Abstract
Hepatorenal syndrome type 1 (HRS-1) is a serious complication of advanced cirrhosis and a potentially reversible form of acute kidney injury that is associated with rapidly deteriorating kidney function. Liver transplantation remains the only curative treatment for decompensated cirrhosis. However, terlipressin, a vasopressin analog, successfully reverses HRS-1, and may improve patient survival while awaiting liver transplantation. Patients with higher baseline serum creatinine have a reduced response to treatment with terlipressin. These post hoc analyses examined pooled data from 352 patients with HRS-1 treated with terlipressin in 3 North American-centric, Phase III, placebo-controlled clinical studies (i.e. OT-0401, REVERSE, and CONFIRM)—across 3 serum creatinine subgroups (i.e. <3, ≥3–<5, and ≥5 mg/dL)—to further delineate their correlation with HRS reversal, renal replacement therapy-free survival, and overall survival. Serum creatinine was significantly associated with HRS reversal in univariate and multivariate logistic regression analyses (P<0.001). The incidence of HRS reversal inversely correlated with serum creatinine subgroup (<3 mg/dL, 49.2%; ≥3–<5 mg/dL, 28.0%; ≥5 mg/dL, 9.1%). At Day 30 follow-up, renal replacement therapy-free survival was significantly higher for patients with HRS-1 in the lower serum creatinine subgroups than in the higher subgroup (<5 vs. >5 mg/dL; p=0.01). Terlipressin-treated patients with HRS-1, with a lower baseline serum creatinine level, had a higher overall survival (p<0.001) and higher transplant-free survival at Day 90 (p=0.04). Patients with HRS-1 and lower serum creatinine levels who were treated with terlipressin had higher HRS reversal and survival outcomes, highlighting the significant need to identify and treat patients with HRS-1 early when they often have lower serum creatinine levels, and likely a greater response to terlipressin.
INTRODUCTION
Hepatorenal syndrome type 1 (HRS-1) is a potentially reversible form of acute kidney injury (AKI), which can develop as a complication of decompensated liver cirrhosis.1,2 HRS-1 is characterized by splanchnic dilatation, reduced renal perfusion pressure, reduced renal blood flow, and rapidly deteriorating kidney function.1,3 HRS-1 was previously defined, per the 2007 International Club of Ascites (ICA) diagnostic criteria, as a serum creatinine doubling to >2.5 mg/dL in <2 weeks.4 In 2015, the definition of HRS-1 (HRS-AKI) was updated by the ICA to include AKI as a diagnostic parameter, which was defined as an increased serum creatinine of at least 0.3 mg/dL within 48 hours, and/or a ≥50% increase from baseline, thus taking into account small acute increases in serum creatinine concentrations.5 By replacing the more rigid requirement of serum creatinine doubling to >2.5 mg/dL in 14 days, the new criteria allows both earlier identification of AKI and earlier treatment for patients with HRS-AKI, which may ultimately lead to better clinical outcomes for patients.6,7 Randomized clinical studies with terlipressin have shown reduced response rates in patients with high serum creatinine.2,8,9 Of note, the rate of HRS reversal from prior clinical trials—that used the 2007 ICA diagnostic criteria for HRS-1—is inferior to those rates currently observed in real-world studies where the more recent definition of HRS-1 (HRS-AKI) was used.2,9–12 Nonetheless, the prognosis for untreated patients diagnosed with HRS-1 remains poor with a median survival time of 8–12 weeks.13
As vasodilation is known to be involved in the pathophysiology of HRS-1 development, vasoconstrictors have been studied as treatment options for HRS-1.7,14 Data from randomized clinical studies and a meta-analysis have demonstrated that treatment with vasoconstrictors are effective in improving kidney function and significantly reduce the risk of mortality for patients with HRS-1, compared with albumin alone or no intervention (risk ratio: 0.82).15 Terlipressin, a synthetic vasopressin analog that binds to vasopressin receptors,16 acts as a systemic vasoconstrictor, thereby counteracting the splanchnic arterial vasodilation associated with HRS-1 and restoring blood flow to the kidneys.2 Terlipressin plus albumin is recommended as the first-line treatment for patients with HRS-1, per the European Association for the Study of the Liver guidelines,17 and is the preferred vasoconstrictor therapy recommended by the American Association for the Study of Liver Diseases guidelines.18 Terlipressin (TERLIVAZ) is currently approved by the US Food and Drug Administration and is indicated to improve kidney function in adult patients with HRS with rapid reduction in kidney function.19
