Abstract
A recently described flow cytometric opsonophagocytic assay (OPA) was adapted to quantify the functional activity of serum antibodies specifically directed against serogroup B inner core lipopolysaccharide (LPS) of Neisseria meningitidis. The percentage of human peripheral polymorphonuclear leukocytes and monocytes (PMNms) ingesting fluorescently labeled, ethanol-fixed N. meningitidis organisms (phagocytic activity) in the presence of human sera was measured to reflect the serum opsonic activity against the bacterium. The contribution to opsonophagocytic activity of antibodies to inner core LPS was estimated by comparing the opsonic activities of adult and infant sera before and after adsorbing anti-LPS antibodies from the sera using purified LPS extracted from an LPS mutant (galE) of N. meningitidis strain MC58 (B:15:P1.7,16:L3). The specificity of the assay was further investigated using monoclonal antibody (MAb) B5, which binds to an inner core LPS epitope of N. meningitidis. A dose-dependent decrease in phagocytic activity was observed when MAb B5 was incubated with LPS from an inner core LPS (galE) mutant. Similarly, the number of PMNms ingesting fluorescently labeled polystyrene beads coated with inner core (galE) LPS decreased in a dose-dependent fashion when MAb B5 was incubated with various concentrations of the homologous inner core LPS. Strong correlations were found between the concentration of serum antibodies to inner core LPS (galE) versus the phagocytic activity using healthy adult sera (r2 = 0.89). There was a correlation between phagocytic ingestion and initiation of intracellular oxidative burst (r2 = 0.99) using polystyrene beads coated with inner core LPS and opsonized with the same sera using the oxidative burst indicator system dihydrorhodamine123/rhodamine 123. OPA results were also found to correlate closely with the results of the serum bactericidal assay using MAb B5 against the N. meningitidis MC58 galE mutant in the presence of human complement (r2 = 0.994, P = 0.003, two-tailed test). These studies demonstrate that functional antibodies are produced in humans against meningococcal inner core LPS and that the OPA is a useful approach to study the opsonic activity of antibodies to inner core LPS in health and disease.
Meningococcal disease remains a global health problem, especially in infants less than 2 years old and young adults. Apart from epidemics, meningococcal disease annually causes an estimated 500,000 cases and 50,000 deaths worldwide (World Health Organization website http://www.who.int/inf-fs/en/fact105.html). Serogroup B causes the majority (50 to 80%) of all Neisseria meningitidis cases in Europe and the United States (2, 32). Whereas capsular polysaccharide-based vaccines are available against meningococcal disease caused by N. meningitidis serogroups A, C, Y, and W-135 (2, 32), we still lack an effective vaccine for routine immunization against serogroup B meningococci. In order to develop effective vaccines against serogroup B meningococcal disease, the relative contributions of human antibodies against serogroup B capsular polysaccharide, lipopolysaccharide (LPS), and the various meningococcal outer membrane components in protection against meningococcal disease need to be established.
An enzyme-linked immunosorbent assay (ELISA) has previously been employed to quantify immunoglobulin M (IgM) and IgG serum antibodies to meningococcal inner core LPS in healthy adults and infants following meningococcal disease (30). Competitive inhibition studies using purified galE LPS have demonstrated the presence of specific galE (inner core) LPS antibodies in these sera (30). These findings suggest that galE (inner core) LPS antibodies might have a functional role in immunity against meningococcal disease. However, until now, the functional activity of the galE (inner core) LPS antibodies has not been investigated.
Historically, serum bactericidal activity (SBA) has been used as the gold standard in vitro correlate of protection against meningococcal disease (9, 15, 25, 44, 45). The amount of high-affinity antimeningococcal antibodies detected by an affinity ELISA (8, 10) has been shown to correlate with SBA (10), and previous ELISA studies using meningococcal serogroup C polysaccharide have also been shown to correlate with SBA results (27). However, a number of other assays have recently been developed to reveal the functional characteristics of antimeningococcal antibodies, including a whole-blood assay (17), an opsonophagocytic killing assay (34, 39), and chemiluminescence- and flow cytometry (FCM)-based species- and antigen-specific opsonophagocytosis assays (OPAs) (11, 14, 20, 23, 24, 38). The traditional SBA is highly dependent on both the complement source (44) and the target strain used and is not ideal since the contribution of these variables to the end point, bacterial killing, cannot be easily distinguished from that of functional antibodies. Although complement also contributes to phagocytic activity in the OPA, the specific antibodies can be quantified as an independent and major factor (23). In the present study, we have therefore modified a flow cytometric OPA (23, 24) to study the functional role of naturally occurring antibodies to meningococcal serogroup B inner core LPS.
Whereas species-specific antimeningococcal OPAs utilize whole bacteria as target cells for opsonizing sera (11, 13, 23, 39), the OPA developed by Lehmann et al. directly identifies the antigen specificity of antimeningococcal opsonic antibodies by using antigen-coated polystyrene beads as targets for functional serum opsonins prior to phagocytosis by human polymorphonuclear leukocytes (PMNs) and monocytes (ms) (20–24). The antigen-specific opsonophagocytosis responses are quantified by flow cytometry (20). Using this method, disease-induced serum opsonins have been detected against serogroup B meningococcal outer membrane vesicles, outer membrane PorA and PorB, and transferrin-binding protein complexes A and B adsorbed to beads (20–24). Furthermore, the OPA results were shown to correlate with the amount of IgG directed against the same meningococcal antigens in the patient sera (21, 22). The aim of this study was to determine whether specific inner core LPS antibodies were functional in species- and antigen-specific OPAs. Previous OPAs were modified using ethanol-fixed wild-type meningococci or fluorescent beads coated with specific meningococcal LPS as targets for human PMNs and monocytes (PMNms) (percent phagocytosis and intracellular oxidative burst). The OPA results were compared to those obtained with SBA.
MATERIALS AND METHODS
Bacterial strains.
Wild-type N. meningitidis group B strain MC58 (isolated from an outbreak of meningococcal disease in Gloucester, United Kingdom [B: 15:P1.7,16:L3; phenotype Cap+ Opa+ Opc+ Pil+]) (16) and MC58 galE mutant (18, 31) were grown overnight on standard BHI agar. The Opa phenotype of MC58 was Opa+, which meant it could have natural opsonic activity through the CD66 receptor on PMNms (41). Therefore, comparative studies were also done with variants that were Opa−, including MC58 c3 (Cap− Opa− Opc+ Pil+ L3), c5 (Cap− Opa− Opc+ Pil− L3), c10 (Cap− Opa− Opc− Pil+ L3), and c12 (Cap− Opa− Opc− Pil− L3) (41) (Table 1). MC58 Cap− mutant was made in the wild-type MC58 Cap+ background by insertion of a kanamycin resistance cassette in the capsule locus (Cap− Opa+ Opc+ Pil+). The LPS (L3) phenotype of the strain was checked on a tricine-sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel, and the Cap− phenotype was checked by immunoblotting with anti-capsule B antibody. A total of 5 × 109 to 5 × 1010 organisms/ml were scraped from the plate, washed once in phosphate-buffered saline (PBS), and suspended in PBS, and ethanol was added to a final concentration of 70% (vol/vol) prior to being labeled with rhodamine green X (RG-X) or rhodamine red-X (RR-X).
TABLE 1.
Opsonophagocytic activity with healthy control adult serum (HC1) and MAb B5 (10× concentrated culture supernatant) and PMNms with different phenotypes of N. meningitidis MC58
| N. meningitidis strain | Phenotype
|
Mean % opsonophagocytic activity
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Capsule | Opa | Opc | Pilin | LPS | Controla | Final bufferb | HC1 | MAb B5 | MAb B5c | |
| MC58 Cap+ | + | + | + | + | L3 | 9.4 | 5.8 | 73.9 | 52.1 | 42.7 |
| MC58 Cap− | − | + | + | + | L3 | 14.7 | 10.9 | 80.9 | 63.3 | 48.7 |
| c3 | − | − | + | + | L3 | 10.4 | 9.5 | 45.8 | 46.0 | 35.7 |
| c5 | − | − | + | − | L3 | 7.1 | 5.6 | 51.0 | 48.35 | 41.2 |
| c10 | − | − | − | + | L3 | 17.9 | 7.8 | 69.6 | 58.95 | 41.0 |
| c12 | − | − | − | − | L3 | 5.9 | 6.1 | 62.9 | 51.8 | 45.9 |
Complement only.
