Abstract
Chronic obstructive pulmonary disease (COPD) has been increasingly accounted for global morbidity and mortality worldwide. Although it is partially reversible, the obstructive ventilatory schema of COPD often causes chronic inflammation that primarily affects peripheral airways, pulmonary parenchyma, and the development of lung lymphoid follicles. Among various T-helper (Th) cell types associated with COPD, Th1, Th2 and Th17 cell numbers are increased in COPD patients, whereas Treg cell number is reduced. Here, we reviewed recent advance in understanding the roles of Th1/Th2 and Th17/Treg in the pathogenesis of COPD and discussed the potential underlying mechanism.
Keywords: lymphocyte, chronic obstructive pulmonary disease, inflammation
Introduction
As a cosmopolitan health issue, chronic obstructive pulmonary disease (COPD) affects around 10% of the population over the age of 40 [1]. It is primarily incurable due to progressive airflow obstruction involving emphysematous destruction of lung parenchyma and mucus hypersecretion with chronic bronchitis [2]. COPD also sets off chronic inflammation in the airways and lung parenchyma, leading to progressive and irreversible airflow limitation. The pathological outcome is reflected in multiple associated disorders, such as small airway obstruction, emphysema andchronic bronchitis [3]. Dyspnea, cough, and sputum production are the most common symptoms of COPD, however patients still can suffer from wheezing, chest tightness, and chest congestion [4].
Smoking used to be the primary pathogenic factor for COPD. Now due to recent social changes, some new factors appear to gradually come into play, featured by indoor and outdoor air pollution, occupational exposure, and chronic infections. In addition, environmental factors, such as dust, smog, haze, particulate matter (PM) 2.5, and pesticide residues (including defoliant and fungicide) are emerging as the pathogenic factor for COPD [5]. Here, we reviewed recent advance in understanding the roles of Th1/Th2 and Th17/Treg in the pathogenesis of COPD and discussed the potential underlying mechanism.
The Inflammatory Response of COPD
Inflammation is critical in the pathogenesis of COPD. The inflammatory response to COPD mainly involves innate immunity (neutrophils, macrophages, eosinophils, mast cells, natural killer cells, γδT cells, innate lymphocytes, and dendritic cells) and adaptive immune responses (T and B lymphocytes). It is also supported by the airways structural cells, alveolar epithelial cells, endothelial cells, fibroblasts, and activated dendritic cells [6].
T Cells Participate in Inflammatory Process
T lymphocytes
Despite the lack of mechanistic understanding, aberrant immune response accounts for the alveolar destruction and associated inflammation, in which T cell-mediated adaptive immunity appears to play important roles [7]. Specifically, CD4 + T-helper lymphocytes can be activated by autologous peptide-major histocompatibility complexes in the presence of co-stimulation signals and produce proinflammatory cytokines that coordinate the inflammatory cell net-works [8].
