Abstract
Oxytocin is a neuropeptide that can produce anxiolytic effects and promote social approach. However, emerging evidence shows that under some conditions, oxytocin can instead induce anxiety-related behaviors. These diverse effects of oxytocin appear to be mediated by circuit-specific actions. Recent data showed that inhibition of oxytocin receptors (OTRs) in the bed nucleus of the stria terminalis (BNST) was sufficient to increase social approach and decrease social vigilance in female California mice (Peromyscus californicus) exposed to social defeat stress. As a member of the G-protein coupled receptor family, OTRs can induce distinct downstream pathways by coupling to different G-protein isoforms. We show that infusion of carbetocin, a biased OTR-Gq agonist, in the BNST reduced social approach in both female and male California mice. In both females and males, carbetocin also increased social vigilance. To gain insight into cell types that could be mediating this effect, we analyzed previously published single-cell RNAseq data from the BNST and nucleus accumbens (NAc). In the NAc, we and others showed that OTR activation promotes social approach behaviors. In the BNST, Oxtr was expressed in over 40 cell types, that span both posterior and anterior subregions of the BNST. The majority of Oxtr-expressing neurons were GABAergic. In the anterior regions of BNST targeted in our carbetocin experiments, Cyp26b1-expressing neurons had high average Oxtr expression. In the NAc, most Oxtr+ cells were D1 dopamine receptor-expressing neurons and interneurons. These differences in Oxtr cell type distribution may help explain how activation of OTR in BNST versus NAc can have different effects on social approach and social vigilance.
Introduction
Oxytocin is traditionally considered to promote affiliative behaviors and has been put forth as a potential treatment for social deficits, such as those associated with autism spectrum disorder (Ford and Young, 2021; MacDonald and MacDonald, 2010; Meyer-Lindenberg et al., 2011; Striepens, 2011). Oxytocin can facilitate pair bonding, parental care, and social play across a wide range of species (Bosch and Neumann, 2012; Bredewold et al., 2014, 2014; Hammock and Young, 2006; Keverne and Kendrick, 1992; Klatt and Goodson, 2013; Leng et al., 2008; Romero et al., 2015). It is also implicated in human research to increase trust, empathy, and in-group cooperation (De Dreu and Kret, 2016; Geng et al., 2018; Kosfeld et al., 2005; Van IJzendoorn and Bakermans-Kranenburg, 2012). Indeed, many studies show that oxytocin signaling can promote social approach behaviors (Dölen et al., 2013; Lukas et al., 2011). However, emerging studies have reported that administration of oxytocin can generate avoidance of social contexts (Beery, 2015). For example, intranasal oxytocin reduced social interaction in female California mice (Steinman et al., 2016) while intracerebroventricular infusion of oxytocin did not increase social approach in female rats exposed to social defeat (Lukas and Neumann, 2014). In humans, intranasal oxytocin increased self-reported perceived social stress among male participants (Eckstein et al., 2014). The mixed results suggest that oxytocin has a more complex role than promoting affiliative behaviors per se.
The social salience hypothesis proposes that oxytocin enhances the salience of both positive or negative social contexts (Shamay-Tsoory and Abu-Akel, 2016). It has been hypothesized that distinct neural circuits may mediate diverse behavioral effects of oxytocin (Steinman et al., 2019). Oxytocin acting in the nucleus accumbens (NAc) and ventral tegmental area (VTA) has been found to promote social reward and enhance social approach (Borland et al., 2018; Dölen et al., 2013; Hung et al., 2017; Peris et al., 2017; Song et al., 2016; Yu et al., 2016). In contrast, oxytocin has been found to produce anxiogenic effects in the bed nucleus of the stria terminalis (BNST) (Duque-Wilckens et al., 2020; Janeček and Dabrowska, 2019). The BNST plays an important role in modulating fear and anxiety-related behaviors (Fox and Shackman, 2019; Walker et al., 2003) and expresses high levels of oxytocin receptors (OTR) (Tribollet et al., 1992). For instance, infusion of an OTR antagonist in the BNST impaired acquisition of cued fear in male rats (Moaddab and Dabrowska, 2017) and attenuated the effects of social defeat stress in female California mice (Duque-Wilckens et al., 2018). While there is a strong literature describing how oxytocin receptors modulate behaviors, less is known about the molecular pathways that mediate these effects.
Oxytocin receptors belong to the G-protein coupled receptor (GPCR) family, which is the target of over one-third of FDA-approved drugs (Rask-Andersen et al., 2011). An important property of these receptors is that they are capable of modulating diverse signaling pathways and pathological processes. Oxytocin receptors can induce distinct downstream pathways through coupling to either excitatory Gq or inhibitory Gi/o subunits (Busnelli et al., 2012; Gimpl and Fahrenholz, 2001; Rosenbaum et al., 2009; Strader et al., 1994). Although the molecular pathways of oxytocin receptor signaling are well-studied in vitro, it is less clear how the differential G-protein signaling translates into behavioral phenotypes (Jurek and Neumann, 2018). A previous study from our group demonstrated that infusion of biased agonists for OTR-Gq but not OTR-Gi pathway in the NAc of stressed female California mice increased social approach and decreased social vigilance, a behavior in which an individual orients towards an unfamiliar conspecific while simultaneously avoiding it (Williams et al., 2020). Recent data showed that infusion of oxytocin into the anteromedial BNST (BNSTam) reduced social approach and increased vigilance (Duque-Wilckens et al., 2020), so we decided to test whether infusion of Gq-biased OTR agonist in the BNST would facilitate behavioral responses related to social anxiety. We also explored whether the oxytocin receptor gene (Oxtr) is expressed in different cell types in the BNST compared to the adjacent NAc. Differences in the cell-type expression of Oxtr across brain regions could contribute to variability in circuit-specific actions of oxytocin.
To address these questions, we first microinjected the functionally selective OTR-Gq agonist carbetocin into the anterior BNST and assessed social interaction behaviors. We used California mice, a species that is unique in that both males and females are aggressive. This has allowed for the study of social defeat stress in both sexes (Kuske and Trainor, 2021). To determine Oxtr cell types we analyzed recently published single cell RNA sequencing datasets from the BNST (Welch et al., 2019) and NAc (Chen et al., 2021) in Mus musculus.
Methods
Animals
All experiments on California mice (Peromyscus californicus) were in accordance with and approval of the Institutional Animal Care and Use Committee (IACUC) at the University of California, Davis. Adult male (n=58) and female (n=26) California mice from our laboratory colony were co-housed in same-sex groups of 2. Mice were kept on a 16:8 Light:Dark cycle and fed ad libitum (2016 Teklad global 16% protein rodent diets). Sani-chip bedding, cotton nestlets, and enviro-dri (Newco Distributors) were provided in all cages. Drug infusion and behavioral tests were performed during the dark cycle. Previous studies have demonstrated that estrus cycle does not affect behaviors during the social interaction test (Trainor et al., 2013, 2011).