Final buffer control only.
Complement background control value subtracted.
LPS preparation.
For inhibition studies in the OPA, LPS was obtained as described previously (31). Briefly, N. meningitidis was grown overnight on 20 brain heart infusion (BHI) agar plates and suspended in 30 ml of 0.05% phenol in PBS for 30 min. Alternatively, batch cultures were prepared in a fermentor using bacteria from overnight growth (six plates) in 50 ml of Bacto Todd-Hewitt broth to inoculate 2.5 liters of the same medium. Following incubation at 37°C for 6 to 8 h, the culture was inoculated into 60 liters of broth in a Brunswick Scientific 1F-75 fermentor, grown overnight (17 h, 37°C), phenol killed (1%, vol/vol), and chilled to 15°C, and bacteria were harvested by centrifugation (13,000 × g for 20 min). In either case, crude LPS was extracted from the bacterial pellet using the standard hot phenol-water method (43) and purified from the aqueous phase by ultracentrifugation (105,000 × g, 4°C, two times for 5 h) (28).
Source of human sera.
Adult sera were obtained from 12 healthy adult volunteers, pooled, aliquoted, and stored at −20°C. Paired sera were obtained from nine children on admission (acute-phase sera) to the John Radcliffe Hospital, Oxford, United Kingdom, and in the convalescent phase (samples obtained at a median of 6 weeks after [range, 4 to 16 weeks]) with culture-confirmed invasive N. meningitidis disease (Table 2).
TABLE 2.
Specific antibody (IgG and IgM) and opsonophagocytic activity of serum from N. meningitidis disease study subjects
| Patient no. (sex) | Infecting N. meningitidis straina | MAb B5 reactivityb (immunotype) | Age (mo) | ELISA titer, AU/mlc
|
Mean % of opsonophagocytic activityd (95% CI)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L2 galE (IgG)
|
L3 galE (IgG)
|
L2 galE (IgM)
|
L3 galE (IgM)
|
||||||||||
| Acute | Convalescent | Acute | Convalescent | Acute | Convalescent | Acute | Convalescent | Acute | Convalescent | ||||
| 1 (F) | B (PCR) | nk | 11 | 1.0 | 1.35 | 0.6 | 6.0 | 2.1 | 3.2 | 1.0 | 6.6 | 10.5 (5–15.9) | 47.5* (29–65.9) |
| 2 (F) | B:NT:P1.14 | + (L1) | 48 | 1.2 | 2.7 | 12.9 | 12.6 | 6.9 | 8.0 | 6.4 | 11.2 | 21.0 (21–21) | 69.8* (57.5–82) |
| 3 (F) | N. meningitidis isolated | nk | 108 | 7.0 | 9.9 | 7.1 | 30.3 | 8.7 | >10 | 2.8 | 6.5 | 35.3 (32.5–38) | 59.9* (56.7–63) |
| 4 (M) | B:15:P1.7 | + (L3,7) | 168 | 2.0 | 4.8 | 1.0 | 13.7 | >10 | >10 | 2.2 | 9.3 | 22.5 (17–28) | 53.8* (50–57.5) |
| 5 (F) | B:NT:P1.15 | nk | 22 | 1.9 | >10 | 0.65 | 6.5 | 4.5 | >10 | nk | >10.0 | 7.4 (7.1–7.7) | 22.2* (17.9–26.5) |
| 6 (M) | C:2a:P1.5 | ± (L2) | 56 | 1.6 | >10 | 4.6 | 7.4 | 2.3 | 10.4 | 2.3 | 10.4 | 25.6 (24.7–26.5) | 39.8* (38.2–41.3) |
| 7 (M) | B (serology) | nk | 82 | 8.7 | 6.5 | 2.1 | 3.8 | 2.5 | 3.6 | 3.4 | 3.0 | 15.0 (14–16) | 13.9 (12.4–15.3) |
| 8 (M) | Clinical diagnosis | nk | 8 | 4.3 | 6.3 | 2.0 | >10 | 4.0 | 6.6 | 0.3 | 3.3 | 14.3 (14.1–14.5) | 29.9 (28–31.8) |
| 9 (M) | C:2a:P1.5 | nk | 95 | 1.5 | >10 | 5.9 | >10 | >10 | >10 | 1.3 | 5.1 | 23.3 (22.1–24.6) | 15.4 (15.2–15.6) |
Patient 1 had positive PCR on cerebrospinal fluid (CSF) (serogroup B), but no isolate grew; patient 3 had cloudy CSF and penicillin-resistant N. meningitidis, but the isolate was nontypeable; patient 7 had antibodies to serogroup B N. meningitidis, but no isolate was cultured; patient 8 had a definite clinical diagnosis of meningococcus, but no group, type, or subtype is available.
nk, not known.
Sera were diluted 1:50 in 1% BSA–PBS, and galE units were determined from adult pooled serum standard curve.
Sera were diluted 1:2 in final buffer with addition of 5 μl of heterologous human complement. *, significant difference between paired sera (P < 0.05, two-tailed t test).
Source of complement.
For the OPA, complement sources were baby rabbit serum (Sigma S7764, rabbit HLA-ABC) and serum from a patient with hypogammaglobulinemia (0.6, 0.2, and <0.1 g/liter for IgG, IgM, and IgA, respectively; normal ranges are 6 to 13, 0.8 to 2.5, and 0.8 to 3 g/liter, respectively).
Murine MAbs to galE LPS.
Monoclonal antibody (MAb) B5 was selected from a set of seven murine MAbs raised to formalin-killed whole cells of galE mutant (H44/76) as described previously (31). Briefly, 6- to 8-week-old BALB/c mice were immunized three times 2 weeks apart intraperitoneally followed by once intravenously with formalin-killed whole cells of galE mutant H44/76. The hybridomas were prepared by fusion of spleen cells with SP2/O-Ag 14 (37) as described by Carlin et al. (4). MAb B5 conjugated to alkaline phosphatase was used to show the specificity of the galE LPS ELISA by direct inhibition using purified galE LPS and pooled adult sera.
Labeling of bacterial strains using RG-X and RR-X.
The labeling method was adapted from the method of Lehmann et al. (23), except RR-X-labeled bacteria were used for the oxidative burst assay. Briefly, a suspension of ethanol-fixed bacteria (total, 1010 organisms in 4 ml) was washed with 0.9% NaCl and allowed to settle on the bench. The saline was removed, and the bacteria were suspended in 3 ml of 0.2 M sodium bicarbonate buffer (pH 8.2). Then 100 μl of stock solution of RG-X (Molecular Probes, R6163) or RR-X (Molecular Probes, succinimidyl ester, mixed isomers, R6160) (5 mg/3 ml of dimethyl sulfoxide stored at −80°C) was added dropwise to the suspension, and the suspension was mixed gently at room temperature (RT) for 1 to 1.5 h and covered in foil to exclude light. The reaction was terminated with 400 μl of hydroxylamine-HCl (pH 8.5) with 5 M NaOH and allowed to mix for a further 1 h at RT. The suspension was centrifuged in a microcentrifuge (13,000 rpm for 10 min) and washed three times in ice-cold 0.9% NaCl. Labeled bacteria were finally suspended in 5 ml of the final buffer used in the OPA, filtered through a Nytal 11-μm pore mesh to remove clumps of bacteria, aliquoted in 250-μl volumes to give approximately 5 × 108 cells per 250 μl, and stored at −80°C.
Preparation of L3 galE LPS-coated latex beads.
The coating method was adapted from the method of Lehmann et al. (23) except the beads were coated with purified galE LPS in bicarbonate buffer. Polystyrene microspheres with a diameter of 1 μm contained a red (Polychromatic Red fluorescent [PC-red]) fluorescent dye within the polymer (Polysciences Fluoresbrite PC-red, 18660) (0.25 ml). These were washed three times in 1 ml of 0.1 M borate buffer (pH 8.5), centrifuged for 6 min at 14,000 rpm, and suspended in 1 ml of coating buffer (pH 9.68). Then 250 μg of galE LPS prepared from stock solution (2.5 mg/ml) was added to the washed beads to give a final volume of 1.25 ml and mixed overnight at RT. The bead mixture was centrifuged for 10 min at 13,000 rpm, and the supernatant was retained for LPS determination. The pellet was resuspended in 1 ml of blocking solution (10 mg of bovine serum albumin [BSA] per ml in borate buffer) and incubated for 30 min at RT with gentle mixing. The mixture was centrifuged for 6 min at 13,000 rpm, and the blocking step was repeated. The pellet was resuspended in 1 ml of storage buffer (0.1 M PBS [pH 7.4] containing BSA [10 mg/ml], 0.1% sodium azide, and 5% glycerol) at 4°C. Then 50 μl of stock solution of beads in storage buffer was added to 0.95 ml of final assay buffer and centrifuged for 6 min at 13,000 rpm. Beads were washed three times in PBS alone. Control beads were coated with 1% BSA for 2 h at RT and stored in storage buffer as described previously.
