Abstract
Mosquito-transmitted diseases account for about 500 000 deaths every year. Blocking these pathogens in the mosquito vector before they are transmitted to humans is an effective strategy to prevent mosquito-borne diseases. Like most higher organisms, mosquitoes harbor a highly diverse and dynamic microbial flora that can be explored for prevention of pathogen transmission. Here we review the structure and function of the mosquito microbiota, including bacteria, fungi, and viruses, and discuss the potential of using components of the microbiota to thwart pathogen transmission.
Mosquito Microbiota and Mosquito-Borne Diseases
Epidemics such as malaria, dengue fever, yellow fever, Zika fever, and chikungunya fever, all transmitted by mosquitoes, account for around 350 million cases and about 500 000 deaths throughout the world each year [1]. As there is no efficient vaccine for most of these diseases [2], vector control remains one of the best strategies to prevent disease. Of concern, overuse of insecticides has caused widespread resistance [3,4], and novel disease-control strategies are urgently demanded. Mosquitoes harbor a highly diverse and dynamic microbial flora, collectively known as the microbiota (see Glossary), mostly in its midgut and on its surface (cuticle), but also in its somatic cells, crop, salivary glands, circulation system, and reproductive organs (Figure 1, Key Figure) [5]. Members of the symbiotic microbiota play a key role in mosquito physiology and immunity [6]. The microbiota of mosquitoes can significantly impact pathogen transmission, and has already displayed valuable potential to combat mosquito-borne diseases such as malaria, Zika fever, dengue fever, yellow fever, and other vector-borne diseases [7].
Figure 1. Key Figure. Mosquitoes and Their Associated Pathogens and Microbes.
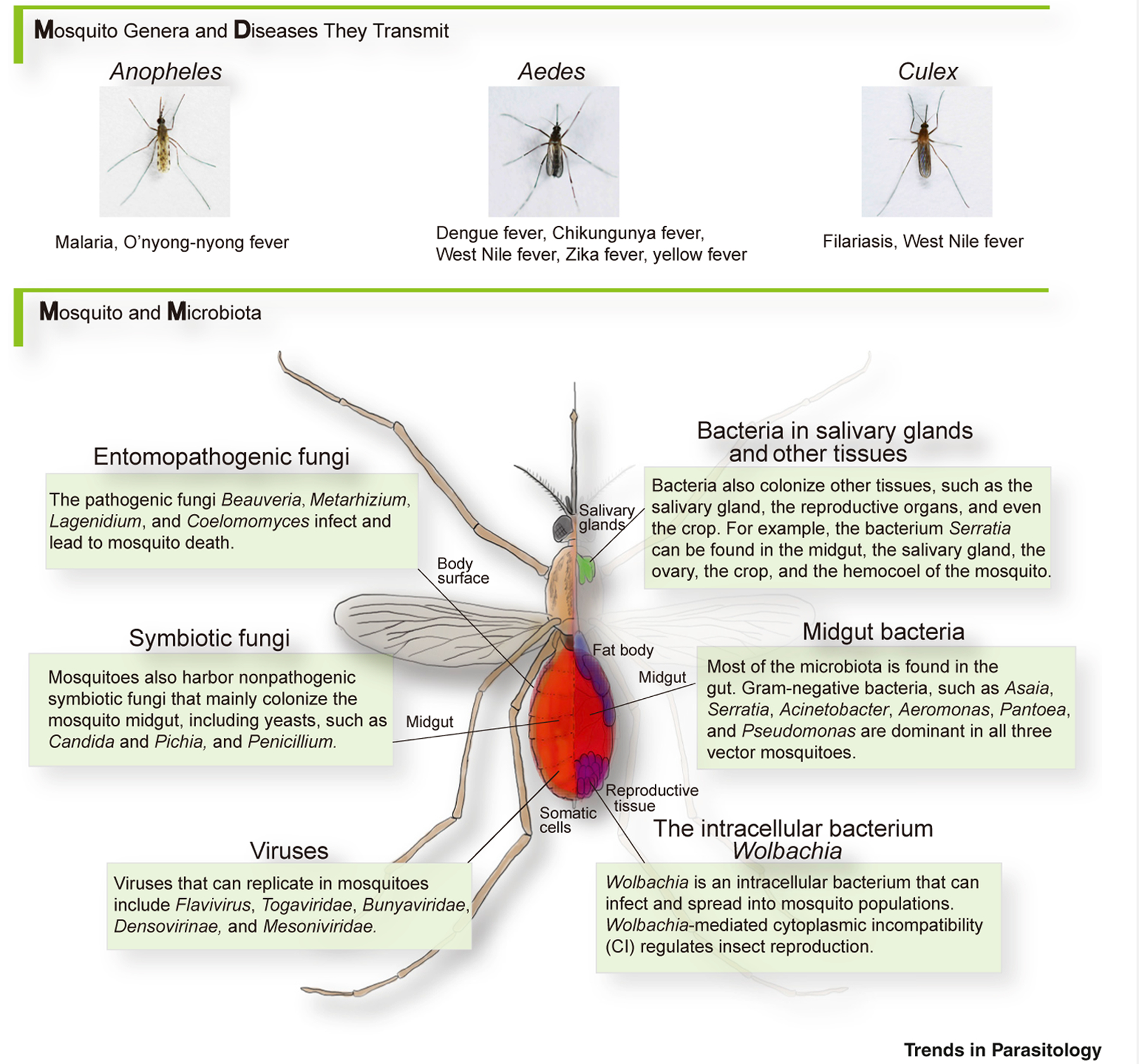
Mosquito species and their transmitted diseases are highly specialized, while they may share similar microbiota. Although the midgut harbors the majority of the microbiota, other tissues may also serve as microbe habitats.
The composition and roles of the gut commensal microbiota in mosquitoes, and its influence on vector competence for malaria parasites and dengue viruses (DENVs), were recently reviewed by others [5,8–10]. In this review, we summarize research progress, made in the last two decades, of interactions between vector mosquitoes and their microbiota, including bacteria, viruses, mosquito-pathogenic fungi, and symbiotic fungi, emphasizing implications for disease control. We also address concerns toward future applications in the field.
Microbiota Composition and Dynamics in Pathogen-Transmitting Mosquitoes
Different Vector Mosquitoes and Diseases Transmitted
Disease-transmitting mosquitoes belong mainly to three genera – Anopheles, Aedes, and Culex. Anopheles transmits malaria and O’nyong-nyong fever [11,12]. Arboviral diseases, including dengue fever, chikungunya fever, West Nile fever, Zika fever, and yellow fever, are transmitted mostly by Aedes [13]. Culex transmits mainly filarial worms and West Nile Virus (WNV) [11]. When the female adult mosquito bites an infected person, pathogens – together with the blood – are taken into the mosquito midgut. The pathogens then infect or traverse the gut epithelial cells, enter the hemolymph, invade the salivary glands, and are then transmitted when the infected mosquito bites another person.
Composition of the Microbiota in Vector Mosquitoes
Prokaryotes
Symbiotic bacteria are the best studied symbionts of mosquitoes. The midgut is where most symbiotic bacteria are located. In both larval and adult stages, Gram-negative bacteria are the majority [8], with Asaia, Acinetobacter, Aeromonas, Pantoea, Pseudomonas, and Serratia being the most common genera present in all vector mosquitoes [11,14,15]. Moreover, Comamonas, Elizabethkingia, Enterobacter, and Klebsiella are common in Anopheles [14]; Sphingomonas, Cupriavidus, and Escherichia–Shigella are common in Aedes [16]; and Staphylococcus, Klebsiella, and Enterobacter are dominant in Culex [11].