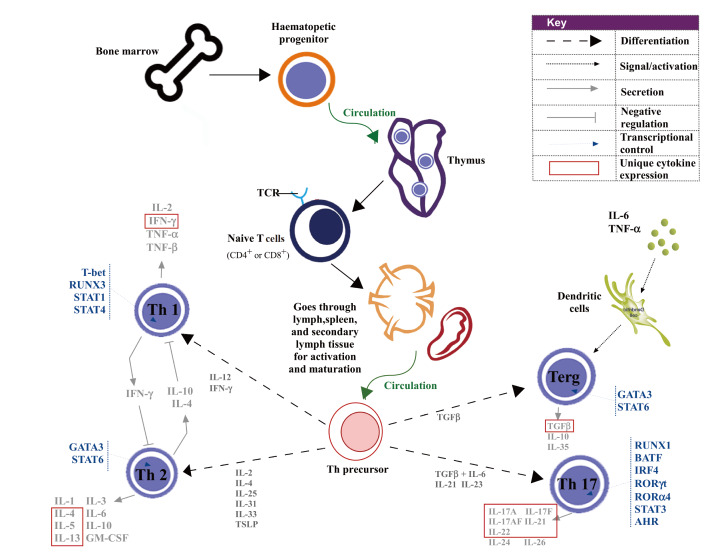
Being distributed throughout the airways and lung parenchyma, lymphocytes consist of thymus-dependent T cells (CD8 + killer cells and CD4 + helper cells) and bone marrow-dependent B cells [9]. CD4 + T cells regulate B cell differentiation and antibody production against viral infection. They are also required for the activation of viral-specific CD8 + T cells, and the activation and recruitment of innate immune cells. Upon activation, CD4 + T cells differentiate into different subsets of functional T cells including T-helper type (Th)1, Th2, Th17, Th22 and regulatory T (Treg) cells ( Figure 1), among which Th1, Th2 cells and Th17 cells are closely associated with COPD [ 10-- 13]. As part of the COPD pathogenesis, smoking can alter the antigens in lung, thereby activating CD4 + T cells for self-targeting and destruction.
Figure1 .
Illustration of the development and differentiation of T cellsOnce activated, CD4+ T cells differentiate into Th1, Th2, Th17 and Treg cells via different signal pathways, and then these Th cells secrete various cytokines to participate in inflammatory response.
T cell subsets
Derived from the α:β lineage of T cells, Th1 cells play important roles against microbial infections such as mycobacterium tuberculosis, mycobacterium leprae, and leishmania. They can identify intracellular pathogens by recognizing major histocompatibility complex (MHC) class I or class II molecules and mediate cellular immunity primarily through secreting interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), which also leads to neutrophilic inflammation and tissue destruction under certain circumstances.
Upon infection or allergy, Th2 cells produce IL-4, IL-5, IL-10 and IL-13 that mediate humoral immunity. Specific subpopulations of immune cells like eosinophils and basophils are recruited by Th2 cells upon infection or responding to allergens or toxins. As a result, tissue eosinophilia sometimes occurs along with mast cell proliferation, which further stimulates mucus production, goblet cell metaplasia and airway hyperresponsiveness. Th2 cells impose significant influence on antibody production and allergic reactions, thereby contributing to the exacerbation of asthma and other allergic inflammatory diseases.
Pro-inflammatory cytokines including IL-17A, IL-17F, IL-21, IL-22 and IL-23 are derived from Th17 cells. Retinoic acid orphan receptor (ROR)-γt expression facilitates the development of Th17 cells in conjunction with IL-6, IL-1β and IL-23 and transforming growth factor-β (TGF-β), whereas it is suppressed by IL-10 [14]. Th17 cells, which are crucial to the pathogenesis of several inflammatory and autoimmune diseases, induce epithelial cells to produce antimicrobial peptides, chemokines, and granulocyte growth factors to promote neutrophil accumulation in the airway [15].
Characterized by Foxp3 and CD25 expressions, Treg cells suppress the proliferation and cytokine production of other T cells through secreting anti-inflammatory cytokines such as IL-10 and TGF-β, therefore they are important for immune tolerance and immune homeostasis [ 11, 16]. Furthermore, Treg cells are imperative in respiratory viral infections by suppressing over-stimulated inflammatory responses and tissue damage introduced by other innate and adaptive immune components [17]. Though Treg cells are associated with the development of autoimmune diseases, their role in COPD is still under debate.
In the respiratory tract, immune system homeostasis is achieved through the balance between the pro-inflammatory cytokines and anti-inflammatory cytokines which are primarily under the control of CD4 + T cells, as well as Th1/Th2 and Th17/Treg cells. Therefore, these cells collectively serve as the major factors in the pathogenesis of COPD. Impaired immunity balance often results in excessive proliferation of effector cells or decreased function of regulatory cells, ultimately leading to inflammation.