Cannulation and Carbetocin Infusion
Males and females were implanted with 26-gauge bilateral cannula guides aimed at the anterior BNST (A-P: +0.45 mm; M-L: ±1.0 mm; D-V: +5.6 mm). The mice were single housed and given a 7-day recovery period after surgery. The animals received daily subcutaneous injection of carprofen as anti-inflammatory from day 1 to 3 and handled daily for 1 minute to get used to scruffing. On the testing day, female mice were randomly assigned to receive 200nL bilateral infusion of either artificial cerebrospinal fluid (aCSF) vehicle, 200ng carbetocin, or 1μg carbetocin. In bioluminescence resonance energy transfer (BRET) assays, carbetocin selectively induces OTR/Gq coupling (Passoni et al., 2016). Although this specificity has never been demonstrated directly in vivo, indirect evidence suggests that carbetocin acts via a similar mechanism in vivo: when microinected in the NAc, atosiban, which blocks OTR/Gq coupling while activating OTR/Gi coupling (Busnelli et al., 2012), reduced social approach in stress naïve mice whereas carbetocin increased social approach in stressed female California mice (Williams et al., 2020). Male mice were randomly assigned to receive 200nL bilateral infusion of either vehicle, 200 ng carbetocin, or 1μg carbetocin. Twenty minutes following the infusion, mice were tested for social interaction. After behavior testing, mice were perfused, and brains were collected for Nissl stain to confirm successful cannula placement.
Behavioral Test
The social interaction test consists of 3 phases, each lasting 3 minutes (Greenberg et al., 2014). Mice were introduced into an empty arena (89 x 63 x 60cm) and allowed to freely explore during the open field phase. During the acclimation phase, an empty wire cage was placed against one side of the arena for habituation. For the social interaction phase, a same-sex unfamiliar target mouse was placed into the wire cage. Distance traveled, time in the center zone (located 14cm from the sides), and time that the focal mouse spent within the interaction zone (within 8cm of the wire cage) were recorded and analyzed using AnyMaze. Time that the focal mouse spent outside of the interaction zone while its head oriented towards the target mouse was defined as social vigilance and scored manually.
Statistical analyses
Behavioral data analyses were performed in RStudio. The Shapiro-Wilk’s test was used to test for data normality and the Fligner-Killeen test was used to assess homogeneity of variance. One-way ANOVA was used to detect group differences in female and male mice, respectively. Pairwise comparisons with Bonferroni correction were used for post-hoc analyses. For data that did not meet the assumptions of normal distribution or homogeneity of variance (i.e., vigilance), Kruskal-Wallis one-way analysis was used followed by Dunn’s test with Bonferroni adjustment. Cohen’s d was calculated to reflect the effect size of the significant results.
Single-cell RNA Sequencing Data Analyses
Single-cell sequencing data were analyzed in RStudio using Seurat v4.0.4 (Hao et al., 2021). For BNST analysis, we accessed previously published data from a Mus musculus BNST single nucleus RNA-seq data containing a total of 204,737 cells across 7 adult female and 8 adult male biological replicates (Welch et al., 2019) from GEO: GSE126836, and loaded these data into a Seurat object (Stuart et al., 2019). We used the Welch et al. 2019 cluster identity, replicate, and sex data as metadata features for each Seurat object. Cells with unique features under 200 and over 7500 were filtered out. To characterize Oxtr cells, cells with > 0 Oxtr counts were considered as Oxtr+ and subsetted from the main Seurat object. The percent expression of the following gene markers: glutamate decarboxylase 1 (Gad1) and glutamate decarboxylase 2 (Gad2) for GABAergic neurons, vesicular glutamate transporter 2 (Slc17a6) and vesicular glutamate transporter 3 (Slc17a8) for glutamatergic neurons and glial fibrillary acidic protein (Gfap) for glial cells were calculated in Oxtr+ cells. These categories made up over 96% of all cell types expressing Oxtr. We also calculated percent expression of dopamine receptor D1 (Drd1) in Oxtr+ cells to make direct comparisons with the NAc data. To visualize Oxtr expressing neurons, gene counts were normalized and scaled from the main Seurat object. Linear dimensionality reduction was performed by principal component analysis (PCA). BNST Oxtr-expressing clusters were visualized with UMAP, using the same number of dimensions as PCA (runUMAP, dims = 10). The expression level of Oxtr across different neuron clusters was quantified and visualized using the DotPlot function. We also visualized the expression of other transcripts including thyrotropin-releasing hormone receptor (Trhr), dopamine receptors (Drd1, Drd2, Drd3), serotonin receptors (Htr1a, Htr1b, Htr2a, Htr2c), neuropeptide Y receptors (Npy1r, Npy2r), opioid receptors (Oprk1, Oprd1, Oprm1), and tachykinin precursor (Tac1) in a Dotplot to compare with Oxtr.
The same approach was used for the NAc analysis. We accessed published data (Chen et al., 2021) from 11 adult male Mus musculus NAc single cell RNA-seq data containing 47,576 total cells, organized into 21,842 neuronal and 25,734 non-neuronal cells from GEO: GSE118020 along with cluster identities. UMAP (runUMAP, dims = 1:10) was used to visualize NAc clustering. A total of 199 Oxtr+ cells were subsetted and analyzed for cell type composition. In addition, the expression level of Oxtr across different NAc cell types and interneuron types (Sst, Pvalb, Chat) was visualized using the DotPlot function.
Results
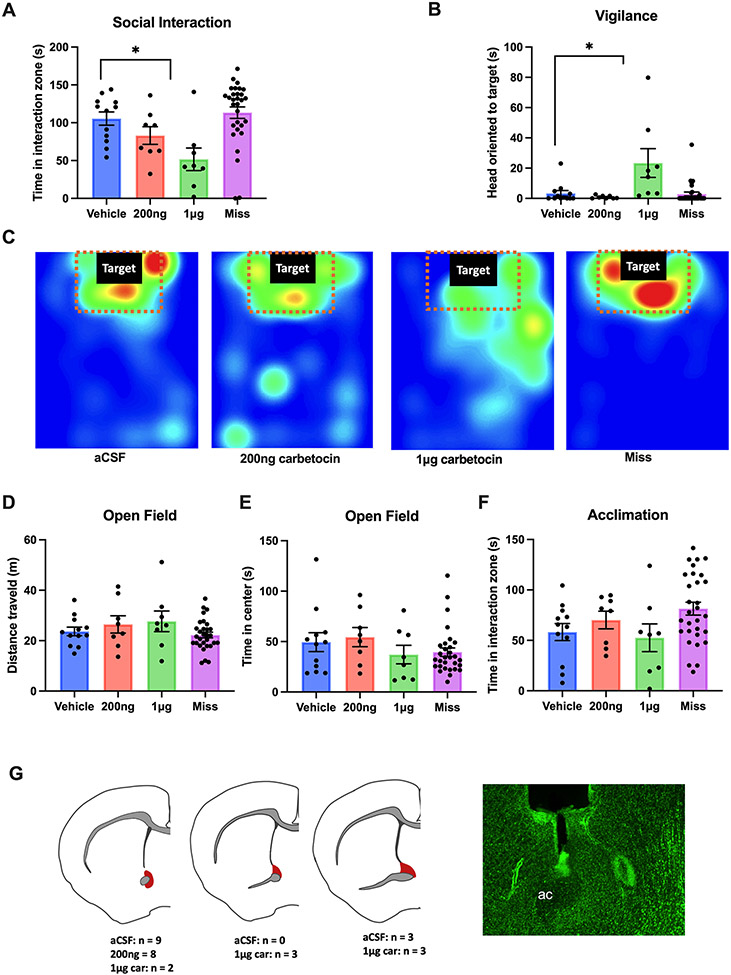
Female California mice that received 1μg carbetocin spent less time interacting with a novel target mouse (p<0.01, d = 2.39, Fig.1A) and showed increased vigilance behavior (p<0.05, d = 1.56, Fig.1B) compared to controls. Females that received 200 ng carbetocin or infusion outside of the BNST did not show significant differences from the controls (all p’s > 0.05, Fig.1A, B). Representative heat maps demonstrated the location of one mouse per treatment group during the interaction phase (Fig.1C). No differences were observed in the distance traveled (F3,22=0.658, p =0.587, Fig.1D), time spent in center during the open field phase (F3,22=0.454, p =0.717, Fig. 1E) or time spent in the interaction zone during the acclimation phase (F3,22=0.745, p =0.537, Fig. 1F) across different treatment groups. Histology was used to confirm successful placement of cannula guides and location of the needle tracts (Fig. 1G)
Figure 1. Intra-BNST carbetocin infusion reduced social approach and increased vigilance in female California mice.