Three North American-centric Phase III, placebo-controlled clinical studies (OT-0401, ClinicalTrials.gov identifier: NCT00089570; REVERSE, ClinicalTrials.gov identifier: NCT01143246; CONFIRM, ClinicalTrials. gov identifier: NCT0277071620–22) have demonstrated the safety and efficacy of terlipressin in patients with liver cirrhosis and HRS-1.2,9,10 All 3 studies showed an improvement in HRS reversal with terlipressin compared with placebo, with 2 studies showing a statistically significant benefit (OT-0401, 33.9% vs. 12.5%, respectively, P<0.01; REVERSE, 19.6% vs. 13.1%, respectively; p=0.22; CONFIRM, 32% vs. 17%, respectively; P<0.01).2,9,10 In OT-0401, REVERSE, and CONFIRM, HRS reversal was defined as at least 1 serum creatinine value of ≤1.5 mg/dL while on treatment (on treatment was defined as up to 24 h after the final dose of study drug) by Day 14 or discharge.
Herein, we report a post hoc pooled analysis of these 3 Phase III clinical studies in patients with cirrhosis, ascites, and HRS-1 who were treated with terlipressin or matched placebo.2,9,10 Post hoc analyses examined the efficacy of terlipressin across 3 baseline serum creatinine level subgroups (i.e. <3 mg/dL, ≥3–<5 mg/dL, and ≥5 mg/dL) and assessed their correlation with HRS reversal, survival without renal replacement therapy (RRT), and overall survival.
METHODS
These post hoc analyses collated data from the intent-to-treat (ITT) populations of 3 Phase III clinical studies: OT-0401,10 REVERSE,2 and CONFIRM.9 As noted in the original publications, these studies were conducted in accordance with the ethical principles of the Declaration of Helsinki and received prior approval by the institutional review board and/or independent ethics committee at each study site. All patients provided written informed consent. The methods for the original studies have been previously published.2,9,10 Briefly, eligible patients were adults aged ≥18 years, with cirrhosis, ascites, and HRS-1.2,9,10
Diagnosis of HRS-1 was per the clinical investigator and was defined as a rapidly progressive worsening in renal function with serum creatinine ≥2.25 mg/dL (CONFIRM) or ≥2.5 mg/dL (OT-0401 and REVERSE) and a doubling of serum creatinine within 14 days before randomization.2,9,10 Patients were excluded if they had sustained improvement in renal function (>20% decrease in serum creatinine, or serum creatinine ≤2.25 mg/dL) at least 48 hours after diuretic withdrawal and plasma volume expansion with albumin.2,9,10 Patients were treated with either terlipressin 1 mg or placebo via slow-push intravenous bolus over 2 minutes.2,9,10 Concomitant administration of albumin [CONFIRM: (9) 1 g/kg bodyweight to ≤100 g followed by 20–40 g/day; REVERSE: (2) 100 g on Day 1 followed by 25 g/day; OT-0401: (10) 20–40 g/day] was recommended.
The pooled ITT population dataset was assessed by both univariate and multivariate logistic regression analyses for any associations between baseline covariates and clinical outcomes (i.e. HRS reversal and overall survival). In both the univariate and multivariate analyses, serum creatinine concentrations were analyzed as a continuous variable. Per the Statistical Analysis Plan for each Phase III study, the term baseline serum creatinine was defined as the serum creatinine concentration on Day 0 of the study period [i.e. just prior to the initiation of treatment (terlipressin or placebo)]. The terlipressin group of the pooled ITT population dataset was also assessed according to their baseline serum creatinine subgroup (i.e. <3 mg/dL, ≥3–<5 mg/dL, and ≥5 mg/dL) for efficacy outcomes including HRS reversal, and alive without RRT at each follow-up visit (i.e. Day 30, 60, 90). Serum creatinine subgroups were selected based on a tertile range for the lower limit (set as the lowest serum creatinine concentration observed in patients enrolled in the terlipressin group) and the upper limit [set as the highest serum creatinine category that can be selected in the chronic liver failure-sequential organ failure assessment score calculation (i.e. 5 mg/dL)]. In addition, the terlipressin group of the pooled ITT population dataset was assessed for overall survival and transplant-free survival through Day 90 via Kaplan-Meier estimates.
