Abstract
GATA2 deficiency is a bone marrow failure syndrome effectively treated with hematopoietic cell transplantation (HCT), which also addresses the predisposition to many infections (prominently mycobacterial). However, many GATA2-deficient persons who come to HCT also have prevalent and refractory human papilloma virus disease (HPVD), which can be a precursor to cancer. We analyzed 75 HCT recipients for the presence of HPVD to identify patient characteristics and transplantation results that influence HPVD outcomes. We assessed the impact of cellular recovery and iatrogenic post-transplantation immunosuppression, as per protocol (PP) or intensified/prolonged (IP) graft-versus-host disease (GVHD) prophylaxis or treatment, on the persistence or resolution of HPVD. Our experience with 75 HCT recipients showed a prevalence of 49% with anogenital HPVD, which was either a contributing or primary factor in the decision to proceed to HCT. Of 24 recipients with sufficient follow-up, 13 had resolution of HPVD, including 8 with IP and 5 with PP. Eleven recipients had persistent HPVD, including 5 with IP and 6 with PP immunosuppression. No plausible cellular recovery group (natural killer cells or T cells) showed a significant difference in HPV outcomes. One recipient died of metastatic squamous cell carcinoma, presumably of anogenital origin, at 33 months post-transplantation after prolonged immunosuppression for chronic GVHD. Individual cases demonstrate the need for continued aggressive monitoring, especially in the context of disease prevalent at transplantation or prior malignancy. HCT proved curative in many cases in which HPVD was refractory and recurrent prior to transplantation, supporting a recommendation that HPVD should be considered an indication rather than contraindication to HCT, but post-transplantation monitoring should be prolonged with a high level of vigilance for new or recurrent HPVD.
Keywords: GATA2 deficiency, Human papillomavirus disease, Hematopoietic cell transplant, Cellular reconstitution
INTRODUCTION
GATA2 deficiency is a congenital immunodeficiency with a predisposition to infections, hematologic malignancy, and manifold associated conditions including pulmonary alveolar proteinosis, deafness, and lymphedema [1–5]. First described a decade ago, the clinical manifestations that led to the discovery of the genetic lesion included the MonoMAC syndrome (monocytopenia and infection with nontuberculous mycobacteria), with bone marrow failure with cytopenias of monocytes, natural killer (NK) cells, and B lymphocytes an integral part of the syndrome, along with a predisposition to myeloid evolution to myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML).
The infectious complications of GATA2 deficiency/MonoMAC, presumably secondary to the associated cytopenias, include infections with mycobacteria (usually nontuberculous species) and warts caused by the human papillomavirus (HPV). Cutaneous common warts and anogenital disease are prevalent; the presentation of disease is HPV-strain related. Both in our series and in the literature, refractory HPV-related disease (HPVD) has been the index condition leading to the diagnosis of GATA2 deficiency in a number of patients [6]. The potential for malignant transformation of HPV lesions appears to be increased by the loss of immunity [7].
Hematopoietic cell transplantation (HCT) is potentially curative of the hematologic manifestations of GATA2 deficiency/MonoMAC. HCT is intended to restore immunity to infections prevalent at the time of transplantation, which is a secondary outcome reported in our previous studies [8,9]. However, the specific evolution of the restoration of cellular immunity in particular and the evolution of prevalent infection and pathology associated with HPV have not been studied systematically.
In this study, we analyzed the outcomes of HPV-related anogenital disease in 37 patients with GATA2 deficiency and HPVD who underwent HCT. We correlated the resolution or persistence of HPVD disease with the restoration or lack of restoration of cellular immunity.
METHODS
Patients
The HCT recipients with HPVD reported here were enrolled on 2 studies, 14 with nonmyeloablative (NMA) conditioning HCT and 59 with myeloablative conditioning (MAC) HCT, to assess different conditioning regimens for the efficacy and safety of HCT in patients with GATA2 deficiency [8,9]. An additional 2 patients underwent HCT, one on a standard of care protocol and the other on a separate, high-intensity protocol. Because these 2 transplantations did not contribute to the 2 outcomes of interest in this series (continuation of our report on a busulfan-based regimen and long-term outcomes related to HPVD prevalent at transplantation), those transplantation regimens are not described here. All studies were approved by the Institutional Review Board of the National Cancer Institute (NCI). These studies were independently monitored for safety and data accuracy (ClinicalTrials.gov identifiers NCT 009233364 and NCT01861106).
Pretransplantation data included age at transplantation; duration of illness; types of infections, including nontuberculous mycobacterial infection and viral infections; other manifestations of disease; cytogenetics; bone marrow biopsy; GATA2 mutation; and presence or absence of a family history of GATA2 deficiency or clinical syndrome consistent with GATA2 deficiency. Pretransplantation and post-transplantation gynecologic and other anogenital disease data were obtained, including physical examination, Pap smear, and other cytologic and anatomic pathology for the presence of HPV via immunohistochemistry or PCR-based detection of high- and low-risk HPV, yielding an assessment of the premalignant or malignant nature of HPV-associated lesions in accordance with standard criteria [10,11]. For some patients, assessment of premalignant lesions led to treatment in the 6 months before transplantation, but in other patients transplantation was considered the most urgent intervention (with HPVD diagnosed only in the course of other complications) and was performed in the presence of disease that otherwise would have been addressed medically or surgically.
Bone Marrow Disease
Descriptive criteria for bone marrow abnormalities have evolved through the years in the 2 protocols reported here. Our first protocol described refractory cytopenia with multilineage dysplasia (RCMD) as a characteristic lesion. In the 2017 updated World Health Organization’s (WHO) Classification of Tumours of the Haematopoietic and Lymphoid Tissues, the terminology was changed from RCMD to myelodysplastic syndrome with multilineage dysplasia (MDS-MLD) [12]. An increasing number of cases with hypocellularity and mild atypia did not meet the WHO criteria for a diagnosis of MDS despite the presence of cellular abnormalities. For these cases, we adopted the term “bone marrow and immunodeficiency disorder with germline GATA2 mutation” (BMID), describing marrow with decreased cellularity and characteristic morphologic features of GATA2 deficiency without frank dysplasia, increased blasts, or cytogenetic abnormalities [13].
Eligibility
The eligibility criterion for patients age 12 years (first protocol) or 8 years (second protocol) to 60 years was at least 1 episode of a life-threatening opportunistic infection or a pathologic mutation in the GATA2 gene. In the absence of an identified GATA2 mutation, a flow cytometry profile on peripheral blood demonstrating severe monocytopenia and CD19+ B cell and CD3−/CD56+ NK cell lymphopenia consistent with the MonoMAC profile was the criterion for eligibility. After the first protocol, in which umbilical cord (UC) products were allowed, all recipients needed to have a 10/10 or 9/10 HLA-matched related donor (MRD), matched unrelated donor (MUD), or haploidentical related donor (HRD) with normal GATA2 gene on sequencing or normal monocyte, NK cell. and B cell counts with no history of mycobacterial or other opportunistic infection. All related (MRD) and unrelated donor-recipient (URD) pairs were matched at 10/10 HLA-A, -B, -C, -DRB1, and -DQB1 loci by high-resolution typing. HRDs shared 1 haplotype with the recipient for a minimum match of 5/10 HLA loci. Blasts in the bone marrow had to be <5% in the absence of granulocyte colony-stimulating factor (GCSF) use.