Infusion of 1μg carbetocin in the anterior BNST (n=8), but not 200ng cabetocin (n=7) or misplaced infusion (“Miss”,n=4), decreased social approach to a novel target mouse (a) and increased social vigilance (b) compared with controls (n=7). Representative heatmaps showed that during the interaction phase, a mouse that received the higher dose of carbetocin spent less time in the interaction zone compared with the other treatment groups (c). There were no significant differences across all treatment groups during the open field or the acclimation phases (d,e,f). Brain slices were Nissl stained to confirm successful injection sites (red shading).
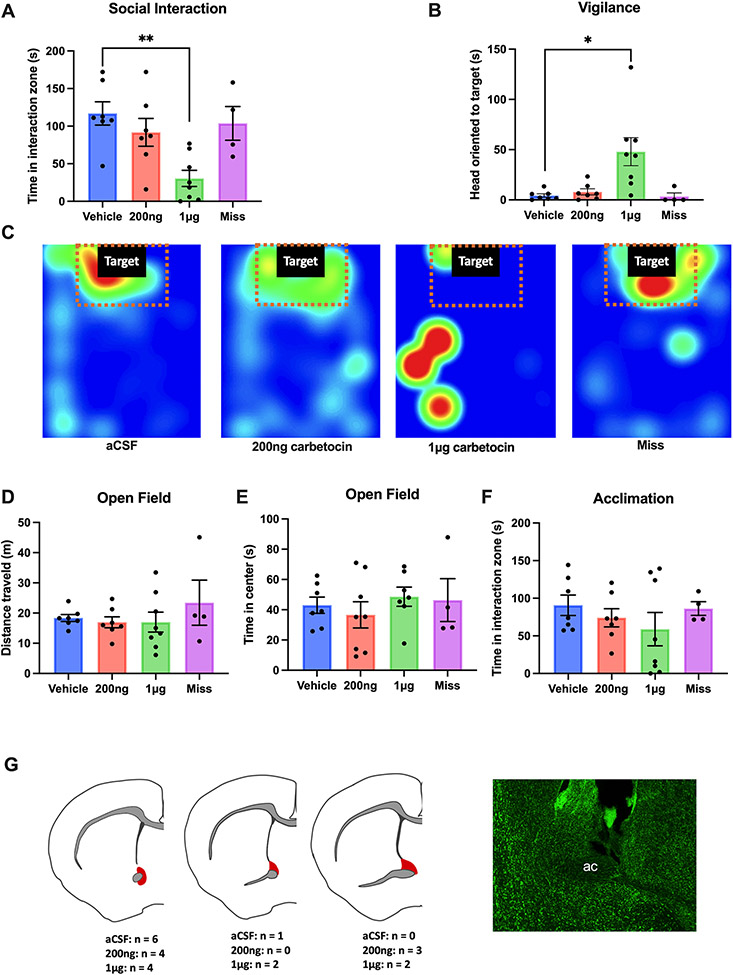
Similar results were observed in males. Infusion of 1μg carbetocin decreased social interaction time (p<0.05, d = 1.47, Fig.2A, C) and increased vigilance behavior (p<0.05, d=1.03, Fig.2b). Males that received 200ng carbetocin or infusion outside of the BNST did not show significant differences from the controls (all p’s> 0.05, Fig. 2A, B). Again, the mice did not show differences in the distance traveled (F3,54=1.462, p =0.235, Fig. 2D), center time (F3,54=1.064, p = 0.372, Fig. 2E) or time investigating the empty cage (F3,54=2.406, p =0.0773, Fig. 2F) across different treatment groups.
Figure 2. Intra-BNST carbetocin infusion reduced social approach and increased vigilance in male California mice.
Infusion of 1μg carbetocin in the anterior BNST (n=8), but not misplaced infusions (n=30), decreased social interaction time with a novel target mouse (a) and increased social vigilance (b) compared with controls (n=12). Representative heatmaps showed that during the interaction phase, a mouse that received the drug spent less time in the interaction zone compared with the other treatment groups(c). There were no significant differences across all treatment groups during the open field or the acclimation phase(d,e,f). Brain slices were Nissl stained to confirm successful injection sites (red shading).