Activation of T cells
Dendritic cells (DCs) serve as both innate lung sentinels and orchestrators of adaptive immunity. They drive the pathological processes through the full course of COPD development, from the initial to the final stages. Derived from adult hematopoietic precursors, DCs are separated into type 1 and type 2 classical/conventional DCs (cDC1 and cDC2, respectively) and plasmacytoid DCs (pDCs) [18]. cDC1s are found in the airway mucosa and vascular walls and crucial for generating regulatory T cells, Th1 immunity, and cytotoxic CD8 + T cells. cDC2s reside in the airway lamina propria and are suggested to activate Th2 and Th17 responses, though the mechanism is elusive [19]. DCs can also present antigen cells and link the innate and adaptive immune responses by phagocytosing microbes and migrating to regional lymph nodes to activate lymphocytes including T cells and B cells [20].
Accurate signal transduction is crucial when facing complex physiological and pathological conditions. It happens primarily in the secondary lymphatic organs, including Pell’s spots, lymph nodes, and spleen in a “face-to-face” manner. However, regional command centers must be close to the menace. Accordingly, chronic immune response induces abnormal tertiary lymphoid structures within organs. In the distal lung parenchyma, tertiary lymphoid structures are better known as lung lymphoid follicles (LLFs) that are central to COPD pathogenesis, though the exact role remains unclear [21]. Encountered by antigens, cDCs migrate to draining lymph nodes to activate naive T cells. Also, they cross-talk with other innate immune cells to initiate adaptive immune responses in the lung as COPD severity progresses [22]. In COPD tissues, increased number of langerin + DCs was observed in the interface between LLF and the alveolar lumen. Such DCs simultaneously contacted both the alveolar surface and lymphocytes within the LLF [23]. Tregs have been shown to limit LLF formation [24]. It is possible that DCs fail to drive Treg differentiation in severe COPD, leading to increased number of lymphoid follicles at later stages of the disease. Since DCs can produce profibrogenic cytokines directly and promote airway inflammation indirectly, it is also likely that they are involved in airway remodeling. Thus, modulating DC recruitment and function provides an attractive therapeutic approach for limiting COPD progression [25].
Aberrant CD4 + T Cell Subsets in COPD Patients
Th1/Th2 imbalance
The balance between Th1 and Th2 cells is critical in regulating cellular and humoral immune responses, which is often disrupted in the case of COPD [ 26-- 28]. As a result, the immune response is disturbed and inflammatory reactions become persistent along with airway remodeling and emphysema [29].
The number of Th1 cells was reported to be progressively increased in stable phase of COPD and acute exacerbation of chronic obstructive pulmonary disease (AECOPD), while IFN-γ expression was elevated. In contrast, Th2 cells and serum IL-4 level were substantially increased. However, in patients with stable COPD, the Th2/Th1 ratio is decreased, which is opposite to that in the AECOPD patients, and the activation and proliferation of Th2 cells is more prominent in the peripheral circulation system. Consequently, the Th1/Th2 ratio is shifted to Th1 responses in stable COPD, whereas it is shifted to Th2 response in AECOPD [ 30, 31]. When the respiratory failure is corrected, the balance of Th1/Th2 begins to be restored [ 27, 32]. In the AECOPD patients’ airway, a large number of pathogen-specific antibodies can be found, indicating active immune response [33]. Meanwhile, Th1s subside and Th2s become dominant in delayed allergy, and the opposite scenario appears in cellular immunity.
Viral infections frequently evoke a strong Th1 cells and/or cytotoxic T lymphocyte response, whereas weak Th1 immunity and robust Th2-biased response is associated with allergic inflammation [29]. In general, Th1 cells promote cell-mediated immunity and phagocyte-dependent inflammation, while Th2 cells support strong antibody responses and eosinophil accumulation. Th1-dominated response is involved in the pathogenesis of organ-specific autoimmune disorders, while Th2-dominated response plays a pathogenic role in both progressive systemic sclerosis and cryptogenic fibrosing alveolitis [34]. Both environmental and genetic factors act in concert to determine the Th1 or Th2 polarization. Aberrant Th2 immune response superimposed on the chronic type 1 immunity of COPD is responsible for further respiratory compromise. However, systematic or topical corticosteroids administration can suppress the production of IL-4 and IL-13, thereby alleviating the airway hyperresponsiveness and mucus hyperproduction induced by these cytokines [35].