In addition to the midgut, symbiotic bacteria may also colonize other mosquito organs or tissues. For example, the common gut bacteria Asaia, Serratia, Acinetobacter and Pseudomonas can also colonize Anopheles and Aedes salivary glands and/or reproductive organs [5,14,17,18]. Bacteria of the genus Serratia can colonize Aedes crops [5]. Another notable prokaryotic genus which colonizes non-gut tissues is the intracellular bacterium Wolbachia. Wolbachia can be vertically transmitted as it infects insect germ cells. Furthermore, many strains of Wolbachia induce cytoplasmic incompatibility (CI) to promote their spread among insect populations. Natural Wolbachia infection is common in over 50% of all insect species [19]. Wolbachia has been identified in Culex pipiens, Aedes albopictus, and Anopheles gambiae in the wild [11]. It is present in various mosquito tissues, including reproductive organs, salivary glands, head, muscle, and Malpighian tubules [20]. Besides Wolbachia, Spiroplasma can also be found in hemolymph, hemocytes, thoracic flight muscle, and nerve cells, although there are not many reports on this genus [20].
Eukaryotes
Although not as well studied as prokaryotes, eukaryotic microorganisms such as fungi are part of the mosquito’s microbiota. The entomopathogenic fungi Beauveria and Metarhizium infect the mosquito cuticle and proliferate in the hemolymph, causing progressive mosquito death [21]. Other parasitic fungi, such as Lagenidium, Coelomomyces, and Culicinomyces – that attack mosquito larvae and adults – are also used as mosquito biological control agents [22].
Mosquitoes also harbor nonpathogenic fungal microbiota; these fungi colonize mainly the mosquito midgut, but they can also be found in other tissues such as salivary glands and reproductive organs [5,23]. Mosquito symbiotic fungi are mainly yeasts such as Candida and Pichia. The filamentous fungus Penicillium has also been reported. These three genera have been found in Anopheles and Aedes [5,23,24].
Viruses
Mosquito-specific viruses can replicate only in mosquitoes and not in vertebrate cells. Previous studies have confirmed that a wide range of wild mosquito strains are infected with this type of virus. Flavivirus (cell-fusing agent virus, Kamiti River virus, and Culex flavivirus), Togaviridae, Bunyaviridae, Densovirinae, and Mesoniviridae are the main taxa that have been reported. Although artificial infection by these viruses is often pathogenic to naïve mosquitoes, it causes nonpathogenic persistent infection in the survivors, which means that viruses stay active in the mosquito and can be transmitted to their offspring [12].
Factors Shaping the Gut Microbiota in Vector Mosquitoes
The composition of symbiotic microbiota is highly dynamic throughout the mosquito’s lifespan. Many factors, including developmental stages, living habitat, feeding habit, and even pathogen infection, can affect microbiota composition.
Mosquito larvae live in the water, so they obtain their gut microbiota mainly from their environment [5]. Changes in the breeding bacterial communities may impact the larval microbiota. For example, higher water temperature favors the growth of Betaproteobacteria, a common gut bacteria phylum, which is thought to be beneficial for the growth of Anopheles larvae [25,26]. Water contaminated by fertilizers rich in ammonium and phosphorus promotes the growth of microbes which can serve as a major source of nutrition for mosquito larvae [26]. Residual antibiotics in water also influence the mosquito larval microbiota as they reduce or eliminate certain bacterial taxa [5].
During pupation, the midgut microbiota is wrapped by the larval peritrophic matrix (PM) to form the meconium. The meconium is egested by the newly hatched adult mosquito, resulting in the loss of the majority of the gut microbiota [14,15]. Moll et al. [27] studied all three vector mosquito genera and found that the bacterial load is high in larvae, less in old larvae, increases in the pupa, and is very low in recently hatched adults. Wang et al. [28] found that gut bacterial diversity is higher in larvae than in adults. They also determined that cyanobacteria are the predominant gut bacteria in larvae and in the pupa, while Proteobacteria and Bacteroidetes dominate in adult guts, with Enterobacteriaceae and Flavobacteriaceae being the core taxa. Blood feeding also changes the gut microbiome composition of adults. Diversity decreases after a blood meal, while some specific taxa, such as Enterobacteriaceae, increase their representation [28–30].
Pathogen infection can affect the composition of the mosquito’s microbiota. For instance, enteric bacteria are favored in Plasmodium- or chikungunya virus (CHIKV)-infected mosquitoes [14,31]. Zika virus infection of Aedes aegypti results in increased representation of Rhodobacteraceae and Desulfuromonadaceae [32]. In Aedes triseriatus and Aedes japonicus, bacterial numbers increase while fungal representation decreases in response to La Crosse virus infection [33].
The Influence of the Microbiota on Mosquito Physiology
Mosquito–microbiota interactions are complex. Mosquito factors can shape the composition and proliferation of the microbiota, and the microbiota contributes to the mosquito’s food digestion, nutrition, growth, fertility, and immunity [34].
Influence of the Microbiota on Mosquito Nutrition
Female mosquitoes acquire nutrition essential for reproduction by feeding on vertebrate blood. Elimination of the female Ae. aegypti gut microbiota with antibiotics slows the digestion of ingested mouse blood; Enterobacter sp. and Serratia sp. residing in the mosquito midgut may have hemolytic activities that contribute to blood digestion [35]. In Ae. albopictus, Acinetobacter baumannii and Acinetobacter johnsonii improve blood protein digestion and nectar assimilation, respectively [36]. In addition to facilitating food digestion, the microbiota can serve as a food source. Ae. aegypti larvae that feed solely on Saccharomyces cerevisiae can develop into adults in a normal way [37]. During a 21-day feeding experiment, Candida glabrata, Candida albicans, Candida pseudolambica, and Wickerhamomyces anomalus isolated from Culex theileri and Cx. pipiens larvae can each separately serve as the sole source of nutrients for Cx. pipiens larval growth and pupation [38]. Wolbachia (wMelPop strain) infection impairs blood-feeding success in Ae. aegypti [39].
Influence of the Microbiota on Mosquito Development
The mosquito microbiota can influence the progress of mosquito development. In Anopheles stephensi, rifampicin-treated larvae showed developmental delay and asynchrony of later instars; supplying antibiotic-treated larvae with rifampicin-resistant Asaia could rescue larval development [40]. Live bacteria or eukaryotes are essential microorganisms for the development of Ae. aegypti larvae to adults [41,42], potentially due to a hypoxia signal in the mosquito gut induced by the micro-biota [43,44]. However, a recent study shows that axenic Ae. aegypti larvae could complete their development to adulthood without live microbiota, suggesting that the main role of the microbiota is to supply nutrition essential for larval development [45]. Removing midgut bacteria shortens longevity of An. stephensi [46]. Wolbachia (wMelPop strain) infection also shortens the Ae. aegypti lifespan [47]. Paraclostridium bifermentans strains isolated in anopheline endemic areas produce a neurotoxin, named PMP1, which cleaves mosquito syntaxin and kills Anopheles mosquitoes [48].
Influence of the Microbiota on Mosquito Reproduction
The microbiota can modulate mosquito mating, preoviposition, and reproduction behavior. A change in the microbiota community composition and number can make anti-Plasmodium transgenic An. stephensi males more attractive mates to wild-type females [49]. Two strains of bacteria isolated from Cx. pipiens, Klebsiella sp. and Aeromonas sp., enhance oviposition [50]. The rearing water of Ae. aegypti larvae infected with Candida pseudoglaebosa enhances the attractiveness of oviposition sites [51]. In Ae. aegypti, antibiotic treatment reduces egg production [45]. Supplementation of germ-free Ae. aegypti with commensal bacteria Paenibacillus, Chryseobacterium, Sphingobacterium, Aquitalea, or Comamonas could restore mosquito fecundity. However, Aedes atropalpus benefits only from Comamonas, while the other microorganisms could not support egg production to equivalent levels as conventionally reared females [52].
