Abstract
BACKGROUND:
Stress-related disorders are among the most prevalent psychiatric disorders, characterized by excess fear and enhanced avoidance of trauma triggers. Elucidating the mechanisms regulating temporally distinct aspects of innate and conditioned fear responses could facilitate novel therapeutic development for stress-related disorders. One potential target that has recently emerged is the endocannabinoid system, which has been reported to mediate the physiological response to stress and represents an important substrate underlying individual differences in stress susceptibility.
METHODS:
Here, we exposed male and female CD-1 mice to an innate predator stressor, 2MT (2-methyl-2-thiazoline), to investigate the ability of endocannabinoid signaling to modulate temporally distinct innate and conditioned fear behaviors.
RESULTS:
We found that 2MT exposure increased amygdala 2-AG (2-arachidonoylglycerol) content and selectively increased excitability in central, but not basolateral, amygdala neurons. We also found that pharmacological 2-AG augmentation during stress exposure exacerbated both acute freezing responses and central amygdala hyperexcitability via cannabinoid receptor type 1– and type 2–dependent mechanisms. Finally, 2-AG augmentation during stress exposure reduced long-term contextual conditioned freezing, and 2-AG augmentation 24 hours after stress exposure reduced conditioned avoidance behavior.
CONCLUSIONS:
Our findings demonstrate a bidirectional effect of 2-AG augmentation on innate and conditioned fear behavior, with enhancement of 2-AG levels during stress promoting innate fear responses but ultimately resulting in long-term conditioned fear reduction. These data could reconcile contradictory data on the role of 2-AG in the regulation of innate and conditioned fear-related behavioral responses.
Stress-related disorders, such as generalized anxiety disorder or posttraumatic stress disorder (PTSD), are among the most prevalent mental health disorders in the world, estimated to affect over 30% of adults in the United States (1,2). Symptoms of these disorders include hyperarousal, excess fear, and enhanced avoidance, even in the absence of immediate threat (3,4). Despite the prevalence of these mental health disorders, current pharmacotherapies are met with heterogeneous outcomes, with a significant portion of patients failing to show symptom improvement or protracted remission (5–9). To guide the development of novel treatment strategies, preclinical studies aimed at elucidating novel signaling systems that regulate distinct aspects of stress-related behavioral adaptations are critical.
The endogenous cannabinoid, or endocannabinoid (eCB), system has emerged as a molecular target for the treatment of mood and stress-related disorders. The eCB system, which consists of cannabinoid receptor type 1 (CB1R), its endogenous ligands AEA (N-arachidonoylethanolamine; also called anandamide) and 2-AG (2-arachidonoylglycerol), and their synthetic and degradative enzymes (10,11), is heavily implicated in the modulation of stress responsivity and conditioned defensive behaviors (12–20). While pharmacological augmentation of AEA signaling has been demonstrated to reduce anxiety-like behaviors in response to acute and chronic stress and facilitate extinction of conditioned fear responses, more recent data have also implicated 2-AG signaling in stress-response physiology and anxiety-like responses (13,18,21–28). For example, genetic or pharmacological inhibition of 2-AG synthesis increases anxiety-like behaviors and worsens behavioral consequences of stress exposure (29–31). In parallel, inhibition of the primary 2-AG degradation enzyme MAGL (monoacylglycerol lipase) has been shown to prevent the negative consequences of stress, promote a stress-resilient phenotype, and reduce freezing to a trauma-associated context (28,32–41). However, the data surrounding the therapeutic potential of 2-AG augmentation are mixed; some studies have reported that pharmacological inhibition or genetic deletion of MAGL results in no effect or even anxiogenesis (27,42). Similarly, enhanced levels of 2-AG have been found to increase conditioned freezing in some models (43,44). These somewhat contradictory data may be partially due to environmental context (low vs. high aversiveness), the interval between stress exposure and behavioral evaluation, and the type of behavior being assessed (active vs. passive).
Here, we used an acute predator odor exposure paradigm to assess the effects of MAGL inhibition on innate and conditioned fear behaviors. Exposure to predator odors or related kairomone-derived compounds, such as 2MT (2-methyl-2-thiazoline), has been shown to induce dose-dependent fear and stress responses, including freezing, avoidance of the odor source, hypothermia, hypometabolism, and increases in corticosterone (45–52). Our data shed new light on the multifaceted behavioral consequences of MAGL inhibition on stress-induced behavioral adaptations and could have implications for ongoing therapeutic development of MAGL inhibitors for stress-related psychiatric disorders.
METHODS AND MATERIALS
Animals
Adult male and female outbred ICR (CD-1) mice were ordered from Envigo at a weight of 30 to 44 g and given at least a 1-week acclimation period in the mouse facilities before behavioral testing.
Drugs
All drugs were administered intraperitoneally (i.p.) 2 hours before initiation of behavioral testing and were injected at the following doses: JZL184, 15 mg/kg (Cayman Chemical); PF3845, 1 mg/kg (Cayman Chemical); DO34, 50 mg/kg (Glixx Laboratories, Inc.); rimonabant, 1 mg/kg (Cayman Chemical); and AM630, 5 mg/kg (Cayman Chemical).
Odor Exposure and Context Testing
For odor exposure, 2MT (40 μL; Tokyo Chemical Industry), butyric acid (38.9 μL; Sigma-Aldrich), or water was pipetted onto a black piece of filter paper taped to the corner of an empty, novel cage. Behavior was recorded and analyzed for 10 minutes using Anymaze behavioral tracking software. For context testing, mice were exposed to 2MT as outlined in the figure legends and then tested for context fear retrieval in a novel cage with filter paper taped to the corner.
eCB Analysis From Brain Tissue
eCBs and related lipids were analyzed in excised brain tissue by liquid chromatography–tandem mass spectrometry analysis. Brain tissue was collected and processed as described in the Supplement, and the resultant samples were analyzed as previously reported (53).
Slice Electrophysiology
Slice electrophysiology experiments were performed as previously described (34).
Statistical Analysis
Statistical analyses were performed as outlined in the figure legends.
Detailed methods and materials are provided in the Supplement, including a table of statistical values (Table S1).
RESULTS
2MT Induced Freezing Behavior Is Associated With Enhanced Amygdala eCB Content
To examine the acute effects of 2MT on fear-related behaviors, we exposed male and female CD-1 mice to 2MT- or water-blotted filter paper (Figure 1A). While 2MT had no effect on total distance traveled (Figure 1B, C), 2MT induced an increase in canonical fear behaviors including freezing time (Figure 1D, E), freezing frequency (Figure 1F), and avoidance of the odor source (Figure 1G–J) in male and female mice (Figure S1). To confirm that these behaviors were specific to predator odor analog, a separate cohort of mice was exposed to the molar equivalent of the pungent odorant butyric acid (Figure S2A). Mice exposed to butyric acid showed no differences in distance traveled (Figure S2B), time spent freezing (Figure S2C), total number of freezing episodes (Figure S2D), or avoidance (Figure S2E, F) relative to water controls. These data support the notion that increases in freezing and avoidance likely reflect increases in fear-like and defensive behaviors because they were only observed in response to predator odor exposure.
Figure 1.
