Abstract
Humanized liver chimeric mice (PXB-mice) are generated by the transplantation of human hepatocytes into mice that have severe combined immunodeficiency and express an albumin-promoted urokinase-type plasminogen activator (cDNA-uPA/SCID) transgene. Human hepatocytes cannot synthesize ascorbic acid (AA; commonly called vitamin C) and humans require supplementation to prevent vitamin C deficiency. PXB-mouse livers contain up to approximately 95% human hepatocytes, which likely affects AA synthesis. To determine whether dietary AA supplementation prevents scurvy-like symptoms and death in PXB-mice, a 12 week study that compared nonsupplemented and supplemented PXB-mice was conducted. Approximately 4 weeks into the study, PXB-mice without dietary supplementation of AA displayed weight loss and clinical signs of hypovitaminosis C, including hunched posture, unkempt appearance, and lameness. Pathologic evaluation of nonsupplemented PXB-mice revealed lesions consistent with hypovitaminosis C. Mean serum AA concentrations in the nonsupplemented PXB-mice were below the limit of quantitation (0.5 µg/mL) and were substantially less than those of controls. AA was also measured in a number of tissues, including adrenal gland, brain, liver, and testis; low AA concentrations were similarly observed in tissues obtained from the nonsupplemented PXB-mice. Collectively, these findings support AA supplementation in PXB-mice to prevent the development of hypovitaminosis C and the potential utility of nonsupplemented PXB-mice as an animal model of scurvy.
Abbreviations and Acronyms: AA, ascorbic acid
Introduction
Vitamin C is a critical nutrient for maintaining proper bone and cardiovascular function in a variety of animal species, from fish to humans.1 However, some species, including humans, have evolutionarily lost the ability to independently synthesize vitamin C. In humans, this is due to mutations in the L-gulono-↖-lactone oxidase (GULO) coding gene that results in the loss of function of the GULO enzyme. The impaired function of this enzyme results in the well-known chronic dietary vitamin C or ascorbic acid (AA) deficiency condition of scurvy. The main clinical manifestations of scurvy are primarily due to impaired collagen formation that leads to hemorrhages in the skin, joints, and oral cavity, impaired wound healing and bone formation, lethargy and fatigue, and if untreated, death. In contrast to humans, mice and most other mammals can synthesize AA from glucose and do not have a dietary requirement for vitamin C and have no known deficiency syndrome similar to that of humans.1,2,4
PXB-mice are a chimeric strain that has a highly humanized liver that is very similar to a human liver both histologically and functionally.9,10 In a preliminary study conducted internally by Inotiv, PXB-mice with high human hepatocyte replacement indices that were fed a commercial western laboratory rodent chow developed marked weight loss and dehydration within 2 to 4 weeks after receipt from the vendor (PhoenixBio, Hiroshima, Japan). Postmortem examination of a small subset of these mice revealed multifocal peri-articular hemorrhages and bone fractures (Figure 1). These observations led us to speculate that these humanized mice might lack the ability to independently synthesize AA, as do humans, and that without dietary supplementation, they develop scurvy-like conditions. The purpose of this study was to determine if morbidity and mortality in the PXB-mice were related to a lack of dietary AA supplementation and resultant systemic hypovitaminosis C.
Figure 1.
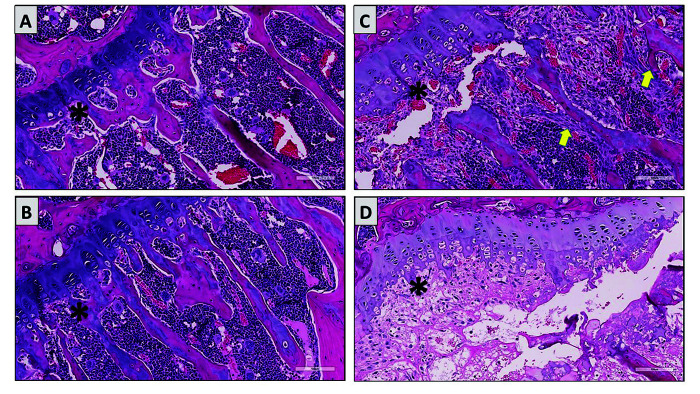
Tibial physis (asterisks) from a SCID mouse (A), a PXB-mouse supplemented with vitamin C (B), and 2 nonsupplemented (vitamin C deficient) mice (C and D). The primary spongiosa adjacent to the physis of the vitamin C deficient mice had markedly less ossification with microfractures, proliferation of spindle cells along partially mineralized trabeculae (yellow arrows), or complete necrosis of the primary spongiosa (D). The primary spongiosa of supplemented mice (B) euthanized on Day 28 were similar in thickness to the SCID mice (A) with no apparent fractures, spindle cell proliferation, or necrosis.
Materials and Methods
All animal procedures were performed under an approved Institutional Animal Care and Use Committee protocol at Saint Louis University Department of Comparative Medicine. All research was conducted in conformance with “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training”.
Experimental design/husbandry.
