Abstract
Objectives
To compare age‐adjusted all‐cause and CVD mortality, relative to the general female population, for women registered for fertility treatment who received it and those who did not.
Design
Prospective cohort study; analysis of Monash IVF clinical registries data, 1975–2018, linked with National Death Index mortality data.
Participants
All women who registered for fertility treatment at Monash IVF (Melbourne, Victoria), 1 January 1975 – 1 January 2014, followed until 31 December 2018.
Main outcome measures
Standardised mortality ratios (SMRs) for all‐cause and CVD mortality, for women who did or did not undergo fertility treatment; SMRs stratified by area‐level socio‐economic disadvantage (SEIFA Index of Relative Socioeconomic Disadvantage [IRSD]) and (for women who underwent treatment), by stimulated cycle number and mean oocytes/cycle categories.
Results
Of 44 149 women registered for fertility treatment, 33 520 underwent treatment (66.4%), 10 629 did not. After adjustment for age, both all‐cause (SMR, 0.58; 95% CI, 0.54–0.62) and CVD mortality (SMR, 0.41; 95% CI, 0.32–0.53) were lower than for the general female population. All‐cause mortality was similar for women registered with Monash IVF who did (SMR, 0.55; 95% CI, 0.50–0.60) or did not undergo fertility treatment (SMR, 0.63; 95% CI, 0.56–0.70). The SMR was lowest for both treated and untreated women in the fifth IRSD quintile (least disadvantage), but the difference was statistically significant only for untreated women. CVD mortality was lower for registered women who underwent fertility treatment (SMR, 0.29; 95% CI, 0.19–0.43) than for those who did not (SMR, 0.58; 95% CI, 0.42–0.81).
Conclusion
Fertility treatment does not increase long term all‐cause or CVD mortality risk. Lower mortality among women registered for fertility treatment probably reflected their lower socio‐economic disadvantage.
Keywords: Reproductive techniques, assisted; Pregnancy; Mortality; Family planning
The known: Fertility therapy may affect the cardiovascular health of women, but this question has not been investigated in Australian women, and the limited data on all‐cause mortality are 20 years old.
The new: Analysis of linked Monash IVF records for 44 149 Australian women who registered for fertility treatment during 1975–2014 and National Death Index data (to the end of 2018) indicate that neither all‐cause nor cardiovascular disease mortality were higher for women treated with fertility drugs than for those who were not.
The implications: Our findings provide important information for clinicians advising women about the long term safety of fertility treatment with respect to cardiovascular health.
More than eight million babies have been born to women using assisted reproduction technologies. 1 Nevertheless, concerns about the long term safety of fertility therapy have been expressed, including its effect on the risk of cardiovascular disease (CVD) in later life. 2 Fertility treatment could lead to adverse cardiovascular events by inducing background thrombosis, activating the renin–angiotensin system, or by vascular injury caused by ovarian hyperstimulation. 3 Fertility treatment is associated with increased risks of pregnancy‐related complications such as maternal metabolic syndromes (eg, gestational diabetes, hypertension), which are in turn associated with higher long term CVD risk for both mothers and their children. 4
A 2001 study found that all‐cause mortality among women who registered for in vitro fertilisation (IVF) in Australia (whether ultimately treated or not) was significantly lower than for other women of the same age. This phenomenon reflects factors that differentiate women who seek fertility treatment from other women, including greater socio‐economic advantage and higher health status (the “healthy patient effect”). 5 However, CVD mortality has not been investigated in Australian women who have undergone fertility treatment, and long term CVD health data for older women (60 years or older), at particular risk of CVD and major coronary events, are also limited. 6
We therefore analysed data collected since the inception of assisted reproduction services in Australia to determine long term all‐cause and CVD mortality, relative to the general female population, for women who registered with Monash IVF (Melbourne, Victoria; https://monashivf.com) and either proceeded to fertility treatment or did not.
Methods
We undertook a retrospective cohort study, analysing clinical records (1975–2018) in the Monash Cancer IVF database (women registered 1975–1990) 7 and the Monash IVF clinical registry (1982–2014). All‐cause and CVD mortality among women who underwent fertility treatment were determined by data linkage with National Death Index (NDI) deaths and cause of death data.
