Abstract
Objectives
The COVID-19 pandemic had a disruptive impact on tuberculosis (TB) and HIV services. We assessed the in-hospital TB diagnostic care among people with HIV (PWH) overall and before and during the pandemic.
Methods
In this prospective study, adult PWH admitted at three hospitals in Ghana were recruited if they had a positive World Health Organization four-symptom screen or one or more World Health Organization danger signs or advanced HIV. We collected data on patient characteristics, TB assessment, and clinical outcomes after 8 weeks and used descriptive statistics and survival analysis.
Results
We enrolled 248 PWH with a median clusters of differentiation 4 count of 80.5 cells/mm3 (interquartile range 24-193). Of those, 246 (99.2%) patients had a positive World Health Organization four-symptom screen. Overall, 112 (45.2%) patients obtained a sputum Xpert result, 66 (46.5%) in the prepandemic and 46 (43.4%) in the pandemic period; P-value = 0.629. The TB prevalence of 46/246 (18.7%) was similar in the prepandemic 28/140 (20.0%) and pandemic 18/106 (17.0%) population; P-value = 0.548. The 8-week all-cause mortality was 62/246 (25.2%), with no difference in cumulative survival when stratifying for the pandemic period; log-rank P-value = 0.412.
Conclusion
The study highlighted a large gap in the access to TB investigation and high early mortality among hospitalized PWH, irrespective of the COVID-19 pandemic.
Keywords: HIV, Tuberculosis, COVID-19, Care cascade, Diagnosis, Xpert MTB/RIF
Introduction
Decades of worldwide scale-up of antiretroviral therapy (ART) and tuberculosis (TB) control efforts have contributed to a significant reduction in TB cases and TB-associated deaths among people with HIV (PWH). However, the progress toward controlling the dual epidemic is slower than envisioned, and the COVID-19 pandemic has had a disproportional negative impact on worldwide TB control [1,2]. Disruptions in health care services like interrupted ART and delayed TB diagnosis and treatment in countries highly burdened by HIV and TB may lead to increasing HIV and TB deaths over the next 5 years compared with if the COVID-19 pandemic never occurred [3].
Similar to the HIV continuum of care, a TB care cascade was constructed to evaluate the patient flow from symptom onset to the end of a successful TB treatment [4]. Population-based evaluations of the cascade in TB endemic areas have shown that patients co-infected with HIV/TB were less likely to complete the TB care cascade than people without HIV [5,6] and that the largest loss of patients from the cascade was due to patients not accessing TB testing [5,7].
Ghana has an estimated adult HIV prevalence of 1.7% [8], an estimated TB incidence rate of 143 per 100,000 population [9], and a TB prevalence of 356 per 100,000 population [10]. Notably, in 2020, only 26% of the estimated 8.100 HIV-associated TB cases were notified in Ghana, implying a TB diagnostic gap in this population [8].
COVID-19 first appeared in Ghana on March 12, 2020 and prompted the banning of public gatherings; school closures; and a 3-week partial lockdown of Accra, Kasoa, and Kumasi [11]. The country's comprehensive COVID-19 test and contact tracing strategy soon expanded to be performed in decentralized public and private laboratories [12], including public chest laboratories, where the existing Cepheid Xpert MTB/RIF® assay (Xpert; Sunnyvale, CA, USA) machines dedicated for TB testing, were used to diagnose COVID-19 [13]. The mild or asymptomatic course of COVID-19 among 80-90% of persons infected and the high rate of self-treatment [12] in Ghana should be taken into consideration when interpreting the national cumulative COVID-19 case rate that reached 118,000, including 1020 COVID-19 deaths, at the end of August 2021 [14]. Up to then, there had been three distinct COVID-19 waves, with peak weekly incidence rates between 15.6 and 18.6 per 100,000 population, including the B.1.1 variant on July 29, 2020, the B.1.1.7 variant (Alpha) on January 31, 2021, and the B.1.617.2 variant (Delta) on August 8, 2021 [14], [15], [16]. Seroprevalence studies suggest high COVID-19 exposure in African communities, including Ghana, with the COVID-19 seroprevalence of 3.0% in April-June 2020 increasing to 65% in July-September 2021 [17]. Disruptions in both HIV and TB service delivery have been reported from Ghana during the pandemic, with decreasing TB notifications [18] and health workers confirming shortages of HIV tests and treatment and missed ART refill appointments [19].
Previous studies from Ghana have described a high TB prevalence and a poor prognosis among hospitalized PWH [20,21] but none in the context of the COVID-19 pandemic. With this prospective study, we aimed to describe the routine in-hospital TB diagnostic care cascade among PWH in Ghana and assess the impact of the COVID-19 pandemic on patient characteristics and early outcomes for TB care. The evaluation of routine in-hospital TB care among PWH may identify gaps in the TB cascade and assist quality improvements of care.
Methods
Study design
We conducted a multisite, prospective cohort study of the in-hospital routine TB care cascade among PWH admitted to the medical wards at three governmental hospitals in the Greater Accra Region of Ghana from October 14, 2019 to July 05, 2021. This study represents a substudy to the Point-of-care strategies to improve tuberculosis care among severely immunosuppressed HIV infected patients (TBPOC) study, an open-label multicenter, stepped wedge cluster randomized controlled study, with implementation of the urine lateral flow lipoarabinomannan (LAM) TB diagnostic test (ClinicalTrials.gov Identifier: NCT04122404). Due to a likely impact of the LAM intervention on the TB diagnostic cascade, this current study is based on data collected before the LAM interventional phase (Figure S1). As a result of the national COVID-19 measures and restrictions in conducting clinical research at the study sites, we did not enroll patients in the study between March and October 2020, representing the period of the first COVID-19 wave in Ghana and the division between the prepandemic and the pandemic study period.
Study population
We recruited PWH consecutively when admitted to the medical wards at Tema General Hospital, Lekma Hospital, and Korle-Bu Teaching Hospital and followed up participants for 8 weeks. The eligibility criteria included: age 18 years or older; able to give written informed consent; and having either a positive World Health Organization (WHO) four-symptom screen (W4SS; at least one of the following: cough, weight loss, fever, or night sweats), or advanced HIV disease (defined as clusters of differentiation 4+ T-lymphocyte count [CD4 count] of less than 200 cells/mm3 or a WHO clinical stage 3 or 4 event at presentation for care), or a WHO danger sign (respiratory rate >30 breaths per minute, temperature >39°C, heart rate >120 beats per minute, or being unable to walk unaided) [22]. Patients were excluded from participation if they had received TB treatment or preventive treatment in the preceding 60 days or had participated in the study before.
Study sites
Patients were enrolled from the medical wards at the following sites: Tema General Hospital, a large-sized hospital providing care for 3000 PWH; Lekma Hospital, a medium-sized district hospital providing care for 1100 PWH; and Korle-Bu Teaching Hospital, a large referral hospital providing care for 6700 PWH. The hospitals offer routine HIV and TB care for an urban population and have access to Xpert for TB diagnosis at on-site laboratories. TB treatment is offered from on-site chest clinics or administrated on the wards by public health nurses. The national TB guidelines recommends screening of PWH with the W4SS at all health facility visits and with chest X-ray twice [23]. Sputum Xpert is the first-line TB diagnostic test in Ghana since 2017 [23] and the next generation Xpert MTB/RIF Ultra has gradually replaced the original cartridge since 2019 [24]. The national policy is to provide all TB and HIV tests and treatments free of charge for patients [23].
