Abstract
Oxidative stress is a key driver in the development and progression of several diseases, including metabolic associated fatty liver disease (MAFLD). This condition includes a wide spectrum of pathological injuries, extending from simple steatosis to inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma. Excessive buildup of lipids in the liver is strictly related to oxidative stress in MAFLD, progressing to liver fibrosis and cirrhosis. The nuclear factor erythroid 2-related factor 2 (NRF2) is a master regulator of redox homeostasis. NRF2 plays an important role for cellular protection by inducing the expression of genes related to antioxidant, anti-inflammatory, and cytoprotective response. Consistent evidence demonstrates that NRF2 is involved in every step of MAFLD deve-lopment, from simple steatosis to inflammation, advanced fibrosis, and ini-tiation/progression of hepatocellular carcinoma. NRF2 activators regulate lipid metabolism and oxidative stress alleviating the fatty liver disease by inducing the expression of cytoprotective genes. Thus, modulating NRF2 activation is crucial not only in understanding specific mechanisms underlying MAFLD progression but also to characterize effective therapeutic strategies. This review outlined the current knowledge on the effects of NRF2 pathway, modulators, and mechanisms involved in the therapeutic implications of liver steatosis, inflammation, and fibrosis in MAFLD.
Keywords: Nonalcoholic fatty liver disease, Metabolic-associated fatty liver disease, Nuclear factor erythroid 2-related factor 2, Oxidative stress, Antioxidants, Liver injury
Core Tip: This updated literature review contributes to the role of nuclear factor erythroid 2-related factor 2 in combating inflammation, oxidative stress, steatosis, and fibrosis in metabolic associated fatty liver disease. There are several reviews that elucidated the advantages of nuclear factor erythroid 2-related factor 2 in human diseases, but this is the first review reporting the broad range of nuclear factor erythroid 2-related factor 2 modulators and their therapeutic implications in metabolic associated fatty liver disease.
INTRODUCTION
Nonalcoholic fatty liver disease is the most frequent chronic liver disease, affecting about 25% of the global population. Due to the reappraisal in its nomenclature, a group of experts changed the terminology from nonalcoholic fatty liver disease to metabolic-associated fatty liver disease (MAFLD), strengthening the link of this disease to metabolic alterations[1]. MAFLD is defined as a condition where hepatic fat accumulation exceeds 5% of the liver weight without alcohol consumption (< 30 g per day). It covers a wide spectrum of pathological conditions, extending from simple steatosis (deposit of fat in hepatocytes) to nonalcoholic steatohepatitis (characterized by the presence of 5% hepatic steatosis and inflammation with hepatocellular damage, with or without fibrosis), cirrhosis, and ultimately leading to hepatocellular carcinoma[2]. MAFLD is emerging with the prevalence of type 2 diabetes mellitus, obesity, and metabolic syndrome[3]. Of note, patients with MAFLD, and particularly with nonalcoholic steatohepatitis, exhibit an increased liver-related mortality rate and higher incidence of cardiovascular-related morbidity and mortality[2].
MAFLD is the hepatic expression of metabolic syndrome, but its pathogenesis is still not clearly known. Insulin resistance (IR) seems to play a key role in the initiation and progression of the disease from simple fatty liver to advanced forms[4]. MAFLD pathogenesis is complex and multifactorial. The first theory was based on a two-hit hypothesis, where the first hit is liver steatosis, which is due to increased hepatic lipogenesis and reduced free fatty acid degradation caused by IR. This alteration is followed by the second hit of oxidative stress, which induces hepatocyte inflammation and cell death[5,6]. However, this simplistic theory has been recently replaced by the multiple hit hypothesis, where many factors including systemic and hepatic IR, intestinal microbiota, genetic predisposition, and oxidative stress act simultaneously resulting in a cascade of detrimental effects such as hepatic inflammation, free radical production from gut and adipose tissue, mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and hepatocyte apoptosis[7]. Among all the contributing factors of MAFLD, oxidative stress plays a major role. Oxidative stress promotes inflammation by activating Kupffer cells and stimulating the release of proinflammatory cytokines, directly leading to lipid, protein, and DNA/RNA damage. Nuclear factor erythroid 2-related factor 2 (NRF2) is the most important tra-nscription factor in preserving redox homeostasis in the cell and counteracting oxidative or electrophilic stress by producing antioxidant and cytoprotective enzymes such as heme oxygenase 1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), and those involved in glutathione (GSH) metabolism[8].
Thus, due to its antioxidative and detoxicant properties, it is currently accepted that NRF2 plays a pivotal role and has been recognized as a potential target to prevent the pathological spectrum of MAFLD. Even though the beneficial role of NRF2 in human diseases has been the topic of several recent reviews, the broad range of NRF2 modulators and their therapeutic implications in MAFLD were not completely summarized in recent literature. In this review, we described the current knowledge on the effects of NRF2-dependent mechanisms involved in the therapeutic implications of liver steatosis, inflammation, and fibrosis in MAFLD.
NRF2 PATHWAY
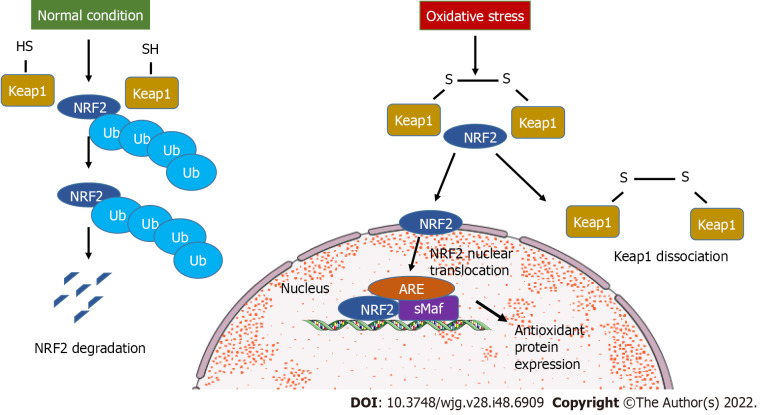
NRF2 belongs to the basic leucine zipper transcription factors in the Cap “n” Collar subfamily including seven functional domains, Nrf2-ECH homology (Neh) 1 to Neh7[9]. Neh2 is important for interaction between NRF2 and Kelch-like ECH-associated protein 1 (Keap1), a negative modulator of NRF2[10]. Keap1 is a substrate for Cullin based E3 ubiquitin ligase. During homeostatic conditions, Keap1 targets NRF2 that is localized in cytoplasm, causing its polyubiquitination and degradation. The binding and regulation of NRF2 by Keap1 has been defined as the “hinge and latch model”[11]. During oxidative stress, hyperactive cysteine residues of Keap1 undergo thiol modification and NRF2 is dissociated from Keap1, preventing ubiquitination and proteasomal degradation (Figure 1). The newly generated NRF2 escaped from Keap1 control translocates to the nucleus and heterodimerizes with the Maf proteins, promoting the expression of antioxidant response element genes like HO-1, superoxide dismutase (SOD), catalase, glutathione-S-transferase, glutathione reductase, glutathione peroxidase (GSH-Px), NQO1, etc[12].
Figure 1.
Kelch-like ECH-associated protein 1-dependent nuclear factor-erythroid 2-related factor 2 signaling. During oxidative stress, nuclear factor-erythroid 2-related factor 2 (NRF2) detaches from kelch-like ECH-associated protein 1 (Keap1) and translocates to the nucleus to bind the target genes. In normal conditions, NRF2 is ubiquitinylated and undergoes degradation. ARE: Antioxidant responsive element; SMaf: Small musculoaponeurotic fibrosarcoma oncogene homologue; Ub: Ubiquitin.
