Abstract
Fabry disease (FD) is a lysosomal storage disorder caused by mutations in the gene for α-galactosidase A, inducing a progressive accumulation of globotriaosylceramide (GB3) and its metabolites in different organs and tissues. GB3 deposition does not fully explain the clinical manifestations of FD, and other pathogenetic mechanisms have been proposed, requiring the identification of new biomarkers for monitoring FD patients. Emerging evidence suggests the involvement of mitochondrial alterations in FD. Here, we propose mitochondrial-related microRNAs (miRs) as potential biomarkers of mitochondrial involvement in FD. Indeed, we demonstate that miRs regulating different aspects of mitochondrial homeostasis including expression and assembly of respiratory chain, mitogenesis, antioxidant capacity, and apoptosis are consistently dysregulated in FD patients. Our data unveil a novel noncoding RNA signature of FD patients, indicating mitochondrial-related miRs as new potential pathogenic players and biomarkers in FD.
SIGNIFICANCE STATEMENT
This study demonstrates for the first time that a specific signature of circulating mitochondrial miRs (mitomiRs) is dysregulated in FD patients. MitomiRs regulating fundamental aspects of mitochondrial homeostasis and fitness, including expression and assembly of the respiratory chain, mitogenesis, antioxidant capacity, and apoptosis are significantly dysregulated in FD patients. Taken together, these new findings introduce mitomiRs as unprecedented biomarkers of FD and point at mitochondrial dysfunction as a novel potential mechanistic target for therapeutic approaches.
Introduction
Fabry disease (FD) is an X-linked inherited disorder of glycosphingolipid metabolism characterized by reduced or absent lysosomal α-galactosidase A (α-GAL A) activity due to mutations of the GLA gene (Germain, 2010), resulting in a progressive accumulation of globotriaosylceramide (GB3) and its metabolites (Germain, 2010; Miller et al., 2020). The clinical presentation of FD patients includes a poor quality of life and a reduced life expectancy due to complications in multiple organs (Morand et al., 2019). Cardiovascular disease remains the leading cause of death in these patients (Sorriento and Iaccarino, 2021). At the molecular level, GB3 deposition is generally considered one of the key pathogenetic mechanisms of this rare pathology, and the actual therapies are aimed at correcting the enzymatic deficiency, either by enzyme replacement therapy (ERT) or enzyme stabilization (e.g., using a chaperone, like Migalastat) (Ioannou et al., 2001; Pisani et al., 2012; Germain et al., 2016; Azevedo et al., 2020; Riccio et al., 2020). However, these therapeutics cannot revert FD pathology and its clinical manifestations, thereby suggesting that other pathogenetic mechanisms are involved. This aspect is further supported by the great variability of clinical phenotypes occurring among patients with the same genetic mutation (Sorriento and Iaccarino, 2021), maybe depending on different triggering mechanisms. For instance, an altered mitochondrial respiration has been suggested in fibroblasts isolated from FD patients (Das and Naim, 2009; Namdar et al., 2012; Rozenfeld and Feriozzi, 2017). An early diagnosis of FD is decisive for reducing the morbidity and mortality and activate organ-specific therapeutic interventions.
We and others have shown that microRNAs (miRs), small noncoding RNAs of ∼22 nucleotides, may serve as reliable biomarkers of various human disorders (Varzideh et al., 2022; Santulli et al., 2014; Sardu et al., 2014; Novak et al., 2015; Santulli, 2015; Santulli, 2016; Cammarata et al., 2018; Mone et al., 2022; Dama et al., 2020; Donati et al., 2021; Jankauskas et al., 2021; Kanach et al., 2021; Kansakar et al., 2022). Therefore, the miR profile could be a promising biomarker candidate for diagnosis and prognosis of FD. Alterations in over 100 circulating miRs were identified in blood samples from FD patients in treatment with ERT compared with untreated patients (Xiao et al., 2019). Increased expression of miR-29a-3p and miR-200a-3p were detected in urinary extracellular vesicles isolated from patients with FD nephropathy (Levstek et al., 2021); miR-let-7a and miR-let-7d were significantly increased in FD after therapy (Maier et al., 2021). These data point at miR profiling as a powerful diagnostic tool of FD progression and drug testing, as well as indicators of the development of specific FD phenotypes. Furthermore, deciphering a specific FD miR signature could help to identify alternative pathogenetic mechanisms and identifying specific therapeutic targets.
Several miRs are specifically associated with the mitochondrial phenotype in human diseases (Zhang et al., 2021). Some of them, also known as mitochondrial miRs (mitomiRs), are produced by the mitochondrial genome to regulate the mRNA transcription in loco. Other mitomiRs can target nuclear-encoded mRNAs localized on the mitochondrial surface or are necessary for mitochondrial homeostasis (Song et al., 2019). The mechanisms underlying mitomiR biogenesis and action sites are still poorly understood, however, they are a fascinating class of miRs that could allow tracking of mitochondrial status and health in pathologic conditions. Indeed, differentially expressed mitomiRs have been observed in heart failure (Pinti et al., 2017; Wang et al., 2017) where mitochondrial dysfunction is evident (Santulli et al., 2015; Sties et al., 2018; Del Campo et al., 2021; Guitart-Mampel et al., 2021; Morciano et al., 2021; Yang et al., 2021; Schwemmlein et al., 2022). Despite their potential power in mirroring systemic mitochondrial homeostasis, the functional contribution of mitomiRs in FD has never been investigated. Here, we evaluated in FD patients a cluster of mitomiRs and miRs with a recognized role in regulating mitochondrial fitness. Our data provide new insights about mitomiRs as biomarkers and therapeutic targets, adding a new mosaic tile in the delineation of the complex FD pathophysiology, potentially implicating mitochondrial dysfunction.
