Abstract
γδ T cells have variously been implicated in the protection against, and the pathogenesis of, malaria, but few studies have examined the γδ T-cell response to malaria in African children, who suffer the large majority of malaria-associated morbidity and mortality. This is unfortunate, since available data suggest that simple extrapolation of conclusions drawn from studies of nonimmune adults ex vivo and in vitro is not always possible. Here we show that both the frequencies and the absolute numbers of γδ T cells are transiently increased following treatment of Plasmodium falciparum malaria in Ghanaian children and they can constitute 30 to 50% of all T cells shortly after initiation of antimalarial chemotherapy. The bulk of the γδ T cells involved in this perturbation expressed Vδ1 and had a highly activated phenotype. Analysis of the T-cell receptors (TCR) of the Vδ1+ cell population at the peak of their increase showed that all expressed Vγ chains were used, and CDR3 length polymorphism indicated that the expanded Vδ1 population was highly polyclonal. A very high proportion of the Vδ1+ T cells produced gamma interferon, while fewer Vδ1+ cells than the average proportion of all CD3+ cells produced tumor necrosis factor alpha. No interleukin 10 production was detected among TCR-γδ+ cells in general or Vδ1+ cells in particular. Taken together, our data point to an immunoregulatory role of the expanded Vδ1+ T-cell population in this group of semi-immune P. falciparum malaria patients.
Human T cells express antigen receptors associated in a molecular complex to CD3. In the majority of T cells, these heterodimer antigen receptors are composed by disulfide-linked α and β chains (39), while a minority instead employ receptors composed of γ and δ chains (8). The latter population, which in healthy Caucasians normally constitutes less than 5% of peripheral T cells, can be subdivided into two largely nonoverlapping subsets (32, 37). The former of these subsets usually comprises more than two-thirds of all the γδ T cells and is characterized by disulfide-linked Vγ9 and Vδ2 chains, whereas the other, smaller subset uses a VδI gene product non-disulfide linked to products of Vγ genes other than Vγ9 (12, 47, 48). In contrast, among healthy individuals in Africa, particularly children, the average frequency of TCR-γδ+ cells may be as high as 10% or more and dominated by Vδ1+ cells rather than Vγ9+ cells (25).
Episodes of clinical Plasmodium falciparum malaria in adults with little or no previous malaria exposure have been reported to induce increased levels of γδ T cells, often persisting for several weeks (23, 44, 45). The Vγ9 subset of γδ T cells was found to dominate the in vivo γδ T-cell response in those studies, and several studies of nonexposed donors have shown preferential outgrowth of Vγ9+ cells following malaria antigen stimulation in vitro (3, 15). In contrast, studies from areas where malaria is endemic have failed to confirm both the Vγ9+ cell dominance of the TCR-γδ+ response to malaria (16, 49) and persistent in vivo increases in the frequency of TCR-γδ+ cells (26). Preliminary data obtained during the latter study rather suggested the presence of very transient but pronounced γδ T-cell perturbations immediately following the patient's admission to a hospital.
Based on the above observations, the present study was undertaken as a detailed examination of the γδ T-cell response to P. falciparum malaria in an area where malaria is endemic.
MATERIALS AND METHODS
Donors.
Children (3 to 10 years old) admitted to the Department of Child Health at Korle-Bu Teaching Hospital with P. falciparum malaria were studied during the peak malaria season, June to August. The general inclusion criteria were asexual P. falciparum parasitemia (>10,000/ml), axillary temperatures that were >37.5°C, and negative sickling (HbS) test (metabisulfite method). In addition, only children with strictly defined cerebral malaria (CM) or uncomplicated malaria (UM) are included in the present report. The specific inclusion criteria for these categories have been described in detail previously (34). Children with severe malarial anemia were specifically excluded, since our previous studies have shown that transfusion affects both frequencies and absolute numbers of T cells in the peripheral blood (27). Clinically healthy and age-matched children from a nearby community (Dodowa, Ghana) were included as control donors.
The study was approved by the Ethical and Protocol Review Committee, University of Ghana Medical School, and by the Minister of Health, Ghana. Children were enrolled in the study only after signed, informed consent had been obtained from parents or guardians.
Blood samples.
Samples (250 to 400 μl) of EDTA-anticoagulated blood were obtained from the patients at admission (day 0) and subsequently on days 1, 2, 4, and 21 or once only (4 to 5 ml; day 2) for the analysis of cytokine production and of Vγ chain usage and CDR3 length polymorphism of Vδ1+ T cells. A single 4- to 5-ml sample was obtained from the healthy control children. Thick and thin Giemsa-stained blood smears were prepared from each sample, and hematological analysis was done using an automatic hematology analyzer (Beckman Coulter, Fullerton, Calif.) before further processing. Analysis of CDR3 length polymorphism was done using peripheral blood mononuclear cells (PBMC) isolated by density centrifugation and cryopreserved in liquid nitrogen as described previously (25).
Monoclonal antibodies.