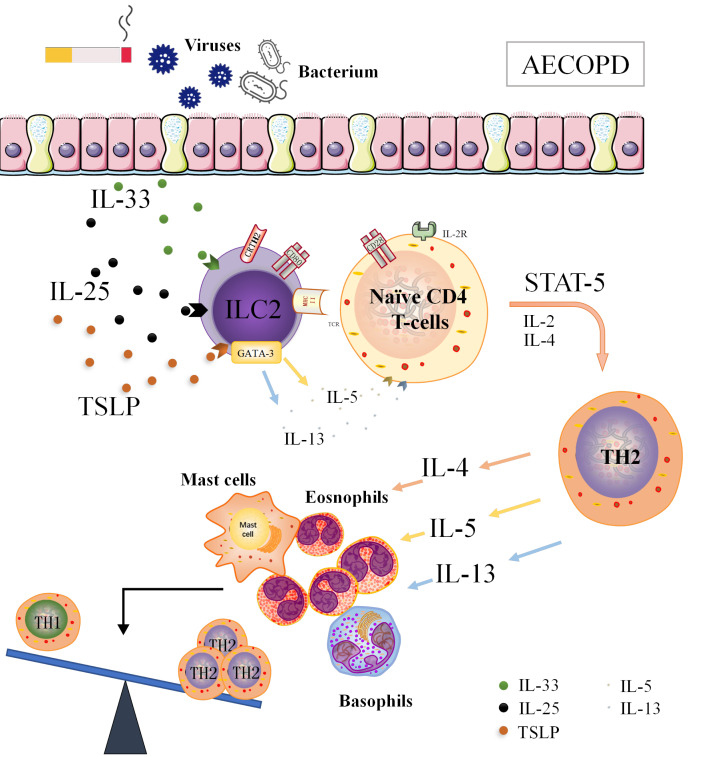
ILC2 is involved in the pathogenesis of COPD [ 36, 37]. In patients with stable COPD, the levels of ILC2-associated transcription factors, including RORα, GATA-3, and CRTH2, were significantly increased and surpassed those in AECOPD patients [30]. We reasoned that ILC2s may promote Th2/Th1 balance towards Th2 in AECOPD patients with high level of MHC II, in which it functions as antigen presenting cells (APCs). Therefore, MHC II + ILC2 can be included in further studies as a new target for clinical immunoassay and treatment of AECOPD ( Figure 2).
Figure2 .
High expression of MHC II, ILC2 may function as APC, promoting Th2/Th1 balance to Th2 shift in AECOPD patientsAECOPD patients with inflammation have rapidly deteriorated lung function, ILC2s which highly express MHC II are likely to perform APC-like functions, promoting the secretion of IL-4, IL-5 and IL-13 through the interaction of MHC II-like molecules with T-cell receptors, leading to elevated differentiation of Th2 cells and ultimately resulting in Th1/Th2 balance to Th2 shift.
Th17/Treg imbalance
Th1 response drives inflammation in COPD. Emerging evidence began to add Th17 response into the inflammatory reaction in COPD. Th17 cells accumulate in the bronchial submucosa, airway epithelium, lung tissue, bronchoalveolar lavage and peripheral blood in COPD patients [ 38, 39]. IL-17 enhances the production of a variety of chemokines, such as IL-1β, IL-6, TNF-α, CXCL8, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage-CSF (GM-CSF) in the lung, thereby orchestrating the recruitment of neutrophils and macrophages [ 40, 41]. In addition, IL-17A was found to contribute to cigarette smoking-induced lymphoid neogenesis of late-stage COPD, suggesting its critical role in chronic inflammation and adaptive immune responses in COPD [42]. Meanwhile, Di Stefano et al. [13] demonstrated that Th17 cells and/or the associated cytokines may participate in the airway inflammation of COPD and tissue remodeling. The activation of Treg secretion has also been suggested to play a role in inflammation [43]. Compromised Treg cell response was found to be associated with persistent inflammation in COPD [44].