Materials and Methods
Study Population
FD patients with a confirmed FD diagnosis and healthy controls were recruited from the FD Clinic of Federico II University Hospital from March 2021 to February 2022. FD patients met the following inclusion criteria: 1) Adult ≥18 years of age, 2) confirmed diagnosis of classic FD, and 3) written informed consent. The diagnostic algorithm was based on the measurement of α-GAL A activity and on the genetic confirmatory testing (Vardarli et al., 2020). The study was officially approved by the Institutional Ethical Committee (protocol n. 181/19).
Blood Collection and Circulating miRs Determination
Peripheral blood was collected in EDTA-tubes and plasma was obtained by centrifugation as previously reported (Gambardella et al., 2020a). We extracted microRNAs using the miRVana miRNA isolation kit (Thermo Fisher Scientific; Waltham, MA) according to the manufacturer’s protocol. The quality of miR was determined using Agilent Small RNA Kit (Matarese et al., 2020; Mone et al., 2021). A custom panel of mitomiRs was quantified as we previously described (Gambardella et al., 2021).
Isolation of Peripheral Blood Mononuclear Cells and Western Blot Analysis
Peripheral blood mononuclear cells (PBMCs) were isolated as previously described (Santulli et al., 2011; Gambardella et al., 2020a; Gambardella et al., 2022). Briefly, PBMC rings were obtained by blood stratification (diluted 1:1 in PBS) on ficoll gradient using HISTOPAQUE-1077 (Merck; Germany). PBMC rings were lysed and the protein concentration was determined spectrophotometrically. Western blot analysis was conducted as previously reported (Gambardella et al., 2020a). Briefly, proteins were separated by 4%–12% SDS-PAGE gel and transferred to an Immobilon-P nitrocellulose filter (Merck); levels of electron transport chain (ETC) subunits were determined by using antibodies for total OXPHOS (abcam), and purified proteins (ETC complexes) as positive control. Actin levels used as loading control were visualized by a specific primary antibody (Santa Cruz Biotechnology, Dallas, TX). Secondary peroxidase-conjugated antibodies (ImmunoReagents; Raleigh, NC) were used to visualize antigen-antibody complexes on nitrocellulose by chemiluminescence. A standard chemiluminescence reaction kit (Thermo Fisher Scientific) was used for autoradiography on film.
Statistical Analysis
All experiments were carried out at least in triplicate by blinded investigators; we performed ANOVA with Bonferroni post hoc test or unpaired t test, as appropriate, where applicable. A significance level of P < 0.05 was assumed for all statistical evaluations. Statistics were computed with GraphPad Prism (v. 9.0) software (San Diego, CA).
Results
Alterations of Several mitomiRs in FD Patients
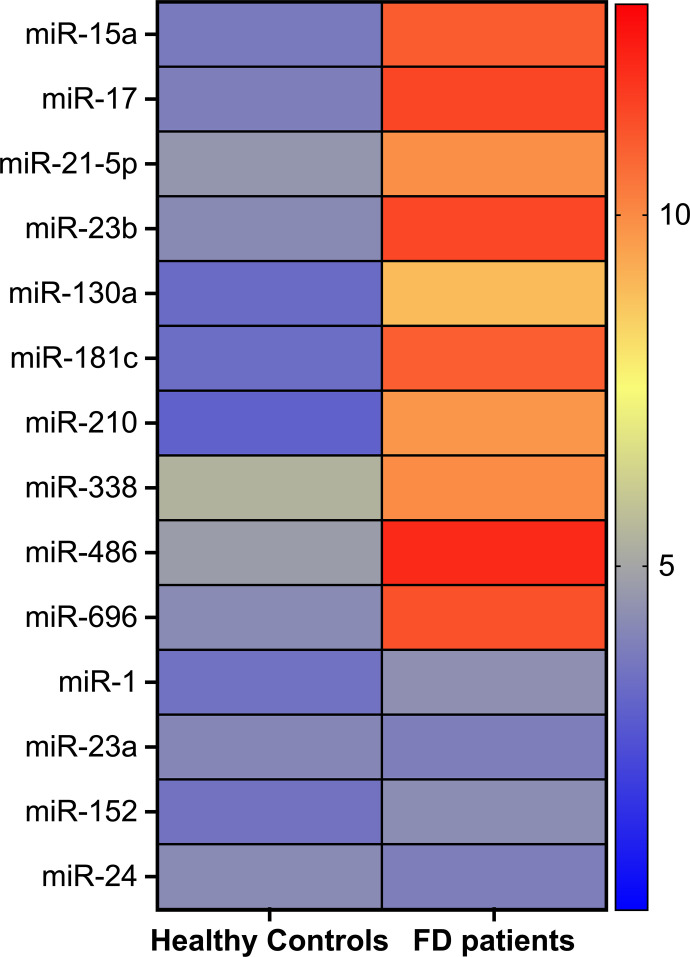
After having excluded 11 patients with late-onset nonclassic variants of FD, we enrolled 63 D and 14 healthy controls. Table 1 summarizes the main clinical characteristics of our population. Circulating miRs were assessed on plasma from FD patients compared with age-matched healthy controls. Specifically, the analysis was focused on a custom panel of key mitomiRs. Overall, a considerable dysregulation of mitomiRs was detected, with FD patients showing a pronounced clusterization (Fig. 1). Indeed, for most of the analyzed mitomiRs, we observed a significant difference in the relative expression between the two groups.
TABLE 1.
Characteristics of FD and control populations
Data are expressed as mean ± S.D. or percent.
| Characteristics | Controls | Patients |
|---|---|---|
| N | 14 | 63 |
| Age (years) | 36 ± 13.4 | 37 ± 13.5 |
| Gender (male, %) | 45.4% | 45.6% |
| Caucasian race (%) | 100% | 100% |
| FD treatment (Migalastat, ERT) |
N/A | 54.2% (43.7%, 56.3%) |
N/A, not applicable.
Fig. 1.