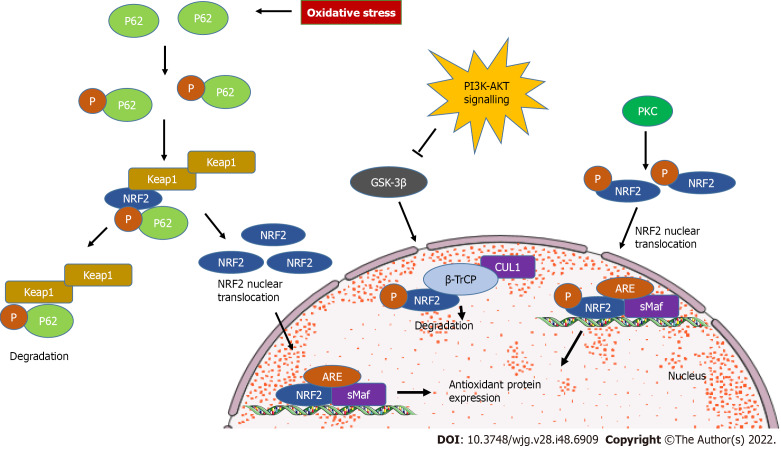
Of note, emerging evidence revealed Keap1-independent novel mechanisms of NRF2 regulation. The phosphatidylinositol 3’-kinase/protein kinase B pathway is protective against oxidative stress and is able to activate NRF2 signaling[13]. Phosphatidylinositol 3’-kinase-protein kinase B-NRF2 signaling pathway involves the glycogen synthase kinase-3β as a key mediator. Glycogen synthase kinase 3β can phosphorylate the NRF2 domain Neh6, containing serine residues that can be recognized by the β-transducin repeats-containing protein. β-transducin repeats-containing protein is a substrate receptor for ubiquitin ligase complex, which targets NRF2 for ubiquitination and proteasomal degradation[14,15]. During autophagy, NRF2 is stabilized by the binding of p62 (autophagy substrate) to Keap1 at the NRF2 binding site, resulting in the transcriptional activation of NRF2-target genes[16,17]. In addition, oxidative stress-induced protein kinase C phosphorylates Neh2 at serine and threonine residue on Ser40, dissociating the Keap1 homodimer and transferring NRF2 to the nucleus, thus binding to the antioxidant response element-mediated cytoprotective genes[18] (Figure 2).
Figure 2.
Kelch-like ECH-associated protein 1-independent nuclear factor-erythroid 2 signaling. During oxidative stress, selective autophagy substrate p62 competes with nuclear factor-erythroid 2 (NRF2) to bind with kelch-like ECH-associated protein 1 (Keap1). As a consequence, NRF2 dissociates from Keap1 and translocates to the nucleus to induce target genes. Glycogen synthase kinase 3β (GSK-3β) phosphorylates the NRF2 subunit Nrf-ECH homology (Neh) 6, leading to degradation by β-transducin repeats containing protein (β-TrCP). Phosphatidylinositol 3’-kinase-protein kinase B (AKT) signaling could inhibit GSK-3β. Protein kinase C phosphorylates Ser40 in Neh2, inducing NRF2 translocation to the nucleus. ARE: Antioxidant responsive element; SMaf: Small musculoaponeurotic fibrosarcoma oncogene homologue.
NRF2 IN THE PATHOGENESIS OF MAFLD
MAFLD is the most widespread chronic liver condition worldwide, potentially leading to end stage disease, which requires liver transplantation[19,20]. MAFLD is a lipotoxic disease characterized by both structural and functional mitochondria abnormalities and oxidative stress. Impairment in mitochondrial electron transport chain causes excessive production of reactive oxygen and nitrogen species (ROS and RNS)[21]. ROS and RNS play a crucial role in cellular signaling, proliferation and differentiation, metabolism, and immune defense mechanisms. Besides mitochondria, ROS and RNS are continuously produced by the ER and peroxisomes as byproducts during their normal physiological processes. Oxidative stress is described as the imbalance between production of ROS/RNS and antioxidant systems[22]. Oxidative stress is intrinsically linked to the pathogenesis of MAFLD, and NRF2 has been found to be a key regulator to protect against the hepatocellular injury. Since MAFLD development and progression are characterized by alterations of redox balance, NRF2 is involved in every stage of disease, from simple steatosis to inflammation, advanced fibrosis, and initiation/progression of hepatocellular carcinoma[8].
NRF2 and liver steatosis
Accumulation of lipids in hepatocytes is the first step characterizing MAFLD development. This process is the result of increased fatty acid uptake/synthesis and decreased fatty acid oxidation/removal[23]. Fatty acid oxidation in peroxisomes produces H2O2, which in turn decreases the expression of enzymes involved in fatty acid oxidation as carnitine palmitoyltransferase 1A and acyl-CoA oxidase through their regulatory factor peroxisome proliferator activated receptor alpha. Besides, H2O2 promotes lipid accumulation by upregulating the expression of sterol regulatory element-binding protein-1c (SREBP-1c), which further activates fatty acid synthase, and stearoyl coenzyme-A desaturase 1, contributing to MAFLD pathogenesis[24]. In addition, ER-stress activates SREBP-1c and increases the expression of hepatic very-low density lipoprotein receptor, leading to deposition of triglycerides (TG)[12,24].
NRF2 is a key player in maintaining cellular homeostasis, suppressing MAFLD promotion and progression. A microarray analysis of mouse hepatic gene expression revealed that pharmacologic and genetic activation of NRF2 suppresses key enzymes involved in lipid synthesis and reduces hepatic lipid storage. NRF2-/- mice fed a high-fat diet (HFD) are more prone to develop steatosis and oxidative stress than wild-type mice[25]. Consistent to this, NRF2-knockout mice fed a methionine- and choline-deficient (MCD) diet developed a severe form of micro- and macrovesicular steatosis and neutrophil recruitment compared to wild-type mice[26-28]. Studies on hepatic protein expression in NRF2-null and wild-type mice found two major groups of NRF2-modulated proteins. One group of proteins in NRF2 wild-type animals was implicated in phase II drug metabolism and antioxidant defense, while the other group of proteins in NRF2-null animals was involved in lipid and fatty acid synthesis and metabolism[29]. Another study in NRF2-null 8-wk old mice revealed a higher expression of SREBP-1c and fatty acid synthase than wild-type mice[30]. Nonetheless, NRF2 has little effect on hepatic fatty acid metabolism in 12-25 wk old mice[31,32].
In addition, flavonoid glycoside scutellarin ameliorates MAFLD pathogenesis by reducing blood lipid levels and enhances antioxidant capacity by activating peroxisome proliferator-activated receptor gamma (PPAR-γ) and its cofactor-1α as well as NRF2-dependent enzymes HO-1 and glutathione-S-transferase. Moreover, scutellarin suppresses nuclear factor κ B (NF-κB) and Keap1 mitigating MAFLD[33]. Another study revealed that scutellarin contains breviscapine as its active component, possibly exerting its antioxidant effects through phosphatidylinositol 3’-kinase/protein kinase B activation and subsequent enhancement of NRF2 nuclear translocation, increasing the expression of HO-1 and NQO1. Thus, breviscapine could be used in MAFLD and hyperlipidemia due to its potential therapeutic effects[34].