The three study sites were all located near several important COVID-19 hotspots in Greater Accra region [11], and during the pandemic period, the medical wards were restructured to accommodate patients with confirmed or suspected COVID-19. A COVID-19 signs and symptoms screening tool was used at the hospital points of entry to decide whether COVID-19 testing and isolation should be performed.
Data collection, study variables, and TB care outcomes
Trained research assistants screened the wards daily for eligible patients and obtained written informed consent. For patients consenting, we used a pretested paper data collection tool to collect baseline information on patient demographics, medical history, initial medical evaluation, and basic blood test results and during admission, information on the acquisition and timing of routine TB investigations, results from other microbiology testing, and outcomes of admission. In addition to the current routine care, we obtained baseline CD4 counts and for patients with sputum availability, one sputum sample was collected for storage and evaluation later in batches, including smear microscopy for acid-fast bacilli and TB liquid culture performed at chest clinic laboratory at Korle-Bu Teaching Hospital. The patients’ results were communicated to the attending physician or provided in the patient's medical folders as soon as the results were known to the study team. Patients underwent an 8-week follow-up assessment, including a basic physical evaluation; record review, and interview for information on HIV; and TB clinical and treatment status. In instances where it was not possible to assess the patient physically at follow-up, we conducted a phone interview with the patient or listed contact person and reviewed the medical records including hospital death records to obtain date and cause of death for deceased patients.
Our primary outcome measure for TB care was the proportion of patients with available sputum Xpert results. The secondary outcomes were the proportion of patients with available chest X-ray, TB prevalence rate, proportion of patients initiating TB treatment, and 8-week all-cause mortality. We recorded the time in days from admission to the outcomes of interest or the end of follow-up. For the Xpert and chest X-ray, we included results obtained from 14 days before admission to the end of follow-up.
The outcomes selected were considered clinically important and key steps in the in-hospital TB care cascade, as described in previous literature and WHO recommendations [25], [26], [27], [28].
For data management, we used REDCap (Research electronic Data Capture) [29].
Definitions and statistical analysis
We used the following TB case definition within follow-up: “confirmed TB” if Mycobacterium tuberculosis was detected in respiratory or extrapulmonary samples by Xpert or liquid culture; “probable TB” if not “confirmed TB” but one of the following: sputum smear microscopy positive, a high clinical suspicion of TB and referred for TB treatment initiation, or recorded dead with or from TB per medical records; and “unlikely TB” if not meeting the criteria for “confirmed” or “probable” TB.
Descriptive analysis was used to characterize the study population. We compared clinical characteristics and study outcomes for the prepandemic and the COVID-19 pandemic population using chi-square or Fisher's exact test for categorical data and unpaired two-sample t-test or Mann-Whitney U test for continuous data.
An adjusted log-binominal generalized linear model was fitted to identify co-variates associated with having an Xpert test performed. We treated age >40 years, male sex, CD4 count ≤100 cells/mm3, self-reported cough, being unable to walk unaided, and admission during the COVID-19 pandemic as clinically relevant co-variates of having an Xpert performed. A cluster variance estimator was used to adjust the model standard error for the hospital.
The cumulative probability of 8-week survival was estimated by means of the Kaplan-Meier method stratified by pandemic period and compared with the log-rank test. To assess the impact of admission during the COVID-19 pandemic on all-cause 8-week mortality, we used Cox proportional hazard regression. We treated age >40 years, male sex, and CD4 count ≤100 cells/mm3 as co-variates of early mortality and included them in the multivariate model. A cluster variance estimator was used to adjust the model standard error for the hospital. Using a global test value based on Schoenfeld residuals, >0.05, we considered the model proportional hazard assumption as fulfilled. In the survival analysis, patients alive and followed up after day 56 were censored to follow-up on day 56. Statistical significance was defined as a two-sided P-value of <0.05. All analyses were performed using STATA/BE 17.0 software.
Results
Patient characteristics
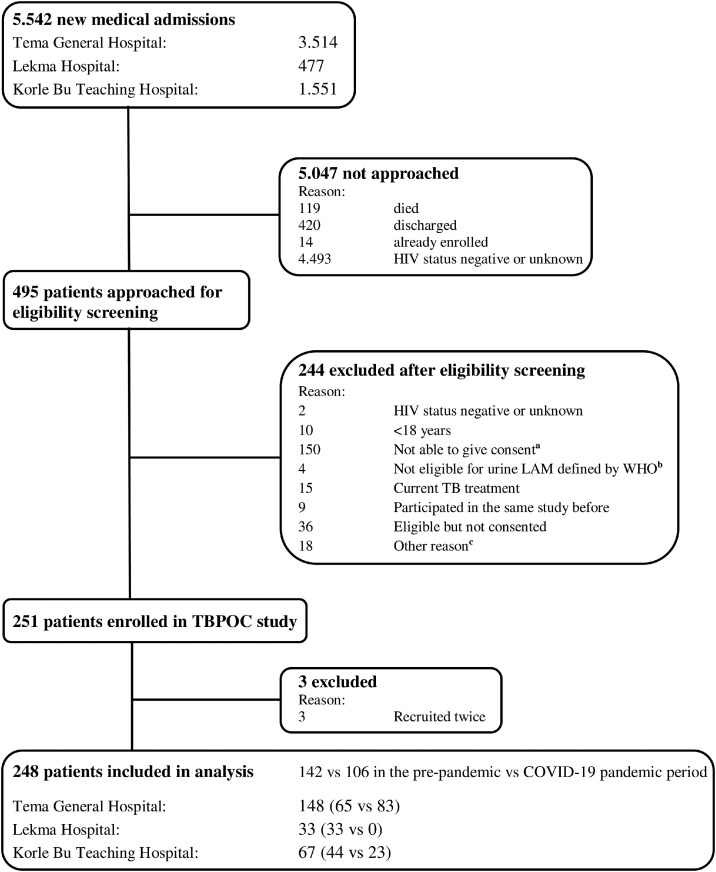
Between October 2019 and July 2021, 248 patients met the inclusion criteria, with 142 (57.3%) patients enrolled in the prepandemic period and 106 (42.7%) patients in the COVID-19 pandemic period (Figure 1 ). Patients were characterized by female sex, 178 (71.8%), a median age of 41.5 (interquartile range [IQR] 34-48) years and a median CD4 count of 80.5 (IQR 24-193) cells/mm3 (Table 1 ). Males had a lower median CD4 count of 52 (17-132) cells/mm3 than females with a median CD4 count of 92 (28-224) cells/mm3; P-value = 0.010. Upon enrollment, 122 (49.2%) patients were newly diagnosed with HIV. Of the 126 (50.8%) patients with known HIV, 79 (62.7%) were taking ART. Of the patients, 243 (98.0%) had a WHO HIV clinical stage III or IV event and 246 (99.2%) had a positive W4SS. Patients enrolled during the COVID-19 pandemic had higher median CD4 count (94.5 [IQR 31-235] vs 62.5 [IQR 22-173], P-value = 0.029), lower prevalence of self-reported cough (63/106 [59.4%] vs 115/142 [81.0%], P-value <0.001), and lower prevalence of abnormalities on chest X-ray (30/48 [62.5%] vs 48/58 [82.8%], P-value = 0.019) than the prepandemic population (Table 1). Among patients enrolled in the pandemic period, 13/106 (12.3%) obtained a SARS-COV-2 polymerase chain reaction test. Of those, one of 13 (7.7%) had confirmed COVID-19.
Figure 1.
Eligibility screening.