Monoclonal antibodies (MAb) directed against the following determinants were used in this study: CD3 (UCHT1; DAKO, Glostrup, Denmark), CD4 (MT310; DAKO), CD8 (DK25; DAKO), CD69 (Leu-23; BD PharMingen, San Diego, Calif.), CD45RA (4KB5; DAKO), CD45R0 (UCHL1; DAKO), TcR-γδ (11F2; BD PharMingen), Vγ2,3,4 (23D12; Beckman Coulter), Vγ3,5 (56.3; a kind gift from Dieter Kabelitz, Hamburg, Germany), Vγ4 (4A11; BIOAdvance, Emerainville, France), Vγ8 (R4.5; Beckman Coulter), Vγ9 (7A5; Endogen, Woburn, Mass.), Vδ1-Jδ1 (δTCS1; Endogen), and HLA-DR (G46-6; BD PharMingen). Appropriate isotype control antibodies were always included. Most MAb were used as direct conjugates to fluorescein isothiocyanate (FITC), phycoerythrin, or Cy5. In the remaining cases, biotinylated or unconjugated primary antibody was used and labeled with FITC-streptavidin (DAKO), FITC-F(ab′)2 (DAKO), or RPE-Cy5-F(ab′)2 (DAKO) as second-step reagents.
Cell phenotyping.
The plasma was removed following centrifugation, and the cell pellet was resuspended to its original volume in phosphate-buffered saline (PBS). Fifty-microliter aliquots were subsequently labeled (20 min; room temperature) with directly conjugated MAb or appropriate nonspecific isotype control antibodies, followed by lysis of erythrocytes (fluorescence-activated cell sorter lysing solution; BD PharMingen). Samples were then washed twice in PBS and analyzed on a FACScan flow cytometer (BD PharMingen). All samples were live gated by forward and side scatter on lymphocytes, and 5,000 to 10,000 events were collected.
Intracellular cytokine detection.
One-milliliter aliquots of whole blood were incubated with monensin (1.5 μM; Sigma, St. Louis, Mo.), ionomycin (1 μM) and phorbol myristate acetate (50 μg/ml) for 90 min. Following surface staining (CD3, CD8, TCR-γδ) and erythrocyte lysis, the cells were washed twice in a freshly made saponin buffer (PBS-bovine serum albumin-NaN3 containing 0.1% [wt/vol] saponin [Sigma]) and finally incubated with anticytokine (gamma interferon [IFN-γ] tumor necrosis factor alpha [TNF-α], or interleukin 10 [IL-10]; BD PharMingen) antibody for 30 min in the dark (4°C). Following cytokine labeling, the cells were washed twice in saponin buffer and twice in staining buffer, resuspended in the same buffer, and analyzed by flow cytometry as described above.
CDR3 size polymorphism analysis.
Total mRNA was prepared from 1 × 106 to 5 × 106 RNAzol-preserved PBMC according to the manufacturer's instructions (Bioprobe Systems, Montreuil, France). Single-stranded cDNA was subsequently synthesized using the superscript inverse transcriptase (Gibco-BRL, Gaithersburg, Md.). The analysis of the CDR3 size distribution of the Vδ1-Cδ rearrangements was done according to the protocol already described (7). The Vδ1- and Cδ-specific primers used, oriented 5′ to 3′, were Vδ1 (CTGTCAACTTCAAGAAAGCAGCGAAATC) and Cδ-fam (ACGGATGGTTTGGTAGAGGCTGA). To compare the Vδ1 CDR3 profiles obtained from patient and control PBMC, we used a clonality index derived from the Nei diversity index (33). This index measures the probability that two randomized Vδ1 T cells have the same CDR3 length. For each sample, the frequency of TVδ1 cells with a given CDR3 length is expressed as xi, corresponding to the fluorescence intensity of peak number i divided by the sum of the fluorescence intensities of all peaks (n). The index of clonality (I) of the TVδ1 cells is then calculated as I = Σ(xi)2.
Data presentation and statistical analysis.
Summary statistics are given as means and standard errors of the means (SEM). Comparison of patient groups at individual time points was done by two-factor analysis of variance (F) supplemented with the Student-Newman-Keuls test. Indices of clonality were compared by the Mann-Whitney test (T).
RESULTS
Initiation of antimalarial therapy causes a rapid, but transient, increase in frequencies and absolute numbers of γδ T cells in the peripheral circulation.