The study consisted of 3 groups of 10 mice (Mus musculus) each. The background (control) group (Group 1) included 10-week-old male SCID (CB17/Icr-Prkdcscid/IcrIcoCrl) mice obtained from Charles River Laboratories (Wilmington, MA). Groups 2 and 3 included 16- to 19-week-old male PXB-mice (human hepatocyte replacement indices ranging from 82% to 95%) that had been acquired from PhoenixBio, Ltd. (Higashi-Hiroshima City, Japan). PXB-mice were produced by transplanting commercially available cryopreserved human hepatocytes (Lot number: BD195, 2-y-old Hispanic female, BD Biosciences, San Jose, CA) into the spleens of cDNA-uPA+/−/SCID mice that are hemizygous for the cDNA-uPA transgene and possess the SCID trait. The replacement index of human hepatocytes engrafted in the mouse liver was assessed by measuring human albumin concentrations in blood using a method established in a previous report.10 Five mice per group were euthanized at 4 weeks for AA analysis and microscopic examination. All remaining mice (up to 5 per group) were euthanized at 12 weeks to analyze the same parameters.
SCID and supplemented PXB-mice were housed individually, while non-supplemented PXB-mice were housed five per cage. All mice were housed in nonventilated, sterile polycarbonate caging containing ALPHA-DriTM bedding (Shepard Specialty Papers, Watertown, TN) and Cotton squaresTM (Ancare Corporation, Bellmore, NY). All mice received irradiated Prolab IsoPro RMH 3000 (5P76) rodent diet (LabDiet, St. Louis, MO) and distilled water. However, Group 3 received water containing 330 mg/L AA (Sigma-Aldrich, St. Louis, MO) and were offered 5 to 10 mg of dietary AA per day by placing irradiated Fruit Crunchies™ (Bio-Serv, Flemington, NJ) containing 0.21 mg AA per pellet on the cage floor.4 The SCID and PXB-mice were free of murine specific pathogens. The room temperature range was 20 to 26°C, the humidity range was 30 to 70%, and the room had a light cycle of 12 hours of light and 12 hours of dark. Sentinel mice were tested quarterly throughout the facility to monitor the pathogen status overall.
The overall health of the mice was monitored by conducting daily cage-side observations and weekly physical examinations. Mice were carefully observed for any changes in gait as this could indicate an abnormality in the leg bones. If an abnormal gait was noted, the mouse was humanely euthanized using carbon dioxide asphyxiation. Mice were weighed daily, and if a mouse lost over 20% body weight as compared with the preshipment weight provided by the vendor, it was euthanized by carbon dioxide asphyxiation. Mice that were euthanized due to a moribund condition or at scheduled end point were evaluated by gross necropsy and a terminal blood sample and tissue samples (adrenal gland, brain, liver, and testes) were collected and snap frozen in liquid nitrogen for subsequent AA analysis. Tissues for histopathology (aorta, femorotibial joint, sternum, femur, bone marrow, brain, epididymis, esophagus, eye, gallbladder, adrenal gland, harderian gland, pituitary gland, prostate gland, salivary gland, seminal vesicle gland, coagulating gland, thyroid gland, parathyroid gland, heart, kidney, cecum, colon, rectum, liver, lung, mandibular lymph node, mesenteric lymph node, mandible with lower incisor, biceps femoris, nasal turbinates, optic nerve, sciatic nerve, pancreas, skin, duodenum, ileum, jejunum, spinal cord [cervical, thoracic, lumbar], spleen, stomach, testis, thymus, trachea, and urinary bladder) were fixed in 10% neutral buffered formalin or Davidson’s Solution (eyes, testis, epididymis) for approximately 72 hours, then transferred to 70% ethanol.
Serum and tissue collection and ascorbic acid bioanalysis.
AA was measured in serum samples that were collected weekly by nicking the tail vein of unanesthetized mice and in liver, kidney, brain, and testes collected following euthanasia. Brain, liver, testes, and adrenal gland tissues were diluted with 8.9 mM aqueous sodium metabisulfite (3:1, w/v). Approximately 10 small, steel homogenization beads were added to deep-well reservoirs containing solid tissue that was subsequently homogenized for 1 minute at ambient temperature in a Mini-BeadBeater-96 (Biospec Products, Bartlesville, OK); the resultant tissue homogenate was prepared for sample extraction and liquid chromatographic tandem mass spectrometry analysis (LC-MS/MS). Serum (10 µL) was diluted with sodium metabisulfite (8.9 mM, 150 µL) containing 13C-labeled AA (internal standard) and vortexed at 500 rpm for 5 minutes. The resultant mixture was vortex-mixed for 5 minutes and centrifuged at 3,000 rpm for 10 minutes. The resulting supernatant (20 µL) was diluted in HPLC grade water (180 µL) and mixed (via vortex) in preparation for LC-MS/MS analysis. Stock solutions of AA were prepared at a concentration of 10 mg/mL in water and serial diluted for the generation of calibration standards (0.5, 1, 2.5, 5, 10, 25, 50, 100, 250, 500, and 1,000 µM); quality control (QC) samples (0.6, 6, 30, 150, and 750 µM) were prepared in duplicate by further diluting the stock solution in water (this provided a surrogate matrix due to endogenous AA concentration in serum).