In addition, the food-derived compound apigenin is a modulator of PPAR-γ, which attenuates the NRF2-associated antioxidative response and hepatocyte lipid metabolism in MAFLD[35]. The specific deletion of NRF2 in mice diminished the signs of MAFLD induced by HFD, decreasing the accumulation of TGs. Hepatic NRF2 deficiency dampens the expression of PPAR-γ, suggesting that the NRF2-dependent expression of PPAR-γ is critical in initiation and progression of MAFLD[36].
Liver X receptors are a family of nuclear receptors implicated in the modulation of lipid homeostasis. Directly or via SREBP-1c, liver X receptor α triggers the expression of lipogenic genes involved in the uptake and synthesis of fatty acids, TGs, cholesterol, and phospholipids. Treatment with the NRF2 activator sulforaphane suppresses T0901317-induced lipogenesis, promoting deacetylation of farnesoid X receptor (FXR) by competitive binding of p300, a protein necessary for the acetylation of FXR. The FXRE chromatin immunoprecipitation assay confirmed that NRF2 may complex with p300 and, as a result, dissociate from the FXR complex[37-39]. Moreover, NRF2 activator inhibits SREBP-1c and lipogenic genes by promoting deacetylation of FXR and inducing small heterodimer partner, which accounts for the repression of liver X receptor α-dependent gene transcription, protecting the liver from excessive fat accumulation[40].
NRF2 and liver inflammation
NRF2 is further involved in the regulation of pro- and anti-inflammatory mediators. NRF2 is known for its anti-inflammatory effects as it inhibits the expression of proinflammatory cytokines like interleukin-6 (IL-6), tumor necrosis factor, and inducible nitric oxide synthase. Moreover, NRF2-dependent antioxidant genes, such as HO-1, NQO1, and glutamate cysteine ligase catalytic and modifier subunits, inhibit the transcription of proinflammatory mediators by blocking NF-κB activation[41-43]. Of note, NRF2 also triggers the NLR family pyrin domain containing 3 inflammasome, which cleaves caspase-1 and initiates the processing of pro-IL-1β to mature IL-1β[44]. NLR family pyrin domain containing 3-dependent production of proinflammatory response can be inhibited by activation of NRF2 through dimethyl fumarate in alcoholic liver disease[45], and 4-acetylantroquinonol B in mice fed with a methionine- and choline-deficient diet[46] inducing the expression of NQO1, which inhibits the ROS/RNS-dependent priming.
NRF2-KO mice fed the methionine- and choline-deficient diet lose the antioxidant and detoxification enzymes and show an increase in steatosis, inflammation, oxidative stress, lipid peroxidation, and fibrinogenesis[26,28]. In line with these results, feeding the NRF2-KO mice with the HFD yielded significantly greater amounts of lipids and inflammation compared to wild-type mice. NRF2-KO mice fed a diet containing 4% soyabean oil and 16% lard for 12 wk exhibited massive lipid accumulation, inflammation, oxidative stress, and iron accumulation when compared to their wild-type counterparts[47]. NRF2-KO mice fed a diet containing 45 kcal% fat (0.02% cholesterol) for 24 wk displayed a higher MAFLD activity score compared to wild-type animals. In HFD-fed NRF2-KO mice, livers scored higher for steatosis, ballooning, inflammation, and fibrosis when compared to Nrf2+/+ mice. The biochemical characterization studies of such mice revealed higher expression of sterol regulatory element binding transcription factor 1 and 2 and carbohydrate response element binding protein also known as MLX-interacting protein-like in HFD-fed NRF2-KO mice, suggesting exaggerated lipogenic transcription[48]. In another study, NRF2-KO mice fed a high-fat plus 30% fructose in drinking water exhibited a higher MAFLD score than wildtype. Moreover, these NRF2-KO mice overexpress lipogenic transcription factor sterol regulatory element binding transcription factor 1, fatty acid synthase, stearoyl coenzyme-A desaturase 1, and CD36 and exhibited higher proinflammatory factors as NF-κB p65 and p50 subunits[49].
In another investigation, NRF2-KO mice fed a chow diet were subjected to scanty inflammation with minimal increases in IL-1β, Cox2, and Nos2 mRNA[26,28]. This is due to the compromised expression of zonula occludens-1 and claudin-1, which are responsible for the translocation of lipopolysaccharides from the gut microbiota to the liver through the portal vein. In addition, the phagocytic ability of Kupffer cells is diminished in NRF2-KO due to lower expression of the macrophage receptor with collagenous structure that restricts TLR4 signaling and boosts the inflammatory response on exposure to lipopolysaccharide[50].
NRF2 and liver fibrosis
Liver fibrosis is a reversible wound healing response and degenerative condition caused by extensive deposition of extracellular matrix proteins like collagen fibrils[51]. Mechanisms underlying liver fibrosis include the activation of both hepatic stellate cells and Kupffer cells, resulting in functional and biological alterations[52]. Oxidative stress is a serious process involved in liver damage, and the activation of the Keap1/NRF2 pathway plays a protective role in liver fibrosis[12]. NRF2 activation triggers the reverse IR and attenuates liver fibrosis by inhibiting hepatic steatosis. These noticeable effects during NRF2 activation are due to the disruption of JAK2/STAT3 signaling and higher expression of suppressor of cytokine signaling 3[53]. Moreover, administration of fibroblast growth factor 1 variants carrying substitutions of heparin-binding sites in 9-mo-old mice inhibited activity and expression of lipogenic genes, improving both steatohepatitis and fibrosis[54].
CCl4-induced hepatic fibrosis is accompanied by elevated serum transaminases, alkaline phosphatase, and bilirubin, decreased albumin, and increased proinflammatory cytokines. In addition, CCl4-intoxicated rats display an increase in NF-κB, p65, malondialdehyde and a decrease in antioxidants. Bone marrow-derived mesenchymal stem cells show favorable effects in ameliorating the hepatic effects of CCl4 through NRF2/HO-1 signaling, suppressing liver fibrosis, inflammation, and oxidative stress[55].
A major bioactive extract from the plant Schisandra chinesis, known as Schisandrin B, exerts anti-inflammatory, anti-tumor, antioxidative, and hepatoprotective properties. Schisandrin B effectively improves liver function and decreases collagen deposition in the CCl4-induced liver fibrosis in rats through the modulation of NRF2-antioxidant response element and transforming growth factor-β/Smad signaling pathways[56]. Tanshinol, a water-soluble compound isolated from Salvia miltiorrhiza Bunge, is known to exert a variety of biological effects, including anti-fibrotic effects. Rats with CCl4-induced liver fibrosis treated intraperitoneally with tanshinol show lower serum levels of aspartate aminotransferase, alanine aminotransferase, and total bilirubin, as well as circulating hyaluronic acid, laminin, type IV collagen, and procollagen III peptide as compared to controls. Tanshinol is also able to suppress the expression of inflammatory cytokines such as transforming growth factor-β, tumor necrosis factor, Cox2, IL-1β, and IL-6 through regulation of the NF-κB pathway. In addition, tanshinol treatment is able to regulate the NRF2/HO-1 signaling pathway increasing SOD and GSH-Px and decreasing malondialdehyde levels. In this regard, tanshinol exerts protective effects on CCl4-induced liver fibrosis by activating the NRF2 pathway[57].