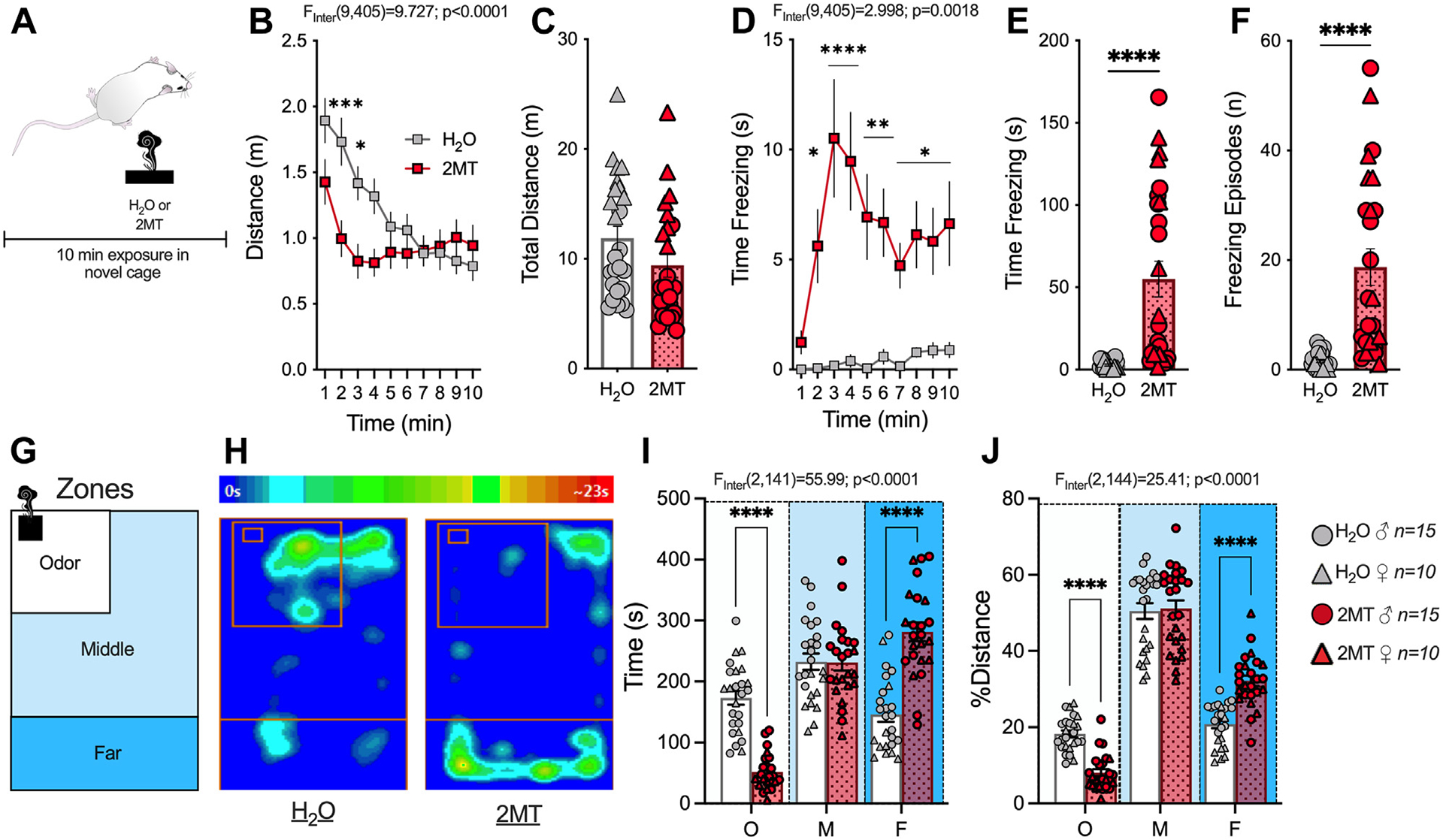
2MT induces freezing to and avoidance of the odor source. (A) Schematic of 2MT (n = 25) or H2O: (n = 25) (no odor control) exposure. Two-way analysis of variance (B, D, I, J) or unpaired t test (C, E, F) performed and effects of interaction shown. (B) 2MT decreases distance traveled during the beginning of the exposure test. (C) 2MT has no effect on total distance traveled. (D) 2MT increases time spent freezing over duration of test. (E) 2MT increases the total time spent freezing during exposure. (F) 2MT increases freezing frequency. (G) Schematic of the zones that the testing apparatus is divided into to assess avoidance of the odor source. (H) Representative heat map of time spent in different zones of mouse exposed to H2O (left) and 2MT (right). (I) 2MT-exposed mice spend significantly less time in the odor zone and more time in the far zone, with no change in the middle zone. (J) 2MT-exposed mice travel significantly less around the odor zone and more in the far zone. *p < .05; **p < .01; ***p < .001; ****p < .0001. 2MT, 2-methyl-2-thiazoline; F, far zone; M, middle zone; O, odor zone.
Given the prominent role of eCB signaling in the regulation of stress-response physiology, we next explored how 2MT affects eCB levels in relevant brain regions. It has been consistently reported that acute and chronic stress exposure results in a short-term increase in 2-AG levels, particularly in the amygdala (AMY) and prefrontal cortex (PFC) (9,12,19,20). Conversely, stress exposure has been reported to reduce AEA levels in these same regions (19,20,54–56). To assess how eCB levels are altered in these brain regions by acute 2MT exposure, we exposed mice to water or 2MT and then immediately collected tissue for liquid chromatography–tandem mass spectrometry analysis (Figure 2A). Within the AMY, we found that 2MT exposure selectively increased levels of 2-AG but not AEA compared with water exposure (Figure 2B–D). We also found no significant changes in the breakdown product of both 2-AG and AEA, arachidonic acid (Figure 2E). To examine the association between AMY eCB levels and the expression of stress coping behaviors, simple linear regressions were performed in both male and female mice (Figure 2F–I). In male mice, AMY 2-AG levels were positively correlated to freezing time but not avoidance (Figure 2F, G); in contrast, AMY AEA levels were found to be positively correlated to avoidance but not freezing (Figure 2H, I). There were no significant associations between AMY 2-AG or AEA and these fear behaviors in female mice (Figure 2F–I).
Figure 2.
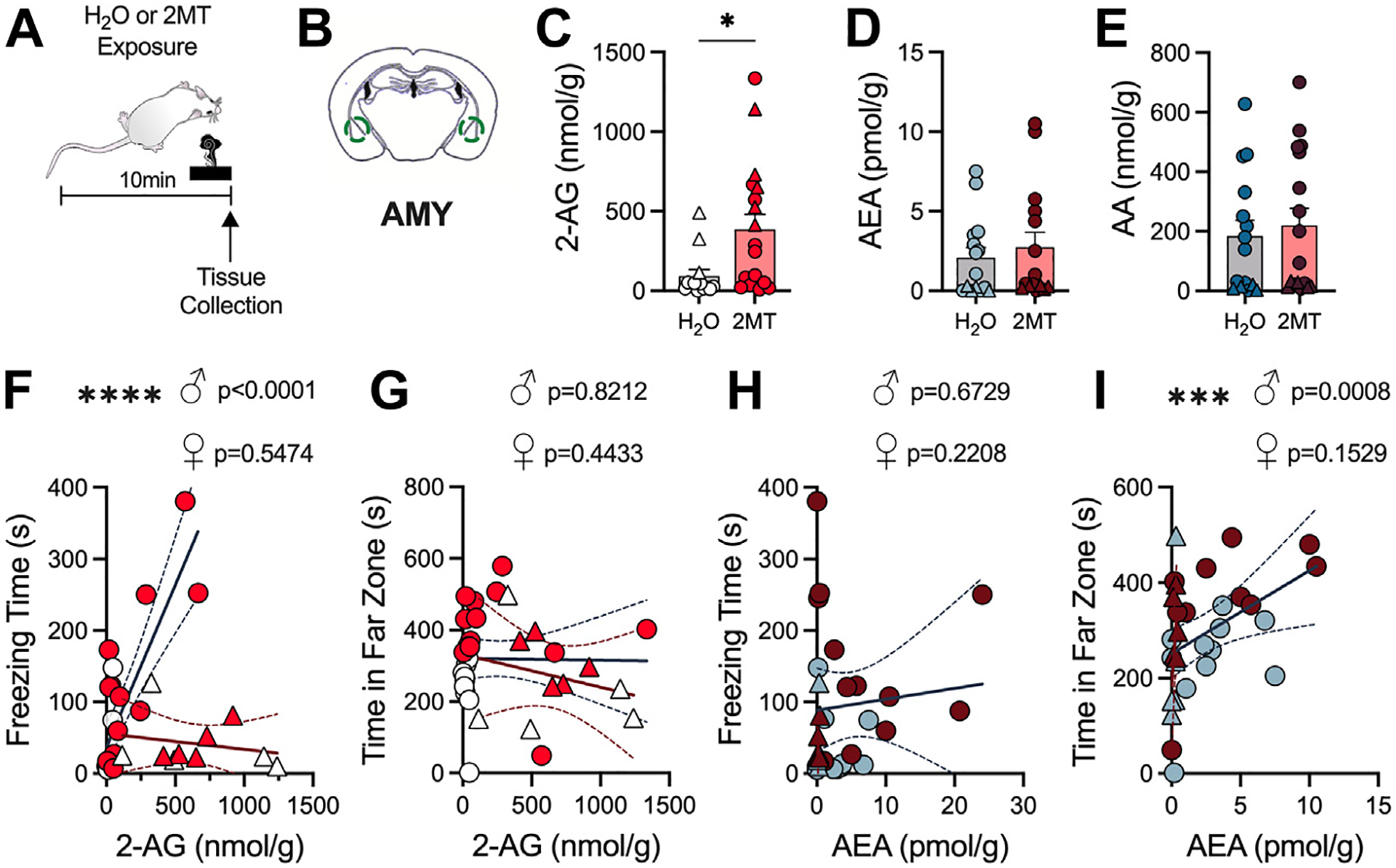
AMY 2-AG levels are increased by 2MT. (A) Experimental timeline for tissue collection used for mass spectrometry. Unpaired, two-tailed t test (C–E) or simple linear regression was performed in males and females (F–I). Circles correspond to males; triangles correspond to females. (B) Schematic of AMY punches. (C) 2MT increases 2-AG levels in the AMY (H2O: n = 13; 2MT: n = 18). (D) 2MT has no effect on AEA content in the AMY (H2O: n = 15; 2MT: n = 15). (E) 2MT has no effect on AA content in AMY (H2O: n = 15; 2MT: n = 17). (F) AMY 2-AG levels positively correlate to freezing time in male mice but not female mice. (G) AMY 2-AG levels are not significantly associated with time spent in the far zone during exposure in male or female mice. (H) AMY AEA levels are not significantly correlated with time spent freezing in either male or female mice. (I) AMY AEA levels significantly correlate with far zone time in male but not female mice. *p < .05; ***p < .001; ****p < .0001. 2-AG, 2-arachidonoylglycerol; 2MT, 2-methyl-2-thiazoline; AA, arachidonic acid; AEA, N-arachidonoylethanolamine; AMY, amygdala.
