Abstract
Since Z‐nucleic acid was identified in the 1970s, much is still unknown about its biological functions and nature in vivo. Recent studies on adenosine deaminase acting on RNA 1 (ADAR1) and Z‐DNA‐binding protein 1 (ZBP1) have highlighted its function in immune responses. Specifically, Z‐RNAs, either endogenous or induced by viral infection, are sensed by ZBP1 and activate necroptosis. Z‐RNAs act as the stimuli that induce innate immune responses through various pathways, including melanoma differentiation‐associated protein 5 (MAD5)‐mitochondrial antiviral‐signaling protein (MAVS)‐mediated type I IFN activation and proteinase kinase R (PKR)‐dependent integrated stress response, and their immunostimulatory potential is curtailed by RNA editing conducted by ADAR1. Aberrant immune responses induced by Z‐RNAs are associated with human diseases. They also induce pathogenesis in mice. Unlike Z‐RNAs, the biological functions of Z‐DNAs were barely studied, especially in mammals. Moreover, the origin or sequence preference of Z‐nucleic acids requires further investigation. Such knowledge will expand our understanding of Z‐nucleic acids, including from which genomic loci and under which circumstances they form, and the mechanisms by which they participate in the physiological activities. In this review, we provide insights in Z‐nucleic acid research and highlight the unsolved puzzles.
Keywords: ADAR1, function, ZBP1, Z‐nucleic acid binding protein, Z‐nucleic acids
This article summarizes the research on Z‐nucleic acid historically and highlights the milestones in this field, including the discovery, characterization of the Z‐nucleic acids and their biological functions mediated by interacting with Z‐nucleic acid binding proteins such as ZBP1, ADAR1, and so on.
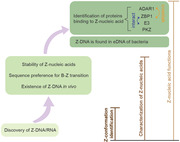
Introduction
The structure of Z‐DNA was first identified and characterized in 1979 by the Alexander Rich laboratory [1], when they crystallized a six‐base pair DNA fragment, d(CpGpCpGpCpG). Z‐DNA is an alternative form of dsDNA with some unique features in comparison with the predominant B‐form DNA. Double‐stranded RNA can also adopt the Z‐form [2]. Z‐nucleic acid is thermodynamically unstable, which makes it challenging to study. Nevertheless, impressive progress has been achieved in the past few years. Here, we summarize the milestones in Z‐nucleic acid research and highlight the significant but still unresolved issues.
Discovery of Z‐DNA and Z‐RNA
In the 1950s, scientists started to notice that DNA fibers undergo structural changes in different solutions. In 1972, this structural transition was systematically studied by Fritz Pohl and Thomas Jovin. They detected an optical rotation of poly(dG‐dC) in increasing salt concentrations, which was defined as R–L transition, also named the Pohl–Jovin transition. This transition is reversible and accompanied by a change in the UV absorption spectrum [3]. This conformational transition was also induced by a high concentration of ethanol (60%) or adding of mitomycin [4, 5]. Pohl et al. applied high‐resolution 1H and 31P NMR spectra to determine the structure of oligo (dG‐dC) in low and high salt solutions, and found that oligo (dG‐dC) in high salt adopted an “alternating B‐DNA” conformation, whereas the oligomer duplex in low salt was the regular B‐DNA type [6]. Subsequently, Wang and Rich et al. successfully crystallized the DNA fragment d(CGCGCG) and revealed the detailed structure of this “alternating B‐DNA” at the atomic level. They first named it “Z‐DNA” and characterized the following features [1]. (1) Unlike B‐form DNA, which is a classic Watson‐Crick right‐handed conformer, Z‐DNA has a left‐handed helical sense. (2) The phosphate backbone of Z‐DNA follows a zig‐zag course in a discontinuous manner, resulting from the alternating residue conformations, thereby receiving the name Z‐DNA. (3) The asymmetric unit of Z‐DNA is two nucleotides rather than a single nucleotide that is found in B‐DNA. This asymmetric unit comprises a syn purine and an anti pyrimidine in Z‐DNA. (4) The Z‐DNA helix has 12 base pair (bp) per turn of helix with a length of 44.6 Å, whereas there is 10 bp per turn occupying a distance of 34 Å in B‐DNA. (5) B‐form DNA contains both major and minor grooves per turn. The major groove is absent in Z‐DNA and only the minor groove remains, which is deeper than that in the B‐form. (6) Z‐DNA is thinner and more extended. The diameter of the Z‐DNA helix is 18 Å compared with 20 Å in B‐DNA. The distance of 12 bp in Z‐DNA can accommodate 13 bp in B‐form [1].
When they first observed the B–Z transition with poly(dG‐dC), Fritz Pohl and Thomas Jovin found that this salt‐induced transition was related to the base composition and sequence because they did not detect a similar transition with synthetic RNA poly(G‐C) or DNA poly(dA‐dT), albeit this was later proved to be untrue [6]. Poly(G‐C) could also be transited to Z‐form RNA but indeed required more extreme conditions than the transition to Z‐form DNA. An increase in the salt concentration, high temperature, and addition of ethanol facilitated the transition of poly(G‐C) to the Z‐form [2, 7]. Accordingly, poly(G‐C) could be induced to a left‐handed Z‐form under 6 M NaClO4, whereas 4 M NaClO4 was sufficient to induce poly(dG‐dC) [2]. The method widely used to distinguish these two different DNA helical forms is that they display different spectra in circular dichroism and ultraviolet absorption with absorption at 295 nm as an indication of the Z‐form [7].
Stability of Z‐nucleic acid
Compared with B‐form DNA, Z‐DNA is thermodynamically unstable, owing to electrostatic repulsion between the negatively charged phosphates on opposite strands, which are closer together in Z‐DNA than in B‐DNA [8]. Under physiological conditions, there is an equilibrium between right‐handed B‐DNA and left‐handed Z‐DNA, and this equilibrium is affected by various environmental factors.
The original finding indicated that high salt favored B–Z transition, which is unsurprising because ions, especially cations, from a high concentration of salt reduce phosphate–phosphate repulsion. Similarly, other chemical modifications that relieve this repulsion also stabilize Z‐DNA, so the equilibrium shifts in this favor. Methylation at the N7 position of guanine stabilizes Z‐DNA because the N7 methylation induces a positive charge that neutralizes the electrostatic repulsion between the phosphate groups on opposite strands [9]. Bromination of poly (dG‐dC) stabilizes this polymer in the Z‐form under a low salt condition. Bromination occurs largely at the C8 position of guanine and to a lesser extent on the C5 position of cytosine. C8‐brominated purines favor the syn conformation as the preferred arrangement for the Z‐form DNA [10]. Various agents, including alcohols, ethanol, methanol, and ethylene glycol, which alter the dielectric constant of water, have also been found to stabilize Z‐DNA [11].
The initial Z‐form transition was observed in synthesized polymers with GC repeats. dCG tracts inserted into plasmids also undergo B–Z transition, even under physiological conditions with a low salt concentration [12, 13]. This transition is induced and stabilized by localized negative supercoiling. When alternating (CG) tracts were inserted either upstream or downstream from the tet gene promoter, Z‐DNA was only detected in (CG) tracts upstream of the promoter. Moreover, this Z‐DNA transition was dependent on transcription because rifampicin treatment, which inhibits transcription in bacteria, blocked this Z‐DNA formation [14]. Therefore, a hypothesis for explaining these phenomena is that translocation of an RNA polymerase elongation complex along the double helix generating negative supercoil waves behind, which induces such formation of Z‐DNA [14]. Altogether, chemical modifications and the negative supercoiling during the process of transcription are believed to stabilize Z‐DNA.
Sequence preference for B–Z transition
The unusual zig–zag phosphate backbone in Z‐DNA comes from the alternating residue conformations that include anti pyrimidine and syn purine as one asymmetric unit. Thus, a perfect d(GC)n containing alternating pyrimidine and purine will form the left‐handed sense most easily because purine adopts the syn conformation more readily than pyrimidine. d(GC)n is the most preferential sequence for Z‐DNA because it fulfills the structural parameter of the Z‐helix with the least energetic penalty, but other sequence combinations also retain potentials for forming Z‐DNA. The detection of the B–Z transition in the insertion of recombinant plasmids, allowed subsequent systematic study of the sequence preference to form a Z‐helix [15]. Apart from alternating d(CG) sequences, which are the most favored to form Z‐DNA, the next most effective sequences are d(CA) or d(TG), whereas the d(AT) sequence favors Z‐DNA least of all [11].