Asiatic acid (AA), a bioactive compound extracted from Centella asiatica, is known to have anti-inflammatory, antioxidative, and hepatoprotective properties[19-22]. Fan et al[34] showed that treatment with AA in the CCl4-induced liver fibrosis dramatically ameliorates oxidative stress, inflammation, and fibrosis in rats. The nuclear NRF2 levels were increased after AA treatment, and the NRF2-dependent proteins like HO-1, NQO-1, and Glutamate cysteine ligase catalytic subunit were significantly increased to counteract oxidative stress. Furthermore, AA inhibited the NF-κB/IkBα and JAK1/STAT3 signaling pathway to suppress the activation of hepatic stellate cells and the production of inflammatory markers, suggesting that AA could be used for the treatment of liver fibrosis[58]. Another water soluble compound, salvianolic acid A, extracted from a traditional Chinese herb Radix Salvia miltiorrhiza, was found to have anti-fibrotic effects. salvianolic acid A is able to modulate the NRF2/HO-1, NF-κB/IkBα, p38 MAPK, and JAK1/STAT3 signaling pathways, and to ameliorate the CCl4-induced liver fibrosis, improve morphology and attenuate collagen deposition in the fibrotic liver. Besides, salvianolic acid A is able to increase the levels of SOD and GSH-Px and decrease the malondialdehyde levels, indicating the effectiveness in preventing liver fibrosis by inhibiting inflammation and oxidative stress[59].
Pharmacological stimulation of NRF2 by acetylenic tricyclic bis (cyano enone) TBE-31 reverses IR in wild-type mice, decreases liver steatosis by increasing hepatic fatty acid oxidation and reducing ER stress, and lessens markers of oxidative stress, apoptosis, and fibrosis. Of note, histology studies showed that TBE-31 decreases the fibrosis score and MAFLD activity score[59]. In another study, NRF2 activator NK-252 (1-(5-(furan-2-yl)-1,3,4-oxadiazol-2-yl)-3-(pyridin-2-ylmethyl)urea) significantly reduced markers of fibrosis like COL1A1, TIMP-1, and transforming growth factor-β in rats, suggesting that this compound could be used as a therapeutic agent to reverse liver fibrosis. In addition, NK-252 attenuated the serum aspartate aminotransferase and alanine aminotransferase levels in male Fischer rats and upregulates NQO1 gene expression[60].
THERAPEUTIC IMPLICATIONS OF NRF2 IN MAFLD
Currently, there is no medicine that can treat MAFLD, but some therapeutic agents are useful in managing the problems associated with the disease (Table 1). Thus, it is necessary to develop and test drugs for the prevention and treatment of MAFLD, and it is conceivable that NRF2-activating compounds can attenuate MAFLD progression. Plant-derived compounds including resveratrol, curcumin, quercetin, and synthetic molecules like oltipraz and pirfenidone could be used to prevent oxidative stress by modulating the NRF2 pathway[12,21].
Table 1.
Modulators of nuclear factor erythroid 2-related factor 2 pathway in metabolic associated fatty liver disease
|
Compound name
|
Species
|
Diet/duration
|
Treatment
|
Key findings
|
Reference
|
| MonoHER | Female C57BL/6J mice (Ldlr-/-) | High fat and high cholesterol/13 wk | Administered daily subcutaneously at a dosage of 500 mg/kg of body weight (25 µL/g of body weight) | NRF2 activation, ↑GSH/GSSG ratio, ↑HO-1, GSH-Px | [62] |
| Scutellarin | Male C57BL/6 mice, hepaG2 cells | High fat/10 wk | Administration of 12.5, 25.0, and 50.0 mg/kg per day | ↑PPARγ, PGC-1α, NRF2, HO-1, NQO1, Keap1, NF-κB | [33] |
| Sprague-Dawley rats | High fat/12 wk | Administered orally 50, 100, and 300 mg/kg/d | NRF2, HO-1, NQO1; PI3K/AKT activation | [34] | |
| Apigenin | Male C57BL/6J mice | High fat/16 wk | Injected intraperitonially 30 mg/kg daily for 3 wk | NRF2 activation; PPARγ inhibition; SOD, CAT, GSH-Px | [35] |
| 7,8-dihydroxyflavone | Male wistar rats | High fat, ethanol/12 wk | Administered intraperitonially at 5 mg/kg/d for 4 wk | Amelioration of liver architecture, vescicular changes, infiltration; restored serum biomarkers like AST, ALT, and TC; ↑NRF2; ↓NF-κB | [63] |
| Resveratrol | Male C57BL/6 mice | High fat/16 wk | Supplemented with 0.4% resveratrol in HFD for 16 wk | Attenuated liver steatosis; ↑NRF2 activation; attenuated HFD induced methylation of NRF2 promoter; ↓oxidative stress | [68] |
| Quecertin | HepG2 cells | - | Treated with quecertin at 5-50 µM concentrations for 0, 10, 30, 60, 120, 240, and 1080 min | ↑GSH, GSH-Px, GCS; p38-MAPK is involved in NRF2 modulation; ↓oxidative stress | [69] |
| Curcumin (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) | Male C57BL/6 mice | High fat and high fructose/8 wk | Administered orally 50 and 100 mg/kg/d for 4 wk | ↑CYP3A, CYP7A; regulation of NRF2/FXR/LXRα pathway; ↓SREBP-1C, FAS | [70] |
| Male Sprague-Dawley rats | High fat/6 wk | Administered orally 50 mg/kg daily for 6 wk | ↓Steatosis and inflammation; ↓Serum aminotransferases, lipids, and insulin resistance; ↓TNF, IL-6, MDA; ↑NRF2, GSH, HO-1, SOD | [71] | |
| Oltipraz | Male Fischer 344 rats | Choline-deficient L-amino acid–defined/10 wk | Administered orally at 60 mg/kg/d for 9 wk | ↑NRF2 activation; antifibrotic and anti-inflammatory; ↓AST and ALT; ↑NQO1 gene expression | [61] |
| GSTD | HL-7702 cells, male C57BL/6J, male Sprague-Dawley rats | Oleic acid (OA)/24 h, high fat/10 wk; high fat and high cholesterol/10 wk | Cells were treated with GSTD for 24 h, administered orally at 10, 20, 50 mg/kg per day for 10 wk, administered orally at 20, 50 mg/kg per day for 10 wk | ↑NRF2, HO-1, SOD; activate AMPK/NRF2; ↓proinflammatory response, and hepatic steatosis; ↓MDA, ROS | [65] |
| NK-252 1-(5-(furan-2-yl)-1,3,4-oxadiazol-2-yl)-3-(pyridin-2-ylmethyl)urea) | Male Fischer 344 rats | Choline-deficient L-amino acid–defined/10 wk | Administered orally at 20, 60 mg/kg/d for 9 wk | Attenuated histological abnormalities; ↑antifibrotic effects; ↓TGF-β1, collagen α1; NRF2 activation; ↑NQO1 expression | [61] |