Clusterization analysis of circulating mitomiRs. Heat map showing the profile of mitomiRs’ relative abundance between healthy controls and patients with FD.
The mitomiRs affected in the FD population regulate several aspects of mitochondrial health and biology.
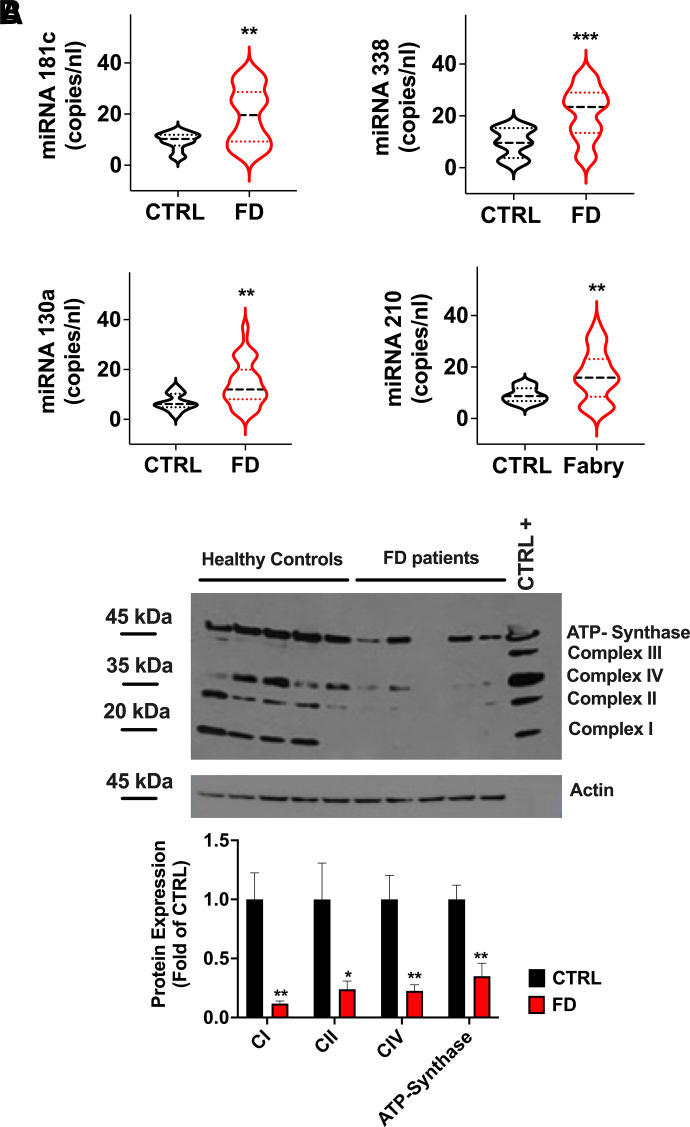
In FD patients, we found the upregulation of miR-181c, miR-338, miR-130, and miR-210, which inhibit mitochondrial ETC expression and assembly (Fig. 2A). Indeed, the expression of ETC complexes was reduced in PBMCs isolated from FD patients (Fig. 2B).
Fig. 2.
Analysis of miRs associated with mitochondrial health and function. (A) Significant dysregulation of circulating miRs related to the expression and assembly of mitochondrial respiratory chain in patients with Fabry disease (FD). Median (dashed line) and quartiles (dotted lines) are indicated in the violin plots. (B) Western blot analysis of mitochondrial ETC in PBMCs revealed a significant downregulation of I-II-IV and ATP synthase complexes in FD; actin was used as loading control; data are mean ± S.E. (C) Purified proteins provided by the supplier, used as molecular weight referrals. *P < 0.05, **P < 0.005, ***P < 0.0005, FD versus control. CTRL +, positive control.
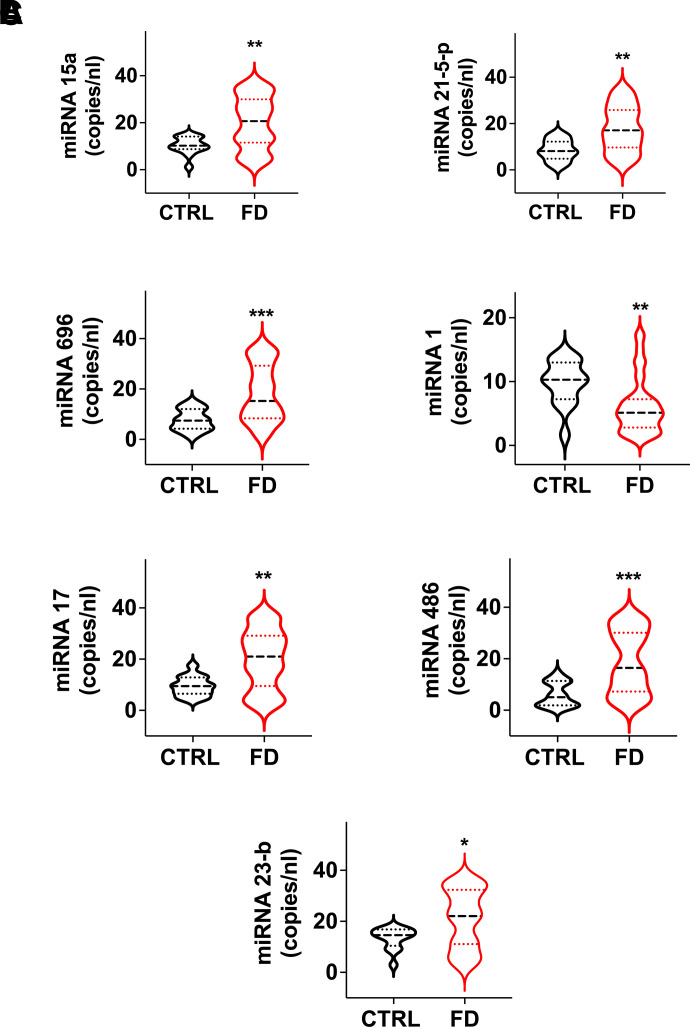
The class of miRs regulating mitochondrial-dependent metabolic pathways was also affected. Levels of miR-15a and miR-21-5p, regulating the expression of Uncoupling Protein 2 (UCP-2) and lipid content and peroxidation (Li et al., 2012; Nasci et al., 2019), were significantly augmented in FD patients compared to healthy controls (Fig. 3A).
