Abstract
We hypothesized that exosomal microRNAs could be implied in the pathogenesis of thromboembolic complications in coronavirus disease 2019 (COVID-19). We isolated circulating exosomes from patients with COVID-19, and then we divided our population in two arms based on the D-dimer level on hospital admission. We observed that exosomal miR-145 and miR-885 significantly correlate with D-dimer levels. Moreover, we demonstrate that human endothelial cells express the main cofactors needed for the internalization of the "Severe acute respiratory syndrome coronavirus 2" (SARS-CoV-2), including angiotensin converting enzyme 2, transmembrane protease serine 2, and CD-147. Interestingly, human endothelial cells treated with serum from COVID-19 patients release significantly less miR-145 and miR-885, exhibit increased apoptosis, and display significantly impaired angiogenetic properties compared with cells treated with non-COVID-19 serum. Taken together, our data indicate that exosomal miR-145 and miR-885 are essential in modulating thromboembolic events in COVID-19.
SIGNIFICANCE STATEMENT
This work demonstrates for the first time that two specific microRNAs (namely miR-145 and miR-885) contained in circulating exosomes are functionally involved in thromboembolic events in COVID-19. These findings are especially relevant to the general audience when considering the emerging prominence of post-acute sequelae of COVID-19 systemic manifestations known as Long COVID.
Introduction
COVID-19 has caused an enormous number of deaths due to the poor information on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its exact mechanisms of action. Substantial progresses have been recently made in science and technology, improving the management of patients with COVID-19 (Kaur and Gupta, 2020; Stasi et al., 2020; Tregoning et al., 2020; Ulinici et al., 2021; Gupta et al., 2022; Närhi et al., 2022; Vincent et al., 2022; Weerakkody et al., 2022). However, the war is not over yet, inasmuch as the COVID-19 pandemic leaves substantial aftermaths due to the long-term complications of the disease, which are often disabling, reducing the quality of life (Crook et al., 2021; Iqbal et al., 2021; Michelen et al., 2021; Desai et al., 2022; Robineau et al., 2022; Whitaker et al., 2022). Several reports suggest that the risk of death for COVID-19 survivors is higher than the risk associated with other conditions, due at least in part to long-term complications (Davido et al., 2020; Basu et al., 2021; Moreno-Pérez et al., 2021; Yang et al., 2021; Zhang et al., 2021b; Comelli et al., 2022; Smith, 2022). Of note, the number of deaths for long-term COVID-19 complications has not necessarily been recorded as deaths due to COVID-19; therefore, the actual situation could be worse than what was reported. In this context, the identification of useful biomarkers of fatal complications is sorely needed.
The symptoms of patients with COVID-19 vary greatly, ranging from an asymptomatic state to debilitating respiratory failure due to bilateral pneumonia (Çalica et al., 2020; Iacobucci, 2022; Kaliszewski et al., 2022). Patients can also develop a systemic inflammatory state that favors multiorgan failure and increases susceptibility to systemic thromboembolic complications that may contribute to a rapid clinical deterioration (Mui et al., 2021). Arterial thrombotic events include end-organ ischemia to systemic organs, cerebrovascular accidents, and limb ischemia, and are associated with high D-dimer, prolonged prothrombin time (PT), and elevated levels of fibrinogen, which indicate activation of coagulation pathways and thrombosis (Li et al., 2020; Gambardella et al., 2021). D-dimer, in particular, is one of the most sensitive coagulation parameters in COVID-19 and indicates a greater risk for the development of thrombosis (Zhang et al., 2020; Conte et al., 2021; Ozen et al., 2021; Poudel et al., 2021). Thus, D-dimer measurement is currently considered a critical approach in the clinical management of COVID-19 (Rostami and Mansouritorghabeh, 2020; Conte et al., 2021; Ghosh and Ghosh, 2022).
A finding that emerged from the intensive research on COVID-19 is that the endothelium is a key target organ of COVID-19. We were among the first groups to describe the involvement of endothelial dysfunction in COVID-19 (Sardu et al., 2020), and successively both clinical and preclinical evidence supported our finding (Otifi and Adiga, 2022). The endothelium is instrumental in thrombosis, fibrinolysis, inflammation, and in maintaining a proper vasodilation/vasoconstriction and antioxidant/pro-oxidant balance (Wu and Thiagarajan, 1996; Libby et al., 2006; Gambardella et al., 2020; Adebayo et al., 2021); therefore, an impaired endothelial function could be the mechanism underlying the systemic complications of COVID-19, especially but not exclusively thromboembolic events (Sardu et al., 2020; Adebayo et al., 2021).
Direct effects of COVID-19 as well as indirect effects of the infection (inflammation, hypoxia) might predispose patients to thrombotic events (Bikdeli et al., 2020) that then play a decisive role in the clinical outcome (Cryer et al., 2022). Thus, an early biomarker of the development of thromboembolic events and predictor of associated clinical outcomes could be useful for a timely intervention with targeted therapies.
In this context, measuring the levels of microRNAs (miRNAs), freely circulating or within extracellular vesicles, represents a useful strategy as diagnostic and prognostic biomarker in numerous disease states (Cho et al., 2021; Guo et al., 2021; He et al., 2021; Ning et al., 2021; Ueta et al., 2021; Ying et al., 2021; Zhang et al., 2021a; Lin et al., 2022; Lyu et al., 2022; Mahmoudi et al., 2022; Wang et al., 2022). The microRNA cargo of extracellular vesicles could not only contribute to the pathogenesis of thrombotic and thromboembolic complications of COVID-19 but most likely also be a diagnostic/prognostic marker. We recently identified a significant association linking endothelial exosomal miR-24 and cerebrovascular disorders, indicating that this approach could be an extremely useful tool for diagnosis and prognosis (Gambardella et al., 2021). Herein, we aim at identifying specific circulating miRNAs associated with thromboembolic events in patients with COVID-19.