Study population
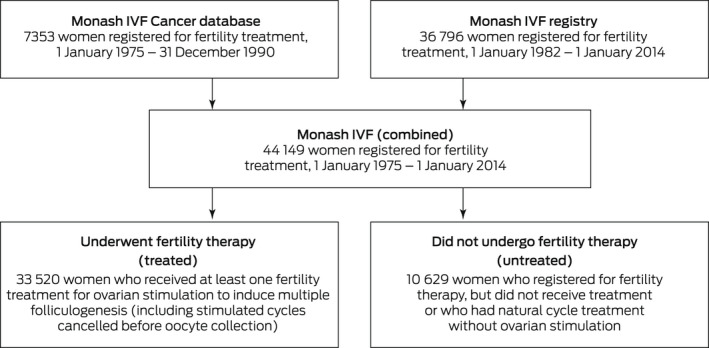
We initially included data for all 44 149 women who registered for fertility treatment at Monash IVF clinics during 1 January 1975 – 1 January 2014. We excluded women whose usual place of residence was outside Australia and those for whom the available information was inadequate for data linkage. Women were classified as “treated” or “untreated” according to whether they were treated with fertility drugs. Women for whom at least one ovarian stimulation to induce multiple folliculogenesis was recorded were classified as “treated”. Stimulated cycles cancelled before oocyte collection were also included. Women who registered for fertility therapy but did not undergo treatment or who had natural cycle treatment without ovarian stimulation were classified as “untreated” (Box 1). Reasons for not proceeding with fertility treatment were not routinely recorded, but included pregnancy while on the waiting list, pursuit of other treatment options, financial or relationship difficulties, and other personal reasons. Data were checked for internal consistency and completeness, and duplicate records removed.
Box 1. Monash IVF clinical database sources for women registered for fertility treatment, 1 January 1975 – 1 January 2014.
Data linkage and analysis
For each woman, we extracted their name and date of birth (for data linkage), residential postcode, and date of registration with the clinic, as well as information about their fertility treatment: type of treatment (stimulated fresh cycle, frozen embryo transfer), fertility drugs used for ovarian stimulation, number of oocytes collected, number of stimulated cycles, and infertility aetiology.
The NDI data linkage process is summarised in the Supporting Information, figure 1. The NDI covers the period 1 January 1980 – 31 December 2018. Monash IVF records indicated that 527 of 539 women who had registered prior to 1980 (98%) were alive on 1 January 1980. Linkage was based on given names (up to three), surname, sex, and date of birth. Deaths were attributed to CVD when the underlying cause of death was coded (International Classification of Diseases, tenth revision) as I10–I15, I20–I25, I46.1, I48, I50–I99, or R96, or (in three cases) when one of these codes was recorded anywhere in the death certificate and the underlying cause of death was uncomplicated diabetes (E109, E119, E149) or unspecified hyperlipidaemia (E785).
Statistical analysis
Statistical analyses were undertaken in Stata/IC 15.1 and SPSS Statistics 26 (IBM). Women were followed until 31 December 2018. Mortality rates (per 1000 person‐years) were calculated for all‐cause and CVD deaths.
For treated women, the mean numbers of oocytes per stimulated cycle (categorised as fewer than six, or six or more) and of stimulated cycles (fewer than three, three or more) were calculated. Data are summarised as medians with interquartile ranges (IQRs), or counts and proportions.
To compare all‐cause and CVD mortality for treated and untreated women, we calculated standardised mortality ratios (SMRs). The SMR is the ratio of the number of deaths to the expected number, based on numbers for the age‐matched general female population derived from Australian Institute of Health and Welfare General Record of Incidence of Mortality (GRIM) books 8 (Supporting Information, table 1). We also calculated SMRs stratified by area‐level socio‐economic disadvantage (Socio‐Economic Indexes for Areas: Index of Relative Socioeconomic Disadvantage [IRSD]; Supporting Information, table 2), based on residential postcode, 9 and, for treated women, by stimulated cycle number and mean oocytes/cycle categories. Confidence intervals (95% CIs) were calculated using Poisson distribution limits. Differences between SMRs were assessed in z tests; P < 0.05 was deemed statistically significant.