Wolbachia can spread through many arthropod populations by a mechanism known as cytoplasmic incompatibility (CI) [53]. CI is manifested by embryonic lethality of progeny from Wolbachia-infected males mated to uninfected females, whereas mating infected males to infected females yields viable progeny [53]. The molecular basis for CI is not completely understood. Mosquito-borne Wolbachia (wPip strain) Type IV Effector WD0830 interacts with the actin cytoskeleton to induce CI [54]. Wolbachia (wPip strain) deubiquitylating enzyme (DUB), CidB and partner CidA, are involved in the CI mechanism [55]. Another cin operon that encodes a nuclease, CinB, and a second protein, CinA, also appear to take part in CI in mosquitoes infected with Wolbachia (wPip strain) [56]. Prophage WO genes from Wolbachia (wMel strain) also participate in and enhance CI [57].
Influence of the Microbiota on Mosquito Physiology and Pathogen Infection
The mosquito gut microbiota proliferates by hundreds of fold for about 24 h after a blood meal [29]. This expansion of the mosquito microbiota could influence pathogen infection through diverse mechanisms. The microbiota may directly interact with mosquito pathogens or modulate pathogen infection by regulating the host mosquito immune defenses and nutrition status. The influence of the microbiota on mosquito physiology and pathogen transmission is summarized in Table 1. The mechanisms are discussed in the next section.
Table 1. Influence of the Microbiota on Mosquito Physiology and Pathogen Transmissiona.
| Mosquito species | Microbiota | Function | Refs |
|---|---|---|---|
| Aedes aegypti | Enterobacter sp., Serratia sp. | Blood digestion | [35] |
| Aedes albopictus | Acinetobacter baumannii, Acinetobacter johnsonii | Blood digestion and nectar assimilation | [36] |
| Ae. aegypti, Culex pipiens | Saccharomyces cerevisiae | Nutrient source | [37,38] |
| Ae. aegypti | Wolbachia (wMelPop) | Blood-feeding success | [39] |
| Anopheles stephensi | Asaia | Larval development | [40] |
| Ae. aegypti | Escherichia coli | Larval development (hypoxia signal) | [43,44] |
| Ae. aegypti | Wolbachia (wMelPop) | Lifespan | [47] |
| Anopheles | Paraclostridium bifermentans | Kills mosquito (neurotoxin PMP1) | [48] |
| Cx. pipiens | Klebsiella sp., Aeromonas sp. | Attracts oviposition | [50] |
| Ae. aegypti | Candida pseudoglaebosa | Attracts oviposition | [51] |
| Ae. aegypti | Paenibacillus, Chryseobacterium, Sphingobacterium, Aquitalea, Comamonas | Fecundity | [52] |
| Aedes atropalpus | Comamonas | Fecundity | [52] |
| Anopheles gambiae | Enterobacter (Esp_Z) | Arrests Plasmodium falciparum (ROS) | [66] |
| Ae. aegypti | Serratia marcescens | Enhances dengue virus (SmEnhancin) | [62] |
| An. stephensi | Wickerhamomyces anomalus | Inhibits Plasmodium berghei (toxin) | [106] |
| An. stephensi | Asaia sp. | Activates antimicrobial peptides | [60] |
| An. stephensi | Serratia marcescens strain Y1 | Inhibits P. berghei | [64] |
| Ae. aegypti | Wolbachia (wMelPop) | Inhibits DENV, YFV, CHIKV, ZIKV | [87,88,110] |
| An. stephensi | Wolbachia (wAlbB) | Represses P. falciparum | [111] |
| An. gambiae | Wolbachia (wMelPop and wAlbB) | Inhibits P. falciparum | [96] |
Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; ROS, reactive oxygen species; YFV, yellow fever virus; ZIKV, Zika virus.
The Potential of the Mosquito Microbiota to Reduce Vector Competence
Much progress has been made in the last two decades in developing procedures to reduce the vector competence of mosquitoes (Figure 2).
Figure 2. Recent Progress in the Use of the Microbiota to Reduce Mosquito Competence.
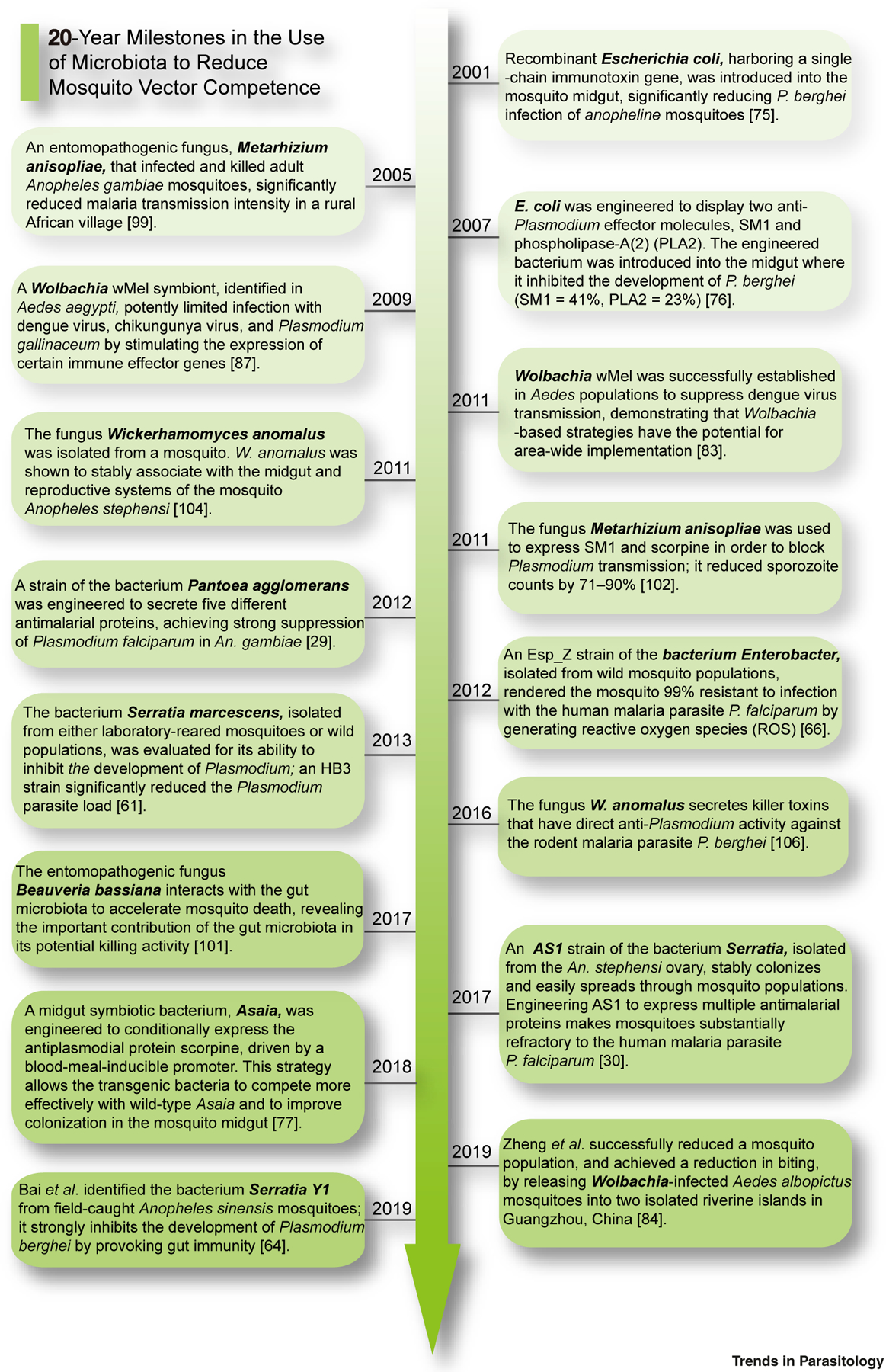
During the last two decades there have been efforts, using various microbes and effectors, to combat different pathogens in specific mosquito species; these studies are summarized in this figure. Certain trends can be seen, including the use of better symbionts, exploring stronger effectors, and designing more efficient expression-delivery methods. See [29,30,61,64,66,75–77,83,84,87,99,101,102,104,106].