Statistical analysis
Statistical analyses for significance were calculated as follows: for logistic regression analyses, the Wald test was used to calculate p values; for HRS reversal, p values were based on a χ2 or the Fisher exact test comparing baseline serum creatinine subgroups; for Kaplan-Meier survival estimates, the accompanying p values were calculated using a log-rank test comparing the 3 baseline categories of serum creatinine; lastly, a Fine and Gray proportional hazards model23 was used to generate cumulative incidence function estimates of transplant-free survival up to 90 days, with transplant as a competing risk for death.
A logistic regression model was developed to predict the probability of HRS reversal based on baseline serum creatinine levels. The resulting equation, which can be used to determine the probability of HRS reversal, is: p=(e(1.9335-0.7810 (baseline serum creatinine)))/[1+(e(1.9335-0.7810(baseline serum creatinine)))]. The model assessed the terlipressin treatment group of the pooled ITT population and excluded any patients who were retreated. The only explanatory variable in the model was baseline serum creatinine levels between 2.25 and 6 mg/dL; for modeling purposes, any values <2.25 and >6 mg/dL were removed.
Safety
A post hoc safety analysis for the pooled safety population (i.e. all randomly assigned patients who received at least 1 dose of study drug; collated from the Phase III studies, OT-0401, REVERSE, and CONFIRM) by baseline serum creatinine subgroup (i.e. <3 mg/dL, ≥3–<5 mg/dL, and ≥5 mg/dL) was performed. Safety assessments included the number of patients with any adverse events (AEs), permanent withdrawal of study drug due to AEs, and serious AEs (SAEs) reported in ≥5% of patients in the terlipressin treatment group.
RESULTS
This study included 352 patients (CONFIRM, n=199; REVERSE, n=97; and OT-0401 n=56) who received terlipressin for the treatment of HRS-1. Of those patients, 126 (35.8%) had a baseline serum creatinine of <3 mg/dL; 182 (51.7%) had a baseline serum creatinine of ≥3–<5 mg/dL; and 44 (12.5%) had a baseline serum creatinine of ≥5 mg/dL. Among the pooled ITT population, most baseline demographics were similar across the serum creatinine subgroups (Table 1). Notably, and as expected, the baseline model for end-stage liver disease (MELD) score was significantly higher among patients with a serum creatinine value of ≥3 mg/dL compared with those with <3 mg/dL [median, 38.0 (serum creatinine ≥5 mg/dL) and 35.5 (serum creatinine ≥3–<5 mg/dL) vs. 31.0 (serum creatinine <3 mg/dL), respectively; p<0.001]. Median total bilirubin levels were numerically higher in patients with serum creatinine ≥5 mg/dL vs. those with <5 mg/dL (10.8 vs. 6.1 mg/dL and 7.1 mg/dL, respectively). The presence of grade 3 ascites was numerically lower in the mid-level serum creatinine subgroup (i.e. ≥3–<5 mg/dL), with grade 3 ascites reported in 36.3% of patients compared with 39.7% and 45.5% in the other 2 subgroups. Lastly, there was a higher incidence of alcoholic hepatitis at baseline among patients in the higher serum creatinine subgroup (≥5 mg/dL, 43.2%) compared with the other 2 subgroups (33%, each).
TABLE 1.