Abbreviations: LAM, lateral flow lipoarabinomannan; TB, tuberculosis; WHO, World Health Organization.
a. Not able to give informed consent due to critical illness including cerebral impairment.
b. Not having advanced HIV disease (WHO HIV stage III or IV or clusters of differentiation 4-cell count <200 cells/mm3) and not being seriously ill (defined by WHO danger signs) and not having a positive WHO TB symptom screening.
c. HIV status not disclosed to the patient and other reasons that hindered an informed consent.
Table 1.
Patient characteristics at enrollment stratified by period before and during the COVID-19 pandemic.
| Overall population | Prepandemic population | Pandemic population | P-valuea | ||
|---|---|---|---|---|---|
| (n = 248) | (n = 142) | (n = 106) | |||
| Age (years) | 41.5 (34-48) | 42 (34-48) | 40 (35-49) | 0.916 | |
| Female sex | 178 (71.8) | 100 (70.4) | 78 (73.6) | 0.584 | |
| Newly diagnosed with HIV (diagnosed <3 months prior current admission) | 122 (49.2) | 64 (45.1) | 58 (54.7) | 0.133 | |
| Antiretroviral therapy coverage among patients with known HIV (diagnosed with HIV ≥3 months prior current admission) | 79/126 (62.7) | 53/78 (68.0) | 26/48 (54.2) | 0.120 | |
| Co-trimoxazole prophylaxis coverage among patients with known HIV | 62/123 (50.4) | 36/75 (48.0) | 26/48 (54.2) | 0.505 | |
| Available HIV RNA result among patients with known HIV | 22/126 (17.5) | 17/78 (21.8) | 5/48 (10.4) | 0.147 | |
| CD4 count (cells/mm3) | 80.5 (24-193) | 62.5 (22-173) | 94.5 (31-235) | 0.029 | |
| WHO clinical stage III or IV HIV disease | 243 (98.0) | 139 (97.9) | 104 (98.1) | 1.000 | |
| Previously treated for TB | 19 (7.8) | 13 (9.4) | 6 (5.7) | 0.277 | |
| Any WHO TB symptom | 246 (99.2) | 142 (100.0) | 104 (98.1) | 0.182 | |
| WHO TB symptom distribution | Cough | 178 (71.8) | 115 (81.0) | 63 (59.4) | <0.001 |
| Fever | 160 (64.5) | 98 (69.0) | 62 (58.5) | 0.087 | |
| Night sweat | 117 (47.6) | 66 (47.1) | 51 (48.1) | 0.880 | |
| Weight loss | 228 (92.7) | 132 (94.3) | 96 (90.6) | 0.267 | |
| Any WHO danger signb | 137 (55.2) | 76 (53.5) | 61 (57.6) | 0.528 | |
| Oxygen saturation on pulse oximeter (%) | 97 (95-98) | 97 (94-98) | 97 (95-98) | 0.189 | |
| BMI (kg/m2) | 19.3 (16.4-22.2) | 19.5 (16.4-21.5) | 18.9 (16.6-23.3) | 0.754 | |
| Hemoglobin (g/dl) | 8.9 (2.9) | 8.8 (2.9) | 9.0 (3.1) | 0.4667 | |
| Any abnormality on chest X-ray | 78/106 (73.6) | 48/58 (82.8) | 30/48 (62.5) | 0.019 | |
Abbreviations: BMI, body mass index; CD, clusters of differentiation; TB, tuberculosis; WHO, World Health Organization.
Data are median (IQR), mean (SD) or n (%).
Two-sided P-value for comparison between participants enrolled before the COVID-19 pandemic and participants enrolled during the COVID-19 pandemic
WHO danger signs are one or more of the following: heart rate >120 beats per minute, respiratory rate >30 breaths per minute, temperature >39°C, or being unable to walk unaided.
Number of participants with missing values per variable excluded from analysis: On co-trimoxazole prophylaxis (3); CD4 count (2); Treated for TB before (4); Night sweat (2); Weight loss (2); BMI (26); Hemoglobin (14)
Routine TB care outcomes
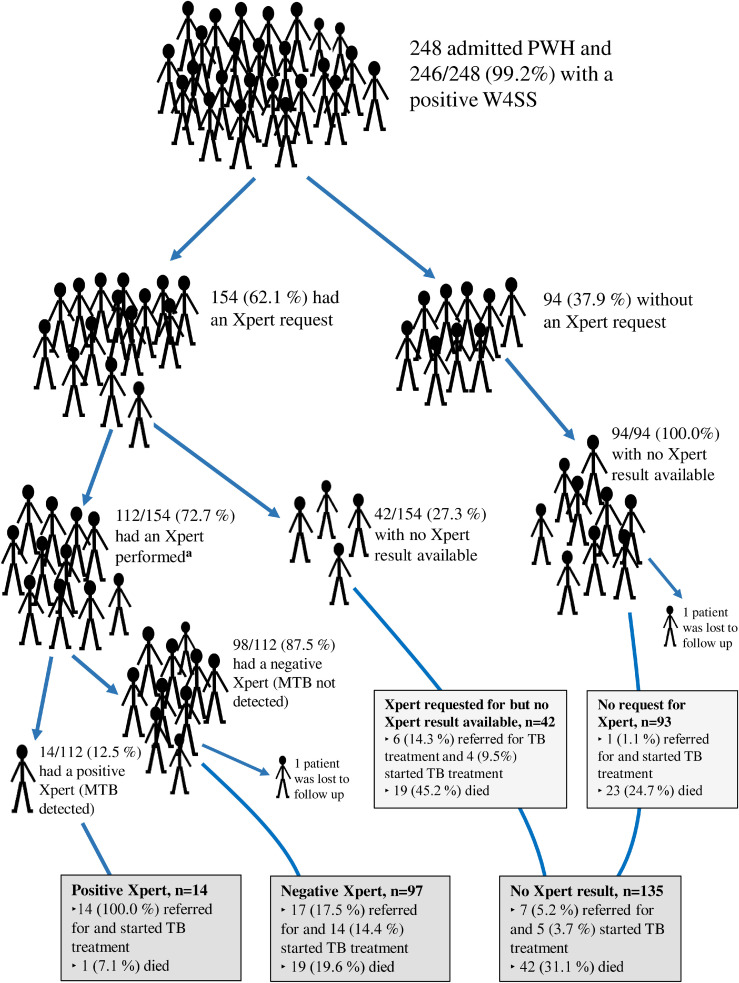
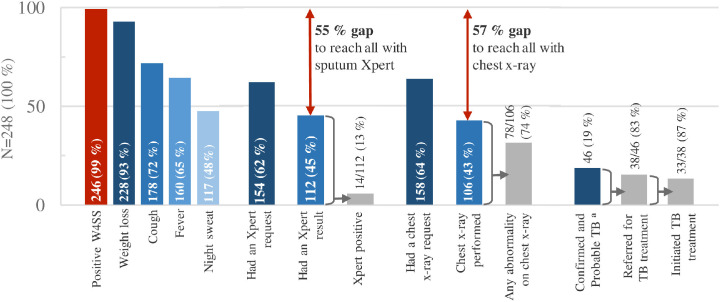
Among all PWH on admission, 154/248 (62.1%) patients had a sputum Xpert request. Of those, 112/154 (72.7%) had an Xpert performed (Figure 2 ) after a median of 4 (IQR 2-6.5) days upon admission (Table S1). The proportion having an Xpert (Table 2 ), the time to Xpert testing (Table S1), and the Xpert positivity (M. tuberculosis complex detected) rate (Table S2) were similar over the two periods. In a multivariate model, being admitted during the COVID-19 pandemic did not alter the chance of having an Xpert test performed (risk ratio [RR] 1.10, 95% confidence interval [CI] 0.96-1.24, P = 0.158), whereas patients were more likely to have Xpert performed if CD4 count ≤100 cells/mm3 (RR 1.31, 95% CI 1.22-1.41, P <0.001) or reporting cough (RR 2.37, 95% CI 1.80-3.13, P <0.001; Table 3 .). Overall, 99/248 (39.9%) patients did not obtain an Xpert result nor a chest X-ray within the follow-up.