Existence of Z‐DNA in vivo
Unlike B‐form DNA, Z‐DNA is highly antigenic. Using brominated poly(dG‐dC) as a stabilized DNA in the Z‐form, specific antibodies targeting Z‐DNA were generated in rabbits and mice [16]. Moreover, natural antibodies that react with Z‐DNA have been found in the sera of mice with lupus and humans with systemic lupus erythematosus [16, 17]. The availability of these antibodies stimulated intensive studies on determining the existence of in vivo Z‐DNA. The first identification of the Z‐DNA conformation in material of biological origin was the interband region of Drosophila polytene chromosomes [18]. Following this discovery, similar staining patterns on Z‐DNA were also exhibited in polytene chromosome in Chironomus [19]. Interbands in polytene chromosome are regions that act as binding sites for RNA polymerase II to initiate replication and to start nucleosome remodeling. Additionally, antibodies against Z‐DNA stained macronuclei specifically, which is transcriptionally active, rather than transcriptionally inactive micronuclei in Stylonychia mytilus [20]. In mammalians, a study also found that Z‐DNA antibodies reacted with fixed metaphase chromosomes of primates to some extent, including humans and Cebus albifrons [21]. Z‐DNA staining was observed in other species, including plants [11]. Such anti‐Z‐DNA antibody staining showed fluorescent signals with various strengths in the nucleus and likely in transcriptionally active regions. Thus, a rational hypothesis is that Z‐DNA is induced in transcriptionally active chromosomes by negative supercoiling generated from the movement of polymerase or chromosome remodelling during transcription. However, concerns were raised when interpreting these staining patterns because the fluorescence intensity was largely affected by the fixation method used for sample preparation. The intense fluorescent signal detected in chromosomes may be the consequence of acid fixation that removed the nucleosome‐forming proteins, released torsional strain, and generated Z‐DNA [22].
The research on staining chromosomes with anti‐Z‐DNA antibodies could not support a firm conclusion on the existence or distribution pattern of Z‐DNA in vivo, but it revealed the presence of segments in chromosome with the potential for Z‐DNA formation. Anti‐Z‐DNA antibodies serve as an important tool to detect DNA in Z‐form in vivo. Additionally, several anti‐sera raised against Z‐DNA cross‐reacted with Z‐RNA [23]. Besides, It was also reported the existence of potential Z‐DNA forming sequences in human genome by mapping of highly repeated sequences, such as the presence of poly (dT‐dG) in one of the intron of a human cardiac muscle actin gene [24].
Identification of Z‐DNA‐binding proteins
The left‐handed conformer may be bound by proteins when it was found that fixation, which potentially removed nucleosome proteins, altered immunofluorescence staining of Z‐DNA. The initial study of Z‐DNA‐binding proteins was performed in Drosophila nuclei. A mixture of large proteins in Drosophila nuclei was retained by binding to either brominated poly (dG‐dC) in Z‐form or negatively supercoiled plasmids carrying Z‐DNA sequences [25]. Herbert et al. identified the first Z‐DNA‐binding protein, namely adenosine deaminase acting on RNA 1 (ADAR1 or DRADA1) [26, 27, 28]. Other Z‐DNA‐binding proteins were subsequently discovered by alignment of the conversed domains capable of binding DNAs in Z‐form, termed the Z‐DNA binding domain (ZBD) or Zα domain.
ADAR1
ADAR1 acts as an RNA‐editing enzyme with dsRNA as a substrate, which converts adenosine to inosine and results in GC pairing because inosine is translated as guanosine. ADAR1 has two isoforms with a longer isoform, P150, which is inducible by IFN and present in the nucleus and cytosol, whereas P110, the shorter isoform, is constitutively expressed and localized in the nucleus. They share identical sequences in the deaminase domain, three dsRNA‐binding domains, and a non‐functional Zβ domain, but with an extra Zα domain in P150, which binds to Z‐DNA/Z‐RNA (Figure 1).
Figure 1.
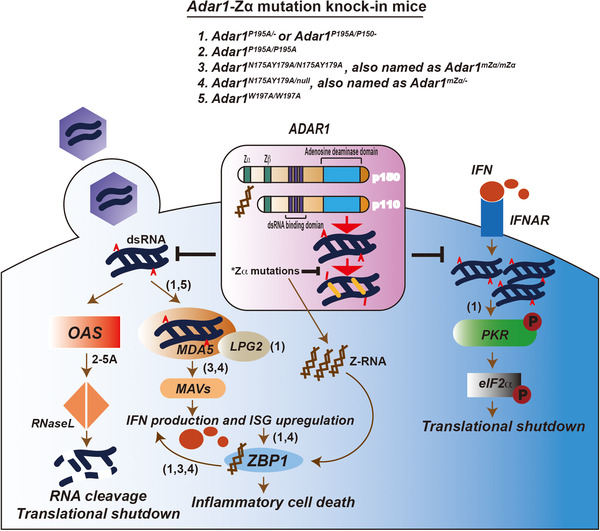
ADAR1‐Zα participates in immune responses. Upon the invasion of pathogens like viruses, dsRNAs are released into cytosol. Acting as non‐self nucleic acids, they are detected by MDA5 to induce the type I IFN signaling and by OAS to activate RNase L‐mediated widespread RNA degradation. Type I IFN binds to its receptor IFNAR which further leads to the upregulation of some endogenous dsRNAs which activate PKR and results in translational shutdown. The adenosine to inosine (A to I) editing of dsRNA by ADAR1 protects them from being sensed by MDA5, OAS or PKR, thus to block the subsequent pathways. Zα domain of ADAR1 contributes to its RNA‐editing efficiency and thus affects the inhibition of the signaling pathways indicated. Mice carrying mutations within Zα domain were characterized by their roles on the hyper‐activation of the downstream signaling pathways, separately. Specifically, P195A mutation in one Adar allele paired with the full Adar null allele (Adar1P195A/‐ ) or the Adar p150 null allele (Adar1P195A/p150– ) leads to both type I IFN pathway activation via MDA5 and LPG2, and the PKR‐mediated integrated stress response. Homozygous mutations including Adar1N175AY179A/N175AY179A and Adar1W197A/W197A in Adar render a spontaneous induction of IFN signaling. Type I IFN signaling activation results in the IFN production and upregulation of IFN‐stimulated gene (ISG), with ZBP1 is one of them. In Adar1P195A/– , Adar1P195A/p150‐ or Adar1N175AY179A/null mice, IFN activation compared with unsupervised Z‐RNA due to the loss‐of‐function of ADAR1‐Zα mutation trigger a ZBP1‐mediated inflammatory cell death, which is responsible partially for the postnasal lethality in those mice. Moreover, ZBP1 seems also play a role in augmenting IFN signaling.
Alternating deoxycytosine and deoxyguanosine are stabilized in the Z‐DNA conformation under physiological salt conditions by bromination at the 5‐position of the deoxycytosine. Herbert et al. labeled this oligodeoxynucleotide with 32P and used it as a probe in a band shift experiment [29]. They purified a 140‐KDa protein from chicken lungs, which specifically bound to this probe in the Z‐form with high affinity, and further identified this 140‐kDa protein as ADAR1 [27]. Subsequently, they screened different regions of human ADAR1 for Z‐DNA‐binding sites and annotated a Zα domain at the N‐terminus [26]. A previous study found a sequence preference for Z‐DNA formation, as discussed above, namely, d(CG)> d(CA) = d(TG)>d(AT). However, the Zα domain in ADAR1 can flip a range of sequences, including d(TA)3, into the Z‐DNA conformation, which implies that Zα is more conformation‐specific rather than sequence‐specific [30]. This finding renewed previous thoughts that B–Z transition is only induced in regions with alternating purines and pyrimidines, which was in fact not widely distributed in metazoans.
How ADAR1 binds to Z‐DNA was subsequently elucidated by co‐crystallization of its Zα domain with left‐handed Z‐DNA [31]. Two Zα domains bind to one Z‐DNA duplex in a way that is markedly different from B‐DNA‐binding proteins. The contacts between Z‐DNA and the Zα domain primarily occur in the DNA backbone and the contact sites form a unique recognition surface [31]. This interaction pattern is highly conserved in all Zα domains including that of other Z‐DNA‐binding proteins which were identified later. Notably, both ADAR1 isoforms (P150 and P110) have a Zβ domain (Figure 1), which share a similar structure, but have a different function with Zα as the Zβ domain is incapable of binding to Z‐DNA [32]. Thus, the Z‐DNA‐binding activity is a unique feature of the longer isoform P150 of ADAR1 that bears an extra Zα domain (Figure 1).
Research on ADAR1 has intensively focused on its RNA‐editing function, converting adenosine to inosine, termed A–I editing, which disrupts the dsRNA duplex (Figure 1). This function is mostly, if not all, dependent on the deaminase domain shared by both isoforms. It is of remarkable importance because substrates of A–I editing by ADAR1 are widely distributed, and the primary substrates edited are inverted transposable elements [33], including Alu elements and short interspersed nuclear elements (SINEs) in humans and mice, respectively. Moreover, exogenously originated RNA duplexes from viruses are extensively edited by ADAR1 [33]. All Adar mice with editing defects, including Adar1–/– mice, Adar1E861A/E861A (deaminase enzymatic inactive) and Adar1P150‐/P150– (lack ADAR1‐P150), are embryonic lethal [34, 35, 36, 37]. For the mechanism, defects in RNA editing of ADAR1 releases unsupervised dsRNA duplexes as typical non‐self nucleic acids that induce various immune response signalings: (1) dsRNA activates oligoadenylate synthetase (OAS), which synthesizes 2’–5’ oligoadenylate, and in turn, triggers widespread RNase L‐mediated RNA degradation [38]. (2) dsRNA activates protein kinase R (PKR), which leads to phosphorylation of eIF2α that represses translation [39, 40]. Activation of either oligoadenylate synthetase‐RNase L or PKR results in cell lethality. (3) dsRNA is sensed by melanoma differentiation‐associated protein 5 (MDA5) that initiates type I IFN signaling via the MDA5‐mitochondrial antiviral‐signaling protein (MAVS) axis [34, 41, 42] (Figure 1).