Baseline demographics and clinical characteristics in the terlipressin group of the ITT populationa
| Baseline Serum Creatinine Subgroup (N=352) | ||||
|---|---|---|---|---|
| Parameters | <3 mg/dL (n=126) | ≥3–<5 mg/dL (n=182) | ≥5 mg/dL (n=44) | p b |
| Age, years | ||||
| Median | 56.8 | 54.2 | 56.2 | 0.27 |
| Minimum, maximum | 26.8, 73.7 | 23.2, 78.0 | 26.7, 77.0 | |
| Sex | ||||
| Male | 74 (58.7) | 111 (61.0) | 28 (63.6) | 0.85 |
| Female | 52 (41.3) | 71 (39.0) | 16 (36.4) | |
| Baseline serum creatinine, mg/dL | ||||
| Median | 2.6 | 3.6 | 5.6 | |
| Minimum, maximum | 1.7, 3.0 | 3.0, 4.9 | 5.0, 11.9 | |
| Baseline MELD score | ||||
| n | 113 | 158 | 41 | <0.001 |
| Median | 31.0 | 35.5 | 38.0 | |
| Minimum, maximum | 16.0, 40.0 | 20.0, 40.0 | 21.0, 40.0 | |
| Child-Pugh Class C | 84 (66.7) | 121 (66.5) | 27 (61.4) | 0.79 |
| International normalized ratio | ||||
| n | 118 | 166 | 41 | 0.83 |
| Median | 2.1 | 2.1 | 2.0 | |
| Minimum, maximum | 1.2, 5.2 | 1.0, 5.8 | 1.1, 3.9 | |
| MAP (mm Hg) | ||||
| Median | 77.8 | 75.2 | 75.7 | 0.20 |
| Minimum, maximum | 52.3, 106.7 | 47.0, 117.7 | 53.3, 100.7 | |
| Lab parameters | ||||
| Albumin (g/dL) | ||||
| n | 121 | 170 | 42 | 0.61 |
| Median | 3.6 (0.76) | 3.4 (0.82) | 3.6 (1.01) | |
| Minimum, maximum | 1.5, 5.3 | 1.2, 5.1 | 1.8, 5.8 | |
| Sodium (mmol/L) | ||||
| n | 125 | 180 | 44 | 0.91 |
| Median | 133 | 132.0 | 133 | |
| Minimum, maximum | 115.0, 154.0 | 116.0, 150.0 | 114.0, 148.0 | |
| Total bilirubin (mg/dL) | ||||
| n | 122 | 173 | 43 | 0.39 |
| Median | 6.1 | 7.1 | 10.8 | |
| Minimum, maximum | 0.4, 51.6 | 0.3, 50.4 | 0.7, 46.7 | |
| History of infections | ||||
| Spontaneous bacterial peritonitis | 22 (20.4) | 19 (12.4) | 4 (11.4) | 0.19 |
| Urinary tract infection | 20 (18.5) | 30 (19.6) | 7 (20.0) | 0.96 |
| Pneumonia | 8 (7.4) | 11 (7.2) | 2 (5.7) | 1.00 |
| Other | 19 (17.6) | 30 (19.6) | 6 (17.1) | 0.59 |
| Ascitesc grade 3 | 50 (39.7) | 66 (36.3) | 20 (45.5) | 0.51 |
| Alcoholic hepatitis | 42 (33.3) | 60 (33.0) | 19 (43.2) | 0.43 |
| Alcoholic hepatitis, baseline MAP <70 mm Hg, or SIRS | 82 (65.1) | 121 (66.5) | 30 (68.2) | 0.93 |
| Esophageal varices | ||||
| Prior history of esophageal variceal hemorrhage | 17 (13.5) | 32 (17.6) | 7 (15.9) | 0.45 |
| Precipitating factors for HRS-1 | 50 (39.7) | 80 (44.0) | 19 (43.2) | 0.77 |
| Prior midodrine and octreotide, ≥3 d | 33 (26.2) | 39 (21.4) | 12 (27.3) | 0.54 |
| SIRS subgroupd | 41 (38.0) | 55 (35.9) | 16 (45.7) | 0.72 |
| Baseline ACLF grade | 0.36 | |||
| 0 | 2 (1.6) | 0 | 0 | |
| 1 | 64 (50.8) | 82 (45.1) | 16 (36.4) | |
| 2 | 38 (30.2) | 61 (33.5) | 17 (38.6) | |
| 3 | 22 (17.5) | 39 (21.4) | 11 (25.0) | |
| Daily exposure to terlipressin (mg/d), median (minimum, maximum) | 3.3 (0.9, 7.2) | 3.2 (0.8, 7.1) | 3.0 (1.0, 6.9) | 0.22 |
| Prior albumin exposure (g) | ||||
| n | 114 | 161 | 37 | 0.62 |
| Median | 300.0 | 300.0 | 300.0 | |
| Minimum, maximum | 25.0, 925.0 | 25.0, 1000.0 | 50.0, 800.0 | |
| Daily concomitant albumin exposure (g/dL) | ||||
| n | 99 | 164 | 36 | 0.15 |
| Median | 45.0 | 46.7 | 50.0 | |
| Minimum, maximum | 12.5, 150.0 | 12.5, 150.0 | 12.5, 100.0 | |
Data are presented as n (%) unless otherwise stated.
Pooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM.
The p values for continuous variables were based on a comparison of baseline serum creatinine categories using the Kruskal-Wallis test; for categorical variables, a Fisher exact test or χ2 test were used.
Every patient had to have a documented history of cirrhosis and ascites to be eligible for the clinical studies in the pooled ITT population.
Criteria to define the SIRS subgroup were not collected for OT-0401; percentages are based on the number of patients in each treatment group for REVERSE and CONFIRM only (<3 mg/dL, n=108, ≥3–<5 mg/dL, n=153; ≥5 mg/dL, n=35).
For patients who received albumin during initial and retreatment periods, albumin exposure data were combined for both periods. Records missing the albumin dose or the date of dosing were excluded.
Abbreviations: ACLF indicates acute-on-chronic liver failure; d, day; HRS-1, hepatorenal syndrome type 1; ITT, intent-to-treat; MAP, mean arterial pressure; max, maximum; MELD, model for end-stage liver disease; min, minimum; n, number of patients; SIRS, systemic inflammatory response syndrome.
Univariate logistic regression analysis identified several baseline characteristics that were associated with HRS reversal in the pooled ITT population, including serum creatinine [odds ratio (95% CI): 0.483 (0.361–0.645), p<0.001], MELD score [0.925 (0.891–0.961), p<0.001], total bilirubin [0.973 (0.954–0.992), P<0.01], international normalized ratio [0.730 (0.537–0.993), p=0.045], and acute-on-chronic liver failure (ACLF) grade [2.411 (1.281–4.536), p=0.006] (Table 2). In the multivariate analysis—which excluded MELD score due to the risk of multicollinearity with total bilirubin and international normalized ratio—only ACLF grade [2.435 (1.260–4.706), p=0.008] and serum creatinine [0.498 (0.369–0.674), p<0.001] remained predictors of HRS reversal (Table 3). With respect to baseline serum creatinine subgroups, HRS reversal was observed in 49.2% of patients in the <3 mg/dL subgroup, compared with 28.0% of patients in the ≥3–<5 mg/dL subgroup, and 9.1% of patients with a baseline serum creatinine level of ≥5 mg/dL (p<0.001) (Figure 1). The rate of liver transplantation at Day 90 was also inversely correlated with serum creatinine subgroup; <10% of patients in the highest subgroup (≥5 mg/dL) received a transplant, compared with 28.6% and 28.0% of patients in the lower 2 serum creatinine subgroups (i.e. <3 mg/dL and ≥3–<5 mg/dL), respectively (Supplemental Table 1, http://links.lww.com/HC9/A20).
TABLE 2.
Univariate logistic regression of baseline characteristics on HRS reversal (terlipressin group, pooled ITT population)a
| Terlipressin | ||||
|---|---|---|---|---|
| Baseline Parameters | nb | Odds Ratio | 95% CI | p |
| Alcoholic hepatitis | 352 | 1.382 | 0.871–2.192 | 0.17 |
| Baseline serum creatininec | 352 | 0.483 | 0.361–0.645 | <0.001 |
| Age <65 years | 352 | 1.099 | 0.590–2.049 | 0.77 |
| Male sex | 352 | 1.126 | 0.714–1.776 | 0.61 |
| Race group (White vs. non-White) | 348 | 1.778 | 0.781–4.048 | 0.17 |
| Baseline MELD Score | 312 | 0.925 | 0.891–0.961 | <0.001 |
| Baseline Child-Turcotte-Pugh Score | 337 | 0.908 | 0.806–1.021 | 0.11 |
| Baseline MAP | 352 | 0.995 | 0.977–1.014 | 0.62 |
| Baseline MAP <65 | 352 | 0.538 | 0.264–1.096 | 0.09 |
| Baseline serum sodium | 349 | 0.985 | 0.950–1.021 | 0.42 |
| Baseline total bilirubin | 338 | 0.973 | 0.954–0.992 | <0.01 |
| Baseline INR | 325 | 0.730 | 0.537–0.993 | 0.045 |
| Baseline ACLF grade (grade 0–2 vs. 3) | 352 | 2.411 | 1.281–4.536 | 0.006 |
| No precipitating factors for HRS | 352 | 1.028 | 0.656–1.611 | 0.90 |
| Prior midodrine or octreotide ≥3 d | 352 | 1.325 | 0.796–2.206 | 0.28 |
Pooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM.