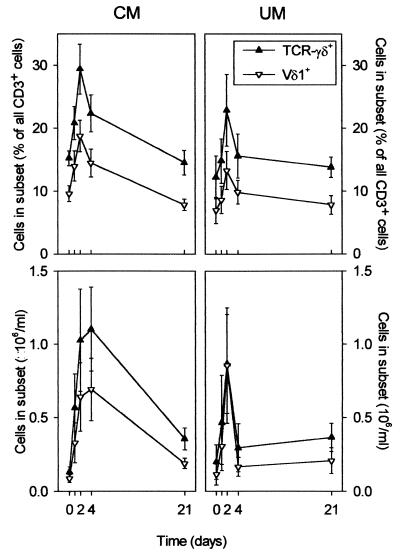
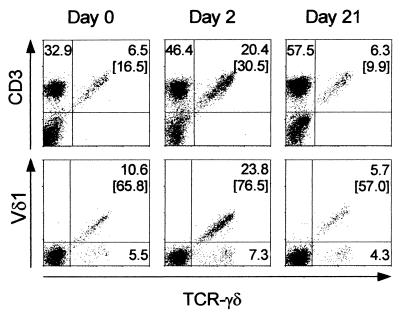
In healthy Caucasians, less than 5% of circulating CD3+ cells express T-cell receptor (TCR)-γδ (17, 36), but the average frequency of TCR-γδ+ cells may be as high as 10% in some African populations (25). It is well established that γδ T cells from nonexposed donors respond to stimulation by malaria antigen preparations in vitro (3, 14, 15), but few studies from areas where malaria is highly endemic have examined malaria-induced changes in γδ T cells in vivo (26). In the present study, about 15% of circulating CD3+ cells from P. falciparum malaria patients carried TCR-γδ at the time of the patient's admission to a hospital prior to initiation of chemotherapy (day 0) (Fig. 1, upper panels, and Fig. 2, left panels). This value is only slightly higher than the steady-state levels found in healthy, age-matched children in the same area (25, 26). However, within the next few days, the proportion of γδ T cells rapidly rose, peaking in most cases around day 2 (P [F] < 0.001), before returning to steady state within 1 week following admission. The increase in the frequency of TCR-γδ+ cells was more pronounced with children with CM (Fig. 1, upper left panel, and Fig. 2) than with children with UM (Fig. 1, upper right panel), but this difference was not significant (P [F] = 0.15). In a few CM patients, more than 50% of the CD3+ cells present in the peripheral blood on day 2 expressed TCR-γδ. Examination of absolute peripheral blood cell numbers showed that the γδ T-cell perturbation persisted longer in CM patients than in UM patients (Fig. 1, lower panels).
FIG. 1.
Proportions (upper panels) and absolute numbers (lower panels) of CD3+ T cells expressing TcR-γδ (▴) or Vδ1 (▿) at various time points after initiation of chemotherapy in 14 patients with CM (left panels) and in 7 patients with UM (right panels). Data are given as means and SEM.
FIG. 2.
Proportions of lymphocytes (FSC/SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] gated) expressing CD3 and TcR-γδ (upper panels) and of CD3+ cells expressing Vδ1 and TCR-γδ (lower panels) in a CM patient. Samples shown were obtained on the day of admission (left panels) and 2 (middle panels) and 21 days (right panels) after initiation of chemotherapy. Numbers indicate percentages of cells in quadrants.
The acquired γδ T-cell response is dominated by Vδ1 cells, with negligible involvement of other γδ T-cell subsets.
While Vγ9+ is by far the dominant γδ T-cell subset in healthy Caucasians, this is not the case in healthy individuals living in areas where malaria is endemic (16, 25). Furthermore, increased proportions of both the Vγ9+ subset and the largely complementary Vδ1+ subset have been reported following P. falciparum malaria in patients from areas where malaria is endemic (23, 49). Only the former of these subsets responds to malaria antigen stimulation in vitro (3, 15). As shown in Fig. 1 and 2, Vδ1+ cells dominated among TCR-γδ+ cells at all time points, whether expressed as fractions of CD3+ cells or as absolute numbers of cells. Furthermore, essentially all the disturbances in the proportions and numbers of TcR-γδ+ cells that were observed in the patients within the first week after admission were due to perturbations within the Vδ1 subset (Fig. 1 and 2) (P [F] < 0.001 [between days]; P [F] = 0.10 [between patient categories]).
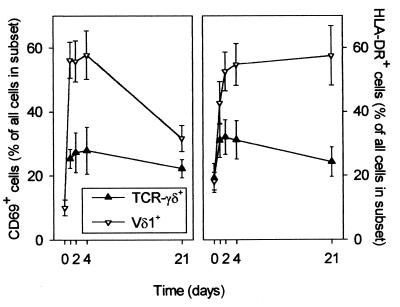
The γδ T cells present in the peripheral circulation initially have an activated phenotype.
We have previously hypothesized that the rapid therapy-induced correction of the lymphopenia in malaria patients reflects the emergence of T cells into the peripheral circulation from sites of disease-induced sequestration and proliferation (11, 27–29). Since T-cell adhesion is a consequence of previous activation, and since our data suggest that γδ T cells are released very rapidly following treatment, we proceeded to examine the level of expression of activation markers on the γδ T cells at various time points. A large proportion of TCR-γδ+ cells, and in particular the Vδ1+ subset of these cells, obtained at various time points after initiation of therapy expressed the early (CD69) and late (HLA-DR) markers of activation (Fig. 3). There was no marked difference in the level of γδ T-cell activation between CM and UM patients, and in Fig. 3 the data from all donors are shown together. The expression of CD69 and HLA-DR on TCR-γδ+ cells and on T cells in general was similar (not shown), with the exception of Vδ1+ cells which showed a much higher level of expression of both markers (Fig. 3).
FIG. 3.
Proportions of TCR-γδ+ (▴) and Vδ1+ (▿) T cells expressing the early activation marker CD69 (left panel) and the late activation marker HLA-DR (right panel) at various time points after initiation of chemotherapy in eight P. falciparum malaria patients. Data are given as means and SEM.