Preparation of human peripheral blood neutrophils.
Peripheral blood leukocytes were prepared from 20 ml of heparinized venous blood from healthy adult donors. Polymorphonuclear leukocytes and monocytes, i.e., the potentially phagocytosing cells (nonlymphocytes), are hereafter referred to as PMNms. The method used was adapted from that of Lehmann et al. (23), except cells were washed in RPMI medium (1640 HEPES modification; Sigma R5886) instead of PBS. Briefly, heparinized blood was diluted 1:10 in lysis buffer (0.15 M ammonium chloride, 0.1 M sodium bicarbonate, 0.002 M EDTA · 2H2O [pH 6.8]), left at RT for exactly 10 min, and then centrifuged at 350 × g for 5 min. The supernatant was discarded, and the pellet of cells was washed with RPMI medium (1640 HEPES modification) and centrifuged as before. The lysis step was repeated for 2 to 5 min if a layer of intact erythrocytes was still present. Cells were washed again in RPMI medium, centrifuged, and suspended in 10 ml of PBS. A differential cell count for total numbers of nonlymphocytes and lymphocytes was determined using the Coulter counter, and the percentage of granulocytes was calculated using flow cytometry. The dilution factor was calculated to give a concentration of 1.25 × 107 PMNms per ml. The PMNms were suspended in final buffer immediately before addition to the assay.
Species- and antigen-specific OPAs.
The OPAs were adapted from the work of Lehmann et al. (23) in 96-well Costar plates (Costar Corporation, no. 3790), except RG-X- or RR-X-stained N. meningitidis serogroup B (MC58) or its galE mutant in the species-specific assay and L3 galE LPS-coated PC-red-labeled polystyrene beads as target particles in the antigen-specific assay were used. The final buffer, diluted sera, bacteria, and PMNms were used in a volume of 25 μl for each assay. Stained bacteria were preopsonized with serum and/or complement in final buffer for 10 min at 37°C in wells in a microtiter plate and mixed on a shaking platform at 500 rpm. PMNms were added to the mixture in the wells and incubated for a further 10 min at 37°C. The reaction was stopped on ice with 150 μl of ice-cold PBS-EDTA per well and transferred to tubes containing 50 μl of trypan blue solution (4 mg/ml) immediately prior to analysis by FCM. A total of 10,000 PMNms (nonlymphocytes) were collected using FCM (Becton Dickinson FACScan and Cellquest software). PMNms can be discriminated and quantified by combined measurements of forward scatter, which is related to the size of the cells, and side scatter, related to granularity of cells. The percent phagocytosis was defined as the percentage of gated PMNms with associated particle (bacterial or bead) fluorescence (RG-X fluorescence collected in FL1 channel between 505 and 545 nm and RR-X fluorescence collected in FL2 channel between 560 and 590 nm [20]). All samples were run in duplicate or triplicate, and accordingly the results presented represent the means of results of two or three measurements.
Variables in OPA.
The optimal assay conditions for the OPA, adapted from Lehmann et al. (23), were determined empirically using MAb B5 (see above). The main sources of variation in the OPA were due to staining of the bacteria, the source of neutrophils, the ratio of bacteria to neutrophils, the temperature of incubation, and the source or storage history of complement and sera (data not shown). The optimal parameters were determined as described above to determine the most practical and reproducible assay conditions (data not shown). Variations in the fluorescent labeling of N. meningitidis bacteria were dependent on growth phase and numbers. The most consistent results were obtained using an overnight growth on solid-phase agar (stationary phase) and 1010 organisms/ml (data not shown). For practical reasons the N. meningitidis bacteria were ethanol fixed (70%), which gave consistently similar results to paraformaldehyde (0.5%, vol/vol) (data not shown). To monitor nonspecific opsonophagocytic activity (percent phagocytosis), negative controls were included in the assay, complement or final buffer with bacteria and PMNms.
OPA-OX.
Intracellular microbicidal oxidative burst production was reflected by supplementing the OPA described above with dihydrorhodamine-123 (DHR-123)/rhodamine 123 (R-123) as an oxidative burst (OX) indicator (Molecular Probes, Eugene, Oreg.). DHR-123 is converted intracellularly to green-fluorescent R-123 by reactive oxygen intermediates (24). Briefly, the same method as for the OPA was followed except RR-X was used instead of RG-X to label the bacteria and the oxidative burst substrate DHR-123 was added to opsonized RR-X-labeled bacteria immediately before addition of PMNms (final dilution of 0.1 mM DHR-123, which facilitated the simultaneous FCM measurement of phagocytosis [red bacterial fluorescence associated with PMNms collected in FL1 channel] and oxidative burst [green R-123 fluorescence of PMNms collected in the FL2 channel]). Electronic color compensations eliminated spectral overlaps between fluorochromes (24). As in the simple OPAs, percent phagocytosis was defined as the percentage of gated PMNms with particle (bacterial or bead) fluorescence (23). The percent oxidative burst was defined as the percentage of gated PMNms with R-123 fluorescence (23). All samples were run in duplicate or triplicate, and accordingly the results presented represent the means of two or three measurements.
Surface labeling of live and ethanol-fixed bacteria using FCM analysis.
The surface labeling method was adapted from Moe et al. (29), except no sodium azide was included in the blocking buffer step. To prepare labeled bacteria, N. meningitidis strain MC58 galE mutant organisms were grown overnight by standard conditions at 37°C on BHI agar plates and gently suspended in PBS. The optical density at 260 nm (OD260) was adjusted to give the required concentration, e.g., 5 × 109/ml. Bacterial cells (100 μl) were added to each tube (5 × 108 organisms), and an equal volume of diluted serum (0.05 μg of MAb B5 per ml in 1% BSA–PBS) was added. Tubes were incubated for 2 h at 4°C, and cells were centrifuged for 5 min at 13,000 rpm. The supernatant was discarded, and cells were washed with 200 μl of 1% BSA–PBS. Then 100 μl of fluorescein isothiocyanate (FITC)-conjugated F(ab)2 goat anti-mouse Ig (Sigma F2772) was added and diluted 1:100 in 1% BSA–PBS, and tubes were incubated for 1 h at 4°C. Cells were centrifuged at 13,000 rpm for 5 min and washed by addition of 200 μl of 1% BSA–PBS. The supernatant was discarded, and the cells were suspended in 1% (vol/vol) formaldehyde. Samples were transferred to tubes and read by FCM. For ethanol-treated bacteria, N. meningitidis bacteria were grown as before and gently suspended in PBS, the OD260 was adjusted to give 5 × 109 organisms/ml, and the bacteria were fixed in 70% ethanol. The same labeling procedure and FCM analysis were used as described previously.
Inhibition studies using L3 galE LPS.
Dilutions of sera or MAb B5 were incubated overnight at 4°C with a range of concentrations of purified N. meningitidis L3 galE LPS (0.5 to 1.0 mg/ml) and centrifuged at 13,000 rpm for 10 min, and the supernatent was used for OPA (30).
Inner core (galE) LPS ELISA.
Inner core LPS antibodies (IgG or IgM) were measured in sera using ELISA as described previously with either L3 galE or L2 galE LPS (30). Dilutions of pooled adult sera or MAb B5 were used as a standard curve. The undiluted pooled adult serum used as a standard was assigned 500 galE arbitrary units (AU) for IgG. The amount of antibody in neat MAb B5 culture supernatant was equivalent to 5.5 μg of IgG per ml determined by protein A purification (200 ml of culture supernatant gave 1.1 mg of protein). Protein concentration was determined using a Coomassie blue protein assay reagent kit (Pierce, Rockford, Ill.) with BSA and bovine gamma globulin standards.
SBA.
MAb B5 was assayed using a standard method described by the Centers for Disease Control and Prevention, Atlanta, Ga. (27), but using bacteria (103 CFU/well) of the target N. meningitidis strain MC58 (wild type) or galE mutant and using either hypogammaglobulinemic human serum or healthy control mouse serum as a complement source.