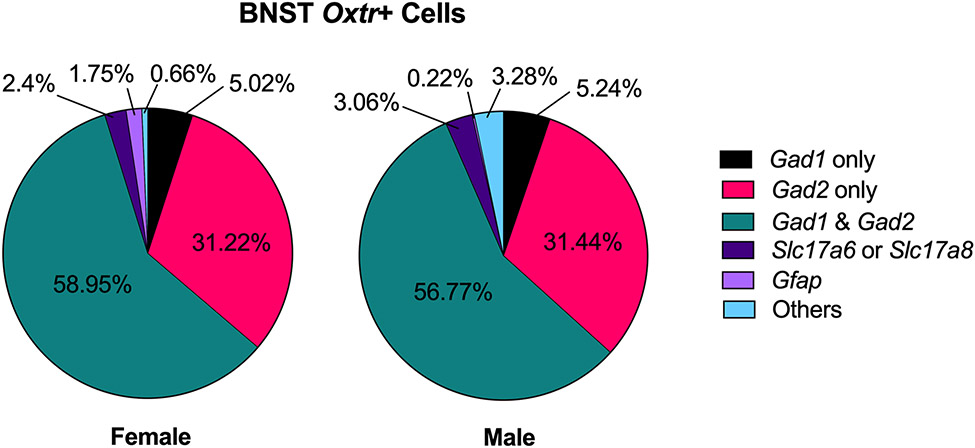
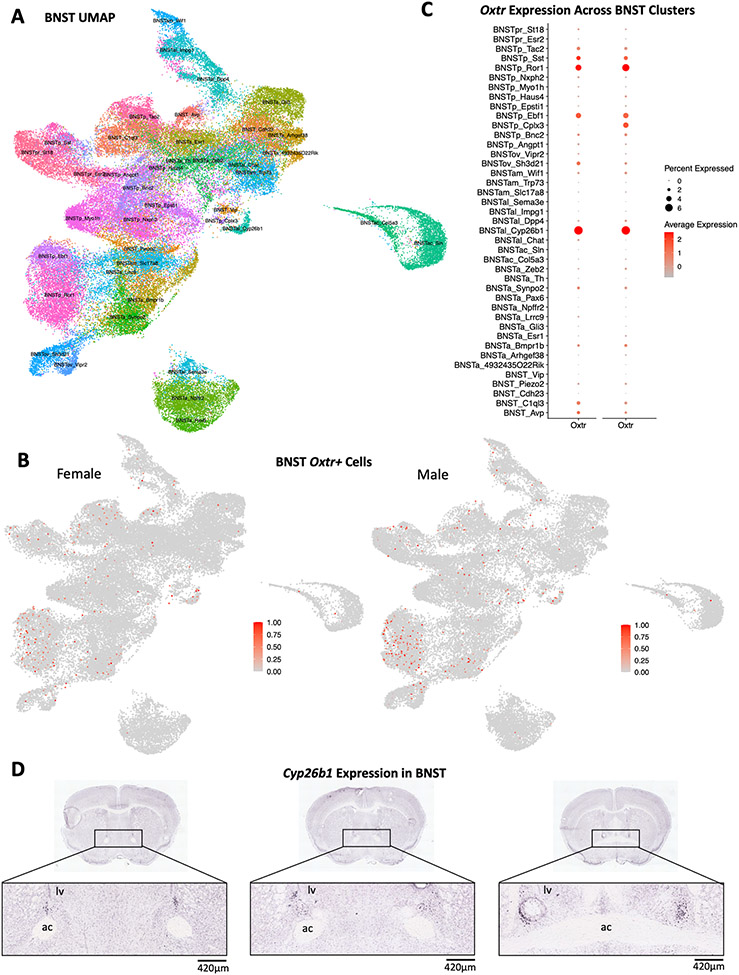
In adult Mus BNST, more than 90% of Oxtr+ cells were GABAergic neurons (Fig 3). In females, 5.02% of Oxtr+ cells only expressed Gad1, 31.22% only expressed Gad2 and 58.96% expressed both. Similarly, in males, 5.24% of Oxtr+ cells only expressed Gad1, 31.44% only expressed Gad2 and 56.77% expressed both. A small percentage of Oxtr+ cells expressed the glutamatergic neuronal markers Slc17a6 or Slc17a8 (2.4% in females and 3.06% in males). An even smaller percentage of Oxtr+ cells expressed glial cell maker (Gfap) or none of the gene markers. To visualize Oxtr-expressing neurons, UMAP was constructed using 41 original cluster IDs acquired from Welch et al.,2019 (Fig.4A). Oxtr was expressed across various neuron types and had similar expression patterns between males and females (Fig.4b). Most Oxtr+ cells were found in the posterior BNST, especially in the Ror1 cluster (Supplementary Data 1). In general, Oxtr expression was less abundant in the BNST (1.18% of transcripts in females and 1.27% in males) compared to other transcripts, such as Htr2c and Oprm1 (Supplementary Fig. 1). In both sexes, Oxtr had the highest average expression (2.5 standard deviation above mean expression) in the BNSTal_Cyp26b1 (Cytochrome P450 Family 26 Subfamily B Member 1) cluster as well as the highest percent expression in the same cluster (6.94% of Cyp261 cells for females and 6.78% for males) (Fig.4C, Supplementary Data 2). In situ hybridization data acquired from the Allen Brain Map showed that Cyp26b1 expression in the anterior BNST overlapped with our microinjection sites (Fig. 4D) (Lein et al., 2007). Oxtr was also highly expressed (over 1.5 SD above mean expression in either sex) in Ror1 (receptor tyrosine kinase-like orphan receptor 1), Sst (somatostatin) and Ebf1 (early B-cell factor 1) clusters in the posterior BNST (Fig. 4C) (Supplementary Data 2).
Figure 3. Characterization of Oxtr+ cells in adult Mus musculus BNST.
Percentage expression of gene markers for inhibitory neurons (Gad1 and Gad2), excitatory neurons (Slac17a6 or Slc17a8) and glial cells (Gfap) in female (left) and male (right) Oxtr-expressing cells.
Figure 4. Oxtr and Cyp26b1 expression in adult Mus musculus BNST.
Oxtr expression across different cell types and BNST subregions (BNSTa, BNSTac, BNSTal, BNSTam, BNSTov, BNSTp, BNSTpr) were visualized with UMAP and Dotplot (a,b,c). Male and female animals showed similar expression patterns of Oxtr (a, b). Oxtr had the highest percent and average expression in the BNSTal Cyp26b1 cluster of both sexes (c). In situ data also showed that Cyp26b1 is expressed in both the oval and anterior BNST. The expression of Cyp26b1 in the anterior region overlaps with our carbetocin microinjection sites (d). Image credit: Allen Institute. URL: https://mouse.brain-map.org/experiment/show/79568022.