Serum and tissue samples were subjected to liquid chromatography tandem mass spectrometry (LC-MS/MS) with samples introduced via electrospray ionization (ESI) into an AB Sciex 4000 triple quadrupole mass spectrometer (Foster City, CA) coupled to a Shimadzu Nexera HPLC dual pump system (Kyoto, Japan) and a Leap Technologies CTC PAL auto-sampler (Carrboro, NC). Chromatographic separation was achieved by employing a binary solvent manager in conjunction with a Luna Phenyl Hexyl column (4.6 × 50mm, 5µm; Phenomenex, Torrance, CA). The mobile phases used were 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The following gradient elution program was employed: 15% B at 0.0 to 0.2 min, 15% to 40% B at 0.21 min, 40% to 85% B at 0.21 to 1.2 minutes, held at 85% B until 1.6 minutes, then returned to 15% B and held until 2 minutes. The flow rate was 0.7 mL/minute and injection volumes were 5 µL. The column temperature was maintained at 40°C and the autosampler was cooled to 10°C.
The AB Sciex 4000 triple quadrupole mass spectrometer (AB Sciex, Ontario, Canada) employed electrospray ionization (ESI) set to negative ionization mode. Multiple reaction monitoring (MRM) parameters were optimized during infusion of working solutions for both parent and internal standard. The MRM transitions monitored were 175.0/115.0 Da and 181.0/119.0 Da for AA and its internal standard, 13C6- ascorbic acid (AA- 13C6), respectively. The mass spectrometer source and ion optic settings were as follows: source temperature, 500°C; curtain gas 20; gas 1:50; gas 2:50; ion spray voltage 4000V; unit resolution for both Q1 and Q3; declustering, −40; EP, −10; CE, −17; CXP, −14; dwell time, 100 ms. Study sample concentrations were determined using a peak-area-ratio of AA:AA-13C6 and applying a linear least squares regression analysis (1/x weighting; Analyst version 1.6.2).
Reagents.
L-Ascorbic acid (AA) and L-Ascorbic acid 13C6 (AA 13C6) were purchased from Toronto Research Chemical (Toronto, Canada). Water and acetonitrile used were HPLC grade and purchased from Fisher Scientific (Waltham, MA). Formic Acid was reagent grade and purchased from Sigma-Aldrich (St. Louis, MO).
Human albumin.
To assess and monitor the viability of the human hepatocytes, serum samples were analyzed approximately every four weeks for human albumin levels using the Human Albumin ELISA Quantitation kit (Bethyl Laboratories, Montgomery, TX).
Histopathology.
Tissues from all mice were trimmed and processed, embedded in paraffin, sectioned at 5 µm, stained with hematoxylin and eosin, and examined microscopically. Microscopic examination was performed by a board-certified veterinary pathologist who was aware of the treatment group. Non-neoplastic microscopic findings were either entered as “present” or assigned a severity grade from 1 to 5 (minimal, mild, moderate, marked, or severe). These severity grades reflected a combination of considerations by the pathologist that included the extent of the process, its distribution, and the degree of alteration in the histologic sections examined.
Micro computed topography (CT) imaging.
For illustrative purposes, the right hind limbs of representative mice (1 mouse from each group) were scanned using micro computed topography (MicroCT 35, ScanCo Medical, Brüttisellen, Switzerland; X-ray tube potential 70 kVp, integration time 300 ms, X-ray intensity 145 µA, isotropic voxel size 10 um, frame averaging 1, projections 1000, high resolution scan). The mice for imaging were chosen based on clinical observations of an impaired gait.
Image acquisition.
Photomicrographs of bone, nasal turbinate, and teeth were obtained from whole slide digital scans (Aperio ImageScope [v12.3.2.5030]) using the software’s preset/standard settings. Edits for white balance and tissue fold/dust removal were performed using Microsoft Photo Editor and composite images and annotations were created using Microsoft PowerPoint for Microsoft 365. Micro CT images were captured using the software installed on the MicroCT 35 and composite images and annotations were created using Microsoft PowerPoint for Microsoft 365.
Statistical analysis.
Statistical analyses were performed using R v.4.2.0. AA concentrations below the limit of quantitation (LOQ) were set to 0.1. AA serum concentrations for the SCID control and supplemented PXB-mice were analyzed with a repeated measures ANOVA and Tukey’s posthoc test to compare differences per week. Nonsupplemented PXB-mice were excluded from this analysis due to all measurements being below the LOQ. AA tissue concentrations were analyzed with a one-way ANOVA and Tukey’s posthoc test. Nonsupplemented PXB-mice were excluded from analysis of adrenal tissue measured at 4 weeks and liver tissue measured at 4 and 12 weeks because all samples were below the LOQ. Histopathology scores were analyzed with the Fisher Exact Test. All statistical tests were evaluated at a significance level of ⟨ = 0.05. Pairwise comparisons of group histopathology scores were analyzed with the Fisher Exact Test and P values were adjusted with a Bonferroni correction for 3 comparisons. All tests were evaluated at a significance level of ⟨ = 0.05.
Results
Nonsupplemented PXB-mice developed notable weight loss beginning on Day 22 (Figure 2). By Day 47, all nonsupplemented PXB-mice had either died or were euthanized due to weight loss greater than or equal to 20% of the preshipment weight, reduced activity, and/or gait abnormalities (Figure 3).9,10 Other clinical observations included distended abdomen, hunched posture, and/or unkempt appearance; these clinical observations were recorded beginning on Day 23. Only 2 AA-supplemented PXB-mice survived to their scheduled euthanasia at 12 weeks. Two supplemented PXB-mice became moribund and were euthanized on Days 32 or 56 due to causes unrelated to vitamin C deficiency (glomerulonephropathy or lymphoma, respectively). One supplemented PXB-mouse died unexpectedly after blood collection on Day 70 (cause of death was likely related to blood collection procedure).