ADAR1 mutations in humans are associated with neuroinflammatory Aicardi‐Goutières syndrome (AGS). Among all mutations in AGS patients, the majority are located in the deaminase domain, whereas one compound heterozygous mutation encoding p.Pro193Ala is found in the Zα domain [43]. Pro193 contributes to Z‐form nucleic acid binding [31], which implying a potential function of Z‐nucleic acid in inducing autoimmune disease. There are recently four studies in parallel, which are trying to address the biological role of Z‐nucleic acid in vivo by mutating the ADAR1‐Zα domain in mice (Figure 1, Table 1). Maurano et al. generated a Adar1p195A mouse that modeled the high‐frequency mutation within the Zα domain found in AGS patients. The homogenous Adar1p195A/p195A mouse is not pathological, whereas Adar1p195A/– or Adar1p195A/p150– causes complete postnatal mortality, which recapitulates the AGS patient phenotype. The lack of pathology in Adar1p195A/p195A mice is consistent with the absence of the known AGS patient who is identified with this homogenous variant. The pathological mice correspond to the compound heterozygous mutant found in AGS patients, with one Adar1 allele carrying the P193A mutant paired with the full Adar1 null allele (Adar1p195A/– ) or Adar1‐p150 null allele (Adar1p195A/p150– ). Therefore, this disease phenotype is a combined consequence from defects in both Zα and deaminase domains. Maurano et al. found that the disease in Adar1p195A/– or Adar1p195A/p150– mice requires MDA5 and RIG‐I‐like receptor LGP2 on the apex for IFN signaling induction and also requires PKR that activates the integrated stress response (ISR) (Figure 1), and the latter is believed to be the main cause of the pathogenesis in mice [44]. They think that it is possible that a null allele of Adar1 in those mice contributes to the upregulation of ISR which leads to severe pathology [44]. Interestingly, it is reported, in the later studies, that the postnasal lethality of Adar1p195A/– or Adar1p195A/p150‐ mice could be rescued by crossing with Zbp1 –/– mice, suggesting a ZBP1‐mediated cell death and inflammatory signature is also involved (Figure 1) [45].The contact sides between the ADAR1‐Zα domain and Z‐DNA form a continuous recognition surface that consists of residues from helix α‐3 and the COOH‐terminal β hairpin. Residues, including N173 and Y177 (homologous to N175 and Y179 in mice), mediate the direct interaction with Z‐DNA, and mutation induction in these two residues abolish Z‐DNA binding [31, 46]. W195 (homologous to W197 in mice) in β hairpin forms hydrogen bonds and contacts with Z‐DNA via the water molecule in an indirect manner [31]. Notably, these residues, including N173, Y177, and W195, are highly conserved across species in ADAR1‐Zα and are conserved with Ζα domains in other proteins such as ZBP1 [47]. The other three reports mainly focused on the biological function that is dependent on its Z‐nucleic acid binding activity. Two groups generated a Adar1mΖα/mΖα mouse with N175AY179A mutations. This mouse develops normally, but only displays a mild phenotype with spontaneous IFN‐stimulated genes (ISG) induction in a MAVS‐MDA5 dependent manner [48, 49] (Figure 1). This ISG induction is also found to be partially contributed by ZBP1, in a Zα domain‐dependent manner, with an unclear mechanism [50]. The viability of the mice enables a subsequent investigation on the endogenous substrates as the Z‐form to which ADAR1 binds for RNA editing. Loss of function in ADAR1‐Zα diminishes editing of a subset of RNAs in vivo, implying that this RNA subset acts as Z‐RNA to which ADAR1 binds and modulates. In line with previous research, the editing sites are heavily enriched in short interspersed nuclear elements (SINEs), but there is no particular member of SINEs is “hypo‐editing” due to Zα mutation [48, 49]. It is of note that N175AY179A mutation paired with a full Adar1 null allele (Adar1 mZα/–) also displays a much more severe disease with postnatal mortality, compared with the milder pathology in homogenous Adar1 mZα/mZα [50, 51] (Figure 1), as the scenario between Adar1 P195A/– and Adar1P195A/P195A. These differences, again, could be simply explained by a combination consequence of defects on both Z‐nucleic acid binding and deaminase activities; but the precise mechanism awaits further study. Compared with Adar1mΖα/mΖα , Adar1W197A/W197A carrying a mutation of residue W197 in the β‐hairpin results in much more severe growth retardation with approximately half of mice dying by up to 6 weeks of age. The mice exhibit encephalopathy and impaired hematopoiesis. For the mechanism, Adar1W197A/W197A is characterized by the MDA5‐mediated ISG signature, presumably due to interference of RNA editing repertoire [52] (Figure 1). The reason why mutation of W197A leads to a much more severe pathology, whereas N175AY179A mutation renders a milder phenotype, requires further study. If the induction of ISR correlates with the disease severity as Maurano et al. proposed, it will be interesting to determine the ISR levels in those Zα‐mutated mice for comparison. If ZBP1 is involved in the phenotype of Adar1W197A/W197A also need to be investigated. Defects on Z‐nucleic acid binding contribute to a reduction of the RNA editing efficiency in some subsets of substrate, but another subset of sites is preferentially edited in mutant mice [48, 49], which presumably due to the heightened level of ADAR1 as an ISG which may recruit more A‐form dsRNAs for editing. But the precise mechanism needs further study. Moreover, the endogenous Z‐RNAs as substrates that the ADAR1‐Zα targets for editing remain unclear.
Table 1.
ADAR1‐Zα mutant mice
| Genotype | Position in Zα | Phenotype | Pathway involved | Reference |
|---|---|---|---|---|
| Adar1 P195A/– or Adar1 P195A/P150– | Conserved residues in β3, homologous to Pro193Ala found in AGS patients |
Postnatal mortality, multiple‐organ pathology. Heightened level of ISG and ISR. |
MDA5, LGP2 mediated IFN signaling. PKR‐dependent activated ISR. Activation of ZBP1‐mediated cell death. |
[44, 45] |
| Adar1 P195A/P195A | Healthy | N/A | [44] | |
| Adar1 N175AY179A/N175AY179A | Conserved residues in α3, directly interact with Z‐DNA | Spontaneous ISG signature in multiple organs | MDA5‐MAVs mediated IFN signaling. | [48, 49] |
| Adar1 N175AY179A/null | Postnatal mortality |
MDA5‐MAVs mediated IFN signaling. Activation of ZBP1‐mediated cell death. |
[49, 50, 51] | |
| Adar1W 197A/W197A | Conserved residues in β3 | Severe growth retardation, encephalopathy and impaired hematopoiesis | MDA5‐MAVs mediated IFN signaling. | [52] |
ZBP1
Z‐DNA‐binding protein 1 (ZBP1), also termed as DLM‐1 and DAI, was initially cloned from stromal tissue in tumor‐bearing mice. Its expression was upregulated upon LPS or IFN‐γ treatment in activated macrophages [53]. Using the Zα domain of ADAR1 (ZαADAR1) as the query model and searching for proteins with a Z‐DNA‐binding potential, ZBP1 was recognized to be a target containing a domain with remarkable sequence similarities to ZαADAR1. The core of the protein–DNA interface is highly conserved between ZαADAR1 and ZαZBP1 in terms of structure [47], which suggests that the Zα family shares Z‐DNA binding as a common feature. It is of note that, unlike ADAR1, which contains one Zα domain and another non‐functional Zβ domain, ZBP1, bears two functional Z‐DNA‐binding domains as Zα1 and Zα2 (Figure 2A) [54]. The Zα2 domain of ZBP1 binds to Z‐DNA in a slightly distinctive mode from the conventional Zα domains. Zα1, similar with ZαADAR1, binds to Z‐DNA through both the α3 recognition helix and β‐loop, whereas Zα2 has a shorter β‐loop in the wing and it is positioned away from Z‐DNA, so Zα2 in contact with Z‐DNA mainly relies on the pivotal residues of the α3 helix [55].
Figure 2.