Exploring the Natural Gut Microbiota to Reduce Vector Competence
The mosquito gut is a major ‘immunity organ’ that plays an important role in fighting pathogen infections [58]. Bacterial strains isolated from mosquito guts, in both laboratory-reared and field-caught mosquitoes, were evaluated for their potential to control pathogen transmission. Bacteria were able to modify the gut environment and inhibit the development of parasites either by inducing reactive oxygen species (ROS) or modulating the expression of mosquito immune genes. In most cases, the introduction of bacteria inhibits pathogens such as Plasmodium, while removal of gut microbiota with antibiotics increases the susceptibility of mosquitoes to infection [59]. For example, Asaia sp. was reported to activate antimicrobial peptide expression in An. stephensi [60]. The presence of the dominant commensal Enterobacteriaceae positively correlates with Plasmodium infection, indicating that the Enterobacteriaceae play a positive role in Plasmodium falciparum infection [61]. Interestingly, a recent study reported that a Serratia marcescens strain, isolated from a laboratory Ae. aegypti strain, facilitates arboviral infection [62]; this bacterium secretes a protein, named SmEnhancin, which digests gut membrane-bound mucins to enhance viral dissemination in mosquitoes. It is important to note that strain-specific activity exists even between bacteria from the same genera or even species, isolated from the same mosquito species. A previous study showed that different strains of S. marcescens species can induce different outcomes in Plasmodium infections [63]. A more recent study by Bai et al. showed that S. marcescens strain Y1, isolated from the gut of field-caught Anopheles sinensis, inhibits Plasmodium development by modulating the immunity-related Plasmodium effector genes such as TEP1 and FBN9 [64]. Interestingly, in this study, another isolated S. marcescens strain J1 had no Plasmodium-inhibiting effect.
While many bacterial strains have different activities relating to pathogen transmission by the mosquito, exploration of the mechanisms of the mode of action is just getting off the ground. Apart from the immunity-related mechanisms mentioned above, gut bacteria can also directly inhibit pathogen development in mosquitoes via their secretions. ROS, metabolites, small peptides, and proteins secreted by gut bacteria may directly influence the transmission and development of pathogens [65]. When cofeeding P. falciparum with the Enterobacter strain Esp_Z, isolated from wild Anopheles arabiensis mosquitoes, Plasmodium development was arrested by bacteria-generated ROS [66]. Many strains of Serratia spp. secrete serralysin proteins and prodigiosin, which have a pathogen-killing effect in vitro [67,68]. Prodigiosin is also a larvicidal agent against Ae. aegypti and An. stephensi [69]. Furthermore, blood ingestion and digestion unleash abundant nutrients, ions, proteins, heme, and lipids that may pose a strong stress on the gut microbiota, resulting in changes in its composition and activities. These factors should be considered when contemplating the introduction of a bacterial strain into a mosquito.
Another interesting perspective in using gut bacteria to inhibit specific pathogens comes from an understanding of the detailed physiological demands of the pathogens. Recently, Zhu et al. revealed that DENV acquisition by Ae. aegypti was inversely correlated with the iron concentration in serum from human donors [70]. This work implies that alterations in iron concentration can be used to interfere with pathogen transmission. Conversely, although host iron is required for Plasmodium parasite development, excess iron during blood digestion in the mosquito gut may be toxic to this pathogen [71]. Also, the iron compound ferric ammonium citrate (FAC) inhibits infections by many viruses, such as influenza A virus, HIV, Zika virus, and Enterovirus 71 (EV71) [72]. Therefore, gut bacteria manipulating the iron concentration and composition after a blood meal could help to develop new methods to block multiple pathogens in the mosquito. Gut bacteria can also cause pH fluctuation. In humans, gut commensals can prevent pathogen infection by altering host pH [73], and a similar phenomenon was also seen in insects [34]. Interestingly, Plasmodium gametocytes require a defined pH for activation and fertilization in the mosquito gut. Therefore, identifying gut bacteria that modulate gut pH has the potential to lead to the development of new methods to block pathogen transmission.
Engineering the Gut Microbiota to Reduce Vector Competence
Genetic engineering has been used to engineer symbiotic bacteria to produce antipathogen effector molecules (termed paratransgenesis). This approach was first tested to control transmission of Trypanosoma cruzi in 1997, when Durvasula et al. engineered an endosymbiont of Rhodnius prolixus to express Cecropin A, a naturally occurring pore-forming peptide lethal to the parasite [74]. Engineering symbiotic bacteria from the mosquito midgut to produce interfering factors has been explored as a promising way to fight various arthropod-borne human pathogens. In the earlier years, Escherichia coli was used to express either a single-chain immunotoxin, or compounds such as salivary gland and midgut peptide 1 (SM1) or phospholipase-A2 (mostly targeting ookinetes) to block Plasmodium development in the mosquito midgut [75,76]. However, E. coli is not a mosquito symbiont and cannot persist in the mosquito gut. Moreover, using a single effector raises the concern of potential development of resistance by the pathogen. Wang et al. addressed these concerns by engineering a mosquito symbiotic Pantoea agglomerans strain to secrete five different antimalarial proteins at the same time, achieving strong suppression (up to 98%) of Plasmodium development [29]. However, forcing symbiotic microbes to constitutively express effectors may cause fitness cost to the microbe, leading to reduced effectiveness. A recent work by Shane et al. addressed such concern. In this work, the midgut symbiont Asaia was engineered to conditionally express the antiplasmodial protein scorpine, driven by a blood meal-inducible promoter, allowing the transgenic bacteria to compete more effectively with wild-type Asaia and improve gut colonization [77].
A central question in using gut microbiota to reduce vector competence is how to introduce the bacteria, and to ensure their persistence in mosquito field populations. This concern was recently addressed by Wang et al. who identified a new Serratia bacterial strain AS1 isolated from an Anopheles ovary. AS1 can stably colonize the mosquito midgut as well as its reproductive organs, and it can be transmitted vertically (from female to offspring), and horizontally (from males to females). These properties allow its fast and stable spread into mosquito populations. When engineered to express antimalarial compounds, AS1 strongly reduced mosquito competence for transmission of the human malaria parasite P. falciparum [30]. This advance provides a promising tool for driving mosquito pathogen refractoriness into the field.
Selection of proper effectors is key in paratransgenesis. In the fight against the malaria parasite, a variety of compounds for expression by gut bacteria were identified [78]. The repertoire for fighting viral infection of mosquitoes is much more restricted. Antiviral effectors are technically difficult to engineer for achieving a satisfactory expression level and delivery efficiency. Finding new delivery approaches may help to solve this problem in the future. Furthermore, concurrent infections of different pathogens are common in mosquitoes. For example, DENV and CHIKV cocirculation and coinfection in humans are frequent [79,80]; there are also reported cases of the presence of both CHIKV and Plasmodium in affected patients [81]. Therefore, finding ways to combat multiple pathogens at the same time is a desirable goal. In 2018, Yen et al. developed an miRNA-based approach which resulted in a dual-resistance phenotype in mosquitoes to DENV-3 and CHIKV viruses [82]. Perhaps it will be possible to engineer mosquito symbionts to block multiple pathogens in the future.