Th17/Treg balance is closely related to the pathophysiological changes of inflammatory response and development of the autoimmune disorders [45]. The ratio of Th17/Treg in both lung tissues and peripheral blood is significantly shifted in COPD patients [ 44, 46, 47]. Smoking can lead to the imbalance of Th17/Treg, which prompts Th17 cells to secrete inflammatory cytokines [ 48, 49]. Smoking also suppresses the protective functions of DCs, airway epithelium, natural killer cells, and damages the balance between mDCs/imDCs and Th17/Treg [49]. Furthermore, inflammatory cytokines inhibit FoxP3 expression, suppress the differentiation of Treg cells, and trigger the conversion to Th17 cells [50].
As a multifunctional cytokine, TGF-β is involved in a variety of human diseases [51], and plays a pivotal role in the differentiation of naive CD4 + T cells into Treg or Th17 cells. This function is dependent on inflammatory microenvironments and epigenetic modifications [52]. In COPD patients, TGF-β1 expression is elevated in the small airway epithelium, lung tissue, and peripheral blood [ 53, 54], and tissues of COPD patients have stronger expression levels of bone morphogenetic protein (BMP) and activin membrane-bound inhibitor (BAMBI) [55] which acts as a competitive receptor antagonist for TGF-β type-I receptors (TGF-β RI) and the subsequent Smad signaling pathways [ 56, 57]. An enhanced plasmaBAMBI level in COPD is positively correlated with increased plasma TGF-β1 level and Th17/Treg ratio, suggesting a functional link between Th17/Treg imbalance and TGF-β-BAMBI signaling pathway alteration. Thus, it is possible that impaired TGF-β/BAMBI pathway fuses inflammation, which leads to the imbalance of Th17/Treg in COPD patients with smoking history, ultimately shifting Th17/Treg balance away from a regulatory role towards an inflammatory role [7].
Conclusions
Research on the function and mechanism of immune balance in COPD provides a novel way for guiding the treatment of COPD. Restoring Th1/Th2 balance may also be beneficial to AECOPD patients where GATA-3 pathway could be primarily targeted to reduce the expression of MHC II + ILC2 and the proliferation and differentiation of Th2 cells. In addition, the repair of the damaged TGF-β/BAMBI pathway could also impose positive impacts on Th17/Treg balance, and thereby alleviating the inflammation of COPD. If effective and safe anti-inflammatory therapies are developed, they should be introduced at the early stages of COPD to prevent disease progression and potentially to reduce the burden of concomitant comorbidities.
Supporting information
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the Natural Science Fund of Shanxi Province (Nos. 201901D111186 and 201901D111190),Open Fund from Key Laboratory of Cellular Physiology (Shanxi Medical University), Ministry of Education, China (No. KLMEC/SXMU-202011), Shanxi ‘1331 Project’ Key Subjects Construction, China (No. 1331KSC), and the “136” Special Fund Project of the First Hospital of Shanxi Medical University (No. 2021-07).