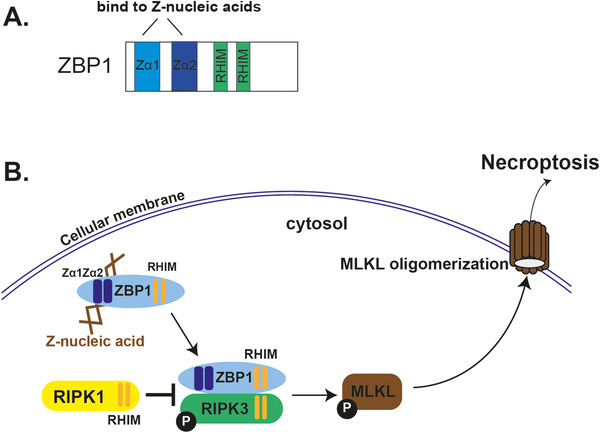
Z‐nucleic acids bind to ZBP1 that triggers necroptosis. (A) ZBP1 has two functional Z‐nucleic acid binding domains, namely Zα1 and Zα2, and two RHIM domains that are responsible for its interaction with other RHIM‐domain‐containing proteins, such as RIPK1 and RIPK3. (B) Once activated by binding Z‐nucleic acids, ZBP1 recruits RIPK3 through RHIM domain, which leads to RIPK3 auto‐phosphorylation and MLKL phosphorylation. Phosphorylated MLKL oligomerizes and translocates to the membrane, where it damages membrane integrity and induces the inflammatory type of cell death, as necroptosis. Another RHIM‐containing protein, RIPK1, prevents ZBP1 from activating RIPK3, and thus inhibits necroptosis.
The alternative name of ZBP1, DAI (DNA‐dependent activator of IFN‐regulatory factors), is based on its function of sensing cytosolic DNA to activate the innate immune response [56]. However, its function as DNA sensor has proven to be controversial and uncertain because ZBP1‐deficient cells and mice respond normally to DNA viruses with considerable levels of IFNs and cytokines are produced [57]. But ZBP1‐deficient cell and mice indeed support a more pronounced replication of some viruses, such as murine CMV (MCMV) and influenza A virus (IAV), which is afforded by the resistance on a ZBP1‐mediated cell death triggered by virus‐generated Z‐RNAs [58, 59, 60].
The cell death triggered by the binding of Z‐nucleic acid and ZBP1 is named as necroptosis. Specifically, ZBP1 binds to Z‐nucleic acids via its two functional Zα domains, Zα1 and Zα2 (Figure 2B). Once bound and activated by sensing Z‐nucleic acids, ZBP1 stimulates receptor‐interacting serine/threonine kinase 3 (RIPK3) that subsequently phosphorylates mixed lineage kinase domain‐like pseudokinase (MLKL). MLKL in turns oligomerizes and translocates to the membrane where it damages the integrity of the membrane and causes necroptosis [23, 60‐62] (Figure 2B). Necroptosis is a potent inducer of inflammation [63]. RIPK1 acts as a negative regulator by preventing ZBP1 from activating RIPK3 [64, 65] (Figure 2B). Deficiency of RIPK1 causes hyperactive of the ZBP1‐RIPK3‐MLKL pathway, which results in perinatal lethality and severe inflammation. The genetic interaction between RIPK1 and ZBP1 was confirmed by rescuing the severe inflammation in RIPK1 and ZBP1 double KO mice [64, 65]. Moreover, endogenous Z‐nucleic acids appear to be the trigger for this inflammatory pathology because mutating Zα1 and Zα2 rescues progressive skin inflammation in RIPK1 KO mice [66, 67]. Similar to RIPK1‐deficient mice, four primary immunodeficiency (PID) patients were found to carry loss‐of‐function mutations in the RIPK1 gene and were characterized by lymphopenia, recurrent viral, bacterial, and fungal infections, early‐onset inflammatory bowel disease (IBD), and arthritis [68] (Figure 3A). Whether Z‐nucleic acids in these PID cells binds and activates ZBP1 similarly to that in RIPK1 KO mice and triggers necroptosis and inflammation, requires further investigation. Z‐nucleic acid‐initiated necroptosis is also associated with human inflammatory bowel disease (IBD) [69] (Figure 3A). IBD patients have been characterized with a decreased level of SETDB1 (a histone H3K9 methyltransferase), which was thought to cause defects in the heterochromatin structure, leading to genome instability and retroelement expression. Intestinal stem cells from these IBD patients had substantially elevated necroptosis, which required Zα domains of ZBP1, and highlighted the role of Z‐nucleic acids in IBD [69]. In the context of sterile inflammation induced by the ZBP1/Z‐nucleic acid interaction, the endogenous ligand remains unclear (Figure 3A). In RIPK1‐deficient mice with hypoactivation of ZBP1‐mediated necroptosis and SETDB1 KO mice that model IBD, a high level of endogenous retroelements (EREs) is observed, which acts as a possible ligand binding to the Zα domain of ZBP1 [67, 69]. In line with that, human retroelement Alu duplex activates ZBP1 in the context of the loss‐of‐function on ADAR1 [51].
Figure 3.
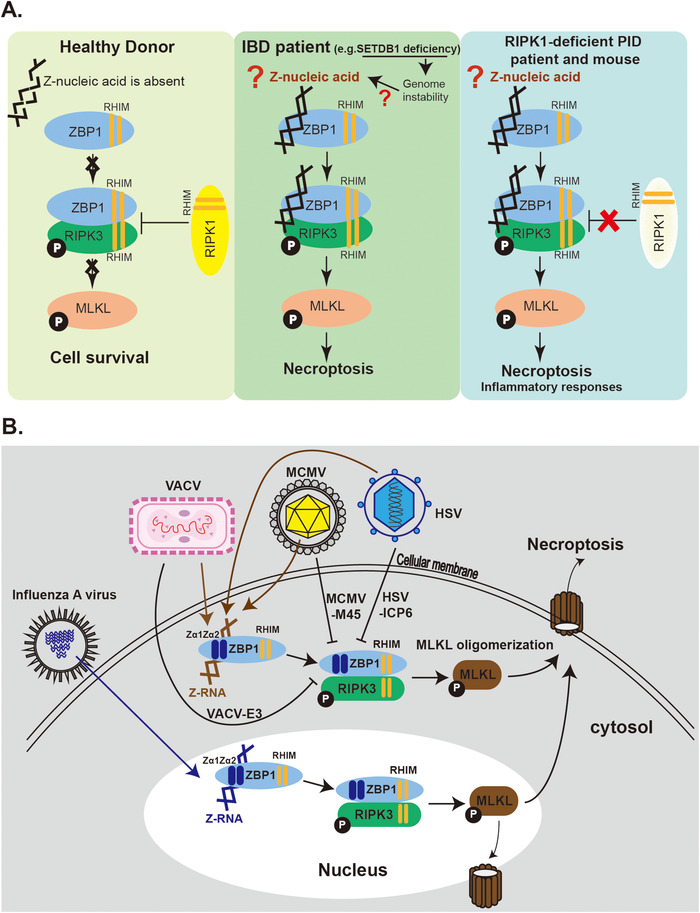
Z‐nucleic acid/ZBP1 interactions are involved in human diseases and antiviral immunity. (A) ZBP1/RIPK3/MLKL pathway is induced by endogenous Z‐nucleic acid in humans and mice. In healthy individuals, Z‐nucleic acid is absent or not accessible to ZBP1; the pathway is not activated and cells survive. In cells of SETDB1‐deficient IBD patients, SETDB1 deficiency leads to genome instability and upregulates endogenous retroelements which act as a possible source of aberrant Z‐nucleic acid to activate ZBP1‐mediated necroptosis. In RIPK1‐deficient mice and PID patients carrying RIPK1‐mutations, the negative regulation of the ZBP1/RIPK3/MLKL pathway is absent, the cells can undergo spontaneous activation of necroptosis which leads to immune disorders and inflammation. (B) The ZBP1/RIPK3/MLKL pathway is activated by exogenous Z‐nucleic acids. Z‐RNAs generated by MCMV and VACV in cytoplasm that activate necroptosis cascade through ZBP1 and this process is blocked by MCMV‐encoding M45 or VACV‐encoding E3, respectively. HSV infection also induces ZBP1‐mediated cell death and the virus employs ICP6 to antagonize this pathway. Influenza A virus produces Z‐RNA that activates ZBP1 in infected nuclei. The downstream MLKL could be recruited for disrupting nuclear envelope or shuttle to cytosol to induce necroptosis.
Z‐nucleic acids bound by ZBP1 could be of endogenous or exogenous origins. Exogenous Z‐nucleic acids, more specifically, Z‐RNA, generated by some viruses, is believed to induce cell death through a ZBP1‐dependent mechanism (Figure 3B). ZBP1 sensitizes cells to virus‐induced necrosis that eliminates infected cells as a strategy for virus clearance. Viruses in turn evolve antagonism to counteract this mechanism. MCMV encodes M45 that disrupts the ZBP1‐RIPK3 interaction and blocks necroptosis activation upon MCMV infection (Figure 3B). Using a mutated MCMV in M45 (MCMV‐M45mutRHIM), ZBP1‐mediated necroptosis is activated upon infection and, more importantly, this activation is induced by the newly transcribed RNA generated upon infection, which binds to the two Zα domains of ZBP1 [60, 61, 70]. Herpes simplex virus 1 (HSV1) triggers necroptosis via ZBP1, encoding ICP6 as the antagonism, with a mechanism reminiscent of MCMV [71] (Figure 3B). The newly transcribed RNAs as ligands that activate ZBP1 in this process, requires further investigation. ZBP1 senses Z‐RNA produced by infection of the vaccinia virus (VACV), which subsequently initiates necroptosis, and similarly, this process is antagonized by VACV encoding E3 [72] (Figure 3B). For the mechanism, VACV E3 completes for Z‐RNA binding with ZBP1 thus to block the initiation of necroptosis [72]. However, the Z‐RNA that E3 sequesters from ZBP1 is in need of identification. Another example of exogenous Z‐nucleic acids is Z‐RNAs generated by influenza A virus (IAV) infection. Unlike other viruses that generate Z‐RNAs in cytosol, IAV produces Z‐RNAs in the nucleus, and these Z‐RNAs are speculated to originate from defective viral genomes [23] (Figure 3B).