Materials and Methods
To test our hypothesis that exosomal miRNAs are a major determinant of thrombosis in COVID-19, we enrolled 26 patients positive for COVID-19 admitted to the Sant’Anna and San Sebastiano Hospital of Caserta and Naples University (Italy). The serum from 10 non-COVID-19 subjects was used as control. All subjects underwent a SARS-CoV-2 test by reverse-transcription polymerase chain reaction to rule out or confirm the COVID-19 diagnosis. The study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments; we obtained a written informed consent from all participants or their legal representatives.
We isolated circulating exosomes from equal amounts of serum, as previously reported by our research group (Gambardella et al., 2021). Purity and absence of contamination were assessed by immunoblot (Wang et al., 2020b), whereas morphology and size distribution were examined by dynamic light scattering and electron microscopy (not shown). Levels of miRNAs were quantified by reverse-transcription polymerase chain reaction (Morelli et al., 2019; Wang et al., 2020b; Gambardella et al., 2021).
In Vitro Experiments
We performed in vitro assays in human umbilical vein endothelial cells (HUVECs), cultured in F12 medium enriched with specific growth factors for endothelial growth (Lonza). The cells were cultured at 37°C in 95% air and 5% CO2. All experiments were performed at least in triplicate using cells between passages 5 and 9. The experimental protocol on HUVECs consisted of 24 h incubation with serum from patients with COVID-19 or from patients negative for SARS-COV-2 as control. The serum was used with a final dilution of 1:50 directly in the medium.
Angiogenesis Assay
The formation of network-like structures by HUVECs on extracellular matrix-like 3D gel was performed as previously described (Santulli et al., 2011; Gambardella et al., 2018). HUVECs (5 × 104) were seeded on Matrigel Matrix and incubated at 37°C for 24 h, in presence of COVID-19 serum or control serum.
Lipid Peroxidation Assay
The level of malondialdehyde was measured by using a lipid peroxidation assay Kit (#ab118970, Abcam, Cambridge, UK), as we previously described (Tang et al., 2021).
Immunoblot Analysis
Total lysates were prepared as we described (Sorriento et al., 2009). Immunoblot analyses were performed as previously reported (Wang et al., 2020b). Briefly, lysates were electrophoresed by SDS-PAGE and transferred to nitrocellulose. Angiotensin converting enzyme 2, transmembrane protease serine 2, CD-147, cleaved caspase 3, and glyceraldehyde-3-phosphate dehydrogenase were visualized by specific antibodies (Cell Signaling Technology, Danvers, MA), whereas fluorochrome-conjugated anti-rabbit and anti-mouse were used as secondary antibodies (LI-COR, Lincoln, NE). The nitrocellulose membrane with fluorescent signal was scanned using the LI-COR imaging system, as we described (Matarese et al., 2020a; Dridi et al., 2022).
Statistical Analysis
Data are expressed as mean ± S.E. All data were analyzed using GraphPad Prism version 9 (GraphPad by Dotmatics, Boston, MA) with a significant difference established at a P value < 0.05. The normal distribution of values was verified by the Shapiro-Wilk test; the Student’s 2-tailed t test was applied to compare values between groups. The correlation between miRNA and D-dimer levels was determined by Pearson's correlation analysis.
Results
miR-145 and miR-885 Downregulation was Associated with Thrombotic Risk and Mortality in Patients with COVID-19
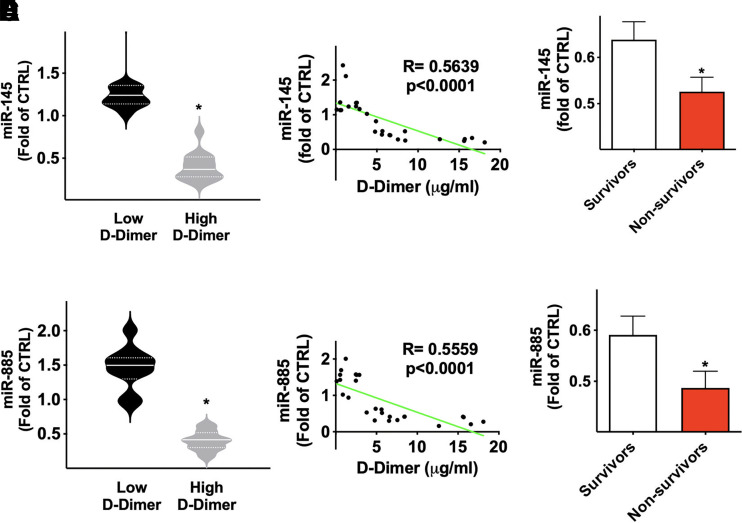
To verify our hypothesis that exosomal miRNAs play a crucial role in the pathogenesis of thrombosis in COVID-19, we divided our population in two groups based on the serum D-dimer level on hospital admission, using a cut-off of 3 μg/ml. We did not detect any significant difference in the main clinical characteristics when comparing patients with low versus high D-dimer. Strikingly, we found that exosomal miR-145 and miR-885 were significantly downregulated in the subjects in the high D-dimer arm compared with subjects in the low D-dimer arm (Fig. 1, A and B).
Fig. 1.
miR-145 and miR-885 are associated with a higher risk of thrombosis and mortality in patients with COVID-19. Expression level of exosomal miRNAs in patients with COVID-19. Different levels of miRNAs (miR-145, miR-885) in patients with high D-dimer and low D-dimer (A–B); in the violin plots, median (solid line) and quartiles (dotted lines) are indicated. The trend was confirmed by linear regression analysis (C–D). In panels E and F, miRNAs levels are quantified as mean ± S.E. among survivors and non survivors (E–F); *P < 0.001.
The Pearson’s correlation analysis confirmed these findings (Fig. 1, C and D). Furthermore, when we dichotomized our COVID-19 population in survivors and non-survivors, we noted that the levels of both miR-145 and miR-885 were significantly lower in the patients who did not survive (Fig. 1, E and F). These data indicate that the downregulation of miR-145 and miR-885 is associated to worst prognosis, correlating with a higher thrombotic risk and mortality in COVID-19. It is noteworthy that miRNAs were assessed on hospital admission; hence, their alterations already at time 0 denote miR-145 and miR-885 as powerful prognostic predictors of adverse outcome.