References
- 1.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, Nair H, et al. Global and regional estimates of COPD prevalence: systematic review and meta–analysis. J glob Health. . 2015;5:020415. doi: 10.7189/jogh.05.020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busch R, Qiu W, Lasky-Su J, Morrow J, Criner G, DeMeo D. Differential DNA methylation marks and gene comethylation of COPD in African-Americans with COPD exacerbations. Respir Res. . 2016;17:143. doi: 10.1186/s12931-016-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellularmechanisms. Eur Respir J. . 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 4.Scoditti E, Massaro M, Garbarino S, Toraldo DM. Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients. . 2019;11:1357. doi: 10.3390/nu11061357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldonyte R, Bagdonas E, Raudoniute J, Bruzauskaite I. Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int J Chron Obstruct Pulmon Dis. . 2015;10:995. doi: 10.2147/COPD.S82518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. . 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JC, Chen G, Chen L, Meng ZJ, Xiong XZ, Liu HJ, Jin Y, et al. TGF-β/BAMBI pathway dysfunction contributes to peripheral Th17/Treg imbalance in chronic obstructive pulmonary disease. Sci Rep. . 2016;6:31911. doi: 10.1038/srep31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth LJC, Starkey C, Vestbo J, Singh D. CD4-regulatory cells in COPD patients. Chest. . 2007;132:156–163. doi: 10.1378/chest.07-0083. [DOI] [PubMed] [Google Scholar]
- 9.Caramori G, Casolari P, Barczyk A, Durham AL, Di Stefano A, Adcock I. COPD immunopathology. Semin Immunopathol. . 2016;38:497–515. doi: 10.1007/s00281-016-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brozyna S, Ahern J, Hodge G, Nairn J, Holmes M, Reynolds PN, Hodge S. Chemotactic mediators of Th1 T-cell trafficking in smokers and COPD patients. COPD-J Chronic Obstructive Pulmonary Dis. . 2009;6:4–16. doi: 10.1080/15412550902724164. [DOI] [PubMed] [Google Scholar]
- 11.Bruzzaniti S, Bocchino M, Santopaolo M, Calì G, Stanziola AA, D’Amato M, Esposito A, et al. An immunometabolic pathomechanism for chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. . 2019;116:15625–15634. doi: 10.1073/pnas.1906303116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, Lenburg ME, et al. Asthma–COPD overlap. clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. . 2015;191:758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. . 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouzic O L, Pichavant M, Frealle E, Guillon A, Si-Tahar M, Gosset P. Th17 cytokines: novel potential therapeutic targets for COPD pathogenesis and exacerbations. Eur Respir J. . 2017;50:1602434. doi: 10.1183/13993003.02434-2016. [DOI] [PubMed] [Google Scholar]
- 15.Rovina N, Koutsoukou A, Koulouris NG. Inflammation and immune response in COPD: where do we stand? Mediators Inflammation. . 2013;2013:1–9. doi: 10.1155/2013/413735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attridge K, Walker LSK. Homeostasis and function of regulatory T cells (Tregs) in vivo : lessons from TCR ‐transgenic Tregs . Immunol Rev. . 2014;259:23–39. doi: 10.1111/imr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S, He Q, Zhou L. T cell responses in respiratory viral infections and chronic obstructive pulmonary disease. Chin Med J. . 2021;134:1522–1534. doi: 10.1097/CM9.0000000000001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. . 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman CM, Curtis JL. It’s complicated: lung dendritic cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. . 2020;202:479–481. doi: 10.1164/rccm.202004-0899ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, Saad M, et al. Inflammatory mechanisms in the lung. J Inflamm Res 2009, 2: 1-11 . [PMC free article] [PubMed]