Figure 2.
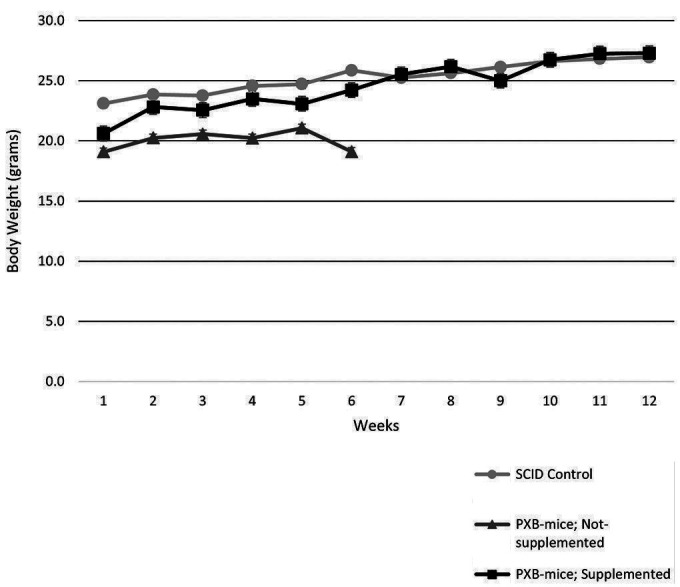
Mice were weighed weekly over the course of the 12-week study. Group mean body weights for supplemented mice were not different from the SCID control group mean body weights. None of the nonsupplemented mice survived to week 7.
Figure 3.
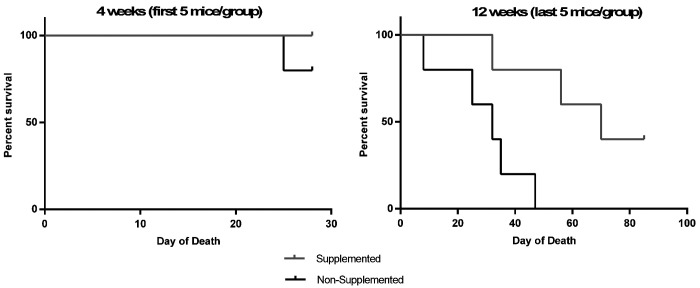
Survival of supplemented and nonsupplemented PXB-mice at scheduled euthanasia (4 and 12-weeks). At 4-weeks (n = 5/group), 100% of the supplemented PXB-mice survived while 80% of the nonsupplemented PXB-mice survived. At 12-weeks (n = 5/group), 40% of the supplemented PXB-mice survived while none of the nonsupplemented PXB-mice survived.
Ascorbic acid analysis.
Serum and tissue from individual groups (Groups 1 to 3) were assessed for the biosynthesis and maintenance of AA throughout the study by using liquid chromatography, tandem mass spectrometry (LC-MS/MS). Group 1 mice (the SCID background strain) had mean serum AA concentrations between 2.5 and 21 µg/mL over the course of the 12 week study (Figure 4). This range of values is similar to the value of 10 µg/mL that was previously reported in aging C57BL/6 mice sampled over a period of 3 to 30 months.3 Furthermore, the AA values in adrenal, brain, liver, and testes tissue of Group 1 mice were similar to those of C57BL/6: adrenal, 720 µg/g (1200 µg/g); brain, 300 µg/g (350 µg/g); liver, 116 µg/g (100 µg/g); and testes, 240 µg/g (175 µg/g).3
Figure 4.
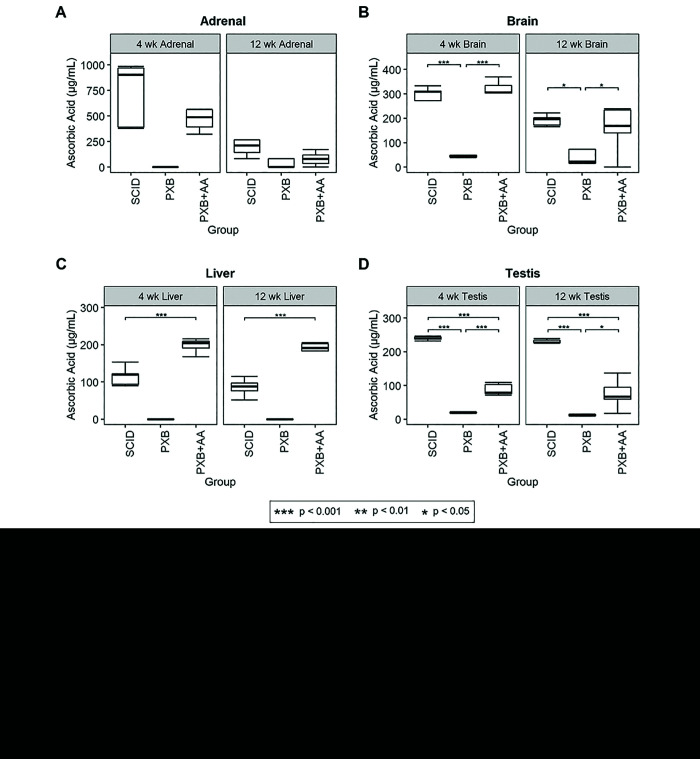
Serum and tissue ascorbic acid (AA) levels in SCID control, nonsupplemented, and supplemented PXB-mice. Serum values below the limit of quantitation (0.5 µg/mL) were reported as 0.1 µg/mL; tissue values below the limit of quantitation (2 µg/g) were reported as 0.1 µg/g.