Fig. 3.
Analysis of circulating miRs associated with mitochondrial health and function. Significant dysregulation in FD patients of miRs regulating mitochondrial metabolism (A), mitochondrial biogenesis and renewal (B), and mitochondrial oxidative damage and apoptosis (C). Medians (dashed line) and quartiles (dotted lines) are indicated in the violin plots. *P < 0.05, **P < 0.005, ***P < 0.0005.
Mitochondrial biogenesis and renewal are essential for a proper mitochondrial function, and are therefore strictly regulated both at the transcriptional and post-transcriptional levels (Jornayvaz and Shulman, 2010); miR-696 regulates mitochondrial biogenesis by targeting peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) (Aoi et al., 2010), while miR-1 has been reported to enhance the transcription of mitochondrial genomes (Zhang et al., 2014). Interestingly, miR-696 was upregulated while miR-1 was downregulated in the FD population (Fig. 3B).
Additionally, the antioxidant capacity and the mitochondrial proapoptotic pathway can be modulated by miRs: miR-17 and miR-23b are able to suppress critical primary mitochondrial antioxidant enzymes (Schmidt, 1990; Xu et al., 2010); miR-486 may regulate apoptosis via the p53-BCL-2–mediated mitochondrial apoptotic pathway (Sun et al., 2017). In our analysis, miR-17, miR-23b, and miR-486 were consistently upregulated in FD patients (Fig. 3C), further supporting our view of mitochondrial stress and/or damage in FD.
Effect of Treatment on mitomiRs Levels
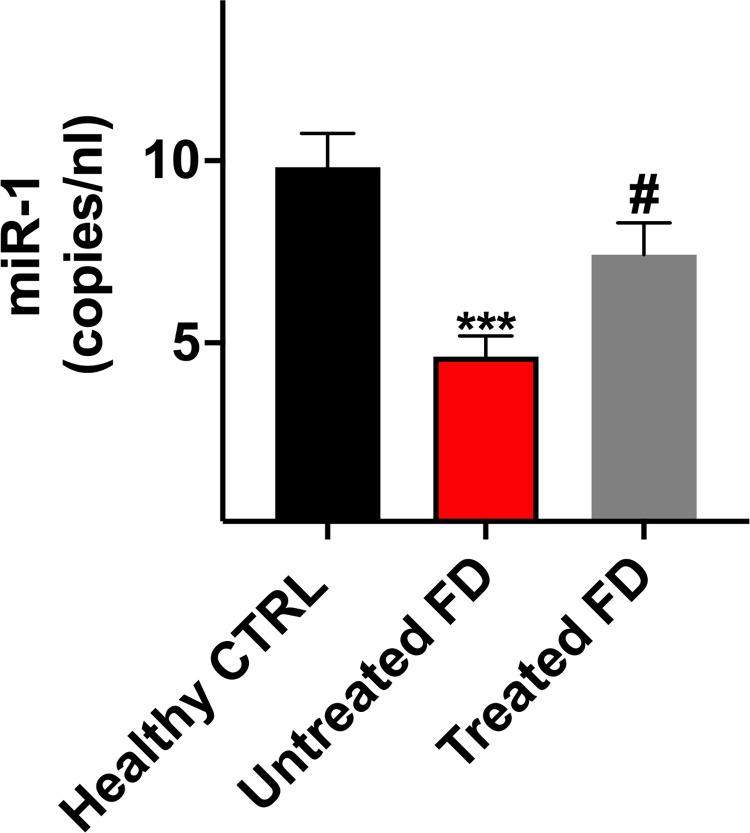
We then dichotomized FD population in naïve and treated patients to test the effect of treatment on the levels of those mitomiRs that were significantly different between FD and controls (miR-181c, miR-338, miR-130a, miR-210, miR-15a, miR-21-5-p, miR-696, miR-1, miR-17, miR-486, miR-23b). Treatment influenced miR-1. In fact, this mitomiR is downregulated in untreated FD patients, and is restored by treatment to the levels observed in control subjects (Fig. 4; Table 2). No effect of treatment was observed for any of the other explored mitomiRs, which remained statistically different from control subjects (Table 2).
Fig. 4.
Effect of treatment on circulating miR-1 in Fabry disease (FD). Evaluation of mitomiRs in untreated and treated FD patients. Untreated FD patients show a reduction in circulating miR-1; treatment restores miR-1 levels in FD patients to the levels observed in control subjects. Data are mean ± S.E. *P < 0.05 versus controls; **P <0.05 versus untreated FD patients.
TABLE 2.
Effects of treatment on circulating mitomiRs in FD
Data are expressed as mean ± SE Data were analyzed by ANOVA with Bonferroni post hoc test.
| Control Subjects | Untreated FD | Treated FD | |
|---|---|---|---|
| miR-1 | 9.81 ± 0.926 | 4.62 ± 0.568* | 7.42 ± 0.872** |
| miR-181c | 9.49 ± 0.887 | 18.98 ± 2.167* | 18.89 ± 1.920* |
| miR-338 | 9.86 ± 1.436 | 19.18 ± 1.586* | 23.07 ± 1.966* |
| miR-130a | 6.81 ± 0.872 | 14.42 ± 1.802* | 15.06 ± 1.563* |
| miR-210 | 9.05 ± 0.788 | 18.86 ± 2.216* | 14.88 ± 1.457* |
| miR-15a | 10.29 ± 0.986 | 20.23 ± 2.021* | 19.49 ± 1.954* |
| miR-21-5-p | 8.51 ± 1.208 | 18.97 ± 1.871* | 16.21 ± 1.777* |
| miR-696 | 7.67 ± 1.123 | 17.92 ± 2.152* | 18.80 ± 2.040* |
| miR-17 | 9.44 ± 1.025 | 19.06 ± 2.258* | 20.21 ± 2.069* |
| miR-486 | 6.10 ± 1.256 | 20.48 ± 2.271* | 18.02 ± 2.250* |
| miR-23b | 13.41 ± 1.116 | 22.82 ± 2.476* | 19.54 ± 1.769* |
*P < 0.05 versus controls.