| Clusterin | Male hCLU-tg mice | MCD/3 wk | Generated hepatocyte-specific clusterin overexpression transgenic mice and fed with MCD diet | ↓Hepatic TGs; less infiltration of macrophages; ↓TNF; ↑NRF2 activation and mRNA of HO-1 | [66] |
| Osteocalcin | Male C57/BL6J mice | High fat/12 wk | Injected intraperitonially at concentration 3 ng/µL/d for 12 wk | ↓Hepatic TG accumulation; ↑NRF2 activation; ↑CAT, SOD, GSH-Px; ↓JNK activation | [67] |
| Orlistat | Male Sprague-Dawley rats | High fat/12 wk | Administered at 10 mg/kg/d for 12 wk | ↑NRF2 activation; protection against insulin resistance, hyperlipidemia, oxidative stress, and liver injury | [72] |
| Garcinia Cambogia | Male C57BL/6N mice | High fat/8 wk | Administered 200, 400 mg/kg/d for 8 wk | ↑NRF2 activation; ↓ROS production; suppressed lipogenic factors C/EBPα and PPARγ; suppressed apoptosis by normalizing Bcl-2/BAX ratio and PARP cleavage | [73] |
| HTT | Male Sprague-Dawley rats, 3T3-L1 murine embryo fibroblast cells | High fat/4 wk, 3T3-L1 cells treated with FBS/DMEM for 8 d | Administered orally HTT at 350, 700, and 1400 mg/kg/d, 3T3-L1 cells treated with HTT at 500 µg/mL for 24 h or 48 h | ↑NRF2-HO-1 activation, antioxidant activities; HTT inhibited liver weight gain; reduced lipid profile; improved liver function; HTT promoted lipolysis and increased antioxidant activities in 3T3-L1 cells | [74] |
| Hesperitin | HepG2 cells, male wistar rats | OA/24 h, high fat/16 wk | Treated cells at 0.25, 0.50, 1.00, 2.50, 5.00, and 10.00 µM; administered 100 mg/kg in 0.5% CMC-Na | Alleviated hepatotoxicity and oxidative stress by increasing SOD, GSH-Px, GCLC, and HO-1; ↑NRF2 activation; suppressed OA induced inflammation; reduced TC, TGs, and LDLC in a dose-dependent manner | [75] |
| Glucoraphanin | Male C57BL/6JSlc mice | High fat/14 wk | Administered 0.3% glucoraphanin orally for 14 wk | Decrease in weight gain; improved insulin resistance; reduced hepatic steatosis and oxidative stress; decrease in circulating LPS; ↑NRF2 activation; ↑energy expenditure and; UCP1 protein expression | [76] |
| Scutellaria baicalensis extract | Male KK-Ay mice | 1% Orotic acid and 33% sugar/7 d | Supplemented with diet for 7 d | Diminished increase in liver weight; attenuated hepatic steatosis; ↑NRF2 expression; suppress SREBP-1c gene and protein expression | [77] |
| Ginkgolide B | Male C57/BL6 ApoE-/--mice, HepG2 cells | High fat/5 wk, 100 µM palmitic acid (PA) and 200 µM OA/24 h | Administered orally at 20, 30, and 1.3 mg/kg/d; treated cells at dosages 0, 1, 2, 4, 8, 16, and 32 µg/mL | NRF2 activation; inhibition of oxidative stress and lipid peroxidation through NRF2 pathway; increase in HO-1 and GSH-Px4 | [78] |
| Scoparone | Male C57BL/6 J mice, AML2 and RAW264.7 cells | MCD/4 wk; AML12/300 µM PA and RAW264.7/10 µM/ Chloroquine | Administered daily intraperitonially for 4 wk at 20, 40, and 80 mg/kg; AML12 and RAW264.7 cells were pretreated with scoparone for 2 h | Ameliorated hepatic inflammation; improved hepatic autophagy; suppressed inflammation by inhibiting ROS/P38/NRF2 axis and PI3K/AKT/mTOR pathway | [79] |
| DA | Male C57BL/6J mice, HL7702 cells | High fat/12 wk, 0.6 mM OA/24 h | Administered by gavage at 10 and 20 mg/kg/d for 9 wk; treated with 2.5, 5.0, and 10.0 µM DA | Ameliorated liver ferroptosis in mice and cells; improved oxidative stress and lipid peroxidation in vivo; ↑NRF2-HO-1 expression; ↑GSH, GSH-Px4 | [80] |
| Silibinin | Male C57BL/6 mice, NCTC-1469 cells | MCD/6 wk, OA plus PA/24 h | Administered by gavage at 10 and 20 mg/kg/d for 6 wk, 0.25 mM/L PA and 0.5 mM/L OA/24 h | Prevented CFLAR-JNK pathway; ↑β-oxidation and efflux of fatty acids; ↑expression of CAT, GSH, GSH-Px, and HO-1; ↓expression of CYP2E1 and CYP4A; ↑NRF2 activation | [81] |
| Chicoric acid | Male C57BL/6 mice | High fat/9 wk | Administered by gavage at 15 and 30 mg/kg/d for 9 wk | Attenuated hyperglycemia, dyslipidemia, and systemic inflammation; alleviated hepatic lipid accumulation and oxidative stress; suppressed hepatic inflammation and NF-κB pathway; ↑NRF2/Keap1 activation; improved gut microbiota | [82] |
| Carbon monoxide releasing molecule-A1 | Male C57BL/6J mice | High fat/16 wk | Administered intraperitonially 2 mg/kg/d for 7 wk | ↑NRF2/ARE activation; improved lipid homeostasis; ↑ATP production; improved mitochondrial biogenesis; ameliorated oxidative stress | [83] |
NRF2: Nuclear factor erythroid 2-related factor 2; GSH: Reduced glutathione; GSSG: Oxidized glutathione; HO-1: Heme oxygenase-1; GSH-Px: Glutathione peroxidase; PPAR-γ: Peroxisome proliferator-activated receptor-γ; PGC-1α: Proliferator-activated receptor gamma coactivator-1α; NQO1: NAD(P)H quinone oxidoreductase 1; DA: Dehydroabietic acid; PA: Palmitic acid; NF-κB: Nuclear factor κ B; PI3K: Phosphatidylinositol 3’-kinase; SOD: Superoxide dismutase; CAT: Catalase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TC: Total cholesterol; HFD: High-fat diet; GCS: Glutamylcysteine-synthetase; MAPK: Mitogen-activated protein kinase; CYP3A: Cytochrome P450, family 3, subfamily A, CYP7A Cytochrome P450, family 7, subfamily A; FXR: Farnesoid-X-receptor; LXRα: Liver X receptor α; SREBP-1C: Sterol regulatory element-binding protein-1c; FAS: Fatty acid synthase; TNF: Tumor necrosis factor; IL-6: Interleukin-6; MDA: Malondialdehyde; AMPK: AMP kinase; ROS: Reactive oxygen species; TGF-β1: Transforming growth factor-β1; TG: Triglycerides; JNK: c-Jun N-terminal kinase; C/EBPα: CCAAT/enhancer binding protein α; Bcl-2: B-Cell Leukemia/Lymphoma 2; BAX: BCL2 associated X protein; PARP: Poly-ADP ribose polymerase; HTT: Hedansanqi Tiaozhi Tang; GCLC: Glutamate cysteine ligase catalytic; LDLC: Low density lipoprotein cholesterol; LPS: Lipopolysaccharide; UCP1: Uncoupling protein 1; GSH-Px4: Glutathione peroxidase 4; mTOR: Mammalian target of rapamycin; CFLAR: CASP8 And FADD like apoptosis regulator; CYP2E1: Cytochrome P450 family 2 subfamily E member 1; CYP4A: Cytochrome P450 family 4 subfamily A; ARE: Antioxidant response element; AKT: Protein kinase B; MCD: Methionine- and choline-deficient; GSTD: Gastrodin; Keap1: Kelch-like ECH-associated protein 1.