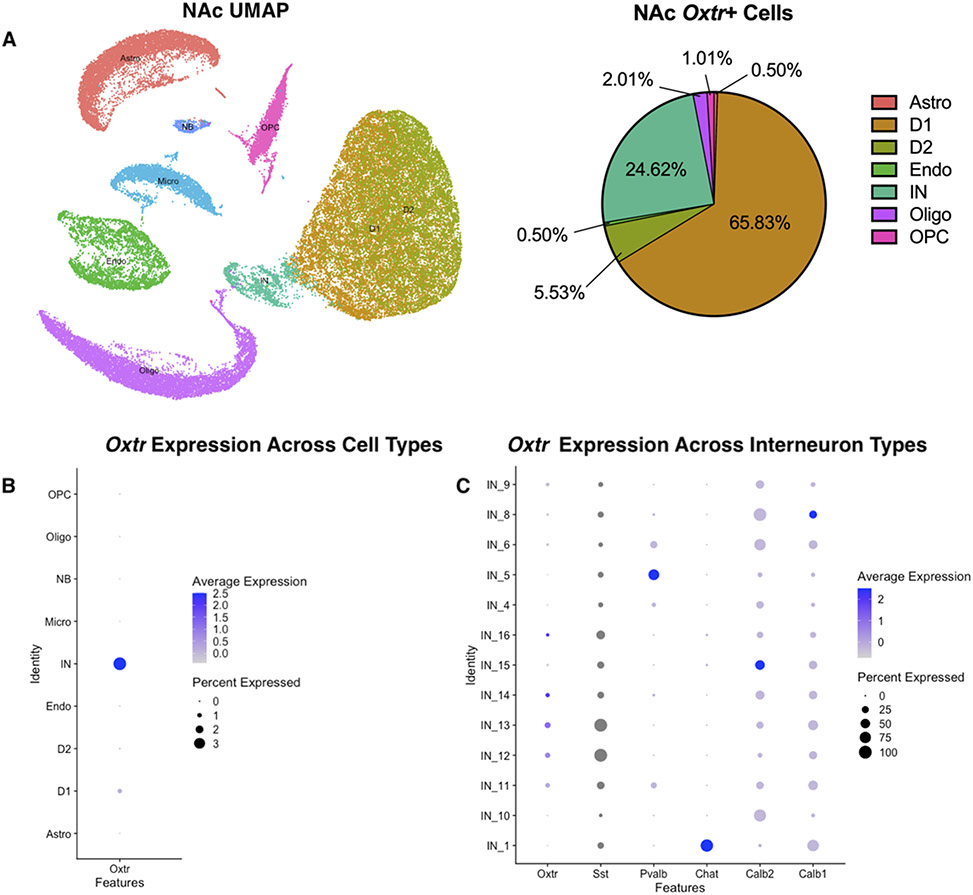
Adult male Mus NAc cells were grouped into 8 different clusters: astrocytes, oligodendrocytes, endothelial cells, interneurons, dopamine receptor 1-expressing cells, dopamine receptor 2-expressing cells, and oligodendrocyte progenitor cells (Fig. 5A). The majority of the Oxtr+ cells were either D1 medium spiny neurons (65.83%) or interneurons (24.62%) (Fig. 5A). These results contrast with cell types in the BNST, where only 15.28% of female and 14.85% of male Oxtr+ cells co-express Drd1. The remaining NAc Oxtr cell types were expressed in D2 medium spiny neurons (5.53%), oligodendrocytes (2.01%), OPC (1.01%), astrocytes (0.5%) and endothelial cells (0.5%). Although most Oxtr+ cells were D1 medium spiny neurons, Oxtr had the highest percent expression (3.68%) and average expression (2.5 standard deviation above mean expression) within the interneurons (Fig. 5B, Supplementary Data 3). We also visualized the expression of Oxtr across different interneuron subtypes (Fig. 5C, Supplementary Data 4) due to the heterogeneity of this cell type. Oxtr expression overlapped with somatostatin (Sst)-expressing interneurons and was generally absent from parvalbumin (Pvalb) and cholingeric (Chat) interneurons (Fig 5D). Calbindin1 (Calb1) and Calb2 were expressed in all interneurons.
Figure 5. NAc Oxtr+ cell type characterization and Oxtr expression across clusters.
The UMAP shows 8 different cell types within the NAc: astrocytes (Astro), oligodendrocytes (Oligo), endothelial cells (Endo), interneurons (IN), dopamine receptor 1-expressing cells (D1), dopamine receptor 2-expressing cells (D2), and oligodendrocyte progenitor cells (OPC) (A). Oxtr is expressed primarily in the dopamine D1 receptor medium spiny neurons and interneurons (A). A Dotplot across all cell types (B) shows Oxtr has the highest average and percent expression in the interneurons (IN). When examining interneuron subtypes (C), a Dotplot was shows that Oxtr expression occurs primarily in somatostatin (Sst) expressing interneurons. Comparatively few Oxtr transcripts were expressed in Pvalb or Chat neurons. Dots for Sst appear gray due to its high abundance relative to other transcripts.
Discussion
We demonstrated that activating OTR via carbetocin in the anterior BNST reduces social approach and increases social vigilance in stress naïve males and females. These behavioral responses mirror behavior patterns observed in mice exposed to social defeat (Duque-Wilckens et al., 2020), suggesting that these are social anxiety-related responses. Although the mechanism of action for carbetocin has never been demonstrated in vivo, prior work in the NAc, suggests that OTR-Gq signaling is most likely the mechanism of action. Our results from the BNST complement observations from the NAc, where social approach is reduced by OTR-Gq antagonists and promoted by carbetocin (Williams et al., 2020), and suggest that oxytocin is more likely to regulate social behaviors in a circuit-dependent manner than through different G-protein coupled signaling. Analyses of single cell RNAseq data from Mus show that in the BNST, Oxtr is expressed by many types of GABAergic neurons. Meanwhile in the NAc, Oxtr is mainly expressed in both D1 dopamine receptor neurons and interneurons. Anatomical variation in Oxtr cell-type expression may be an important factor contributing to circuit-specific effects of Oxtr on behavior.
Effects of Oxytocin Receptors in the BNST on Behavior
Microinjection of the highest dose of carbetocin (1 μg) induced social avoidance and social vigilance in females and males. In vitro, carbetocin can activate V1a receptors at high concentrations (Passoni et al., 2016). However, it is unlikely that decreases in social approach and increases in social vigilance were driven by V1a receptors. Infusion of a highly selective V1a receptor antagonist into the BNST reduced social approach in unstressed males and females (Duque-Wilckens et al., 2016). On the contrary, infusion of a selective oxytocin receptor antagonist into the BNST increased social approach and reduced social vigilance in stressed females (Duque-Wilckens et al., 2018). If carbetocin infusions activated V1a receptors, we would expect to see enhanced social approach, which is the opposite of what we observed. Together, these results suggest that the anxiogenic behavioral effects of carbetocin in the anterior BNST are mediated by oxytocin receptors. Similarly, in a non-social context, OTR neurotransmission in the dorsolateral BNST facilitated acquisition of cued fear response in male rats (Martinon et al., 2019; Moaddab and Dabrowska, 2017). We also observed that intra-BNST administration of carbetocin induced similar behavioral phenotypes in both sexes. Sex differences have been reported in neural and behavioral responses to intranasal oxytocin in human (Domes et al., 2010; Rilling et al., 2014) and animal research (Duque-Wilckens et al., 2018; Steinman et al., 2016). However, sex differences were not observed in OTR expression across a wide range of brain regions and oxytocin infusions into anterior BNST of California mice had similar behavioral effects in both sexes (Duque-Wilckens et al., 2020). These results suggest that sex differences in oxytocin release may be a key driver for sex differences in stress responses.
Previous studies have demonstrated that systemic and central administration of carbetocin could reverse stress-induced depression and anxiety-related behaviors (Chaviaras et al., 2010; Klenerova et al., 2010, 2009; Meng et al., 2016). In one study, two weeks of intraperitoneal (i.p.) injection of carbetocin blocked social withdrawal, sucrose anhedonia and learned helplessness in stressed tree shrews (Meng et al., 2016). In another study, either acute intravenous, intraperitoneal or intracerebroventricular injection of carbetocin reduced immobility during forced swim tests in male rats (Chaviaras et al., 2010). These studies indicated that both chronic and acute administration of carbetocin could induce anti-depressant or anxiolytic effects. Instead, our results showed that intra-BNST infusion of carbetocin decreased social approach and increased social vigilance. There is growing evidence that the behavioral effects of OTR are circuit-specific (Steinman et al., 2019). For example, microinjection of carbetocin in the NAc, which is adjacent to the anterior BNST, induced an opposite behavioral phenotype by increasing social approach and decreasing vigilance in stressed female California mice (Williams et al., 2020). These results were consistent with previous findings that oxytocin acting in the NAc could interact with serotonin and dopamine receptor signaling to enhance social approach responses (Dölen et al., 2013; Liu and Wang, 2003). Similarly, oxytocin infusion into the dorsal lateral septum blocked social fear responses after conditioning (Zoicas et al., 2014). Taken together, our results support the hypothesis that the behavioral effects of OTR signaling are brain region-specific. Although many mechanisms, likely contribute to circuit specific actions of OTR, one contributing factor could be Oxtr expression in different cell types across the brain. To examine Oxtr cell types in a more systematic way than has been performed previously, we analyzed single-cell RNAseq data from the BNST and NAc.