**P < 0.05 versus untreated.
Discussion
Our data reveals for the first time a dysregulation of mitomiRs in FD. In particular, miRs regulating different aspects of mitochondrial biology including oxidative phosphorylation capacity and energetic metabolism, mitochondrial biogenesis, mitochondrial oxidative damage, and apoptosis, were consistently affected. The upregulation of miRs targeting ETC complexes, thus inhibiting their expression and/or assembly, was particularly interesting. Indeed, miR-181c has been reported to directly bind the 3' untranslated region (3′UTR) of mitochondrial respiratory chain complex 1, determining its downregulation (Das et al., 2012). Similarly, miR-338 induces the downregulation of mitochondrial transport chain complex IV, and its overexpression decreases mitochondrial activity (Aschrafi et al., 2008). A potential interaction between miR-130 and ETC complex III was described (Kren et al., 2009) as well. Additionally, the protein involved in the complex IV assembly, Cytochrome C Oxidase Assembly Factor Heme A:Farnesyltransferase (COX10), is the target of miR-210 (Colleoni et al., 2013).
Consistently, we observed the downregulation of ETC complexes in cells derived from FD patients compared with controls. According with the reduced mitochondrial respiration recorded in fibroblasts from FD patients (Lucke et al., 2004), our data indicate that the oxidative phosphorylation capacity could be affected in FD cells and indicate mitomiR dysregulation as a possible underlying mechanism.
Fundamental miRs regulating mitochondrial biogenesis were also affected in FD patients. Indeed, miR-696 can target and downregulate PGC-1α, the master regulator of mitochondrial biogenesis (Aoi et al., 2010), whereas miR-1 is able to modulate the expression of transcripts encoded by the mitochondrial genome (Zhang et al., 2014). Specifically, miR-1 has been reported to enter the mitochondria, where it can stimulate, rather than repress, the translation of specific mitochondrial transcripts, playing a protective role for mitochondria. Consistently, in our analysis, miR-696 was upregulated in FD patients while miR-1 was downregulated, supporting the hypothesis that both mitochondrial and nuclear factors orchestrating the synthesis of mitochondrial components could be compromised in FD patients.
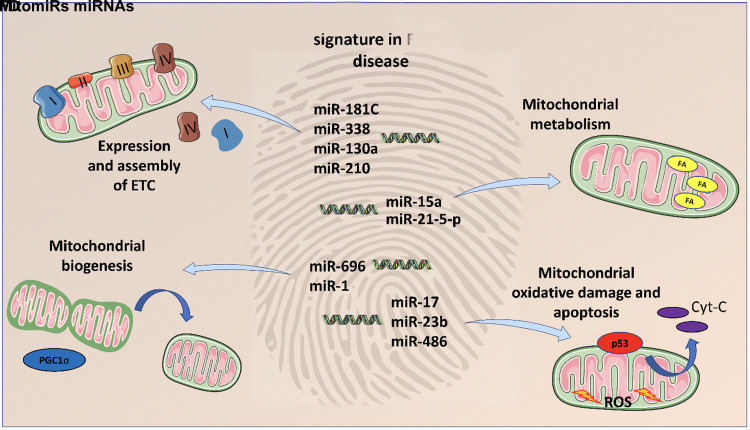
Altogether, our data strongly suggest the implication of mitochondrial dysfunction in FD and shed light on mitomiRs as potential functional players in contributing to aberrant mitochondrial homeostasis. MitomiR dysregulation could represent a new signature of FD underlying the alterations of mitochondrial homeostasis in FD (Fig. 5).
Fig. 5.
MitomiR dysregulation as signature of FD. MitomiRs are significantly dysregulated in FD. The altered miRs are involved in fundamental mechanisms required for the maintenance of mitochondrial homeostasis, including ETC expression and assembly, mitochondrial metabolism, mitogenesis, mitochondrial oxidative damage and apoptosis. This emphatic pattern could represent a new FD footprint. Cyt-C, Cytochrome C; ETC, electron transport chain; FD, Fabry disease.
Consistent with our results, alterations of mitochondrial function and regulation are common in lysosome storage disorders (de la Mata et al., 2016) but they have not been fully investigated in FD. Moreover, our findings are in agreement with a recent report indicating that miR-184 levels are altered in FD patients (Salamon et al., 2021). Indeed, miR-184 is known to target another gene that is crucial for mitochondrial function, namely Slc25a22 (Morita et al., 2013), a mitochondrial carrier that transports glutamate (Goubert et al., 2017) and asymmetric dimethyl L-arginine (Gambardella et al., 2020b). Our results call for future investigations in this direction, unveiling the essential role of mitochondria as a new frontier in FD research.