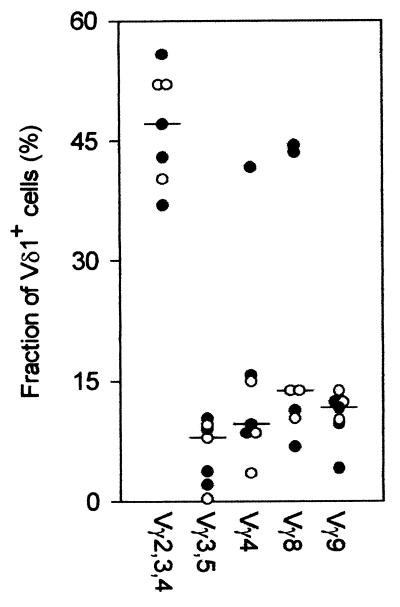
Vδ1+ T cells use all expressed Vγ chains.
The very high frequencies and absolute numbers of Vδ1+ cells around day 2 after initiation of therapy prompted us to examine the TCR usage of these cells in more detail. As seen in Fig. 4, Vδ1+ cells expressing all Vγ gene products could be identified in all children examined this way at day 2, without marked differences between UM and CM patients. The most common phenotype among the Vδ1+ cells was cells labeled by MAb 23D12, which reacts with cells expressing Vγ2, Vγ3, or Vγ4, but all expressed Vγ chains were identified among the Vδ1+ cells, as observed previously (25). The data did not suggest expansion of Vδ1+ cells bearing particular Vγ chains. Cocktails of all the Vγ antibodies labeled only about 85% of the Vδ1+ cells, in contrast to experiments done in parallel on healthy children, where essentially all Vδ1+ cells were labeled by the antibody cocktail. The reason for this difference is unclear and is under investigation.
FIG. 4.
Expression of Vγ gene products by Vδ1+ T cells obtained at day 2 following initiation of chemotherapy from four patients with cerebral (●) and three patients with uncomplicated ( ) P. falciparum malaria. Individual data points and medians are given.
The high proportion of Vδ1+ cells is not caused by conventional antigen-driven proliferation.
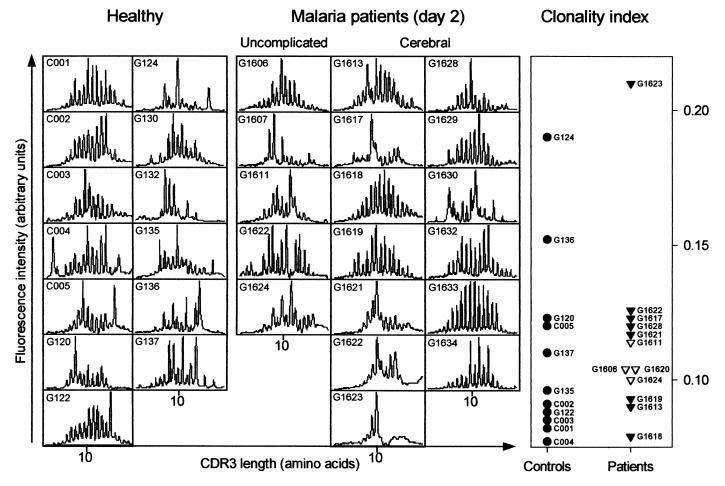
In the absence of evidence of unusual dominance of particular Vγ-Vδ combinations, we proceeded to investigate whether the high proportion of Vδ1+ cells was driven by antigen recognized by Vδ1 alone. This was done by size spectratyping of the CDR3 antigen recognition domain of Vδ1 (43). This analysis was completed for day-2 samples from 5 UM and 13 CM patients, in addition to samples from 13 healthy control children, and revealed no obvious differences between patients and control donors or between patient groups (Fig. 5). Supplementary clonality index analysis, taking into consideration both the degree of CDR3 length polymorphism and the percentage of Vδ1+ cells, did not show significant differences (P [T] = 0.29) between patients and controls or among patient groups (Fig. 5).
FIG. 5.
Vδ1 CDR3 characterization of T cells obtained from 13 healthy children and from 18 P. falciparum malaria patients (5 with UM and 13 with CM) on day 2 following initiation of chemotherapy. Individual CDR3 length polymorphism (Immunoscope profiles; left panel) and Vδ1 clonality indices (right panel) from healthy children (●) (two data points missing) and patients with UM (▾) (one data point missing) and CM (▿) (five data points missing) are shown.
Vδ1+ cells have a cytokine profile different from that of CD3+ cells in general.
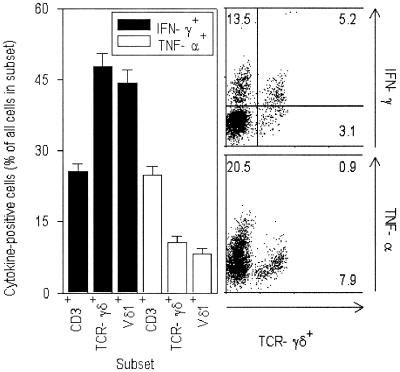
As a final element in our characterization of the Vδ1+ cells involved in the perturbation of this subset following malaria, we evaluated subset-specific production of three major T-cell cytokines, IFN-γ, TNF-α, and IL-10. Figure 6 shows that the proportions of cytokine-producing CD3+ cells, TCR-γδ+ cells, and Vδ1+ cells were significantly different (P [F] < 0.001). Thus, intracellular IFN-γ could be detected in almost twice as many TCR-γδ+ cells and Vδ1+ cells as among CD3+ cells in general. Conversely, TCR-γδ+ cells and Vδ1+ cells produced substantially less TNF-α than CD3+ cells in general (P [F] < 0.001). We did not detect any IL-10 production by TCR-γδ+ cells (not shown).