The association of fertility treatment with all‐cause and CVD mortality was assessed in Cox proportional hazards regression models by estimating hazard ratios (HRs) with 95% CIs. The follow‐up period for all‐cause and CVD mortality was to the date of death or 31 December 2018. The assumption of proportional hazards was satisfied in graphs of log‐negative log plots of the relative hazards by time and by scaled Schoenfeld residuals. Initial models were adjusted for age at treatment and data source (Monash IVF Cancer database or registry). Models were adjusted for socio‐economic disadvantage (IRSD), number of stimulated cycles, and number of oocytes per stimulated cycle.
Ethics approval
The study was approved by the Monash Health Human Research Ethics Committee (LNR/17/MonH/385), and by the Australian Institute of Health and Welfare Ethics Committee (EO2018/2/447), which waived the requirement for individual consent.
Results
Of 44 149 women registered for fertility treatment at Monash IVF during 1 January 1975 – 1 January 2014, 33 519 had undergone fertility treatment (66.4%). Data for two women (one treated, one untreated) were excluded from our analysis because their registration dates were not reliable.
Because of the difference in registration periods, the median age at follow‐up of women in the Monash IVF Cancer database (65 years; IQR, 62–69 years) was higher than for those in the Monash IVF clinical registry (49 years; IQR, 44–55 years). The median age at registration was slightly higher for treated (34 years; IQR, 31–38 years) than for untreated women (33 years; IQR, 29–37 years); median follow‐up time was 14 years (IQR, 9–21 years; range, 0–43 years) for treated women, 27 years (IQR, 16–33 years; range, 0–44 years) for untreated women. An aetiology for infertility was recorded for 26 447 women (60%), most frequently “unexplained” (7234 women, 27% of recorded aetiologies) or tubal origin (6387 women, 24%). Women who received fertility treatment underwent a median of two (IQR, 1–4) stimulated cycles and produced a median of seven (IQR, 3–11) oocytes per stimulated cycle. Follicle‐stimulating hormone was the most frequent drug therapy (29 637 treated women, 88.4%); hormone replacement therapy was used by 6267 women (18.7%), clomiphene citrate by 2419 (7.2%) (Box 2).
Box 2. Characteristics of 44 147 women registered for fertility treatment at Monash IVF, 1 January 1975 – 1 January 2014, by fertility drug treatment* .
| Characteristic | Treated | Untreated |
|---|---|---|
| Number of women | 33 519 | 10 628 |
| Monash IVF Cancer database | 3085 | 4267 |
| Monash IVF registry | 30 434 | 6361 |
| Age (years) | ||
| At entry, median (IQR) | 34 (31–38) | 33 (29–37) |
| At follow‐up, median (IQR) | 49 (44–56) | 59 (50–65) |
| < 40 years | 3868 (11.5%) | 609 (5.7%) |
| 40–49 years | 14 126 (42.1%) | 1935 (18.2%) |
| 50–59 years | 10 831 (32.3%) | 3321 (31.2%) |
| 60–69 years | 4030 (12.0%) | 4024 (37.9%) |
| > 70 years | 664 (2.0%) | 739 (7.0%) |
| Aetiology | ||
| Aetiology recorded | 20 428 (60.9%) | 6018 (56.6%) |
| Unexplained | 5453 [26.7%] | 1781 [29.6%] |
| Tubal | 4274 [20.9%] | 2112 [35.1%] |
| Endometrial | 2402 [11.8%] | 462 [7.7%] |
| Ovarian | 2139 [10.5%] | 378 [6.3%] |
| Multiple factors | 3045 [14.9%] | 140 [2.3%] |
| Male factor | 1534 [7.5%] | 1036 [17.2%] |
| Other | 1581 [7.7%] | 109 [1.8%] |
| No aetiology recorded | 13 091 (39.1%) | 4610 (43.4%) |
| Stimulated cycles, median (IQR) | 2 (1–4) | — |
| 1 or 2 | 17 412 (51.9%) | — |
| 3 or more | 16 107 (48.1%) | — |
| Oocytes/cycle, median (IQR) | 7.0 (3–11) | — |
| 1–5 | 12 913 (38.5%) | — |
| 6 or more | 17 470 (52.1%) | — |
| Missing data | 3136 (9.4%) | — |
| Fertility drug type † | ||
| Follicle‐stimulating hormone | 29 637 (88.4%) | — |
| Hormone replacement therapy | 6267 (18.7%) | — |
| Clomiphene citrate | 2419 (7.2%) | — |
| Unknown | 3068 (9.1%) | — |
IQR = interquartile range.