This represents the evaluable number of patients for each baseline parameter.
Serum creatinine is used as a continuous variable and is not split by subgroup for this analysis.
Abbreviations: ACLF indicates acute-on-chronic liver failure; HRS hepatorenal syndrome; INR, International normalized ratio; ITT, intent-to-treat; MAP, mean arterial pressure; MELD, model for end-stage liver disease; n, number of patients.
TABLE 3.
Multivariate logistic regression of baseline characteristics on HRS reversal (terlipressin group, pooled ITT population)a
| Terlipressin | ||||
|---|---|---|---|---|
| Baseline Parametersb c | nd | Odds Ratio | 95% CI | p |
| Baseline ACLF grade (grade 0–2 vs. grade 3) | 320 | 2.435 | 1.260–4.706 | 0.008 |
| Baseline serum creatinine | 320 | 0.498 | 0.369–0.674 | <0.001 |
Pooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM.
Significant univariate results were added to the model, and stepwise selection was used to obtain the final model.
MELD score was excluded from the multivariate logistic regression model due to the risk of multicollinearity with MELD score components.
This represents the evaluable number of patients for each baseline parameter.
Abbreviations: ACLF indicates acute-on-chronic liver failure; HRS, hepatorenal syndrome; ITT, intent-to-treat; MELD, model for end-stage liver disease; n, number of patients.
FIGURE 1.
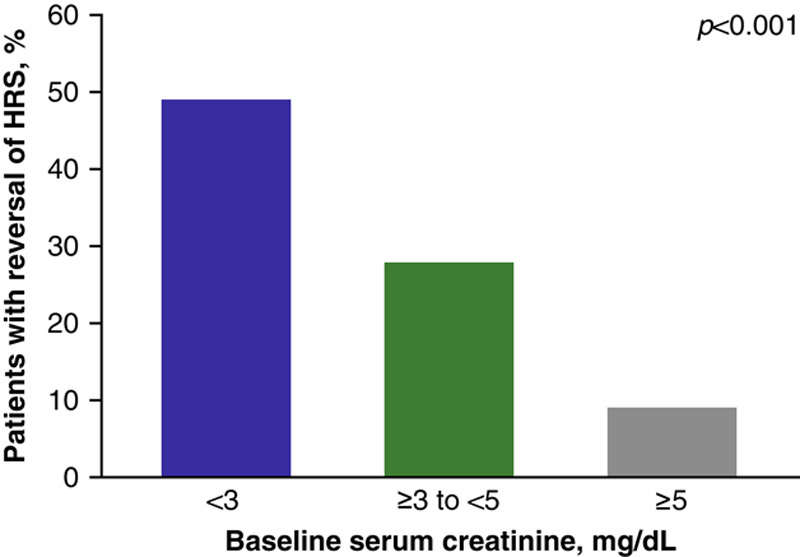
Percent of patients in the terlipressin treatment group who had HRS reversala by baseline serum creatinine subgroup (terlipressin group, pooled ITT populationb). aThe incidence of HRS reversal across the 3 Phase III studies was defined as at least 1 serum creatinine value of ≤1.5 mg/dL while on treatment (on treatment was defined as up to 24 h after the final dose of study drug) by Day 14 or discharge. †Pooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM. Abbreviations: HRS indicates hepatorenal syndrome; ITT, intent-to-treat.