Figure 2.
TB treatment initiation and 8 weeks all-cause mortality among admitted patients with HIV and high prevalence of WHO TB symptoms in Ghana, stratified by access to Xpert.
Abbreviations: MTB, Mycobacterium tuberculosis; PWH, people with HIV; TB, Tuberculosis; WHO, World Health Organization; W4SS, WHO four-symptom screening; Xpert, sputum Xpert MTB/RIF.
a. Xpert results from 14 days before the admission and until maximum 70 days post-enrollment were included in analysis.
Table 2.
TB care outcomes among people with HIV at 8 weeks follow-up stratified by period before and during the COVID-19 pandemic.
| Overall population (n = 246) | Prepandemic population (n = 140) | Pandemic population (n = 106) | P-valuea | |
|---|---|---|---|---|
| TB assessment | ||||
| Xpert performed, n = 248b | 112 (45.2) | 66 (46.5) | 46 (43.4) | 0.629 |
| Xpert performed among those with an Xpert request, n = 154b | 112/154 (72.7) | 66/85 (77.7) | 46/69 (66.7) | 0.128 |
| Chest X-ray | ||||
| Chest X-ray performed, n = 248b | 106 (42.7) | 58 (40.9) | 48 (45.3) | 0.485 |
| Chest X-ray performed among those with a chest X-ray request, n = 158b | 106/158 (67.1) | 58/89 (65.2) | 48/69 (69.6) | 0.560 |
| TB prevalence | ||||
| Confirmed TB | 15 (6.1) | 9 (6.4) | 6 (5.7) | 0.803 |
| Probable TB | 31 (12.6) | 19 (13.6) | 12 (11.3) | 0.598 |
| All TB cases | 46 (18.7) | 28 (20.0) | 18 (17.0) | 0.548 |
| TB treatment | ||||
| Referred for TB treatment among probable and confirmed TB, n = 46 | 38/46 (82.6) | 24/28 (85.7) | 14/18 (77.8) | 0.693 |
| Initiated TB treatment among those referred, n = 38 | 33/38 (86.8) | 22/24 (91.7) | 11/14 (78.6) | 0.337 |
| Mortality rate | ||||
| Eight weeks all-cause mortality | 62 (25.2) | 38 (27.1) | 24 (22.6) | 0.421 |
Abbreviations: TB, tuberculosis; Xpert, sputum Xpert MTB/RIF.
Data are presented as n (%).
Two-sided P-value for comparison between participants enrolled before the COVID-19 pandemic and participants enrolled during the COVID-19 pandemic
Two patients were lost to follow-up after discharge. During admission, one of them had a normal chest X-ray and no Xpert and the other patient had an abnormal chest X-ray and a negative Xpert. Both patients were included in the analyses of access to Xpert and chest X-ray. They were excluded from the other outcome analyses due to missing information after discharge.
Table 3.
Adjusted log-binominal generalized linear model fitted to identify co-variates associated with having an Xpert test performed during 8 weeks follow-up among patients with HIV on medical admission in Ghana.
| Multivariate modela |
|||
|---|---|---|---|
| Variable | Adjusted risk ratio | 95% confidence interval | P-value |
| Age >40 years | 1.31 | 0.88-1.95 | 0.190 |
| Male sex | 1.02 | 0.85-1.22 | 0.866 |
| CD4 count ≤100 cells/mm3 | 1.31 | 1.22-1.41 | <0.001 |
| Self-reported cough | 2.37 | 1.80-3.13 | <0.001 |
| Unable to walk unaided | 0.78 | 0.65-0.94 | 0.010 |
| COVID-19 pandemic period | 1.10 | 0.96-1.24 | 0.158 |
Abbreviations: CD, clusters of differentiation; Xpert, sputum Xpert MTB/RIF.
In the multivariate model a cluster variance estimator was used to adjust the standard error for three clusters (hospitals). The multivariate model included all clinically relevant co-variates (age >40 years, male sex, CD4 count ≤100 cells/mm3, self-reported cough, being unable to walk unaided, and being admitted during the COVID-19 pandemic).
The overall TB prevalence of 46 of 246 (18.7%; 95% CI 14.0-24.1) remained stable over the two periods (Table 2), including 15 patients with confirmed TB (6.1%; 95% CI 3.5-9.9) and 31 patients with probable TB (12.6%; 95% CI 8.7-17.4). Among patients with confirmed TB or probable TB, 17 (36.9%) were males and 29 (63.0%) were females. Patients with TB were severely immunosuppressed, with a prepandemic median CD4 count of 55 (IQR 17-126) cells/mm3 and a pandemic median CD4 count of 57.5 (IQR 9-101) cells/mm3; P-value = 0.845. Of patients diagnosed with TB, 38/46 (82.6%) were referred for TB treatment initiation (Figure 3). Of those 33/38 (86.8%) initiated TB treatment (Table 2) after a median 6 (IQR 4-8) days (Table S1). Patients with confirmed TB were initiated on TB treatment at a higher rate (14 of 15 [93.3%]) than patients with probable TB (19/31 [61.3%], P-value = 0.024).
Figure 3.
The routine in-hospital TB diagnostic care cascade among 248 people with HIV at three hospitals in Ghana.
Abbreviations: TB, Tuberculosis; WHO, World Health Organization; W4SS, WHO four-symptom screening; Xpert, sputum GeneXpert MTB/RIF.
Percentages are rounded to zero decimal places.
a.Two patients were lost to follow-up after discharge but were included in the denominator as ”Unlikely TB”.
Among patients not on ART at enrollment, 122 of 154 (79.2%) initiated ART during follow-up after a median 12 (IQR 6-20) days in the prepandemic period and after a median 6.5 (IQR 3-18) days in the pandemic period; P-value = 0.031 (Table S1).
Mortality
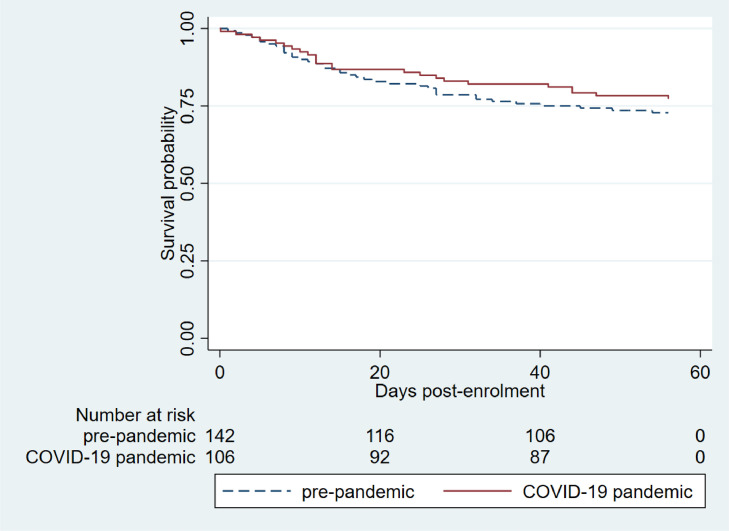
The 8-week all-cause mortality rate was 62/246 (25.2%; 95% CI 20.0-31.1) (Table 2). The median days from admission to death was 17.5 days (IQR 10-33; Table S1). There was no difference in the 8-week cumulative survival when stratifying for the pandemic period (log-rank P-value = 0.412; Figure 4 ), and the admission during the pandemic period was not a predictor of 8-week all-cause mortality in a multivariate model (hazard ratio 0.94, 95% CI 0.48-1.83, P = 0.861; Table 4 ).