The Intracellular Bacterium Wolbachia
In addition to the gut microbiota, mosquitoes also harbor microbes in other tissues. Wolbachia is an intracellular bacterium that infects most insect species, including mosquitoes. Wolbachia-mediated CI regulates insect reproduction and has been used to modify or reduce mosquito populations [53]. Population modification was demonstrated in the wild first in Australia in 2011 [83]. More recently, an Ae. albopictus field population was nearly eliminated in Guangzhou, China, by releasing Wolbachia-infected mosquitoes in an area-wide application [84].
Besides restricting mosquito populations, Wolbachia can also affect pathogen transmission in several ways. Wolbachia infection of Ae. aegypti alters blood meal excretion and delays oviposition without affecting trypsin activity [85]. Importantly, Wolbachia can affect viral replication via a combination of competition for host resources and activation of host immunity. In Ae. aegypti, Wolbachia (wMelPop strain) infection induces upregulation of the mosquito’s innate immune response against filarial nematodes and pathogenic bacterial infection [86]. Wolbachia wMel infection of Ae. aegypti has been reported to block mosquito-borne viruses, including DENV, CHIKV, yellow fever virus, and Zika virus, but not WNV [87–90]. Interestingly, the wMelPop strain of Wolbachia significantly reduced the replication of WNV in Ae. aegypti [90].
There are reports that Wolbachia may also facilitate transmission of certain viruses. Surprisingly, Wolbachia wMel was reported to increase the mean and the variance in Ae. aegypti susceptibility to dengue infection when introgressed into Brazil and Vietnam genetic backgrounds [91]. Wolbachia wAlbB strain enhanced WNV infection in Culex tarsalis mosquitoes via downregulating the Toll immune pathway [92]. Recently, a Wolbachia wPip strain was reported to enhance vertical transmission of Cx. pipiens densovirus (CpDV) when bacteria and viruses co-exist in ovaries of Cx. pipiens [93]. There is also a report showing no difference in prevalence of infection and viral load between Wolbachia wFlu-infected and -uninfected Aedes fluviatilis mosquitoes [94]. These bewildering results suggest that strain-specific effects of both the bacteria and vectors also exist in the case of Wolbachia.
It is worth mentioning that malaria-transmitting Anopheles mosquitoes are not usually naturally infected by Wolbachia, but infection can be accomplished in the laboratory [95]. The effects of Wolbachia introduced into Anopheles mosquitoes are not clear-cut and seem to be species-specific. In 2011, Hughes et al. characterized somatic infections of two Wolbachia strains (wMelPop and wAlbB) in An. gambiae. Both significantly inhibited P. falciparum oocyst levels in the mosquito midgut [96]. However, Wolbachia strain wAlbB enhanced An. gambiae infection by the rodent malaria parasite Plasmodium berghei [97]. Since Plasmodium malariae, Plasmodium ovale, Plasmodium knowlesi, and Plasmodium vivax, the four other human malaria parasites, are more closely related to rodent malaria parasites phylogenetically, it raises the possibility that Wolbachia infections would enhance transmission of these parasite species.
Unlike most gut bacteria, the intracellular bacterium Wolbachia cannot presently be engineered for paratransgenesis proposes. However, recently Reveillaud et al. identified a putative 9.2 kb circular plasmid – pWCP – carried by Wolbachia from field-caught Cx. pipiens in France, raising the possibility of future paratransgenesis with Wolbachia [98].
The Use of Fungi for the Control of Pathogen Transmission
Mosquito fungi can also be used to fight pathogen transmission, whether used directly or combined with paratransgenic approaches. Two fungi that can infect and spread in the mosquito population, Beauveria bassiana and Metarhizium anisopliae, have the advantage of being able to survive in the field for months in the form of spores, and of being able to infect various mosquito species [21,99]. These fungi infect mosquitoes through the cuticle and proliferate in the hemolymph. Fungus infection causes progressive mosquito death, so those fungi with high vector virulence (either selected or engineered to achieve this purpose) can be used as biopesticides to control mosquito populations [99]. Recently, it was shown that B. bassiana generated a cross-kingdom microRNA-like RNA (bba-milR1) that attenuates mosquito immunity and accelerates insecticidal action [100]. Interestingly, mosquito fungi can manipulate the gut microbiota to accelerate vector mortality [101]. Moreover, fungi can also be engineered to express pathogen-killing effectors. In 2011, M. anisopliae was engineered to express SM1 and scorpine to block Plasmodium transmission, and reduced sporozoite counts by 71–90%, suggesting that mosquito fungi can be engineered as a powerful weapon for combating malaria [102].
Early studies indicated that the adult mosquito midgut environment is not compatible with fungal survival. The larval midgut may contain a few fungi as symbionts or even pathogens [103]. However, in 2011, the fungus W. anomalus was isolated from the midgut and reproductive system of different mosquito species, suggesting that symbiotic relationships between mosquitoes and fungi can truly exist in the gut [104]. W. anomalus acts via secretion of killer toxins (KTs). KTs have an enzymatic activity with broad-spectrum antimicrobial activities targeting the cell-wall glucan components of bacteria, yeasts, and protozoa [105]. The purified W. anomalus KT WaF17.12 acts against different developmental stages of the rodent malaria parasite P. berghei [106].
In recent years, with the help of high-throughput sequencing approaches, more mosquito midgut fungal communities have been characterized [33]. These studies indicate that some fungi, though in much smaller quantities, can survive the midgut environment and coexist with gut bacteria. These fungi can play important roles. A fungus – Penicillium chrysogenum – isolated from the gut of a field-caught An. gambiae mosquito, renders the mosquito more susceptible to Plasmodium infection via suppression of the mosquito’s innate immune system [33]. More recently, a Talaromyces (Tsp_PR) fungus, isolated from the midgut of Ae. aegypti, was shown to enhance susceptibility to DENV by modulating gut trypsin activity [107]. These observations, together with the species- or strain-specific phenomenon in gut bacteria mentioned above, call for an in-depth study of microbiota–vector–pathogen interactions to find ‘perfect symbionts’ while avoiding the enhancement of other pathogen transmission.
Viruses in Pathogen Transmission Control
Mosquitoes carry many viruses, both mosquito-specific viruses lacking human pathogenicity and viruses pathogenic to humans. The densonucleosis viruses (DNVs) can infect arthropods, including many mosquito species. Infection with DNVs is largely avirulent to mosquitoes [108]. In 2008, a DNV (AgDNV) was shown to infect An. gambiae and spread enhanced green fluorescent protein eGFP reporter gene into the mosquito population [109]. These properties indicate that DNVs can serve as an effective tool for a paratransgenic control strategy.
The use of mosquito-specific viruses to control pathogen transmission was largely conceptual until now. However, mosquito-specific viruses have interesting characteristics. First, mosquito-specific viruses are host-specific. Second, virus infection often results in spreading into mosquito populations. Viruses can have a broad impact on mosquito immunity. They compete with other microbes for nutrition and can even shape midgut bacterial and fungal microbiota [33]. However, their restrictive genome capacity severely restricts the size of potential effector genes and limits the use of viruses for pathogen control.
Concluding Remarks
Driving mosquito refractoriness to pathogens with microbiota has made much progress in the last two decades (Figure 2). Importantly, this strategy is also compatible with current mosquito-control tools (insecticides) and genetically modified mosquitoes. However, several key questions restrict this approach (see Outstanding Questions). Challenges for moving the use of gut microbiota to the field is summarized in Figure 3. A high priority should be given to address regulatory, ethical, and public acceptance issues. The insect gut provides favorable conditions for bacterial conjugation, so the possibility of gene transfer needs to be addressed. An alternative approach is to identify naturally occurring bacteria that naturally produce pathogen-limiting effectors.
Outstanding Questions.
What are the underlying mechanisms by which the gut microbiota blocks pathogen transmission?
What roles does the gut microbiota play in commensalism?
How do some bacteria inhibit one pathogen in a mosquito species while facilitating infection of a different pathogen in a different mosquito vector?