We further assessed 2MT-induced changes in eCB levels in the PFC and the periaqueductal gray, two brain regions heavily implicated in stress responding (Figure S3). Within the PFC, we found that 2MT decreased 2-AG levels (Figure S3B) but did not significantly alter AEA or arachidonic acid levels (Figure S3C, D). However, PFC eCB content did not correlate with either freezing or avoidance (Figure S3E–H). In the periaqueductal gray, we found no significant changes in 2-AG, AEA, or arachidonic acid (Figure S3I–L) and no significant association of these eCB levels with fear expression (Figure S3M–P). Ultimately, these data demonstrate that 2MT exposure significantly increases 2-AG content in the AMY, and AMY 2-AG and AEA predict passive (freezing) and active (avoidance) fear responding in male mice, respectively.
2-AG Augmentation Exacerbates 2MT-Induced Freezing
We next used behavioral pharmacology to examine the role of eCB signaling in regulating 2MT-induced fear behaviors. It has been proposed that stress-induced elevations in 2-AG serve to counteract increases in avoidance behavior induced by previous stress exposure (12,18,33); we thus hypothesized that augmenting 2-AG levels would also decrease fear behaviors and avoidance during 2MT exposure. Increasing 2-AG levels with the MAGL inhibitor JZL184 (15 mg/kg i.p.), which has previously been shown to significantly enhance brain 2-AG levels (33), increased the total time spent freezing during 2MT exposure in both male and female mice (Figure 3A–D; Figure S4A–D). JZL184 did not alter freezing behavior in mice exposed to water (Figure 3C), demonstrating that MAGL inhibition augments fear expression selectively during stress exposure. The effects of 2-AG augmentation on defensive behaviors were specific to freezing, because MAGL inhibition had no effect on odor avoidance (Figure 3E, F; Figure S4E, F).
Figure 3.
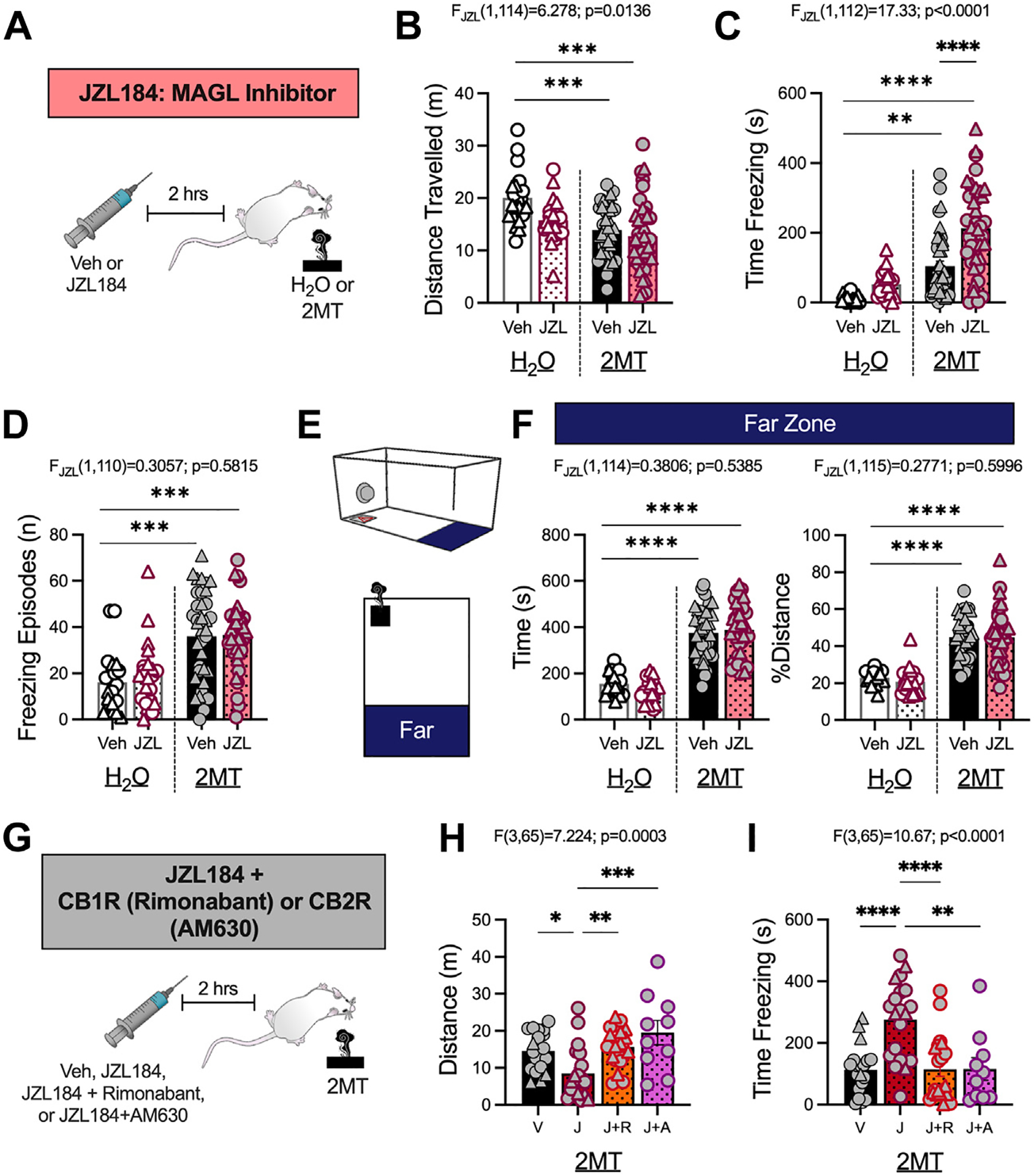
2-AG augmentation with JZL184 enhances 2MT-induced freezing via CB1R and CB2R. (A) Experimental timeline. Male (circles) and female (triangles) mice were administered Veh (DMSO) or JZL184 (15 mg/kg) 2 hours before H2O or 2MT exposure (H2O, Veh: n = 20; H2O, JZL184: n = 21; 2MT, Veh: n = 39; 2MT, JZL184: n = 39). Two-way analysis of variance, JZL184 effect (B–D, F), or one-way analysis of variance (H, I) results shown above graphs. (B) JZL184 has significant effect on total distance traveled, but post hoc analysis reveals no significance between Veh- or JZL184-injected mice. (C) JZL184 significantly increases freezing in 2MT-exposed mice but not H2O-exposed mice. (D) JZL184 has no effect on freezing frequency. (E) Schematic of far zone during exposure. (F) JZL184 has no effect on time (left) or % distance (right) traveled in the far zone. (G) Experimental design for coadministration experiment (Veh: n = 19; JZL184: n = 20; JZL184+rimonabant: n = 20; JZL184+AM630: n = 10). (H) JZL184 decreases distance traveled; this effect is prevented by coadministration with rimonabant or AM630. (I) Coadministration of JZL184 with rimonabant or AM630 prevents JZL184-induced increases in 2MT-driven freezing. *p < .05; **p < .01; ***p < .001; ****p < .0001. 2-AG, arachidonoylglycerol; 2MT, 2-methyl-2-thiazoline; CB1R, CB1 receptor; CB2R, CB2 receptor; MAGL, monoacylglycerol lipase; Veh, vehicle.
To determine whether JZL184 enhancement of 2MT-induced freezing was mediated by the CB1R or CB2R, mice were coadministered JZL184 with either the CB1R inverse agonist rimonabant (1 mg/kg i.p.) (57) or the CB2R antagonist AM630 (5 mg/kg i.p.) (58,59) (Figure 3G). Neither rimonabant nor AM360 affected any behavioral responses when given alone in mice exposed to either water or 2MT (Figure S5), but both rimonabant and AM630 blocked the increase in freezing elicited by JZL184 (Figure 3H, I). These experiments demonstrate that activation of both the CB1R and CB2R is required for the behavioral effects of JZL184.
Based on our data that elevating 2-AG levels increased 2MT-induced freezing, we next tested whether we could decrease 2MT-induced freezing by decreasing 2-AG levels. Mice were administered the 2-AG synthesis enzyme (diacylglycerol lipase) inhibitor DO34 (50 mg/kg i.p.) and exposed to water or 2MT (Figure S6A). DO34 had no significant effect on freezing (Figure S6B–D) or avoidance (Figure S6E, F) in either water- or 2MT-exposed animals, indicating that 2-AG levels cannot bidirectionally control innate fear responses to acute 2MT exposure.
Finally, given that AMY AEA levels were positively correlated to 2MT-induced avoidance behavior, we next examined whether pharmacological AEA augmentation would also affect behavioral responses to 2MT. We increased levels of AEA by inhibiting the AEA degradation enzyme fatty-acid amide hydrolase (Figure S7A). Increasing AEA levels with the fatty-acid amide hydrolase inhibitor PF3845 (1 mg/kg i.p.) had no effect on freezing (Figure S7B–D) or avoidance (Figure S7E, F), demonstrating that although AMY AEA content was correlated with the magnitude of avoidance behavior, facilitation of AEA signaling does not affect 2MT-induced behavioral responses.
2MT-Induced Freezing Is Associated With Increased Central AMY Excitability
Given that 2MT induces changes in 2-AG levels in the AMY but not the PFC or periaqueductal gray, we next assessed the effects of 2MT exposure on specific subnuclei in the AMY. Here, mass spectrometry analysis of 2-AG content in the AMY could not differentiate between distinct subnuclei; therefore, to investigate 2MT-induced changes in specific AMY regions, electrophysiological recordings were performed. Within the AMY, several subnuclei have been implicated in the regulation of fear-related behavior, including the central AMY (CeA) and basolateral AMY (BLA) (60–62). Within the CeA, the lateral subnucleus of the CeA (CeL) has been found to regulate defensive behaviors and appears to be critical for innate fear responding (45,63,64). 2MT also induces upregulation of the immediate early gene c-fos selectively in the CeA, and especially the CeL, but not the BLA (45). Based on these reports, we hypothesized that 2MT would alter CeL neuronal activity (Figure 4; Figures S8 and S9).
Figure 4.
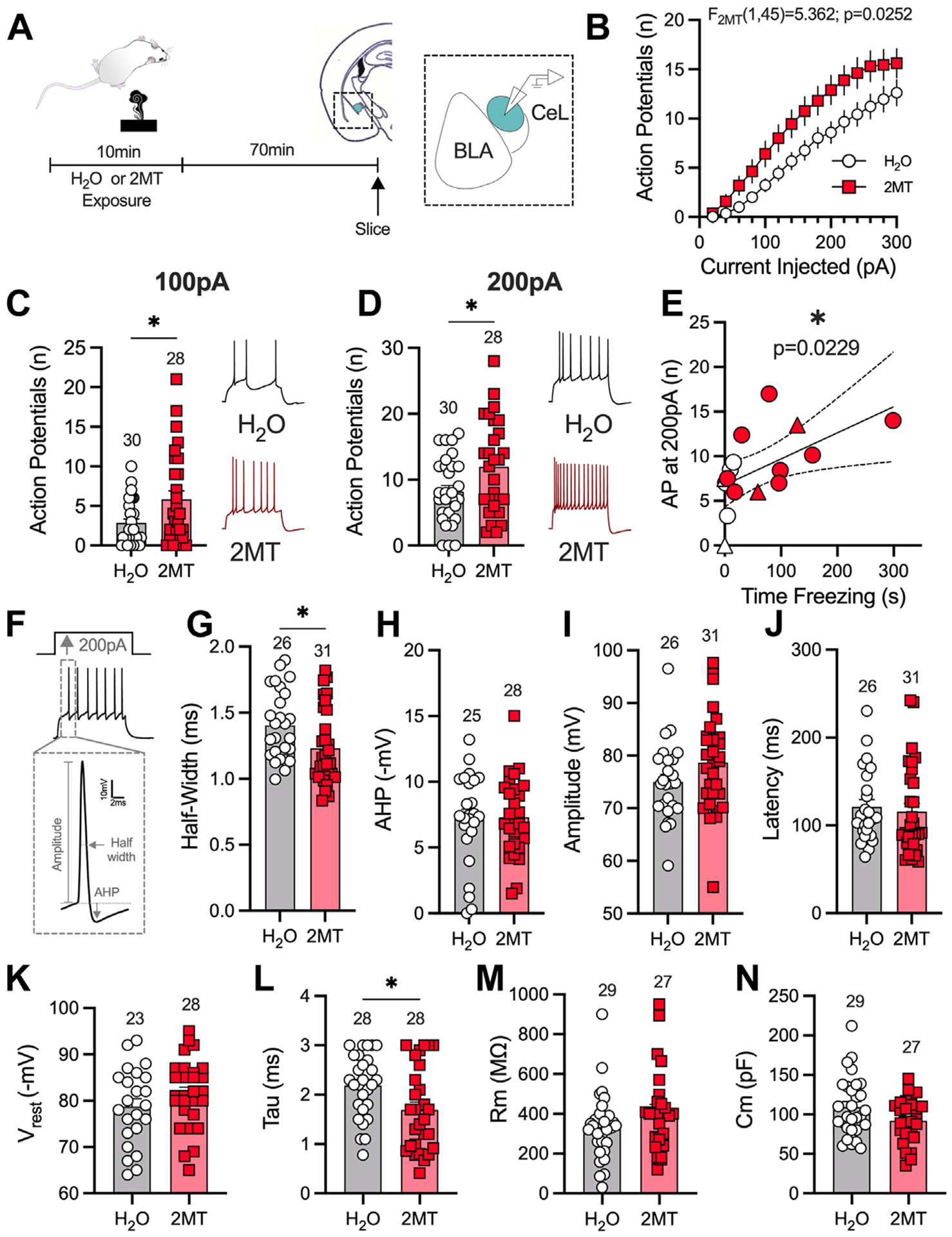
2MT enhances CeL intrinsic excitability. (A) Experimental timeline for slice electrophysiology recordings in the CeL. Analysis by two-way analysis of variance (2MT effect) (B), unpaired, two-tailed t test (C, D; G–N), or linear regression (E) performed. H2O: n = 7; 2MT: n = 7. (B) 2MT significantly enhances intrinsic excitability of the CeL. (C) 2MT enhances the number of action potentials fired per 100 pA of injected current. (D) 2MT increases the number of APs fired at 200-pA current injection. (E) Enhanced CeL excitability is positively correlated to freezing behavior (circles are males, triangles are females). (F) The first AP fired at 200-pA current step was used for analysis. Representative image of analysis of half-width and afterhyperpolarization potential. (G) 2MT decreases the half-width of the first AP fired. (H) 2MT has no effect on AHP. (I) 2MT has no effect on the amplitude of the first AP fired at 200-pA current step. (J) 2MT has no effect on the latency of the first AP fired. (K–N) Passive membrane properties of CeL neurons. (K) 2MT has no effect on resting membrane potential (Vrest). (L) 2MT significantly decreases the membrane constant, tau. (M) 2MT has no effect on the membrane resistance (Rm). (N) 2MT has no effect on membrane capacitance (Cm). *p < .05. 2MT, 2-methyl-2-thiazoline; AHP, afterhyperpolarization potential; AP, action potential; BLA, basolateral amygdala; CeL, lateral subnucleus of the central amygdala.
In the CeL, 2MT exposure resulted in an increase in cell excitability (Figure 4A, B). Specifically, 2MT increased the number of action potentials (APs) fired in response to 100-pA and 200-pA current injections (Figure 4C, D). We found a significant correlation between the amount of time that mice spent freezing during 2MT exposure and CeL excitability (Figure 4E). This enhanced excitability was associated with changes in AP firing kinetics as the half-width of the first AP fired at 200 pA was decreased (Figure 4F, G); there were no differences in afterhyperpolarization following this first AP or the amplitude or latency of the first AP (Figure 4H–J). We also assessed spontaneous excitatory postsynaptic currents (Figure S8A–C) and spontaneous inhibitory postsynaptic currents (Figure S8D, E) after 2MT exposure and found no changes in the amplitude or the frequency of these spontaneous events, suggesting that this 2MT-induced increase in intrinsic excitability was not driven by changes in synaptic transmission. We also found a significant change in passive membrane properties, because 2MT exposure decreased the membrane constant, tau, with no significant effects on resting membrane potential, membrane resistance, or capacitance (Figure 4K–N). 2MT-induced changes in excitability were absent in the BLA (Figure S9A–E), and no effects on passive membrane properties (Figure S9F–I) or synaptic transmission (Figure S9J–M) were detected in the BLA. Together, these data demonstrate that 2MT causes an increase in CeL but not BLA intrinsic excitability and that these changes predict freezing behavior, with greater CeL excitability associated with more freezing.
JZL184 Augments 2MT-Induced Increases in Lateral Subnucleus of the CeA Neuron Excitability
Given that 2MT increases excitability of CeL neurons, which was positively correlated with freezing, we hypothesized that 2-AG augmentation with JZL184, which exacerbates 2MT-induced freezing, would also augment 2MT-induced increases in CeL excitability. To test this hypothesis, mice were administered JZL184 or vehicle in vivo prior to 2MT exposure; slice electrophysiology experiments were then conducted to assess changes in CeL excitability (Figure 5A). In vivo JZL184 administration augmented 2MT-driven neuronal hyperexcitability in the CeL compared with vehicle-injected mice (Figure 5B–D). JZL184 administration to water-exposed mice, which we showed to have no effect on freezing, also had no effect on CeL excitability (Figure S10A–D) or passive membrane properties (Figure S10E–H). We further compared CeL excitability in mice that received injections of JZL184 with the CB1R antagonist rimonabant or the CB2R antagonist AM630, both of which were shown to block JZL184 augmentation of 2MT-induced freezing behavior. Coadministration of JZL184 with either rimonabant or AM630 prevents JZL184-induced increases in CeL excitability (Figure 5B–D). However, we found no significant changes between vehicle- and JZL184-injected mice in either AP firing kinetics (Figure 5E–H) or passive membrane properties (Figure 5I–L). Ultimately, these data further support the CeL as a central locus associated with 2MT-induced freezing; these results also demonstrate that in vivo 2-AG elevation can augment 2MT-induced increases in CeL excitability.
Figure 5.
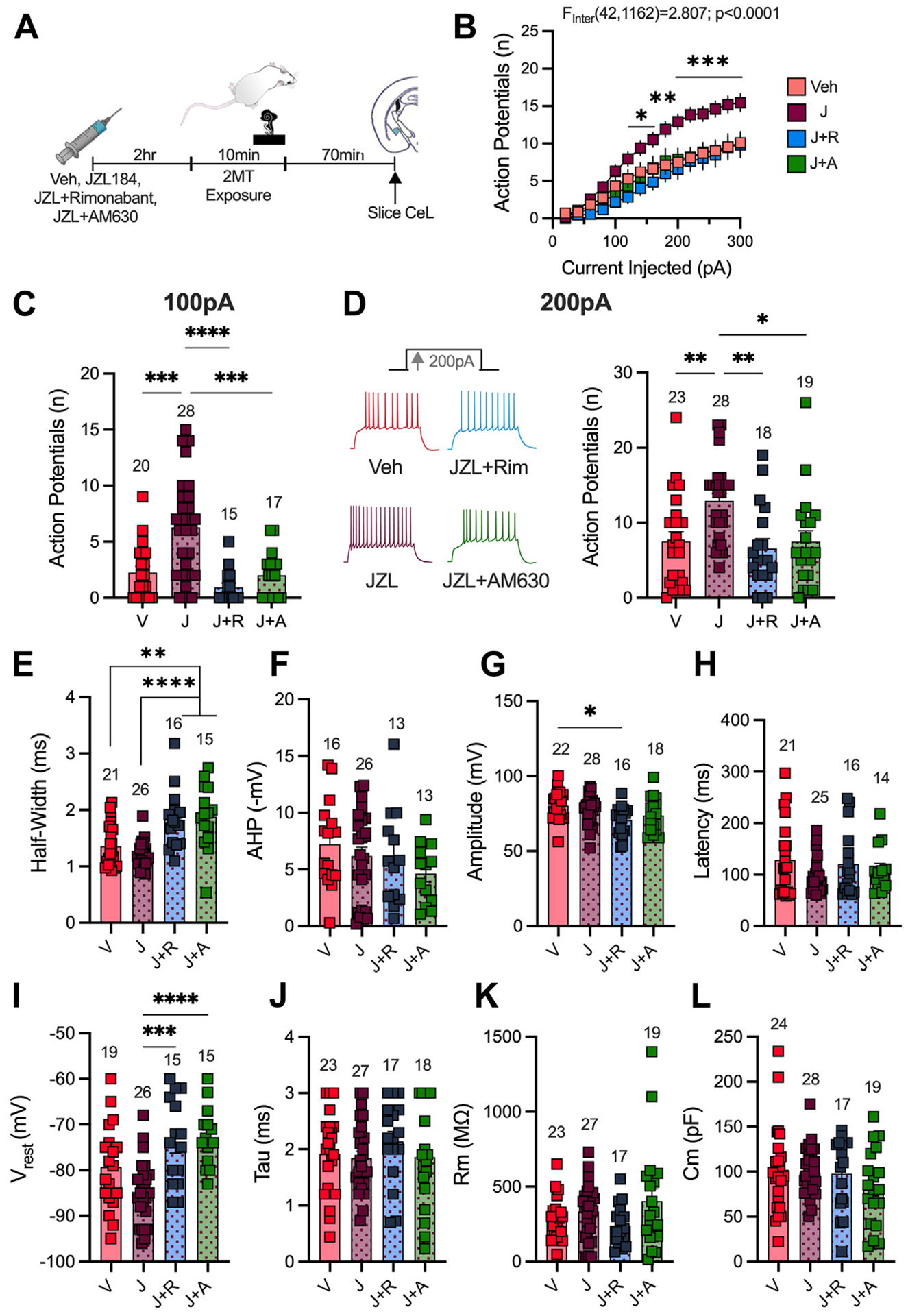
JZL184 augments 2MT-induced increases in CeL excitability. (A) Experimental timeline. Mice are injected with Veh (DMSO), JZL184, JZL184+rimonabant, or JZL184+AM630 2 hours before 2MT exposure. Analysis by two-way analysis of variance (drug × current interaction effect shown) (B) or ordinary one-way analysis of variance (C–L) performed. Veh: n = 7, JZL184: n = 7, J+R: n = 5, J+A: n = 5. (B) JZL184 increases CeL excitability compared with mice administered Veh or coadministered JZL184 with rimonabant or AM630. Significance shown compares JZL184-injected mice to Veh-injected mice. (C) In vivo JZL184 administration enhances excitability at 100-pA injection of current compared with Veh-injected mice or mice coadministered JZL184 with rimonabant or AM630. (D) Representative traces of action potential firing at 200-pA current injection (left). JZL184 enhances excitability per 200-pA injection of current; this effect is blocked by coadministration with rimonabant or AM630. (E) JZL184 has no effect on the half-width of the first action potential fired at 200 pA compared with Veh-injected mice. Coadministration of JZL184 with rimonabant or AM630 enhanced the half-width compared with Veh- and JZL184-injected mice. (F) There is no significant effect of any drug on afterhyperpolarization potential of the first action potential fired at 200 pA. (G) JZL184 administration does not have a significant effect on the amplitude of the first action potential fired at 200 pA compared with Veh-injected mice. Coadministration of JZL184 and rimonabant decreases the amplitude. (H) There is no significant effect of any drug on the latency of the first action potential fired at 200-pA current step. (I–L) Passive membrane properties of CeL neurons. (I) Coadministration of JZL184 with rimonabant or AM630 decreased the resting membrane potential (Vrest) compared with JZL184 administration alone. (J) There are no differences in the membrane constant (tau) of mice injected with either drug. (K) Membrane resistance (Rm) is unaltered by drug administration. (L) Capacitance (Cm) is not changed by drug injection. *p < .05; **p < .01; ***p < .001; ****p < .0001. 2MT, 2-methyl-2-thiazoline; AHP, afterhyperpolarization potential; CeL, lateral subnucleus of the central amygdala; J, JZL184; J+A, JZL184+AM630; J+R, JZL184+rimonabant; V, vehicle; Veh, vehicle.
Administration of JZL184 Results in Mitigation of Contextual Fear Expression
A large volume of preclinical data indicates that 2-AG augmentation can reduce stress-induced avoidance behavior and may have anxiolytic therapeutic potential (12,13,18,32,33). Despite this, some recent reports have indicated that 2-AG augmentation can increase acute fear behavior, including freezing (43,44). In an initial attempt to reconcile this apparent discrepancy, we tested whether 2-AG augmentation could have differential effects depending on the time of treatment, type of freezing being examined (i.e., innate vs. conditioned), or type of stress coping being assessed (i.e., active [avoidance] vs. passive [freezing]).
First, mice were administered JZL184 prior to 2MT exposure and, 2 to 6 days later, were tested for contextual fear expression by placing mice back into the cage environment previously associated with 2MT exposure (Figure 6A). Mice that were given JZL184 during 2MT exposure exhibited decreased freezing time and decreased number of freezing episodes during context fear recall (Figure 6B–D) with no effect on avoidance (Figure 6E, F). These data demonstrate that 2-AG augmentation, which we showed enhanced acute innate freezing to 2MT, can also promote a long-term alleviation of contextually dependent freezing behaviors several days later.
Figure 6.
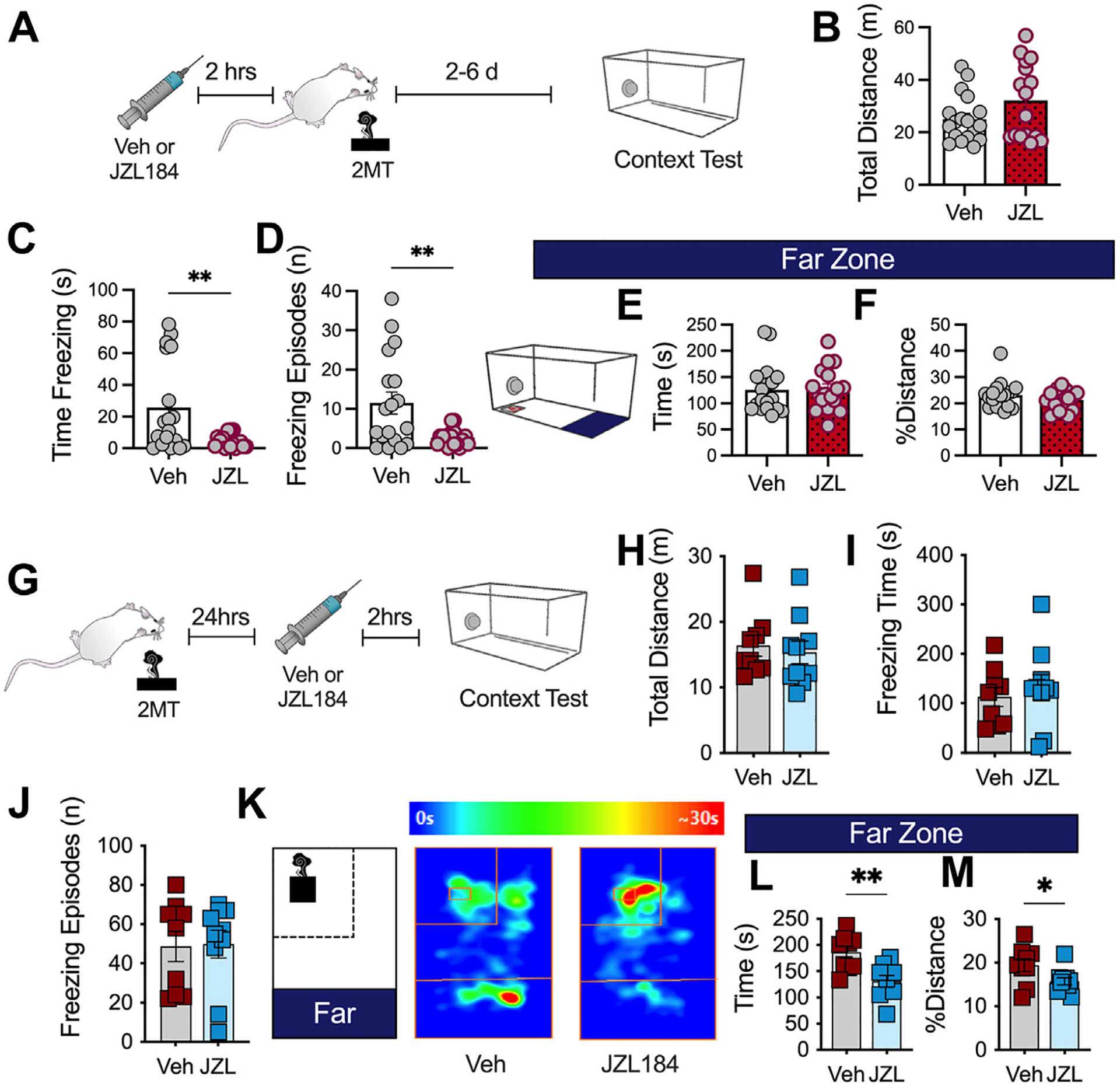
JZL184 results in long-term alleviation of conditioned fear responses. (A) Timeline of experiment. Mice received Veh or JZL184 injections 2 hours before 2MT exposure. Mice were tested for contextual fear expression 2 to 6 days later (Veh: n = 18; JZL184: n = 17). Unpaired, two-tailed t test performed for all analyses. (B) JZL184 has no effect on total distance traveled. (C) JZL184 during 2MT exposure decreases conditioned freezing. (D) JZL184 decreases the total number of conditioned freezing episodes. (E) JZL184 has no effect on time spent in the far zone. (F) JZL184 has no effect on % distance spent in the far zone. (G) Schematic of experimental timeline. All mice were exposed to 2MT. Mice were administered Veh or JZL184. One day later, they were tested for contextual fear expression (Veh: n = 9; JZL184: n = 10). (H) JZL184 before context testing has no effect on total distance traveled. (I) JZL184 has no effect on conditioned freezing during context test. (J) JZL184 does not alter the total number of freezing episodes. (K) Schematic of zones and representative heat map of Veh-treated (left) and JZL184-treated (right) animal, demonstrating that JZL184 administration before context testing decreases conditioned avoidance. (L) JZL184 decreases time spent in the far zone. (M) JZL184 decreases % distance traveled in the far zone. *p < .05; **p < .01. 2MT, 2-methyl-2-thiazoline; Veh, vehicle.
Furthermore, it has been shown that 2-AG augmentation can mitigate or alleviate stress effects on anxiety-like behaviors (32,33,36–40). For example, JZL184 administered after TMT exposure has been shown to decrease anxiety-like behavior in the elevated plus maze (35,59); similarly, augmenting 2-AG levels after stress has also been shown to alleviate the negative consequences of restraint stress in the light-dark box (33). To determine the effects of MAGL inhibition after stress exposure on conditioned fear expression, we exposed all mice to 2MT without drug treatment. Mice were administered vehicle or JZL184 24 hours later and tested for contextually conditioned fear responses (Figure 6G). As expected, 2-AG augmentation after stress exposure decreased conditioned avoidance of the filter paper (Figure 6H–M).
Taken together, these data indicate that while 2-AG elevation during predator odor exposure increases innate freezing behaviors, subsequent conditioned freezing appears to be significantly mitigated. Furthermore, 2-AG augmentation can reduce conditioned avoidance behavior after 2MT exposure, consistent with established literature (28,41). These data reveal a complex and multidimensional behavioral effect of MAGL inhibition on distinct aspects of fear responses (innate vs. conditioned and freezing vs. avoidance), which are dependent on the timing of MAGL inhibition relative to stress exposure.
DISCUSSION
Stress plays a prominent role in the development and exacerbation of most psychiatric disorders, including major depression, general anxiety disorder, and PTSD. Elucidating the mechanisms by which stress exposure can affect brain structure and function could reveal novel approaches to mitigate the effects of stress on mental health outcomes. Over the past decade, eCB signaling has emerged as a key neuro-modulatory system regulating stress responsivity, and components of this system serve as ongoing targets for novel therapeutics development (9,12,13). One such target is the 2-AG degradation enzyme MAGL, which tightly regulates 2-AG signaling in the brain (11). Indeed, pharmacological inhibition of MAGL elevates 2-AG levels and enhances 2-AG–mediated eCB signaling at central synapses (33,40). Moreover, MAGL inhibition has been shown to reduce anxiety-like behaviors and stress responses in preclinical models, while 2-AG depletion increases anxiety-like and fear-related behaviors and physiological stress responses in rodents (12,23,29–40,65). Despite these compelling data implicating 2-AG as an antistress and anxiolytic signaling system, some contradictory data demonstrating that MAGL inhibition can enhance fear responding have been reported (27,28,41–44,66). Here, we shed light on the multidimensional effects of MAGL inhibition on acute fear, which we suggest depends on the timing of treatment relative to stress exposure and the imminence of threat.
We showed that 2MT exposure increases 2-AG levels in the AMY, an area heavily linked to the processing of fear (60,62). Other forms of acute and chronic homotypic stress have also been demonstrated to increase AMY 2-AG levels (18–20,54–56,67–72). We further show that these AMY 2-AG levels positively correlate with passive fear coping (freezing) in male mice, in line with previous results indicating that AMY 2-AG levels positively correlate with anxiety-like behaviors (33). However, this correlation was absent in female mice, suggesting potential sex differences in 2MT-induced mobilization of eCB signaling. While other studies have reported sex-dependent effects of predator odor-induced eCB content in different subnuclei of the AMY, our tissue collection procedure did not discriminate between the BLA and the CeA (73). Future studies will need to be performed to explicitly examine sex effects on predator odor eCB production and correlations with fear responding.
In our model of acute predator odor exposure, we demonstrate that 2-AG augmentation increases innate freezing responses without overtly affecting avoidance behaviors. Increases in freezing behavior to threat-predictive auditory cues and increased flight behavior in response to robo-beetle exposure have also been reported with MAGL inhibition (28,41,43,44,66). While published studies to date have examined the role of CB1R and CB2R in MAGL inhibition effects on protracted avoidance behavior induced by stress, our studies focused on the acute innate defensive responses induced by predator odor exposure (33,59). We found that both CB1R and CB2R are required for the enhanced freezing observed after MAGL inhibition during 2MT exposure. Previous studies have implicated CB1R, and to a lesser degree CB2R, in the anxiolytic effects of MAGL inhibition, but a role for CB2R in the promotion of fear responses has not been demonstrated (33,59). In fact, some previous studies have shown that CB2R activation or overexpression has anxiolytic effects in mice (74–76). Future studies using convergent genetic and pharmacological approaches will be required to confirm our observations and evaluate any potential therapeutic implications of CB2R antagonists for stress-related disorders.
Our electrophysiological studies revealed a potential mechanism through which 2MT and MAGL inhibition alter behavioral responding, specifically by increasing excitability of CeL neurons. While both CeL and BLA subnuclei are involved in fear processing, our data is consistent with previous reports implicating the CeL but not the BLA in the behavioral responses to predator odor exposure (45). These effects were independent of changes in synaptic transmission and could rely on changes in postsynaptic, voltage-gated potassium channel expression or function (77–79). Furthermore, in vivo administration of JZL184 augmented 2MT-incued increases in CeL excitability, which we show relies on CB1R and CB2R activation, paralleling our behavior data. However, it remains to be determined how 2-AG augmentation alters cellular excitability. One potential mechanism relies on 2-AG’s ability to inhibit A-type potassium channels, which in turn can increase the AP firing rate (80); however, future studies will need to explicitly test this hypothesis. Furthermore, the firing rate of CeL neurons was positively correlated with freezing behavior, suggesting the possibility that changes in CeL activity could underlie the increase in freezing observed after MAGL inhibition during 2MT exposure. Use of in vivo neuronal activity modulation approaches could be used to test this hypothesis.
While mice that are treated with the MAGL inhibitor JZL184 during 2MT exposure display enhanced freezing, we further demonstrate that MAGL inhibition can reduce active or passive stress coping under conditions of moderate environmental aversiveness depending on the timing of JZL184 administration; enhancement of 2-AG signaling during odor presentation results in long-term alleviation of passive conditioned fear expression (freezing), but 2-AG enhancement after odor exposure reduces active conditioned stress responding (avoidance). These divergent effects of JZL184 may rely on differential activation of CB1R on glutamatergic versus GABAergic (gamma-aminobutyric acidergic) neurons. For example, it has been shown that CB1R deletion selectively from glutamatergic neurons results in more passive stress coping and enhanced freezing behavior, while deletion from GABAergic neurons promotes active behaviors and decreased freezing (81). Thus, during conditions of imminent threat when 2-AG levels are increased, such as during 2MT presentation (Figure 2), further augmentation of 2-AG may result in activation of CB1R on GABA neurons, promoting freezing. However, when 2-AG is augmented during periods of moderate environmental aversiveness (context re-exposure), CB1R activity on glutamatergic neurons may promote decreases in active coping behaviors, such as escape or avoidance, consistent with a large volume of published work that MAGL inhibition can reduce basal and stress-induced avoidance behaviors (22,24,27,32,33,35,37,40).
Mice that receive JZL184 during odor presentation exhibit reduced contextual freezing behavior when returned to the odor-paired context 2 to 6 days later. While the mechanisms accounting for this phenomenon are not known, one intriguing possibility relates to the ability of MAGL inhibition to prolong stress-induced corticosterone release (25,82). Corticosterone augmentation after acute stress has been shown to reduce stress-induced avoidance behavior (83). Thus, it is possible that MAGL inhibition–induced augmentation of corticosterone release during stress may be required for reductions in freezing behavior observed 2 to 6 days later. It also remains possible that 2-AG augmentation affects memory acquisition and consolidation; numerous studies have demonstrated that activation of the eCB system, either directly by CB1R agonism or indirectly through MAGL inhibition, can impair learning and memory processes (84–89). Ultimately, future studies will be needed to examine the mechanism for the long-term effects of 2-AG augmentation on contextual fear expression.
Finally, we report that decreasing 2-AG levels via pharmacological inhibition of diacylglycerol lipase was unable to affect acute fear responses to 2MT exposure. These data are surprising given previous work demonstrating that diacylglycerol lipase inhibition can increase fear and anxiety-like responses in rodents (29–31). The reasons for these results are not clear at this time but could be an important area of future investigation.
In summary, our data provide advancing insight into the therapeutic potential of 2-AG augmentation in the regulation of conditioned and unconditioned fear behaviors, with MAGL inhibition promoting freezing behaviors during imminent threat but ultimately reducing conditioned passive and active fear behaviors when the threat is no longer present. Mounting literature suggests that psychiatric disorders are associated with alterations in eCB levels; for example, some studies have revealed that patients with PTSD have reduced levels of circulating 2-AG (90,91). While speculative, our data combined with previous work suggest that this deficiency (if associated with decreased brain 2-AG signaling) could contribute to persistent avoidance and conditioned fear behaviors after trauma exposure and that 2-AG augmentation after trauma could represent a novel approach to the treatment of several dimensions of PTSD symptomology.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | JZL184 | Cayman Chemical | CAS number: 1101854-58-3 | |
| PF3845 | Cayman Chemical | CAS number: 1196109-52-0 | ||
| DO34 | Cayman Chemical | CAS number: 1848233-58-8 | ||
| Rimonabant | Cayman Chemical | CAS number: 168273-06-1 | ||
| AM630 | Cayman Chemical | CAS number: 164178-33-0 | ||
| 2-methyl-2-thiazoline [2-methylthiazoline] (2MT) | Tokyo Chemical Industry | CAS number: 2346-00-1 | ||
| Butyric Acid | Sigma-Aldrich | B103500 | ||
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | ICR (CD-1) outbred mice males and females | Envigo | Hsd:ICR (CD-1) | |
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | ||||
| Transfected Construct | ||||
| Other |
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported by the National Institutes of Health (Grant Nos. MH107435 and MH119817 [to SP]).
SP is a scientific consultant for Psy Therapeutics, Janssen, and Jazz Pharmaceuticals. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2022.05.012.
REFERENCES
- 1.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. (1994): Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National comorbidity Survey. Arch Gen Psychiatry 51:8–19. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU (2012): Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calhoon GG, Tye KM (2015): Resolving the neural circuits of anxiety. Nat Neurosci 18:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, Washington, DC: American Psychiatric Publishing. [Google Scholar]
- 5.Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, Figueira I (2009): Pharmacologic alternatives to antidepressants in post-traumatic stress disorder: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry 33:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW (2016): Psychotherapy versus pharmacotherapy for post-traumatic stress disorder: Systemic review and meta-analyses to determine first-line treatments. Depress Anxiety 33:792–806. [DOI] [PubMed] [Google Scholar]
- 7.Stein DJ, Ipser JC, Seedat S (2006): Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006: CD002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koen N, Stein DJ (2011): Pharmacotherapy of anxiety disorders: A critical review. Dialogues Clin Neurosci 13:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondev V, Winters N, Patel S (2021): Cannabis use and post-traumatic stress disorder comorbidity: Epidemiology, biology and the potential for novel treatment approaches. Int Rev Neurobiol 157:143–193. [DOI] [PubMed] [Google Scholar]
- 10.Blankman JL, Cravatt BF (2013): Chemical probes of endocannabinoid metabolism. Pharmacol Rev 65:849–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M (2009): Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89:309–380. [DOI] [PubMed] [Google Scholar]
- 12.Bedse G, Hill MN, Patel S (2020): 2-Arachidonoylglycerol modulation of anxiety and stress adaptation: From grass roots to novel therapeutics. Biol Psychiatry 88:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill MN, Campolongo P, Yehuda R, Patel S (2018): Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology 43:80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ney LJ, Matthews A, Bruno R, Felmingham KL (2019): Cannabinoid interventions for PTSD: Where to next? Prog Neuropsychopharmacol Biol Psychiatry 93:124–140. [DOI] [PubMed] [Google Scholar]
- 15.Dow-Edwards D, Silva L (2017): Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res 1654:157–164. [DOI] [PubMed] [Google Scholar]
- 16.Riebe CJ, Wotjak CT (2011): Endocannabinoids and stress. Stress 14:384–397. [DOI] [PubMed] [Google Scholar]
- 17.Morena M, Campolongo P (2014): The endocannabinoid system: An emotional buffer in the modulation of memory function. Neurobiol Learn Mem 112:30–43. [DOI] [PubMed] [Google Scholar]
- 18.Morena M, Patel S, Bains JS, Hill MN (2016): Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41:80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS (2010): Functional interactions between stress and the endocannabinoid system: From synaptic signaling to behavioral output. J Neurosci 30:14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill MN, Tasker JG (2012): Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience 204:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A (2013): Amygdala FAAH and anandamide: Mediating protection and recovery from stress. Trends Pharmacol Sci 34:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel S, Hill MN, Cheer JF, Wotjak CT, Holmes A (2017): The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev 76:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH (2011): Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav 98:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aliczki M, Balogh Z, Tulogdi A, Haller J (2012): The temporal dynamics of the effects of monoacylglycerol lipase blockade on locomotion, anxiety, and body temperature. Behav Pharmacol 23:348–357. [DOI] [PubMed] [Google Scholar]
- 25.Aliczki M, Zelena D, Mikics E, Varga ZK, Pinter O, Bakos NV, et al. (2013): Monoacylglycerol lipase inhibition-induced changes in plasma corticosterone levels, anxiety and locomotor activity in male CD1 mice. Horm Behav 63:752–758. [DOI] [PubMed] [Google Scholar]
- 26.Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, et al. (2003): Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81. [DOI] [PubMed] [Google Scholar]
- 27.Sciolino NR, Zhou W, Hohmann AG (2011): Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res 64:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morena M, Berardi A, Colucci P, Palmery M, Trezza V, Hill MN, Campolongo P (2018): Enhancing endocannabinoid neurotransmission augments the efficacy of extinction training and ameliorates traumatic stress-induced behavioral alterations in rats. Neuropsychopharmacology 43:1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, et al. (2016): Anxiety, stress, and fear response in mice with reduced endocannabinoid levels. Biol Psychiatry 79:858–868. [DOI] [PubMed] [Google Scholar]