Endothelial Dysfunction as Responsible for miR-145 and miR-885 Downregulation
Endothelial cells are leading actors in producing and releasing tissue factor (TF) and von Willebrand Factor (vWF), thus regulating the thrombotic cascade. We hypothesized that the endothelial dysfunction occurring during SARS-CoV-2 infection determines a dysregulation of this mechanism, reducing the capability of endothelial cells to release miR-145 and miR-885, eventually resulting in an uncontrolled coagulation. To test this hypothesis we employed human endothelial cells as an in vitro model and exposed them to serum from patients with COVID-19, compared with cells exposed to serum from patients negative for SARS-CoV-2.
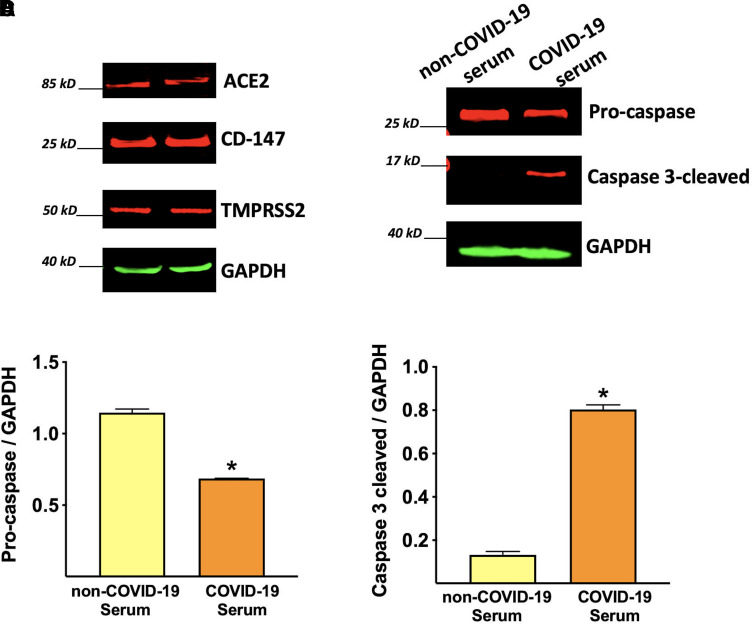
Importantly, HUVECs expressed all the major SARS-CoV-2 receptors, including angiotensin converting enzyme 2, transmembrane protease serine 2, and CD-147 (Matarese et al., 2020b; Wang et al., 2020a; Zipeto et al., 2020; Evans and Liu, 2021), providing further evidence of the susceptibility of endothelial cells to SARS-CoV-2 infection (Fig. 2A). This expression pattern of viral receptors also confirms that our in vitro setting is a valuable model to study endothelial response and damage in COVID-19.
Fig. 2.
Endothelial cell damage induced by COVID-19 environment. Representative immunoblots showing the expression of the main viral receptors on human endothelial cells (A). The exposure of HUVECs to COVID-19 serum induces the cleavage of caspase 3, indicating apoptosis activation (B), quantified in panels C and D. Data are from triplicate experiments; *P < 0.001.
The exposure to COVID-19 serum induces apoptosis of endothelial cells, as indicated by caspase 3 activation (Fig. 2, B–D), supporting the hypothesis of a significant endothelial damage.
We further explored the endothelial dysfunction evaluating a key feature of viable endothelial cells, angiogenic competence. Consistently, we observed an impaired capacity of forming network-like structures on Matrigel, indicating that COVID-19 serum exposure compromises the angiogenic capacity of endothelial cells (Fig. 3, A and B).
Fig. 3.
Endothelial cell angiogenic capacity is impaired in COVID-19. Angiogenesis assay on Matrigel. COVID-19 serum significantly affects the angiogenic capacity of endothelial cells. Representative pictures of network-like formation (dimensional bar: 100 μm) from independent triplicate experiments (A) and relative quantification (B); *P < 0.001.
To assess the effects of COVID-19 on lipid peroxidation, an established hallmark of severity in patients with COVID-19 (Martín-Fernández et al., 2021; Žarković et al., 2021; Lage et al., 2022; Soto et al., 2022), we measured malondialdehyde level in HUVEC lysate, and we observed a significantly increased peroxidation in the lysate of endothelial cells incubated for 24 hours with COVID-19 serum compared to cells incubated with non-COVID-19 serum (Fig. 4).
Fig. 4.
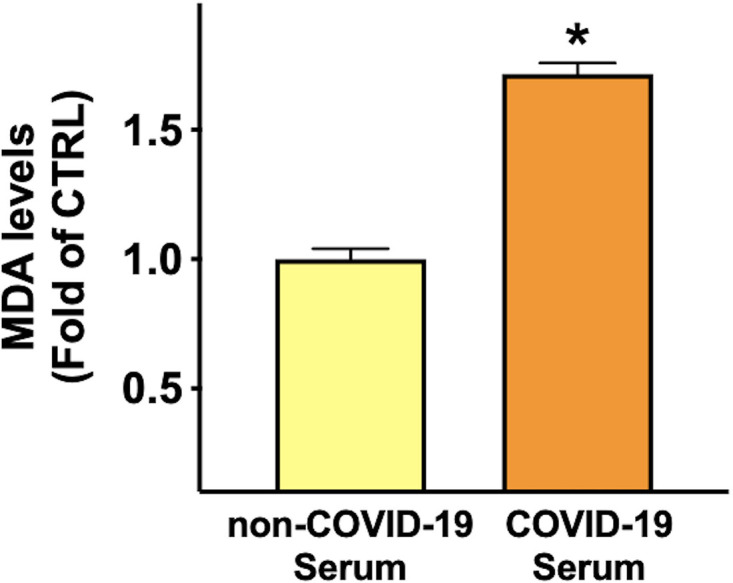
COVID-19 environment induces lipid peroxidation. We evaluated lipid peroxidation by measuring the level of malondialdehyde (MDA) in HUVEC lysate. Data are from triplicate experiments; *P < 0.001.