Details of the NMA and MAC regimens have been reported previously [8,9]. High-dose (50 mg/kg i.v. on days 3 and 4) post-transplantation cyclophosphamide (PTCy) was increasingly used for recipients from all donors through the course of the protocol.
Immune Reconstitution of T, B, and NK Cells and Monocytes
CD14+ monocytes, CD3−CD56+ NK cells, CD19+ B lymphocytes, and CD3+CD4+ and CD3+CD8+ T lymphocytes were quantified by flow cytometry using subset-specific monoclonal antibodies pretransplantation and at designated intervals post-transplantation. Baseline cell counts are reported as those immediately before the start of conditioning.
Analysis of Chimerism
Engraftment of donor cells was assessed using polymorphisms in regions known to contain short tandem repeats. In addition, CD14+CD15+ myeloid cells and CD3+ lymphocytes were selected using immunobeads, and chimerism was assessed on the selected cells.
HPV Testing
Hybrid capture with signal amplification for the detection of low-risk (6, 11, 42, 43, 44) and high-risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) HPV types were performed at Quest Diagnostics Nichols Institute (Chantilly, VA). Immunoperoxidase and in situ hybridization was performed at the Department of Pathology, National Cancer Institute with assays that have not been cleared or approved by the US Food and Drug Administration (FDA). The FDA has determined that such clearance and approval is not necessary.
Supportive Care
Standard guidelines for supportive care established at the National Institutes of Health Clinical Center for patients undergoing allogeneic HCT were followed. These guidelines are in agreement with international guidelines for preventing infectious complications among HCT recipients [14].
Statistical Analysis
Data are summarized as proportion (binary) and median with interquartile range (IQR; 25th to 75th percentile) (continuous) and compared between those with and without HPV using Fisher’s exact test and Wilcoxon’s rank-sum test, respectively. The time to recovery of each relevant cell subset was compared among groups (group 1, persistent disease and prolonged/intensified [PI] immunosuppression; group 2, persistent disease without PI; group 3, resolved disease without PI; group 4, resolved disease with PI) using Kaplan-Meier plots and the log-rank test.
RESULTS
Transplantation Outcomes
HCT for MonoMAC/GATA2 deficiency was performed in 73 patients on 2 protocols. In addition, 2 recipients, who contribute only to the description of prevalent disease pre-HCT, underwent transplantation on standard of care or other protocols. Our initial NMA conditioning protocol (14 recipients) and 22 recipients of a busulfan-based regimen were reported previously. Thus, an additional 37 recipients who underwent HCT on the second protocol were included in the total number of patients. We report the outcomes of the patients with HPVD who underwent HCT.
Overall survival in the second protocol was 88.1% at a median follow-up of 34 months (IQR, 16 to 52 months), with an event-free survival of 86%. Table 1 presents data for patients with anogenital disease from both protocols, and Supplementary Table S1 presents data for recipients without anogenital disease from both protocols. Table 2 compares recipients with and without anogenital disease, demonstrating significant differences in age and CD8 T cell counts, with a trend toward an association with sex.
Table 1.
GATA2-Deficient HCT Recipients with Historical or Prevalent HPV Anogenital Disease
| Patient | Age, yr/Sex | GATA2-MonoMAC Disease/Protocol*, Donor/Product, PTCy | Age at Onset of HPV (Interval to HCT), History | Cell Profile at HCT† | ||
|---|---|---|---|---|---|---|
| NK/B, cells/μL | Monocytes, k cells/μL | CD4+/CD8+, cells/μL | ||||
| 1 | 46/F | MonoMAC-AML*, MUD/PBSC | Biopsy with dysplasia at age 37 yr (9 yr), cervical cancer at age 43 yr | 4/6 | 140 | 73/39 |
| 2 | 27/F | MonoMAC-AML **, MUD/PBSC | Recurrent VIN since age 23 yr (4 yr), vulvectomy | 62/27 | 80 | 412/479 |
| 3 | 33/M | RCMD/1, MRD/PBSC | Unknown, penile condyloma | 2/1 | 0 | 494/269 |
| 4 | 49/F | RCMD/1, MRD/PBSC | Unknown, recurrent abnormal Pap, LEEP × 4 | 2/3 | 0 | 140/86 |
| 5 | 33/M | RCMD/1, MRD/PBSC | Unknown, anogenital condyloma | 105/5 | 10 | 188/220 |
| 6 | 38/F | RAEB1/1, MRD/PBSC | 32 (7 yr), condyloma, diverting colostomy, excision VIN II/III | 0/0 | 50 | 242/219 |
| 7 | 41/F | RAEB2/1, double UC | Hysterectomy age 23 yr (18 yr), >30 procedures for recurrent VV HPV | 0/0 | 740 | 251/136 |
| 8 | 28/M | RCMD/1, double UC | Unknown, penile/anal condyloma, debulked | 0/1 | 40 | 154/78 |
| 9 | 26/F | RCMD/1, double UC | Unknown, excised CIN III/VIN III | 4/12 | 20 | 178/119 |
| 10 | 29/F | MDS/2, MRD/BM | 22 (7 yr), recurrent condylomas, excision of VIN II, laser ablations | 10/7 | 0 | 315/243 |
| 11 | 28/F | MonoMAC- MDS/2, MUD/PBSC | 16 (12 yr), refractory VIN III | 10/27 | 40 | 785/537 |
| 12 | 22/M | MDS/2, MUD/PBSC | Unknown, penile condyloma | 0/0 | 10 | 154/90 |
| 13 | 24/F | MDS/2, MUD/BM | Age 17 yr (7 yr), partial vulvectomy, yearly surgery × 5, VIN III, CIN | 2/25 | 20 | 165/382 |
| 14 | 28/F | MDS/2, MUD/BM | Age 26 yr (2 yr), multiple cold knife procedures, vulvar condyloma | 0/10 | 0 | 211/142 |
| 15 | 38/F | MDS/2, MUD/PBSC | Unknown, VIN III,? invasion | 7/21 | 0 | 509/418 |
| 16 | 24/F | MDS/2, MUD/BM, PTCy | Age 19 yr (5 yr), CIN III, high Ki-67 index and p16+, severe dysplasia | 7/11 | 10 | 487/405 |
| 17 | 27/F | MDS/2, HRD/BM, PTCy | Age 21 yr (6 yr), multiple laser surgeries, refractory vulvar/anal | 0/1 | 20 | 168/72 |
| 18 | 27/ F | MDS/2, HRD/BM, PTCy | Age 23 yr (4 years), excised VIN II | 10/1 | 124 | 124/28 |
| 19 | 26/M | MDS/2, HRD/BM, PTCy | Unknown, chronic penile warts | 67/3 | 10 | 720/343 |
| 20 | 34/F | BMID/2, HRD/BM, PTCy | Age 26 yr (8 yr), anal condyloma, CIN III, vulvovaginal dysplasia | 53/15 | 0 | 837/392 |
| 21 | 17/F | MDS/2, HRD/BM, PTCy | First diagnosis at transplantation, LGSIL | 15/23 | 0 | 544/663 |