Consistent with the scurvy-like pathology described for PXB-mice with humanized livers (Group 2), no measurable AA in serum was detected from these mice over 5 weeks of sampling, as all samples measured by LC-MS/MS were below the lower limit of quantitation (0.5 µg/mL). However, PXB-mice that received AA supplementation in drinking water (330 mg/L) or dietary supplements (5 to 10 mg, solid) had serum concentrations of AA in samples from all 12 weeks, with a range of 4 to 14 µg/mL; this range is consistent with the aforementioned C57BL/6 strain aging investigation (that is, 10 µg/mL).3 Serum AA concentrations were significantly higher in supplemented PXB-mice than in SCID control mice at weeks 2 and 3, and significantly lower in supplemented PXB-mice as compared with SCID control mice at weeks 7, 10, and 12; however, the overall range of AA in serum from supplemented PXB-mice is consistent with the overall range in SCID control mice (Figure 4).
Supplemented PXB-mice had measurable AA in adrenal, brain, liver, and testis tissue, whereas the nonsupplemented PXB-mice had no measurable AA concentrations in adrenal or liver tissue at 4 and weeks and had significantly lower AA concentrations in brain and testes tissue as compared with SCID control mice and supplemented PXB-mice. AA concentrations in adrenal and brain tissue were not significantly different between SCID control mice and supplemented PXB-mice. However, the AA concentration in liver tissue at 4 and 12 weeks was significantly higher in supplemented PXB-mice as compared with SCID control mice, and the AA concentration in testis tissue at 4 and 12 weeks were significantly lower in supplemented PXB-mice than in SCID control mice (Figure 4).
Human albumin.
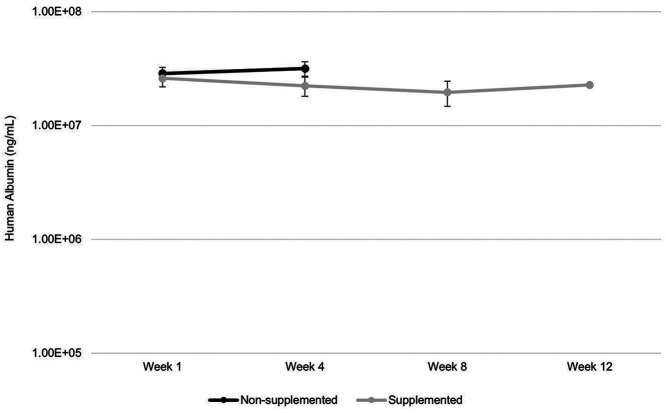
Serum human albumin levels were stable throughout the study (Figure 5), indicating healthy human hepatocytes; this suggested maintenance of high humanization for the duration of the study. Serum albumin concentrations greater than 1.0E7 ng/mL have been previously correlated with humanization of over 75% of cells.10 Values for the nonsupplemented group ranged from 2.3E7 to 3.4E7 ng/mL, and values for the supplemented group ranged from 1.2E7 to 2.7E7 ng/mL.
Figure 5.
Human albumin levels over the course of 12-week. Human albumin levels were stable over the course of the study indicating healthy human hepatocytes and a high degree of repopulation of human hepatocytes.
Pathology.
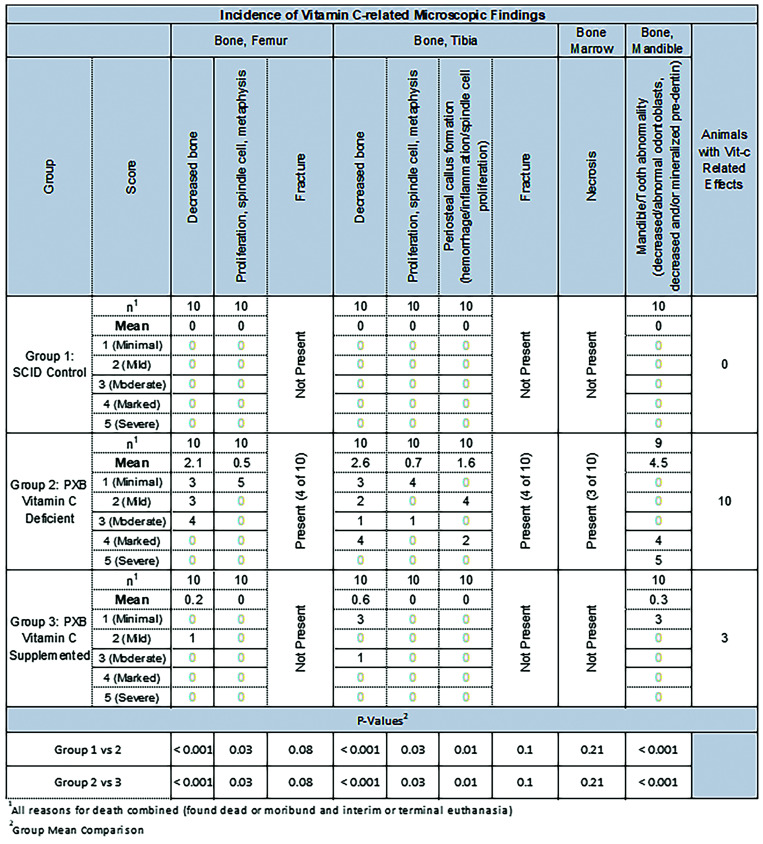
Histopathology findings in nonsupplemented PXB-mice consistent with a scurvy-like syndrome due to hypovitaminosis C were most notable in the hind limbs (tibia/femur), nasal turbinates, and pulp cavity of the mandibular incisors.