FIG. 6.
Cytokine expression by T-cell subsets following 90 min of stimulation in vitro. Left panel, proportion of subsets producing IFN-γ (filled bars) and TNF-α (open bars). Right panels, production of IFN-γ (upper panel) and TNF-α (lower panel) by CD3+ cells from a UM patient (G1611). Numbers indicate percentages of all CD3+ cells in quadrants.
DISCUSSION
γδ T cells have been implicated in the immune response to malaria (reviewed in reference 35), but few studies have examined individuals exposed to malaria parasites in areas where the disease is endemic. This is problematic, since the limited evidence available from such studies indicates that a simple extrapolation from studies of nonexposed individuals may not always be possible (16, 26, 49). Thus, the sustained and Vγ9+-cell-dominated responses seen in nonexposed individuals caused by cells activated by phosphorylated nonpeptide antigens present in malaria parasites (4) were not seen in the above-cited studies in Africa.
Here we present evidence that children from areas where malaria is endemic show a very pronounced, but transient, γδ T-cell response during treatment of P. falciparum malaria. This perturbation peaked around day 2 after initiation of antimalarial chemotherapy and thus occurred considerably earlier than the TCR-αβ lymphopenia-to-lymphocytosis-to-homeostasis sequence reported from the same area (27). It is likely that the perturbation is induced by a therapy-mediated alleviation of tissue inflammation and that the transiently increased frequency and absolute numbers represent cells emerging from sites of malaria-induced sequestration and expansion (reviewed in reference 24).
Subset analysis at the time of peak frequency and the number of TCR-γδ+ cells revealed that the peak was totally dominated by changes in cells using Vδ1 in their TCR, while only very minor perturbations were seen among Vδ1-negative γδ T cells. In contrast to the healthy children that we have previously studied in the same area (25), the Vδ1+ cells obtained at day 2 of treatment from the malaria patients studied here showed marked evidence of activation. The expanded Vδ1+ population at this time was not characterized by particular Vγ-chain usage and did not show significant dominance of certain CDR3 lengths. In all these respects, our patients resemble those from other studies of infectious diseases, such as HIV infection and autoimmune disorders (6, 10, 21, 38, 41).
The transient increase in circulating Vδ1+ T cells in the absence of evidence of conventional antigen-driven expansion of Vδ1+ T cells during P. falciparum malaria raises the question of its cause. In vitro stimulation of T cells from unexposed donors by crude P. falciparum antigen causes expansion of Vγ9+ Vδ2+ cells but not of Vδ1+ cells (3, 15). In line with this, we did not find a significant relation between in vivo frequencies of Vδ1+ cells and asymptomatic parasitemia (25).
The Vγ9+ Vδ2+ T-cell response to stimulation by P. falciparum preparations is caused by recognition of nonpeptide phosphorylated antigens found in numerous bacteria and protozoa, including P. falciparum (4, 9). While the antigens that are recognized by these cells are thus well characterized, little is known about the antigens recognized by Vδ1+ cells (reviewed in reference 40). It has been proposed that these cells respond to stress- or infection-induced molecules on a variety of cell types, including epithelial cells and B cells. Specifically, Vδ1+ cells have been reported to recognize the weakly polymorphic major histocompatibility complex class I-like molecules MICA and MICB (18, 19). However, the same authors have recently shown that it is NKG2D rather than TCR-γδ that is the MICA and MICB receptor (2). Other recent work has demonstrated recognition of CD1c molecules expressed on dendritic cells and B cells by Vδ1+ clones expressing various Vγ chains (46).
Finally, it should be noted that although the conditions that have been described as being characterized by locally or systemically increased levels of Vδ1+ T cells are seemingly very heterogeneous, they all share evidence of polyclonal B-cell activation. This is not least the case in malaria (1, 13), and it is thus of interest that activated B cells are an antigenic target of Vδ1+ T cells (20, 30, 42). Although T-cell activation is a general feature of P. falciparum malaria (11, 29), we found particularly high levels of activation among Vδ1+ T cells. The lack of evidence of particular antigens driving the expansion of Vδ1+ cells, and their preponderance in situations where B-cell activation is a major feature, make it tempting to speculate that they are in fact regulatory cells responding to self antigens expressed by activated B cells. An autoregulatory role for γδ T cells has been proposed in several recent reviews (5, 22). The antigens recognized by such putative B-cell-regulatory Vδ1+ T cells remain unknown, but the observation that certain cytotoxic T cells expressing TCR-γδ recognize idiotype is intriguing in this context (31, 50). A B-cell-cytotoxic role for the Vδ1+ T cells in our malaria patients is consistent with the observed ex vivo cytokine production profile of these cells, which resembles that of CD8+ cells, and is furthermore in line with our preliminary in vitro experiments (unpublished data). We are currently characterizing a panel of Vδ1+ γδ T-cell clones from an individual from our study area to pursue this issue further.