The diagnosis of FD is usually obtained by detecting GB3 in urine and plasma and is confirmed by genetic analysis. However, GB3 levels and genetic information often are not reliable prognostic indicators, probably because other GB3-independent mechanisms could contribute to organ damage and dysfunction in FD. Several alternative strategies have been proposed in recent years to monitor FD patients (Simonetta et al., 2020). Although they require further investigation, mitomiRs could represent a new class of biomarkers in FD. Our data show that most mitomiRs don’t seem to be sensitive to currently available FD therapies, suggesting that their dysregulation is probably an intimate mechanism of FD, probably independent from GB3 accumulation (Weidemann et al., 2013; Braun et al., 2019), which is reduced by treatment. In this view, their levels could be extremely useful to monitor the onset of GB3-independent damage, highlighting once again the urgent need for new therapeutic targets, beyond current therapies. Intriguingly, miR-1 levels were instead significantly different between untreated and treated patients. In particular, the treatment seems to restore miR-1 levels in FD patients, indicating this miR as a valuable candidate marker of FD, useful also to monitor the response to therapies. Our research has several limitations. The effect of treatment, for instance, was tested in different patients, as our study had not been designed to compare pre- and post- effects. It is likely that our observations are limited to the Caucasian population, since no other ethnicities were investigated. Also, the relative abundance of mitomiRs should be confirmed in a larger population of FD patients and controls.
In conclusion, our results support the involvement of mitochondria in FD. In this view, mitomiRs could have a pathogenic role to induce the mitochondrial alterations described in FD, and therefore could also represent a potential therapeutic target. Furthermore, miR-1 could be a useful biomarker to monitor the response to treatment in FD.
Acknowledgments
The authors kindly thank the trainees of the Sport Medicine School and of Urgent Medicine School: Dr. Rosita Mottola, Dr. Luca Allocca, Dr. Amos Cocola, Dr. Giada Annarumma, Dr. Pasquale Perrella, Dr. Vincenza Notarangelo, and Dr. Marco Rumolo. The authors thank Dr. Stanislovas S. Jankauskas, Dr. Urna Kansakar, and Dr. Xujun Wang for insightful discussion and technical assistance.
Abbreviations
- ERT
enzyme replacement therapy
- ETC
electron transport chain
- FD
Fabry disease
- α-GAL A
α-galactosidase A
- GB3
globotriaosylceramide
- miR
microRNA (miRNA)
- mitomiR
mitochondrial microRNA
- PBMCs
peripheral blood mononuclear cells
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator-1α
- UCP-2
uncoupling protein-2
Authorship Contributions
Participated in research design: Gambardella, Santulli, and Iaccarino.
Conducted experiments: Gambardella, Fiordelisi, Sorriento, Cerasuolo, Buonaiuto, Avvisato, Pisani, Varzideh, Riccio.
Contributed new reagents or analytic tools: Gambardella, Fiordelisi, Sorriento, Cerasuolo, Buonaiuto, Avvisato, Pisani, Varzideh, Riccio.
Performed data analysis: Gambardella, Fiordelisi, Santulli, and Iaccarino.
Wrote or contributed to the writing of the manuscript: Gambardella, Sorriento, Pisani, Santulli, Iaccarino.
Footnotes
The Iaccarino’s Laboratory is supported in part by PRIN [Grant 2017HTKLRF] and Campania Bioscience [Grant PON03PE_00060_8] (to G.I.), and 54 2020 FRA (to D.S.). The Santulli’s Laboratory is supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL159062], [Grant R01-HL146691], and [Grant T32-HL144456]; National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK123259] and [Grant R01-DK033823] (to G.S.), by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). F.V. is supported by a postdoctoral fellowship of the American Heart Association [AHA-21POST836407].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
J.G. and A.F. contributed equally to this work as first authors.
G.S. and G.I. contributed equally to this work as senior authors.
References
- Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ichikawa H, Yoshikawa T (2010) The microRNA miR-696 regulates PGC-1alpha in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab 298,E799–E806. [DOI] [PubMed] [Google Scholar]
- Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB (2008) MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci 28,12581–12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo O, Gago MF, Miltenberger-Miltenyi G, Sousa N, Cunha D (2020) Fabry Disease Therapy: State-of-the-Art and Current Challenges. Int J Mol Sci 22:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun F, Blomberg L, Brodesser S, Liebau MC, Schermer B, Benzing T, Kurschat CE (2019) Enzyme Replacement Therapy Clears Gb3 Deposits from a Podocyte Cell Culture Model of Fabry Disease but Fails to Restore Altered Cellular Signaling. Cell Physiol Biochem 52:1139–1150. [DOI] [PubMed] [Google Scholar]
- Cammarata G, Scalia S, Colomba P, Zizzo C, Pisani A, Riccio E, Montalbano M, Alessandro R, Giordano A, Duro G (2018) A pilot study of circulating microRNAs as potential biomarkers of Fabry disease. Oncotarget 9:27333–27345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleoni F, Padmanabhan N, Yung HW, Watson ED, Cetin I, Tissot van Patot MC, Burton GJ, Murray AJ (2013) Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: a role for miRNA-210 and protein synthesis inhibition. PLoS One 8:e55194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dama E, Melocchi V, Mazzarelli F, Colangelo T, Cuttano R, Di Candia L, Ferretti GM, Taurchini M, Graziano P, Bianchi F (2020) Non-Coding RNAs as Prognostic Biomarkers: A miRNA Signature Specific for Aggressive Early-Stage Lung Adenocarcinomas. Noncoding RNA 6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AM, Naim HY (2009) Biochemical basis of Fabry disease with emphasis on mitochondrial function and protein trafficking. Adv Clin Chem 49:57–71. [DOI] [PubMed] [Google Scholar]