Flavonoids represent a class of bioactive antioxidants extracted from vegetables, plants, and fruits known to exhibit therapeutic properties in MAFLD. The flavonoid 7-mono-O-(β-hydroxyethyl)-rutoside activates NRF2 and improves the ratio of GSH/glutathione disulfide and increases the expression of HO-1 and GSH-Px3[61,62]. The flavonoid scutellarin (4′,5,6-trihydroxy flavonoid-7-glucuronide) increases NRF2 protein in C57BL/6J mice, increases the expression of HO-1, glutathione-S-transferase, and NQO1, and inhibits both NF-κB and Keap1[33]. Furthermore, 7,8-dihydroxyflavone upregulates NRF2 activity to counteract alcohol-induced and HFD-induced liver toxicity[63]. Apigenin (4′,5,7-trihydroxyflavone), a flavonoid derived from fruits, inhibits lipid peroxidation and exerts protective effects against hepatic steatosis. Moreover, apigenin increases the activities of SOD, CAT, and GSH-Px[35,64].
Gastrodin is a water-soluble extract of Gastrodia elata BI that exerts antioxidative activity and improves lipid metabolism in MAFLD mice by promoting NRF2 nuclear translocation[65]. Clusterin, a glycoprotein extracted from ram rete testis fluid, improves steatosis and hepatitis induced by methionine and choline-deficient diet by triggering NRF2 and HO-1 expression[66]. Osteocalcin treatment improves hepatic TG accumulation, promotes NRF2 nuclear translocation, and inhibits phosphorylation of c-Jun N-terminal kinase pathway[67].
In addition, compounds like scutellarin containing breviscapine, hesperitin, apigenin, scoparone, Schisandrin B, tanshinol, and AA and other tabulated compounds are known to exert antioxidative and hepatoprotective activity by modulating the NRF2 pathway.
CONCLUSION
Oxidative stress can be a potent inducer of inflammation and fibrosis in the spectrum of chronic liver diseases. Among them, MAFLD is the most widespread chronic liver condition worldwide. The transcription factor NRF2 has gained importance in recent years as a possible therapeutic target for the treatment of liver diseases. The expression of antioxidant protective genes through the NRF2 pathway counteracts oxidative stress and prevents progression of liver damage in MAFLD. The different antioxidative molecules modulating the NRF2 pathway have exerted beneficial effects in ameliorating liver damage. Currently, there is no efficient treatment to counteract the complex pathophysiology of liver diseases. Thus, compounds having antioxidative properties could be useful candidates for the treatment of liver diseases by modulating the NRF2 signaling pathway. NRF2 activators could improve and prevent the advanced stages of MAFLD such as liver fibrosis and liver cirrhosis. Natural plant-derived and synthetic NRF2 activators require further experimental validation to be promoted as efficient therapeutic agents. Some drugs have entered clinical trials, and further attempts are ongoing to find NRF2 inducers with high bioavailability, safety, and specificity.
Footnotes
Conflict-of-interest statement: All authors declare no conflicts of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 18, 2022
First decision: October 30, 2022
Article in press: November 22, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdelbasset WK, Saudi Arabia; Sangineto M, Italy S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
Contributor Information
Vidyasagar Naik Bukke, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy.
Archana Moola, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy.
Gaetano Serviddio, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy.
Gianluigi Vendemiale, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy.
Francesco Bellanti, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy. francesco.bellanti@unifg.it.
References
- 1.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Abd El-Kader SM, El-Den Ashmawy EM. Non-alcoholic fatty liver disease: The diagnosis and management. World J Hepatol. 2015;7:846–858. doi: 10.4254/wjh.v7.i6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD) J Diabetes Res. 2020;2020:3920196. doi: 10.1155/2020/3920196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Serviddio G, Bellanti F, Vendemiale G. Free radical biology for medicine: learning from nonalcoholic fatty liver disease. Free Radic Biol Med. 2013;65:952–968. doi: 10.1016/j.freeradbiomed.2013.08.174. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Zheng Q, Chen Z. The Nrf2 Pathway in Liver Diseases. Front Cell Dev Biol. 2022;10:826204. doi: 10.3389/fcell.2022.826204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird L, Yamamoto M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol Cell Biol. 2020;40 doi: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galicia-Moreno M, Lucano-Landeros S, Monroy-Ramirez HC, Silva-Gomez J, Gutierrez-Cuevas J, Santos A, Armendariz-Borunda J. Roles of Nrf2 in Liver Diseases: Molecular, Pharmacological, and Epigenetic Aspects. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9100980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med. 2006;41:1079–1091. doi: 10.1016/j.freeradbiomed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci U S A. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fei YD, Li W, Hou JW, Guo K, Chen XM, Chen YH, Wang Q, Xu XL, Wang YP, Li YG. Oxidative Stress-Induced Afterdepolarizations and Protein Kinase C Signaling. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18040688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377–386. doi: 10.1038/s41575-019-0144-8. [DOI] [PubMed] [Google Scholar]
- 20.Sangineto M, Bukke VN, Bellanti F, Tamborra R, Moola A, Duda L, Villani R, Romano AD, Serviddio G. A Novel Nutraceuticals Mixture Improves Liver Steatosis by Preventing Oxidative Stress and Mitochondrial Dysfunction in a NAFLD Model. Nutrients. 2021;13 doi: 10.3390/nu13020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y, Jiang J, He B, Shi Z. Chemical Activators of the Nrf2 Signaling Pathway in Nonalcoholic Fatty Liver Disease. Nat Prod Commun . 2021;16:1934578X20987095. [Google Scholar]
- 22.Ma Y, Lee G, Heo SY, Roh YS. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants (Basel) 2021;11 doi: 10.3390/antiox11010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int. 2021;15:21–35. doi: 10.1007/s12072-020-10121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, Liby KT, Sporn MB, Sutter TR, Kensler TW. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhry S, Nazmy MH, Meakin PJ, Dinkova-Kostova AT, Walsh SV, Tsujita T, Dillon JF, Ashford ML, Hayes JD. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med. 2010;48:357–371. doi: 10.1016/j.freeradbiomed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Okada K, Warabi E, Sugimoto H, Horie M, Tokushige K, Ueda T, Harada N, Taguchi K, Hashimoto E, Itoh K, Ishii T, Utsunomiya H, Yamamoto M, Shoda J. Nrf2 inhibits hepatic iron accumulation and counteracts oxidative stress-induced liver injury in nutritional steatohepatitis. J Gastroenterol. 2012;47:924–935. doi: 10.1007/s00535-012-0552-9. [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto H, Okada K, Shoda J, Warabi E, Ishige K, Ueda T, Taguchi K, Yanagawa T, Nakahara A, Hyodo I, Ishii T, Yamamoto M. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G283–G294. doi: 10.1152/ajpgi.00296.2009. [DOI] [PubMed] [Google Scholar]