All nonsupplemented PXB-mice had minimally to markedly less cortical and subphyseal trabecular bone in the distal femur (Scores ranged from 1 to 3) and/or proximal tibia (scores ranged from 1 to 4) with decreased osteoid formation, whereas SCID control mice had normal bone formation. One supplemented PXB-mouse had less bone formation in the distal femur and 4 supplemented PXB-mice had less bone formation in the proximal tibia. The Fisher exact test revealed significant differences in bone formation between SCID control mice and nonsupplemented PXB-mice and between nonsupplemented and supplemented PXB-mice, but not between SCID control mice and supplemented PXB-mice (Figure 6).
Figure 6.
Microscopic findings related to vitamin C. Statically significant effects in vitamin C deficient PXB mice compared with SCID or vitamin C supplemented mice included femoral and tibial bone and mandibular tooth abnormalities (P < 0.001) and changes associated with bone fractures including metaphyseal spindle cell proliferation and periosteal callus formation (P < 0.05).
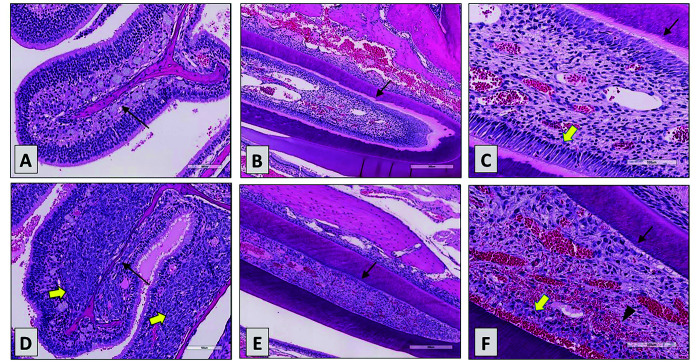
Seven of 10 AA deficient mice had complete fractures of the subphyseal trabecular bone in the distal femur and/or the proximal tibia. SCID control and supplemented PXB-mice had no fractures of the subphyseal trabecular bone in the distal femur or the proximal tibia, yet the Fisher exact test revealed no significant differences between SCID control and nonsupplemented PXB-mice (femur = 0.26; tibia = 0.26) or between nonsupplemented and supplemented PXB-mice (femur = 0.26; tibia = 0.26). In some mice, tibial fractures were complete and were accompanied by widespread regional bone marrow necrosis, hemorrhage, and inflammation (Figure 7).
Figure 7.
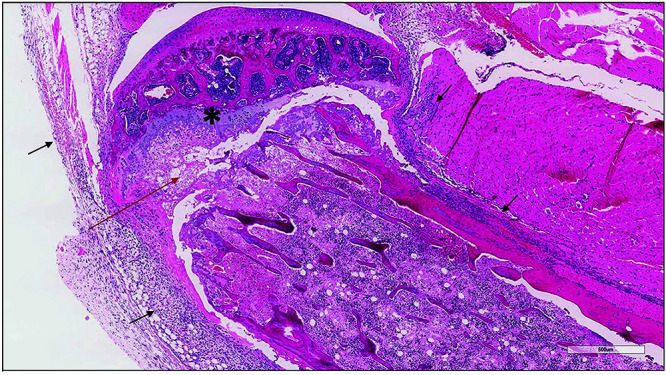
Proximal tibia from a vitamin C-deficient PXB-mouse with necrosis and a pathologic fracture at the physeal/metaphyseal region (red arrow). The entire marrow cavity was necrotic with marked periosteal hemorrhage and inflammation (black arrows). Asterisk marks the remaining visible physeal cartilage.
Bone marrow necrosis, periosteal inflammation, hemorrhage, and callus formation (recorded as proliferation, spindle cell, metaphysis, or periosteal hemorrhage/inflammation/spindle cell proliferation) were observed at the fracture sites in mice with complete femoral or tibial fractures. SCID and supplemented PXB-mice had no fractures of the subphyseal trabecular bone in the distal femur or the proximal tibia; the Fisher exact test showed no significant differences between SCID mice and nonsupplemented PXB-mice (femur = 0.26; tibia = 0.26) or between nonsupplemented and supplemented PXB-mice (femur = 0.26; tibia = 0.26). Likewise, SCID and supplemented PXB-mice had no bone marrow necrosis or femoral or tibial proliferation, yet the Fisher exact test showed no significant differences between SCID mice and nonsupplemented PXB-mice (P = 0.63, P = 0.10, P = 0.10, respectively) or between nonsupplemented and supplemented PXB-mice (P = 0.63, P = 0.10, P = 0.10, respectively). Conversely, differences in periosteal hemorrhage/inflammation/spindle cell proliferation were significantly different between SCID mice and nonsupplemented PXB-mice (P = 0.03) and between nonsupplemented and supplemented PXB-mice (P = 0.03).