- Das S, Ferlito M, Kent OA, Fox-Talbot K, Wang R, Liu D, Raghavachari N, Yang Y, Wheelan SJ, Murphy E, et al. (2012) Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 110:1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mata M, Cotán D, Villanueva-Paz M, de Lavera I, Álvarez-Córdoba M, Luzón-Hidalgo R, Suárez-Rivero JM, Tiscornia G, Oropesa-Ávila M (2016) Mitochondrial Dysfunction in Lysosomal Storage Disorders. Diseases 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo A, Perez G, Castro PF, Parra V, Verdejo HE (2021) Mitochondrial function, dynamics and quality control in the pathophysiology of HFpEF. Biochim Biophys Acta Mol Basis Dis 1867:166208. [DOI] [PubMed] [Google Scholar]
- Donati S, Aurilia C, Palmini G, Miglietta F, Falsetti I, Iantomasi T, Brandi ML (2021) MicroRNAs as Potential Biomarkers in Pituitary Adenomas. Noncoding RNA 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Coppola A, Izzo R, Fiorentino G, Trimarco B, Santulli G (2021) Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit Care 25:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Jankauskas SS, D’Ascia SL, Sardu C, Matarese A, Minicucci F, Mone P, Santulli G (2022) Glycation of ryanodine receptor in circulating lymphocytes predicts the response to cardiac resynchronization therapy. J Heart Lung Transplant 41:438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Sorriento D, Bova M, Rusciano M, Loffredo S, Wang X, Petraroli A, Carucci L, Mormile I, Oliveti M, et al. (2020a) Role of Endothelial G Protein-Coupled Receptor Kinase 2 in Angioedema. Hypertension 76:1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, and Trimarco V (2020b) Arginine and Endothelial Function. Biomedicines 8:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain DP (2010) Fabry disease. Orphanet J Rare Dis 5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain DP, Hughes DA, Nicholls K, Bichet DG, Giugliani R, Wilcox WR, Feliciani C, Shankar SP, Ezgu F, Amartino H, et al. (2016) Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N Engl J Med 375:545–555. [DOI] [PubMed] [Google Scholar]
- Goubert E, Mircheva Y, Lasorsa FM, Melon C, Profilo E, Sutera J, Becq H, Palmieri F, Palmieri L, Aniksztejn L, et al. (2017) Inhibition of the Mitochondrial Glutamate Carrier SLC25A22 in Astrocytes Leads to Intracellular Glutamate Accumulation. Front Cell Neurosci 11:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart-Mampel M, Urquiza P, Borges JI, Lymperopoulos A, Solesio ME (2021) Impact of Aldosterone on the Failing Myocardium: Insights from Mitochondria and Adrenergic Receptors Signaling and Function. Cells 10:1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou YA, Zeidner KM, Gordon RE, Desnick RJ (2001) Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzyme-deficient mice. Am J Hum Genet 68:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankauskas SS, Gambardella J, Sardu C, Lombardi A, Santulli G (2021) Functional Role of miR-155 in the Pathogenesis of Diabetes Mellitus and Its Complications. Noncoding RNA 7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanach C, Blusztajn JK, Fischer A, Delalle I (2021) MicroRNAs as Candidate Biomarkers for Alzheimer’s Disease. Noncoding RNA 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansakar U, Varzideh F, Mone P, Jankauskas SS, Santulli G (2022) Functional Role of microRNAs in Regulating Cardiomyocyte Death. Cells 11:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kren BT, Wong PY, Sarver A, Zhang X, Zeng Y, Steer CJ (2009) MicroRNAs identified in highly purified liver-derived mitochondria may play a role in apoptosis. RNA Biol 6:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levstek T, Mlinšek T, Holcar M, Goričar K, Lenassi M, Dolžan V, Vujkovac B, Trebušak Podkrajšek K (2021) Urinary Extracellular Vesicles and Their miRNA Cargo in Patients with Fabry Nephropathy. Genes (Basel) 12:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Jiao J, Gao G, Prabhakar BS (2012) Control of mitochondrial activity by miRNAs. J Cell Biochem 113:1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücke T, Höppner W, Schmidt E, Illsinger S, Das AM (2004) Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab 82:93–97. [DOI] [PubMed] [Google Scholar]
- Maier N, Gatterer C, Haider P, Salzmann M, Kaun C, Speidl WS, Sunder-Plassmann G, Podesser BK, Wojta J, Graf S, et al. (2021) MiRNA Let-7a and Let-7d Are Induced by Globotriaosylceramide via NF-kB Activation in Fabry Disease. Genes (Basel) 12:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese A, Gambardella J, Lombardi A, Wang X, Santulli G (2020) miR-7 Regulates GLP-1-Mediated Insulin Release by Targeting β-Arrestin 1. Cells 9:1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JJ, Kanack AJ, Dahms NM (2020) Progress in the understanding and treatment of Fabry disease. Biochim Biophys Acta, Gen Subj 1864:129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Gambardella J, Wang X, Jankauskas SS, Matarese A, Santulli G (2021) miR-24 Targets the Transmembrane Glycoprotein Neuropilin-1 in Human Brain Microvascular Endothelial Cells. Noncoding RNA 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Lombardi A, Kansakar U, Varzideh F, Jankauskas SS, Pansini A, De Gennaro S, Famiglietti M, Macina G, Frullone S et al. (2022) Empagliflozin improves the microRNA signature of endothelial dysfunction in patients with HFpEF and diabetes. J Pharmacol Exp Ther, in press DOI: 10.1124/jpet.121.001251. [DOI] [PMC free article] [PubMed]