Oxtr cell types in BNST and NAc
In the Welch et al. 2019 BNST data, the overwhelming majority of Oxtr+ cells were GABAergic neurons expressing either Gad1, Gad2, or both transcripts. In both the Mus RNAseq data and in situ hybridization analyses of Oxtr in BNSTam California mice (Duque-Wilckens et al., 2020), about 60% of Oxtr+ neurons co-expressed Gad1. Interestingly, almost all Gad1 expressing cells also expressed Gad2, while an additional 31% of Oxtr+ neurons in the BNST only expressed Gad2. This suggests that Gad2 may be a better marker for GABAergic neurons in the BNST. It is also important to consider the heterogeneity of neuronal subtypes in the BNST (Beyeler and Dabrowska, 2020). Over 40 cell types were identified in Welch et al. 2019 dataset, and many of these cell types expressed low or moderate levels of Oxtr mRNA. Several Oxtr+ cell types are predominant in more anterior regions of BNST, which were targeted in our carbetocin experiment. One cell type that had the most abundant Oxtr expression was Cyp26b1-expressing neurons. Cyp26b1 encodes a type of retinoic acid degradation enzyme (White et al., 2000). A recent sequencing study of BNST observed increased expression of Cyp26b1 in male mice that exhibited increased social approach after chronic social defeat (resilient) compared to mice that exhibited reduced social approach (susceptible) (Gururajan et al., 2022). Although clinical and preclinical research suggests that retinoic acid signaling can modulate depression and anxiety-related behaviors (Bremner and McCaffery, 2008), no studies have manipulated Cyp26b-1 function in the extended amygdala. This transcript could be an interesting target for functional studies, especially given that Cyp26b1 is part of a group of transcripts that distinguish the anterior basolateral amygdala (BLA) from the posterior BLA (Hintiryan and Dong, 2022). Intriguingly, retrograde tracing studies show a strong connection between the caudal anterior BLA and the anteromedial BNST, the primary target of the carbetocin experiments.
Our analyses also showed that Oxtr is expressed in several cell types located in the posterior BNST. Posterior subregions of BNST are sexually dimorphic (Campi et al., 2013), and well known for modulating sexual behavior and aggression (Flanigan and Kash, 2020). The Welch et al. 2019 dataset is unusual in that cells from both males and females were included. Oxtr had similar expression patterns across different neuron clusters in males and females. While these observations are consistent with a lack of reported sex differences in OTR binding within the anterior BNST subnuclei (Duque-Wilckens et al., 2018), previous studies have reported sex differences in OTR binding in the posterior BNST (Dumais and Veenema, 2016; Smith et al., 2017). It is possible that RNA transcript expression level may not be linearly correlated with the protein expression level. This may help explain how Oxtr in the BNST is behaviorally active even though the relative abundance of Oxtr transcripts was relatively low compared with transcripts for TRH, dopamine receptors, NPY and opioid receptors in the Mus BNST.
In the NAc, Oxtr was primarily observed in D1 medium spiny neurons and interneurons. Conversely, few BNST Oxtr+ neurons expressed D1 receptors. This result provides new insights into previous behavioral pharmacology experiments that identified coordination between oxytocin and dopamine signaling in the NAc. For example, in female prairie voles the formation of pair bonds requires activation of both OTR and D2 receptors (Liu and Wang, 2003). The fact that almost no D2 neurons express Oxtr suggests that pair bond formation in voles could be mediated by the coordinated action of D2 neurons and either D1 neurons or interneurons. Although there are many fewer interneurons than medium spiny neurons, interneurons have significant effects on behavior (Castro and Bruchas, 2019; Robison and Nestler, 2011). Increased activity of somatostatin interneurons enhances the rewarding effects of cocaine in a place preference assay (Ribeiro et al., 2018). Interestingly, optogenetic manipulations of somatostatin interneurons in the absence of cocaine or another salient context had no effects on place preference or locomotor behavior. Behavioral effects of oxytocin are also generally stronger in social contexts (e.g., enhancing social salience), suggesting that somatostatin interneurons may enhance the salience of biologically important experiences. Also notable is that in male mice Oxtr positive somatostatin interneurons in the medial prefrontal cortex promote interest in females during estrus (Nakajima et al., 2014) . We also observed Oxtr positive somatostatin neurons in the BNST, which suggests that Oxtr/somatostatin cell types may be present more broadly across the brain.
Conclusions
Oxytocin can promote diverse behavioral effects by acting in different neural circuits. The availability of single cell RNA-seq methods allows for the ability to determine how Oxtr is expressed in different cell types in different brain regions. In the BNST, Oxtr was distributed across several types of GABAergic neurons while in the NAc, Oxtr was confined primarily to D1 medium spiny neurons and interneurons. While some BNST neurons have electrophysiological properties (inward rectification in response to current injection) that are similar to medium spiny neurons, they have different input resistances and resting membrane potentials (Egli and Winder, 2003). Taken together, Oxtr is expressed in substantially different cell types in the BNST versus NAc. Our analyses of Oxtr mRNA have important implications for interpreting mechanisms of oxytocin action. Previous work highlighted the role of pre-synaptic OTR on dorsal raphe terminals in the NAc in promoting salience in a social reward task (Dölen et al., 2013). That Oxtr mRNA was detected in single cell (NAc) and single nucleus (BNST) datasets implies the importance of post-synaptic OTR action may also be important. For example, in male rats, oxytocin excites Type I interneurons within the dorsolateral BNST through a post-synaptic mechanism (Francesconi et al., 2021). Excitation of these interneurons suppresses the activity of adjacent Type II neurons that project to the central nucleus of the amygdala. This observation is especially interesting as optical excitation of different populations of BNST projection neurons can produce different behavioral effects (Kim et al., 2013). The complexities of how oxytocin modulates different brain circuits likely contribute to the mixed results observed in clinical studies using intranasal oxytocin as a therapeutic treatment for anxiety (Leppanen et al., 2018; MacDonald and MacDonald, 2010) or other social deficits (Ford and Young, 2021). Future studies should explore potential interaction between region-and-cell-type-specific effects of OTR signaling. Development of therapeutics that could target specific cell types or circuits could be more effective than existing systemic treatments.
