi: 10.1128/iai.66.6.2943-2950.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 71.Tufano M A, Rossano F, Catalanotti P, Liguori G, Capasso C, Ceccarelli M T, Marinelli P. Immunobiological activities of Helicobacter pylori porins. Infect Immun. 1994;62:1392–1399. doi: 10.1128/iai.62.4.1392-1399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner S, Beil W, Westermann J, Logan R P, Bock C T, Trautwein C, Bleck J S, Manns M P. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Fan X, Lindholm C, Bennett M, O'Connoll J, Shanahan F, Brooks E G, Reyes V E, Ernst P B. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect Immun. 2000;68:4303–4311. doi: 10.1128/iai.68.7.4303-4311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang K, Yin X M, Chao D T, Milliman C L, Korsmeyer S J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 75.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 76.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3–3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 77.Zhu J, Meinersmann R J, Hiett K L, Evans D L. Apoptotic effect of outer-membrane proteins from Campylobacter jejuni on chicken lymphocytes. Curr Microbiol. 1999;38:244–249. doi: 10.1007/pl00006795. [DOI] [PubMed] [Google Scholar]
- 78.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 79.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 80.Zychlinsky A, Thirumalai K, Arondel J, Cantey J, Aliprantis A, Sansonetti P. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]