Nonsupplemented PXB-mice had fractures, periosteal or periodontal hemorrhage, or other lesions of the nasal turbinates and mandible, with the incidence of mandible or tooth abnormalities significantly different as compared with SCID control mice (P < 0.05) or supplemented PXB-mice (P < 0.05). In the nasal cavity, notable findings associated with AA deficiency included atrophy and malformation of the nasal turbinate bone with higher numbers (proliferation) of periosteal mesenchymal cells that were similar to those previously described as dysplastic osteoblasts (Figure 6).5 Secondary disruption of the olfactory epithelium and a reduced size and number of Bowman’s glands (Figure 8) were also seen in mice with proliferating periosteal mesenchymal cells. In the pulp cavity of the teeth of nonsupplemented mice (primarily in the mandibular incisors and less frequently in the maxillary molars), odontoblasts were severely decreased (Max Score 5,), mean 4.5, SD 0.5 compared with SCID control (mean 0; SD 0) and supplemented PXB-mice (minimally decreased, Max Score 1, Mean 0.3, SD 0.5) Fewer supplemented PXB-mice had microscopic evidence of decreased nasal or mandibular bone (2 out of 10) or abnormalities of the dental pulp cavities (3 out of 10) and when present, the findings were generally minimal (Score 1). Scores of SCID control and supplemented PXB-mice were not statistically different (P = 0.63). When present, odontoblasts were often disorganized, abnormally shaped, and intermingled with unidentifiable mesenchymal cells. The predentin of all nonsupplemented PXB-mice was scant and abnormally mineralized (Figure 8). The pulp cavity and periodontal tissues occasionally exhibited acute hemorrhage.
Figure 8.
Nasal turbinate and mandibular incisor from a SCID mouse (A – C) and a vitamin-C deficient PXB-mouse (D – F). In the PXB-mouse, vitamin C deficiency was associated with marked thinning of the nasal turbinate (black arrow, panel D) and proliferation of periosteal mesenchymal cells (yellow arrows, panel D) as well as secondary loss of Bowman’s glands and mucosal nerves, and disruption of the olfactory epithelium. Vitamin C deficiency in the PXB-mouse resulted in abnormal mineralization and thinning of the predentin (black arrows B – C, E – F), disruption and decrease of odontoblasts (yellow arrows, panels D and F) and hemorrhage within the pulp cavity (arrowhead).
One supplemented PXB-mouse that became moribund and was euthanized had mild and moderate bone loss in the femur and tibia, respectively; the mouse also had severe glomerulonephropathy that was considered to be the cause of the moribund condition. Another supplemented PXB-mouse that was euthanized in a moribund condition had multicentric lymphoma. Both conditions (glomerulonephropathy and lymphoma) have been previously reported in mice with a uPA/SCID or SCID background and were considered unrelated to the dietary vitamin C intake.8,10 A cause of death was not determined for the supplemented PXB-mouse that died after blood collection; however, vitamin C deficiency was an unlikely contributor to death in that this mouse had no bone, tooth or nasal turbinate abnormalities, and its AA levels in serum and tissue were similar to those of controls.
Micro computed topography (CT) imaging.
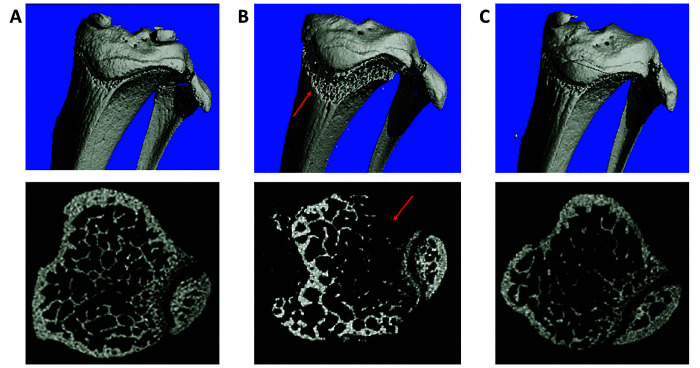
Nonsupplemented PXB-mice had qualitatively less trabecular bone, whereas vitamin C supplemented mice had bone structure similar to that of SCID controls (Figure 9).
Figure 9.
Micro CT from a SCID mouse (Panel A), nonsupplemented PXB-mouse (Panel B) and a PXB-mouse supplemented with ascorbic acid (AA) (Panel C). Nonsupplemented PXB-mice had less trabecular bone (red arrows), resulting in physeal fractures in some mice (bottom middle image). Vitamin C supplementation in PXB-mice was associated with bone structure similar to that of SCID controls.
Discussion
AA is a cofactor in many biologic and physiologic metabolic functions essential for survival including but not limited to hydroxylation reactions necessary for normal connective tissue formation and function, amidation reactions required in catecholamine synthesis, and antioxidant and free-radical scavenging functions.6 Most mammals, including mice, endogenously synthesize AA from glucose. Synthesis occurs primarily in the liver by a complex enzymatic process including dehydrogenation, reduction, and enzymatic conversion of glucose and uridine triphophopshate.5 The rate limiting enzyme L‐gulono‐↖‐lactone oxidase (GULO) is required for the final step in the synthesis of AA. In mice, GULO is expressed at low levels in other tissues including, muscle, bone, kidney, adrenal gland, brain, thymus, and spleen, however the liver is the primary source of GULO-mediated AA production.5
In humans, nonhuman primates, guinea pigs, some bats, birds, and fish, AA is an essential dietary nutrient because of mutations that have inactivated the GULO gene. The clinical symptoms of hypovitaminosis C in humans include lethargy, weakness, anemia, tooth loss, poor wound healing, and spontaneous hemorrhages in the skin, joints, and oral mucous membranes. In guinea pigs, bone fracture and periosteal hemorrhage are common clinical signs of hypovitaminosis C. Most of the signs and symptoms of scurvy (hypovitaminosis C) can be attributed to the improper hydroxylation of procollagen, leading to weak and unstable collagen. Dietary intake of AA has been known for decades to prevent development of scurvy, and vitamin C is readily available in a variety of foods; however, cases occasionally occur in the context of malnourishment.