Figure 4.
Kaplan-Meier cumulative probability of 8 weeks survival among people with HIV stratified by admission to hospital in the prepandemic and the COVID-19 pandemic period.
Table 4.
Cox regression analysis with unadjusted and adjusted predictors of 8 weeks all-cause mortality among patients with HIV on medical admission in Ghana.
| Univariate analysis |
Multivariate analysisa |
|||||
|---|---|---|---|---|---|---|
| Variable | Unadjusted HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value |
| Age >40 years | 1.06 | 0.64-1.74 | 0.825 | 1.10 | 0.91-1.33 | 0.337 |
| Male sex | 2.14 | 1.29-3.55 | 0.004 | 1.93 | 1.17-3.18 | 0.010 |
| CD4 count ≤100 cells/mm3 | 2.00 | 1.14-3.51 | 0.012 | 1.81 | 1.02-3.20 | 0.041 |
| COVID-19 pandemic period | 0.81 | 0.48-1.35 | 0.410 | 0.94 | 0.48-1.83 | 0.861 |
Abbreviations: CI, confidence interval; HR, hazard ratio; Xpert, sputum Xpert MTB/RIF.
In the multivariate model, a cluster variance estimator was used to adjust the standard error for the three hospitals. The multivariate model included all proposed co-variates (age >40 years, male sex, CD4 count ≤100 cells/mm3) and the proposed risk factor (COVID-19 pandemic period) from univariate analysis.
In the subgroup analyses for patients with TB, the mortality rate was 19/46 (41.3%; 95% CI 27.0-56.8); 2/15 (13.3%; 95% CI 1.7-40.5) for confirmed TB cases and 17/31 (54.8%; 95% CI 36.0-72.7) for probable TB cases (P-value = 0.010), and there was no difference in the mortality rate over the prepandemic (13/28, 46.4%) and the pandemic (6/18, 33.3%) period (P-value = 0.379).
Discussion
This study prospectively describes the in-hospital TB diagnostic care and early clinical outcomes among severely ill PWH before and during the COVID-19 pandemic in Ghana. We identified a major gap in reaching all patients eligible for TB investigation with sputum Xpert and chest X-ray, which remained similarly large over the two periods. The TB prevalence and 8-week all-cause mortality were comparably high before and during the COVID-19 pandemic.
Less than half of patients hospitalized at sites with available tests accessed a sputum Xpert, despite being eligible for TB investigation, according to the national [23] and international guidelines [28]. This is in line with findings from population-based evaluations [5,7] and a systematic review of facility based TB care [30] in TB-burdened countries, concluding that the largest gap in the cascade was due to patients not accessing TB testing. Although Xpert may be available and offered free of charge, several barriers persist, such as difficulties to produce sputum, lack of trained staff to collect extrapulmonary specimens for testing, reliance on patients or relatives to pick up Xpert results from the laboratory, and disrupted availability of Xpert cartridges, as has been reported from Ghana [31].
We found that patients with cough had an increased chance of having an Xpert performed, which may indicate a gap in TB testing among patients not presenting with cough. We further found that only 112/246 (45.5%) produced a sputum sample for Xpert testing as part of routine care. Of all cases with TB, only 15/46 (32.6%) were confirmed by a positive Xpert result. This is similar to findings from other studies in sub-Saharan Africa (SSA), where a high proportion of inpatients were unable to produce sputum [32], [33], [34]. Sputum scarcity may be particularly high in a population like our study population characterized by low CD4 count, high rate of WHO danger signs, weight loss, and anemia, suggesting progressed HIV and late presentation to health care. The same population may further experience high rates of extrapulmonary and disseminated TB [35,36] unidentified by sputum Xpert.
To improve TB case detection, increased efforts should be made to offer all medical PWH inpatients Xpert for TB screening, as recommended by WHO [28]. Furthermore, to cover extrapulmonary TB, disseminated TB, and sputum-scarce pulmonary TB, methods such as Xpert on extrapulmonary specimens and urine LAM testing should be considered [28,37].
In addition, we found a gap in the chest X-ray performed. This may partly be explained by a low request rate for chest X-ray, difficulties to move unstable patients to the radiology department because more than half of patients in our study had at least one WHO danger sign, and “out-of-pocket” payment for a chest X-ray [38]. Free-of-charge digital chest X-ray offered through the national TB program in Ghana is currently being rolled out but was not standard of care at the research sites at the time of this study.
During the COVID-19 pandemic, WHO and others reported reduced overall TB case finding services [1,25,27]. In Ghana, a 30% drop in TB case detection was observed in routine data during the first year of the pandemic compared with a 15-month prepandemic period [18]. Factors contributing to delayed or interrupted TB diagnosis during the pandemic may include an overlap in TB and COVID-19 symptoms [39], limited concomitant TB and COVID-19 testing using Xpert machines [40], overworked laboratories, and delayed TB test performance [41]. In our study of severely ill PWH, the observed gaps in the in-hospital TB diagnostic cascade did not alter between the prepandemic and the pandemic period.
The TB prevalence of 18.7% was expectedly high, comparable to a previous study among PWH from Ghana [20], and remained constant over the two periods. We had expected that the pandemic population would be characterized by markers of more progressed HIV and TB disease than the prepandemic population but found the opposite, such as higher CD4 count, lower rate of cough, and fewer abnormalities on chest X-ray. This may be explained by alterations in the wards open for medical admission of PWH during the pandemic. The pretest probability of having TB may as such have been lower among patients admitted during the pandemic period. However, we do speculate that a subpopulation of very ill patients with HIV may have stayed or died at home without reaching hospital admission during the pandemic, thus underestimating the difference in TB prevalence and mortality rate between prepandemic period and the COVID-19 pandemic period. The reasons for not coming on admission despite severe illness may include the patients’ fear of contracting COVID-19 or increased transportation costs during the pandemic [42], among other things. Under-reporting of cough and changed health-seeking behavior may be an important issue to address, especially during the pandemic with the risk of stigmatization due to both TB and COVID-19 [1]. Furthermore, due to a forced halt in clinical research activities, patients admitted during the first half year of the pandemic were not included in our study and may have had a different presentation and TB prevalence.
The next gap in the TB care cascade concerns those who are successfully diagnosed but not registered in treatment [4]. Although all patients with a positive Xpert initiated TB treatment, a noteworthy gap of 13/46 (28.3%) of TB cases overall never initiated TB treatment. The gap in TB treatment initiation can be partly explained by the high early mortality rate among probable TB cases because the cases with TB at death were included in the TB case definition, limiting the chances of successful early HIV/TB management in this population. Others have explained this gap with the health care systems’ failure to link patients to TB clinics, experienced stigma around the diagnosis [43], patient transfer/dropout, and deteriorating clinical condition [44].
Reduced access to TB treatment during the pandemic has been described [1,25]. In our study, we found that 91.7% of the referred patients initiated TB treatment in the prepandemic period compared with 78.6% in the pandemic period, which is a nonsignificant difference; although, the numbers are small. The median time to TB treatment initiation was 6 days, which was within the WHO recommended 14 days [28], and comparable to findings from other SSA studies [44,45].