In conclusion, we have shown that patients with previous parasite exposure and who are undergoing treatment for P. falciparum malaria transiently show markedly increased levels of highly activated Vδ1+ T cells having a proinflammatory cytokine profile. These cells are not restricted by particular Vγ chains and do not appear to expand in response to a specific antigen recognized by a limited set of Vδ1+ clones. Taken together, the data point to an immunoregulatory role of these cells. This is the first detailed longitudinal study of the γδ T-cell response to P. falciparum malaria in children from Africa, i.e., those who suffer most of the morbidity and essentially all the mortality from this disease. Our findings are at variance with most previous data from ex vivo and in vitro studies of nonimmune adults and thus emphasize the extreme caution necessary when extrapolating from model laboratory investigations to the situation in the main target population of this major health problem.
ACKNOWLEDGMENTS
We thank Marc M. Addae, Alex Coffie, Abdelrahman Hammond, Enid Owusu, Gitte Pedersen, and John Tsakpo for excellent technical assistance. The cooperation of the donors and their guardians is gratefully acknowledged.
This study received financial support from the ENRECA program of the Danish International Development Assistance (Danida; grant no. 14.Dan.8.L.306), the International Co-operation with Developing Countries (INCO-DC) program of the European Commission (grant no. IC18-CT98-0370), the Danish Medical Research Council (SSVF; grant no. 9802405), and the Danish Research Council for Development Research (RUF; grant no. 90900).
REFERENCES
- 1.Banic D M, Viana-Martins F S, De Souza J M, Peixoto T D, Daniel-Ribeiro C. Polyclonal B-lymphocyte stimulation in human malaria and its association with ongoing parasitemia. Am J Trop Med Hyg. 1991;44:571–577. doi: 10.4269/ajtmh.1991.44.571. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, Groh V, Wu J, Steinle A, Phillips J H, Lanier L L, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 3.Behr C, Dubois P. Preferential expansion of Vγ9Vδ2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol. 1992;4:361–366. doi: 10.1093/intimm/4.3.361. [DOI] [PubMed] [Google Scholar]
- 4.Behr C, Poupot R, Peyrat M A, Poquet Y, Constant P, Dubois P, Bonneville M, Fournie J J. Plasmodium falciparumstimuli for human γδ T cells are related to phosphorylated antigens of mycobacteria. Infect Immun. 1996;64:2892–2896. doi: 10.1128/iai.64.8.2892-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 6.Boullier S, Cochet M, Poccia F, Gougeon M-L. CDR3-independent γδ Vδ1+T cell expansion in the peripheral blood of HIV-infected persons. J Immunol. 1995;154:1418–1431. [PubMed] [Google Scholar]
- 7.Boullier S, Dadaglio G, Lafeuillade A, Debord T, Gougeon M-L. Vδ1 T cells expanded in the blood throughout HIV infection display a cytotoxic activity and are primed for TNF-α and IFN-γ production but are not selected in lymph nodes. J Immunol. 1997;159:3629–3637. [PubMed] [Google Scholar]
- 8.Brenner M B, McLean J, Dialynas D P, Strominger J L, Smith J A, Owen F L, Seidman J G, Ip S, Rosen F, Krangel M S. Identification of a putative second T-cell receptor. Nature. 1986;322:145–149. [PubMed] [Google Scholar]
- 9.Constant P, Davodeau F, Peyrat M-A, Poquet Y, Puzo G, Bonneville M, Fournié J-J. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 10.De Maria A, Ferrazin A, Ferrini S, Ciccone E, Terragna A, Moretta L. Selective increase of a subset of T cell receptor γδ T lymphocytes in the peripheral blood of patients with human immunodeficiency virus type 1 infection. J Infect Dis. 1992;165:917–919. doi: 10.1093/infdis/165.5.917. [DOI] [PubMed] [Google Scholar]
- 11.Elhassan I M, Hviid L, Satti G, Akerström B, Jakobsen P H, Jensen J B, Theander T G. Evidence of endothelial inflammation, T cell activation, and T cell reallocation in uncomplicated Plasmodium falciparummalaria. Am J Trop Med Hyg. 1994;51:372–379. doi: 10.4269/ajtmh.1994.51.372. [DOI] [PubMed] [Google Scholar]
- 12.Faure F, Jitsukawa S, Triebel F, Hercend T. Characterization of human peripheral lymphocytes expressing the CD3-γ/δ complex with anti-receptor monoclonal antibodies. J Immunol. 1988;141:3357–3360. [PubMed] [Google Scholar]
- 13.Freeman R R, Parish C R. Polyclonal B-cell activation during rodent malarial infections. Clin Exp Immunol. 1978;32:41–45. [PMC free article] [PubMed] [Google Scholar]