| 22 | 26/M | MonoMAC- MDS/2, MUD/BM, PTCy | Unknown, penile condyloma | 93/6 | 130 | 300/339 |
| 23 | 32/F | MDS/2, MUD/BM, PTCy | Unknown, vulvar resection | 3/5 | 10 | 283/203 |
| 24 | 32/F | MDS/2, MUD/BM, PTCy | Age 16 yr (16 yr), SCC vulva at 19 yr | 1/9 | 10 | 146/187 |
| 25 | 45/M | MDS/2, MRD/BM, PTCy | Anorectal cancer age 40 yr (5 yr), debulking, radiation, and chemotherapy | 18/2 | 10 | 201/360 |
| 26 | 36/F | MDS/2, MRD/BM, PTCy | Age 18 yr (18 yr), CIS, laser × 3 | 3/14 | 10 | 379/191 |
| 27 | 28/F | BMID/2, MUD/PBSC, PTCy | Age 18 yr (10 yr), vulvar CA, 6 surgical procedures, CIN II, VIN II/III, AIN III | 95/14 | 30 | 278/344 |
| 28 | 34/F | MDS/2, MRD/BM, PTCy | Age 27 yr (7 yr), vulvar carcinoma in situ, vulvar/perianal ablation | 15/61 | 0 | 390/457 |
| 29 | 32/F | BMID/2, HRD/BM, PTCy | Age 20 yr (12 yr), 2 vulvectomies, HGSIL anal resection | 0/6 | 19 | 460/513 |
| 30 | 34/F | MDS/2, MUD/BM, PTCy | Early 20s (~14 yr), anal precancer, bilateral vulvectomy | 0/2 | 10 | 212/175 |
| 31 | 24/F | MDS/2, MUD/BM, PTCy | Age 20 yr (4 yr), multiple lasers and vulvectomy, SCC vulva | 1/1 | 0 | 166/91 |
| 32 | 52/M >30 yr | MDS/2, MUD/BM | Age ~22 yr (>30 yr), SCC inguinal fold | 131/10 | 0 | 150/205 |
| 33 | 23/F | MDS/2, MUD/BM, PTCy | Age 21 yr (2 yr), HPV+ abnormal Pap, LGSIL | 37/5 | 10 | 362/279 |
| 34 | 29/F | MDS/2, MUD/BM, PTCy | Age 23 yr (6 yr), vulvectomy at 24 yr, cryosurgery of cervix and vulva at 26 yr | 130 | 10 | 393/183 |
| 35 | 38/F | MDS/2, HRD/BM, PTCy | Age 33 yr (5 yr), repeated surgical procedures with recurrence | 75/15 | 20 | 397/216 |
| 36 | 39/F | MDS/2, MUD/BM, PTCy | Age 26 yr (13 yr), multiple vulvar, vaginal, and cervical carcinoma in situ, resection and laser | 54/1 | 30 | 130/193 |
| 37 | 31/F | BMID/2, MRD/BM, PTCy | Unknown, recurrent vulvar condyloma, vulvectomy at age 26 yr | 5/36 | 0 | 348/588 |
PTCy indicates post-transplantation cyclophosphamide; RCMD, refractory cytopenia with multilineage dysplasia; RAEB, refractory anemia with excess blasts; BMID, bone marrow disorder with immune deficiency; SCC, squamous cell carcinoma; LGSIL, low-grade squamous intraepithelial lesion; HGSIL, high-grade squamous intraepithelial lesion; CIN, cervical intraepithelial neoplasia; VIN, vulvar intraepithelial neoplasia; AIN, anal intraepithelial neoplasia; LEEP, loop electrosurgical excision procedure; BM, bone marrow; PBSC, peripheral blood stem cells.
Protocol 1, NMA conditioning; protocol 2, MAC.
Cell counts at the pretransplantation immunophenotype.
Table 2.
Age, Sex, and Immunologic Comparison of GATA2-Deficient HCT Recipients by HPV Anogenital Disease Status
| Parameter | No HPV Disease (N = 38) | HPV Disease (N = 37)* | P Value† |
|---|---|---|---|
| Age, yr, median (IQR) | 22.5 (18–32) | 31 (27–35) | .001 |
| Sex, male/female, n | 16/22 | 8/29 | .083 |
| Monocytes k/μL, median (IQR) | 20 (10–48) | 10 (0–20) | .074 |
| NK cells/μL, median (IQR) | 12 (5–42) | 7 (1–53) | .378 |
| B cells/μL, median (IQR) | 10 (2–31) | 6 (2–15) | .186 |
| CD4 cells/μL, median (IQR) | 322 (155–518) | 278 (166–397) | .515 |
| CD8 cells/μL, median (IQR) | 322 (258–737) | 219 (142–382) | .008 |
n = 2 HPV, no protocol, excluded.
P values were computed using Fisher’s exact test for dichotomous variables and Wilcoxon’s rank-sum test for continuous variables.
HPV Disease
Some form of HPVD (warts and/or anogenital) was present in 59 of 75 (78.6%) HCT recipients. Cutaneous disease alone was present in 22 recipients (Supplementary Table S1), and anogenital disease alone was present in 17 recipients. Five recipients had undergone medical or surgical treatment for squamous cell carcinoma before HCT, including 2 with vulvar lesions and 1 each with cervical, inguinal fold, and anal lesions. Sites of disease present at transplantation were cervix (n = 13), vulva (n = 23), vagina (n = 5), anal (n = 12), and penile (n = 8). Carcinoma in situ was diagnosed in 20 recipients, 9 of whom had undergone 1 or more vulvectomies.
Data for 37 HCT recipients with anogenital HPVD are presented in Table 1 (>1 year follow-up; n = 27; males, n = 8; females, n = 29). Among these patients, 23 could report a date of onset, yielding a median duration of disease by the time of transplantation of 7 years (IQR, 5 to 12 years). All recipients had a bone marrow abnormality, and 27 had mycobacterial disease. All had characteristic deficient NK and/or B cell count, and 36 had monocytopenia. The median NK cell count was 7/μL (IQR, 52.5; 1 to 53.5), and the count was low at transplantation in 35 of the 37 patients. The median cell counts at transplantation were 6/μL (IQR, 13.5; 1 to 14.5/μL) for B cells, 10 k/μL (IQR, 30; 0 to 30 k/μL) for monocytes, 278/μL (IQR, 239; 165.5 to 404.5/μL) for CD3+CD4+ T cells (below normal in 22 patients), and 219/μL (IQR, 248; 139 to 387/μL) for CD3+CD8+ T cells (below normal in 10 patients).
Twenty-four HCT recipients had sufficient follow-up and pretransplantation prevalent disease to enable further characterization of post-transplantation HPVD (Table 3). One male recipient (patient 3) with a history of penile condyloma had no recorded examination prior to transplant, and 1 male recipient and 1 female recipient had no disease at transplantation (patients 1 and 19) (Tables 1 and 3). Supplementary Table S2 presents the initial presentation and transplantation outcomes of recipients without sufficient follow-up. Recipients could be characterized by persistent or resolved disease and by the use of immunosuppression related to the protocol standard of 6 months of calcineurin inhibitor and/or sirolimus therapy (per protocol [PP] or prolonged/intensified [PI] immunosuppression). Kaplan-Meier analysis of the days to resolution of cytopenias showed significance for monocyte recovery (driven by a single recipient with prolonged post-transplantation cytopenias on protocol 1) and for B cell recovery among all groups (Supplementary Figure S1).