Considering the symptom overlap between TB and COVID-19, the COVID-19 test rate in our pandemic population was surprisingly low, with only 12.3% patients having a COVID-19 polymerase chain reaction test result. The low COVID-19 test rates may be explained by periodic shortages in the equipment needed for sample collection, such as cotton swabs, or for COVID-19 testing (data not collected), high workload with missed opportunities for COVID-19 testing, which was performed by dedicated COVID-19 teams, including mobile laboratory technicians (data not collected), and challenges linking the laboratories with the care institution [12], leading to an underestimation of COVID-19 test rate and COVID-19 prevalence in this study.
Our study population was characterized by a majority of female sex (71.8%). It has been proposed that the reported HIV prevalence is higher among females in Ghana due to earlier sexual debut than males [46] and that more females are tested for HIV because of HIV screening services being part of reproductive health programs [47].
The 8-week all-cause mortality at 25.2% in our cohort remained high over the two periods. However, studies from Ghana have in previous years observed even higher mortality rates among PWH with an in-hospital mortality rate of 40.6% [21] and a 6-month mortality rate of 48.6% [48]. In line with previous literature, we found that low CD4 count [49,50] and male sex [49,51] were predictors of all-cause mortality. A meta-analysis of PWH initiating ART in Africa confirms that fewer males reached health care and that males had higher risk of mortality than females [51]. The poorer prognosis among males may in our study further be explained by significantly lower CD4 count than females. A meta-analysis of PWH in SSA evidenced that males had lower ART coverage, poorer viral suppression, and suboptimal disease knowledge than females, highlighting that, in particular, men are missed out in the HIV care cascade [52].
We did not find that admission during the pandemic was associated with a higher risk of mortality. This may be due to the limited number of cases included in the analysis but may also reflect that we studied a severely ill population with a priori high risk of mortality, regardless of COVID-19. Moreover, although we reported the outcomes for a population that has reached hospital admission, WHO more broadly reported that TB-related deaths among PWH overall has increased for the first time in 13 years during the first year of the COVID-19 pandemic [53]. The mortality rates in our study increased to 41.3% among patients co-infected with HIV/TB, with probable TB cases having a four-fold higher mortality rate than confirmed TB cases. These high mortality rates are alarming and confirm the findings from prepandemic studies among patients co-infected with HIV/TB in Ghana [20,21,54]. The majority of deaths occurred after the first 10 days on hospital admission, highlighting a potential time window for point-of-care interventions to guide early management.
Our study complement previously described gaps in the HIV-associated TB care cascade in Ghana, such as suboptimal TB screening [55] and TB treatment outcomes [56]. Such evaluations may guide the continuous work toward improved HIV and TB care in Ghana and in similar settings.
This study provides a rare opportunity to prospectively describe clinical characteristics and the in-hospital routine care among a hard-to-reach study population of severely ill PWH at three large government hospitals in Ghana before and during a pandemic. We had a very low loss to follow-up rate that minimized bias. Our study had several limitations. Because access to routine TB testing was suboptimal already before the COVID-19 pandemic, further disruption during the pandemic may be difficult to demonstrate when the sample size is small. The pandemic led to structural changes at the study sites, including alterations in the wards open for medical admission of PWH because some wards were transformed to COVID-19 units. This may have caused a variation in study population characteristics and subsequently confounded the comparison of study outcomes. We sought to overcome this limitation by standardized screening of all medical wards admitting PWH, ensuring inclusion of our target population, regardless of the in-hospital location. The national and hospital restrictions in conducting clinical research during the first 6 months of the pandemic prevented us to include data from the most critical pandemic phase, including the first COVID-19 wave, with possible serious disruptions in health care services. In fact, the disruptions of the TB care cascade may have been considerable during this period and we may have underestimated gaps in the routine TB diagnostic cascade during the pandemic. After the approval of a study amendment, including COVID-19 measures, at the relevant ethical boards, we restarted the study enrollment. To avoid any impact of the by hospital stepwise rollout of the LAM intervention when describing the routine TB diagnostic cascade, the Lekma Hospital contributed only with prepandemic data and the Korle-Bu Teaching Hospital contributed partly with pandemic data. We included a cluster variance estimator to adjust the standard error for hospitals in the adjusted log-binominal generalized linear model and in the multivariate Cox regression model. Furthermore, we cannot exclude a calendar effect given that the two pandemic periods are not matched by season. Finally, generalizability to other hospitals in West Africa may be limited.
Conclusion
In this cohort of hospitalized PWH who are severely immunosuppressed, we describe a high TB prevalence and poor prognosis. We detail the HIV-associated TB diagnostic care cascade and quantify a significant gap with less than half of patients accessing routine TB investigations, regardless of being admitted before or during the COVID-19 pandemic. Missed or delayed TB diagnosis may be critical in this population, with 25% of the patients dying within 8 weeks from hospital admission.
Funding
J.Å. received grants from Julie von Müllens fond, Læge Agnethe Løvgreens legat, Torben og Alice Frimodts fond, A.P. Møller fonden, University of Southern Denmark Faculty of Health Sciences Ph.D. scholarship, University of Southern Denmark traveling fund, the Region of Southern Denmark, and Odense University Hospital Internationalization fund. I.S.J. received grants from the Strategic Research Council, Region of Southern Denmark. The funding sources had no influence on the study design, data collection, analysis, interpretation of the data, or the writing of the manuscript.
Ethical approval
The study was approved by the Ghana Health Service Ethics Review Committee for research conducted at Tema General Hospital and Lekma Hospital (GHS-ERC 006/06/19, August 19, 2019) and by the Scientific and Technical Committee and the Institutional Review Committee at Korle-Bu Teaching Hospital (KBTH-IRB /00052/2019, September 9, 2019). The study was approved by the Danish Data Protection Agency through the Region of Southern Denmark for the handling of personal research data. An amendment of the study protocol, including procedures to ensure adherence to national and local COVID-19 safety measures and to minimize the risk for patients and staff to get COVID-19 as a result of the study, was approved on August 7, 2020 from the institutional review committee at Korle-Bu Teaching Hospital and on September 9, 2020 from the review committee for research at Ghana Health Service.
Author contributions
J.Å., S.B., Å.B.A., E.K., M.L., and I.S.J. conceptualized and designed the study. J.Å., V.J.G., A.K., J.O.C., Y.A.P., and P.P. contributed to the acquisition of data. J.Å. performed the data analysis, and the writing of the original draft. S.B. and I.S.J. edited the original draft. All authors (J.Å., S.B., V.J.G., A.K., J.O.C., Y.A.P., P.P., Å.B.A., E.K., M.L., and I.S.J.) revised, read, and approved the final manuscript.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
The authors are most grateful to the patients, hospital management, and staff of Lekma Hospital, Tema General Hospital and Korle-Bu Teaching Hospital who participated in this study. The authors thank research assistants at the study sites; the staff at the Chest Clinic and Fevers Unit Laboratories, Korle-Bu Teaching Hospital; staff at the National Public Health and Reference Laboratory, Korle-Bu; key personnel at the National TB Control Programme in Ghana; and Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark for their support during the study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.12.044.
Appendix. Supplementary materials
Figure S1. Patient study enrollment by research site and COVID-19 pandemic period
Table S1. Time from the first day of admission to key steps in the in-hospital TB diagnostic care cascade among people with HIV stratified by period before and during the COVID-19 pandemic.
Table S2. Results from TB investigation during admission and 8 weeks follow-up stratified by period before and during the COVID-19 pandemic.