- 14.Goerlich R, Häcker G, Pfeffer K, Heeg K, Wagner H. Plasmodium falciparummerozoites primarily stimulate the Vγ9 subset of human γ/δ T cells. Eur J Immunol. 1991;21:2613–2616. doi: 10.1002/eji.1830211045. [DOI] [PubMed] [Google Scholar]
- 15.Goodier M, Fey P, Eichmann K, Langhorne J. Human peripheral blood γδ T cells respond to antigens of Plasmodium falciparum. Int Immunol. 1992;4:33–41. doi: 10.1093/intimm/4.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Goodier M, Krause-Jauer M, Sanni A, Massougbodji A, Sadeler B-C, Mitchell G H, Modolell M, Eichmann K, Langhorne J. γδ T cells in the peripheral blood of individuals from an area of holoendemic Plasmodium falciparumtransmission. Trans R Soc Trop Med Hyg. 1993;87:692–696. doi: 10.1016/0035-9203(93)90299-6. [DOI] [PubMed] [Google Scholar]
- 17.Groh V, Porcelli S, Fabbi M, Lanier L L, Picker L J, Anderson T, Warnke R A, Bhan A K, Strominger J L, Brenner M B. Human lymphocytes bearing T cell receptor γ/δ are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein K H, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 20.Hacker G, Kromer S, Falk M, Heeg K, Wagner H, Pfeffer K. Vδ1+subset of human γδ T cells responds to ligands expressed by EBV-infected Burkitt lymphoma cells and transformed B lymphocytes. J Immunol. 1992;149:3984–3989. [PubMed] [Google Scholar]
- 21.Halstensen T S, Scott H, Brandtzaeg P. Intraepithelial T cells of the TcR γ/δ+ CD8− Vδ1/Jδ1+phenotypes are increased in coeliac disease. Scand J Immunol. 1989;30:665–672. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- 22.Hayday A, Geng L. γδ cells regulate autoimmunity. Curr Opin Immunol. 1997;9:884–889. doi: 10.1016/s0952-7915(97)80193-8. [DOI] [PubMed] [Google Scholar]
- 23.Ho M, Tongtawe P, Kriangkum J, Wimonwattrawatee T, Pattanapanyasat K, Bryant L, Shafiq J, Suntharsamai P, Looareesuwan S, Webster H K, Elliott J F. Polyclonal expansion of peripheral γδ T cells in human Plasmodium falciparummalaria. Infect Immun. 1994;62:855–862. doi: 10.1128/iai.62.3.855-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hviid L. Peripheral T-cell non-responsiveness in individuals exposed to Plasmodium falciparummalaria. APMIS. 1995;103(Suppl. 53):1–46. [PubMed] [Google Scholar]
- 25.Hviid L, Akanmori B D, Loizon S, Kurtzhals J A L, Ricke C H, Lim A, Koram K A, Nkrumah F K, Mercereau-Puijalon O, Behr C. High frequency of circulating γδ T cells with dominance of the Vδ1 subset in a healthy population. Int Immunol. 2000;12:797–805. doi: 10.1093/intimm/12.6.797. [DOI] [PubMed] [Google Scholar]
- 26.Hviid L, Kurtzhals J A L, Dodoo D, Rodrigues O, Ronn A, Commey J O O, Nkrumah F K, Theander T G. The γ/δ T-cell response to Plasmodium falciparummalaria in a population in which malaria is endemic. Infect Immun. 1996;64:4359–4362. doi: 10.1128/iai.64.10.4359-4362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hviid L, Kurtzhals J A L, Goka B Q, Oliver-Commey J O, Nkrumah F K, Theander T G. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparummalaria. Infect Immun. 1997;65:1090–1093. doi: 10.1128/iai.65.10.4090-4093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hviid L, Theander T G, Abdulhadi N H, Abu-Zeid Y A, Bayoumi R A, Jensen J B. Transient depletion of T cells with high LFA-1 expression from peripheral circulation during acute Plasmodium falciparummalaria. Eur J Immunol. 1991;21:1249–1253. doi: 10.1002/eji.1830210523. [DOI] [PubMed] [Google Scholar]
- 29.Hviid L, Theander T G, Abu-Zeid Y A, Abdulhadi N H, Jakobsen P H, Saeed B O, Jepsen S, Bayoumi R A L, Jensen J B. Loss of cellular immune reactivity during acute Plasmodium falciparummalaria. FEMS Microbiol Immunol. 1991;76:219–228. doi: 10.1111/j.1574-6968.1991.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 30.Hyjek E M, Bartkowiak J, Drozdz R, Wasik T J, Jasinski M, Kaneko Y, Lischner H W, Kozbor D. Evidence for B cell-mediated activation of Vδ1+T lymphocytes during progression of HIV infection. J Immunol. 1997;158:464–474. [PubMed] [Google Scholar]
- 31.Kim H T, Nelson E L, Clayberger C, Sanjanwala M, Sklar J, Krensky A M. γδ T cell recognition of tumor Ig peptide. J Immunol. 1995;154:1614–1623. [PubMed] [Google Scholar]
- 32.Krangel M S, Band H, Hata S, McLean J, Brenner M B. Structurally divergent human T cell receptor gamma proteins encoded by distinct Cγ genes. Science. 1987;237:64–67. doi: 10.1126/science.2955517. [DOI] [PubMed] [Google Scholar]