Significantly more patients with a baseline serum creatinine level of <5 mg/dL were alive without the need for RRT through Day 30 compared with the ≥5 mg/dL subgroup (<3 mg/dL, 77.3%; ≥3–<5 mg/dL, 76.5%; ≥5 mg/dL, 40.0%; p=0.01) (Figure 2). Patients with lower serum creatinine levels (i.e. <3 mg/dL at baseline) had higher overall survival at Day 90 than those with mid-level serum creatinine levels (i.e. ≥3–<5 mg/dL), who in turn had longer overall survival than those patients with the highest baseline serum creatinine levels (i.e. ≥5 mg/dL; P<0.001) (Figure 3; Supplemental Table 1, http://links.lww.com/HC9/A20). Similarly, after accounting for liver transplant as a competing risk for death, lower serum creatinine levels at baseline correlated with higher transplant-free survival at Day 90 (Figure 4).
FIGURE 2.
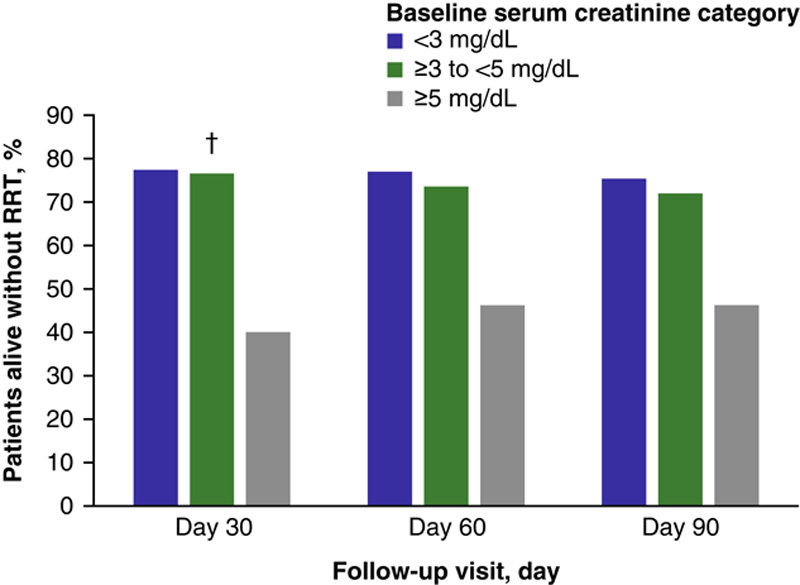
Percent of patients at each follow-up visit (Day 30, 60, 90) who were alive without RRT by baseline serum creatinine subgroup (terlipressin group, pooled ITT populationa). aPooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM. bThe p value is based on comparison of baseline serum creatinine categories at Day 30; p=0.01. Abbreviations: ITT indicates intent-to-treat; RRT, renal replacement therapy.
FIGURE 3.
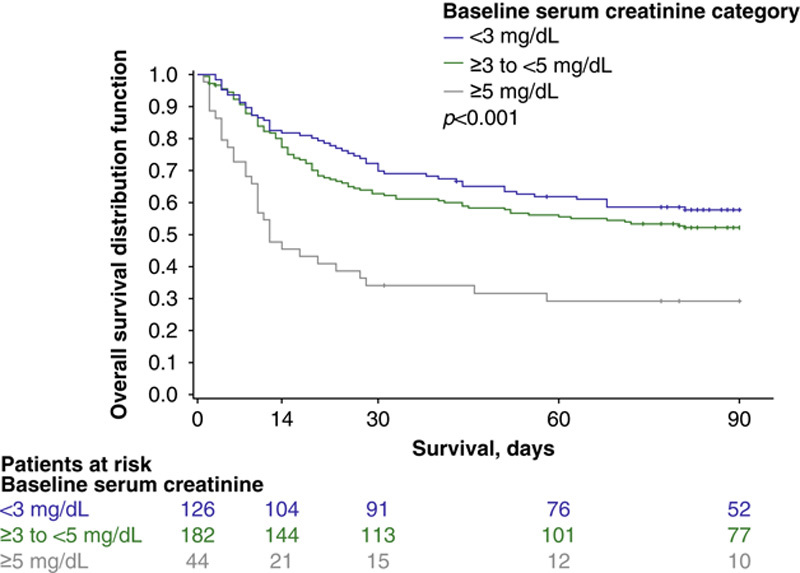
Overall survival up to 90 days by baseline serum creatinine subgroup (terlipressin group, pooled ITT populationa). aPooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM. The p value was calculated using a log-rank test comparing the 3 baseline categories of serum creatinine. Abbreviations: ITT indicates intent-to-treat.