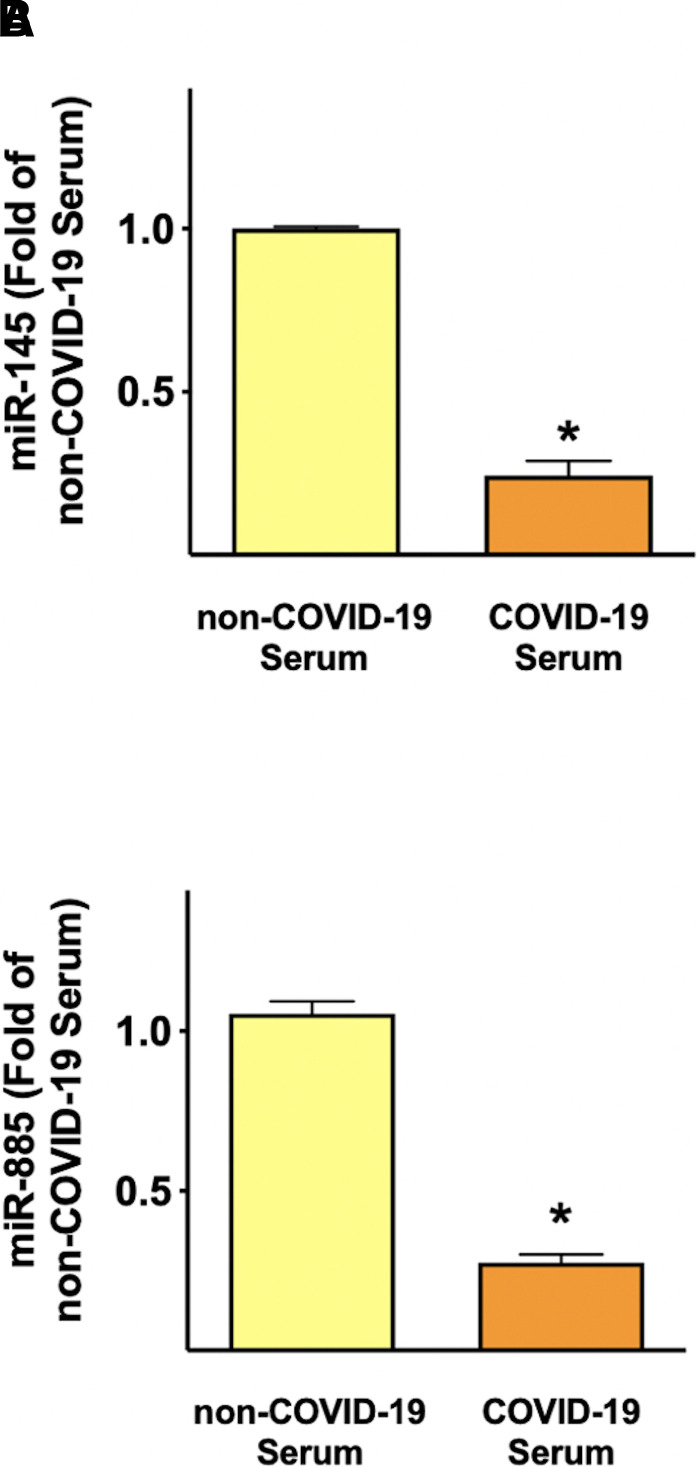
Then, we verified whether human endothelial cells were able to produce miR-145 and miR-885 and whether such production was regulated by COVID-19. We detected both miRNAs in the HUVEC lysate; interestingly, the levels of both miRNAs were reduced in response to COVID-19 serum exposure (Fig. 5, A and B). This phenomenon confirms that endothelial cells are able to actively release these miRNAs and that stress conditions can affect miR-145 and miR-885 production by endothelial cells.
Fig. 5.
Endothelial cells produce miR-145 and miR-885, and COVID-19 serum affects their levels. Quantification of miR-145 and miR-885 in HUVEC lysate, after incubation with the indicated sera for 24 h. Data are from triplicate experiments; *P < 0.001.
Discussion
One of the main findings of the present study is the identification of two miRNAs, miR-145 and miR-885, as potential predictors of thrombotic risk in patients with COVID-19. Indeed, miR-145 and miR-885 are significantly downregulated in COVID-19 patients with elevated circulating levels of D-dimer, and our correlation analysis confirms that miR-145 and miR-885 inversely correlate with D-dimer. Moreover, the downregulation of these miRNAs predicts mortality in patients with COVID-19. Indeed, the group of patients that died for COVID-19 exhibited lower levels of both miR-145 and miR-885 at baseline. These data unveil the clinically relevant predictor value of these miRNAs, also suggesting a causal implication of these molecules in determining higher thrombotic risk and mortality in patients with COVID-19. Consistently, these two miRNAs are implicated in the coagulation pathway; indeed, tissue factor (TF) has been identified as a direct target of miR-145 (Sahu et al., 2017), whereas miR-885 targets the von Willebrand Factor (vWF) (Zhang et al., 2019). In agreement with our findings, the downregulation of these miRNAs should promote higher levels of TF and vWF, thus evoking a prothrombotic state. By modulating these two factors, endothelial cells can regulate the activation of the coagulation cascade. One limitation of our study is that we did not determine the exact source of exosomes in our population; nevertheless, since endothelial dysfunction is a prominent feature of COVID-19, functionally contributing to the proinflammatory and prothrombotic state of the vasculature (Bikdeli et al., 2020), we speculate that at least one of the main sources of exosomes could be represented by endothelial cells, which indeed do express these miRNAs in normal conditions (Santulli, 2016). To test this hypothesis, we performed in vitro experiments in human endothelial cells. Specifically, we set an in vitro model in which we verified the expression of cofactors needed for the internalization of SARS-CoV-2 in host cells, and we explored endothelial stress responses to a “COVID-like environment” represented by serum from patients with COVID-19. We found that COVID-19 serum (collected on hospital admission) induces endothelial damage, including cell apoptosis and alterations of a specialized endothelial feature like angiogenic capacity. These findings are in line with numerous reports from us and others supporting the endothelial involvement in COVID-19 clinical manifestations (Libby and Lüscher, 2020; Gu et al., 2021; Teuwen et al., 2020; Fiorentino et al., 2021; Gambardella and Santulli, 2021; Mesquida et al., 2021; Mone et al., 2021; Perea Polak et al., 2021; Qin et al., 2021; Schmaier et al., 2021; Yin et al., 2021; Kelliher et al., 2022; Mone et al., 2022; Otifi and Adiga, 2022; Robles et al., 2022).