Statistical methods.
FCM results are presented as means of triplicate measurements. A two-tailed Student t test and Wilcoxon's signed rank test were used to determine differences between the data using Prism software (GraphPad Software Inc, San Diego, Calif.).
RESULTS
We have investigated the role of antibodies specific for the inner core LPS of N. meningitidis by adapting the OPA of Lehmann et al. (23). These studies used MAb B5 (31), N. meningitidis labeled with RG-X or RR-X, and PMNs. Despite the many variables noted (detailed in Materials and Methods), reproducible results could be obtained using ethanol-fixed N. meningitidis and a pool of regular human donors as the source of the PMNms.
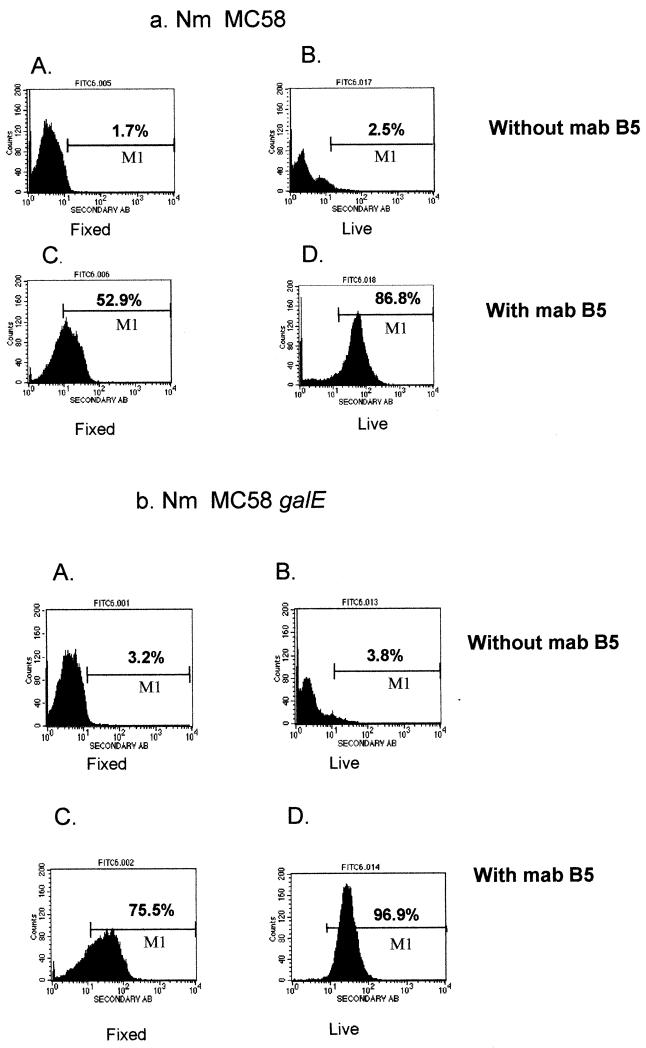
FCM surface labeling was used as a tool to compare the levels of binding of MAb B5 to ethanol-fixed and live N. meningitidis wild-type (MC58) and its galE mutant bacteria. The results suggested that MAb B5 binds live N. meningitidis better than ethanol-fixed organisms (Fig. 1). The FCM profiles using anti-mouse Ig-FITC showed that MAb B5 binds live and ethanol-fixed galE bacteria better than wild-type MC58. There are quantitatively more N. meningitidis bacteria labeled with the live (86.8 and 96.9% positive for MC58 and the galE mutant, respectively) compared to fixed N. meningitidis (52.9 and 75.5% positive, respectively) when equivalent numbers of bacteria were used (5 × 108 organisms per tube). MAb B5 binding was dependent on bacterial numbers and concentration of MAb B5 for both live and fixed N. meningitidis (data not shown).
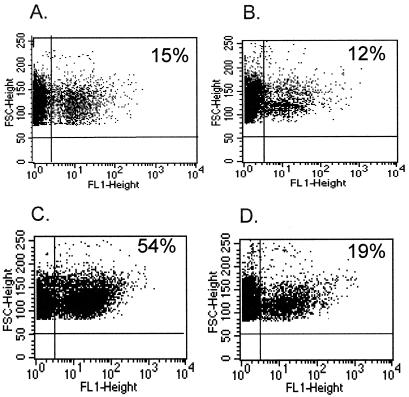
FIG. 1.
FCM profile comparing surface labeling of ethanol-fixed and live bacteria, 5 × 108 organisms per tube. (a) N. meningitidis MC58 wild type. (b) N. meningitidis MC58 galE mutant. Bacteria were opsonized with (C and D) or without (A and B) MAb B5 (approximately 0.11 μg/ml), and antibody binding was detected using anti-mouse IgG-FITC. The percentage of fluorescent organisms is indicated above gate M1 (see text).
Antibodies to inner core LPS are opsonic.
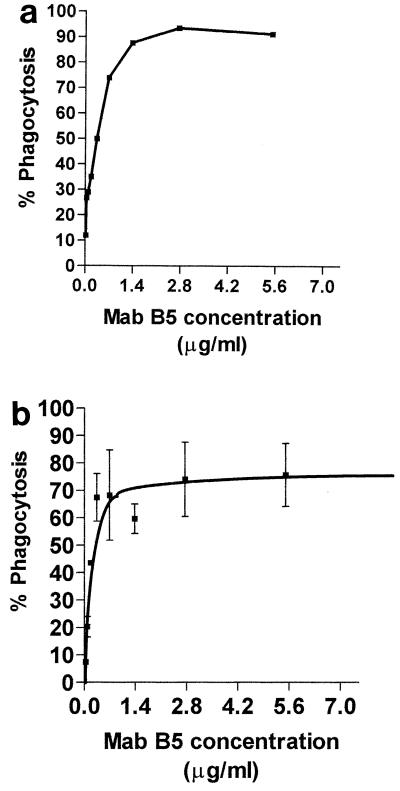
We determined the opsonic potential of MAb B5 using fluorescently labeled N. meningitidis organisms. With increasing concentrations of MAb B5 in the presence of human complement, the percentage of PMNs taking up labeled bacteria also increased up to 90% at an MAb B5 concentration of approximately 0.14 μg/ml (Fig. 2a). The role of antibodies to inner core LPS as opsonins was further investigated using fluorescently labeled latex beads coated with inner core LPS extracted from an L3 galE mutant. LPS-coated but not uncoated plain beads (data not shown) were ingested by PMNms in a dose-dependent fashion when opsonized with MAb B5 or adult human sera, but only in the presence of a source of complement (Fig. 2b). The complement source was from a hypogammaglobulinemic human (see Materials and Methods). The phagocytosis of coated beads with neat MAb B5 but with no complement and in the presence of complement alone was 6.3 and 5.9%, respectively (data not shown). Optimal phagocytosis of LPS-coated beads (75% phagocytosis) was observed with a concentration of MAb B5 of approximately 0.28 μg of IgG per ml (Fig. 2b). Control beads (BSA coated) in the presence of MAb B5 and complement gave approximately 5% phagocytosis (data not shown).
FIG. 2.
(a) Phagocytosis of ethanol-fixed N. meningitidis strain MC58 by PMNms from healthy adult donor opsonized with MAb B5 in the presence of a human complement source. (b) Effect of increasing the concentration of MAb B5 on phagocytosis of N. meningitidis L3 galE LPS-coated beads by PMNms from a healthy adult donor.
Specificity of OPA for inner core LPS antibodies.