Excludes two women (one in each group) for whom registration dates were not reliable.
Women who have ever been treated with drug combination; multiple treatments possible.
During 785 995 person‐years of follow‐up (44 147 women registered for fertility therapy), 753 all‐cause deaths (0.96 [95% CI, 0.89–1.0] deaths per 1000 person‐years) and 59 CVD deaths (0.07 [95% CI, 0.06–0.09] per 1000 person‐years) were recorded. For treated women, 427 all‐cause deaths (0.81 [95% CI, 0.73–0.88] deaths per 1000 person‐years) and 24 CVD deaths (0.05 [95% CI, 0.03–0.06] deaths per 1000 person‐years) were recorded during 529 990 person‐years of follow‐up. For untreated women, 326 all‐cause deaths (1.3 [95% CI, 1.1–1.4] deaths per 1000 person‐years) and 35 CVD deaths (0.14 [95% CI, 0.09–0.18] deaths per 1000 person‐years) were recorded during 256 005 person‐years of follow‐up.
All‐cause mortality
The all‐cause SMR for all women registered with Monash IVF for fertility treatment was 0.58 (95% CI, 0.54–0.62). All‐cause mortality was similar for women registered with Monash IVF who did (SMR, 0.55; 95% CI, 0.50–0.60) or did not undergo fertility treatment (SMR, 0.63; 95% CI, 0.56–0.70), and also by data source (Monash IVF Cancer database or registry). The SMR was lower for treated women (combined data sources) who underwent three or more stimulated cycles (0.48; 95% CI, 0.42–0.56) than for those who underwent fewer cycles (0.60; 95% CI, 0.53–0.68), but the difference in SMR by oocyte production category was not statistically significant (Box 3). Stratified by IRSD level, the SMR was lowest for treated and untreated women in the fifth quintile (least disadvantage), but the difference between the first and fifth quintiles was statistically significant only for untreated women (Box 4).
Box 3. Standardised mortality ratios (SMRs) for all‐cause and cardiovascular disease mortality for 44 147 women registered for fertility treatment at Monash IVF, 1 January 1975 – 1 January 2014* .
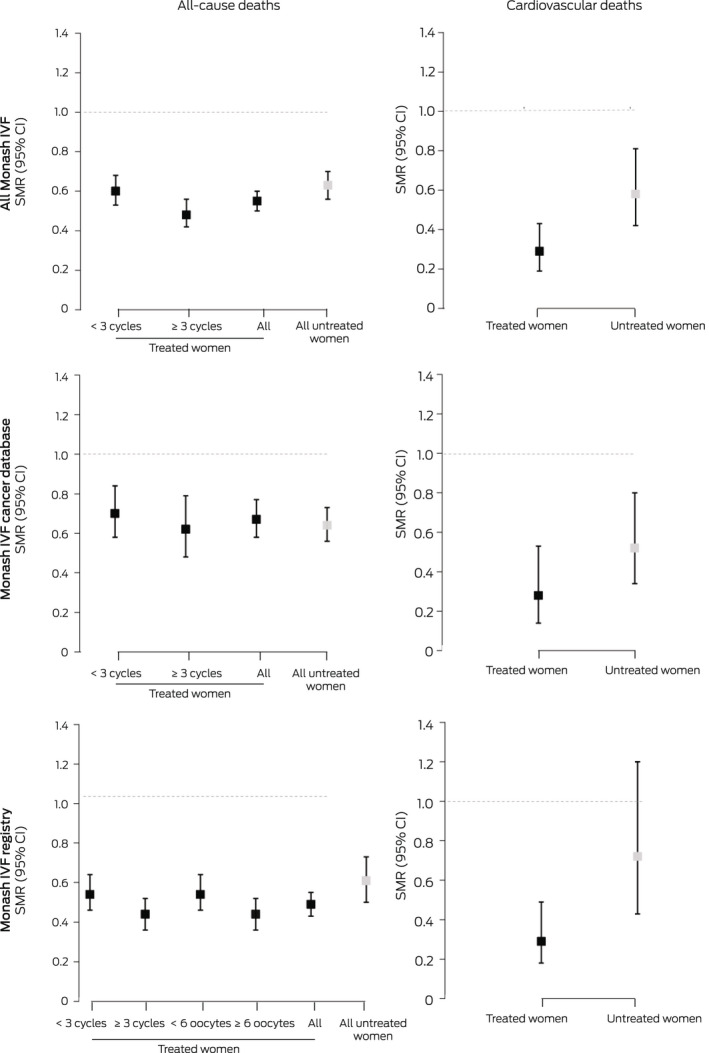
CI = confidence interval. * A total of 33 519 underwent fertility treatment, 10 628 women did not. For cardiovascular mortality, the number of deaths was too small for stratification by stimulated cycle or oocyte production numbers. The data for these graphs are included in the Supporting Information, tables 3–5.