Supplementary Material
Acknowledgements
This work supported by R01MH113628 to JT and R01MH121829 to BCT
Bibliography
- Beery AK, 2015. Antisocial oxytocin: complex effects on social behavior. Current Opinion in Behavioral Sciences 6, 174–182. 10.1016/j.cobeha.2015.11.006 [DOI] [Google Scholar]
- Beyeler A, Dabrowska J, 2020. Chapter 3 - Neuronal diversity of the amygdala and the bed nucleus of the stria terminalis, in: Urban JH, Rosenkranz JA (Eds.), Handbook of Behavioral Neuroscience, Handbook of Amygdala Structure and Function. Elsevier, pp. 63–100. 10.1016/B978-0-12-815134-1.00003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland JM, Grantham KN, Aiani LM, Frantz KJ, Albers HE, 2018. Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology 95, 128–137. 10.1016/j.psyneuen.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID, 2012. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and Behavior 61, 293–303. 10.1016/j.yhbeh.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Bredewold R, Smith CJW, Dumais KM, Veenema AH, 2014. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front. Behav. Neurosci 8. 10.3389/fnbeh.2014.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, McCaffery P, 2008. The neurobiology of retinoic acid in affective disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry 32, 315–331. 10.1016/j.pnpbp.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, Bulgheroni E, Manning M, Kleinau G, Chini B, 2013. Selective and Potent Agonists and Antagonists for Investigating the Role of Mouse Oxytocin Receptors. Journal of Pharmacology and Experimental Therapeutics 346, 318–327. 10.1124/jpet.113.202994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, Saulière A, Manning M, Bouvier M, Galés C, Chini B, 2012. Functional Selective Oxytocin-derived Agonists Discriminate between Individual G Protein Family Subtypes*. Journal of Biological Chemistry 287, 3617–3629. 10.1074/jbc.M111.277178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Jameson CE, Trainor BC, 2013. Sexual dimorphism in the brain of the monogamous california mouse (peromyscus californicus). Brain, Behavior and Evolution 81. https://doi.org/10.1159000353260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Bruchas MR, 2019. A Motivational and Neuropeptidergic Hub: Anatomical and Functional Diversity within the Nucleus Accumbens Shell. Neuron 102, 529–552. 10.1016/j.neuron.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaviaras S, Mak P, Ralph D, Krishnan L, Broadbear JH, 2010. Assessing the antidepressant-like effects of carbetocin, an oxytocin agonist, using a modification of the forced swimming test. Psychopharmacology 210, 35–43. 10.1007/s00213-010-1815-x [DOI] [PubMed] [Google Scholar]
- Chen R, Blosser TR, Djekidel MN, Hao J, Bhattacherjee A, Chen W, Tuesta LM, Zhuang X, Zhang Y, 2021. Decoding molecular and cellular heterogeneity of mouse nucleus accumbens. Nat Neurosci 24, 1757–1771. 10.1038/s41593-021-00938-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CKW, 2012. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Hormones and Behavior, Oxytocin, Vasopressin and Social Behavior 61, 419–428. 10.1016/j.yhbeh.2011.12.009 [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Kret ME, 2016. Oxytocin Conditions Intergroup Relations Through Upregulated In-Group Empathy, Cooperation, Conformity, and Defense. Biological Psychiatry, Oxytocin and Psychiatry: From DNA to Social Behavior 79, 165–173. 10.1016/j.biopsych.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC, 2013. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, 2010. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 35, 83–93. 10.1016/j.psyneuen.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH, 2016. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Frontiers in Neuroendocrinology 40, 1–23. 10.1016/j.yfrne.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, Trainor BC, 2018. Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biological Psychiatry 83, 203–213. 10.1016/j.biopsych.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Laredo SA, Hao R, Perkeybile AM, Bales KL, Trainor BC, 2016. Inhibition of vasopressin V1a receptors in the medioventral bed nucleus of the stria terminalis has sex- and context-specific anxiogenic effects. Neuropharmacology 110, 59–68. 10.1016/j.neuropharm.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, Hao R, Ramos-Maciel S, Rios RA, Jackson K, Flores-Ramirez FJ, Garcia-Carachure I, Pesavento PA, Iñiguez SD, Grinevich V, Trainor BC, 2020. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc Natl Acad Sci USA 117, 26406–26413. 10.1073/pnas.2011890117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Weber K, Stoffel-Wagner B, Maier W, Hurlemann R, 2014. Oxytocin facilitates the sensation of social stress. Human Brain Mapping 35, 4741–4750. 10.1002/hbm.22508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Winder DG, 2003. Dorsal and Ventral Distribution of Excitable and Synaptic Properties of Neurons of the Bed Nucleus of the Stria Terminalis. Journal of Neurophysiology 90, 405–414. 10.1152/jn.00228.2003 [DOI] [PubMed] [Google Scholar]
- Flanigan ME, Kash TL, 2020. Coordination of social behaviors by the bed nucleus of the stria terminalis. European Journal of Neuroscience in press. 10.1111/ejn.14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CL, Young LJ, 2021. Refining oxytocin therapy for autism: context is key. Nat Rev Neurol 1–2. 10.1038/s41582-021-00602-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shackman AJ, 2019. The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters 693, 58–67. 10.1016/j.neulet.2017.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Olivera-Pasilio V, Dabrowska J, 2021. Oxytocin excites BNST interneurons and inhibits BNST output neurons to the central amygdala. Neuropharmacology 192, 108601. 10.1016/j.neuropharm.2021.108601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Zhao W, Zhou F, Ma X, Yao S, Hurlemann R, Becker B, Kendrick KM, 2018. Oxytocin Enhancement of Emotional Empathy: Generalization Across Cultures and Effects on Amygdala Activity. Front. Neurosci 12. 10.3389/fnins.2018.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F, 2001. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiological Reviews 81, 629–683. 10.1152/physrev.2001.81.2.629 [DOI] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC, 2014. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci 7, 223. 10.3389/fnbeh.2013.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan A, Bastiaanssen TFS, Ventura Silva AP, Moloney GM, Cryan JF, 2022. The impact of psychosocial defeat stress on the bed nucleus of the stria terminalis transcriptome in adult male mice. European Journal of Neuroscience 55, 67–77. 10.1111/ejn.15567 [DOI] [PubMed] [Google Scholar]
- Hammock EAD, Young LJ, 2006. Oxytocin, vasopressin and pair bonding: implications for autism. Phil. Trans. R. Soc. B 361, 2187–2198. 10.1098/rstb.2006.1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R, 2021. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29. 10.1016/j.cell.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintiryan H, Dong H-W, 2022. Brain Networks of Connectionally Unique Basolateral Amygdala Cell Types. J Exp Neurosci 17, 26331055221080176. 10.1177/26331055221080175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dölen G, Malenka RC, 2017. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411. 10.1126/science.aan4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM, 2010. Oxytocin Enhances Amygdala-Dependent, Socially Reinforced Learning and Emotional Empathy in Humans. J. Neurosci 30, 4999–5007. 10.1523/JNEUROSCI.5538-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeček M, Dabrowska J, 2019. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies—potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res 375, 143–172. 10.1007/s00441-018-2889-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, Neumann ID, 2018. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiological Reviews 98, 1805–1908. 10.1152/physrev.00031.2017 [DOI] [PubMed] [Google Scholar]