- 33.Krener A, Petit R J, Pons O. Measures of polymorphism within and among populations. In: Karp A, Isaac P G, Ingram D S, editors. Molecular tools for screening biodiversity. London, England: Chapman & Hall; 1998. pp. 301–311. [Google Scholar]
- 34.Kurtzhals J A L, Rodrigues O, Addae M, Commey J O O, Nkrumah F K, Hviid L. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparummalaria. Br J Haematol. 1997;97:169–174. doi: 10.1046/j.1365-2141.1997.82654.x. [DOI] [PubMed] [Google Scholar]
- 35.Langhorne J, Goodier M, Behr C, Dubois P. Is there a role for γδ cells in malaria ? Immunol Today. 1992;13:298–300. doi: 10.1016/0167-5699(92)90041-5. [DOI] [PubMed] [Google Scholar]
- 36.Lanier L L, Ruitenberg J, Bolhuis R L, Borst J, Phillips J H, Testi R. Structural and serological heterogeneity of γ/δ T cell antigen receptor expression in thymus and peripheral blood. Eur J Immunol. 1988;18:1985–1992. doi: 10.1002/eji.1830181218. [DOI] [PubMed] [Google Scholar]
- 37.Littman D R, Newton M, Crommie D, Ang S L, Seidman J G, Gettner S N, Weiss A. Characterization of an expressed CD3-associated Ti gamma-chain reveals C gamma domain polymorphism. Nature. 1987;326:85–88. doi: 10.1038/326085a0. [DOI] [PubMed] [Google Scholar]
- 38.Meliconi R, Pitzalis C, Kingsley G H, Panayi G S. γ/δ T cells and their subpopulations in blood and synovial fluid from rheumatoid arthritis and spondyloarthritis. Clin Immunol Immunopathol. 1991;59:165–172. doi: 10.1016/0090-1229(91)90090-w. [DOI] [PubMed] [Google Scholar]
- 39.Meuer S C, Acuto O, Hussey R E, Hodgdon J C, Fitzgerald K A, Schlossman S F, Reinherz E L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983;303:808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- 40.Morita C T, Mariuzza R A, Brenner M B. Antigen recognition by human γδ T cells: pattern recognition by the immune system. Springer Semin Immunopathol. 2000;22:191–218. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 41.Ohta M, Sato N. The cytotoxic analysis of T cell receptor Vδ1+T cell lines derived from the synovial fluid of rheumatoid arthritis patients. Clin Exp Immunol. 1994;97:193–199. doi: 10.1111/j.1365-2249.1994.tb06067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orsini D L, van Gills M, Kooy Y M, Struyk L, Klein G, van-den Elsen P, Koning F. Functional and molecular characterization of B cell-responsive Vδ1+γδ T cells. Eur J Immunol. 1994;24:3199–3204. doi: 10.1002/eji.1830241243. [DOI] [PubMed] [Google Scholar]
- 43.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor β chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roussilhon C, Agrapart M, Ballet J-J, Bensussan A. T lymphocytes bearing the γδ T cell receptor in patients with acute Plasmodium falciparummalaria. J Infect Dis. 1990;162:283–285. doi: 10.1093/infdis/162.1.283-a. [DOI] [PubMed] [Google Scholar]
- 45.Roussilhon C, Agrapart M, Guglielmi P, Bensussan A, Brasseur P, Ballet J J. Human TcRγδ+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol. 1994;95:91–97. doi: 10.1111/j.1365-2249.1994.tb06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spada F M, Grant E P, Peters P J, Sugita M, Melián A, Leslie D S, Lee H K, Van Donselaar E, Hanson D A, Krensky A M, Majdic O, Porcelli S A, Morita C T, Brenner M B. Self-recognition of CD1 by γ/δ T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Triebel F, Faure F, Graziani M, Jitsukawa S, Lefranc M P, Hercend T. A unique V-J-C-rearranged gene encodes a γ protein expressed on the majority of CD3+ T cell receptor-α/β−circulating lymphocytes. J Exp Med. 1988;167:694–699. doi: 10.1084/jem.167.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Triebel F, Faure F, Mami-Chouaib F, Jitsukawa S, Griscelli A, Genevee C, Roman R S, Hercend T. A novel human Vδ gene expressed predominantly in the TiγA fraction of γ/δ+peripheral lymphocytes. Eur J Immunol. 1988;18:2021–2027. doi: 10.1002/eji.1830181223. [DOI] [PubMed] [Google Scholar]
- 49.Worku S, Björkman A, Troye-Blomberg M, Jemaneh L, Färnert A, Christensson B. Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct γδ+ T cell patterns in Plasmodium falciparum and P. vivaxinfections. Clin Exp Immunol. 1997;108:34–41. doi: 10.1046/j.1365-2249.1997.d01-981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright A, Lee J E, Link M P, Smith S D, Carroll W, Levy R, Clayberger C, Krensky A M. Cytotoxic T lymphocytes specific for self tumor immunoglobulin express T cell receptor delta chain. J Exp Med. 1989;169:1557–1564. doi: 10.1084/jem.169.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]