Chimeric mice with a largely humanized liver were developed in the early 2000’s to support research aimed at understanding human hepatitis virus infection, liver function, and drug metabolism. The livers of PXB-mice are highly repopulated with human hepatocytes and exhibit gene expression levels similar to those of human livers. PXB-mice that are not supplemented with vitamin C develop scurvy-like conditions that include weight loss, early death, tooth abnormalities, and decreased bone formation occasionally resulting in pathologic fractures of the femur, tibia, and/or nasal turbinates. In this study, nonsupplemented PXB-mice were housed 5 per cage, whereas SCID mice and supplemented PXB-mice were individually housed. As such, any differences between nonsupplemented PXB-mice and SCID or supplemented PXB-mice are potentially confounded by housing effects that could not be controlled in a post hoc analysis. However, effects seen in the nonsupplemented PXB-mice are consistent vitamin C deficiency. The nonsupplemented PXB-mice had significantly greater incidences of decreased bone formation compared to SCID mice and supplemented PXB-mice; although fracture incidence was not significant between the SCID mice, nonsupplemented PXB-mice or supplemented PXB-mice. In addition, the genetically modified rodents that were developed specifically to study skeletal and nonskeletal effects of vitamin C also require dietary supplementation to prevent bone loss (trabecular and cortical), spontaneous fractures, tooth abnormalities, proliferation of periosteal spindle cells in the nasal turbinates, weight loss and death, which are the clinical and pathologic observations identified in nonsupplemented PXB-mice.2,5,7
Acknowledgments
We thank the following individuals and groups: Inlife staff for collecting data and samples from the mice and for taking great care of them; the Histology, Bioanalysis, and Mechanistic Pharmacology & Toxicology labs at Inotiv for sample analysis; Kim To from Predictive Toxicology and Information Science at Inotiv for her help and expertise in statistical analysis; Sara McBride-Gagyi at Saint Louis University for her assistance with micro CT imaging; and John Long and Kathleen Donovan at Saint Louis University for their guidance along the way. PhoenixBio provided the mice; other supplies were provided by Inotiv.
References
- 1.Drouin G, Godin J, Pagé B. 2011. The genetics of vitamin C loss in vertebrates. Curr Genomics 12:371–378. 10.2174/138920211796429736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabbay KH, Bohren KM, Morello R, Bertin T, Liu J, Vogel P. 2010. Ascorbate synthesis pathway dual role of ascorbate in bone homeostasis. J Biol Chem 285:19510–19520. 10.1074/jbc.M110.110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwama M, Amano A, Shimokado K, Maruyama N, Ishigami A. 2012. Ascorbic acid levels in various tissues, plasma and urine of mice during aging. J Nutr Sci Vitaminol (Tokyo) 58:169–174. 10.3177/jnsv.58.169. [DOI] [PubMed] [Google Scholar]
- 4.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. 2000. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA 97:841–846. 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohan S, Kapoor A, Singgih A, Zhang Z, Taylor T, Yu H, Chadwick RB, Chung YS, Donahue LR, Rosen C, Crawford GC, Wergedal J, Baylink DJ. 2005. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C defiency. J Bone Miner Res 20:1597–1610. 10.1359/JBMR.050406. [DOI] [PubMed] [Google Scholar]
- 6.Padayatty SJ, Levine M. 2016. Vitamin C: The known and the unknown and Goldilocks. Oral Dis 22:463–493. 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitts AT. 1935. Vitamin C and its effect on the structure of teeth. Arch Dis Child 10:295–308. 10.1136/adc.10.58.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuel R. 2015. Spontaneous development of neoplasms in severe combined immunodeficient mice. SAGE Open Med Case Rep 3:2050313X1456869. 10.1177/2050313X14568698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tateno C, Miya F, Wake K, Kataoka M, Ishida Y, Yamasaki C, Yanagi A, Kakuni M, Wisse E, Verheyen F, Inoue K, Sato K, Kudo A, Arii S, Itamoto T, Asahara T, Tsunoda T, Yoshizato K. 2013. Morphological and microarray analyses of human hepatocytes from xenogenic host livers. Lab Invest 93:54–71. 10.1038/labinvest.2012.158. [DOI] [PubMed] [Google Scholar]
- 10.Tateno C, Kawase Y, Tobita Y, Hamamura S, Oshita H, Yokomichi H, Sanada H, Kakuni M, Shiota A, Kojima Y, Ishida Y, Shitara H, Wada NA, Tateishi H, Sudoh M, Nagatsuka S, Jishage K, Kohara M. 2015. Generation of novel chimeric mice with humanized livers by using hemizygous cDNA-uPA/SCID mice. PLoS One 10:e0142145. 10.1371/journal.pone.0142145. [DOI] [PMC free article] [PubMed] [Google Scholar]