- Keverne EB, Kendrick KM, 1992. Oxytocin Facilitation of Maternal Behavior in Sheepa. Annals of the New York Academy of Sciences 652, 83–101. 10.1111/j.1749-6632.1992.tb34348.x [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K, 2013. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223. 10.1038/nature12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt JD, Goodson JL, 2013. Oxytocin-like receptors mediate pair bonding in a socially monogamous songbird. Proc. R. Soc. B 280, 20122396. 10.1098/rspb.2012.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S, 2010. Oxytocin and carbetocin ameliorating effects on restraint stress-induced short- and long-term behavioral changes in rats 9. [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S, 2009. Oxytocin and carbetocin effects on spontaneous behavior of male rats: Modulation by oxytocin receptor antagonists. Neuro endocrinology letters 30, 335–42. [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E, 2005. Oxytocin increases trust in humans. Nature 435, 673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Kuske JX, Trainor BC, 2021. Mean Girls: Social Stress Models for Female Rodents, in: Current Topics in Behavioral Neurosciences. Springer, Berlin, Heidelberg, pp. 1–30. 10.1007/7854_2021_247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen Lin, Chen Li, Chen T-M, Chi Chin M, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong H-W, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf K-R, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Feng Yuan X, Zhang B, Zwingman TA, Jones AR, 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- Leng G, Meddle S, Douglas A, 2008. Oxytocin and the maternal brain. Current Opinion in Pharmacology 8, 731–734. 10.1016/j.coph.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Leppanen J, Ng KW, Kim Y-R, Tchanturia K, Treasure J, 2018. Meta-analytic review of the effects of a single dose of intranasal oxytocin on threat processing in humans. Journal of Affective Disorders 225, 167–179. 10.1016/j.jad.2017.08.041 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX, 2003. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544. 10.1016/S0306-4522(03)00555-4 [DOI] [PubMed] [Google Scholar]
- Lukas M, Neumann ID, 2014. Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvment of brain oxytocin and vasopressin. J. Neurosci Methods 234, 101–107. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID, 2011. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36, 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM, 2010. The Peptide That Binds: A Systematic Review of Oxytocin and its Prosocial Effects in Humans. Harv Rev Psychiatry 22. [DOI] [PubMed] [Google Scholar]
- Martinon D, Lis P, Roman AN, Tornesi P, Applebey SV, Buechner G, Olivera V, Dabrowska J, 2019. Oxytocin receptors in the dorsolateral bed nucleus of the stria terminalis (BNST) bias fear learning toward temporally predictable cued fear. Transl Psychiatry 9, 140. 10.1038/s41398-019-0474-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Shen F, Li C, Li Y, Wang X, 2016. Depression-like behaviors in tree shrews and comparison of the effects of treatment with fluoxetine and carbetocin. Pharmacology Biochemistry and Behavior 145, 1–8. 10.1016/j.pbb.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M, 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience 12, 524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Moaddab M, Dabrowska J, 2017. Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear-potentiated startle paradigm in rats. Neuropharmacology 121, 130–139. 10.1016/j.neuropharm.2017.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Görlich A, Heintz N, 2014. Oxytocin Modulates Female Sociosexual Behavior through a Specific Class of Prefrontal Cortical Interneurons. Cell 159, 295–305. 10.1016/j.cell.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passoni I, Leonzino M, Gigliucci V, Chini B, Busnelli M, 2016. Carbetocin is a functional selective Gq agonist that does not promote oxytocin receptor recycling after inducing b-arrestin-independent internalisation. J. Neuroendocrinol 28, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, MacFadyen K, Smith JA, Kloet A.D. de, Wang L, Krause EG, 2017. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. Journal of Comparative Neurology 525, 1094–1108. 10.1002/cne.24116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Andersen M, Almén MS, Schiöth HB, 2011. Trends in the exploitation of novel drug targets. Nature Reviews Drug Discovery 10, 579–590. 10.1038/nrd3478 [DOI] [PubMed] [Google Scholar]
- Ribeiro EA, Salery M, Scarpa JR, Calipari ES, Hamilton PJ, Ku SM, Kronman H, Purushothaman I, Juarez B, Heshmati M, Doyle M, Lardner C, Burek D, Strat A, Pirpinias S, Mouzon E, Han M-H, Neve RL, Bagot RC, Kasarskis A, Koo JW, Nestler EJ, 2018. Transcriptional and physiological adaptations in nucleus accumbens somatostatin interneurons that regulate behavioral responses to cocaine. Nat Commun 9, 3149. 10.1038/s41467-018-05657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G, 2014. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39, 237–248. 10.1016/j.psyneuen.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ, 2011. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci 12, 623–637. 10.1038/nrn3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero T, Nagasawa M, Mogi K, Hasegawa T, Kikusui T, 2015. Intranasal administration of oxytocin promotes social play in domestic dogs. Communicative & Integrative Biology 8, e1017157. 10.1080/19420889.2015.1017157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SGF, Kobilka BK, 2009. The structure and function of G-protein-coupled receptors. Nature 459, 356–363. 10.1038/nature08144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A, 2016. The Social Salience Hypothesis of Oxytocin. Biological Psychiatry 79, 194–202. 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Smith CJW, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH, 2017. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct 222, 981–1006. 10.1007/s00429-016-1260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE, 2016. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 74, 164–172. 10.1016/j.psyneuen.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EM, Walch K, Bales KL, Trainor BC, 2016. Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biological Psychiatry 80, 406–414. 10.1016/j.biopsych.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Trainor BC, 2019. Complementary Neural Circuits for Divergent Effects of Oxytocin: Social Approach Versus Social Anxiety. Biological Psychiatry 85, 792–801. 10.1016/j.biopsych.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader CD, Fong TM, Tota MR, Underwood D, Dixon RAF, 1994. Structure and Function of G Protein-Coupled Receptors 32. [DOI] [PubMed] [Google Scholar]
- Striepens N, 2011. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Frontiers in Neuroendocrinology 25. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ, 2012. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology 37, 438–443. 10.1016/j.psyneuen.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M, 2003. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology 463, 199–216. 10.1016/S0014-2999(03)01282-2 [DOI] [PubMed] [Google Scholar]
- Welch JD, Kozareva V, Ferreira A, Vanderburg C, Martin C, Macosko EZ, 2019. Single-Cell Multi-omic Integration Compares and Contrasts Features of Brain Cell Identity. Cell 177, 1873–1887.e17. 10.1016/j.cell.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Ramshaw H, Taimi M, Stangle W, Zhang A, Everingham S, Creighton S, Tam S-P, Jones G, Petkovich M, 2000. Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proceedings of the National Academy of Sciences 97, 6403–6408. 10.1073/pnas.120161397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Duque-Wilckens N, Ramos-Maciel S, Campi KL, Bhela SK, Xu CK, Jackson K, Chini B, Pesavento PA, Trainor BC, 2020. Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacol. 45, 1423–1430. 10.1038/s41386-020-0657-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CJ, Zhang SW, Tai FD, 2016. Effects of nucleus accumbens oxytocin and its antagonist on social approach behavior. Behavioural Pharmacology 27, 672–680. 10.1097/FBP.0000000000000212 [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID, 2014. Brain Oxytocin in Social Fear Conditioning and Its Extinction: Involvement of the Lateral Septum. Neuropsychopharmacology 39, 3027–3035. 10.1038/npp.2014.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.