Table 3.
Anogenital HPV Status at Transplantation, and Post-Transplantation Evaluation, Immunosuppression, and Transplant Outcome
| Patient | Age, yr/Sex | Disease at HCT | Days to Achieve Normal* | Cytopathology/ Molecular | Anatomic Pathology | GVHD/ Immunosuppression | HPV-related Outcome/ Duration Transplant Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| NK/B Cells | Monocytes | CD4+/CD8+T Cells | |||||||
| 5 | 33/M | Anogenital condyloma, rectal LGSIL, possible HG | Never low/97 | 10 | 201/201 | ASCUS through 169 wk | No disease 143 wk; p16+ through 129 wk | Cryptogenic organizing pneumonia, tacrolimus until 169 wk, intermittent prednisone | 243 wk negative anal mapping Alive >8 yr |
| 6 | 38/F | Vulvar/perianal condyloma, anal SCC in situ | 14/60 | 8 | Never low/never low | Rare ASCUS through 262 wk/12 wk HR, thereafter all negative | Perianal dysplasia with HPV through 131 wk | Tacrolimus through 262 wk for proteinuria/hypoalbuminemia | Anal disease resolved, rare cervical ASCUS 262 wk Alive >8 yr |
| 7 | 41/F | HGSIL | 181/181 | 76 | 559/371 | HGSIL 80 wk/ HR detected through 80 wk | VaIN III, VIN II, p16+, anal SCC 106 wk | None | No reassessment after 106 wk Died at 2.5 yr from Donor-derived AML |
| 10 | 29/F | HPV lesions | 52/300 | 12 | 1109/179 | Resolved 79 wk, recurrent 153 wk/ HR through week 106 Negative week 153 | VIN p16− 52 wk, 79 wk CC HPV neg | Grade III aGVHD GI and skin; VV GVHD; topical corticosteroids | No disease week 210 Alive >5 yr |
| 11 | 28/F | Vulvar Bowen’s, VAIN III, AIN III | 14/267 | 10 | 14/14 | Resolved 104 wk/ HR through week 134 | Resolved dysplasia 176 wk CC through 197 wk | aGVHD GI and skin; moderate-severe cGVHD; systemic corticosteroids/ ruxolitinib/ibrutinib | No disease 296 wk Alive >6 yr |
| 12 | 22/M | Penile condyloma | 29/217 | 16 | 217/362 | ND/ ND | ND | cGVHD, limited skin, eyes | Resolved disease Alive >4 yr |
| 13 | 24/F | VIN I | 1148/520 | 14 | 722/1148 | Resolved 192 wk/ Resolved 139 wk | Resolved 139 wk | aGVHD; systemic corticosteroids/ ruxolitinib | No disease 192 wk Alive >4 yr |
| 17 | 27/F | Minimal | 101/181 | 16 | 362/362 | ASCUS resolved 75 wk/ LR through 51 wk | CC, focal p16, VIN 75 wk, normal 102 wk | aGVHD grade III skin; systemic corticosteroids/ruxolitinib | Resolved disease Alive >5 yr |
| 18 | 27/F | VIN III | 745/111 | 17 | 382/214 | Resolved 83 wk/ HR at 136 wk | VIN III p16+ 55 wk | aGVHD grade II GI and skin; systemic corticosteroids | No disease 156 wk Alive >5 yr |
| 20 | 34/F | VAIN I, VIN I/III, CIN I (p16−) | 182/182 | 19 | 710/ never low | LGSIL through 172 wk/ LR and HR through 172 wk | CIN I/II, severe dysplasia 77 wk | aGVHD grade III GI moderate cGVHD; tacrolimus to 77 wk, prednisone, ruxolitinib, ibrutinib off 95 wk | Exam 172 wk with small vulvar condyloma Alive >4 yr |
| 21 | 17/F | LGSIL, LR/HR | 1102/96 | 18 | 360/31 | LGSIL through 156 wk/ HR through 156 wk | Cervical dysplasia resolved 104 wk | Persistent disease 158 wk Alive >4 yr | |
| 22 | 26/M | Penile condyloma | 574/194 | 15 | 574/369 | ND/ ND | ND | aGVHD grade II skin, GI | Condyloma gone 168 wk Alive >4 yr |
| 23 | 32/F | Vulvar condyloma | 80/80 | 18 | 1123/80 | LGSIL through 161 wk/ LR and HR through 54 wk | Condyloma resolved 161 wk | Possible liver aGVHD; azathioprine through 39 wk | LGSIL persists Alive >3 yr |
| 24 | 32/F | Vulvar condyloma | Never/101 | 20 | Never/101 | 26 wk LGSIL/HR | Persistent dysplasia 104 wk | Moderate cGVHD; systemic corticosteroids and sirolimus | Metastatic anorectal or cervical CA, 131 wk Died 2.75 yr |
| 26 | 36/F | VIN II/III, CIN I, VaIN II/III condyloma | 787/368 | 16 | Never/ 787 | Resolved 123 wk/ negative 123 wk | Negative 123 wk Normal exam 123 wk | None | Resolved 123 wk Alive >3 yr |
| 27 | 28/F | AIN III | 28/60 | 14 | 739/60 | Resolved 95 wk | None | Normal exam 107 wk Alive >3 yr | |
| 28 | 34/F | Condyloma pubis to anus | Never/59 | 17 | 724/ 185 | ASCUS through 104 wk | Resolved 104 wk | None | Only small nonhealing ulcer 104 wk, VIN II 136 wk Alive >2 yr |
| 29 | 32/F | Warts | 731/361 | 16 | 731/never | 78 wk ASCUS/36 wk LR and HR | 2 year neg | aGVHD grade II skin; systemic corticosteroids | 1.75 yr negative exam and pathology Alive >2.5 yr |
| 30 | 34/F | LGSIL HR | 122/never | 44 | never/never low | 52 wk verruca vulgaris or condyloma acuminatum with cytopathic effect | Anorectal condyloma HPV+ through 61 wk | aGHVD grade I; cGVHD moderate oral, vulvovaginal; BOS; tacrolimus through 76 wk | Persistent anal condyloma through 71 wk Alive >1.5 yr |
| 31 | 24/F | LGSIL, LR | 63/63 | 30 | Never/364 | LGSIL 83 wk/ HR 83 wk | Vulvar and anal condyloma 83 wk | Intermittent corticosteroids without diagnosis through 52 wk | Persistent disease 83 wk Alive >1.5 yr |
| 32 | 52/M | Penile wart | 61/103 | 20 | Never/61 | 104 wk genital condyloma persists Alive >2.5 yr | |||
| 33 | 23/F | Condyloma | Never/62 | 17 | 370/62 | LGSIL 26 wk/ LR and HR 26 wk | None | 52 wk single condyloma Alive >1.3 yr | |
| 34 | 29/F | CIN I | 60/60 | 15 | 368/ never low | Negative 51 wk/ LR 104 wk | None | 78 wk normal exam Alive >3 yr | |
| 35 | 38 F | Vulvar and anal warts, CC, LGSIL, CIN, HPV+ | 362/60 | 15 | 362/362 | ASCUS 87 wk/105 wk HR | 52 wk CIN I | None | 105 wk resolved vulvar warts Alive >2.5 yr |
VaIN indicates vaginal intraepithelial neoplasia; LR, low-risk HPV type; HR, high-risk HPV type; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; VV, vulvovaginal; CC, condylomatous cervicitis; ASCUS, atypical squamous cells of undetermined significance; BOS, bronchiolitis obliterans syndrome
NK, B, and T cells expressed per μL; monocytes expressed as k/μL.