FIGURE 4.
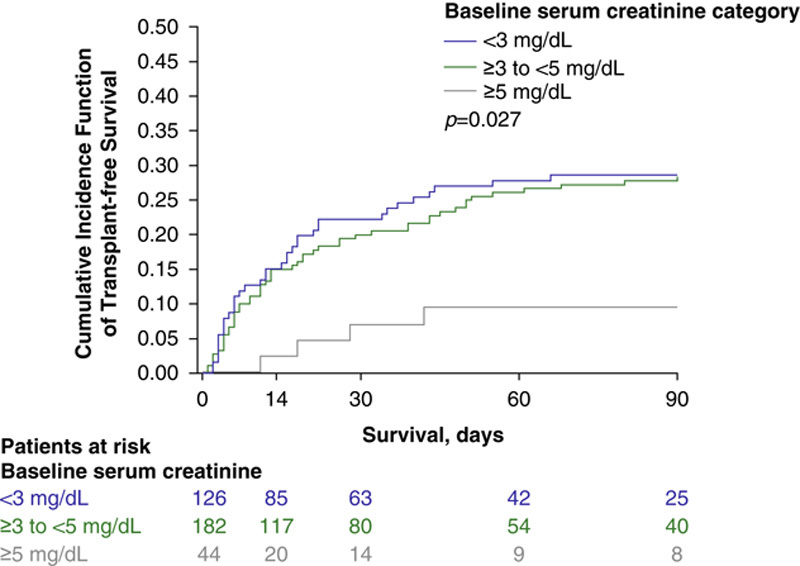
Transplant-free survivala up to 90 days using cumulative incidence function with competing risks of transplant and death by baseline serum creatinine subgroup (terlipressin group, pooled ITT populationb). aA Fine and Gray proportional hazards model was used to produce cumulative incidence function estimates of transplant-free survival, with transplant as a competing risk for death. bPooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM. ITT indicates intent-to-treat.
A model developed to predict the probability of HRS reversal by serum creatinine levels at baseline, showed an inverse correlation between serum creatinine levels and HRS reversal (Supplemental Table 2, http://links.lww.com/HC9/A21).
Safety
Most patients in the pooled population experienced at least 1 AE. The incidence of AEs of any grade were similar across baseline serum creatinine subgroups, as was study drug withdrawal due to an AE (Table 4). However, the incidence of many of the most commonly reported SAEs (≥5% by system organ class) trended higher as the baseline serum creatinine subgroup concentrations increased; these included gastrointestinal disorders (12.2%, 13.7%, and 18.2%, respectively), general disorders and administration site conditions (5.7%, 8.8%, and 15.9%, respectively), and hepatobiliary disorders (17.1%, 20.3%, 36.4%, respectively). The incidence of infections, infestations, respiratory, thoracic, and mediastinal disorders were generally similar across baseline serum creatinine subgroups (Table 4). Also, the incidence of any AE, SAEs, and withdrawals due to AEs were similar across baseline serum creatinine subgroups, regardless of the daily exposure to terlipressin (Supplemental Table 3, http://links.lww.com/HC9/A22).
TABLE 4.
Summary of adverse events in patients with HRS-1 (pooled safety population)a
| Terlipressin | |||
|---|---|---|---|
| Baseline Serum Creatinine Subgroup (N=349) | |||
| Parameter, n (%) | <3 mg/dL (n=123) | ≥3–<5 mg/dL (n=182) | ≥5 mg/dL (n=44) |
| Any AEb | 108 (87.8) | 168 (92.3) | 42 (95.5) |
| Permanent withdrawals due to AEsc | 15 (12.2) | 27 (14.8) | 5 (11.4) |
| Any SAEd | 72 (58.5) | 118 (64.8) | 37 (84.1) |
| SAEs reported by ