Abstract
Anti-IgLON5 disease is a recently defined autoimmune disorder of the nervous system associated with autoantibodies against IgLON5. Given its broad clinical spectrum and extremely complex pathogenesis, as well as difficulties in its early diagnosis and treatment, anti-IgLON5 disease has become the subject of considerable research attention in the field of neuroimmunology. Anti-IgLON5 disease has characteristics of both autoimmunity and neurodegeneration due to the unique activity of the anti-IgLON5 antibody. Neuropathologic examination revealed the presence of a tauopathy preferentially affecting the hypothalamus and brainstem tegmentum, potentially broadening our understanding of tauopathies. In contrast to that seen with other autoimmune encephalitis-related antibodies, basic studies have demonstrated that IgLON5 antibody-induced neuronal damage and degeneration are irreversible, indicative of a potential link between autoimmunity and neurodegeneration in anti-IgLON5 disease. Herein, we comprehensively review and discuss basic and clinical studies relating to anti-IgLON5 disease to better understand this complicated disorder.
Key Words: anti-IgLON5 disease, autoimmune encephalitis, human leukocyte antigen, IgG4-related diseases, IgLON5 antibody, IgLONs, immunotherapy, inflammation, neurodegeneration, neuroimmunology, tauopathy
Introduction
Anti-IgLON5 disease is a rare autoimmune neurological disorder associated with autoantibodies against the IgLON5 protein. In 2014, Sabater et al. reported six patients presenting with IgLON5 antibodies, unique rapid eye movement and non-rapid eye movement sleep disorders, and tau deposition. A growing number of cases have since been reported and have revealed the heterogeneity of the disease. Most patients experience chronic onset and no gender differences have been identified (Grüter et al., 2022). Patients may present with sleep disorders, bulbar syndrome, gait instability, cognitive impairment, and movement disorders (Sabater et al., 2014). The human leukocyte antigen (HLA)-DRB1*10:01-DQB1*05:01 haplotype is strongly associated with the disease, indicating that genetic factors may contribute to anti-IgLON5 disease susceptibility (Gaig et al., 2019).
Tau deposits mainly involving the hypothalamus and brainstem tegmentum have been found in several patients (Sabater et al., 2014; Gelpi et al., 2016), hinting at a correlation between autoimmunity and neurodegeneration in this disease. Basic studies have investigated the long-term effects of the anti-IgLON5 antibody, which may include inflammation and neuronal damage (Sabater et al., 2016; Ni et al., 2022a). Disruption of the blood-brain barrier and intrathecal lymphocytosis have also been identified, confirming the importance of autoimmunity (Strippel et al., 2022). Additionally, the anti-IgLON5 antibody seems to play a central role in this disease, implying that neurodegeneration in anti-IgLON5 disease may be a consequence of autoimmunity.
Reviews focusing on anti-IgLON5 disease are scarce and mostly concerned with clinical manifestations. Herein, we review published clinical and basic research on anti-IgLON5 disease, mainly focusing on newly discovered features, including clinical and imaging features, diagnostic clues, and treatment prognosis. We also analyze the unique tauopathy and experiments relating to anti-IgLON5 disease aiming to understand its pathogenesis and discuss the challenges and directions for future study.
Literature Search Strategy
In this review, PubMed was searched for articles published until June 2022. The following keywords were used for the literature search: IgLON5, anti-IgLON5 disease, IgLON5 antibody, IgLON, IgG4-related diseases, tauopathy, neurodegeneration, and inflammation. Only publications in English were screened.
The Structure and Function of IgLON5
IgLON5 is a cell adhesion molecule belonging to the IgLON family. Members of this family are characterized by an N-terminal signal peptide, three Ig-like domains, and a glycosylphosphatidylinositol (GPI) anchor (Vanaveski et al., 2017). The IgLON family comprises five members, namely, opioid binding protein/cell adhesion molecule (OPCML), neurotrimin (NTM), limbic system associated membrane protein (LSAMP), neural growth regulator 1 (NEGR1), and IgLON family member 5 (IgLON5). IgLONs form homo- or heterodimers both in cis (on the same membrane) and in trans (between cells) (Reed et al., 2004; Vanaveski et al., 2017; Venkannagari et al., 2020). Ranaivoson et al. (2019) and Venkananagari et al. (2020) analyzed the crystal structure of IgLONs and identified several possible configurations (Figure 1). IgLONs participate in neural development and neural circuit formation (Karis et al., 2018), and may also be involved in tumor inhibition, depression, and obesity (Karis et al., 2018).
Figure 1.
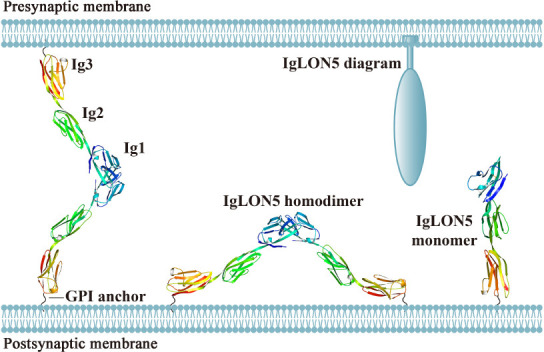
The crystal structure and possible configurations of IgLON5.
The possible trans-dimers (between cells), cis-dimers (homodimers or heterodimers within the same membrane), and monomers in the synaptic cleft. Adapted from Venkannagari et al. (2020). The crystal structures of the IgLON5 monomer and homodimer were downloaded from Protein Data Bank Japan (PDBj) (https://pdbj.org/). GPI: Glycosylphosphatidylinositol.
In humans, IgLON5 proteins are mainly expressed in the brain and testis. In the brain, the highest IgLON5 protein levels are found in the cerebellum. Meanwhile, IgLON5 transcripts are distributed in a wide variety of tissues, but particularly in the brain, retina, and testis (https://www.proteinatlas.org/). However, the distribution of IgLON5 transcripts in mice differs from that in humans (Vanaveski et al., 2017), with mice displaying the highest IgLON5 RNA expression in the thalamus and pons. IgLON5 transcripts are also abundantly present in mouse skeletal muscle but are absent in the liver (Vanaveski et al., 2017).
The function of IgLON5 remains mostly obscure. Lim et al. (2021) demonstrated that IgLON5 regulates the adhesion and differentiation of myoblasts and promotes myogenesis and regeneration. Interestingly, the authors reported that the antibody-mediated inhibition of IgLON5 in myoblasts led to a compensatory increase in IgLON5 mRNA and protein expression levels. Additionally, IgLON5 transcript levels were found to be markedly lower in the dorsolateral prefrontal cortex of schizophrenic patients who committed suicide than in those who did not, suggestive of a potential role for IgLON5 in central nervous systems development and regulation (Karis et al., 2018). IgLON5 levels may also be negatively correlated with the prognosis of colon cancer (Chen et al., 2021) and positively associated with limonin-mediated cardiac repair after myocardial infarction (Xiong et al., 2021). More studies on the role of anti-IgLON5 disease are needed to reveal the functions of IgLON5, the last member of the IgLON family to be identified.
Current Knowledge of Anti-IgLON5 Disease: Clinical Features, Diagnosis, and Treatment
Clinical features and imaging characteristics
Several cases of anti-IgLON5 disease have been reported since its discovery and they display significant heterogeneity. Relevant clinical studies performed from 2014 to 2022 are summarized in Additional Table 1. Previous reviews have fully described the core clinical phenotypes, which include sleep disorders, bulbar syndrome, gait instability, and cognitive impairment (Madetko et al., 2022). Dysautonomia, oculomotor abnormalities, and neuropsychiatric symptoms are also frequently reported (Grüter et al., 2022), as are movement disorders, with 87% (63/72) of patients having at least one type of dyskinesia (Gaig et al., 2021).
Additional Table 1.
Clinical research of anti-IgLON5 disease from 2014 to 2022
| Study | Patient information | Clinical presentation | Antibody Serum/CSF | CSF | MRI | Neuropathology | Treatment | Outcome | HLA DRB1*1001/ DQB1*0501 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Age (yr) | Sex (Male/Female) | Typicalsyndromes | Atypicalsymptoms | ||||||||||||
|
| |||||||||||||||
| S | B | G | C | M | |||||||||||
| Sabater et al., 2014 | 59 (52-76) | 3/5 | 8/8 | 8/8 | 5/8 | 2/8 | 5/8 | Oculomotor disroders(5/8), dysautonomia(7/8) | +/+(5/8) +/NK(3/8) | N(8/8) | N(8/8) | taudeposition in the tegmentum and hypothalamus(2/8) | Steroids(1/8), IVIg(1/8), steroids+CTX(3/8), steroids+IVIg+CTX(1/8), steroids+RTX(1/8), steroids+IVIg+RTX(1/8) | Not improved(2/8), not improved+deceased(5/8), improved+deceased(1/8) | +/+(4/4) |
|
| |||||||||||||||
| Högl et al., 2015 | 54 | 0/1 | + | + | Mild bilateral ptosis, mild broadbased gait | +/+ | NK | NK | NK | CPAP,modafinil, laterofixation of left vocal cord | Partially improved deceased | +/+ | |||
|
| |||||||||||||||
| Simabukuro et al., 2015 | 71 | 0/1 | + | + | + | + | Oculomotor disorders, depression | +/+ | Mild pleocytosis, increased protein | N | NK | CPAP,haloperidol, IVIg, RTX | Not improved | +/+ | |
|
| |||||||||||||||
| Brüggemannet al., 2016 | 64 | 1/0 | + | + | + | + | Oculomotor disorders, dysautonomia | +/- | Mild increase of total tau and p-tau | N | NK | BiPAP,steroids, IVIg, RTX, baclofen | Partially improved deceased | +/+ | |
|
| |||||||||||||||
| Haitaoet al., 2016 | 64 | 0/1 | + | + | + | + | Burningpainof buccalcavity and tongue | +/+ | Increased protein | N | NK | Levodopa, benserazide, IVIg, MMF | Substantially improved | -/+ | |
|
| |||||||||||||||
| Montojoet al., 2016 | 70 | 0/1 | + | + | + | Oculomotor disorders, dysautonomia | +/NK | NK | mesencephalic atrophy | NK | IVIg | Stable deceased | NK | ||
|
| |||||||||||||||
| Bonello et al., 2017 | 45 | 1/0 | + | + | + | + | Neck pain, depression, weight gain | +/+ | Pleocytosis,increased protein | N | NK | Prednisolone, IVIg, plasmapheresis, CTX | Substantially improved | +/+ | |
|
| |||||||||||||||
| Gaiget al., 2017 | 64.5 (46-83) | 10/12 | 22/22 | 20/22 | 16/22 | 9/22 | 14/22 | Oculomotor disorders(13/22), dysautonomia(14/22) | +/+(14/16) +/-(2/16) | pleocytosis(6/20), increased protein(10/20), OB(+)(1/14) | Mild brainstem atrophy(3/22), bilateral hippocampal atrophy(1/22) | NK | Methylprednisolone(13/20),IVIg(11/20), RTX(9/20),plasmapheresis(6/20), CTX(5/20) | Partially improved(2/20), not improved(18/20), deceased(13/22) | +/+(13/15) |
|
| |||||||||||||||
| Haitaoet al., 2017 | 61 | 1/0 | + | + | + | Vitiligo, paroxysm unconsciousness, visual hallucinations | +/+ | N | NK | NK | IVIg, prednisone, MMF | Partially improved | +/+ | ||
|
| |||||||||||||||
| Honorat et al., 2017 | 62 (46–75) | 9/11 | 12/20 | 12/20 | 14/20 | 6/20 | 13/20 | Oculomotor disorders(8/20), neuropsychiatric disorders(8/20), dysautonomia(9/20), electrographic seizures (1/20), peripheral neuropathy(8/20) | +/+(7/20) +/NK(12/20) NK/+(1/20) | Increased protein(5/8) | Leukoariotic changes(6/20), cerebral atrophy(6/20), cerebellar atrophy(2/20) | NK | CPAP(3/20), steroids(3/20), steroids+AZA(1/20), steroids+MMF(1/20), steroids+CTX+AZA(1/20), steroids+plasmapheresis(1/20), Ster+IVIg+MMF+RTX(1/20), IVIg+plasmapheresis (1/20), plasmapheresis(1/20) | Substantially improved (2/10) partially improved (5/10) initially improved with subsequent worsening(1/10) not improved(1/10) not improved+deceased(1/10) deceased(3/20) | NK |
|
| |||||||||||||||
| Schröder et al., 2016 | 77 | 0/1 | + | + | Right-sided ptosis, peripheral facial palsy,weight loss | +/NK | NK | N | NK | Tracheotomy,methylprednisolone, steroids, plasmapheresis | Substantially improved deceased | NK | |||
|
| |||||||||||||||
| Wenninger,2017 | 58 | 1/0 | + | + | + | +/+ | N | N | NK | Prednisolone, physiotherapy | Partially improved deceased | NK | |||
|
| |||||||||||||||
| Macher et al., 2018 | 71 (64-76) | 0/3 | + | + | + | Vertigo, seizure | +/+(3/3) | Increased protein(3/3) | T2 abnormalities in hippocampal (1/3),T2 abnormalities in globus pallidusbilateral (1/3) | NK | Steroids, IVIg, immunoadsorption, RTX, AZA, CTX | One patient diagnosed after 7 years of bilateral vocal cord palsy had improved | NK | ||
|
| |||||||||||||||
| Montagna et al., 2018 | 75 | 0/1 | + | + | Neuropsychiatric disorders, fever, seizure | +/+ | Mildly increased protein, OB(+) | Spottyenhancement in the right temporal and frontal lobes, focal leptomeningeal enhancement and edema | severe white matter destruction with many macrophages and lymphocytosis. no tau deposition | Steroids, plasmapheresis, levetiracetam, AZA | Substantially improved | +/+ | |||
|
| |||||||||||||||
| Morales-Briceño et al., 2018 | 49 | 1/0 | + | + | + | + | Cold intolerance, hypersalivation | +/+ | N | N | frontal cortex: meningeal thickening, lymphocyte and microglia infiltration, edema, gliosis. cerebellum: edema, gliosis, loss of Purkinje cells. no tau deposition | Plasmapheresis, IVIg, RTX | NK | +/+ | |
|
| |||||||||||||||
| Moreno-Estébanez et al., 2018 | 65 | 1/0 | + | + | + | + | Emotionallability | +/+ | Pleocytosis | Paramagneticdeposits in the basalganglia, substantia nigra, red nucleus | NK | Tetrabenazine, quetiapine, trazodone, CPAP, methylprednisolone, RTX | Partially improved | +/+ | |
|
| |||||||||||||||
| Ramanan et al., 2018 | 73 | 1/0 | + | + | + | Constipation, confusion, emotionallability headache, chills, fever,renal oncocytoma | +/+ | Increased protein, pleocytosis | Subtle T2 hyperintensities in hypothalamus | NK | Outside hospital: antimicrobials, antiepileptics, dexamethasone Mayo Clinic: supportive care | Rapidly improved | NK | ||
|
| |||||||||||||||
| Schöberl et al., 2018 | 70 | 0/1 | + | + | + | Oculomotor disorders, cerebellar symptoms, depression | +/+ | Pleocytosis,increased protein | N | tau-PET: increased taudeposits in cerebellar hemispheres and midline, upper and lower brainstem. TSPO-PET:microglia activationin leptomeninges | Steroids,AZA | Partially improved | NK | ||
|
| |||||||||||||||
| Taoet al.,2018 | 57 | 1/0 | + | + | + | Limb weakness, muscle atrophy | +/+ | N | N | NK | Mechanical ventilation, prednisolone, IVIg, plasmapheresis | Not improved | +/+ | ||
|
| |||||||||||||||
| Vetter et al.,2018 | 79 | 0/1 | + | + | + | +/+ | Intrathecalantibody synthesis,elevated tau and p-tau | N | NK | NK | NK | NK | |||
|
| |||||||||||||||
| Bhatia and Singh, 2020 | 58 | 1/0 | + | + | + | + | + | Cerebellar symptoms | +/NK | NK | T2 hyperintensities in bilateral ventricles | NK | CPAP,ropirinole, BiPAP | Partially improved | +/+ HLA-DR*14 |
|
| |||||||||||||||
| Brunetti et al., 2019 | 69 | 1/0 | + | + | + | Dysautonomia | +/+ | NK | N | NK | Tracheotomy,IVIg, prednisolone,AZA | Substantially improved | +/+ | ||
|
| |||||||||||||||
| Chen et al., 2020 | 40+ | 0/1 | + | Psychobehavioral disorders, headache, fever, vomit, generalized seizures | +/NK | Increased protein | Reduced diffusion in bilateral dorsal midbrain, deep cerebellar white matter, superior cerebellar peduncles, superior cerebellar decussation, ventrolateral thalamus | NK | Paroxetine, buspirone, methylprednisolone, plasmapheresis | Partially improved deceased | +/+ | ||||
|
| |||||||||||||||
| Chunget al., 2019 | 58 | 1/0 | + | + | + | + | + | Psychobehavioral disorders, weight loss | +/+ (IgLON5) +/-(GABABR) | NK | N | NK | Methylprednisolone, plasmapheresis, CTX | Partially improved | +/+ |
|
| |||||||||||||||
| Gaiget al., 2019 | 63 (42-81) | 17/18 | Parasomina(18/ 35), sleep breathing disorder(27/35) | 25/35 | 21/35 | 21/35 | chorea(5/35), ataxia(1/35) , stiff-person(1/35) | Oculomotor disorders(18/35), dysautonomia(16/35) | NK | NK | NK | NK | NK | Substantially improved(5/19) | +/+(20/35), -/+(7/35) MAPT H1(22/27) |
|
| |||||||||||||||
| Logmin et al., 2019 | 56 | 1/0 | + | + | + | Oculomotor disorders | +/+ | Increased protein, increased p-tau | Macroadenoma of the pituitary gland | NK | Tiapride, corticosteroids, IVIg | Substantially improved | -/+ | ||
|
| |||||||||||||||
| Nissen and Blaabjerg, 2019 | 61 | 1/0 | + | + | + | + | + | Oculomotor disorders | +/+ | Mild pleocytosis | T2 hyperintensities in white matter of brainstem | NK | CPAP,steroids, plasmapheresis, RTX, tracheotomy | Partially improved | +/+ |
|
| |||||||||||||||
| Aslam and Shill, 2020 | 58 | 1/0 | + | + | + | + | Anxiety,dysautonomia,cold intolerance, headache | Positive (details were unknown) | N | N | NK | IVIg, steroids, CTX, olanzapine, tetrabenazine, plasmapheresis, RTX | Worsening | NK | |
|
| |||||||||||||||
| Erro et al., 2019 | 71 | 1/0 | + | + | + | + | + | Weight loss | +/+ | N | N | few perivascular CD8+T-cell and microglial activation in posterior hypothalamus,amygdala, hippocampus, brainstem; pTau deposition consistent with AD, no pTauin brainstem | IVIg | Not improved deceased | +/+ |
|
| |||||||||||||||
| Fuseya et al., 2020 | 78 | 0/1 | + | + | + | Corticalsensory deficit | +/- | NK | CORTICAl atrophy mainly in right parietal lobe | NK | Methylprednisolone, IVIg | Partially improved | -/+ HLA-DRB1*01:01 | ||
|
| |||||||||||||||
| Grüter et al.,2020 | 82 | 0/1 | + | + | + | + | Dysesthesia, dysautonomia, right-side ptosis | +/NK | NK | N | NK | IVIg | Complete recovery | -/+ | |
|
| |||||||||||||||
| Hansenet al., 2020 | 65 | 1/0 | + | + | + | + | Axonal neuropathy | +/+ | Pleocytosis,mildly increased phosphorylatedTau-18 1,BBB disturbance | Right-sidefrontal subcortical lesions,unspecific periventricular white-matter lesions,mild globalatrophy | NK | Methylprednisolone | Partially improved | NK | |
|
| |||||||||||||||
| Peeters et al., 2020 | 65 | 0/1 | + | + | + | + | + | +/NK | N | N | NK | Steroids, plasmapheresis, RTX | Worsening | +/+ | |
|
| |||||||||||||||
| Aghelan et al., 2022 | 37.5(33-48) | M+F=4 | Isolated chronic insomnia disease | +/NK(4/4) | NK | NK | NK | NK | NK | NK | |||||
|
| |||||||||||||||
| Asioli et al., 2021 | 68 | 0/1 | + | + | + | + | Fragmented ocular pursuit, apathy,depression | +/+ | Mild pleocytosis | Calcification inbilateral pallidalnuclei | NK | Pramipexole, IVIg, plasmapheresis | Not Improved deceased | +/+ | |
|
| |||||||||||||||
| Chen andVasani, 2021 | 70 | 1/0 | + | + | +/NK | N | N | NK | Methylprednisolone, tracheostomy | Substantially improved | NK | ||||
|
| |||||||||||||||
| El Shazly et al., 2021 | 67 | 0/1 | + | + | + | Stroke, depression, diabetes mellitus type 1 | +/+ | N | N | NK | CPAP,gastric bypass surgery, methylprednisolone, IVIg, plasmapheresis, AZA | Partially improved | NK | ||
|
| |||||||||||||||
| Gaiget al., 2021 | 62 (42-91) | 40/32 | 63/72 | 53/72 | 52/72 | 38/72 | 27/72 | Neuromuscular(10/72), oculomotor disorders(45/72), dysautonomia(38/72) | +/+(52/72) +/-(6/72) +/NK(14/72) | Pleocytosis(17/63), increased protein(29/63), OB(+)(5/28) | brainstem atrophy(6/70), cerebellar atrophy(3/70), other abnormalities(3/70) | NK | Immunotherapy(55/72) | Sustained improvement of movement disorders(7/55) | +/+(35/36) +/-(1/36) |
|
| |||||||||||||||
| González-Ávila et al., 2021 | 66 | 1/0 | + | + | + | + | + | Oculomotor disorders, diabetes mellitus type 2 | NK/+ | N | frontalatrophy | NK | NK | NK | NK |
|
| |||||||||||||||
| Helmchen et al., 2021 | 74 | 0/1 | + | + | Bilateral vestibulopathy, tinglingand paresthesia in lower legs, broad-based gait | +/+ | NK | N | NK | Doxycycline, IVIg, RTX | NK | +/+ | |||
|
| |||||||||||||||
| Liu et al., 2021 | 62 | 1/0 | + | + | +/+ | NK | N | NK | Carbamazepine, escitalopram, prednisolone, tacrolimus | Substantially improved | NK | ||||
|
| |||||||||||||||
| Macher et al., 2021 | 72(64-77) (3/4) | 1/3 | 4/4 | 3/4 | 1/4 | 4/4 | 3/4 | Oculomotor disorders(4/4), dysautonomia(2/4), polyneuropathy(3/4), seizure(1/4), vertigo(1/4) | +/+(3/4) | Mild pleocytosis(1/4), slightly increased protein(4/4) | Bilateral hippocampal atrophy(1/4), leukoencephalopathy(2/4), ischemicarea thalamus(1/4) | NK | Steroids, IVIg, plasmapheresis(3/4); AZA, RTX, CTX(2/4) | Stable(2/4) deceased(1/4) | NK |
|
| |||||||||||||||
| Park et al., 2021 | 84 | 0/1 | + | + | + | + | + | Oculomotor disorders, psychobehavioral disorders, dysautonomia | NK/+ | N | Left temporal meningioma, persistent leptomeningeal enhancement in cerebellum and upper cervical spinal cord | NK | Warfarin,sertraline, hydrocodone, acetaminophen (refused steroid and immunosuppressive therapygiven the risks) | Worsening | NK |
|
| |||||||||||||||
| Pi et al., 2021 | 37 | 1/0 | + | + | Left abducent paralysis | +/- | OB(+) | Diffusion restriction in left tegmentum of midbrain and occipital horn of rightlateral ventricle | NK | Steroids, IVIg, MMF | Complete recovery | HLA-DRB1*11:01, *15:01 HLA-DQB1*03:01, *06:02 | |||
|
| |||||||||||||||
| Shambrook et al., 2021 | 59 | 0/1 | + | + | + | + | Oculomotor disorders, psychobehavioral disorders, cerebellar symptoms, weight loss | +/+ | N | N | NK | Clonazepam,steroids, plasmapheresis, RTX, CTX, neuroleptics | Substantially improved | NK | |
|
| |||||||||||||||
| Stoyanov et al., 2021 | 50 | 1/0 | Visual hallucinations, musical hallucinations of mainstream popular music, speech difficulties, headache, seizures | +/NK | NK | Righttemporal lobe changes | NK | Anticonvulsants,AZA, prednisone | Complete recovery | NK | |||||
|
| |||||||||||||||
| Swayne et al., 2021 | 70 | NK | + | + | + | Speech disturbance, dysautonomia,flu-like illness | +/+ | Increased protein | NK | NK | IVIg, RTX | Partially improved | NK | ||
|
| |||||||||||||||
| Tagliapietra et al., 2021 | 65 | 0/1 | + | + | + | + | Oculomotor disorders, constipation, cough, fatigue, headache, diabetes mellitus type 2 | +/- | Increased protein | T2-hypeintenseinbrainstem tegmentum from open medulla oblongata to the caudalpons and hypothalamus | NK | IVIg, prednisone,AZA, tracheostomy | Partially improved | DQB1*05 DQA1*01:01, *01:02 DRB1*01, *15 MAPT H1 | |
|
| |||||||||||||||
| Villacieros-Álvarez et al., 2021 | 74 | 0/1 | + | + | + | Oculomotor disorders | +/+ | N | N | NK | Methylprednisolone, IVIg | Partially improved | -/+ | ||
|
| |||||||||||||||
| Wang et al., 2021 | 62 | 1/0 | + | + | + | Psychobehavioral disorders, dysautonomia,fever, headache | +/- | Increased pressure, increased protein, pleocytosis | N | NK | Cefoxitinsodium, oseltamivir,acyclovir, mannitol(no immunotherapy) | Rapidly improved | +/+ | ||
|
| |||||||||||||||
| Werner et al., 2021 | 70 (52-77) | 5/0 | 4/5 | 5/5 | 2/5 | 2/5 | 4/5 | Dysautonomia(4/5) | +/+(2/5) +/-(2/5) +/NK(1/5) | mild-to-moderate increased protein(5/5) | N(5/5) | NK | CPAP+ BiPAP(2/5), CPAP+ASV(2/5), mercaptopurine(1/5), methylprednisolone(3/5), IVIg (1/5), plasmapheresis(4/5), RTX(4/5) | Partially improved(2/5), not improved(3/5) | +/+(3/5) Patient 1: DQB1*05:01, DRB1*01:01, DRB1*04:04 Patient 3: DRB1*03: 01 |
|
| |||||||||||||||
| Yeet al., 2021 | 2 | 1/0 | + | + | Langerhans cell histiocytosis, nystagmus | +/- | N | Enhancement of the meninges and spinal cord | NK | Chemotherapy,immunotherapy | NK | -/- | |||
|
| |||||||||||||||
| Grüter et al.,2022 | 63.8±10.3 (40-82) | 32/21 | 27/53 | 29/53 | 21/53 | 15/53 | Neuromuscular hyperexcitability (18/53), hyperkinetic(11/53), hypokinetic(5/53) | Psychobehavioral disorders(9/53), dysautonomia(19/53) | +/+(43/53) +/-(6/53) +/NK(3/53) -/+(1/53) | Pleocytosis(17/51), OB(+)(6/47), blood-CSF-barrier dysfunction(21/46), increased protein(24/51), increased total-tau(1/25), increased p-tau(3/20), decreasedAβ1-42(2/27), decreased Aβ1-42/1-40(1/16) | NK | NK | First-line short-term immunotherapy(27/53), long-term immunotherapy(36/53) | Improved after short-term therapy(11/27), stable after long-term therapy(27/36), deceased (10/53) | +/+(22/44) -/+(10/44) -/-(10/44) +/-(1/44) +/NK(1/44) |
|
| |||||||||||||||
| Ni et al., 2022 | 60 (33-73) | 7/6 | 8/13 | 4/13 | 4/13 | 7/13 | 6/13 | Diplopia(2/13), psychobehavioral disorders (7/13), dysautonomia(4/13), seizure(1/13), signs of infection(2/13) | +/+(6/13), +/-(6/13), -/+(1/13) | Increased protein(5/10), pleocytosis(2/10) | Brainatrophy(2/13), white matter lesions(2/13), diffuse leukoencephalopathy(1/13), focalswelling in rightfrontal cortex(1/13),bilateral lesions inhippocampus(1/13) | NK | Steroids+IVIg(5/10), IVIg(2/10), steroid(2/10), IVIg+CTX+MMF(1/10), antiviral+immunotherapy(1/10) | Responded well(4/10), responded poorly(6/10) | +/+(4/7) -/-(3/7) |
|
| |||||||||||||||
| Videnovic et al., 2022 | 67 | 1/0 | + | + | + | + | + | Dysautonomia, diabetes mellitus type 2 | +/NK | NK | Moderate bilateral neural foraminalnarrowing in C3–C4, punctatesubacute infarctin the left posterior centrum semiovale, chronic infarcts in left middle frontal gyrus and right cerebellum, moderate-to-severe white-matter changes | NK | Melatonin,trazodone, riluzole, CPAP, modafinil, methylprednisolone, IVIg, RTX | Partially improved | NK |
|
| |||||||||||||||
| Strippel et al., 2022 | 68 (49-81) | M+F= 11 | 8/11 | 9/11 | 7/11 | 2/11 | Oculomotor disorders(7/11), neuropathy(2/11), neuroendocrinetumor(1/11) | +/+(8/11) +/NK(3/11) | Increased protein, pleocytosis(3/10), Blood-CSF-barrier impairment(6/10), OB(+)(1/10), increased intrathecal B cells and plasma cells | NK | NK | Methylprednisolone(11/11), plasmapheresis(9/11),AZA(1/11), RTX(4/11), immunoadsorption(3/11) | Stable(patients with increased CSF plasma cells, treated with RTX)(4/11) | NK | |
|
| |||||||||||||||
| Wang et al., 2022 | 51 | 1/0 | + | + | Fever,coma, seizure, psychobehavioral disorders | NK/+ | Increased protein, pleocytosis | T2 hyperintensities in left temporallobe and hippocampus | NK | Acyclovir,IVIg, methylprednisolone, antiepileptic | Partially improved | +/+ | |||
|
| |||||||||||||||
| Bhatti, 2022 | 75 | 0/1 | + | + | + | Oculomotor disorders | +/NK | NK | Incidental rightmiddle cranial fossa arachnoidcyst | NK | Botulinum toxin, IVIg | NK | NK | ||
|
| |||||||||||||||
| Caoet al., 2022 | 77 | 1/0 | + | + | + | + | + | Oculomotor disorders | +/+ | Pleocytosis,increased protein | N | NK | Plasmapheresis, steroids,AZA | Not improved deceased | NK |
|
| |||||||||||||||
| Fu et al., 2022 | 61 | 0/1 | + | + | Epilepticseizures, acute ischemic stroke | +/+ | N | Abnormal signals and increased volumeof the right hippocampus and a few unspecificperiventricular whitematter lesions | NK | Intravenous thrombolysis, IVIg, levetiracetam,RTX | Complete recovery | -/+ | |||
|
| |||||||||||||||
| Urso et al., 2022 | 63 | 1/0 | + | + | + | + | Dysautonomia, oculomotor disorders | +/+ | N | Temporal atrophy, high-convexity tight sulci(hyperperfusion in this area), midbrain atrophy | NK | NK | NK | NK | |
ASV: Adaptive servo-venti; AZA: azathioprine; B: bulbar syndrome; BBB: blood brain barrier; BiPAP: bilevel positive airway pressure; C: cognitive impairment; CPAP: continuous positive airway pressure; CSF: cerebrospinal fluid; CTX: cyclophosphamide; G: gait instability; HLA: human leukocyte antigen; IgLON5: immunoglobulin-like cell adhesion molecule 5; IVIg: intravenous immunoglobulin; M: movement disorders; MMF: mycophenolate mofetil; N: normal; NK: not known; OB: oligoclonal band; PEG: percutaneous endoscopic gastrostomy; RTX: rituximab; S: sleep disorders.
The range of symptoms has gradually expanded as more patients are identified. One patient with tau deposition, mainly in the cerebellum, displayed obvious symptoms associated with cerebellar dysfunction, such as intention tremors and nystagmus (Schöberl et al., 2018). Peripheral neuropathy has also been observed in several patients (Honorat et al., 2017; Hansen et al., 2020; Macher et al., 2021), implying that the effects of anti-IgLON5 antibodies may extend to the peripheral nervous system. Some patients had paresthesia, which manifested as tongue neuralgia (Haitao et al., 2016), peripheral facial paralysis (Schröder et al., 2016), and cold intolerance (Morales-Briceño et al., 2018; Aslam and Shill, 2020). Some cases may present as motor neuron disease. For instance, Tao et al. (2018) described a patient who displayed progressive dysphagia and limb weakness with muscle atrophy. Electromyography showed extensive denervation in limb and thoracic paraspinal muscles. Epilepsy has also been observed in patients with anti-IgLON5 disease, although only rarely. One case series identified electrographic seizures without clinical manifestation in one patient (Honorat et al., 2017); however, only a few cases of epilepsy have been described since then (Macher et al., 2018, 2021; Montagna et al., 2018; Chen et al., 2020; Stoyanov et al., 2021; Fu et al., 2022; Ni et al., 2022b; Wang et al., 2022). Recently, Fu et al. (2022) found signs of inflammation in the hippocampus of a patient who experienced seizures as the main symptom, suggesting inflammation-induced hyperexcitability. Ni et al. (2022b) identified some unusual manifestations, such as “dancing belly and restless legs syndrome”. Takotsubo cardiomyopathy, a fatal condition, was observed in one patient during stridor (Montojo et al., 2016). Another case reported the occurrence of pop music hallucinations, which had never been observed before (Stoyanov et al., 2021).
New cases can provide novel insights into the occurrence and development of the disease. A few patients demonstrated influenza-like illnesses and exhibited headache, fever, and vomiting (Chen et al., 2020; Swayne et al., 2021; Wang et al., 2021). Both the Epstein-Barr virus (Ni et al., 2022b) and the herpes virus (Wang et al., 2022) have been reported in patients with anti-IgLON5 disease. A few patients also had other autoimmune diseases, such as vitiligo, Hashimoto’s thyroiditis, and connective tissue disease (Haitao et al., 2017; Honorat et al., 2017; Ni et al., 2022b; Grüter et al., 2022). Notably, a 2-year-old boy with Langerhans cell histiocytosis showed IgLON5 antibodies in serum (Ye et al., 2021). He was the youngest patient reported. Furthermore, the IgLON5 antibody was found to coexist with other antibodies, such as LGI1 (Honorat et al., 2017; Ni et al., 2022b), GAD65 (Honorat et al., 2017), gamma-aminobutyric acid type B receptor (Chung et al., 2019), amphiphysin (Grüter et al., 2022), and MOG (Ni et al., 2022b), indicative of B-lymphocyte dysfunction. The role of these coexisting antibodies in anti-IgLON5 disease pathogenesis requires further study. Importantly, diagnosis should be carefully managed because it is difficult to determine the primary ‘culprit’ antibody.
Gaig et al. (2021) documented that 76% (55/76) of patients with anti-IgLON5 disease had some form of chronic manifestation (> 4 months) while another clinical study reported that approximately 28% (15/53) of patients had acute to subacute manifestations (≤ 4 weeks) (Grüter et al., 2022). Subacute manifestations are associated with inflammation-related changes in the cerebrospinal fluid (CSF) and are regarded as part of the early period of the disease (Grüter et al., 2022). Pathologies previously described by Gelpi et al. (2016) included a lack of inflammation and the presence of p-tau deposition mainly in the hypothalamus and brainstem tegmentum; however, subsequent cases did not involve tauopathy (Montagna et al., 2018; Morales-Briceño et al., 2018). The differences in clinical features and pathological changes highlight the heterogeneous nature of this disorder.
Patients with anti-IgLON5 disease can display diverse magnetic resonance imaging (MRI) changes. In one study, 12.5% (9/72) patients had distinct alterations on MRI (Tagliapietra et al., 2021). Brain atrophy (Gaig et al., 2017; Honorat et al., 2017; Macher et al., 2021), T2 hyperintensity (Macher et al., 2018; Ramanan et al., 2018; Wang et al., 2022), and white matter changes (Honorat et al., 2017; Macher et al., 2021; Ni et al., 2022b; Videnovic et al., 2022) have all been observed, in addition to other signs (Additional Table 1) mainly involving the hippocampus, brainstem, white matter, cerebellum, and cortex. Recently, “high-convexity tight sulci” (defined as the compression of sulci at the vertex, enlarged CSF spaces in the Sylvian fissure, and ventriculomegaly) on MRI, a marker of CSF dynamics problems, was reported in one patient (Urso et al., 2022). Hyperperfusion was also seen in the high-convexity area on single-photon emission computed tomography and arterial spin labeling-MRI, which may reflect the increased gray matter density of the convexity (Urso et al., 2022). Additionally, single-photon emission computed tomography imaging using dopamine transporter radioligands showed decreased ligand uptake mostly in the striatum (Montojo et al., 2016; Fuseya et al., 2020; González-Ávila et al., 2021), suggesting that the dopaminergic pathway had had been affected (González-Ávila et al., 2021). Meanwhile, on 18F-FDG positron emission tomography scans, some patients displayed hypermetabolism in the basal ganglia (Haitao et al., 2016, 2017; Zhang et al., 2016; Morales-Briceño et al., 2018; Ni et al., 2022b), cerebellum (Haitao et al., 2016; Zhang et al., 2016), brainstem (Morales-Briceño et al., 2018), and other areas (Haitao et al., 2017; Wang et al., 2022). White matter and left temporal lobe hypometabolism has also been observed in some patients (Ni et al., 2022b).
Diagnosis
At present, anti-IgLON5 disease diagnosis is mainly based on clinical manifestations and the detection of IgLON5 antibodies in serum and/or CSF of patients. The most common symptoms at diagnosis are sleep disorders, bulbar syndrome, movement disorders, cognitive impairment, oculomotor abnormalities, and dysautonomia. Most patients have IgLON5 antibodies in both serum and CSF, which can help confirm diagnosis (Grüter et al., 2022). The serum antibody titer increases with disease duration and decreases after long-term immunotherapy. IgG4 is the most commonly identified subtype, followed by IgG1 and IgG2 (Gaig et al., 2017; Grüter et al., 2022).
Other tests may be helpful for diagnosis. Recently, signs of inflammation in the CSF or on MRI have been reported in some patients (Montagna et al., 2018; Hansen et al., 2020; Grüter et al., 2022; Strippel et al., 2022), and may be a predictor of the initial stage of anti-IgLON5 disease. Meanwhile, HLA genotyping may represent a means of identifying susceptible patients. The HLA-DRB1*10:01 and HLA-DQB1*05:01 alleles are highly present in patients with anti-IgLON5 disease (Sabater et al., 2014), with HLA-DRB1*10:01 confirmed as having a stronger correlation with the disease than HLA-DQB1*05:01 (Gaig et al., 2017). Employing prediction algorithms, Gaig et al. (2019) determined a strong binding affinity between the IgLON5 peptide and HLA-DRB1 molecules. They also found that patients carrying HLA-DRB1*10:01 were more prone to sleep abnormalities, medulla oblongata dysfunction, and autonomic dysfunction; however, cognitive impairment was associated with being HLA-DRB1*10:01-negative. In contrast, Grüter et al. (2022) did not report finding the same associations, except for the link between sleep disorders and the HLA-DRB1*10:01 and HLA-DQB1*05:01 alleles. Notably, they also observed younger ages in both HLA-DRB1*10:01 and HLA-DQB1*05:01 carriers than HLA-DRB1*non-10:01 and HLA-DQB1*non-05:01 carriers, and anti-IgLON5 IgG titers were higher in patients with HLA-DRB1*10:01 than in those with HLA-DRB1*non-10:01. This evidence links genotype and phenotype, providing guidance for the diagnosis and treatment of anti-IgLON5 disease.
Regarding pathology, the detection of hyperphosphorylated tau protein can serve as important evidence in anti-IgLON5 disease diagnosis. Gelpi et al. (2016) proposed the establishment of levels of certainty-definite, probable, and possible—as criteria for the diagnosis of anti-IgLON5-related tauopathy. The levels of certainty are mainly based on pathology, autoantibody, HLA genotype/clinical history. Although these criteria are helpful for diagnosis, they have limitations. Evidence from both clinical and basic studies has shown that tau deposition occurs in the late stages of anti-IgLON5 disease; however, not all patients can be diagnosed at this stage, which may result in pathological changes going undetected.
The mortality rate is higher for anti-IgLON5 disease than for other types of autoimmune encephalitis (AE) (Sabater et al., 2014), particularly in patients with respiratory disorders. It can be inferred from existing cases that laryngeal spasm (Tagliapietra et al., 2021), respiratory failure (Honorat et al., 2017), central hypoventilation (Grüter et al., 2022), aspiration pneumonia (Högl et al., 2015), and cardiac complications (Cagnin et al., 2017; Gaig et al., 2017; Grüter et al., 2022) are the most likely causes of death. Extra care must be taken when treating patients with these symptoms.
Treatment progress
The prognosis for patients with anti-IgLON5 disease, even with treatment, is assumed to be poor (Sabater et al., 2014; Gaig et al., 2017). Nevertheless, recent studies have suggested that patients may benefit from immunotherapy, although whether immunotherapy can improve the long-term prognosis of the disease remains unknown (Grüter et al., 2022). The main treatment consists of first- and second-line immunotherapy as well as symptomatic therapies. First-line therapy is the treatment of choice and comprises steroids, intravenous immunoglobulin, and plasmapheresis (Tagliapietra et al., 2021). The response rate to first-line therapy was reported to be 55.8% (39/70), while that to second-line therapy, such as rituximab, cyclophosphamide, azathioprine, and mycophenolate mofetil, was 63% (17/27) (Fu et al., 2022). Moreover, one study revealed that 52.9% (27/51) patients had relapse-like exacerbations during the course of the disease (Grüter et al., 2022). Among these, 41% (11/27) showed improvement after first-line therapy. In the same study, long-term immunotherapy, including first- and second-line therapy, was found to be effective in 75% (27/36) patients. In general, immunotherapy seems to be more effective than was previously thought and can be effective in half of the patients. Long-term use may benefit an even greater number of patients.
Factors other than drug selection may also affect prognosis. Patients who develop anti-IgLON5 disease and already have a chronic disease were reported to be more likely to relapse or deteriorate due to late discovery, while those with acute and subacute attacks were typically diagnosed earlier (Grüter et al., 2022). Patients with cognitive impairment and atypical phenotypes showed better outcomes than those with typical symptoms such as sleep and bulbar disorders (Cabezudo-García et al., 2020). Early treatment is crucial for patients (Grüter et al., 2020; Pi et al., 2021). Grüter et al. (2022) identified that the initiation of short-term, first-line treatment within 6 weeks of initial deterioration, or the initiation of long-term treatment within 1 year of onset, were predictors of clinical improvement; moreover, a low serum level of neurofilament light chain before treatment was found to be correlated with a better prognosis.
The presence of inflammation may be indicative of early-stage disease and its identification is likely to be associated with better outcomes (Montagna et al., 2018; Cabezudo-García et al., 2020; Hansen et al., 2020). Grüter et al. (2022) reported that 37.3% (19/51) patients exhibited inflammation-related changes in the CSF, while the CSF cell count was negatively correlated with disease duration. Approximately 8.1% (4/49) of patients had IgLON5 antibodies only in serum (Tagliapietra et al., 2021) and some of these patients recovered after immunotherapy (Pi et al., 2021; Wang et al., 2021). Several studies reported poor outcomes in patients with IgG4 subtype predominance (Högl et al., 2015; Simabukuro et al., 2015; Haitao et al., 2017; Ni et al., 2022b). However, this effect seemed to be associated with HLA-DRB1*10:01 negativity, which is related to better outcomes (Gaig et al., 2019; Werner et al., 2021). Additional studies are needed to clarify the relationship between antibody subtypes, genotypes, and prognosis.
A recent study revealed an increase in B cell numbers and the presence of plasma cells in the CSF of patients with IgLON5 disease. Patients with plasma cells in the CSF responded well to rituximab (Strippel et al., 2022). This observation explains the key role of B cells and antibodies in the pathogenesis of the disease and confirms the effectiveness of anti-B cell drugs. In addition, anti-B cell drugs, such as rituximab, have been confirmed to be effective in treating IgG4-related diseases (Dalakas, 2021, 2022) and, thus, may be a better choice for treatment. Additional strategies may be developed once the underlying mechanisms have been determined.
Research Progress on the Pathogenicity of the IgLON5 Antibody
Auto-IgLON5 antibody-related tauopathy: Broadening our understanding of tauopathies
Anti-IgLON5 disease has been associated with tau deposition since its discovery. Sabater et al. (2014) were the first to report tau deposition in patients with anti-IgLON5 disease in 2014. Neuropathological examination revealed p-tau deposition mainly in the hypothalamus and brainstem tegmentum. Gelpi et al. (2016) proposed the concept of anti-IgLON5 disease-related tauopathy and summarized its possible pathological features, including neuronal loss, gliosis, and lack of inflammatory infiltration. Consistent with the pathological analysis, a few patients had increased tau and p-tau levels in the CSF (Brüggemann et al., 2016; Vetter et al., 2018; Logmin et al., 2019; Grüter et al., 2022; Hansen et al., 2020).
However, some patients do not exhibit the above typical pathology. One patient showed tau deposition mainly involving the cerebellum (Schöberl et al., 2018). Notably, this patient had inflammation in the leptomeningeal area (Schöberl et al., 2018). Another patient showed tau deposition primarily in the hippocampus as well as inflammation-related changes, such as lymphocyte infiltration and microglia activation (Erro et al., 2019). Interestingly, one case reported TDP-43 aggregation, in addition to tau deposition, in various microglial populations, which suggested the possibility of microglial-induced neuronal damage (Cagnin et al., 2017). In these patients, tauopathy was accompanied by inflammation or an abnormal immune microenvironment. Furthermore, in two patients, biopsy results showed no evidence of tau deposition (Montagna et al., 2018; Morales-Briceño et al., 2018). One of these patients had severe white matter lesions with increased macrophage and lymphocyte infiltration (Montagna et al., 2018), while the other showed infiltration of histiocytes and lymphocytes, increased numbers of microglia, microglial activation, and edema (Morales-Briceño et al., 2018). These patients had immune and inflammatory activation in the brain, but no evidence of tauopathy. Combined with the finding of increased B cell numbers in the CSF, these observations suggest that inflammation may independently dominate the disease in the early stages. These distinct pathological manifestations highlight the need for clinicians and researchers to carefully consider the correlation between autoimmunity, inflammation, and neurodegeneration in the treatment of anti-IgLON5 disease.
Anti-IgLON5 disease-related tauopathy may involve a novel neurodegenerative disease process that differs from that of traditional tauopathies. Tau isoforms and deposition sites in anti-IgLON5 disease are different from those in other traditional tauopathies, such as Alzheimer’s disease (AD), progressive supranuclear palsy, and corticobasal degeneration (Arendt et al., 2016). The tau isoforms identified in anti-IgLON5 disease to date include three-repeat (3R) and four-repeat (4R) isoforms (Gelpi et al., 2016). These are mainly found in neurons, and only seldom in glia and white matter (Gelpi et al., 2016). Moreover, the complex pathology of this disorder makes it difficult to classify it as a traditional tauopathy. More work is needed to determine the connection between tau and anti-IgLON5 antibodies. The neuropathological findings since 2014 are summarized in Table 1.
Table 1.
Neuropathological findings from patients with anti-IgLON5 disease since 2014
| Reference | Patient | Age (yr) | Sex | Neuropathology | Autopsy/biopsy/PET |
|---|---|---|---|---|---|
| Sabater et al., 2014 | 1 | 53 | Male | P-tau deposition mainly involving the hypothalamus and brainstem tegmentum. | Autopsy |
| 2 | 76 | Female | |||
| Gelpi et al., 2016 | 3 | 53 | Male | Tau pathology with predominant involvement of the hypothalamus and brainstem tegmentum, with a rostrocaudal gradient of severity to include the upper cervical cord. | Autopsy |
| 4 | 76 | Female | |||
| 5 | 54 | Female | |||
| 6# | 77 | Female | |||
| 7# | 48 | Male | |||
| 8# | 49 | Male | |||
| Cagnin et al., 2017 | 9 | 69 | Female | Tau deposition in the nucleus basalis, amygdala, hypothalamus, hippocampus, and locus coeruleus. TDP-43 aggregation in various microglial populations. |
Autopsy |
| Montagna et al., 2018 | 10 | 75 | Female | Extensive white matter destruction and the presence of numerous macrophages and lymphocytosis. No tau deposition. |
Biopsy |
| Morales-Briceño et al., 2018 | 11 | 49 | Male | Frontal cortex: meningeal thickening, lymphocyte and microglia infiltration, edema, gliosis. Cerebellum: edema, gliosis, and loss of Purkinje cells. No tau deposition. |
Biopsy |
| Schöberl et al., 2018 | 12 | 70 | Female | Tau-PET: increased tau deposition in the cerebellar hemispheres and midline, upper and lower brainstem. TSPO-PET: microglia activation in the leptomeninges |
PET |
| Erro et al., 2019 | 13 | 71 | Male | Few perivascular CD8+ T cell infiltrates and increased microglial activation in the posterior hypothalamus, amygdala, hippocampus, and brainstem. P-tau deposition consistent with AD, no p-tau found in the brainstem. |
Autopsy |
#: IgLON5 antibody test was not done. Patients 3 and 4 are the same as the patients 1 and 2, respectively. AD: Alzheimer’s disease; CSF: cerebrospinal fluid; PET: positron emission tomography; TSPO: translocator protein.
Anti-IgLON5 Antibody as a ‘Culprit’ Antibody: the Link between Neuroimmunology and Neurodegeneration
Because anti-IgLON5 disease was only recently described, research relating to this condition is still limited. However, important insights and information can still be obtained from related studies.
In vitro experiments
Sabater et al. (2014) detected IgLON5 antibodies and tau deposition in patients with anti-IgLON5 disease. They further demonstrated that the IgG1 subtype, which targets the Ig-like domain 2 of IgLON5, induced the time-dependent, irreversible internalization of IgLON5 in rat hippocampal neurons (Sabater et al., 2016). Two studies have provided evidence for neurodegeneration in vitro. Landa et al. (2020) revealed that the IgLON5 antibody disrupted the cytoskeleton in rat hippocampal neurons, resulting in dystrophic axons and axon swelling after 3 weeks. They also discovered an increased concentration of neurofilament light chain in CSF, which is a marker of axonal damage. One clinical study reported that a correlation may exist between high serum concentrations of neurofilament light chain and the risk of death (Grüter et al., 2022). Although this study demonstrated damage to the cytoskeleton, which has a close association with tau protein (Mietelska-Porowska et al., 2014), p-tau deposition was not observed. Ryding et al. (2021) demonstrated that anti-IgLON5 antibodies induced p-tau accumulation in differentiated human neural stem cells and further reported that the proportion of p-tau-positive neurons was increased, while synaptic structure and function were disrupted. Additionally, increased neuronal death was observed after 21 days of exposure to anti-IgLON5 antibodies compared with exposure to control IgG (Ryding et al., 2021). Finally, the authors found that neurodegeneration was accompanied by a continuous reduction in IgLON5 concentrations (Ryding et al., 2021). Combined, these studies confirmed that anti-IgLON5 antibodies can directly damage nerve cells.
In vivo experiments
A pilot study in mice confirmed p-tau deposition in vivo (Alvente et al., 2022). Humanized transgenic hTau mice and C57BL/6J wild-type (WT) mice, which served as controls, were infused intracerebroventricularly with human IgLON5 antibody or control antibody for 14 days. Neuropathological examination showed p-tau deposition in the hippocampal CA4 region, mossy fibers, and posterior periependymal areas in both WT and transgenic mice. Furthermore, a longer ventilatory period during sleep and a shorter inter-lick interval during wakefulness were observed in IgLON5 antibody-treated mice with no additional differences in sleep, respiration, or motor function. These results were suggestive of an increase in airway resistance and possible behavioral stress following the infusion of the IgLON5 antibody. Ni et al. (2022a) revealed long-term cognitive impairment and anxiety-like behavior in mice infused with anti-IgLON5 antibodies. They established two models of anti-IgLON5 disease using C57BL/6 mice—stereotactic injection in the hippocampal CA1 region and ventricular cannula embedment—and noted that cognition-related neural circuits and synaptic homeostasis were both disrupted, which could explain the behavioral results.
The most recent study has provided clues regarding the dual effects of the IgLON5 antibody (Ni et al., 2022a). First, the IgLON5 antibody was found to exert long-term pathogenic effects in vivo. The authors reported the extended binding between anti-IgLON5 antibodies from patients and the mouse brain (Ni et al., 2022a), which was consistent with the findings of Sabater et al. (2016). Three other studies also directly revealed the neuronal damage and degeneration induced by the IgLON5 antibody, thereby consolidating the linkage between the antibody and neurodegeneration. Secondly, IgLON5 antibodies may induce brain inflammation. Neuroinflammation was observed in vivo, and included the gradual activation of microglia and astrocytes, as well as an increase in the relative mRNA expression levels of several inflammatory factors, including TGF-β, CCL5, and CXCL13 (Ni et al., 2022a). Inflammation-related changes in the CSF and pathological findings have also been reported in several cases. These observations indicate that the IgLON5 antibody can affect neurons both directly and indirectly by inducing inflammation. Ni et al. (2022a) reported neuronal loss and morphological changes on day 30 after IgLON5 antibody injection in the presence of both antibody and inflammation. Nevertheless, the function of the IgLON5 antibody is still largely unknown, and the crucial pathways involved in how IgLON5 antibodies promote neuronal damage remain to be determined. The possible mechanisms underlying the pathophysiology of anti-IgLON5 disease based on current knowledge are depicted in Figure 2. We hypothesize that the deposition of tau protein seen in some of the patients was the result of the long-term effect of the IgLON5 antibody.
Figure 2.
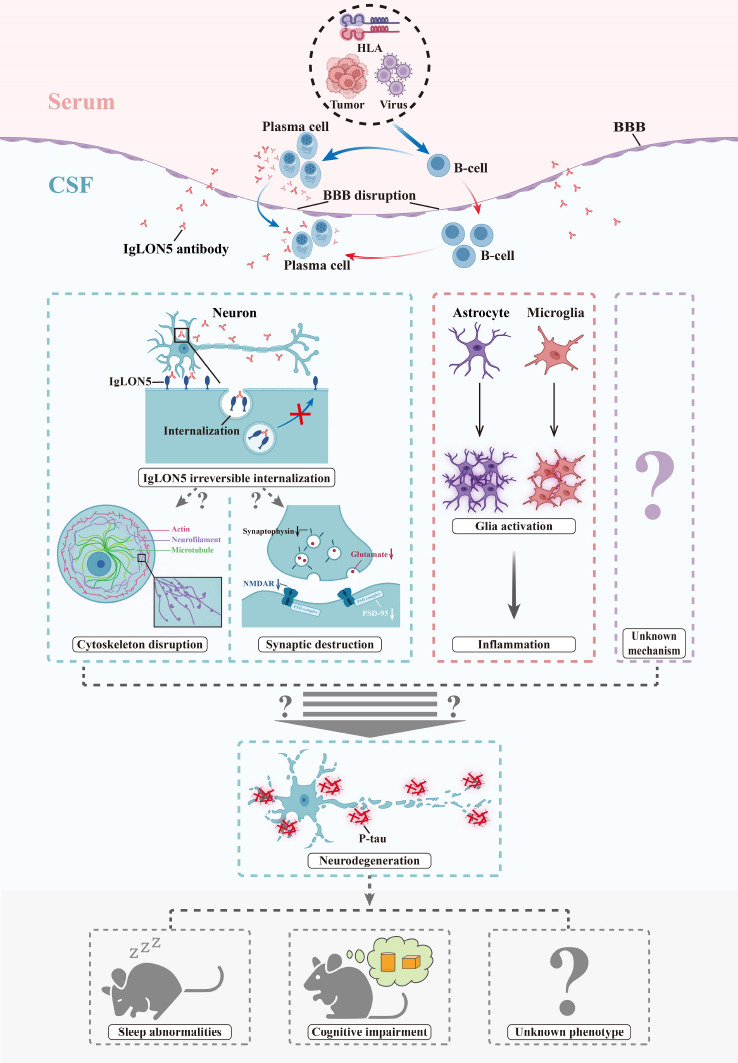
The possible mechanisms underlying the pathophysiology of anti-IgLON5 disease.
HLA genotypes, viruses, and tumors are the probable triggers of anti-IgLON5 disease in clinical cases; these may induce B cell immunity. B cells may differentiate into plasma cells and produce IgLON5 antibodies in the peripheral blood. Moreover, lymphocytes may infiltrate the central nervous systems through the disrupted BBB. Peripheral infiltration or intrathecal synthesis of IgLON5 antibodies can further cause neuronal damage via two or more possible mechanisms. These include the irreversible internalization of IgLON5 and the induction of inflammation in the microenvironment, such as glia activation. The internalization of IgLON5 may lead to the disruption of the cytoskeleton and synapses through unknown mechanisms. Finally, the resulting neuronal damage may be the cause of the neurodegeneration and p-tau deposition observed during pathological examination. In addition to the already identified pathways, there may be other as yet unknown mechanisms that could lead to neurodegeneration. BBB: Blood-brain barrier; CSF: cerebrospinal fluid; HLA: human leukocyte antigen.
Perspective: Challenges and Future Directions
Anti-IgLON5 disease was only recently described; accordingly, several important questions regarding this condition remain to be answered.
First, what is the definite cause of anti-IgLON5 disease? Abnormal immunity, viral infection, tumor, and genetic susceptibility are all possible etiologies. Some patients exhibited vitiligo (Haitao et al., 2017) or renal oncocytoma (Ramanan et al., 2018) before disease onset. Also, it is known that the skin and adrenal glands express IgLON5 RNA. Meanwhile, the Epstein-Barr virus (Ni et al., 2022b) and herpes virus (Wang et al., 2022) have been detected in some patients, and either virus could induce cross-reactions. HLA-DRB1 was proposed to present sequences in the signal peptide and Ig-like domain 2 of IgLON5 to CD4+ T cells and induce antibody production (Gaig et al., 2019). Furthermore, some patients display fasciculation (Wenninger, 2017) and cardiac symptoms (Montojo et al., 2016). Given that IgLON5 was reported to be present in skeletal muscle and myocardium, it should also be clarified whether organs outside the central nervous system can be directly targeted.
Second, what are the differences between the IgG1 and IgG4 subtypes? Current evidence only explains the effect of internalization of the IgG1 subtype in anti-IgLON5 disease. The IgG1 antibody is bivalent and Fc-dependent, which causes cross-linking between antigens and the complement cascade in other diseases, such as anti-NMDAR autoimmune encephalitis (Hughes et al., 2010) and neuromyelitis optica spectrum disorder (Duan et al., 2019). It seems that the IgG4 subtype mainly disrupts protein-protein interactions because of the univalent nature of Fab-arm exchange, which affects signal transduction and cell adhesion (Koneczny, 2020). IgG4-mediated autoimmune diseases are insidious and fatal (Perugino and Stone, 2020). These characteristics are highly consistent with anti-IgLON5 disease, suggesting the important role of IgG4. As both subtypes are present simultaneously in patients, it should be further clarified whether these subtypes exert dominant or synergistic effects in this disease.
Third, which pathways mediate the effects of the antibodies on neurodegeneration? As discussed above, the IgLON5 antibody acts in concert with IgLON5 to initiate the neurodegenerative process. However, the specific signaling pathways involved need to be further explored. IgLON5 antibodies may exist in the body for extended periods due to genetic abnormality, thereby exerting long-term effects on neurons. In addition, inflammation has been observed in vivo and may be another cause of neuronal damage. Appropriate animal models must be established, such as those relating to active immunity, to better explore the natural processes of the disease.
Finally, how can the diagnosis and treatment of anti-IgLON5 disease be improved? Given the complex phenotypes and potentially poor long-term outcomes, early diagnosis and treatment are needed to improve patient survival and prognosis. As the IgLON5 antibody may cause irreversible damage to neurons, exploring new strategies in addition to traditional immunotherapy, such as small-molecule targeted therapy (requires further study of antibody structure), as well as developing neuroprotective therapy, will be important for the future treatment of this disease.
Additional file:
Additional Table 1: Clinical research of anti-IgLON5 disease from 2014 to 2022.
Footnotes
Conflicts of interest: There are no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
C-Editor: Zhao M, S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
Funding: This work was supported by Shanghai Shuguang Plan Project, No. 18SG15, Shanghai Outstanding Young Scholars Project, Shanghai Talent Development Project, No. 2019044, Clinical Research Plan of SHDC, No. SHDC 2020CR2027B (all to SC).
References
- 1.Alvente S, Matteoli G, Molina-Porcel L, Landa J, Alba M, Bastianini S, Berteotti C, Graus F, Lo Martire V, Sabater L, Zoccoli G, Silvani A. Pilot study of the effects of chronic intracerebroventricular infusion of human anti-IgLON5 disease antibodies in mice. Cells. 2022;11:1024. doi: 10.3390/cells11061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull. 2016;126(Pt 3):238–292. doi: 10.1016/j.brainresbull.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Aslam S, Shill H. Chorea in IgLON5-mediated autoimmune encephalitis. Mov Disord Clin Pract. 2020;7(Suppl 3):S83–84. doi: 10.1002/mdc3.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brüggemann N, Wandinger KP, Gaig C, Sprenger A, Junghanns K, Helmchen C, Münchau A. Dystonia, lower limb stiffness, and upward gaze palsy in a patient with IgLON5 antibodies. Mov Disord. 2016;31:762–764. doi: 10.1002/mds.26608. [DOI] [PubMed] [Google Scholar]
- 5.Cabezudo-García P, Mena-Vázquez N, Estivill Torrús G, Serrano-Castro P. Response to immunotherapy in anti-IgLON5 disease: A systematic review. Acta Neurol Scand. 2020;141:263–270. doi: 10.1111/ane.13207. [DOI] [PubMed] [Google Scholar]
- 6.Cagnin A, Mariotto S, Fiorini M, Gaule M, Bonetto N, Tagliapietra M, Buratti E, Zanusso G, Ferrari S, Monaco S. Microglial and neuronal TDP-43 pathology in anti-IgLON5-related tauopathy. J Alzheimers Dis. 2017;59:13–20. doi: 10.3233/JAD-170189. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Wu J, Irani SR. Distinctive magnetic resonance imaging findings in IgLON5 antibody disease. JAMA Neurol. 2020;77:125–126. doi: 10.1001/jamaneurol.2019.3638. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Huang J, Xiong J, Fu P, Chen C, Liu Y, Li Z, Jie Z, Cao Y. Identification of a tumor microenvironment-related gene signature indicative of disease prognosis and treatment response in colon cancer. Oxid Med Cell Longev 2021. 2021 doi: 10.1155/2021/6290261. 6290261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung HY, Wickel J, Voss A, Ceanga M, Sell J, Witte OW, Geis C. Autoimmune encephalitis with anti-IgLON5 and anti-GABAB-receptor antibodies: A case report. Medicine (Baltimore) 2019;98:e15706. doi: 10.1097/MD.0000000000015706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalakas MC. IgG4-mediated neurologic autoimmunities: understanding the pathogenicity of IgG4, ineffectiveness of IVIg, and long-lasting benefits of anti-B cell therapies. Neurol Neuroimmunol Neuroinflamm. 2021;9:e1116. doi: 10.1212/NXI.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalakas MC. Autoimmune neurological disorders with IgG4 antibodies: a distinct disease spectrum with unique IgG4 functions responding to anti-B cell therapies. Neurotherapeutics. 2022;19:741–752. doi: 10.1007/s13311-022-01210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan T, Smith AJ, Verkman AS. Complement-independent bystander injury in AQP4-IgG seropositive neuromyelitis optica produced by antibody-dependent cellular cytotoxicity. Acta Neuropathol Commun. 2019;7:112. doi: 10.1186/s40478-019-0766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erro ME, Sabater L, Martínez L, Herrera M, Ostolaza A, García de Gurtubay I, Tuñón T, Graus F, Gelpi E. Anti-IGLON5 disease: A new case without neuropathologic evidence of brainstem tauopathy. Neurol Neuroimmunol Neuroinflamm. 2019;7:e651. doi: 10.1212/NXI.0000000000000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Zou X, Liu L. Epileptic seizures and right-sided hippocampal swelling as presenting symptoms of anti-IgLON5 disease: a case report and systematic review of the literature. Front Neurol. 2022;13:800298. doi: 10.3389/fneur.2022.800298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuseya K, Kimura A, Yoshikura N, Yamada M, Hayashi Y, Shimohata T. Corticobasal syndrome in a patient with anti-IgLON5 antibodies. Mov Disord Clin Pract. 2020;7:557–559. doi: 10.1002/mdc3.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaig C, Graus F, Compta Y, Högl B, Bataller L, Brüggemann N, Giordana C, Heidbreder A, Kotschet K, Lewerenz J, Macher S, Martí MJ, Montojo T, Pérez-Pérez J, Puertas I, Seitz C, Simabukuro M, Téllez N, Wandinger KP, Iranzo A, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88:1736–1743. doi: 10.1212/WNL.0000000000003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaig C, Ercilla G, Daura X, Ezquerra M, Fernández-Santiago R, Palou E, Sabater L, Höftberger R, Heidbreder A, Högl B, Iranzo A, Santamaria J, Dalmau J, Graus F. HLA and microtubule-associated protein tau H1 haplotype associations in anti-IgLON5 disease. Neurol Neuroimmunol Neuroinflamm. 2019;6:e605. doi: 10.1212/NXI.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaig C, Compta Y, Heidbreder A, Marti MJ, Titulaer MJ, Crijnen Y, Högl B, Lewerenz J, Erro ME, Garcia-Monco JC, Nigro P, Tambasco N, Patalong-Ogiewa M, Erdler M, Macher S, Berger-Sieczkowski E, Höftberger R, Geis C, Hutterer M, Milán-Tomás A, et al. Frequency and characterization of movement disorders in anti-IgLON5 disease. Neurology. 2021;97:e1367–1381. doi: 10.1212/WNL.0000000000012639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelpi E, Höftberger R, Graus F, Ling H, Holton JL, Dawson T, Popovic M, Pretnar-Oblak J, Högl B, Schmutzhard E, Poewe W, Ricken G, Santamaria J, Dalmau J, Budka H, Revesz T, Kovacs GG. Neuropathological criteria of anti-IgLON5-related tauopathy. Acta Neuropathol. 2016;132:531–543. doi: 10.1007/s00401-016-1591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-Ávila C, Casado L, Muro García I, Villacieros-Álvarez J, Vivancos J, Quintas S. Altered ioflupane single-photon emission computed tomography in anti-IgLON5 disease: A new case mimicking probable progressive supranuclear palsy and review of the literature. Eur J Neurol. 2021;28:1392–1395. doi: 10.1111/ene.14634. [DOI] [PubMed] [Google Scholar]
- 21.Grüter T, Behrendt V, Bien CI, Gold R, Ayzenberg I. Early immunotherapy is highly effective in IgG1/IgG4 positive IgLON5 disease. J Neurol. 2020;267:2151–2153. doi: 10.1007/s00415-020-09924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grüter T, Möllers FE, Tietz A, Dargvainiene J, Melzer N, Heidbreder A, Strippel C, Kraft A, Höftberger R, Schöberl F, Thaler FS, Wickel J, Chung HY, Seifert F, Tschernatsch M, Nagel M, Lewerenz J, Jarius S, Wildemann BC, de Azevedo L, et al. Clinical, serological and genetic predictors of response to immunotherapy in anti-IgLON5 disease. Brain: awac090. 2022 doi: 10.1093/brain/awac090. [DOI] [PubMed] [Google Scholar]
- 23.Haitao R, Yingmai Y, Yan H, Fei H, Xia L, Honglin H, Chaiyan L, Stöcker W, Liying C, Hongzhi G. Chorea and parkinsonism associated with autoantibodies to IgLON5 and responsive to immunotherapy. J Neuroimmunol. 2016;300:9–10. doi: 10.1016/j.jneuroim.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Haitao R, Huiqin L, Tao Q, Xunzhe Y, Xiaoqiu S, Wei L, Jiewen Z, Liying C, Hongzhi G. Autoimmune encephalitis associated with vitiligo? J Neuroimmunol. 2017;310:14–16. doi: 10.1016/j.jneuroim.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Hansen N, Hirschel S, Stöcker W, Manig A, Falk HS, Ernst M, Vukovich R, Zerr I, Wiltfang J, Bartels C. Figural memory impairment in conjunction with neuropsychiatric symptoms in IgLON5 antibody-associated autoimmune encephalitis. Front Psychiatry. 2020;11:576. doi: 10.3389/fpsyt.2020.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Högl B, Heidbreder A, Santamaria J, Graus F, Poewe W. IgLON5 autoimmunity and abnormal behaviours during sleep. Lancet. 2015;385:1590. doi: 10.1016/S0140-6736(15)60445-7. [DOI] [PubMed] [Google Scholar]
- 27.Honorat JA, Komorowski L, Josephs KA, Fechner K, St Louis EK, Hinson SR, Lederer S, Kumar N, Gadoth A, Lennon VA, Pittock SJ, McKeon A. IgLON5 antibody: Neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm. 2017;4:e385. doi: 10.1212/NXI.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J, Balice-Gordon RJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karis K, Eskla KL, Kaare M, Täht K, Tuusov J, Visnapuu T, Innos J, Jayaram M, Timmusk T, Weickert CS, Väli M, Vasar E, Philips MA. Altered expression profile of IgLON family of neural cell adhesion molecules in the dorsolateral prefrontal cortex of schizophrenic patients. Front Mol Neurosci. 2018;11:8. doi: 10.3389/fnmol.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koneczny I. Update on IgG4-mediated autoimmune diseases: New insights and new family members. Autoimmunity Reviews. 2020;19:102646. doi: 10.1016/j.autrev.2020.102646. [DOI] [PubMed] [Google Scholar]
- 31.Landa J, Gaig C, Plagumà J, Saiz A, Antonell A, Sanchez-Valle R, Dalmau J, Graus F, Sabater L. Effects of IgLON5 antibodies on neuronal cytoskeleton: a link between autoimmunity and neurodegeneration. Ann Neurol. 2020;88:1023–1027. doi: 10.1002/ana.25857. [DOI] [PubMed] [Google Scholar]
- 32.Lim JH, Beg MMA, Ahmad K, Shaikh S, Ahmad SS, Chun HJ, Choi D, Lee WJ, Jin JO, Kim J, Jan AT, Lee EJ, Choi I. IgLON5 regulates the adhesion and differentiation of myoblasts. Cells. 2021;10:417. doi: 10.3390/cells10020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logmin K, Moldovan AS, Elben S, Schnitzler A, Groiss SJ. Intravenous immunoglobulins as first-line therapy for IgLON5 encephalopathy. J Neurol. 2019;266:1031–1033. doi: 10.1007/s00415-019-09221-3. [DOI] [PubMed] [Google Scholar]
- 34.Macher S, Zimprich F, De Simoni D, Höftberger R, Rommer PS. Management of autoimmune encephalitis: an observational monocentric study of 38 patients. Front Immunol. 2018;9:2708. doi: 10.3389/fimmu.2018.02708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macher S, Milenkovic I, Zrzavy T, Höftberger R, Seidel S, Berger-Sieczkowski E, Berger T, Rommer PS, Wiest G. Ocular motor abnormalities in anti-IgLON5 disease. Front Immunol. 2021;12:753856. doi: 10.3389/fimmu.2021.753856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madetko N, Marzec W, Kowalska A, Przewodowska D, Alster P, Koziorowski D. Anti-IgLON5 disease - The current state of knowledge and further perspectives. Front Immunol. 2022;13:852215. doi: 10.3389/fimmu.2022.852215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G. Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci. 2014;15:4671–4713. doi: 10.3390/ijms15034671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montagna M, Amir R, De Volder I, Lammens M, Huyskens J, Willekens B. IgLON5-associated encephalitis with atypical brain magnetic resonance imaging and cerebrospinal fluid changes. Front Neurol. 2018;9:329. doi: 10.3389/fneur.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montojo T, Piren V, Benkhadra F, Codreanu A, Diederich NJ. Gaze palsy, sleep and gait disorder, as well as Tako-Tsubo syndrome in a patient with IgLON5 antibodies. Mov Disord Clin Pract. 2016;4:441–443. doi: 10.1002/mdc3.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales-Briceño H, Cruse B, Fois AF, Lin MW, Jiang J, Banerjee D, Grunstein R, Varikatt W, Rodriguez M, Shepherd C, Fung VSC. IgLON5-mediated neurodegeneration is a differential diagnosis of CNS Whipple disease. Neurology. 2018;90:1113–1115. doi: 10.1212/WNL.0000000000005679. [DOI] [PubMed] [Google Scholar]
- 41.Ni Y, Feng Y, Shen D, Chen M, Zhu X, Zhou Q, Gao Y, Liu J, Zhang Q, Shen Y, Peng L, Zeng Z, Yin D, Hu J, Chen S. Anti-IgLON5 antibodies cause progressive behavioral and neuropathological changes in mice. J Neuroinflammation. 2022a;19:140. doi: 10.1186/s12974-022-02520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni Y, Shen D, Zhang Y, Song Y, Gao Y, Zhou Q, He L, Yin D, Wang Y, Song F, Chen M, Lian Y, Chen Y, Zhao X, Zhang X, Chen X, Wang Y, Zhang L, Mo N, Lv D, Liu J, Mao Z, Peng L, Chen S. Expanding the clinical spectrum of anti-IgLON5 disease: A multicenter retrospective study. Eur J Neurol. 2022b;29:267–276. doi: 10.1111/ene.15117. [DOI] [PubMed] [Google Scholar]
- 43.Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol. 2020;16:702–714. doi: 10.1038/s41584-020-0500-7. [DOI] [PubMed] [Google Scholar]
- 44.Pi Y, Zhang LL, Li JC. Anti-IgLON5 disease with distinctive brain MRI findings responding to immunotherapy: A case report. Medicine (Baltimore) 2021;100:e24384. doi: 10.1097/MD.0000000000024384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramanan VK, Crum BA, McKeon A. Subacute encephalitis with recovery in IgLON5 autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2018;5:e485. doi: 10.1212/NXI.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranaivoson FM, Turk LS, Ozgul S, Kakehi S, von Daake S, Lopez N, Trobiani L, De Jaco A, Denissova N, Demeler B, Özkan E, Montelione GT, Comoletti D. A proteomic screen of neuronal cell-surface molecules reveals IgLONs as structurally conserved interaction modules at the synapse. Structure. 2019;27:893–906.e9. doi: 10.1016/j.str.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed J, McNamee C, Rackstraw S, Jenkins J, Moss D. Diglons are heterodimeric proteins composed of IgLON subunits, and Diglon-CO inhibits neurite outgrowth from cerebellar granule cells. J Cell Sci. 2004;117(Pt 17):3961–3973. doi: 10.1242/jcs.01261. [DOI] [PubMed] [Google Scholar]
- 48.Ryding M, Gamre M, Nissen MS, Nilsson AC, Okarmus J, Poulsen AAE, Meyer M, Blaabjerg M. Neurodegeneration induced by anti-IgLON5 antibodies studied in induced pluripotent stem cell-derived human neurons. Cells. 2021;10:837. doi: 10.3390/cells10040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, Contreras A, Giometto B, Compta Y, Embid C, Vilaseca I, Iranzo A, Santamaría J, Dalmau J, Graus F. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13:575–586. doi: 10.1016/S1474-4422(14)70051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabater L, Planagumà J, Dalmau J, Graus F. Cellular investigations with human antibodies associated with the anti-IgLON5 syndrome. J Neuroinflammation. 2016;13:226. doi: 10.1186/s12974-016-0689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schöberl F, Levin J, Remi J, Goldschagg N, Eren O, Okamura N, Unterrainer M, Rominger A, Albert N, Brendel M. IgLON5: A case with predominant cerebellar tau deposits and leptomeningeal inflammation. Neurology. 2018;91:180–182. doi: 10.1212/WNL.0000000000005859. [DOI] [PubMed] [Google Scholar]
- 52.Schröder JB, Melzer N, Ruck T, Heidbreder A, Kleffner I, Dittrich R, Muhle P, Warnecke T, Dziewas R. Isolated dysphagia as initial sign of anti-IgLON5 syndrome. Neurol Neuroimmunol Neuroinflamm. 2016;4:e302. doi: 10.1212/NXI.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simabukuro MM, Sabater L, Adoni T, Cury RG, Haddad MS, Moreira CH, Oliveira L, Boaventura M, Alves RC, Azevedo Soster L, Nitrini R, Gaig C, Santamaria J, Dalmau J, Graus F. Sleep disorder, chorea, and dementia associated with IgLON5 antibodies. Neurol Neuroimmunol Neuroinflamm. 2015;2:e136. doi: 10.1212/NXI.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoyanov A, McDougall A, Urriola N. Musical hallucinations: a rare and atypical presentation of anti-IgLON5 disease responsive to immunosuppressive therapy. BMJ Case Rep. 2021;14:e236963. doi: 10.1136/bcr-2020-236963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strippel C, Heidbreder A, Schulte-Mecklenbeck A, Korn L, Warnecke T, Melzer N, Wiendl H, Pawlowski M, Gross CC, Kovac S. Increased intrathecal B and plasma cells in patients with anti-IgLON5 disease: a case series. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1137. doi: 10.1212/NXI.0000000000001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swayne A, Warren N, Prain K, Gillis D, O'Gorman C, Tsang BK, Muller C, Broadley S, Adam RJ, McCombe P, Wong RC, Blum S. An Australian state-based cohort study of autoimmune encephalitis cases detailing clinical presentation, investigation results, and response to therapy. Front Neurol. 2021;12:607773. doi: 10.3389/fneur.2021.607773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tagliapietra M, Frasson E, Cardellini D, Mariotto S, Ferrari S, Zanusso G, Plebani M, Monaco S. Hypothalamic-bulbar MRI hyperintensity in anti-IgLON5 disease with serum-restricted antibodies: a case report and systematic review of literature. J Alzheimers Dis. 2021;79:683–691. doi: 10.3233/JAD-201105. [DOI] [PubMed] [Google Scholar]
- 58.Tao QQ, Wei Q, Song SJ, Yin XZ. Motor neuron disease-like phenotype associated with anti-IgLON5 disease. CNS Neurosci Ther. 2018;24:1305–1308. doi: 10.1111/cns.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urso D, De Blasi R, Anastasia A, Gnoni V, Rizzo V, Nigro S, Tafuri B, Iacolucci CM, Zecca C, Dell'Abate MT, Andreetta F, Logroscino G. Neuroimaging findings in a patient with anti-IgLON5 disease: cerebrospinal fluid dynamics abnormalities. Diagnostics (Basel) 2022;12:849. doi: 10.3390/diagnostics12040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanaveski T, Singh K, Narvik J, Eskla KL, Visnapuu T, Heinla I, Jayaram M, Innos J, Lilleväli K, Philips MA, Vasar E. Promoter-specific expression and genomic structure of IgLON family genes in mouse. Front Neurosci. 2017;11:38. doi: 10.3389/fnins.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venkannagari H, Kasper JM, Misra A, Rush SA, Fan S, Lee H, Sun H, Seshadrinathan S, Machius M, Hommel JD, Rudenko G. Highly conserved molecular features in IgLONs contrast their distinct structural and biological outcomes. J Mol Biol. 2020;432:5287–5303. doi: 10.1016/j.jmb.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vetter E, Olmes DG, Linker R, Seifert F. Teaching video NeuroImages: Facial myokymia and myorhythmia in anti-IgLON5 disease: The bitten lip. Neurology. 2018;91:e1659. doi: 10.1212/WNL.0000000000006388. [DOI] [PubMed] [Google Scholar]
- 63.Videnovic A, Babu S, Zhao B, Reda HM, Linnoila JJ. Case 1-2022: A 67-year-old man with motor neuron disease and odd behaviors during sleep. N Engl J Med. 2022;386:173–180. doi: 10.1056/NEJMcpc2115844. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Wu X, Lu B. Improvement in mild anti-IgLON5 encephalopathy without immunotherapy: a case report. BMC Neurol. 2021;21:120. doi: 10.1186/s12883-021-02145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Li R, Sun X, Liao J, Li J, Xia H, Peng L, Qiu W, Shu Y. Anti-IgLON5 encephalopathy with concomitant herpes virus encephalitis. Neuroimmunomodulation. 2022:1–5. doi: 10.1159/000522234. [DOI] [PubMed] [Google Scholar]
- 66.Wenninger S. Expanding the clinical spectrum of IgLON5-syndrome. J Neuromuscul Dis. 2017;4:337–339. doi: 10.3233/JND-170259. [DOI] [PubMed] [Google Scholar]
- 67.Werner J, Jelcic I, Schwarz EI, Probst-Müller E, Nilsson J, Schwizer B, Bloch KE, Lutterotti A, Jung HH, Schreiner B. Anti-IgLON5 disease: a new bulbar-onset motor neuron mimic syndrome. Neurol Neuroimmunol Neuroinflamm. 2021;8:e962. doi: 10.1212/NXI.0000000000000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong W, Feng S, Wang H, Qing S, Yang Y, Zhao Y, Zeng Z, Gong J. Identification of candidate genes and pathways in limonin-mediated cardiac repair after myocardial infarction. Biomed Pharmacother. 2021;142:112088. doi: 10.1016/j.biopha.2021.112088. [DOI] [PubMed] [Google Scholar]
- 69.Ye F, Fan C, Peng M, Liu S, Yu Y, Yang L. Anti-IgLON5 disease in a pediatric patient with Langerhans cell histiocytosis. Clin Chim Acta. 2021;521:212–214. doi: 10.1016/j.cca.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Zhang W, Niu N, Cui R. Serial 18F-FDG PET/CT findings in a patient with IgLON5 encephalopathy. Clin Nucl Med. 2016;41:787–788. doi: 10.1097/RLU.0000000000001339. [DOI] [PubMed] [Google Scholar]