Patients 7, 20, 24, 30, and 31 had persistent disease at a median of 131 weeks (IQR, 81.5; 75 to 156.5 weeks) with either prolonged systemic or topical (genital) corticosteroid use (group 1; n = 5). Six patients (21, 28, 32, 33, 34, and 35) had prolonged evidence of HPVD even after the cessation of immunosuppression at a median of 120 weeks (IQR, 114.5; 42.75 to 157.25 weeks) (group 2; n = 6). Thirteen patients had resolution of HPV-related disease. Patients 12 (with resolution before cessation of immunosuppression), 18, 22, 26, and 27 stopped protocol immunosuppression by 26 weeks and had HPVD resolution at a median of 123 weeks (IQR, 101.5; 60.5 to 162 weeks), with no disease detected at the last follow-up (group 3; n = 5). Patients 5, 6, 10, 11, 13, 17, 23, and 29 had resolution of disease at median of 217.5 weeks (IQR, 131; 139.5 to 270.5 weeks) in the presence of PI immunosuppression beyond 26 weeks (group 4; n = 8).
Immune Reconstitution
The days to achievement of normal cell counts in the patients with HPV anogenital disease are shown in Table 3. Of the 24 patients of interest (HPVD present at transplantation, >1 year of follow-up), 3 had not recovered NK cells to normal values at 3, 24, and 24 months (including 1 patient with secondary graft failure and no post-transplantation immunophenotype). The median time to reconstitution for those evaluable was 181 days (IQR, 447.5; 30 to 477.5 days). B cell recovery in 22 recipients occurred at a median of 197.5 days (IQR, 178.5; 181.75 to 360.25 days). Monocyte recovery occurred in all these recipients at median of 16 days (IQR, 4; 14 to 18 days). CD3+CD4+ cells (n = 19) and CD3+CD8+ cells (n = 18; 5 without ever experiencing a level below normal in the CD3+CD8+ compartment) recovered at median of 367 days (IQR, 367; 354 to 721 days) and 207.5 days (IQR, 210.75; 153.5 to 364.25 days), respectively.
HPV and Immune Reconstitution
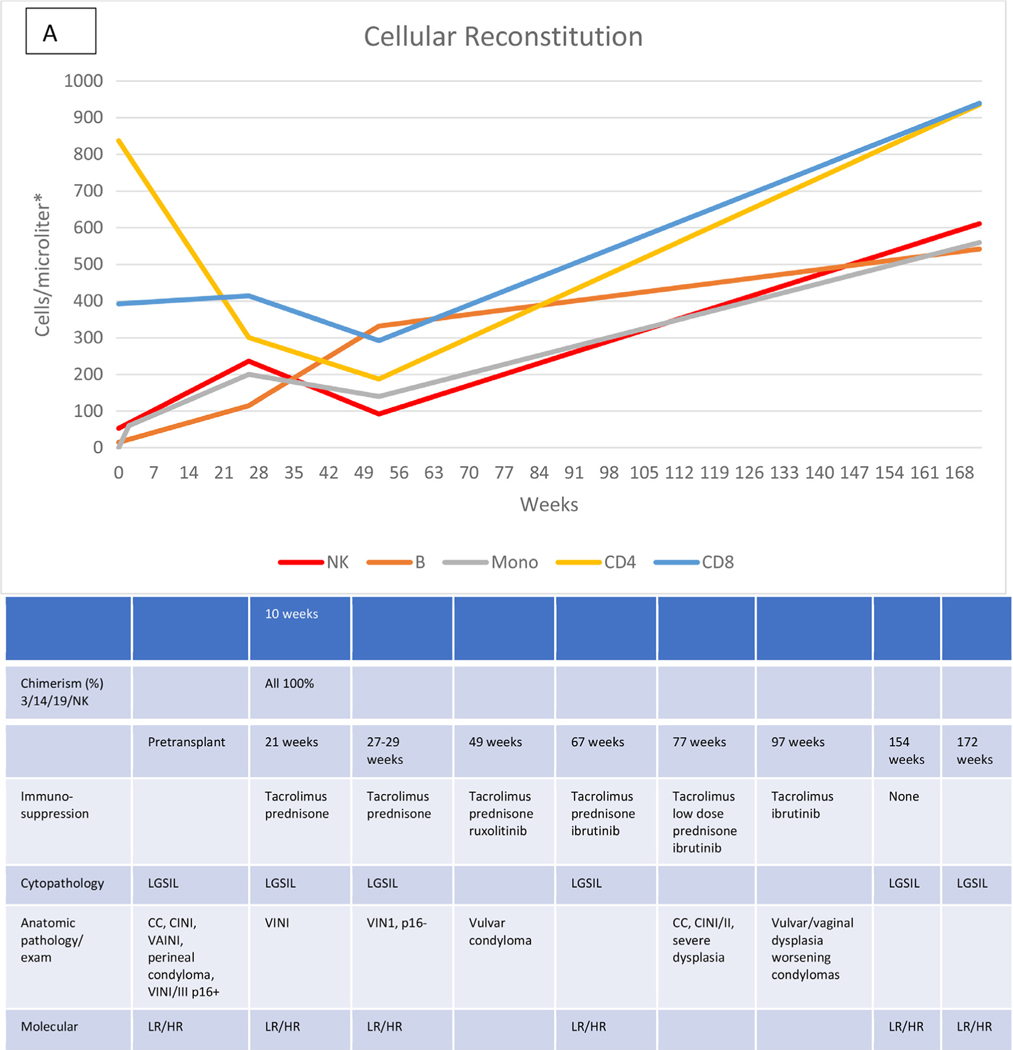
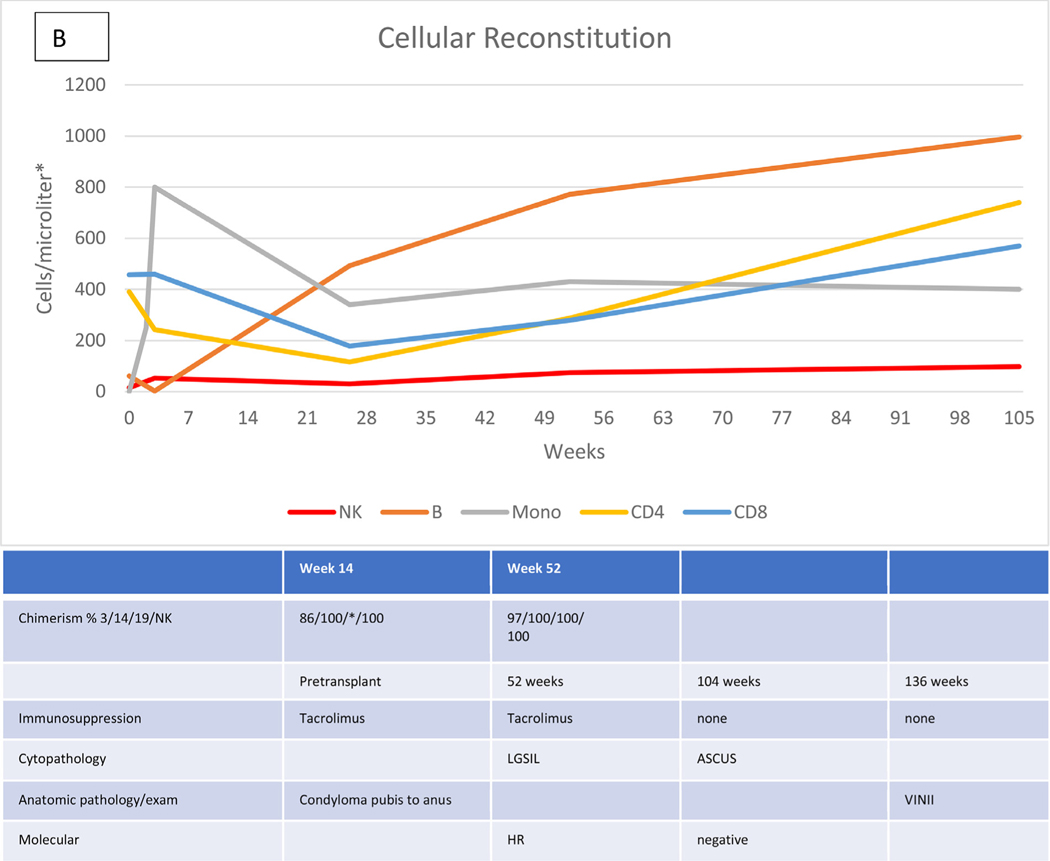
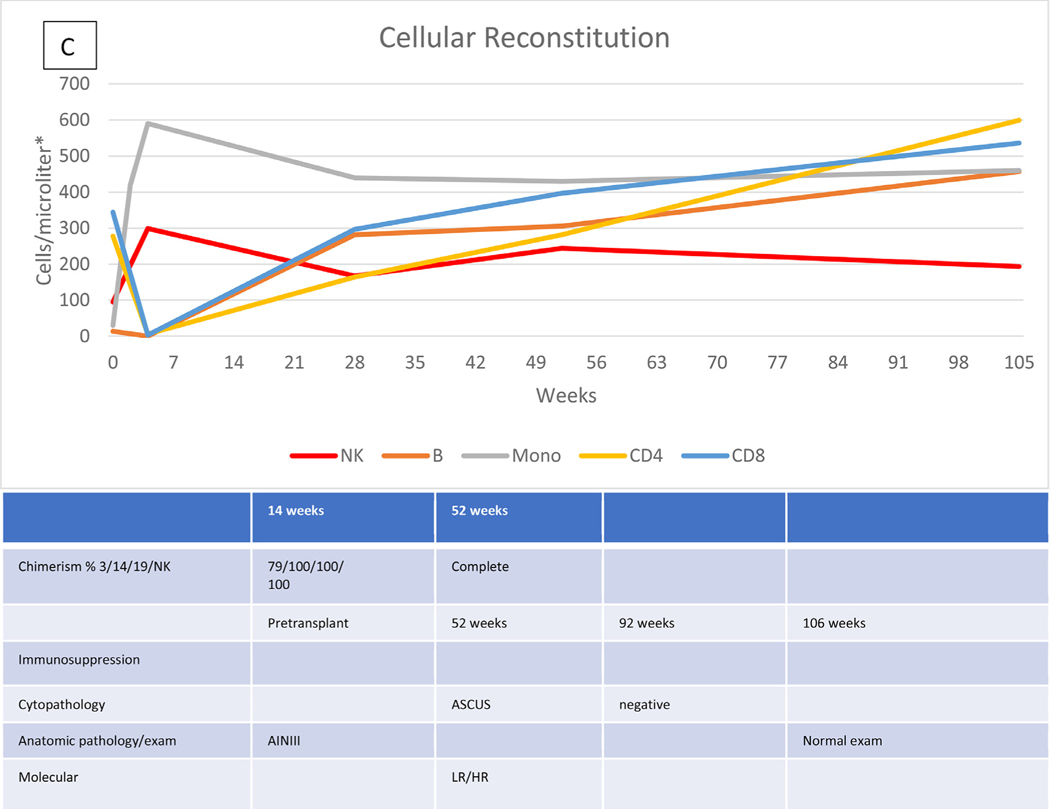
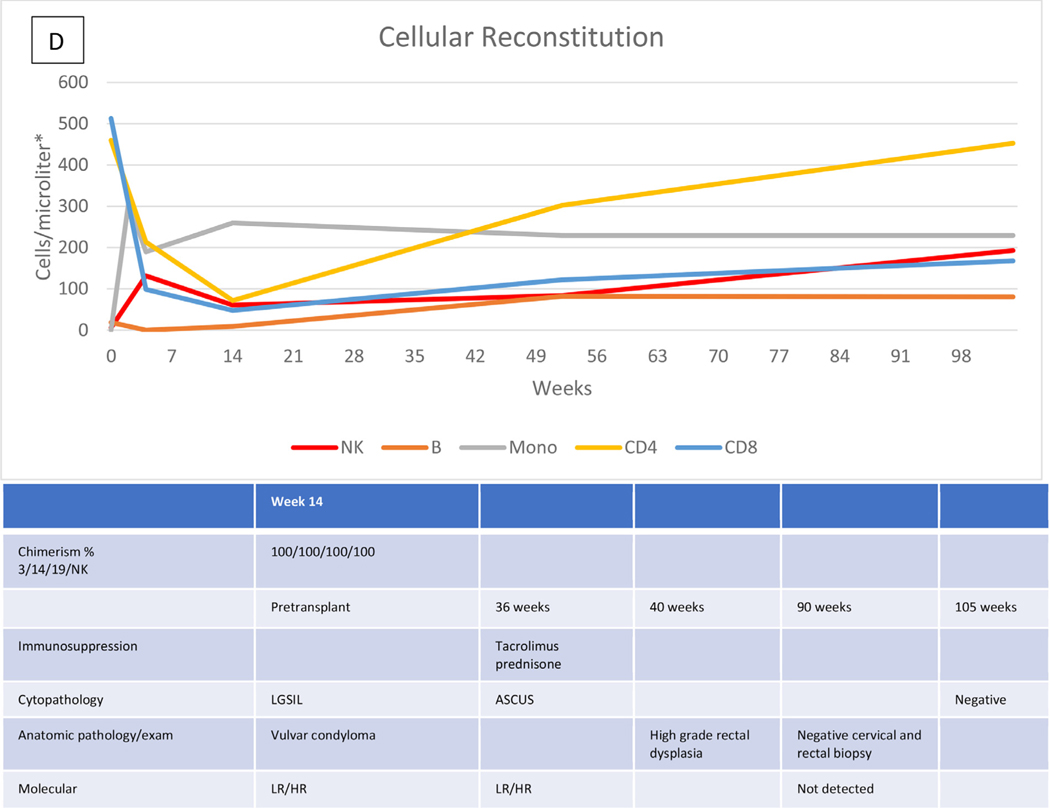
Characteristic data describing the immunologic recovery (absolute cell counts and chimerism) in the context of HPVD for 4 HCT recipients are shown in Figure 1A to D. Figure 1A shows the course of patient 20 with evolving disease after the cessation of prolonged immunosuppression for both acute and chronic GVHD, but represented at week 172 by only a small vulvar condyloma after vulvar, vaginal, and anal disease pretransplantation. Figure 1B shows the course of patient 28 with persistent disease in the absence of GVHD or prolonged immunosuppression. Pretransplantation disease was extensive, with condylomas from the mons pubis to the anus, which resolved except for a single small ulcer that persisted through follow-up to week 136, at which time a biopsy revealed vulvar intraepithelial neoplasia (VIN) II, necessitating partial vulvectomy. Figure 1C shows the course of patient 27, who had previous vulvar cancer and underwent at least 6 surgical procedures for refractory/recurrent disease and anal intraepithelial neoplasia III at the time of transplantation. Cytopathologic examination at week 95 showed no abnormalities. There was no GVHD, and immunosuppression was stopped at week 26. Her examination remained normal at last follow-up (106 weeks). The course of patient 29, whose history included 2 vulvectomies and vulvar and perianal ablation for high-grade intraepithelial neoplasia, with warts present at transplantation, is shown in Figure 1D. Topical and systemic therapy for cutaneous GVHD (topical corticosteroids, prednisone, and tacrolimus) were continued through 10 months post-transplantation. Examination and pathology studies were negative for persistent disease at 21 months post-transplantation.
Figure 1.
Cellular reconstitution, immunosuppression, and HPV evaluations post-transplantation. (A) Recipient with mild persistent HPV disease after prolonged immunosuppression. (B) Recipient with persistent disease in the absence of continued immunosuppression. (C) Recipient with resolution of HPV disease without prolonged immunosuppression. (D) Recipient with resolution of HPV disease with prolonged immunosuppression. *Lymphocytes expressed as cells per microliter; monocytes, as thousands per microliter. 3/14/19/NK, CD3+/CD14+/CD19+/NK cells; LGSIL, low-grade squamous intraepithelial lesion; LR/HR, low-risk/high-risk HPV types; ASCUS, atypical squamous cells of undetermined significance; VIN, vulvar intraepithelial neoplasia; AIN, anal intraepithelial neoplasia.
DISCUSSION
Warts, a manifestation of HPV infection, have appeared in many accounts of the phenotype of GATA2 deficiency [15]. However, HPVD spans a continuum from common warts (usually involving the hands and feet) through infection with oncogenic HPV types that cause anogenital and other epithelial malignancies.
HCT has proven curative in GATA2 deficiency, as described previously [8,9]. HCT for GATA2 deficiency is modeled on the treatment of hematologic malignancies, because bone marrow failure is a major component of the condition. However, the need to restore normal immunity also plays an important role in the decision to progress to transplantation. Mycobacterial infection contributed to this decision in 27 of 75 patients.
Several factors necessitate the reanalysis of GATA2 deficiency through the lens of HPV-related disease. Only 16 of 75 patients were free of this manifestation. The indication for HCT in several cases was refractory anogenital HPVD. Almost one-third of the women in our second series had undergone vulvectomy, sometimes multiple times, a procedure that can have devastating consequences for sexual function and mental health [16]. Two older patients had both undergone complex procedures to treat HPV-related malignancies: cervical cancer in a 46-year-old woman and anal cancer in a 45-year-old man. Both were cancer-free at the time of HCT, but both had extensive complications (surgical diversions) related to their cancers. Thus, relapsed/refractory precancerous or malignant disease due to HPV should be an indication for HCT to prevent these events [17].
Our results in this series provide reason for hope and for caution. Analysis of this group preselected on the basis of recognition of GATA2 deficiency, referral to our center, and their indications for HCT supports older age and CD8+ T cell deficiency as contributing to the occurrence of HPVD in GATA2 deficiency, which may reflect the sexual acquisition of certain HPV types (although more than 1 patient in our cohort reported the onset of anogenital disease before sexual activity) and progressive immunodeficiency in a cell type responsible for the control of chronic viral infections [18].
We have not found a linear correlation with resolution of HPVD and immunologic recovery, perhaps related to the unusual and combined cellular deficiency. In fact, post-transplantation immunosufficiency, as measured by the available but flawed index of recovery to normal cell counts, finds significance only in 2 comparisons for monocytes and 1 comparison for B cells, neither of which we would associate with the physiology likely to contribute to the control of HPVD. Both humoral immunity and CD3+ T cells are implicated in the protective responses generated naturally and by HPV vaccines [19]. However, vaccines and vaccine-related immunity are not relevant to the results presented here, because these data address prevalent disease and its response to transplantation, and there is no current consensus that antibody responses or vaccination have a therapeutic role in this area [20]. Our data also are insufficient to address the complexity of stromal and immune cells in HPV-related malignancy, although they do provide some suggestive characteristics of the disease, as demonstrated in the single patient reports provided here [21]. NK cells are singled out as a major contributor in the control of viral disease when describing GATA2 patients as NK-deficient, but low NK cell count in GATA2 deficiency is always associated with decreases in other cell lines, such as monocytes and B cells [22,23].
PTCy GVHD prophylaxis is associated with an increased risk of post-transplantation viral complications, perhaps related to some degree to its elimination of NK cells [24]. Intriguingly, an NK cell “add-back” (ie, donor expanded NK cells before and after haploidentical transplantation for high-risk myeloid malignancies) has been reported [25]. It is unclear how to balance these effects of PTCy with the competing virtue of decreasing GVHD, the treatment of which impedes long-term viral immunity.
Perhaps for the Perhaps for the same reasons that we cannot identify a cellular deficiency that leads to HPVD, an analysis of cellular recovery fails to distinguish the elements that lead to resolution post-transplantation. Our MAC protocol has a fairly uniform and characteristic immunologic recovery. Monocytes recover first (monitored daily). The immunophenotype at 2 to 4 weeks demonstrates NK recovery, and CD3+ and B cells lag. In 3 HCT recipients (patients 11, 15, and 37), large genital warts (VIN III in 1 case) actually just fell off before a 1-month evaluation. Conversely, 2 of 3 recipients (patients 24 and 28) without NK recovery at the last analysis had continued or progressive HPV-related abnormalities, 1 with GVHD and 1 without GVHD.
Numerous factors may contribute to the lack of sensitivity of cell counts, and post-transplantation immunosuppression is a major confounder. The recovery of cell numbers might not be linearly correlated with a return in function. Chronic immune dysregulation has been identified as a contributor to secondary genital neoplasms peri-transplantation and post-transplantation, and continued monitoring is recommended [26]. Gynecologic manifestations of GVHD and their treatment require attention and caution in the application of topical immunosuppressants in the presence of HPVD [27,28].
Our indications of resolution may be insensitive or nonspecific. Multiple gynecologic/dermatologic measures can be taken into consideration (eg, visible abnormalities, histopathology, molecular detection of oncogenic HPV), and they might not all have equal weight in determining the propensity to HPV-related premalignant and malignant complications [29,30].