One of the main new findings unveiled here is that the dysfunctional endothelium could participate in thrombotic manifestations by changing its miRNA profile. As proposed here, endothelial cells physiologically produce miR-145 and miR-885 to target vWF and TF, blocking their release and controlling coagulation. Indeed, we observed that endothelial cells are able to produce these miRNAs, and under stress conditions (i.e., when exposed to COVID-19 serum), their levels significantly decrease. Consistently, in patients in which these adaptive mechanisms are compromised, the resultant lower availability of miR-145 and miR-885 predisposes to a detrimental prothrombotic status, denoted by high D-dimer, and eventually high mortality. Our data in COVID-19 patients combined with in vitro experiments are highly suggestive of this view. Our report further exposes the endothelium as a central player in orchestrating the adaptative and maladaptive response to SARS-CoV-2 infection, acting as a main trigger of thrombotic manifestations.
Consistent with these findings, the importance of miRs and other noncoding RNAs in the management of patients with COVID-19 has been emphasized by several investigators (Amini-Farsani et al., 2021; Battaglia et al., 2021; Dash et al., 2021; Farr et al., 2021; Lukiw, 2021; Narożna and Rubiś, 2021; Plowman and Lagos, 2021; Saha et al., 2021).
Our data can be also useful for the management and treatment of Long COVID; in fact, the so-called “Long COVID-19 Syndrome”, indicating the set of disorders and clinical manifestations that remain or appear de novo months after COVID-19 infection and SARS-CoV-2 negativization, is emerging as the next challenge in biomedical research (Yan et al., 2021; Antoniou et al., 2022; Cattadori et al., 2022; Desai et al., 2022; Martínez-Salazar et al., 2022; Murray et al., 2022; Oikonomou et al., 2022; Thye et al., 2022). Henceforward, the deep understanding of the molecular mechanisms underlying the major complications of COVID-19 will allow us to intervene and thwart Long COVID.
Acknowledgments
The authors thank Dr. Varzideh for insightful discussion.
Abbreviations
- COVID-19
coronavirus disease 2019
- HUVEC
human umbilical vein endothelial cell
- miRNA
microRNA
- TF
tissue factor
- vWF
von Willebrand factor
Authorship Contributions
Participated in research design: Gambardella, Santulli.
Conducted experiments: Gambardella, Kansakar, Messina, Jankauskas, Marfella, Maggi, Mone, Paolisso, Sorriento.
Contributed new reagents or analytic tools: Gambardella, Kansakar, Messina, Jankauskas, Marfella, Maggi, Mone, Paolisso, Sorriento.
Performed data analysis: Gambardella, Kansakar, Santulli.
Wrote or contributed to the writing of the manuscript: Gambardella, Kansakar, Sorriento, Santulli.
Footnotes
The Santulli’s Laboratory is supported in part by National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [Grant R01-DK123259] and [Grant R01-DK033823] and National Heart, Lung, and Blood Institute (NHLBI) [Grant R01-HL159062], [Grant R01-HL146691], and [Grant T32-HL144456] (to G.S.); the Diabetes Action Research and Education Foundation (to G.S.); and the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). J.G. and S.S.J. are supported by a postdoctoral fellowship of the American Heart Association [AHA-20POST35211151] and [AHA-21POST836407], respectively.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Adebayo AVarzideh FWilson SGambardella JEacobacci MJankauskas SSDonkor KKansakar UTrimarco VMone P, et al. (2021) l-Arginine and COVID-19: an update. Nutrients 13:3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini-Farsani Z, Yadollahi-Farsani M, Arab S, Forouzanfar F, Yadollahi M, Asgharzade S (2021) Prediction and analysis of microRNAs involved in COVID-19 inflammatory processes associated with the NF-kB and JAK/STAT signaling pathways. Int Immunopharmacol 100:108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou KMVasarmidi ERussell AMAndrejak CCrestani BDelcroix MDinh-Xuan ATPoletti VSverzellati NVitacca M, et al. (2022) European respiratory society statement on long COVID-19 follow-up. Eur Respir J DOI: 10.1183/13993003.02174-2021 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Agwu JC, Barlow N, Lee B (2021) Hypertension is the major predictor of poor outcomes among inpatients with COVID-19 infection in the UK: a retrospective cohort study. BMJ Open 11:e047561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia RAlonzo RPennisi CCaponnetto AFerrara CStella MBarbagallo CBarbagallo DRagusa MPurrello M, et al. (2021) MicroRNA-mediated regulation of the virus cycle and pathogenesis in the SARS-CoV-2 disease. Int J Mol Sci 22:13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli BMadhavan MVJimenez DChuich TDreyfus IDriggin ENigoghossian CAgeno WMadjid MGuo Y, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function (2020) COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çalica Utku A, Budak G, Karabay O, Güçlü E, Okan HD, Vatan A (2020) Main symptoms in patients presenting in the COVID-19 period. Scott Med J 65:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattadori G, Di Marco S, Baravelli M, Picozzi A, Ambrosio G (2022) Exercise training in post-COVID-19 patients: the need for a multifactorial protocol for a multifactorial pathophysiology. J Clin Med 11:2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho O, Kim DW, Cheong JY (2021) Plasma exosomal miRNA levels after radiotherapy are associated with early progression and metastasis of cervical cancer: a pilot study. J Clin Med 10:2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli AViero GBettini GNobili ATettamanti MGalbussera AAMuscatello AMantero MCanetta CMartinelli Boneschi F, et al. (2022) Patient-reported symptoms and sequelae 12 months after COVID-19 in hospitalized adults: a multicenter long-term follow-up study. Front Med (Lausanne) 9:834354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte G, Cei M, Evangelista I, Colombo A, Vitale J, Mazzone A, Mumoli N (2021) The meaning of D-dimer value in Covid-19. Clin Appl Thromb Hemost 27:10760296211017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook H, Raza S, Nowell J, Young M, Edison P (2021) Long covid-mechanisms, risk factors, and management. BMJ 374:n1648. [DOI] [PubMed] [Google Scholar]