Box 4. All‐cause standardised mortality ratios (SMRs) for 44 147 women registered for fertility treatment at Monash IVF, 1 January 1975 – 1 January 2014, by area‐based Index of Relative Socioeconomic Disadvantage quintile*.
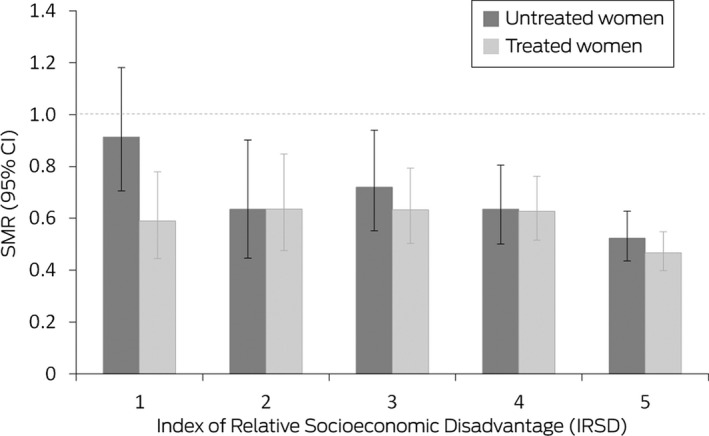
CI = confidence interval. * Level 1 = most disadvantaged, level 5 = least disadvantaged). A total of 33 519 women underwent fertility treatment, 10 628 women did not. The data for these graphs are included in the Supporting Information, table 6.
Cardiovascular disease mortality
The CVD SMR for all women registered with Monash IVF for fertility therapy was 0.41 (95% CI, 0.32–0.53). The SMR was lower for treated than untreated women, both overall (0.29; 95% CI, 0.19–0.43 v 0.58; 95% CI, 0.42–0.81) and for each data source (Box 3). As the number of CVD deaths was small, particularly among treated women, stratification by area level socio‐economic disadvantage, number of stimulated cycles, and oocyte production was not undertaken.
Cox regression analysis
In Cox proportional hazards models with age as the time scale and adjusted for data source, the risk of all‐cause death was similar for women who received fertility treatment and those who did not (adjusted HR, 0.92; 95% CI, 0.79–1.07); CVD mortality risk was lower for women who had received fertility therapy (adjusted HR, 0.47; 95% CI, 0.27–0.81). Similar results were yielded after adjusting for area‐based socio‐economic disadvantage, number of stimulated cycles, and number of oocytes per stimulated cycle (data not shown).
Discussion
We found that all‐cause and CVD mortality, after adjustment for age, were each lower among women registered for fertility treatment with Monash IVF than for the general female population, consistent with the previously reported “healthy patient” phenomenon. 5 Further, fertility therapy was not associated with higher all‐cause or CVD mortality among registered women.
In an earlier study of mortality among women registered for fertility treatment in Australia, the median follow‐up time was eight years. 5 Our study included a broader age range (53% of registered women, and 46% of those who underwent fertility treatment, were at least 50 years old at follow‐up) with an overall median follow‐up time of 15 years. Median follow‐up time was shorter for women who underwent fertility therapy than for those who did not because their proportion was larger in the more recent Monash IVF registry than in the older IVF Cancer database. This difference may reflect advances in treatment options, with greater affordability, success, and accessibility leading to more women deciding to proceed with fertility treatment.
Mortality among women who received fertility therapy, after adjusting for age, was not significantly higher than for those who did not. Further, the SMR was significantly lower for women who had undergone three or more stimulated cycles than for those who had undergone fewer cycles, and the number of oocytes produced was not associated with differences in all‐cause mortality. This suggests that all‐cause mortality does not increase with the intensity of fertility therapy or the follicular response. Our findings are similar to those of overseas cohort studies that found no differences in all‐cause mortality risk (after adjusting for age, educational level, treatment year, partnership status, parity, and comorbidity) between treated and untreated women five 10 or ten years 11 after fertility treatment.