Although how the ZBP1/Z‐nucleic acid interaction contributes to inflammatory diseases and anti‐viral immunity has been extensively studied in the past few years, the nature of Z‐nucleic acids that ZBP1 senses in these processes remain largely unknown, especially the Z‐RNAs exogenously generated upon viral infections.
E3 and PKZ
The critical residues forming the Z‐DNA‐binding surface are highly conserved among all the described Zα domains from different species. E3 is encoded by vaccina viral gene E3L, acting as an innate immune evasion protein [73]. E3 contains a C‐terminal dsRNA binding domain that functions by sequestering dsRNA from activating innate immune sensors such as PKR [73]. Moreover, it contains sequences highly similar to the well‐known Zα domain at the N‐terminus [26]. E3 is necessary for the pathogenicity of the virus. As mentioned above, by completing for Z‐RNA binding with ZBP1, Zα domain of E3 protein prevents the initiation of necroptosis [72, 73]. As a consequence, in the absence of E3 protein or its Zα domain, immune surveillances on viral infection including PKR signaling and ZBP1‐RIPK3‐MLKL mediated necroptosis is released to be evoked to restrict the viral replication [72, 73]. The replacement with the Zα domain from ADAR1 or ZBP1 rescues its virulence, indicating Zα domains from ADAR1, ZBP1, and E3 are functionally exchangeable [74]. Moreover, E3L‐like protein in Yaba‐like disease virus belonging to Yatapoxvirus family contains a Zα domain, namely yabZαE3L, which shares an extremely similar Z‐DNA‐binding surface with the Zα domains of mammalian proteins [75], reinforcing the importance of its Z‐nucleic acid‐binding activity for poxvirus infection.
Using the Zα domain from rat ZBP1 (ratZαZBP1), a protein in zebrafish was identified with two Zα‐like domains [76]. It also contains a conserved kinase domain in the‐C terminus and was named protein kinase‐containing Z‐DNA‐binding domains (PKZ). PKZ is closely related to mammalian PKR, which senses dsRNA and initiates translational shutdown and cell death by modulating eIF2α, a translational initiation factor. Moreover, PKZ likely has the same function as PKR, but with substitution of the Z‐binding domain for the dsRNA‐binding domain in PKR. Therefore, PKZ is a functional ortholog of PKR, but it recognizes Z‐DNA instead of dsRNA [77] and its orthologues have been identified in several fish species [76, 77, 78, 79].
Z‐DNA in bacteria
Unlike Z‐RNAs, which participate largely in immune responses, the biological role of Z‐DNA is barely studied. Even though it has been speculated to be relevant to transcriptional activation as mentioned above, but the in‐depth characterization of Z‐DNA in vivo is lacking. Very recently, Z‐DNA was found as a structural component in bacteria [80]. Specifically, Z‐form DNA accumulates as extracellular DNA (eDNA) in bacteria biofilm. The biofilm is comprised of extracellular DNAs, proteins, lipids, polysaccharides, biopolymers, and divalent cations as a polymeric substance matrix. And the mature biofilms contained eDNA is resistant to nuclease digestion. The eDNA favors the Z‐conformation that confers this nuclease‐resistant property. Moreover, the bacterial DNABII family of proteins stabilizes these eDNAs in the Z‐form which contributes to the structural integrity of the biofilm matrix. By stabilizing Z‐form DNA in biofilm, bacterial DNABII proteins inactivated neutrophil extracellular traps (NET)‐mediated bacterial killing [80]. Altogether, this study highlighted the existence of Z‐DNA as the bacterial eDNA and its role in biofilm pathogenesis and immune evasion. This study also proposed that bacterial‐derived Z‐DNA within biofilms is a major contributor to the reservoir of Z‐DNA in the host. Whether it interacts with host immune system directly and what is the clinical relevance of bacteria‐reserved Z‐DNA with human disease will be interesting to investigate.
Conclusion
In recent years, extensive studies on Z‐nucleic acid‐binding proteins, including ZBP1 and ADAR1, have been gradually clarifying the biological function of Z‐nucleic acids. This alternative nucleic acid conformer, specifically Z‐RNA, primarily participates in immune responses by interacting with ADAR1 or ZBP1. Loss of function in the Z‐RNA‐binding of ADAR1 induces activation of type I IFN signaling via MDA5 [48, 49, 52]. Z‐RNAs, rather than Z‐DNAs, modulated by ADAR1 because MDA5 senses RNAs. It is mainly Z‐RNAs, from endogenous and exogenously originating from viral infection, activate necroptosis via ZBP1 [23, 60, 61, 66, 67, 69, 72]. Whether Z‐DNA or DNA‐RNA hybrids are also involved in these functions mediated by ZBP1 or ADAR1 is yet to be determined.
The origin of virus‐generated Z‐nucleic acids has not been fully characterized. Viruses, such as MCMV, IAV, and VACV, generate Z‐RNAs that trigger ZBP1‐mediated necroptosis [23, 60, 61, 72]. Interestingly, even though it remains unclear which exact Z‐RNAs are produced by viruses, it is clear that Z‐RNA rather than Z‐DNA, in most cases, activates ZBP1. Zhang et al. synthesized a Z‐RNA duplex to screen antibodies previously designed to target Z‐DNA (anti‐Z‐NA) for their ability to cross‐react with Z‐RNAs [23]. Using an antibody that reacted with Z‐RNA rather than A‐RNA, they detected IAV‐generated Z‐RNAs inside the nucleus, which was potentially generated from defective viral genomes [23]. ZBP1, which normally localizes in cytosol, translocates into nucleus in this process [23]. However, it is not excluded that IAV may also produce foreign nucleic acids in Z‐form in cytoplasm. Because anti‐Z‐NA is also capable of detecting Z‐DNA, it is hard to distinguish detection of Z‐RNA from Z‐DNA by immunostaining only. In the context of MCMV infection, actinomycin D, which inhibits de novo RNA synthesis, prevents ZBP1 activation, suggesting it is derived from RNA in Z‐form produced during virus infection. The binding of endogenous Z‐RNA rather than Z‐DNA to ZBP1‐Zα domains was confirmed by CLIP experiments (cross‐linking and immunoprecipitation) [60]. VACA infection accumulates RNA in Z‐form in cytosol, which binds to ZBP1 and viral protein E3. However, the RNA species that interacts with them wer not characterized [72].
Both DNA (e.g., MCMV and VACV) and RNA (e.g., IAV) viruses produce Z‐RNAs during their replication, suggesting there is no particular virus family with this ability. If other virus families are involved in Z‐nucleic acid‐mediated immune responses and if Z‐nucleic acid surveillance is a general strategy that the host exploits to inhibit viruses will be interesting to explore.
While this manuscript is under review, there are four papers pointing out the cross‐talk between ADAR1 and ZBP1 [45, 50, 51, 81]. Adar1–/– mice are embryonic lethal [34, 41]. The mutation induced in the Zα domain of Adar1 such as Adar1p195A/p195A [44] and Adar1mΖα/mΖα mice (N175AY179A) [48, 49]are not pathological; whereas the mice carrying a combination of the mutation in Zα domain and a null Adar1 allele, including Adar1p195A/–, Adar1 p195A/p150null [44], or Adar1mZα/– [49] display a much server phenotype, with pronounced ISG upregulation and inflammatory signature [45, 50, 51], and those mice died postnatally. Interestingly, ZBP1‐deficiency, especially deficiency on its Z‐nucleic acid binding activity, could rescue the postnatal lethality of them, to the various degrees [45, 50, 51], suggesting that ADAR1 prevents the spontaneous activation of ZBP1. Moreover, this activation of ZBP1 induced by ADAR1 deficiency, not only results in cell death and inflammation [45, 50, 51], but only contributes to a ZBP1‐dependent IFN signaling augment [45, 50]. The interaction between ZBP1 and ADAR1 is very likely mediated by the endogenous Z‐nucleic acid as their shared ligand. Alu‐Alu hybrids, the major substrates of ADAR1 to edit, could induce ZBP1‐dependent cell death by transfection [51], suggesting they function as the Z‐RNA ligands in this scenario. In line with this, in human cells, ADAR1‐deficiency leads to the accumulation of endogenous Z‐RNAs, enriched in the 3’ untranslated region of IFN‐stimulated mRNAs, which in turn activates ZBP1‐dependent necroptosis [81]. Activation of ZBP1‐mediated necroptosis pharmacologically could be applied in antitumor therapy [81]. Surprisingly, the ZBP1‐dependent cell death induced by ADAR1 deficiency seems not, or only partially, dependent on the known cascades downstream of ZBP1, such as RIPK3‐MLKL‐mediated necroptosis, or FADD‐caspase9‐mediated apoptosis [45, 50, 51]. Which factors that ZBP1 employs for the inflammation signature in those ADAR1‐deficient mice need to be determined and the mechanism by which ZBP1 augments IFN responses is still unknown [45, 50]. Studies that underline the precise mechanisms on this ZBP1–ADAR1 interaction need to be conducted in the future.
Conflict of interest
The author declares no commercial or financial conflict of interest.
Abbreviations
- A to I
adenosine to inosine
- ADAR1
adenosine deaminase acting on RNA 1
- Adar1P195A/‐
Adar null allele
- Adar1P195A/p150–
Adar p150 null allele
- AGS
Aicardi‐Goutières syndrome
- anti‐Z‐NA
antibodies previously designed to target Z‐DNA
- eDNA
extracellular DNA
- EREs
endogenous retroelements
- HSV1
Herpes simplex virus 1
- IAV
influenza A virus
- IBD
inflammatory bowel disease
- ISR
integrated stress response
- MAD5
melanoma differentiation‐associated protein 5
- MAVS
MDA5‐mitochondrial antiviral‐signaling protein
- MAVS
mitochondrial antiviral‐signaling protein
- MDA5
melanoma differentiation‐associated protein 5
- MLKL
mixed lineage kinase domain like pseudokinase
- NET
neutrophil extracellular traps
- OAS
oligoadenylate synthetase
- PID
primary immunodeficiency
- PKR
protein kinase R
- PKR
proteinase kinase R
- PKZ
protein kinase‐containing Z‐DNA‐binding domains
- ratZαZBP1
Zα domain from rat ZBP1
- RIPK3
receptor‐interacting serine/threonine kinase 3
- SINEs
short interspersed nuclear elements
- VACV
vaccinia virus
- ZBD
Z‐DNA binding domain
- ZBP1
Z‐DNA‐binding protein 1
- ZαADAR1
Zα domain of ADAR1
Acknowledgments
The author thanks Professor Jan Rehwinkel for providing suggestions on the initial draft of this article.
Tang Qiannan. Z‐nucleic acids: uncovering the functions from past to present. Eur. J. Immunol. 2022;00:00–00. 10.1002/eji.202249968
References
- 1. Wang, A. H. , Quigley, G. J. , Kolpak, F. J. , Crawford, J. L. , van Boom, J. H. , van der Marel, G. and Rich, A. , Molecular structure of a left‐handed double helical DNA fragment at atomic resolution. Nature 1979. 282: 680–686. [DOI] [PubMed] [Google Scholar]
- 2. Hall, K. , Cruz, P. , Tinoco I., Jr , Jovin, T. M. and van de Sande, J. H. : ‘ Z‐RNA’–a left‐handed RNA double helix. Nature 1984. 311: 584–586. [DOI] [PubMed] [Google Scholar]
- 3. Pohl, F. M. and Jovin, T. M. , Salt‐induced co‐operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG‐dC). J Mol Biol 1972. 67: 375–396. [DOI] [PubMed] [Google Scholar]
- 4. Mercado, C. M. and Tomasz, M. , Circular dichroism of mitomycin‐DNA complexes. Evidence for a conformational change in DNA. Biochemistry 1977. 16: 2040–2046. [DOI] [PubMed] [Google Scholar]
- 5. Pohl, F. M. , Polymorphism of a synthetic DNA in solution. Nature 1976. 260: 365–366. [DOI] [PubMed] [Google Scholar]
- 6. Patel, D. J. and Canuel, L. L. , Pohl FM: “Alternating B‐DNA” conformation for the oligo(dG‐dC) duplex in high‐salt solution. Proc Natl Acad Sci U S A 1979. 76: 2508–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis, P. W. , Hall, K. , Cruz, P. , Tinoco I., Jr. and Neilson, T. , The tetraribonucleotide rCpGpCpG forms a left‐handed Z‐RNA double‐helix. Nucleic Acids Res 1986. 14: 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang, A. J. , Quigley, G. J. , Kolpak, F. J. , van der Marel, G. , van Boom, J. H. and Rich, A. , Left‐handed double helical DNA: variations in the backbone conformation. Science 1981. 211: 171–176. [DOI] [PubMed] [Google Scholar]
- 9. Moller, A. , Nordheim, A. , Nichols, S. R. and Rich, A. , 7‐Methylguanine in poly(dG‐dC).poly(dG‐dC) facilitates z‐DNA formation. Proc Natl Acad Sci U S A 1981. 78: 4777–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moller, A. , Nordheim, A. , Kozlowski, S. A. , Patel, D. J. and Rich, A. , Bromination stabilizes poly(dG‐dC) in the Z‐DNA form under low‐salt conditions. Biochemistry 1984. 23: 54–62. [DOI] [PubMed] [Google Scholar]
- 11. Rich, A. , Nordheim, A. and Wang, A. H. , The chemistry and biology of left‐handed Z‐DNA. Annu Rev Biochem 1984. 53: 791–846. [DOI] [PubMed] [Google Scholar]
- 12. Singleton, C. K. , Klysik, J. , Stirdivant, S. M. and Wells, R. D. , Left‐handed Z‐DNA is induced by supercoiling in physiological ionic conditions. Nature 1982. 299: 312–316. [DOI] [PubMed] [Google Scholar]
- 13. Klysik, J. , Stirdivant, S. M. , Larson, J. E. , Hart, P. A. and Wells, R. D. , Left‐handed DNA in restriction fragments and a recombinant plasmid. Nature 1981. 290: 672–677. [DOI] [PubMed] [Google Scholar]
- 14. Rahmouni, A. R. and Wells, R. D. , Stabilization of Z DNA in vivo by localized supercoiling. Science 1989. 246: 358–363. [DOI] [PubMed] [Google Scholar]
- 15. McLean, M. J. and Wells, R. D. , The role of sequence in the stabilization of left‐handed DNA helices in vitro and in vivo. Biochim Biophys Acta 1988. 950: 243–254. [DOI] [PubMed] [Google Scholar]
- 16. Lafer, E. M. , Moller, A. , Nordheim, A. , Stollar, B. D. and Rich, A. , Antibodies specific for left‐handed Z‐DNA. Proc Natl Acad Sci U S A 1981. 78: 3546–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lafer, E. M. , Valle, R. P. , Moller, A. , Nordheim, A. , Schur, P. H. , Rich, A. and Stollar, B. D. , Z‐DNA‐specific antibodies in human systemic lupus erythematosus. J Clin Invest 1983. 71: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nordheim, A. , Pardue, M. L. , Lafer, E. M. , Moller, A. , Stollar, B. D. and Rich, A. , Antibodies to left‐handed Z‐DNA bind to interband regions of Drosophila polytene chromosomes. Nature 1981. 294: 417–422. [DOI] [PubMed] [Google Scholar]
- 19. Robert‐Nicoud, M. , Arndt‐Jovin, D. J. , Zarling, D. A. and Jovin, T. M. , Immunological detection of left‐handed Z DNA in isolated polytene chromosomes. Effects of ionic strength, pH, temperature and topological stress. EMBO J 1984. 3: 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lipps, H. J. , Nordheim, A. , Lafer, E. M. , Ammermann, D. , Stollar, B. D. and Rich, A. , Antibodies against Z DNA react with the macronucleus but not the micronucleus of the hypotrichous ciliate stylonychia mytilus. Cell 1983. 32: 435–441. [DOI] [PubMed] [Google Scholar]
- 21. Viegas‐Pequignot, E. , Derbin, C. , Malfoy, B. and Taillandier, E. , Leng, M. , Dutrillaux B: Z‐DNA immunoreactivity in fixed metaphase chromosomes of primates. Proc Natl Acad Sci U S A 1983. 80: 5890–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hill, R. J. and Stollar, B. D. , Dependence of Z‐DNA antibody binding to polytene chromosomes on acid fixation and DNA torsional strain. Nature 1983. 305: 338–340. [DOI] [PubMed] [Google Scholar]
- 23. Zhang, T. , Yin, C. , Boyd, D. F. , Quarato, G. , Ingram, J. P. , Shubina, M. , Ragan, K. B. , Ishizuka, T. , Crawford, J. C. and Tummers, B. , et al., Influenza Virus Z‐RNAs Induce ZBP1‐Mediated Necroptosis. Cell 2020. 180: 1115–1129.e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamada, H. and Kakunaga, T. , Potential Z‐DNA forming sequences are highly dispersed in the human genome. Nature 1982. 298: 396–398. [DOI] [PubMed] [Google Scholar]
- 25. Nordheim, A. , Tesser, P. , Azorin, F. , Kwon, Y. H. and Moller, A. , Rich A: Isolation of Drosophila proteins that bind selectively to left‐handed Z‐DNA. Proc Natl Acad Sci U S A 1982. 79: 7729–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herbert, A. , Alfken, J. , Kim, Y. G. , Mian, I. S. , Nishikura, K. and Rich, A. , A Z‐DNA binding domain present in the human editing enzyme, double‐stranded RNA adenosine deaminase. Proc Natl Acad Sci U S A 1997. 94: 8421–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herbert, A. , Lowenhaupt, K. , Spitzner, J. , Rich, A. , Chicken double‐stranded RNA adenosine deaminase has apparent specificity for Z‐DNA. Proc Natl Acad Sci U S A 1995. 92: 7550–7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herbert, A. G. , Spitzner, J. R. , Lowenhaupt, K. and Rich, A. , Z‐DNA binding protein from chicken blood nuclei. Proc Natl Acad Sci U S A 1993. 90: 3339–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herbert, A. G. and Rich, A. , A method to identify and characterize Z‐DNA binding proteins using a linear oligodeoxynucleotide. Nucleic Acids Res 1993. 21: 2669–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herbert, A. , Schade, M. , Lowenhaupt, K. , Alfken, J. , Schwartz, T. , Shlyakhtenko, L. S. , Lyubchenko, Y. L. and Rich, A. , The Zalpha domain from human ADAR1 binds to the Z‐DNA conformer of many different sequences. Nucleic Acids Res 1998. 26: 3486–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz, T. , Rould, M. A. , Lowenhaupt, K. , Herbert, A. and Rich, A. , Crystal structure of the Zalpha domain of the human editing enzyme ADAR1 bound to left‐handed Z‐DNA. Science 1999. 284: 1841–1845. [DOI] [PubMed] [Google Scholar]
- 32. Athanasiadis, A. , Placido, D. , Maas, S. , Brown B. A., 2nd , Lowenhaupt, K. and Rich, A. , The crystal structure of the Zbeta domain of the RNA‐editing enzyme ADAR1 reveals distinct conserved surfaces among Z‐domains. J Mol Biol 2005. 351: 496–507. [DOI] [PubMed] [Google Scholar]
- 33. Pfaller, C. K. , Donohue, R. C. , Nersisyan, S. , Brodsky, L. and Cattaneo, R. , Extensive editing of cellular and viral double‐stranded RNA structures accounts for innate immunity suppression and the proviral activity of ADAR1p150. PLoS Biol 2018. 16: e2006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liddicoat, B. J. , Piskol, R. , Chalk, A. M. , Ramaswami, G. , Higuchi, M. , Hartner, J. C. , Li, J. B. , Seeburg, P. H. and Walkley, C. R. , RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science 2015. 349: 1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ward, S. V. , George, C. X. , Welch, M. J. , Liou, L. Y. , Hahm, B. , Lewicki, H. , de la Torre, J. C. and Samuel, C. E. , Oldstone MB: RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc Natl Acad Sci U S A 2011. 108: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang, Q. , Miyakoda, M. , Yang, W. , Khillan, J. , Stachura, D. L. , Weiss, M. J. and Nishikura, K. , Stress‐induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem 2004. 279: 4952–4961. [DOI] [PubMed] [Google Scholar]
- 37. Hartner, J. C. , Schmittwolf, C. , Kispert, A. , Muller, A. M. , Higuchi, M. and Seeburg, P. H. , Liver disintegration in the mouse embryo caused by deficiency in the RNA‐editing enzyme ADAR1. J Biol Chem 2004. 279: 4894–4902. [DOI] [PubMed] [Google Scholar]
- 38. Li, Y. , Banerjee, S. , Goldstein, S. A. , Dong, B. , Gaughan, C. , Rath, S. , Donovan, J. , Korennykh, A. , Silverman, R. H. and Weiss, S. R. , Ribonuclease L mediates the cell‐lethal phenotype of double‐stranded RNA editing enzyme ADAR1 deficiency in a human cell line. Elife 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chung, H. , Calis, J. J. A. , Wu, X. , Sun, T. , Yu, Y. , Sarbanes, S. L. , Dao Thi, V. L. , Shilvock, A. R. , Hoffmann, H. H. , Rosenberg, B. R. and Rice, C. M. , Human ADAR1 Prevents Endogenous RNA from Triggering Translational Shutdown. Cell 2018. 172: 811–824.e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li, Z. , Wolff, K. C. and Samuel, C. E. , RNA adenosine deaminase ADAR1 deficiency leads to increased activation of protein kinase PKR and reduced vesicular stomatitis virus growth following interferon treatment. Virology 2010. 396: 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pestal, K. , Funk, C. C. , Snyder, J. M. , Price, N. D. , Treuting, P. M. and Stetson, D. B. , Isoforms of RNA‐Editing Enzyme ADAR1 Independently Control Nucleic Acid Sensor MDA5‐Driven Autoimmunity and Multi‐organ Development. Immunity 2015. 43: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mannion, N. M. , Greenwood, S. M. , Young, R. , Cox, S. , Brindle, J. , Read, D. , Nellaker, C. , Vesely, C. , Ponting, C. P. , McLaughlin, P. J. , et al., The RNA‐editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep 2014. 9: 1482–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rice, G. I. , Kasher, P. R. , Forte, G. M. , Mannion, N. M. , Greenwood, S. M. , Szynkiewicz, M. , Dickerson, J. E. , Bhaskar, S. S. , Zampini, M. , Briggs, T. A. , et al., Mutations in ADAR1 cause Aicardi‐Goutieres syndrome associated with a type I interferon signature. Nat Genet 2012. 44: 1243–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maurano, M. , Snyder, J. M. , Connelly, C. , Henao‐Mejia, J. , Sidrauski, C. and Stetson, D. B. , Protein kinase R and the integrated stress response drive immunopathology caused by mutations in the RNA deaminase ADAR1. Immunity 2021. 54: 1948–1960.e1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hubbard, N. W. , Ames, J. M. , Maurano, M. , Chu, L. H. , Somfleth, K. Y. , Gokhale, N. S. , Werner, M. , Snyder, J. M. , Lichauco, K. , Savan, R. , et al., ADAR1 mutation causes ZBP1‐dependent immunopathology. Nature 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feng, S. , Li, H. , Zhao, J. , Pervushin, K. , Lowenhaupt, K. , Schwartz, T. U. and Droge, P. , Alternate rRNA secondary structures as regulators of translation. Nat Struct Mol Biol 2011. 18: 169–176. [DOI] [PubMed] [Google Scholar]
- 47. Schwartz, T. , Behlke, J. , Lowenhaupt, K. , Heinemann, U. and Rich, A. , Structure of the DLM‐1‐Z‐DNA complex reveals a conserved family of Z‐DNA‐binding proteins. Nat Struct Biol 2001. 8: 761–765. [DOI] [PubMed] [Google Scholar]
- 48. Tang, Q. , Rigby, R. E. , Young, G. R. , Hvidt, A. K. , Davis, T. , Tan, T. K. , Bridgeman, A. , Townsend, A. R. , Kassiotis, G. and Rehwinkel, J. , Adenosine‐to‐inosine editing of endogenous Z‐form RNA by the deaminase ADAR1 prevents spontaneous MAVS‐dependent type I interferon responses. Immunity 2021. 54: 1961–1975.e1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Reuver, R. , Dierick, E. , Wiernicki, B. , Staes, K. , Seys, L. , De Meester, E. , Muyldermans, T. , Botzki, A. , Lambrecht, B. N. , Van Nieuwerburgh, F. , et al., ADAR1 interaction with Z‐RNA promotes editing of endogenous double‐stranded RNA and prevents MDA5‐dependent immune activation. Cell Rep 2021. 36: 109500. [DOI] [PubMed] [Google Scholar]
- 50. Jiao, H. , Wachsmuth, L. , Wolf, S. , Lohmann, J. , Nagata, M. , Kaya, G. G. , Oikonomou, N. , Kondylis, V. , Rogg, M. , Diebold, M. , et al., ADAR1 averts fatal type I interferon induction by ZBP1. Nature 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Reuver, R. , Verdonck, S. , Dierick, E. , Nemegeer, J. , Hessmann, E. , Ahmad, S. , Jans, M. , Blancke, G. , Van Nieuwerburgh, F. , Botzki, A. , et al., ADAR1 prevents autoinflammation by suppressing spontaneous ZBP1 activation. Nature 2022. [DOI] [PubMed] [Google Scholar]
- 52. Nakahama, T. , Kato, Y. , Shibuya, T. , Inoue, M. , Kim, J. I. , Vongpipatana, T. , Todo, H. , Xing, Y. and Kawahara, Y. , Mutations in the adenosine deaminase ADAR1 that prevent endogenous Z‐RNA binding induce Aicardi‐Goutieres‐syndrome‐like encephalopathy. Immunity 2021. 54: 1976–1988.e1977. [DOI] [PubMed] [Google Scholar]
- 53. Fu, Y. , Comella, N. , Tognazzi, K. , Brown, L. F. , Dvorak, H. F. and Kocher, O. , Cloning of DLM‐1, a novel gene that is up‐regulated in activated macrophages, using RNA differential display. Gene 1999. 240: 157–163. [DOI] [PubMed] [Google Scholar]
- 54. Deigendesch, N. , Koch‐Nolte, F. and Rothenburg, S. , ZBP1 subcellular localization and association with stress granules is controlled by its Z‐DNA binding domains. Nucleic Acids Res 2006. 34: 5007–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ha, S. C. , Kim, D. , Hwang, H. Y. , Rich, A. , Kim, Y. G. and Kim, K. K. , The crystal structure of the second Z‐DNA binding domain of human DAI (ZBP1) in complex with Z‐DNA reveals an unusual binding mode to Z‐DNA. Proc Natl Acad Sci U S A 2008. 105: 20671–20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takaoka, A. , Wang, Z. , Choi, M. K. , Yanai, H. , Negishi, H. , Ban, T. , Lu, Y. , Miyagishi, M. , Kodama, T. , Honda, K. , et al., DAI (DLM‐1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007. 448: 501–505. [DOI] [PubMed] [Google Scholar]
- 57. Ishii, K. J. , Kawagoe, T. , Koyama, S. , Matsui, K. , Kumar, H. , Kawai, T. , Uematsu, S. , Takeuchi, O. , Takeshita, F. , Coban, C. and Akira, S. , TANK‐binding kinase‐1 delineates innate and adaptive immune responses to DNA vaccines. Nature 2008. 451: 725–729. [DOI] [PubMed] [Google Scholar]
- 58. Thapa, R. J. , Ingram, J. P. , Ragan, K. B. , Nogusa, S. , Boyd, D. F. , Benitez, A. A. , Sridharan, H. , Kosoff, R. , Shubina, M. , Landsteiner, V. J. , et al., DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3‐Dependent Cell Death. Cell Host Microbe 2016. 20: 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Upton, J. W. , Kaiser, W. J. and Mocarski, E. S. , DAI/ZBP1/DLM‐1 complexes with RIP3 to mediate virus‐induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 2012. 11: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maelfait, J. , Liverpool, L. , Bridgeman, A. , Ragan, K. B. , Upton, J. W. and Rehwinkel, J. , Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sridharan, H. , Ragan, K. B. , Guo, H. , Gilley, R. P. , Landsteiner, V. J. , Kaiser, W. J. and Upton, J. W. , Murine cytomegalovirus IE3‐dependent transcription is required for DAI/ZBP1‐mediated necroptosis. EMBO Rep 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wallach, D. , Kang, T. B. , Dillon, C. P. and Green, D. R. , Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 2016. 352: aaf2154. [DOI] [PubMed] [Google Scholar]
- 63. Pasparakis, M. and Vandenabeele, P. , Necroptosis and its role in inflammation. Nature 2015. 517: 311–320. [DOI] [PubMed] [Google Scholar]
- 64. Newton, K. , Wickliffe, K. E. , Maltzman, A. , Dugger, D. L. , Strasser, A. , Pham, V. C. , Lill, J. R. , Roose‐Girma, M. , Warming, S. , Solon, M. , et al., RIPK1 inhibits ZBP1‐driven necroptosis during development. Nature 2016. 540: 129–133. [DOI] [PubMed] [Google Scholar]
- 65. Lin, J. , Kumari, S. , Kim, C. , Van, T. M. , Wachsmuth, L. , Polykratis, A. and Pasparakis, M. , RIPK1 counteracts ZBP1‐mediated necroptosis to inhibit inflammation. Nature 2016. 540: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Devos, M. , Tanghe, G. , Gilbert, B. , Dierick, E. , Verheirstraeten, M. , Nemegeer, J. , de Reuver, R. , Lefebvre, S. , De Munck, J. , Rehwinkel, J. , et al., Sensing of endogenous nucleic acids by ZBP1 induces keratinocyte necroptosis and skin inflammation. J Exp Med 2020. 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jiao, H. , Wachsmuth, L. , Kumari, S. , Schwarzer, R. , Lin, J. , Eren, R. O. , Fisher, A. , Lane, R. , Young, G. R. , Kassiotis, G. , et al., Z‐nucleic‐acid sensing triggers ZBP1‐dependent necroptosis and inflammation. Nature 2020. 580: 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cuchet‐Lourenco, D. , Eletto, D. , Wu, C. , Plagnol, V. , Papapietro, O. , Curtis, J. , Ceron‐Gutierrez, L. , Bacon, C. M. , Hackett, S. , Alsaleem, B. , et al., Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science 2018. 361: 810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang, R. , Li, H. , Wu, J. , Cai, Z. Y. , Li, B. , Ni, H. , Qiu, X. , Chen, H. , Liu, W. , Yang, Z. H. , et al., Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature 2020. 580: 386–390. [DOI] [PubMed] [Google Scholar]
- 70. Assil, S. and Paludan, S. R. , Live and let die: ZBP1 senses viral and cellular RNAs to trigger necroptosis. EMBO J 2017. 36: 2470–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guo, H. , Gilley, R. P. , Fisher, A. , Lane, R. , Landsteiner, V. J. , Ragan, K. B. , Dovey, C. M. , Carette, J. E. , Upton, J. W. , Mocarski, E. S. and Kaiser, W. J. , Species‐independent contribution of ZBP1/DAI/DLM‐1‐triggered necroptosis in host defense against HSV1. Cell Death Dis 2018. 9: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koehler, H. , Cotsmire, S. , Zhang, T. , Balachandran, S. , Upton, J. W. , Langland, J. , Kalman, D. , Jacobs, B. L. and Mocarski, E. S. , Vaccinia virus E3 prevents sensing of Z‐RNA to block ZBP1‐dependent necroptosis. Cell Host Microbe 2021. 29: 1266–1276.e1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koehler, H. , Cotsmire, S. , Langland, J. , Kibler, K. V. , Kalman, D. , Upton, J. W. , Mocarski, E. S. and Jacobs, B. L. , Inhibition of DAI‐dependent necroptosis by the Z‐DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proc Natl Acad Sci U S A 2017. 114: 11506–11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim, Y. G. , Muralinath, M. , Brandt, T. , Pearcy, M. , Hauns, K. , Lowenhaupt, K. and Jacobs, B. L. , Rich A: A role for Z‐DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci U S A 2003. 100: 6974–6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ha, S. C. , Lokanath, N. K. , Van Quyen, D. , Wu, C. A. , Lowenhaupt, K. , Rich, A. , Kim, Y. G. and Kim, K. K. , A poxvirus protein forms a complex with left‐handed Z‐DNA: crystal structure of a Yatapoxvirus Zalpha bound to DNA. Proc Natl Acad Sci U S A 2004. 101: 14367–14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rothenburg, S. , Deigendesch, N. , Dittmar, K. , Koch‐Nolte, F. , Haag, F. , Lowenhaupt, K. and Rich, A. , A PKR‐like eukaryotic initiation factor 2alpha kinase from zebrafish contains Z‐DNA binding domains instead of dsRNA binding domains. Proc Natl Acad Sci U S A 2005. 102: 1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bergan, V. , Jagus, R. , Lauksund, S. , Kileng, O. and Robertsen, B. , The Atlantic salmon Z‐DNA binding protein kinase phosphorylates translation initiation factor 2 alpha and constitutes a unique orthologue to the mammalian dsRNA‐activated protein kinase R. FEBS J 2008. 275: 184–197. [DOI] [PubMed] [Google Scholar]
- 78. Su, J. , Zhu, Z. and Wang, Y. , Molecular cloning, characterization and expression analysis of the PKZ gene in rare minnow Gobiocypris rarus. Fish Shellfish Immunol 2008. 25: 106–113. [DOI] [PubMed] [Google Scholar]
- 79. Hu, C. Y. , Zhang, Y. B. , Huang, G. P. , Zhang, Q. Y. and Gui, J. F. , Molecular cloning and characterisation of a fish PKR‐like gene from cultured CAB cells induced by UV‐inactivated virus. Fish Shellfish Immunol 2004. 17: 353–366. [DOI] [PubMed] [Google Scholar]
- 80. Buzzo, J. R. , Devaraj, A. , Gloag, E. S. , Jurcisek, J. A. , Robledo‐Avila, F. , Kesler, T. , Wilbanks, K. , Mashburn‐Warren, L. , Balu, S. , Wickham, J. , et al., Z‐form extracellular DNA is a structural component of the bacterial biofilm matrix. Cell 2021. 184: 5740–5758.e5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang, T. , Yin, C. , Fedorov, A. , Qiao, L. , Bao, H. , Beknazarov, N. , Wang, S. , Gautam, A. , Williams, R. M. , Crawford, J. C. , et al., ADAR1 masks the cancer immunotherapeutic promise of ZBP1‐driven necroptosis. Nature 2022. 606: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]