- 29.Kitteringham NR, Abdullah A, Walsh J, Randle L, Jenkins RE, Sison R, Goldring CE, Powell H, Sanderson C, Williams S, Higgins L, Yamamoto M, Hayes J, Park BK. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J Proteomics. 2010;73:1612–1631. doi: 10.1016/j.jprot.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YK, Yeager RL, Tanaka Y, Klaassen CD. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol. 2010;245:326–334. doi: 10.1016/j.taap.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka Y, Ikeda T, Yamamoto K, Ogawa H, Kamisako T. Dysregulated expression of fatty acid oxidation enzymes and iron-regulatory genes in livers of Nrf2-null mice. J Gastroenterol Hepatol. 2012;27:1711–1717. doi: 10.1111/j.1440-1746.2012.07180.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang YK, Wu KC, Liu J, Klaassen CD. Nrf2 deficiency improves glucose tolerance in mice fed a high-fat diet. Toxicol Appl Pharmacol. 2012;264:305–314. doi: 10.1016/j.taap.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Ji R, Sun H, Peng J, Ma X, Wang C, Fu Y, Bao L, Jin Y. Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARγ/PGC-1α-Nrf2 pathway. Free Radic Res. 2018;52:198–211. doi: 10.1080/10715762.2017.1422602. [DOI] [PubMed] [Google Scholar]
- 34.Fan H, Ma X, Lin P, Kang Q, Zhao Z, Wang L, Sun D, Cheng J, Li Y. Scutellarin Prevents Nonalcoholic Fatty Liver Disease (NAFLD) and Hyperlipidemia via PI3K/AKT-Dependent Activation of Nuclear Factor (Erythroid-Derived 2)-Like 2 (Nrf2) in Rats. Med Sci Monit. 2017;23:5599–5612. doi: 10.12659/MSM.907530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng X, Yu W, Li X, Zhou F, Zhang W, Shen Q, Li J, Zhang C, Shen P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem Pharmacol. 2017;136:136–149. doi: 10.1016/j.bcp.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Fu J, Liu D, Sun J, Hou Y, Chen C, Shao J, Wang L, Wang X, Zhao R, Wang H, Andersen ME, Zhang Q, Xu Y, Pi J. Hepatocyte-specific Nrf2 deficiency mitigates high-fat diet-induced hepatic steatosis: Involvement of reduced PPARγ expression. Redox Biol. 2020;30:101412. doi: 10.1016/j.redox.2019.101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang S, Tsang S, Jones R, Ponugoti B, Yoon H, Wu SY, Chiang CM, Willson TM, Kemper JK. The p300 acetylase is critical for ligand-activated farnesoid X receptor (FXR) induction of SHP. J Biol Chem. 2008;283:35086–35095. doi: 10.1074/jbc.M803531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kay HY, Kim WD, Hwang SJ, Choi HS, Gilroy RK, Wan YJ, Kim SG. Nrf2 inhibits LXRα-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxid Redox Signal. 2011;15:2135–2146. doi: 10.1089/ars.2010.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellezza I, Tucci A, Galli F, Grottelli S, Mierla AL, Pilolli F, Minelli A. Inhibition of NF-κB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity. J Nutr Biochem. 2012;23:1583–1591. doi: 10.1016/j.jnutbio.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Brigelius-Flohé R, Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, Shen J, Chen CT, Huo L, Hsu MC, Li CW, Ding Q, Liao TL, Lai CC, Lin AC, Chang YH, Tsai SF, Li LY, Hung MC. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sogawa Y, Nagasu H, Iwase S, Ihoriya C, Itano S, Uchida A, Kidokoro K, Taniguchi S, Takahashi M, Satoh M, Sasaki T, Suzuki T, Yamamoto M, Horng T, Kashihara N. Infiltration of M1, but not M2, macrophages is impaired after unilateral ureter obstruction in Nrf2-deficient mice. Sci Rep. 2017;7:8801. doi: 10.1038/s41598-017-08054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sangineto M, Grabherr F, Adolph TE, Grander C, Reider S, Jaschke N, Mayr L, Schwärzler J, Dallio M, Moschen AR, Moschetta A, Sabbà C, Tilg H. Dimethyl fumarate ameliorates hepatic inflammation in alcohol related liver disease. Liver Int. 2020;40:1610–1619. doi: 10.1111/liv.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yen IC, Tu QW, Chang TC, Lin PH, Li YF, Lee SY. 4-Acetylantroquinonol B ameliorates nonalcoholic steatohepatitis by suppression of ER stress and NLRP3 inflammasome activation. Biomed Pharmacother. 2021;138:111504. doi: 10.1016/j.biopha.2021.111504. [DOI] [PubMed] [Google Scholar]
- 47.James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495–501. doi: 10.1016/s0168-8278(98)80073-1. [DOI] [PubMed] [Google Scholar]
- 48.Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ, Dinkova-Kostova AT, Dillon JF, Hayes JD, Ashford ML. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol. 2014;34:3305–3320. doi: 10.1128/MCB.00677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma R, Sharma A, Kumari A, Kulurkar PM, Raj R, Gulati A, Padwad YS. Consumption of green tea epigallocatechin-3-gallate enhances systemic immune response, antioxidative capacity and HPA axis functions in aged male swiss albino mice. Biogerontology. 2017;18:367–382. doi: 10.1007/s10522-017-9696-6. [DOI] [PubMed] [Google Scholar]
- 50.Akiyama K, Warabi E, Okada K, Yanagawa T, Ishii T, Kose K, Tokushige K, Ishige K, Mizokami Y, Yamagata K, Onizawa K, Ariizumi SI, Yamamoto M, Shoda J. Deletion of both p62 and Nrf2 spontaneously results in the development of nonalcoholic steatohepatitis. Exp Anim. 2018;67:201–218. doi: 10.1538/expanim.17-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512–10522. doi: 10.3748/wjg.v22.i48.10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu C, Xu W, Shao J, Zhang F, Chen A, Zheng S. Nrf2 induces lipocyte phenotype via a SOCS3-dependent negative feedback loop on JAK2/STAT3 signaling in hepatic stellate cells. Int Immunopharmacol. 2017;49:203–211. doi: 10.1016/j.intimp.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Lin Q, Huang Z, Cai G, Fan X, Yan X, Liu Z, Zhao Z, Li J, Shi H, Kong M, Zheng MH, Conklin DJ, Epstein PN, Wintergerst KA, Mohammadi M, Cai L, Li X, Li Y, Tan Y. Activating Adenosine Monophosphate-Activated Protein Kinase Mediates Fibroblast Growth Factor 1 Protection From Nonalcoholic Fatty Liver Disease in Mice. Hepatology. 2021;73:2206–2222. doi: 10.1002/hep.31568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khadrawy SM, Mohamed HM, Mahmoud AM. Mesenchymal stem cells ameliorate oxidative stress, inflammation, and hepatic fibrosis via Nrf2/HO-1 signaling pathway in rats. Environ Sci Pollut Res Int. 2021;28:2019–2030. doi: 10.1007/s11356-020-10637-y. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q, Zhang H, Cao Y, Li Y, Sun S, Zhang J, Zhang G. Schisandrin B attenuates CCl4-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways. Drug Des Devel Ther. 2017;11:2179–2191. doi: 10.2147/DDDT.S137507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang R, Wang J, Song F, Li S, Yuan Y. Tanshinol ameliorates CCl4-induced liver fibrosis in rats through the regulation of Nrf2/HO-1 and NF-κB/IκBα signaling pathway. Drug Des Devel Ther. 2018;12:1281–1292. doi: 10.2147/DDDT.S159546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan J, Chen Q, Wei L, Zhou X, Wang R, Zhang H. Asiatic acid ameliorates CCl4-induced liver fibrosis in rats: involvement of Nrf2/ARE, NF-κB/IκBα, and JAK1/STAT3 signaling pathways. Drug Des Devel Ther. 2018;12:3595–3605. doi: 10.2147/DDDT.S179876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, Wang R, Song F, Chen P, Gu Y, Chen C, Yuan Y. Salvianolic acid A suppresses CCl4-induced liver fibrosis through regulating the Nrf2/HO-1, NF-κB/IκBα, p38 MAPK, and JAK1/STAT3 signaling pathways. Drug Chem Toxicol. 2022:1–10. doi: 10.1080/01480545.2022.2028822. [DOI] [PubMed] [Google Scholar]