What are the bases for mosquitoes to differentiate the commensal microbiota from pathogenic bacteria? By which mechanisms do mosquitoes support commensal micro-biota and suppress harmful bacteria?
How can we increase the efficiency of microbial-based mosquito-borne disease control while ensuring biosafety?
Figure 3. Prospects for the Use of the Mosquito Microbiota to Reduce Vector Competence.
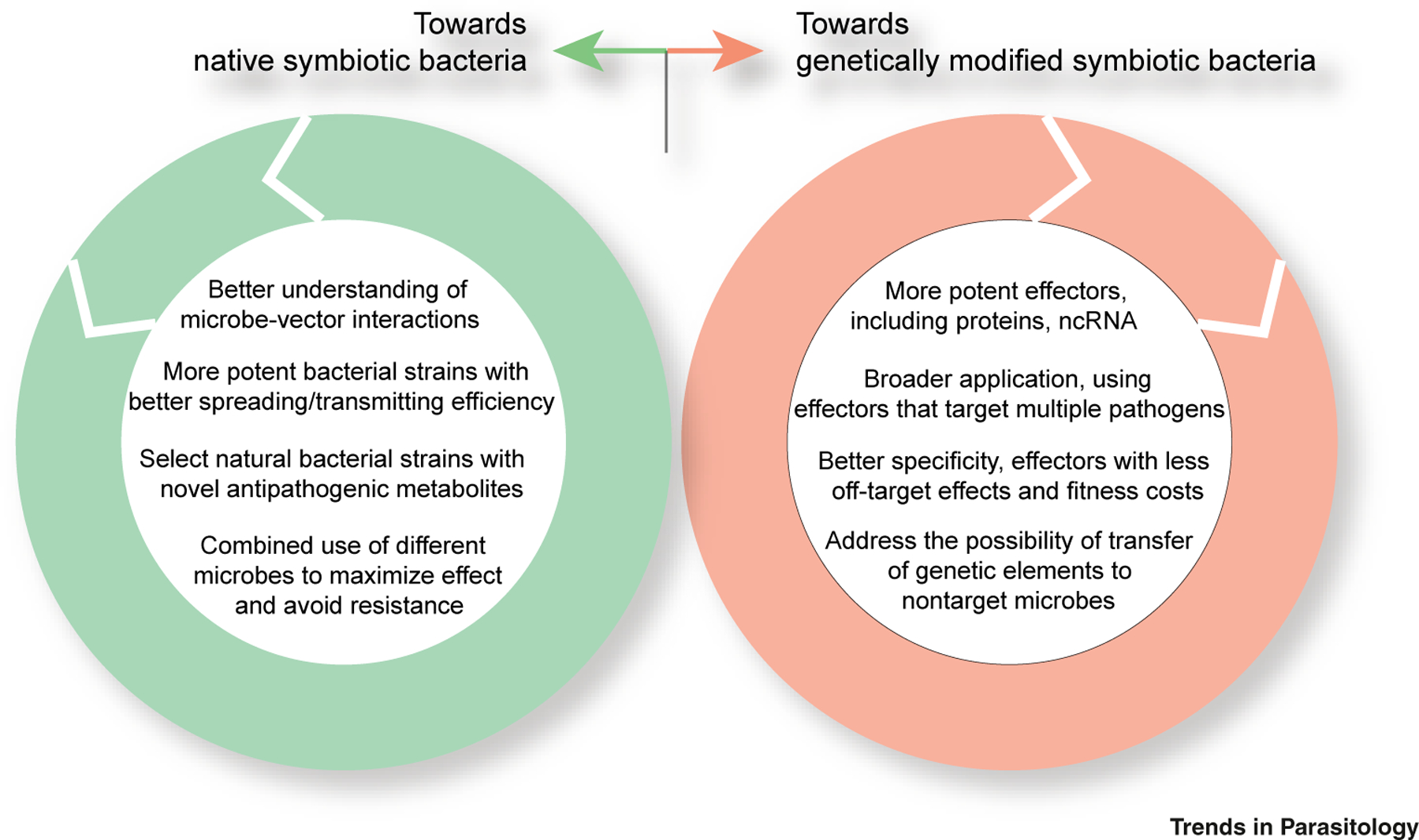
While the two alternative strategies of using native or symbiotic bacteria to reduce mosquito competence address different challenges, they share common concerns such as improving inhibitory efficiency, avoiding fitness cost, and solving ecosafety concerns. Abbreviations: ncRNA, noncoding RNA.
Another consideration relates to the concurrent infections of different pathogens that can occur in mosquitoes. The targeting of one pathogen may facilitate the transmission of another. Thus, utilizing a symbiotic microbe with multipathogen inhibitory activities would be ideally sought. Finally, it is important to keep in mind that no tool is 100% effective. In the fight against mosquito-borne diseases, a ‘magic bullet’ does not exist. Diseases can be controlled only by the coordinated and simultaneous deployment of as many tools as possible.
Highlights.
Mosquitoes are vectors of pathogens that cause many fatal diseases; they harbor a diverse and dynamic microbial flora, including (prokaryotic) bacteria, (eukaryotic) fungi, and viruses.
The microbiota of the mosquito gut plays important roles in host physiology, such as blood digestion, development, fertility, and immunity.
The mosquito microbiota has promising applications for blocking pathogen transmission. Natural or engineered gut microbiota and Wolbachia have demonstrated the capacity to block transmission. Mosquito-pathogenic fungi and Wolbachia can be used to control mosquito populations.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (grants 31830086, 31772534, 31830086, 31472044), the National Key R&D Program of China (2017YFD0200400, 2018YFA0900502), the Strategic Priority Research Program of Chinese Academy of Sciences (grant XDB11010500), the Key Research Program of the Chinese Academy of Sciences (KFZD-SW-219), National Institutes of Health (NIH) grant R01AI031478, and the Bloomberg Philanthropies.
Glossary
- Arbovirus
an arthropod-borne virus that shares a cycle of transmission between vertebrate hosts and hematophagous arthropod vectors.
- Cytoplasmic incompatibility (CI)
CI is manifested by embryonic lethality resulting from Wolbachia-infected males mating with uninfected females, whereas mating between infected males and infected females yields viable progeny. CI promotes Wolbachia spread into mosquito populations.
- Effectors
proteins or noncoding RNAs (ncRNAs), produced by microbes, that block pathogens in vector mosquitoes. The microbes can be either natural or genetically modified.
- Entomopathogenic fungi
fungal pathogens that infect insects, mainly through the cuticle, and proliferate in the hemolymph.
- Intracellular bacteria
bacteria that invade and reside within their host cells. They can induce their uptake by host cells.
- Microbiota
collectively, all the microbes that live in and on the host body; they comprise bacteria, archaea, viruses, fungi, and protozoans. Collectively, their genes are known as the ‘microbiome’. The microbiota may associate with the host in a mutualistic/commensal or opportunistic/parasitic manner.
- Midgut
the main digestive organ of invertebrates which digests a blood meal and assimilates nutrition. The midgut harbors a diverse microbial flora.
- Paratransgenesis
a method used to genetically engineer symbiotic bacteria to produce antipathogen effector molecules.
- Peritrophic matrix (PM)
an extracellular layer, composed of chitin and glycoproteins, that lines the insect intestinal lumen. It is a physical barrier that protects the gut epithelium from the microbiota, pathogens, and blood.
- Plasmodium
a protozoan pathogen and the causal agent of malaria. It infects its mammalian hosts via the bite of anopheline mosquitoes.
- Symbiotic microbiota
microorganisms that colonize the gut of insects and mammals. They are essential to the health of the host and play a role in nutrition, development, metabolism, pathogen resistance, and regulation of immune responses.
- Vector-borne diseases
illnesses caused by pathogens transmitted by blood-feeding arthropods such as mosquitoes, ticks, and fleas.