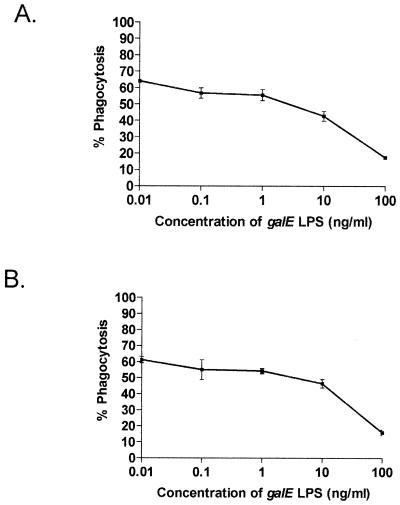
Ingestion by PMNms of ethanol-fixed wild-type or galE mutant N. meningitidis organisms decreased in a dose-dependent manner when MAb B5 was preadsorbed with increasing concentrations of L3 galE LPS (0.01 to 100 ng/ml) (Fig. 3). These data provided further evidence of the specificity of the opsonic activity for inner core LPS epitopes. We were concerned that the LPS used to preadsorb MAb B5 might affect PMN ingestion through toxic effects of lipid A. However, preincubation of MAb B5 with Salmonella or Escherichia coli LPS did not alter the percent ingestion by PMNms, nor did a highly truncated source of N. meningitidis LPS (lacking the more terminal inner core sugars; data not shown). Finally, MAb B5 was preincubated with equivalent molar amounts of O-deacylated galE (lacking O-acyl groups), and similar inhibition was observed compared to that with intact lipid A (data not shown). Thus, preincubation of MAb B5 with L3 galE LPS resulted in a 30 to 40% reduction in phagocytic ingestion of N. meningitidis by PMNms compared to that with untreated MAb B5. Controls using Opa+ and Opa− phenotypes of MC58 suggested that MAb B5 opsonophagocytic activities were similar (strains MC58 Cap+, Cap−, and c3), and therefore the opsonophagocytic activity observed was not significantly affected by Opa or other surface factors such as capsule, Opc, or pilin (Table 1). For studies with human serum antibodies, the background nonspecific opsonophagocytic activity can be assessed with complement controls, and if necessary opsonophagocytic activity with different phenotypes of N. meningitidis MC58 lacking capsule, Opa, Opc, and pilin can be determined.
FIG. 3.
Effect of prior incubation of MAb B5 (0.55 μg/ml) with various amounts of purified N. meningitidis (galE) LPS on phagocytosis of ethanol-fixed N. meningitidis organisms by PMNms. (A) Wild-type strain MC58. (B) MC58 galE mutant.
Inner core LPS antibodies in acute- and convalescent-phase human sera are opsonic.
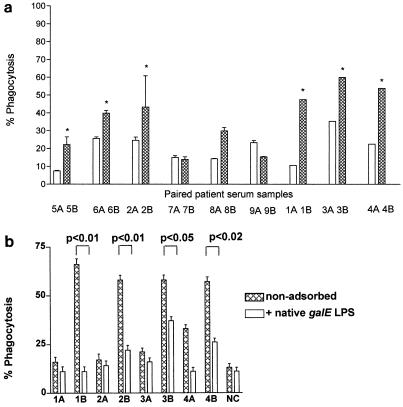
Acute- and convalescent-phase infant sera have previously been shown to contain antibodies specific for inner core LPS epitopes (IgG and IgM) by ELISA (30). The concentrations of L3 galE and L2 galE inner core LPS IgG antibodies, detected by LPS ELISA in nine paired sera, were greater in the convalescent-phase than the acute-phase sera in each individual patient except patient 2 for L3 galE LPS antibodies (Table 2). There was a good correlation between the immunotype and MAb B5 reactivity of the infecting N. meningitidis strain and increase in L3 galE LPS antibodies in convalescent- compared to acute-phase sera, for example, patients 2 (L1, B5 reactive) and 4 (L3, B5 reactive) but not 6 (L2, B5 nonreactive) (Table 2). Patient 6, who was infected by L2 immunotype (B5 nonreactive) had a higher L2 galE LPS antibody titer in the convalescent-phase sample (mean titer >10 L2 galE AU/ml) (Table 2). The phagocytosis in acute- and convalescent-phase sera with N. meningitidis wild-type strain MC58 correlated positively with the concentration of inner core LPS antibodies in paired sera, with mean values of 17.65% (95% confidence interval [CI], 12.9 to 26.0%) for the acute phase and 33.98% (95% CI, 23.7 to 54.6%) for the convalescent phase (Table 2, Fig. 4a). There was a significant difference between opsonophagocytic activities in acute- and convalescent-phase sera (P < 0.05, two-tailed t test) (Table 2, Fig. 4a). The functional opsonophagocytic activity that could be attributed to inner core (L3 galE) LPS antibodies in four paired infant sera taken early (acute) or later (convalescent) during N. meningitidis invasive disease was demonstrated by inhibition of phagocytosis when sera were preincubated with L3 galE LPS (Fig. 4b). There was a significant difference in levels of phagocytosis of ethanol-fixed N. meningitidis strain MC58 when opsonized with four paired infant sera taken early and later during invasive N. meningitidis disease (P < 0.05) (Fig. 4b). When convalescent-phase sera were preadsorbed with one dose of N. meningitidis L3 galE native LPS (2 ng/ml) and compared to nonadsorbed sera from the same infant, there was a significant reduction in opsonophagocytic activity (P < 0.05, one-tailed t test) (Fig. 4b). In one representative pair of sera (patient 3), there was a 33% reduction in inhibition when opsonophagocytic activity in acute-phase sera (A and B) was compared to that in convalescent-phase sera (C and D) (Fig. 5). The low-level background opsonophagocytic activity in human sera remaining after adsorption with purified LPS could be attributed to the presence of opsonic antibodies to other outer membrane components. This was demonstrated by opsonophagocytic studies with healthy control sera and a series of MC58 mutants deficient in Opa, Opc, Cap, or pilin (Table 1).
FIG. 4.
(a) Phagocytosis by PMNms of fluorescently stained ethanol-fixed N. meningitidis strain MC58 opsonized with nine paired infant sera taken early (open bars) (A) and later (hatched bars) (B) in invasive N. meningitidis disease (Table 2). Heterologous human complement was added to all sera in equal amounts. ∗, significant difference (P < 0.05, one-tailed t test) between pairs of sera. (b) Phagocytosis of fluorescently labeled ethanol-fixed N. meningitidis strain MC58 opsonized with four paired infant sera taken during acute (A) and convalescent (B) phases of N. meningitidis disease with PMNms. Sera were either nonadsorbed (hatched bars) or adsorbed (open bars) with native L3 galE LPS. Negative control serum (NC) was the human complement source. OP activity of convalescent-phase sera preadsorbed with galE LPS was significantly lower than that of the same nonadsorbed sera (P < 0.05, one-tailed t test).
FIG. 5.
Typical dot plot analysis of phagocytosis of N. meningitidis serogroup B strain MC58 opsonized with paired acute- or convalescent-phase sera from an infant during (A and B) or following (C and D) invasive meningococcal disease with PMNms. Sera in panels B and D were preadsorbed with purified N. meningitidis LPS (2 ng/ml) from the L3 galE mutant of MC58.
Correlation between ELISA and OPA for inner core LPS antibodies.
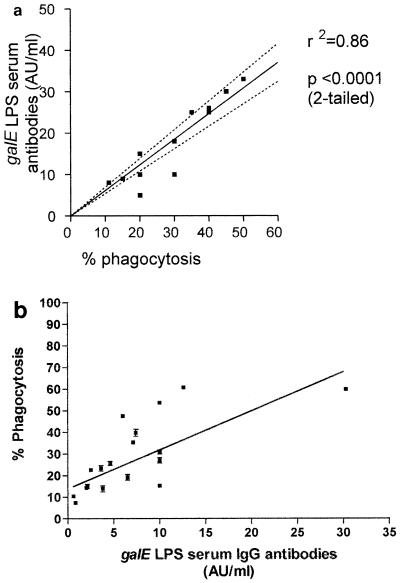
There was a correlation between the concentration of antibodies to inner core LPS of 12 healthy adult sera measured by ELISA and phagocytosis of ethanol-fixed N. meningitidis serogroup B (strain MC58) in the OPA (r2 = 0.89, two-tailed t test, P < 0.001) (Fig. 6a). There was a trend for the concentrations of inner core LPS antibodies in infant sera taken during N. meningitidis disease to reflect phagocytosis of wild-type serogroup B N. meningitidis bacteria (strain MC58) (Fig. 6b), but insufficient sera were available to determine a statistically significant correlation. However, there were examples of low concentrations of specific antibody in convalescent-phase sera resulting in high phagocytosis (e.g., <10 AU/ml) and higher concentrations (>10 AU/ml) in acute-phase sera resulting in low phagocytosis (Fig. 6b), which may reflect different affinities or specificities of these antibodies, as demonstrated in Table 2. Taken together, these data provide evidence that antibodies to inner core in sera from healthy individuals or individuals with invasive N. meningitidis disease had functional activity in the OPA.
FIG. 6.
(a) Correlation between galE LPS serum IgG antibodies (ELISA titers) and phagocytosis of MC58 with normal adult sera by PMNms from healthy adult donors. (b) Correlation between phagocytosis with N. meningitidis MC58 and galE LPS serum IgG antibodies (ELISA titers) (L3 galE) against adult pooled serum standard. Serum samples were taken from nine children with culture-confirmed invasive meningococcal disease during acute and convalescent phases of disease (Table 2). A human complement source was used.
Correlation between oxidative burst-killing assay (dihydrorhodamine) and OPA with inner core LPS antibodies.
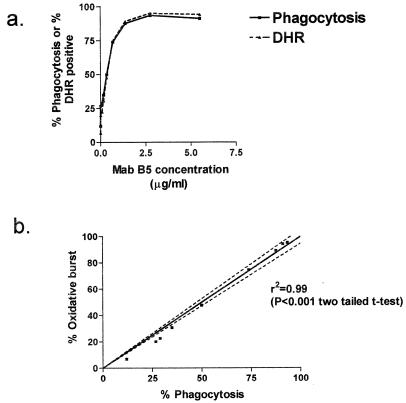
The OPA was adapted for monitoring oxidative burst within neutrophils, using DHR-123 (FL1 green channel) and fluorescently RR-X-labeled N. meningitidis bacteria (FL2 red channel). There was a significant correlation between oxidative burst (intracellular killing) using DHR-123 and phagocytosis of whole ethanol-fixed N. meningitidis strain MC58 bacteria with dilutions of MAb B5 (in the presence of a fixed concentration of complement) (r2 = 0.99, P < 0.0001, two-tailed t test) (Fig. 7a and b). A similar correlation between phagocytosis and oxidative burst was observed using L3 galE LPS-coated beads opsonized with dilutions of MAb B5 (r2 = 0.79 to 0.99) (data not shown). The phagocytosis of the coated beads was apparently not affected by the presence of the DHR-123, since the results were identical in the absence of DHR-123 (r2 = 0.78 to 0.96 and 0.79 to 1.0, respectively) (data not shown).
FIG. 7.
(a) Simultaneous measurement of phagocytosis (solid line) and quantitation of oxidative burst within the PMNm using DHR (oxidative burst) (dashed line) with fluorescently labeled (RR-X) N. meningitidis serogroup B (strain MC58) opsonized with different concentrations of MAb B5, human complement, and PMNms. (b) Correlation between phagocytosis of N. meningitidis strain MC58 by PMNms and quantitation of oxidative burst within PMNm with DHR (oxidative burst).
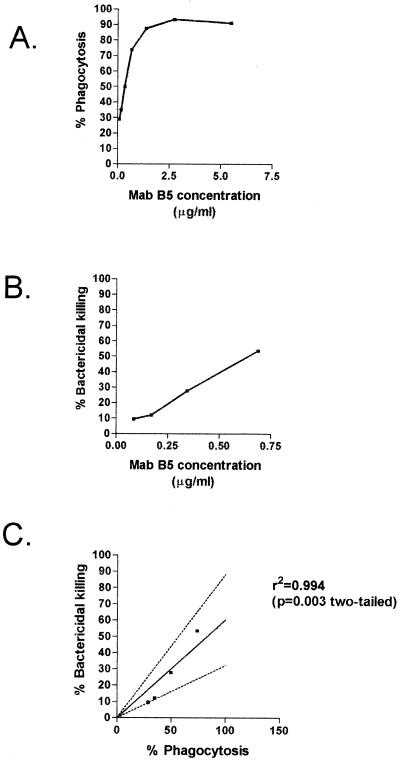
Correlation between results of opsonic and SBA assays using MAb B5.
There was a correlation between OPA and SBA with reciprocal twofold dilutions of MAb B5 against N. meningitidis serogroup B MC58 galE strain using a human complement source (r2 = 0.994, P = 0.003, two-tailed) (Fig. 8). This trend in SBA was also shown with increasing concentrations of MAb B5 and normal mouse serum as the complement source against N. meningitidis MC58 galE mutant (data not shown).
FIG. 8.
Correlation between phagocytosis by PMNms in OPA (A) and serum bactericidal killing by SBA (B) of reciprocal dilutions of MAb B5 against N. meningitidis MC58 galE mutant and human complement source. (C) Correlation between serum bactericidal killing and phagocytosis with dilutions of MAb B5 against N. meningitidis MC58 galE mutant.
DISCUSSION
Adaption of a flow cytometric OPA (23) with ethanol-fixed fluorescently stained N. meningitidis serogroup B bacteria and LPS-coated beads has demonstrated that inner core LPS antibodies are opsonic with human complement and PMNms. Inhibition studies with (i) MAb B5 preadsorbed with purified galE LPS and (ii) human sera taken during or following N. meningitidis disease preadsorbed with purified galE LPS have demonstrated that opsonic activity can be inhibited in a dose-dependent manner.
The specificity of the OPA for galE LPS antibodies has been demonstrated using MAb B5 (specific for galE LPS) and prior adsorption of MAb B5 or test sera with purified specific galE LPS. This was shown for ethanol-fixed N. meningitidis bacteria and galE LPS-coated beads. The short opsonization and incubation times (10 min) and the initial ratio of beads or bacteria to PMNms in the OPA were selected to favor antigen-specific, rather than nonspecific opsonophagocytosis for both bacteria and beads (20, 23). If phagocytes are exposed to a high quantity of beads without antigens on the surface (for example, 100 beads per PMN) and longer incubation times are used (for example, 1 h), a certain amount of beads will be taken up nonspecifically (7). In addition to the incubation conditions, the blocking step with BSA (after attaching antigen) further minimizes the possibility of other antibodies, other than those recognizing bead-attached antigen, attaching to the bead. Further evidence that the OPA is measuring antigen-specific phagocytosis is demonstrated using control beads (BSA coated), which in the presence of MAb B5 and complement give approximately 5% phagocytosis (data not shown). Lehmann et al. (23) clearly demonstrate that serum from patients in the convalescent phase of meningococcal disease induce higher phagocytosis of meningococcal outer membrane vesicle beads than acute-phase serum from the same person, which indicates that this OPA using similar incubation conditions was antigen specific (23).
The differences observed in specificity of naturally occurring antibodies produced during different stages of invasive N. meningitidis disease may be due to the quality of antibody response, e.g., avidity and isotype. Other factors such as how the serum was stored and treated could also affect the functional response.
This study suggested that specific galE LPS antibodies (IgG) are opsonic in sera from healthy and diseased individuals and are in part responsible for the uptake of wild-type N. meningitidis serogroup B bacteria by PMNms. Therefore, OP activity could be used as an important in vitro correlate of protection that can be used alongside SBA. There was a good correlation between phagocytosis of bacteria and LPS-coated beads with oxidative burst within the PMNm. This agreed with previous studies using N. meningitidis and protein-coated beads (22) and demonstrated that bacteria and beads were not only taken up by PMNms but also trigger the oxidative burst within the cells. MAb B5 has been shown to be opsonic and to have bactericidal activity against MC58 galE mutant using a human complement source (Fig. 8).
The OPA was very reproducible over repeated experiments using human donor peripheral blood PMNms (data not shown). High and low standards enabled assay performance to be compared on consecutive runs. The batch-to-batch variation in the efficiency of labeling of N. meningitidis bacteria was a major variable in OPA under standard conditions. Therefore it was important to use the same high and low standards to standardize the assay. This is one of the potential advantages of using antigen-coated fluorescent beads that are uniformly fluorescently labeled within the latex bead. Semiquantitation of LPS coating is possible by dissociation of LPS from beads by boiling and estimating the concentration on silver-stained tricine gels against a standard of known concentration. The purity of the LPS has been shown by silver-stained gels (data not shown), and the LPS samples have been characterized by electrospray mass spectrometric analysis as described previously (31). Therefore, there is no significant protein contamination in these preparations that would give falsely elevated antibody responses.
OPAs using pneumococci as target cells have been developed for the quantitation of antipneumococcal antibodies (26), and good correlations between OPA and passive protection in an infant rat model for pneumococcal disease have been shown (19). For pneumococcal antibodies in adults, there is a correlation between reduced functional OP antibody and reduced avidity as measured by ELISA (33). In vitro OPAs using pneumococci and cultured phagocytic cells (HL-60) correlated with point estimates of vaccine efficacy with a pneumococcal multivalent vaccine and were therefore suggested to be a better indicator of immune response to vaccination than ELISA IgG measurements (34). Also, Guy et al. have recently adapted the antigen-specific OPA method (23, 24) to evaluate functional OP activity after vaccination with pneumococcal polysaccharide conjugates (12). Cultured cell lines of phagocytes have been preferred to facilitate reproducibility in the OPAs evaluating antipneumococcal antibodies. However, the cultured cells may not express the appropriate Fc receptor phenotype to mimic human PMNms in vivo and are technically difficult to prepare. Therefore, we employ freshly prepared PMNms from humans in our OPA studies.
Individual donor variations in PMNms with the same standard sera and N. meningitidis bacteria may be due to the Fcy receptor type (3). However, there is no direct evidence to suggest that the homozygous or heterologous Fcy R type alone had an effect on OP ability of the PMNms with different serogroups of N. meningitidis.
A human complement source from a hypogammaglobulinemic patient was used for the complement-dependent OPA The advantages in using human complement rather than baby rabbit complement are that the results are more reliable and reproducible and there is less nonspecific background (44).
FCM surface labeling studies of MAb B5 binding to live compared to ethanol-fixed N. meningitidis galE mutant cells suggested variations in antibody binding. The basis of this variation is not clear. It could be due to the number of bacteria (live exponentially dividing N. meningitidis bacteria could bind MAb B5 better) or the growth phase (live bacteria in the exponential phase compared to fixed N. meningitidis in the stationary phase). Another possibility is that variation represents differences in the availability of the MAb B5 epitope. In an accessibility assay using monolayers of human umbilical vein endothelial cells infected with N. meningitidis, MAb B5 binds close to 100% of N. meningitidis strain MC58 (ethanol fixed) (31). The percentage of live N. meningitidis bacteria that bind MAb B5 in galE mutant (96.9%) compared to wild-type MC58 (86.8%) may be significant in vivo. Further studies are required to determine if this difference in binding with fixed and live N. meningitidis reflects the growth phase, i.e., whether exponentially dividing N. meningitidis bind MAb B5 to a greater extent or, alternatively, the number of bacteria is an independent variable. Previous studies using fixed N. meningitidis have suggested that the availability of the PorB epitope may be altered by ethanol fixation compared to native, live meningococci (A. Aase, A. E. Hoiby, and T. Michelsen, Abstr. Int. Pathogenic Neisseria Conf., p. 284, 1998). A flow cytometric assay to measure serum opsonins to serogroup B N. meningitidis demonstrated optimal sensitivity using viable bacteria compared to that using heat-, formalin-, or ethanol-inactivated N. meningitidis (39). Other workers standardizing flow cytometric and chemiluminescence assays for N. meningitidis opsonins in human sera have demonstrated highly reproducible and sensitive methods using ethanol-fixed N. meningitidis (11, 13). Future studies will compare levels of epitope (MAb B5) accessibility by other methods of fixation, e.g., UV irradiation against live N. meningitidis.
Previous studies by our group have shown that the MAb B5 epitope is consistently available to antibodies in wild-type N. meningitidis and during disease (30, 31). In addition to FCM surface labeling experiments (Fig. 1), the accessibility of the MAb B5 epitope in ethanol-fixed wild-type N. meningitidis, in the presence of capsule, has been demonstrated by immunofluorescence microscopy (31). Using electron microscopy, MAb B5 binding to both N. meningitidis wild-type and galE mutant cells has also been shown (data not shown). MAb B5 binds to both N. meningitidis wild-type and galE LPS bands by Western blot analysis (data not shown). SBAs with N. meningitidis wild-type strain MC58 have demonstrated that MAb B5 does have bactericidal activity but that a higher concentration of antibody than that needed by the galE mutant is required (data not shown). Together, these data demonstrate that although binding of MAb B5 to the galE mutant was greater than wild-type binding, the same trends were observed in both OPAs and SBAs, and that the MAb B5 epitope N. meningitidis is accessible in the wild type.
Previous studies have correlated IgG1 and IgG2 with functional OP antibody activity with N. meningitidis (21). In this study we have looked at the effect of total IgG only. Future studies will look at the effect of isotype on OP activity. IgM may be responsible for nonspecific uptake in the OPA. The importance of IgG compared to IgM in the OPA is not difficult to understand, as the Fcy receptors are the major receptors involved in opsonization (3). To determine whether IgM is contributing to uptake in the phagocytosis assay, sera could be semipurified with protein G and IgG-depleted, IgM-rich sera could be used in an OPA. Preliminary data suggested that IgM may be having a nonspecific effect, and OP activity was primarily due to IgG (data not shown). Similar studies with outer membrane proteins of N. meningitidis PorA and PorB antibodies demonstrated that IgG1 and IgG3 are the important isotypes for OP activity and antiporin IgM did not correlate with OP activity (22).
Our studies demonstrate that specific inner core LPS antibodies (IgG) are functional in human serum and that the OPA may provide a useful correlate of in vitro protection to complement the existing SBA. Studies are under way to look at the functional activity of inner core LPS antibodies in vivo using the infant rat model (adapted from Moe et al [29]). Preliminary experiments demonstrate that MAb B5 is able to passively protect 5-day-old infant rats against intraperitoneal challenge with a dose of 108 N. meningitidis MC58 galE mutant organisms (data not shown). The functional data from OPAs and SBAs and an in vivo model demonstrate the importance of MAb B5 epitope (L3 galE) in combination with other inner core LPS glycoforms (L2/L4 galE) in an LPS-based vaccine against N. meningitidis serogroup B invasive disease.
ACKNOWLEDGMENTS
This work was supported by programme grants from the Medical Research Council (E.R.M.) and Wellcome Trust (E.R.M. and K.M.). Funding was also provided by the National Meningitis Trust (E.R.M. and J.P.) and Chiron Vaccines (E.R.M. and J.R.).
We acknowledge David J. Ferguson for expert technical help with the electron microscopy images.
REFERENCES
- 1.Aase A, Bjune G, Hoiby E A, Rosenqvist E, Pedersen A K, Michaelsen T E. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect Immun. 1995;63:3531–3536. doi: 10.1128/iai.63.9.3531-3536.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. Meningococcal disease—New England, 1993–1999. Morb Mortal Wkly Rep. 1999;48:629–633. [PubMed] [Google Scholar]
- 3.Bredius R G M, Derkx B H F, Fijen A P, de Wit T P M, de Haas M, Weening R S, van de Winkel J G J, Out T A. Fcy receptor IIa (CD32) polymorphism in fulminant meningococcal shock in children. J Infect Dis. 1994;170:848–853. doi: 10.1093/infdis/170.4.848. [DOI] [PubMed] [Google Scholar]
- 4.Carlin N I, Gidney M A, Lindberg A A, Bundle D R. Characterisation of Shigella flexneri-specific murine monoclonal antibodies by chemically defined glycoconjugates. J Immunol. 1986;137:2361–2366. [PubMed] [Google Scholar]
- 5.Delvig A A, Michaelsen T E, Aase A, Hoiby E A, Rosenqvist E. Vaccine-induced IgG antibodies to the linear epitope on the PorB outer membrane protein promote opsonophagocytosis of Neisseria meningitidis by human neutrophils. Clin Immunol Immunopathol. 1997;84:27–35. doi: 10.1006/clin.1997.4360. [DOI] [PubMed] [Google Scholar]
- 6.Drogari-Apiranthitou M, Fijen C A P, Thiel S, Platonov A, Jensen L, Dankert J, Kuijper E J. The effect of mannan-binding lectin on opsonophagocytosis of Neisseria meningitidis. Immunopharmacology. 1997;38:93–99. doi: 10.1016/s0162-3109(97)00081-7. [DOI] [PubMed] [Google Scholar]
- 7.Dunn P A, Tyrer H W. Quantitation of neutrophil phagocytosis, using fluorescent latex beads: correlation of microscopy and flow cytometry. J Lab Clin Med. 1981;98:374–381. [PubMed] [Google Scholar]
- 8.Goldblatt D, Vaz A R, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis. 1998;177:1112–1115. doi: 10.1086/517407. [DOI] [PubMed] [Google Scholar]
- 9.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granoff D M, Maslanka S E, Carlone G M, Plikaytis B D, Santos G F, Mokatrin A, Raff H V. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol. 1998;5:479–485. doi: 10.1128/cdli.5.4.479-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttormsen H-K, Bjerknes R, Naess A, Lehmann V, Halstensen A, Sørnes S, Solberg C O. Cross-reacting opsonins in patients with meningococcal disease. Infect Immun. 1992;60:2777–2783. doi: 10.1128/iai.60.7.2777-2783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy B, Testart C, Gimenez S, Sanchez V, Lheritier P, Rossin D, Mignon M, Danve B, Trannoy E. Comparison of polymorphonuclear cells from healthy donors and differentiated HL-60 cells as phagocytes in an opsonophagocytosis assay using antigen-coated fluorescent beads. Clin Diagn Lab Immunol. 2000;7:314–317. doi: 10.1128/cdli.7.2.314-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halstensen A, Haneberg B. Standardization of a chemiluminescence method for the measurement of meningococcal opsonins using ethanol-fixed meningococci. APMIS Scand. 1987;95:155–160. doi: 10.1111/j.1699-0463.1987.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 14.Halstensen A, Haneberg B, Glette J, Sandberg S, Solberg C O. Factors important for the measurements of chemiluminescence production by polymorphonuclear leukocytes. J Immunol Methods. 1986;88:121–128. doi: 10.1016/0022-1759(86)90060-8. [DOI] [PubMed] [Google Scholar]
- 15.Høiby E A, Rosenqvist E, Froholm L O, Bjune G, Ferling B, Nokleby H, Ronnild E. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann (Oslo) 1991;14:147–156. [PubMed] [Google Scholar]
- 16.Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol. 1979;9:186–188. doi: 10.1128/jcm.9.2.186-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ison C A, Anwar N, Cole M J, Galassin R, Heyderman R S, Klein N J, West J, Pollard A J, Morley S, Levin M. Assessment of immune response to meningococcal disease: comparison of a whole-blood assay and the serum bactericidal assay. Microb Pathog. 1999;27:207–214. doi: 10.1006/mpat.1999.0296. [DOI] [PubMed] [Google Scholar]
- 18.Jennings M P, van der Ley P, Wilks K E, Maskell D J, Poolman J T, Moxon E R. Cloning and molecular analysis of the galE gene of Neisseria meningitidis and its role in lipopolysaccharide biosynthesis. Mol Microbiol. 1993;10:361–369. [PubMed] [Google Scholar]
- 19.Johnson S E, Rubin L, Romero-Steiner S, Dykes J K, Pais L B, Rizvi A, Ades E, Carlone G M. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J Infect Dis. 1999;180:133–140. doi: 10.1086/314845. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann A K, Sørnes S, Halstensen A. Phagocytosis: measurement of flow cytometry. J Immunol Methods. 2000;243:229–242. doi: 10.1016/s0022-1759(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann A K, Gorringe A R, Reddin K M, West K, Smith I, Halstensen A. Human opsonins induced during meningococcal disease recognize transferrin binding protein complexes. Infect Immun. 1999;67:6526–6532. doi: 10.1128/iai.67.12.6526-6532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann A K, Halstensen A, Aaberge I S, Hoist J, Michaelson T E, Sornes S, Wetzler L M, Guttormsen H K. Human opsonins induced during meningococcal disease recognize outer membrane proteins PorA and PorB. Infect Immun. 1999;67:2532–2560. doi: 10.1128/iai.67.5.2552-2560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann A K, Halstensen A, Hoist J, Bassoe C-F. Functional assays for evaluation of serogroup B meningococcal structures as mediators of human opsonophagocytosis. J Immunol Methods. 1997;200:55–68. doi: 10.1016/s0022-1759(96)00185-8. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann A K, Halstensen A, Bassoe C-F. Flow cytometric quantitation of human opsonin-dependent phagocytosis and oxidative burst responses to meningococcal antigens. Cytometry. 1998;33:406–413. doi: 10.1002/(sici)1097-0320(19981201)33:4<406::aid-cyto3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Mandrell R E, Azmi F H, Granoff D M. Complement mediated bactericidal activity of human antibodies to poly α 2–8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis group B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- 26.Martinez J E, Romero-Steiner S, Pilishvilli T, Barnard S, Schinsky J, Goldblatt D, Carlone G M. A flow cytometric opsonophagocytosis assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin Diagn Lab Immunol. 1999;6:581–586. doi: 10.1128/cdli.6.4.581-586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maslanka S E, Gheesling L L, Libutti D E, Donaldson K B, Harakeh H S, Dykes J K, Arhin F F, Devi S J, Frasch C E, Huang J C, Kriz-Kuzemenska P, Lemmon R D, Lorange M, Peeters C C, Quataert S, Tai J Y, Carlone G M. Standardization and multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4:156–167. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masoud H, Moxon E R, Martin A, Krajcarski D, Richards J C. Structure of the variable and conserved lipopolysaccharide oligosaccharide epitopes expressed by Haemophilus influenzae serotype b Eagan. Biochemistry. 1997;36:2091–2103. doi: 10.1021/bi961989y. [DOI] [PubMed] [Google Scholar]
- 29.Moe G R, Tan S, Granoff D M. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect Immun. 1999;67:5664–5675. doi: 10.1128/iai.67.11.5664-5675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plested J S, Gidney M-A, Coull P A, Griffiths H G, Herbert M A, Bird A G, Richards J C, Moxon E R. Enzyme linked immunosorbent assay (ELISA) for the detection of serum antibodies to the inner core lipopolysaccharide of Neisseria meningitidis group B. J Immunol Methods. 2000;237:73–84. doi: 10.1016/s0022-1759(00)00142-3. [DOI] [PubMed] [Google Scholar]
- 31.Plested J S, Makepeace K, Jennings M P, Gidney M-A, Lacelle S, Brisson J-R, Cox A D, Martin A, Bird A G, Tang C M, Mackinnon F G, Richards J C, Moxon E R. Conservation and accessibility of an inner core lipopolysaccharide epitope of Neisseria meningitidis. Infect Immun. 1999;67:5417–5426. doi: 10.1128/iai.67.10.5417-5426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racoosin J A, Whitney C G, Conover C S, Diaz P S. Serogroup Y meningococcal disease in Chicago, 1991–1997. JAMA. 1998;280:2094–2098. doi: 10.1001/jama.280.24.2094. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Steiner S, Musher D M, Cetron M S, Pais L B, Groover J E, Fiore A E, Plikaytis B D, Carlone G M. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Investig Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross S C, Rosenthal P J, Berberich H M, Densen P. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987;155:1266–1275. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- 36.Rothe G, Oser A, Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil activity in neutrophil granulocytes. Naturwissenschaften. 1988;75:354–453. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- 37.Shulman M, Wilde C D, Kohler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978;276:269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- 38.Sjursen H, Wedege E, Rosenqvist E, Naess A, Halstenen A, Matre R, Solberg C O. IgG subclass antibodies to serogroup B meningococcal outer membrane antigens following infections and vaccinations. APMIS. 1990;98:1061–1069. doi: 10.1111/j.1699-0463.1990.tb05035.x. [DOI] [PubMed] [Google Scholar]
- 39.Sjursen H, Bjerknes R, Halstensen A, Naess A, Sørnes S, Solberg C O. Flow cytometric assay for the measurement of serum opsonins to Neisseria meningitidis serogroup B, serotype 15. J Immunol Methods. 1989;116:235–243. doi: 10.1016/0022-1759(89)90209-3. [DOI] [PubMed] [Google Scholar]
- 40.Soderstrom C, Braconier J H, Kayhty H, Sjoholm A G, Thuresson B. Immune response to a tetravalent meningococcal vaccine: opsonic and bactericidal functions of normal and properdin deficient sera. Eur J Clin Microbiol Infect Dis. 1989;8:220–224. doi: 10.1007/BF01965264. [DOI] [PubMed] [Google Scholar]
- 41.Virji M, Watt S M, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 42.Virji M, Makepeace K, Peak I R A, Ferguson D J P, Jennings M P, Moxon E R. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol Microbiol. 1995;18:741–754. doi: 10.1111/j.1365-2958.1995.mmi_18040741.x. [DOI] [PubMed] [Google Scholar]
- 43.Westphal O, Jann J K. Bacterial lipopolysaccharides extraction with water-phenol and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 44.Zollinger W D, Mandrell R E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zollinger W D, Moran E E, Sarvamangala J N D, Frasch C E. Bactericidal antibody responses of juvenille rhesus monkeys immunized with group B Neisseria meningitidis capsular polysaccharide-protein conjugate vaccines. Infect Immun. 1997;65:1053–1060. doi: 10.1128/iai.65.3.1053-1060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]