- Cryer MJ, Farhan S, Kaufmann CC, Jäger B, Garg A, Krishnan P, Mehran R, Huber K (2022) Prothrombotic milieu, thrombotic events and prophylactic anticoagulation in hospitalized COVID-19 positive patients: a review. Clin Appl Thromb Hemost 28:10760296221074353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S, Dash C, Pandhare J (2021) Therapeutic significance of microRNA-mediated regulation of PARP-1 in SARS-CoV-2 infection. Noncoding RNA 7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davido B, Seang S, Tubiana R, de Truchis P (2020) Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect 26:1448–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AD, Lavelle M, Boursiquot BC, Wan EY (2022) Long-term complications of COVID-19. Am J Physiol Cell Physiol 322:C1–C11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dridi HSantulli GGambardella JJankauskas SSYuan QYang JReiken SWang XWronska ALiu X, et al. (2022) IP3 receptor orchestrates maladaptive vascular responses in heart failure. J Clin Invest 132:e152859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP, Liu SL (2021) Role of host factors in SARS-CoV-2 entry. J Biol Chem 297:100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr RJRootes CLRowntree LCNguyen THOHensen LKedzierski LCheng ACKedzierska KAu GGMarsh GA, et al. (2021) Altered microRNA expression in COVID-19 patients enables identification of SARS-CoV-2 infection. PLoS Pathog 17:e1009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino G, Coppola A, Izzo R, Annunziata A, Bernardo M, Lombardi A, Trimarco V, Santulli G, Trimarco B (2021) Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine 40:101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Coppola A, Izzo R, Fiorentino G, Trimarco B, Santulli G (2021) Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit Care 25:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, De Rosa M, Sorriento D, Prevete N, Fiordelisi A, Ciccarelli M, Trimarco B, De Luca N, Iaccarino G (2018) Parathyroid hormone causes endothelial dysfunction by inducing mitochondrial ROS and specific oxidative signal transduction modifications. Oxid Med Cell Longev 2018:9582319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V (2020) Arginine and endothelial function. Biomedicines 8:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Santulli G (2021) What is linking COVID-19 and endothelial dysfunction? Updates on nanomedicine and bioengineering from the 2020 AHA Scientific Sessions. Eur Heart J Cardiovasc Pharmacother 7:e2–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Ghosh K (2022) D-dimer: an analyte with increasing application in Covid-19 infection. Expert Rev Hematol 15:243–251. [DOI] [PubMed] [Google Scholar]
- Gu SXTyagi TJain KGu VWLee SHHwa JMKwan JMKrause DSLee AIHalene S, et al. (2021) Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol 18:194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TWang YJia JMao XStankiewicz EScandura GBurke EXu LMarzec JDavies CR, et al. (2021) The identification of plasma exosomal miR-423-3p as a potential predictive biomarker for prostate cancer castration-resistance development by plasma exosomal miRNA sequencing. Front Cell Dev Biol 8:602493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AGonzalez-Rojas YJuarez ECrespo Casal MMoya JRodrigues Falci DSarkis ESolis JZheng HScott N, et al. ; COMET-ICE Investigators (2022) Effect of Sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 327:1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Park S, Chen Y, Lee H (2021) Extracellular vesicle-associated miRNAs as a biomarker for lung cancer in liquid biopsy. Front Mol Biosci 8:630718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci G (2022) Covid-19: UK adds sore throat, headache, fatigue, and six other symptoms to official list. BMJ 377:o892. [DOI] [PubMed] [Google Scholar]
- Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A (2021) Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine 36:100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliszewski KDiakowska DNowak ŁTokarczyk USroczyński MSępek MDudek ASutkowska-Stępień KKiliś-Pstrusińska KMatera-Witkiewicz A, et al. (2022) Assessment of gastrointestinal symptoms and dyspnea in patients hospitalized due to COVID-19: contribution to clinical course and mortality. J Clin Med 11:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur SP, Gupta V (2020) COVID-19 vaccine: a comprehensive status report. Virus Res 288:198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher SWeiss LCullivan SO’Rourke EMurphy CAToolan SLennon ÁSzklanna PBComer SPMacleod H, et al. (2022) Non-severe COVID-19 is associated with endothelial damage and hypercoagulability despite pharmacological thromboprophylaxis. J Thromb Haemost 20:1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage SLAmaral EPHilligan KLLaidlaw ERupert ANamasivayan SRocco JGalindo FKellogg AKumar P, et al. (2022) Persistent oxidative stress and inflammasome activation in CD14highCD16- monocytes from COVID-19 patients. Front Immunol 12:799558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B (2020) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 5:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Aikawa M, Jain MK (2006) Vascular endothelium and atherosclerosis. Handb Exp Pharmacol 176 Pt 2:285–306. [DOI] [PubMed] [Google Scholar]
- Libby P, Lüscher T (2020) COVID-19 is, in the end, an endothelial disease. Eur Heart J 41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Wong A, Alam S (2022) Extraction of exosomes and exosomal miRNA from mesenchymal stem cells. Methods Mol Biol 2455:333–341. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ (2021) microRNA heterogeneity, innate-immune defense and the efficacy of SARS-CoV-2 infection-a commentary. Noncoding RNA 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu L, Li H, Chen C, Yu Y, Wang L, Yin S, Hu Y, Jiang S, Ye F, Zhou P (2022) Exosomal miRNA profiling is a potential screening route for non-functional pituitary adenoma. Front Cell Dev Biol 9:771354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi A, Butler AE, Jamialahmadi T, Sahebkar A (2022) The role of exosomal miRNA in nonalcoholic fatty liver disease. J Cell Physiol 237:2078–2094. [DOI] [PubMed] [Google Scholar]