- Vector competence
refers to the capacity of a vector to transmit pathogens.
- Wolbachia
a Gram-negative, maternally transmitted, endosymbiotic, intracellular bacterium that infects more than half of all arthropod species.
References
- 1.WHO. (2017) Global Vector Control Response (2017–2030), WHO [Google Scholar]
- 2.Ferguson NM (2018) Challenges and opportunities in controlling mosquito-borne infections. Nature 559, 490–497 [DOI] [PubMed] [Google Scholar]
- 3.Ranson H and Lissenden N (2016) Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol 32, 187–196 [DOI] [PubMed] [Google Scholar]
- 4.Dusfour I et al. (2019) Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis 13, e0007615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scolari F et al. (2019) Aedes spp. and their microbiota: a review. Front. Microbiol 10, 2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas AE (2014) The molecular basis of bacterial–insect symbiosis. J. Mol. Biol 426, 3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennison NJ et al. (2014) The mosquito microbiota influences vector competence for human pathogens. Curr. Opin. Insect Sci 3, 6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strand MR (2018) Composition and functional roles of the gut microbiota in mosquitoes. Curr. Opin. Insect Sci 28, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guegan M et al. (2018) The mosquito holobiont: fresh insight into mosquito–microbiota interactions. Microbiome 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caragata EP et al. (2019) Curious entanglements: interactions between mosquitoes, their microbiota, and arboviruses. Curr. Opin. Virol 37, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilke AB and Marrelli MT (2015) Paratransgenesis: a promising new strategy for mosquito vector control. Parasit. Vector 8, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kean J et al. (2015) Fighting arbovirus transmission: natural and engineered control of vector competence in Aedes mosquitoes. Insects 6, 236–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraemer MUG et al. (2019) Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol 4, 854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gendrin M and Christophides GK (2013) The Anopheles mosquito microbiota and their impact on pathogen transmission. In Anopheles Mosquitoes: New Insights into Malaria Vectors (Manguin S ed), pp. 525–548, Intech [Google Scholar]
- 15.Wang S and Jacobs-Lorena M (2017) Chapter 13. Paratransgenesis applications: fighting malaria with engineered mosquito symbiotic bacteria. In Arthropod Vector: Controller of Disease Transmission (vol. 1) (Wikel SK et al. eds), pp. 219–234, Academic Press [Google Scholar]
- 16.Mancini MV et al. (2018) Estimating bacteria diversity in different organs of nine species of mosquito by next generation sequencing. BMC Microbiol 18, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P et al. (2014) Salivary glands harbor more diverse microbial communities than gut in Anopheles culicifacies. Parasit. Vectors 7, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchioffo MT et al. (2015) Dynamics of bacterial community composition in the malaria mosquito’s epithelia. Front. Microbiol 6, 1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sazama EJ et al. (2019) Bacterial endosymbionts are common among, but not necessarily within, insect species. Environ. Entomol 48, 127–133 [DOI] [PubMed] [Google Scholar]
- 20.Jupatanakul N et al. (2014) The insect microbiome modulates vector competence for arboviruses. Viruses 6, 4294–4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bukhari T et al. (2011) Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit. Vectors 4, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst-Jan Scholte BGJK et al. (2004) Entomopathogenic fungi for mosquito control: a review. J. Insect Sci 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricci I et al. (2012) Mosquito/microbiota interactions: from complex relationships to biotechnological perspectives. Curr. Opin. Microbiol 15, 278–284 [DOI] [PubMed] [Google Scholar]
- 24.Romoli O and Gendrin M (2018) The tripartite interactions between the mosquito, its microbiota and Plasmodium. Parasit. Vectors 11, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hörtnagl P et al. (2010) The bacterial community composition of the surface microlayer in a high mountain lake. FEMS Microbiol. Ecol 73, 458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onchuru TO et al. (2016) Chemical parameters and bacterial communities associated with larval habitats of Anopheles, Culex and Aedes mosquitoes (Diptera: Culicidae) in western Kenya. Int. J. Trop. Insect Sci 36, 146–160 [Google Scholar]
- 27.Moll RM et al. (2001) Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J. Med. Entomol 38, 29–32 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y et al. (2011) Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One 6, e24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S et al. (2012) Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl. Acad. Sci. U. S. A 109, 12734–12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S et al. (2017) Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 357, 1399–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zouache K et al. (2012) Chikungunya virus impacts the diversity of symbiotic bacteria in mosquito vector. Mol. Ecol 21, 2297–2309 [DOI] [PubMed] [Google Scholar]
- 32.Villegas LEM et al. (2018) Zika virus infection modulates the bacterial diversity associated with Aedes aegypti as revealed by metagenomic analysis. PLoS One 13, e0190352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muturi EJ et al. (2016) Midgut fungal and bacterial microbiota of Aedes triseriatus and Aedes japonicus shift in response to La Crosse virus infection. Mol. Ecol 25, 4075–4090 [DOI] [PubMed] [Google Scholar]
- 34.Engel P and Moran NA (2013) The gut microbiota of insects – diversity in structure and function. FEMS Microbiol. Rev 37, 699–735 [DOI] [PubMed] [Google Scholar]
- 35.Gaio Ade O et al. (2011) Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (diptera: culicidae) (L.). Parasit. Vectors 4, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minard G et al. (2013) Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. FEMS Microbiol. Ecol 83, 63–73 [DOI] [PubMed] [Google Scholar]
- 37.Sirot LK et al. (2011) Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS Negl. Trop. Dis 5, e989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steyn A et al. (2016) Yeasts associated with Culex pipiens and Culex theileri mosquito larvae and the effect of selected yeast strains on the ontogeny of Culex pipiens. Microb. Ecol 71, 747–760 [DOI] [PubMed] [Google Scholar]
- 39.Turley AP et al. (2009) Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Negl. Trop. Dis 3, e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chouaia B et al. (2012) Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol 12 (Suppl. 1 ), S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coon KL et al. (2014) Mosquitoes rely on their gut microbiota for development. Mol. Ecol 23, 2727–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valzania L et al. (2018) Both living bacteria and eukaryotes in the mosquito gut promote growth of larvae. PLoS Negl. Trop. Dis 12, e0006638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coon KL et al. (2017) Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc. Natl. Acad. Sci. U. S. A 114, E5362–E5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valzania L et al. (2018) Hypoxia-induced transcription factor signaling is essential for larval growth of the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A 115, 457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Correa MA et al. (2018) Generation of axenic Aedes aegypti demonstrate live bacteria are not required for mosquito development. Nat. Commun 9, 4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma A et al. (2013) Gut microbes influence fitness and malaria transmission potential of Asian malaria vector Anopheles stephensi. Acta Trop 128, 41–47 [DOI] [PubMed] [Google Scholar]
- 47.McMeniman CJ et al. (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323, 141–144 [DOI] [PubMed] [Google Scholar]
- 48.Contreras E et al. (2019) A neurotoxin that specifically targets Anopheles mosquitoes. Nat. Commun 10, 2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pike A et al. (2017) Changes in the microbiota cause genetically modified Anopheles to spread in a population. Science 357, 1396–1399 [DOI] [PubMed] [Google Scholar]
- 50.Díaz-Nieto LM et al. (2016) Culex pipiens development is greatly influenced by native bacteria and exogenous yeast. PLoS One 11, e0153133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reeves WK (2004) Oviposition by Aedes aegypti (Diptera: Culicidae) in relation to conspecific larvae infected with internal symbiotes. J. Vector Ecol 29, 159–163 [PubMed] [Google Scholar]