- Morand O, Johnson J, Walter J, Atkinson L, Kline G, Frey A, Politei J, Schiffmann R (2019) Symptoms and Quality of Life in Patients with Fabry Disease: Results from an International Patient Survey. Adv Ther 36:2866–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morciano G, Vitto VAM, Bouhamida E, Giorgi C, Pinton P (2021) Mitochondrial Bioenergetics and Dynamism in the Failing Heart. Life (Basel) 11:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Horii T, Kimura M, Hatada I (2013) MiR-184 regulates insulin secretion through repression of Slc25a22. PeerJ 1:e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdar M, Gebhard C, Studiger R, Shi Y, Mocharla P, Schmied C, Brugada P, Lüscher TF, Camici GG (2012) Globotriaosylsphingosine accumulation and not alpha-galactosidase-A deficiency causes endothelial dysfunction in Fabry disease. PLoS One 7:e36373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasci VL, Chuppa S, Griswold L, Goodreau KA, Dash RK, Kriegel AJ (2019) miR-21-5p regulates mitochondrial respiration and lipid content in H9C2 cells. Am J Physiol Heart Circ Physiol 316:H710–H721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák J, Olejníčková V, Tkáčová N, Santulli G (2015) Mechanistic Role of MicroRNAs in Coupling Lipid Metabolism and Atherosclerosis. Adv Exp Med Biol 887:79–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti MV, Hathaway QA, Hollander JM (2017) Role of microRNA in metabolic shift during heart failure. Am J Physiol Heart Circ Physiol 312:H33–H45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Visciano B, Roux GD, Sabbatini M, Porto C, Parenti G, Imbriaco M (2012) Enzyme replacement therapy in patients with Fabry disease: state of the art and review of the literature. Mol Genet Metab 107:267–275. [DOI] [PubMed] [Google Scholar]
- Riccio E, Zanfardino M, Ferreri L, Santoro C, Cocozza S, Capuano I, Imbriaco M, Feriozzi S, Pisani A; AFFIINITY Group (2020) Switch from enzyme replacement therapy to oral chaperone migalastat for treating fabry disease: real-life data. Eur J Hum Genet 28:1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld P, Feriozzi S (2017) Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab 122:19–27. [DOI] [PubMed] [Google Scholar]
- Salamon I, Biagini E, Kunderfranco P, Roncarati R, Ferracin M, Taglieri N, Nardi E, Laprovitera N, Tomasi L, Santostefano M, et al. (2021) Circulating miR-184 is a potential predictive biomarker of cardiac damage in Anderson-Fabry disease. Cell Death Dis 12:1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G (2015) Exploiting microRNA Specificity and Selectivity: Paving a Sustainable Path Towards Precision Medicine. Adv Exp Med Biol 888:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G (2016) MicroRNA: From Molecular Biology to Clinical Practice, Springer Nature, New York. [Google Scholar]
- Santulli G, Campanile A, Spinelli L, Assante di Panzillo E, Ciccarelli M, Trimarco B, Iaccarino G (2011) G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol 107:1125–1130. [DOI] [PubMed] [Google Scholar]
- Santulli G, Iaccarino G, De Luca N, Trimarco B, Condorelli G (2014) Atrial fibrillation and microRNAs. Front Physiol 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G, Xie W, Reiken SR, Marks AR (2015) Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA 112:11389–11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C, Marfella R, Santulli G, Paolisso G (2014) Functional role of miRNA in cardiac resynchronization therapy. Pharmacogenomics 15:1159–1168. [DOI] [PubMed] [Google Scholar]
- Schmidt J (1990) Comparative studies on the anticonvulsant effectiveness of nootropic drugs in kindled rats. Biomed Biochim Acta 49:413–419. [PubMed] [Google Scholar]
- Schwemmlein J, Maack C, Bertero E (2022) Mitochondria as Therapeutic Targets in Heart Failure. Curr Heart Fail Rep 19:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta I, Tuttolomondo A, Daidone M, Pinto A (2020) Biomarkers in Anderson-Fabry Disease. Int J Mol Sci 21:8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Hu XQ, Zhang L (2019) Mitochondrial MiRNA in Cardiovascular Function and Disease. Cells 8:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorriento D, Iaccarino G (2021) The Cardiovascular Phenotype in Fabry Disease: New Findings in the Research Field. Int J Mol Sci 22:1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sties SW, Andreato LV, de Carvalho T, Gonzáles AI, Angarten VG, Ulbrich AZ, de Mara LS, Netto AS, da Silva EL, Andrade A (2018) Influence of exercise on oxidative stress in patients with heart failure. Heart Fail Rev 23:225–235. [DOI] [PubMed] [Google Scholar]
- Sun Y, Su Q, Li L, Wang X, Lu Y, Liang J (2017) MiR-486 regulates cardiomyocyte apoptosis by p53-mediated BCL-2 associated mitochondrial apoptotic pathway. BMC Cardiovasc Disord 17:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardarli I, Rischpler C, Herrmann K, Weidemann F (2020) Diagnosis and Screening of Patients with Fabry Disease. Ther Clin Risk Manag 16:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varzideh F, Kansakar U, Donkor K, Wilson S, Jankauskas SS, Mone P, Wang X, Lombardi A, and Santulli G (2022) Cardiac Remodeling After Myocardial Infarction: Functional Contribution of microRNAs to Inflammation and Fibrosis. Front Cardiovasc Med 9:863238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Song C, Zhou X, Han X, Li J, Wang Z, Shang H, Liu Y, Cao H (2017) Mitochondria Associated MicroRNA Expression Profiling of Heart Failure. BioMed Res Int 2017:4042509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann F, Sanchez-Niño MD, Politei J, Oliveira JP, Wanner C, Warnock DG, Ortiz A (2013) Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis 8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Lu D, Hoepfner J, Santer L, Gupta S, Pfanne A, Thum S, Lenders M, Brand E, Nordbeck P, et al. (2019) Circulating microRNAs in Fabry Disease. Sci Rep 9:15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Fang F, Zhang J, Josson S, St Clair WH, St Clair DK (2010) miR-17* suppresses tumorigenicity of prostate cancer by inhibiting mitochondrial antioxidant enzymes. PLoS One 5:e14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Wang X, Wang T (2021) Regulation of Mitochondrial Function by Noncoding RNAs in Heart Failure and Its Application in Diagnosis and Treatment. J Cardiovasc Pharmacol 78:377–387. [DOI] [PubMed] [Google Scholar]
- Zhang GQ, Wang SQ, Chen Y, Fu LY, Xu YN, Li L, Tao L, Shen XC (2021) MicroRNAs Regulating Mitochondrial Function in Cardiac Diseases. Front Pharmacol 12:663322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zuo X, Yang B, Li Z, Xue Y, Zhou Y, Huang J, Zhao X, Zhou J, Yan Y, et al. (2014) MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell 158:607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]