- Martín-Fernández MAller RHeredia-Rodríguez MGómez-Sánchez EMartínez-Paz PGonzalo-Benito HSánchez-de Prada LGorgojo ÓCarnicero-Frutos ITamayo E, et al. (2021) Lipid peroxidation as a hallmark of severity in COVID-19 patients. Redox Biol 48:102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Salazar B, Holwerda M, Stüdle C, Piragyte I, Mercader N, Engelhardt B, Rieben R, Döring Y (2022) COVID-19 and the vasculature: current aspects and long-term consequences. Front Cell Dev Biol 10:824851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese A, Gambardella J, Lombardi A, Wang X, Santulli G (2020a) miR-7 regulates GLP-1-mediated insulin release by targeting β-Arrestin 1. Cells 9:1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese A, Gambardella J, Sardu C, Santulli G (2020b) miR-98 regulates TMPRSS2 expression in human endothelial cells: key implications for COVID-19. Biomedicines 8:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquida JCaballer ACortese LVila CKaradeniz UPagliazzi MZanoletti MPacheco APCastro PGarcía-de-Acilu M, et al. ; HEMOCOVID-19 Consortium (2021) Peripheral microcirculatory alterations are associated with the severity of acute respiratory distress syndrome in COVID-19 patients admitted to intermediate respiratory and intensive care units. Crit Care 25:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelen MManoharan LElkheir NCheng VDagens AHastie CO’Hara MSuett JDahmash DBugaeva P, et al. (2021) Characterising long COVID: a living systematic review. BMJ Glob Health 6:e005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Gambardella J, Wang X, Jankauskas SS, Matarese A, Santulli G (2021) miR-24 targets the transmembrane glycoprotein neuropilin-1 in human brain microvascular endothelial cells. Noncoding RNA 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Izzo R, Marazzi G, Manzi MV, Gallo P, Campolongo G, Cacciotti L, Tartaglia D, Caminiti G, Varzideh F, Santulli G, Trimarco V (2022) L-Arginine enhances the effects of cardiac rehabilitation on physical performance: new insights for managing cardiovascular patients during the COVID-19 pandemic. J Pharmacol Exp Ther 381:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli MB, Shu J, Sardu C, Matarese A, Santulli G (2019) Cardiosomal microRNAs are essential in post-infarction myofibroblast phenoconversion. Int J Mol Sci 21:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Pérez OMerino ELeon-Ramirez JMAndres MRamos JMArenas-Jiménez JAsensio SSanchez RRuiz-Torregrosa PGalan I, et al. ; COVID19-ALC research group (2021) Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect 82:378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui LW, Lau JF, Lee HK (2021) Thromboembolic complications of COVID-19. Emerg Radiol 28:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EGoodfellow HBindman JBlandford ABradbury KChaudhry TFernandez-Reyes DGomes MHamilton FLHeightman M, et al. (2022) Development, deployment and evaluation of digitally enabled, remote, supported rehabilitation for people with long COVID-19 (Living With COVID-19 Recovery): protocol for a mixed-methods study. BMJ Open 12:e057408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Närhi FMoonesinghe SRShenkin SDDrake TMMulholland RHDonegan CDunning JFairfield CJGirvan MHardwick HE, et al. ; ISARIC4C investigators (2022) Implementation of corticosteroids in treatment of COVID-19 in the ISARIC WHO Clinical Characterisation Protocol UK: prospective, cohort study. Lancet Digit Health 4:e220–e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narożna M, Rubiś B (2021) Anti-SARS-CoV-2 strategies and the potential role of miRNA in the assessment of COVID-19 morbidity, recurrence, and therapy. Int J Mol Sci 22:8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning W, Li S, Yang W, Yang B, Xin C, Ping X, Huang C, Gu Y, Guo L (2021) Blocking exosomal miRNA-153-3p derived from bone marrow mesenchymal stem cells ameliorates hypoxia-induced myocardial and microvascular damage by targeting the ANGPT1-mediated VEGF/PI3k/Akt/eNOS pathway. Cell Signal 77:109812. [DOI] [PubMed] [Google Scholar]
- Oikonomou ESouvaliotis NLampsas SSiasos GPoulakou GTheofilis PPapaioannou TGHaidich ABTsaousi GNtousopoulos V, et al. (2022) Endothelial dysfunction in acute and long standing COVID-19: a prospective cohort study. Vascul Pharmacol 144:106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otifi HM, Adiga BK (2022) Endothelial dysfunction in Covid-19 infection. Am J Med Sci 363:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen M, Yilmaz A, Cakmak V, Beyoglu R, Oskay A, Seyit M, Senol H (2021) D-dimer as a potential biomarker for disease severity in COVID-19. Am J Emerg Med 40:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea Polak A, Romero Madrid B, PP, Alvarez G, Pilar L, -Moyano E (2021) Complement-mediated thrombogenic vasculopathy in COVID-19. Int J Dermatol 60:229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman T, Lagos D (2021) Non-coding RNAs in COVID-19: emerging insights and current questions. Noncoding RNA 7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel A, Poudel Y, Adhikari A, Aryal BB, Dangol D, Bajracharya T, Maharjan A, Gautam R (2021) D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS One 16:e0256744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin ZLiu FBlair RWang CYang HMudd JCurrey JMIwanaga NHe JMi R, et al. (2021) Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics 11:8076–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robineau OWiernik ELemogne Cde Lamballerie XNinove LBlanché HDeleuze JFRibet CKab SGoldberg M, et al. (2022) Persistent symptoms after the first wave of COVID-19 in relation to SARS-CoV-2 serology and experience of acute symptoms: a nested survey in a population-based cohort. Lancet Reg Health Eur 17:100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles JP, Zamora M, Adan-Castro E, Siqueiros-Marquez L, Martinez de la Escalera G, Clapp C (2022) The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling. J Biol Chem 298:101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami M, Mansouritorghabeh H (2020) D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol 13:1265–1275. [DOI] [PubMed] [Google Scholar]
- Saha C, Laha S, Chatterjee R, Bhattacharyya NP (2021) Co-regulation of protein coding genes by transcription factor and long non-coding RNA in SARS-CoV-2 infected cells: an in silico analysis. Noncoding RNA 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu AJha PKPrabhakar ASingh HDGupta NChatterjee TTyagi TSharma SKumari BSingh S, et al. (2017) MicroRNA-145 impedes thrombus formation via targeting tissue factor in venous thrombosis. EBioMedicine 26:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G (2016) MicroRNA: From Molecular Biology to Clinical Practice, Springer Nature, New York. [Google Scholar]