- 52.Coon KL et al. (2016) Gut bacteria differentially affect egg production in the anautogenous mosquito Aedes aegypti and facultatively autogenous mosquito Aedes atropalpus (Diptera: Culicidae). Parasit. Vectors 9, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flores HA and O’Neill SL (2018) Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol 16, 508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheehan KB et al. (2016) Identification and characterization of a candidate Wolbachia pipientis type IV effector that interacts with the actin cytoskeleton. mBio 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beckmann JF et al. (2017) A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol 2, 17007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen H et al. (2019) A Wolbachia nuclease and its binding partner provide a distinct mechanism for cytoplasmic incompatibility. Proc. Natl. Acad. Sci. U. S. A 116, 22314–22321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LePage DP et al. (2017) Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543, 243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saraiva RG et al. (2016) Mosquito gut antiparasitic and antiviral immunity. Dev. Comp. Immunol 64, 53–64 [DOI] [PubMed] [Google Scholar]
- 59.Dong Y et al. (2009) Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5, e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Capone A et al. (2013) Interactions between Asaia, Plasmodium and Anopheles: new insights into mosquito symbiosis and implications in malaria symbiotic control. Parasit. Vectors 6, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boissiere A et al. (2012) Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog 8, e1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu P et al. (2019) A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe 25, 101–112.e5 [DOI] [PubMed] [Google Scholar]
- 63.Bando H et al. (2013) Intra-specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity. Sci. Rep 3, 1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai L et al. (2019) A gut symbiotic bacterium Serratia marcescens renders mosquito resistance to Plasmodium infection through activation of mosquito immune responses. Front. Microbiol 10, 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azambuja P et al. (2005) Gut microbiota and parasite transmission by insect vectors. Trends Parasitol 21, 568–572 [DOI] [PubMed] [Google Scholar]
- 66.Cirimotich CM et al. (2011) Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332, 855–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castro AJ (1967) Antimalarial activity of prodigiosin. Nature 213, 903–904 [DOI] [PubMed] [Google Scholar]
- 68.Welch RA (1991) Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol 5, 521–528 [DOI] [PubMed] [Google Scholar]
- 69.Patil CD et al. (2011) Prodigiosin produced by Serratia marcescens NMCC46 as a mosquito larvicidal agent against Aedes aegypti and Anopheles stephensi. Parasitol. Res 109, 1179–1187 [DOI] [PubMed] [Google Scholar]
- 70.Zhu Y et al. (2019) Host serum iron modulates dengue virus acquisition by mosquitoes. Nat. Microbiol 4, 2405–2415 [DOI] [PubMed] [Google Scholar]
- 71.Portugal S et al. (2011) Superinfection in malaria: Plasmodium shows its iron will. EMBO Rep 12, 1233–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H et al. (2018) Antiviral effects of ferric ammonium citrate. Cell Discov 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamada N et al. (2013) Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol 14, 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Durvasula RV et al. (1997) Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc. Natl. Acad. Sci. U. S. A 94, 3274–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shigeto Yoshida DI et al. (2001) Bacteria expressing single-chain immunotoxin inhibit malaria parasite development in mosquitoes. Mol. Biochem. Parasit 113, 89–96 [DOI] [PubMed] [Google Scholar]
- 76.Riehle MA et al. (2007) Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int. J. Parasitol 37, 595–603 [DOI] [PubMed] [Google Scholar]
- 77.Shane JL et al. (2018) Blood meal-induced inhibition of vector-borne disease by transgenic microbiota. Nat. Commun 9, 4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang S and Jacobs-Lorena M (2013) Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol 31, 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chahar HS et al. (2009) Co-infections with chikungunya virus and dengue virus in Delhi, India. Emerg. Infect. Dis 15, 1077–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chipwaza B et al. (2014) Dengue and Chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl. Trop. Dis 8, e3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baba M et al. (2013) Evidence of arbovirus co-infection in suspected febrile malaria and typhoid patients in Nigeria. J. Infect. Dev. Countries 7, 51–59 [DOI] [PubMed] [Google Scholar]
- 82.Yen PS et al. (2018) Synthetic miRNAs induce dual arboviral-resistance phenotypes in the vector mosquito Aedes aegypti. Commun. Biol 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoffmann AA et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457 [DOI] [PubMed] [Google Scholar]
- 84.Zheng X et al. (2019) Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572, 56–61 [DOI] [PubMed] [Google Scholar]
- 85.Pimenta de Oliveira S et al. (2017) Wolbachia infection in Aedes aegypti mosquitoes alters blood meal excretion and delays oviposition without affecting trypsin activity. Insect Biochem. Molec 87, 65–74 [DOI] [PubMed] [Google Scholar]
- 86.Kambris Z et al. (2009) Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326, 134–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moreira LA et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 [DOI] [PubMed] [Google Scholar]
- 88.van den Hurk AF et al. (2012) Impact of Wolbachia on infection with Chikungunya and Yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis 6, e1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caragata EP et al. (2016) Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microb. Cell 3, 293–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hussain M et al. (2013) Effect of Wolbachia on replication of West Nile virus in a mosquito cell line and adult mosquitoes. J. Virol 87, 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King JG et al. (2018) Variation in Wolbachia effects on Aedes mosquitoes as a determinant of invasiveness and vectorial capacity. Nat. Commun 9, 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dodson BL et al. (2014) Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl. Trop. Dis 8, e2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Altinli M et al. (2018) Sharing cells with Wolbachia: the transovarian vertical transmission of Culex pipiens densovirus. Environ. Microbiol Published online December 25, 2018. 10.1111/1462-2920.14511 [DOI] [PubMed] [Google Scholar]
- 94.Silva JBL et al. (2017) Wolbachia and dengue virus infection in the mosquito Aedes fluviatilis (Diptera: Culicidae). PLoS One 12, e0181678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hughes GL and Rasgon JL (2014) Transinfection: a method to investigate Wolbachia-host interactions and control arthropod-borne disease. Insect Mol. Biol 23, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hughes GL et al. (2011) Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog 7, e1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hughes GL et al. (2012) Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl. Environ. Microbiol 78, 1491–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reveillaud J et al. (2019) The Wolbachia mobilome in Culex pipiens includes a putative plasmid. Nat. Commun 10, 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scholte E-J et al. (2005) An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308, 1641–1642 [DOI] [PubMed] [Google Scholar]
- 100.Cui C et al. (2019) A fungal pathogen deploys a small silencing RNA that attenuates mosquito immunity and facilitates infection. Nat. Commun 10, 4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei G et al. (2017) Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. U. S. A 114, 5994–5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fang W et al. (2011) Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 331, 1074–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tajedin L et al. (2009) Study on fungal flora in the midgut of the larva and adult of the different populations of the malaria vector Anopheles stephensi. Iran J. Arthropod-Bor 3, 36–40 [PMC free article] [PubMed] [Google Scholar]
- 104.Ricci I et al. (2011) The yeast Wickerhamomyces anomalus (Pichia anomala) inhabits the midgut and reproductive system of the Asian malaria vector Anopheles stephensi. Environ. Microbiol 13, 911–921 [DOI] [PubMed] [Google Scholar]
- 105.Walker GM (2011) Pichia anomala: cell physiology and biotechnology relative to other yeasts. Antonie Leeuw. Int. J. G 99, 25–34 [DOI] [PubMed] [Google Scholar]
- 106.Cappelli A et al. (2019) Killer yeasts exert anti-plasmodial activities against the malaria parasite Plasmodium berghei in the vector mosquito Anopheles stephensi and in mice. Parasit. Vectors 12, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anglero-Rodriguez YI et al. (2017) An Aedes aegypti-associated fungus increases susceptibility to dengue virus by modulating gut trypsin activity. eLife 6, e28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Neill SL et al. (1995) Insect densoviruses may be widespread in mosquito cell lines. J. Gen. Virol 76, 2067–2074 [DOI] [PubMed] [Google Scholar]
- 109.Ren X et al. (2008) Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog 4, e1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dutra HL et al. (2016) Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19, 771–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bian G et al. (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340, 748–751 [DOI] [PubMed] [Google Scholar]