- 21.Curtis JL, Freeman CM, Huffnagle GB. “B” for bad, beneficial, or both? Lung lymphoid neogenesis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. . 2015;192:648–651. doi: 10.1164/rccm.201506-1230ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. . 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 23.Mori M, Andersson CK, Svedberg KA, Glader P, Bergqvist A, Shikhagaie M, Löfdahl CG, et al. Appearance of remodelled and dendritic cell-rich alveolar-lymphoid interfaces provides a structural basis for increased alveolar antigen uptake in chronic obstructive pulmonary disease. Thorax. . 2013;68:521–531. doi: 10.1136/thoraxjnl-2012-202879. [DOI] [PubMed] [Google Scholar]
- 24.Foo SY, Zhang V, Lalwani A, Lynch JP, Zhuang A, Lam CE, Foster PS, et al. Regulatory T cells prevent inducible BALT formation by dampening neutrophilic inflammation. J Immunol. . 2015;194:4567–4576. doi: 10.4049/jimmunol.1400909. [DOI] [PubMed] [Google Scholar]
- 25.Freeman CM, Curtis JL. Lung dendritic cells: shaping immune responses throughout COPD progression. Am J Respir Cell Mol Biol. . 2017:152–156. doi: 10.1165/rcmb.2016-0272TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi Y, Di Q, Han G, Li M, Sun B. Mir-29b mediates the regulation of Nrf2 on airway epithelial remodeling and Th1/Th2 differentiation in COPD rats. Saudi J Biol Sci. . 2019;26:1915–1921. doi: 10.1016/j.sjbs.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Liu T, Yan Y, Huo K, Zhang W, Liu H, Shi Z. The role of Th1/Th2 cytokines played in regulation of specific CD4 + Th1 cell conversion and activation during inflammatory reaction of chronic obstructive pulmonary disease . Scand J Immunol. . 2018;88:e12674. doi: 10.1111/sji.12674. [DOI] [PubMed] [Google Scholar]
- 28.Chung KF. Cytokines as targets in chronic obstructive pulmonary disease. Curr Drug Targets. . 2006;7:675–681. doi: 10.2174/138945006777435263. [DOI] [PubMed] [Google Scholar]
- 29.Singh M, Lee SH, Porter P, Xu C, Ohno A, Atmar RL, Greenberg SB, et al. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. . 2010;125:1369–1378.e2. doi: 10.1016/j.jaci.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang M, Liu H, Li Z, Wang J, Zhang F, Cao K, Li F, et al. ILC2s induce adaptive Th2-type immunity in acute exacerbation of chronic obstructive pulmonary disease. Mediators Inflammation. . 2019;2019:1–12. doi: 10.1155/2019/3140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsoumakidou M, Tzanakis N, Chrysofakis G, Kyriakou D, Siafakas NM. Changes in sputum T-lymphocyte subpopulations at the onset of severe exacerbations of chronic obstructive pulmonary disease. Respiratory Med. . 2005;99:572–579. doi: 10.1016/j.rmed.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J ZX, Zhang J, Wang L, Cong L. Study on trend of Th1/Th2 in respiratory failure stage of COPD and regulation of Chinese medicine treatment according to syndrome differentiation. Chinese Archives of Traditional Chinese Medicine 2016, 34: 451455
- 33.Staples KJ, Taylor S, Thomas S, Leung S, Cox K, Pascal TG, Ostridge K, et al. Relationships between mucosal antibodies, non-typeable haemophilus influenzae (NTHi) infection and airway inflammation in COPD. PLoS ONE. . 2016;11:e0167250. doi: 10.1371/journal.pone.0167250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romagnani S. T-cell subsets (Th1 versus Th2) Ann Allergy Asthma Immunol. . 2000;85:9–21. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- 35.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. . 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Grove KC, Provoost S, Verhamme FM, Bracke KR, Joos GF, Maes T, Brusselle GG. Characterization and quantification of innate lymphoid cell subsets in human lung. PLoS ONE. . 2016;11:e0145961. doi: 10.1371/journal.pone.0145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L, Pritchard GH, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. . 2016;17:626–635. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paats MS, Bergen IM, Hoogsteden HC, van der Eerden MM, Hendriks RW. Systemic CD4+ and CD8+ T-cell cytokine profiles correlate with GOLD stage in stable COPD. Eur Respir J. . 2012;40:330–337. doi: 10.1183/09031936.00079611. [DOI] [PubMed] [Google Scholar]