Although our small patient number limits our statistical analysis, individual cases retain the power to inform this work. Patient 24, a 32-year-old woman, underwent transplantation for MDS and had a history of genital HPV-related malignancy. Although HCT was successful, she died just short of 3 years post-transplantation from metastatic squamous cell carcinoma, presumably of anogenital origin. She had developed chronic GVHD and had been successfully treated through 2 years post-transplantation with corticosteroids and sirolimus. Examination during her GVHD treatment noted persistence of HPV-related disease until symptoms led to the diagnosis of metastatic cancer at 2.5 years post-transplantation and her death shortly thereafter. This case further illustrates the need for comprehensive examination of the genital and anal sites for involvement and treatment of disease when found, since anal involvement is common and contributed to this death and extensive disease in other patients in this series (patients 5, 11, 20, 27, 28, 29, 30, and 35) and as reported previously [31,32].
Fortunately, many HPV-affected recipients cleared their disease, some in the presence of continued immunosuppression. Some cleared their disease after undergoing surgical treatment post-transplantation. Conversely, a small number continue to be monitored for manifestations of HPVD without GVHD or immunosuppression beyond that mandated by protocol. Disease related to HPV infection is common in GATA2 deficiency. HCT may reverse the immunologic defect that pre-disposes patients to refractory and recurrent disease and its risk of malignancy; however, incomplete immune reconstitution and persistent iatrogenic immunosuppression after HCT may attenuate the effects of successful HCT. This result may mirror that obtained in HPV vaccination post-HCT, in which iatrogenic immunosuppression leads to a decreased proportion of recipients developing antibody responses [33]. Although no data exist to support the practice, we support vaccination for HPV in donors when medically appropriate in the hope that the transplanted immune response might mirror that related to donor Epstein-Barr virus and cytomegalovirus status. Vigorous evaluation for HPV-related disease must occur before transplantation and should continue post-transplantation so that surgical and other therapeutic measures can be undertaken in cases with new or persistent disease. HPVD, refractory and recurrent, should not exclude HCT and, consistent with results here, should be an indication for transplantation.
Supplementary Material
ACKNOWLEDGMENTS
Financial disclosure:
This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and [in part] by the National Institute of Allergy and Infectious Diseases. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E and Contract No. 75N910D00024, Task Order No. 75N91019F00131. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Financial disclosure: See Acknowledgments on page 435.e11.
Conflict of interest statement: There are no conflicts of interest to report.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2020.12.028.
REFERENCES
- 1.Vinh DC, Patel SY, Uzel G, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickinson RE, Griffin H, Bigley V, et al. Exome sequencing identifies GATA2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43:929–931. [DOI] [PubMed] [Google Scholar]
- 5.Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Álvarez-Chinchilla P, Poveda I, Marco FM, et al. Vulvar lymphedema and refractory VIN-III heralding GATA2 deficiency syndrome. Eur J Obstet Gynecol Reprod Biol. 2017;218:138–140. [DOI] [PubMed] [Google Scholar]
- 7.de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. [DOI] [PubMed] [Google Scholar]
- 8.Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biol Blood Marrow Transplant. 2014;20:1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parta M, Shah NN, Baird K, et al. Allogeneic hematopoietic stem cell transplantation for GATA2 deficiency using a busulfan-based regimen. Biol Blood Marrow Transplant. 2018;24:1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674–686. [DOI] [PubMed] [Google Scholar]
- 11.Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Genit Tract Dis. 2019;23:87–101. [DOI] [PubMed] [Google Scholar]
- 12.Polyatskin IL, Artemyeva AS, Krivolapov YA. [Revised WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 2017 (4th edition): lymphoid tumors]. Arkh Patol. 2019;81:59–65. [in Russian]. [DOI] [PubMed] [Google Scholar]
- 13.Ganapathi KA, Townsley DM, Hsu AP, et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015;125:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West ES, Kingsbery MY, Mintz EM, et al. Generalized verrucosis in a patient with GATA2 deficiency. Br J Dermatol. 2014;170:1182–1186. [DOI] [PubMed] [Google Scholar]
- 16.Shah SH, Parameswaran S, Hickey N, Zetler S, Nathan M. Multifocal intraepithelial neoplasia and the psychological consequence of vulvectomy. BMJ Case Rep. 2011:2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donadieu J, Lamant M, Fieschi C, et al. Natural history of GATA2 deficiency in a survey of 79 French and Belgian patients. Haematologica. 2018;103:1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacopoulou F, Karakitsos P, Kottaridi C, et al. Genital HPV in children and adolescents: does sexual activity make a difference? J Pediatr Adolesc Gynecol. 2016;29:228–233. [DOI] [PubMed] [Google Scholar]
- 19.Mboumba Bouassa RS, Pere H, Jenabian MA, et al. Natural and vaccine-induced B cell-derived systemic and mucosal humoral immunity to human papillomavirus. Expert Rev Anti Infect Ther. 2020;18:579–607. [DOI] [PubMed] [Google Scholar]
- 20.Choi CH, Choi HJ, Lee JW, et al. Phase I study of a B cell-based and monocyte-based immunotherapeutic vaccine, BVAC-C, in human papillomavirus type 16- or 18-positive recurrent cervical cancer. J Clin Med. 2020;9:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rego Barros M Jr, Lagos de Melo CML, Rego Barros MLC, Pereira de Lima RDC, de Freitas AC, Venuti A. Activities of stromal and immune cells in HPV-related cancers. J Exp Clin Cancer Res. 2018;37:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon WY, Powis SJ. Does natural killer cell deficiency (NKD) increase the risk of cancer? NKD may increase the risk of some virus-induced cancer. Front Immunol. 2019;10:1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson RE, Milne P, Jardine L, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Elssen CHM, Ciurea SO. NK cell alloreactivity in acute myeloid leukemia in the post-transplant cyclophosphamide era. Am J Hematol. 2020;95:1590–1598. [DOI] [PubMed] [Google Scholar]
- 25.Ciurea SO, Schafer JR, Bassett R, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130:1857–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang HA, Armenian SH, Dellinger TH. Secondary neoplasms of the female lower genital tract after hematopoietic cell transplantation. J Natl Compr Canc Netw. 2018;16:211–218. [DOI] [PubMed] [Google Scholar]
- 27.Shanis D, Anandi P, Grant C, et al. Risks factors and timing of genital human papillomavirus (HPV) infection in female stem cell transplant survivors: a longitudinal study. Bone Marrow Transplant. 2018;53:78–83. [DOI] [PubMed] [Google Scholar]
- 28.Sri T, Merideth MA, Klepac Pulanic T, Childs R, Stratton P. Human papillomavirus reactivation following treatment of genital graft-versus-host disease. Transpl Infect Dis. 2013;15:E148–E151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiffman M, Kinney WK, Cheung LC, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Natl Cancer Inst. 2018;110:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuzick J, Adcock R, Carozzi F, et al. Combined use of cytology, p16 immunostaining, and genotyping for triage of women positive for high-risk human papillomavirus at primary screening. Int J Cancer. 2020;147:1864–1873. [DOI] [PubMed] [Google Scholar]
- 31.Stier EA, Sebring MC, Mendez AE, Ba FS, Trimble DD, Chiao EY . Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: a systematic review. Am J Obstet Gynecol. 2015;213:278–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toboni MD, Bevis KS. Vulvar cancer as a result of GATA2 deficiency, a rare genetic immunodeficiency syndrome. Obstet Gynecol. 2018;132: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 33.Stratton P, Battiwalla M, Tian X, et al. Immune response following quadrivalent human papillomavirus vaccination in women after hematopoietic allogeneic stem cell transplant: a nonrandomized clinical trial. JAMA Oncol. 2020;6:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.