- Santulli GBasilicata MFDe Simone MDel Giudice CAnastasio ASorriento DSaviano MDel Gatto ATrimarco BPedone C, et al. (2011) Evaluation of the anti-angiogenic properties of the new selective αVβ3 integrin antagonist RGDechiHCit. J Transl Med 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G (2020) Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? a comprehensive evaluation of clinical and basic evidence. J Clin Med 9:1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaier AAPajares Hurtado GMManickas-Hill ZJSack KDChen SMBhambhani VQuadir JNath AKCollier AYNgo D, et al. ; MGH COVID-19 Collection and Processing Team (2021) Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19. JCI Insight 6:e151527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MP (2022) Estimating total morbidity burden of COVID-19: relative importance of death and disability. J Clin Epidemiol 142:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorriento D, Campanile A, Santulli G, Leggiero E, Pastore L, Trimarco B, Iaccarino G (2009) A new synthetic protein, TAT-RH, inhibits tumor growth through the regulation of NFkappaB activity. Mol Cancer 8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto ME, Guarner-Lans V, Díaz-Díaz E, Manzano-Pech L, Palacios-Chavarría A, Valdez-Vázquez RR, Aisa-Álvarez A, Saucedo-Orozco H, Pérez-Torres I (2022) Hyperglycemia and loss of redox homeostasis in COVID-19 patients. Cells 11:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi C, Fallani S, Voller F, Silvestri C (2020) Treatment for COVID-19: an overview. Eur J Pharmacol 889:173644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XH, Gambardella J, Jankauskas S, Wang X, Santulli G, Gudas LJ, Levi R (2021) A retinoic acid receptor β2 agonist improves cardiac function in a heart failure model. J Pharmacol Exp Ther 379:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuwen LA, Geldhof V, Pasut A, Carmeliet P (2020) COVID-19: the vasculature unleashed. Nat Rev Immunol 20:389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thye AY, Law JW, Tan LT, Pusparajah P, Ser HL, Thurairajasingam S, Letchumanan V, Lee LH (2022) Psychological symptoms in COVID-19 patients: insights into pathophysiology and risk factors of long COVID-19. Biology (Basel) 11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning JS, Brown ES, Cheeseman HM, Flight KE, Higham SL, Lemm NM, Pierce BF, Stirling DC, Wang Z, Pollock KM (2020) Vaccines for COVID-19. Clin Exp Immunol 202:162–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta E, Tsutsumi K, Kato H, Matsushita H, Shiraha H, Fujii M, Matsumoto K, Horiguchi S, Okada H (2021) Extracellular vesicle-shuttled miRNAs as a diagnostic and prognostic biomarker and their potential roles in gallbladder cancer patients. Sci Rep 11:12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulinici M, Covantev S, Wingfield-Digby J, Beloukas A, Mathioudakis AG, Corlateanu A (2021) Screening, diagnostic and prognostic tests for COVID-19: a comprehensive review. Life (Basel) 11:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Levi M, Hunt BJ (2022) Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir Med 10:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DHuang TRen TLiu QZhou ZGe LChen ZLiu JNie HMa W, et al. (2022) Identification of blood exosomal miRNA-1246, miRNA-150-5p, miRNA-5787 and miRNA-8069 as sensitive biomarkers for hepatitis B virus infection. Clin Lab 68. [DOI] [PubMed] [Google Scholar]
- Wang KChen WZhang ZDeng YLian JQDu PWei DZhang YSun XXGong L, et al. (2020a) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Morelli MB, Matarese A, Sardu C, Santulli G (2020b) Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo. ESC Heart Fail 7:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody S, Arina P, Glenister J, Cottrell S, Boscaini-Gilroy G, Singer M, Montgomery HE (2022) Non-invasive respiratory support in the management of acute COVID-19 pneumonia: considerations for clinical practice and priorities for research. Lancet Respir Med 10:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M, Elliott J, Chadeau-Hyam M, Riley S, Darzi A, Cooke G, Ward H, Elliott P (2022) Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat Commun 13:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KK, Thiagarajan P (1996) Role of endothelium in thrombosis and hemostasis. Annu Rev Med 47:315–331. [DOI] [PubMed] [Google Scholar]
- Yan Z, Yang M, Lai CL (2021) Long COVID-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines 9:966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Liu F, Liu W, Cao G, Liu J, Huang S, Zhu M, Tu C, Wang J, Xiong B (2021) Myocardial injury and risk factors for mortality in patients with COVID-19 pneumonia. Int J Cardiol 326:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Wang S, Liu Y, Chen J, Li D, Xu T (2021) Coronary microvascular dysfunction pathophysiology in COVID-19. Microcirculation 28:e12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Gao H, Dos Reis FCG, Bandyopadhyay G, Ofrecio JM, Luo Z, Ji Y, Jin Z, Ly C, Olefsky JM (2021) MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab 33:781–790.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žarković NOrehovec BMilković LBaršić BTatzber FWonisch WTarle MKmet MMataić AJakovčević A, et al. (2021) Preliminary findings on the association of the lipid peroxidation product 4-hydroxynonenal with the lethal outcome of aggressive COVID-19. Antioxidants 10:1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DCai GLiu KZhuang ZJia KPei SWang XWang HXu SCui C, et al. (2021a) Microglia exosomal miRNA-137 attenuates ischemic brain injury through targeting Notch1. Aging (Albany NY) 13:4079–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z (2020) D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 18:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Guo J, Gu J, Wang Z, Wang G, Li H, Wang J (2019) Identifying the key genes and microRNAs in colorectal cancer liver metastasis by bioinformatics analysis and in vitro experiments. Oncol Rep 41:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XBHu LMing QWei XJZhang ZYChen LDWang MHYao WZHuang QFYe ZQ, et al. (2021b) Risk factors for mortality of coronavirus disease-2019 (COVID-19) patients in two centers of Hubei province, China: a retrospective analysis. PLoS One 16:e0246030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipeto D, Palmeira JDF, Argañaraz GA, Argañaraz ER (2020) ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front Immunol 11:576745. [DOI] [PMC free article] [PubMed] [Google Scholar]