- 60.Sharma RS, Harrison DJ, Kisielewski D, Cassidy DM, McNeilly AD, Gallagher JR, Walsh SV, Honda T, McCrimmon RJ, Dinkova-Kostova AT, Ashford MLJ, Dillon JF, Hayes JD. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2) Cell Mol Gastroenterol Hepatol. 2018;5:367–398. doi: 10.1016/j.jcmgh.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimozono R, Asaoka Y, Yoshizawa Y, Aoki T, Noda H, Yamada M, Kaino M, Mochizuki H. Nrf2 activators attenuate the progression of nonalcoholic steatohepatitis-related fibrosis in a dietary rat model. Mol Pharmacol. 2013;84:62–70. doi: 10.1124/mol.112.084269. [DOI] [PubMed] [Google Scholar]
- 62.Lemmens KJ, van de Wier B, Koek GH, Köhler E, Drittij MJ, van der Vijgh WJ, Bast A, Haenen GR. The flavonoid monoHER promotes the adaption to oxidative stress during the onset of NAFLD. Biochem Biophys Res Commun. 2015;456:179–182. doi: 10.1016/j.bbrc.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 63.Kumar D, Dwivedi DK, Lahkar M, Jangra A. Hepatoprotective potential of 7,8-Dihydroxyflavone against alcohol and high-fat diet induced liver toxicity via attenuation of oxido-nitrosative stress and NF-κB activation. Pharmacol Rep. 2019;71:1235–1243. doi: 10.1016/j.pharep.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Panda S, Kar A. Apigenin (4',5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J Pharm Pharmacol. 2007;59:1543–1548. doi: 10.1211/jpp.59.11.0012. [DOI] [PubMed] [Google Scholar]
- 65.Qu LL, Yu B, Li Z, Jiang WX, Jiang JD, Kong WJ. Gastrodin Ameliorates Oxidative Stress and Proinflammatory Response in Nonalcoholic Fatty Liver Disease through the AMPK/Nrf2 Pathway. Phytother Res. 2016;30:402–411. doi: 10.1002/ptr.5541. [DOI] [PubMed] [Google Scholar]
- 66.Park JS, Shim YJ, Kang BH, Lee WK, Min BH. Hepatocyte-specific clusterin overexpression attenuates diet-induced nonalcoholic steatohepatitis. Biochem Biophys Res Commun. 2018;495:1775–1781. doi: 10.1016/j.bbrc.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 67.Du J, Zhang M, Lu J, Zhang X, Xiong Q, Xu Y, Bao Y, Jia W. Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of Nrf2 and inhibition of JNK. Endocrine. 2016;53:701–709. doi: 10.1007/s12020-016-0926-5. [DOI] [PubMed] [Google Scholar]
- 68.Hosseini H, Teimouri M, Shabani M, Koushki M, Babaei Khorzoughi R, Namvarjah F, Izadi P, Meshkani R. Resveratrol alleviates non-alcoholic fatty liver disease through epigenetic modification of the Nrf2 signaling pathway. Int J Biochem Cell Biol. 2020;119:105667. doi: 10.1016/j.biocel.2019.105667. [DOI] [PubMed] [Google Scholar]
- 69.Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem Biol Interact. 2012;195:154–164. doi: 10.1016/j.cbi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Yan C, Zhang Y, Zhang X, Aa J, Wang G, Xie Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed Pharmacother. 2018;105:274–281. doi: 10.1016/j.biopha.2018.05.135. [DOI] [PubMed] [Google Scholar]
- 71.Li B, Wang L, Lu Q, Da W. Liver injury attenuation by curcumin in a rat NASH model: an Nrf2 activation-mediated effect? Ir J Med Sci. 2016;185:93–100. doi: 10.1007/s11845-014-1226-9. [DOI] [PubMed] [Google Scholar]
- 72.Zakaria Z, Othman ZA, Bagi Suleiman J, Jalil NAC, Ghazali WSW, Mohamed M. Protective and Therapeutic Effects of Orlistat on Metabolic Syndrome and Oxidative Stress in High-Fat Diet-Induced Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) in Rats: Role on Nrf2 Activation. Vet Sci. 2021;8 doi: 10.3390/vetsci8110274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han JH, Park MH, Myung CS. Garcinia cambogia Ameliorates Non-Alcoholic Fatty Liver Disease by Inhibiting Oxidative Stress-Mediated Steatosis and Apoptosis through NRF2-ARE Activation. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10081226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu M, Xiao F, Wang T, Piao S, Zhao W, Shao S, Yan M, Zhao D. Protective effect of Hedansanqi Tiaozhi Tang against non-alcoholic fatty liver disease in vitro and in vivo through activating Nrf2/HO-1 antioxidant signaling pathway. Phytomedicine. 2020;67:153140. doi: 10.1016/j.phymed.2019.153140. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Wang T, Liu P, Yang F, Wang X, Zheng W, Sun W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021;12:3898–3918. doi: 10.1039/d0fo02736g. [DOI] [PubMed] [Google Scholar]
- 76.Nagata N, Xu L, Kohno S, Ushida Y, Aoki Y, Umeda R, Fuke N, Zhuge F, Ni Y, Nagashimada M, Takahashi C, Suganuma H, Kaneko S, Ota T. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes. 2017;66:1222–1236. doi: 10.2337/db16-0662. [DOI] [PubMed] [Google Scholar]
- 77.Chen Q, Liu M, Yu H, Li J, Wang S, Zhang Y, Qiu F, Wang T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J Nat Med. 2018;72:655–666. doi: 10.1007/s11418-018-1199-5. [DOI] [PubMed] [Google Scholar]
- 78.Yang Y, Chen J, Gao Q, Shan X, Wang J, Lv Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology. 2020;445:152599. doi: 10.1016/j.tox.2020.152599. [DOI] [PubMed] [Google Scholar]
- 79.Liu B, Deng X, Jiang Q, Li G, Zhang J, Zhang N, Xin S, Xu K. Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in macrophages. Biomed Pharmacother. 2020;125:109895. doi: 10.1016/j.biopha.2020.109895. [DOI] [PubMed] [Google Scholar]
- 80.Gao G, Xie Z, Li EW, Yuan Y, Fu Y, Wang P, Zhang X, Qiao Y, Xu J, Hölscher C, Wang H, Zhang Z. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med. 2021;75:540–552. doi: 10.1007/s11418-021-01491-4. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Xu W, Zhai T, You J, Chen Y. Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Pharm Sin B. 2019;9:745–757. doi: 10.1016/j.apsb.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding X, Jian T, Li J, Lv H, Tong B, Meng X, Ren B, Chen J. Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NFκB Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxid Med Cell Longev. 2020;2020:9734560. doi: 10.1155/2020/9734560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Upadhyay KK, Jadeja RN, Vyas HS, Pandya B, Joshi A, Vohra A, Thounaojam MC, Martin PM, Bartoli M, Devkar RV. Carbon monoxide releasing molecule-A1 improves nonalcoholic steatohepatitis via Nrf2 activation mediated improvement in oxidative stress and mitochondrial function. Redox Biol. 2020;28:101314. doi: 10.1016/j.redox.2019.101314. [DOI] [PMC free article] [PubMed] [Google Scholar]