- 39.Vargas-Rojas MI, Ramírez-Venegas A, Limón-Camacho L, Ochoa L, Hernández-Zenteno R, Sansores RH. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respiratory Med. . 2011;105:1648–1654. doi: 10.1016/j.rmed.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. . 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. . 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 42.Roos AB, Sandén C, Mori M, Bjermer L, Stampfli MR, Erjefält JS. IL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke–induced lymphoid neogenesis. Am J Respir Crit Care Med. . 2015;191:1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- 43.Hou J, Sun Y, Hao Y, Zhuo J, Liu X, Bai P, Han J, et al. Imbalance between subpopulations of regulatory T cells in COPD. Thorax. . 2013;68:1131–1139. doi: 10.1136/thoraxjnl-2012-201956. [DOI] [PubMed] [Google Scholar]
- 44.Zheng X, Zhang L, Chen J, Gu Y, Xu J, Ouyang Y. Dendritic cells and Th17/Treg ratio play critical roles in pathogenic process of chronic obstructive pulmonary disease. Biomed Pharmacother. . 2018;108:1141–1151. doi: 10.1016/j.biopha.2018.09.113. [DOI] [PubMed] [Google Scholar]
- 45.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. AutoImmun Rev. . 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Ying H, Wang S, Gu X, Weng Y, Peng W, Xia D, et al. Imbalance of peripheral blood Th17 and Treg responses in patients with chronic obstructive pulmonary disease. Clin Respiratory J. . 2015;9:330–341. doi: 10.1111/crj.12147. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Peng W, Weng Y, Ying H, Li H, Xia D, Yu W. Imbalance of Th17/Treg cells in mice with chronic cigarette smoke exposure. Int Immunopharmacol. . 2012;14:504–512. doi: 10.1016/j.intimp.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Larsson L, Pehrson C, Dechen T, Crane-Godreau M. Microbiological components in mainstream and sidestream cigarette smoke. Tob Induced Dis. . 2012;10:13. doi: 10.1186/1617-9625-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect. . 2013;67:169–184. doi: 10.1016/j.jinf.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. . 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akhurst RJ. TGFβ signaling in health and disease. Nat Genet. . 2004;36:790–792. doi: 10.1038/ng0804-790. [DOI] [PubMed] [Google Scholar]
- 52.Li MO, Flavell RA. TGF-β: a master of all T Cell trades. Cell. . 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mak JCW, Chan-Yeung MMW, Ho SP, Chan KS, Choo K, Yee KS, Chau CH, et al. Elevated plasma TGF-β1 levels in patients with chronic obstructive pulmonary disease. Respiratory Med. . 2009;103:1083–1089. doi: 10.1016/j.rmed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Hodge SJ, Hodge GL, Reynolds PN, Scicchitano R, Holmes M. Increased production of TGF-β and apoptosis of T lymphocytes isolated from peripheral blood in COPD. Am J Physiol-Lung Cell Mol Physiol. . 2003;285:L492–L499. doi: 10.1152/ajplung.00428.2002. [DOI] [PubMed] [Google Scholar]
- 55.Drömann D, Rupp J, Rohmann K, Osbahr S, Ulmer AJ, Marwitz S, Röschmann K, et al. The TGF-beta-pseudoreceptor BAMBI is strongly expressed in COPD lungs and regulated by nontypeable Haemophilus influenzae. Respir Res. . 2010;11:67. doi: 10.1186/1465-9921-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shangguan L, Ti X, Krause U, Hai B, Zhao Y, Yang Z, Liu F. Inhibition of TGF-β/smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells. . 2012;30:2810–2819. doi: 10.1002/stem.1251. [DOI] [PubMed] [Google Scholar]
- 57.Sekiya T, Oda T, Matsuura K, Akiyama T. Transcriptional regulation of the TGF-β pseudoreceptor BAMBI by TGF-β signaling. Biochem Biophysl Res Commun. . 2004;320:680–684. doi: 10.1016/j.bbrc.2004.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.