We also found that lower socio‐economic disadvantage was associated with lower mortality among women who had received fertility treatment. This conclusion is consistent with the association between social disadvantage and higher mortality in the general population 12 and the negative association between the affordability and use of assisted reproduction technologies. 13 Women who seek fertility therapy may be healthier than other women of the same age, having modified their behaviour to maximise the chance of conception. 12 Socio‐economic advantage is consequently a major determinant of the “healthy patient” effect with respect to fertility treatment in Australia.
CVD mortality was not higher among women registered for fertility treatment than for the general female population, and among registered women it was lower for women who had undergone treatment. Whether this reduction is biological or explained by selection bias associated with fertility treatment is unclear, as the number of CVD deaths was too low for robust analyses. In Cox proportional hazards models adjusted for age and area‐based socio‐economic disadvantage, the risk of CVD mortality was lower for women who had undergone fertility treatment. However, as we did not include key risk factors for CVD mortality (eg, diabetes, hypertension, physical activity levels, body mass index, individual socio‐economic position), residual confounding was likely in our minimally adjusted models, and inferences about fertility treatment reducing CVD mortality risk should be cautious. Nonetheless, our findings provide reassurance that CVD mortality is not increased, consistent with the findings of a smaller Canadian study (6979 women) of lower risk of a composite outcome of CVD mortality and morbidity among women who underwent successful fertility therapy (adjusted HR, 0.55; 95% CI, 0.41–0.74). 14
Our findings have important clinical implications. About 55 000 women use assisted reproduction technologies in Australia each year. They are counselled about fertility treatment decisions with only limited understanding and discussion of its impact on their longer term health. Our study provides information for discussions by women and their clinicians of the long term safety of fertility therapy, and provides some reassurance for women who have previously undergone such treatment.
Limitations
As the lack of information on CVD risk factors and chronic disease conditions for more than 50% of registered women precluded a comprehensively adjusted analysis, we compared mortality risk with age‐standardised population values to maximise statistical power. Further, as CVD risk was not assessed at registration for fertility treatment, we could not determine its specific contribution to risk. Healthy lifestyle behaviours, greater socio‐economic advantage, and other factors may have led to women undergoing fertility treatment having lower CVD risk prior to therapy, so that any rise in risk linked with treatment may have been less apparent. Nevertheless, socio‐economic status can serve as a proxy for CVD risk, 15 and our adjusting for it may have partially accounted for prior CVD risk. As the year of cycle treatment was available, but not the exact date, exposure was defined as a fixed term (ie, ever received fertility treatment), possibly introducing mortal time bias, and strengthening our caveat regarding associations with reduced CVD mortality.
Both pregnancy and parity contribute to CVD risk. 16 Fertility treatment would have led, for some women, to successful pregnancies and live births, but we could not adjust for this potential confounder because the information was not available in the Monash IVF Cancer database, nor for the untreated women. The comparator group in our analysis comprised women who had registered with Monash IVF for fertility therapy but did not receive it there. We selected this control group to minimise confounding by characteristics and comorbid conditions that are common causes of infertility and CVD (eg, polycystic ovaries, type 2 diabetes, chronic hypertension). However, these women may have received fertility treatment elsewhere during the follow‐up period. Further, the comparator group may have included less healthy women who were advised against pregnancy or to adjust their lifestyle as a first step to conceiving. Finally, the analysed data were derived from a single IVF clinic in Victoria, and our findings may not be generalisable to all women receiving fertility treatment in Australia.
Conclusions
Fertility therapy did not increase the risks of all‐cause or CVD death beyond those of other Australian women of the same age. Among women who registered for fertility therapy, all‐cause and CVD mortality was lower than for the general female population of the same age, which may reflect confounding by socio‐economic status and other health determinants. Our findings provide reassurance about the long term safety of fertility therapy.
Open access
Open access publishing facilitated by Monash University, as part of the Wiley – Monash University agreement via the Council of Australian University Librarians.
Competing interests
Monash IVF supported our investigation through the Monash Research and Education Fund. Luk Rombauts was employed at Monash IVF during the period covered by the study.