References
- 1.World Health Organization. Global tuberculosis report 2022, https://apps.who.int/iris/rest/bitstreams/1474924/retrieve; 2022 [accessed 27 October 2022].
- 2.Joint United Nations programme on HIV/AIDS. Global AIDS Update 2020: Seizing the Moment - Tackling entrenched inequalities to end epidemics, https://aids2020.unaids.org/report/; 2020 [accessed 19 July 2022].
- 3.Hogan AB, Jewell BL, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbaraman R, Nathavitharana RR, Mayer KH, Satyanarayana S, Chadha VK, Arinaminpathy N, Pai M. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lungu P, Kerkhoff AD, Kasapo CC, Mzyece J, Nyimbili S, Chimzizi R, et al. Tuberculosis care cascade in Zambia - identifying the gaps in order to improve outcomes: a population-based analysis. BMJ, (Open) 2021;11 doi: 10.1136/bmjopen-2020-044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbaraman R, Nathavitharana RR, Satyanarayana S, Pai M, Thomas BE, Chadha VK, et al. The tuberculosis cascade of care in India's public sector: a systematic review and meta-analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Keshavjee S, Atun R. Health systems performance in managing tuberculosis: analysis of tuberculosis care cascades among high-burden and non-high-burden countries. J Glob Health. 2019;9 doi: 10.7189/jogh.09.010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joint United Nations programme on HIV/AIDS. UNAIDS 2021 estimates, HIV estimates with uncertainty bounds 1990-Present, https://www.unaids.org/sites/default/files/media_asset/HIV_estimates_from_1990-to-present.xlsx; 2021 [accessed 27 October 2022].
- 9.World Health Organization. Tuberculosis profile: Ghana, https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22GH%22; 2020 [accessed 19 July 2022].
- 10.Bonsu F, Addo KK, Alebachew Z, Gyapong J, Badu-Peprah A, Gockah R, et al. National population-based tuberculosis prevalence survey in Ghana 2013. Int J Tuberc Lung Dis. 2020;24:321–328. doi: 10.5588/ijtld.19.0163. [DOI] [PubMed] [Google Scholar]
- 11.Kenu E, Frimpong JA, Koram KA. Responding to the COVID-19 pandemic in Ghana. Ghana Med J. 2020;54:72–73. doi: 10.4314/gmj.v54i2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandoh DA, Frimpong JA, Malm KL, Asiedu-Bekoe F, Kenu E. Strategies adopted by Ghana during first and second waves of COVID-19 in Ghana. J Interv Epidemiol Public Health. 2022;5:3. doi: 10.11604/JIEPH.supp.2022.5.1.1206. [DOI] [Google Scholar]
- 13.Acheampong G, Owusu M, Nkrumah B, Obeng-Boadi P, Opare DA, Sambian DJ, et al. Laboratory capacity in COVID-19 diagnosis and the need to enhance molecular testing in Ghana. Glob Sec Health Sci Policy. 2021;6:10–17. doi: 10.1080/23779497.2021.1908157. [DOI] [Google Scholar]
- 14.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, Macdonald B, Dattani S, Beltekian D, Ortiz-Ospina E, Roser M. Coronavirus pandemic (COVID-19). England: Our World in Data; 2020.
- 16.Morang'a CM, Ngoi JM, Gyamfi J, Amuzu DSY, Nuertey BD, Soglo PM, Appiah V, Asante IA, Owusu-Oduro P, Armoo S, Adu-Gyasi D, Amoako N, Oliver-Commey J, Owusu M, Sylverken A, Fenteng ED, M'cormack VV, Tei-Maya F, Quansah EB, Ayivor-Djanie R, Amoako EK, Ogbe IT, Yemi BK, Osei-Wusu I, Mettle DNA, Saiid S, Tapela K, Dzabeng F, Magnussen V, Quaye J, Opurum PC, Carr RA, Ababio PT, Abass AK, Akoriyea SK, Amoako E, Kumi-Ansah F, Boakye OD, Mibut DK, Odoom T, Ofori-Boadu L, Allegye-Cudjoe E, Dassah S, Asoala V, Asante KP, Phillips RO, Osei-Atweneboana MY, Gyapong JO, Kuma-Aboagye P, Ampofo WK, Duedu KO, Ndam NT, Bediako Y, Quashie PK, Amenga-Etego LN, Awandare GA. Genetic diversity of SARS-CoV-2 infections in Ghana from 2020–2021. Nat Commun. 2022;13:2494. doi: 10.1038/s41467-022-30219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis HC, Ware H, Whelan M, Subissi L, Li Z, Ma X, et al. SARS-CoV-2 infection in Africa: a systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021. BMJ Glob Health. 2022;7 doi: 10.1136/bmjgh-2022-008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arsenault C, Gage A, Kim MK, Kapoor NR, Akweongo P, Amponsah F, et al. COVID-19 and resilience of healthcare systems in ten countries. Nat Med. 2022;28:1314–1324. doi: 10.1038/s41591-022-01750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abraham SA, Berchie GO, Doe PF, Agyare E, Addo SA, Obiri-Yeboah D. Effects of COVID-19 pandemic on art service delivery: perspectives of healthcare workers in a Teaching Hospital in Ghana. BMC Health Serv Res. 2021;21:1295. doi: 10.1186/s12913-021-07330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjerrum S, Kenu E, Lartey M, Newman MJ, Addo KK, Andersen AB, Johansen IS. Diagnostic accuracy of the rapid urine lipoarabinomannan test for pulmonary tuberculosis among HIV-infected adults in Ghana-findings from the DETECT HIV-TB study. BMC Infect Dis. 2015;15:407. doi: 10.1186/s12879-015-1151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saavedra A, Campinha-Bacote N, Hajjar M, Kenu E, Gillani FS, Obo-Akwa A, et al. Causes of death and factors associated with early mortality of HIV-infected adults admitted to Korle-Bu Teaching Hospital. Pan Afr Med J. 2017;27:48. doi: 10.11604/pamj.2017.27.48.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV: policy update 2019, https://apps.who.int/iris/handle/10665/329479; 2019 [accessed 19 July 2022].
- 23.Ghana National Tuberculosis Programme, Bonsu FA, Adusi-Poku Y, Kwami Afutu F, Hanson-Nortey NN, Asare-Baah M, Alebachew Z. Standard operating procedures for systematic screening for active TB at health facility and different settings. Retrieved personally as an unpublished document from the Ghana National Tuberculosis Programme 16 September 2019, Accra. Ghana, 2018.
- 24.Global Laboratory Initiative. Planning for country transition to Xpert® MTB/RIF Ultra Cartridges, www.stoptb.org/wg/gli, http://www.who.int/tb/publications/2017/XpertUltra; 2017 [accessed 18 June 2022].