- 30.Shonesy BC, Bluett RJ, Ramikie TS, Báldi R, Hermanson DJ, Kingsley PJ, et al. (2014): Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep 9:1644–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavener VS, Gaulden A, Pennipede D, Jagasia P, Uddin J, Marnett LJ, Patel S (2018): Inhibition of diacylglycerol lipase impairs fear extinction in mice. Front Neurosci 12:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedse G, Bluett RJ, Patrick TA, Romness NK, Gaulden AD, Kingsley PJ, et al. (2018): Therapeutic endocannabinoid augmentation for mood and anxiety disorders: Comparative profiling of FAAH, MAGL and dual inhibitors. Transl Psychiatry 8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bedse G, Hartley ND, Neale E, Gaulden AD, Patrick TA, Kingsley PJ, et al. (2017): Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biol Psychiatry 82:488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bluett RJ, Báldi R, Haymer A, Gaulden AD, Hartley ND, Parrish WP, et al. (2017): Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun 8:14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim J, Igarashi M, Jung KM, Butini S, Campiani G, Piomelli D (2016): Endocannabinoid modulation of predator stress-induced long-term anxiety in rats. Neuropsychopharmacology 41:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosch-Bouju C, Larrieu T, Linders L, Manzoni OJ, Layé S (2016): Endocannabinoid-mediated plasticity in nucleus accumbens controls vulnerability to anxiety after social defeat stress. Cell Rep 16:1237–1242. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Wang W, Zhong P, Liu SJ, Long JZ, Zhao L, et al. (2015): Blockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticity. Hippocampus 25:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, et al. (2014): Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology 39:1763–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Gu N, Duan T, Kesner P, Blaskovits F, Liu J, et al. (2017): Monoacylglycerol lipase inhibitors produce pro- or antidepressant responses via hippocampal CA1 GABAergic synapses. Mol Psychiatry 22:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumislawski JJ, Ramikie TS, Patel S (2011): Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: A potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology 36:2750–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balogh Z, Szente L, Biro L, Varga ZK, Haller J, Aliczki M (2019): Endocannabinoid interactions in the regulation of acquisition of contextual conditioned fear. Prog Neuropsychopharmacol Biol Psychiatry 90:84–91. [DOI] [PubMed] [Google Scholar]
- 42.Imperatore R, Morello G, Luongo L, Taschler U, Romano R, De Gregorio D, et al. (2015): Genetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor CB1R signaling and anxiety-like behavior. J Neurochem 135:799–813. [DOI] [PubMed] [Google Scholar]
- 43.Llorente-Berzal A, Terzian ALB, di Marzo V, Micale V, Viveros MP, Wotjak CT (2015): 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology (Berl) 232:2811–2825. [DOI] [PubMed] [Google Scholar]
- 44.Hartley ND, Gunduz-Cinar O, Halladay L, Bukalo O, Holmes A, Patel S (2016): 2-arachidonoylglycerol signaling impairs short-term fear extinction. Transl Psychiatry 6:e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isosaka T, Matsuo T, Yamaguchi T, Funabiki K, Nakanishi S, Kobayakawa R, Kobayakawa K (2015): Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell 163:1153–1164. [DOI] [PubMed] [Google Scholar]
- 46.Matsuo T, Isosaka T, Tang L, Soga T, Kobayakawa R, Kobayakawa K (2021): Artificial hibernation/life-protective state induced by thiazoline-related innate fear odors. Commun Biol 4:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace KJ, Rosen JB (2000): Predator odor as an unconditioned fear stimulus in rats: Elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci 114:912–922. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi LK, Nakashima BR, Hong H, Watanabe K (2005): The smell of danger: A behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev 29:1157–1167. [DOI] [PubMed] [Google Scholar]
- 49.Endres T, Apfelbach R, Fendt M (2005): Behavioral changes induced in rats by exposure to trimethylthiazoline, a component of fox odor. Behav Neurosci 119:1004–1010. [DOI] [PubMed] [Google Scholar]
- 50.Rosen JB, Asok A, Chakraborty T (2015): The smell of fear: Innate threat of 2,5-dihydro-2,4,5-trimethylthiazoline, a single molecule component of a predator odor. Front Neurosci 9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen JB, Pagani JH, Rolla KLG, Davis C (2008): Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: A model for animal phobias. Neurosci Biobehav Rev 32:1267–1276. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Cao L, Lee CY, Matsuo T, Wu K, Asher G, et al. (2018): Large-scale forward genetics screening identifies Trpa1 as a chemosensor for predator odor-evoked innate fear behaviors. Nat Commun 9:2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan AJ, Kingsley PJ, Mitchener MM, Altemus M, Patrick TA, Gaulden AD, et al. (2018): Detection of cyclooxygenase-2-derived oxygenation products of the endogenous cannabinoid 2-Arachidonoylglycerol in mouse brain. ACS Chem Neurosci 9:1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel S, Roelke CT, Rademacher DJ, Hillard CJ (2005): Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci 21:1057–1069. [DOI] [PubMed] [Google Scholar]
- 55.Rademacher DJ, Meier SE, Shi L, Ho WSV, Jarrahian A, Hillard CJ (2008): Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology 54:108–116. [DOI] [PubMed] [Google Scholar]
- 56.Dubreucq S, Matias I, Cardinal P, Häring M, Lutz B, Marsicano G, Chaouloff F (2012): Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology 37:1885–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. (2005): Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis [published correction appears in Proc Natl Acad Sci U S A 2006; 103:2465]. Proc Natl Acad Sci U S A 102:18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soethoudt M, Grether U, Fingerle J, Grim TW, Fezza F, de Petrocellis L, et al. (2017): Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun 8:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivy D, Palese F, Vozella V, Fotio Y, Yalcin A, Ramirez G, et al. (2020): Cannabinoid CB2 receptors mediate the anxiolytic-like effects of monoacylglycerol lipase inhibition in a rat model of predator-induced fear. Neuropsychopharmacology 45:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LeDoux JE (2000): Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. [DOI] [PubMed] [Google Scholar]
- 61.Davis M, Shi C (2000): The amygdala. Curr Biol 10:R131. [DOI] [PubMed] [Google Scholar]
- 62.Maren S, Quirk GJ (2004): Neuronal signalling of fear memory. Nat Rev Neurosci 5:844–852. [DOI] [PubMed] [Google Scholar]
- 63.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B (2013): Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 16:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. (2010): Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lutz B, Marsicano G, Maldonado R, Hillard CJ (2015): The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 16:705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heinz DE, Genewsky A, Wotjak CT (2017): Enhanced anandamide signaling reduces flight behavior elicited by an approaching robo-beetle. Neuropharmacology 126:233–241. [DOI] [PubMed] [Google Scholar]
- 67.Gray JM, Vecchiarelli HA, Morena M, Lee TTY, Hermanson DJ, Kim AB, et al. (2015): Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci 35:3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill MN, Hillard CJ, McEwen BS (2011): Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex 21:2056–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TTY, Gray JM, et al. (2010): Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A 107:9406–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TTY, et al. (2011): Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 31:10506–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE (2012): Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol 26:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP (2010): Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 151:4811–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Albrechet-Souza L, Nastase AS, Hill MN, Gilpin NW (2021): Amygdalar endocannabinoids are affected by predator odor stress in a sex-specific manner and modulate acoustic startle reactivity in female rats. Neurobiol Stress 15:100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bahi A, Al Mansouri S, Al Memari E, Al Ameri M, Nurulain SM, Ojha S (2014): β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav 135:119–124. [DOI] [PubMed] [Google Scholar]
- 75.García-Gutiérrez MS, Pérez-Ortiz JM, Gutiérrez-Adán A, Manzanares J (2010): Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol 160:1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.García-Gutiérrez MS, Manzanares J (2011): Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol 25:111–120. [DOI] [PubMed] [Google Scholar]
- 77.Bean BP (2007): The action potential in mammalian central neurons. Nat Rev Neurosci 8:451–465. [DOI] [PubMed] [Google Scholar]
- 78.Boddum K, Hougaard C, Lin JXY, von Schoubye NL, Jensen HS, Grunnet M, Jespersen T (2017): Kv3.1/Kv3.2 channel positive modulators enable faster activating kinetics and increase firing frequency in fast-spiking GABAergic interneurons. Neuropharmacology 118:102–112. [DOI] [PubMed] [Google Scholar]
- 79.Erisir A, Lau D, Rudy B, Leonard CS (1999): Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons [published correction appears in J Neurophysiol 2000; 84:followi]. J Neurophysiol 82:2476–2489. [DOI] [PubMed] [Google Scholar]
- 80.Gantz SC, Bean BP (2017): Cell-autonomous excitation of midbrain dopamine neurons by endocannabinoid-dependent lipid signaling. Neuron 93:1375–1387.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Metna-Laurent M, Soria-Gómez E, Verrier D, Conforzi M, Jégo P, Lafenêtre P, Marsicano G (2012): Bimodal control of fear-coping strategies by CB1 cannabinoid receptors. J Neurosci 32:7109–7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts CJ, Stuhr KL, Hutz MJ, Raff H, Hillard CJ (2014): Endocannabinoid signaling in hypothalamic-pituitary-adrenocortical axis recovery following stress: Effects of indirect agonists and comparison of male and female mice. Pharmacol Biochem Behav 117:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chakraborty P, Datta S, McEwen BS, Chattarji S (2020): Corticosterone after acute stress prevents the delayed effects on the amygdala. Neuropsychopharmacology 45:2139–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen R, Zhang J, Fan N, Teng ZQ, Wu Y, Yang H, et al. (2013): Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling [published correction appears in Cell 2014; 156:618]. Cell 155:1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Griebel G, Pichat P, Beeské S, Leroy T, Redon N, Jacquet A, et al. (2015): Selective blockade of the hydrolysis of the endocannabinoid 2-arachidonoylglycerol impairs learning and memory performance while producing antinociceptive activity in rodents. Sci Rep 5:7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. (2012): Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148:1039–1050. [DOI] [PubMed] [Google Scholar]
- 87.Mallet PE, Beninger RJ (1998): The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by Δ9-tetrahydrocannabinol or anandamide. Psychopharmacology (Berl) 140:11–19. [DOI] [PubMed] [Google Scholar]
- 88.Wise LE, Long KA, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH (2012): Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS Chem Neurosci 3:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heyser CJ, Hampson RE, Deadwyler SA (1993): Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: Alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther 264:294–307. [PubMed] [Google Scholar]
- 90.Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, et al. (2013): Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 38:2952–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, et al. (2013): Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol Psychiatry 18:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