Supporting information
Appendix S1.
Acknowledgements
This investigation was funded by a Monash IVF Research and Education Foundation grant and by the Victorian Government Operational Infrastructure Support Program. Stephanie Yiallourou was supported by an Eleanor Shaw and Alice Baker Fellowship from the Baker Foundation. We gratefully acknowledge statistical support from the Baker Institute, and the Monash IVF research administrative team for study support. We also acknowledge Alison Venn (University of Tasmania), who provided access to the Monash IVF Cancer database and reviewed the final version of our manuscript.
References
- 1. European Society of Human Reproduction and Embryology . Eight million IVF babies since the birth of the world’s first in 1978 [media release]. 4 July 2018. https://www.focusonreproduction.eu/article/ESHRE‐News‐GlobalIVF18 (viewed Feb 2022).
- 2. Dayan N, Filion KB, Okano M, et al. Cardiovascular risk following fertility therapy: systematic review and meta‐analysis. J Am Coll Cardiol 2017; 70: 1203‐1213. [DOI] [PubMed] [Google Scholar]
- 3. Henriksson P, Westerlund E, Wallén H, et al. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilisation: cross sectional study. BMJ 2013; 346: e8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fraser A, Nelson SM, Macdonald‐Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation 2012; 125: 1367‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venn A, Hemminki E, Watson L, et al. Mortality in a cohort of IVF patients. Hum Reprod 2001; 16: 2691‐2696. [DOI] [PubMed] [Google Scholar]
- 6. Australian Institute of Health and Welfare . Heart, stroke and vascular disease: Australian facts [web report]. Updated 29 Sept 2021. https://www.aihw.gov.au/getmedia/88095d15‐88f7‐4b64‐a954‐846d2f1cdd26/Heart‐stroke‐and‐vascular‐disease‐Australian‐facts.pdf.aspx?inline=true (viewed Feb 2022).
- 7. Venn A, Watson L, Lumley J, et al. Breast and ovarian cancer incidence after infertility and in vitro fertilisation. Lancet 1995; 346: 995‐1000. [DOI] [PubMed] [Google Scholar]
- 8. Australian Institute of Health and Welfare . General record of incidence of mortality (GRIM) books. Updated 25 June 2021. https://www.aihw.gov.au/reports/life‐expectancy‐deaths/grim‐books/contents/general‐record‐of‐incidence‐of‐mortality‐grim‐data (viewed Feb 2022).
- 9. Australian Bureau of Statistics . Census of population and housing: Socio‐Economic Indexes for Areas (SEIFA), Australia, 2016 (Cat no. 2033.0.55.001). Mar 2018. https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2033.0.55.0012016?OpenDocument#Publications (viewed Feb 2022).
- 10. Coddington CC, Gopal D, Cui X, et al. Influence of subfertility and assisted reproductive technology treatment on mortality of women after delivery. Fertil Steril 2020; 113: 569‐577.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vassard D, Schmidt L, Pinborg A, et al. Mortality in women treated with assisted reproductive technology‐addressing the healthy patient effect. Am J Epidemiol 2018; 187: 1889‐1895. [DOI] [PubMed] [Google Scholar]
- 12. Australian Institute of Health and Welfare . Health across socioeconomic groups. 23 July 2020. https://www.aihw.gov.au/reports/australias‐health/health‐across‐socioeconomic‐groups (viewed Feb 2022).
- 13. Chambers GM, Hoang VP, Sullivan EA, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril 2014; 101: 191‐198.e4. [DOI] [PubMed] [Google Scholar]
- 14. Udell JA, Lu H, Redelmeier DA. Long‐term cardiovascular risk in women prescribed fertility therapy. J Am Coll Cardiol 2013; 62: 1704‐1712. [DOI] [PubMed] [Google Scholar]
- 15. Australian Institute of Health and Welfare . Indicators of socioeconomic inequalities in cardiovascular disease, diabetes and chronic kidney disease (Cat. no. CDK 12). Canberra: AIHW, 2019. https://www.aihw.gov.au/getmedia/01c5bb07‐592e‐432e‐9fba‐d242e0f7e27e/aihw‐cdk‐12.pdf.aspx?inline=true (viewed Feb 2022). [Google Scholar]
- 16. Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ 2020; 371: m3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