- 25.Dheda K, Perumal T, Moultrie H, Perumal R, Esmail A, Scott AJ, et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. 2022;10:603–622. doi: 10.1016/S2213-2600(22)00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jassat W, Cohen C, Tempia S, Masha M, Goldstein S, Kufa T, Murangandi P, Savulescu D, Walaza S, Bam JL, Davies MA, Prozesky HW, Naude J, Mnguni AT, Lawrence CA, Mathema HT, Zamparini J, Black J, Mehta R, Parker A, Chikobvu P, Dawood H, Muvhango N, Strydom R, Adelekan T, Mdlovu B, Moodley N, Namavhandu EL, Rheeder P, Venturas J, Magula N, Blumberg L. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV. 2021;8:e554–e567. doi: 10.1016/S2352-3018(21)00151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mbithi I, Thekkur P, Chakaya JM, Onyango E, Owiti P, Njeri NC, et al. Assessing the real-time impact of COVID-19 on TB and HIV services: the experience and response from selected health facilities in Nairobi, Kenya. Trop Med Infect Dis. 2021;6 doi: 10.3390/tropicalmed6020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO consolidated guidelines on tuberculosis: module 2: screening: systematic screening for tuberculosis disease, https://www.who.int/publications/i/item/9789240022676; 2021a [acessed 18 June 2022]. [PubMed]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divala TH, Lewis J, Bulterys MA, Lutje V, Corbett EL, Schumacher SG, MacPherson P. Missed opportunities for diagnosis and treatment in patients with TB symptoms: a systematic review. Public Health Action. 2022;12:10–17. doi: 10.5588/pha.21.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eboreime O. Master of Public health dissertation). Legon: University of Ghana; 2017. Factors Associated with the Utilization of GeneXpert in the Diagnosis of Drug Resistant Tuberculosis in the Greater Accra Region. [Google Scholar]
- 32.Gupta-Wright A, Corbett EL, van Oosterhout JJ, Wilson D, Grint D, Alufandika-Moyo M, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet. 2018;392:292–301. doi: 10.1016/S0140-6736(18)31267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huerga H, Cossa L, Manhiça I, Bastard M, Telnov A, Molfino L, Systematic Sanchez-Padilla E. point-of-care urine lipoarabinomannan (Alere TB-LAM) assay for diagnosing tuberculosis in severely immunocompromised HIV-positive ambulatory patients. Am J Trop Med Hyg. 2020;102:562–566. doi: 10.4269/ajtmh.19-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawn SD, Kerkhoff AD, Burton R, Schutz C, Boulle A, Vogt M, et al. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med. 2017;15:67. doi: 10.1186/s12916-017-0822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29:1987–2002. doi: 10.1097/QAD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhana A, Hamada Y, Kengne AP, Kerkhoff AD, Rangaka MX, Kredo T, et al. Tuberculosis screening among HIV-positive inpatients: a systematic review and individual participant data meta-analysis. Lancet HIV. 2022;9:e233–e241. doi: 10.1016/S2352-3018(22)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedrazzoli D, Siroka A, Boccia D, Bonsu F, Nartey K, Houben R, Borghi J. How affordable is TB care? Findings from a nationwide TB patient cost survey in Ghana. Trop Med Int Health. 2018;23:870–878. doi: 10.1111/tmi.13085. [DOI] [PubMed] [Google Scholar]
- 39.Narita M, Hatt G, Gardner Toren K, Vuong K, Pecha M, Jereb JA, Goswami ND. Delayed tuberculosis diagnoses during the coronavirus disease 2019 (COVID-19) pandemic in 2020-king county, Washington. Clin Infect Dis. 2021;73:S74–S76. doi: 10.1093/cid/ciab387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmer AJ, Klinton JS, Oga-Omenka C, Heitkamp P, Nawina Nyirenda C, Furin J, Pai M. Tuberculosis in times of COVID-19. J Epidemiol Community Health. 2022;76:310–316. doi: 10.1136/jech-2021-217529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aznar ML, Espinosa-Pereiro J, Saborit N, Jové N, Sánchez Martinez F, Pérez-Recio S, et al. Impact of the COVID-19 pandemic on tuberculosis management in Spain. Int J Infect Dis. 2021;108:300–305. doi: 10.1016/j.ijid.2021.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraham SAA, Doe PF, Osei Berchie G, Agyare E, Ayisi Addo S, Obiri-Yeboah D. Explorative-descriptive study on the effects of COVID-19 on access to antiretroviral therapy services: the case of a teaching hospital in Ghana. BMJ. 2022;12 doi: 10.1136/bmjopen-2021-056386. (Open) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zawedde-Muyanja S, Manabe YC, Cattamanchi A, Castelnuovo B, Katamba A. Patient and health system level barriers to and facilitators for tuberculosis treatment initiation in Uganda: a qualitative study. BMC Health Serv Res. 2022;22:831. doi: 10.1186/s12913-022-08213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Floridia M, Ciccacci F, Andreotti M, Hassane A, Sidumo Z, Magid NA, et al. Tuberculosis case finding with combined rapid point-of-care assays (xpert MTB/RIF and determine TB LAM) in HIV-positive individuals starting antiretroviral therapy in Mozambique. Clin Infect Dis. 2017;65:1878–1883. doi: 10.1093/cid/cix641. [DOI] [PubMed] [Google Scholar]
- 45.Akanbi MO, Achenbach C, Taiwo B, Idoko J, Ani A, Isa Y, et al. Evaluation of gene xpert for routine diagnosis of HIV-associated tuberculosis in Nigeria: a prospective cohort study. BMC Pulm Med. 2017;17:87. doi: 10.1186/s12890-017-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sia D, Onadja Y, Hajizadeh M, Heymann SJ, Brewer TF, Nandi A. What explains gender inequalities in HIV/AIDS prevalence in sub-Saharan Africa? Evidence from the demographic and health surveys. BMC Public Health. 2016;16:1136. doi: 10.1186/s12889-016-3783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yawson AE, Appiah LK, Yawson AO, Bonsu G, Aluze-Ele S, Amanhyia NA, et al. Sex differences in perceived risk and testing experience of HIV in an urban fishing setting in Ghana. Int J Equity Health. 2014;13:109. doi: 10.1186/s12939-014-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjerrum S, Oliver-Commey J, Kenu E, Lartey M, Newman MJ, Addo KK, et al. Tuberculosis and non-tuberculous mycobacteria among HIV-infected individuals in Ghana. Trop Med Int Health. 2016;21:1181–1190. doi: 10.1111/tmi.12749. [DOI] [PubMed] [Google Scholar]
- 49.Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, Bisson GP, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One. 2011;6:e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Druyts E, Dybul M, Kanters S, Nachega J, Birungi J, Ford N, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. AIDS. 2013;27:417–425. doi: 10.1097/QAD.0b013e328359b89b. [DOI] [PubMed] [Google Scholar]
- 52.Nardell MF, Adeoti O, Peters C, Kakuhikire B, Govathson-Mandimika C, Long L, et al. Men missing from the HIV care continuum in sub-Saharan Africa: a meta-analysis and meta-synthesis. J Int AIDS Soc. 2022;25:e25889. doi: 10.1002/jia2.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization. Global tuberculosis report 2021, https://www.who.int/publications/i/item/9789240037021; 2021b [accessed 10 February 2022].
- 54.Osei E, Oppong S, Der J. Trends of tuberculosis case detection, mortality and co-infection with HIV in Ghana: a retrospective cohort study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjerrum S, Bonsu F, Hanson-Nortey NN, Kenu E, Johansen IS, Andersen AB, et al. Tuberculosis screening in patients with HIV: use of audit and feedback to improve quality of care in Ghana. Glob Health Action. 2016;9:32390. doi: 10.3402/gha.v9.32390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayibor KM, Bandoh DA, Asante-Poku A, Kenu E. Predictors of adverse TB treatment outcome among TB/HIV patients compared with non-HIV patients in the Greater Accra Regional Hospital from 2008 to 2016. Tuberc Res Treat. 2020;2020 doi: 10.1155/2020/1097581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient study enrollment by research site and COVID-19 pandemic period
Table S1. Time from the first day of admission to key steps in the in-hospital TB diagnostic care cascade among people with HIV stratified by period before and during the COVID-19 pandemic.
Table S2. Results from TB investigation during admission and 8 weeks follow-up stratified by period before and during the COVID-19 pandemic.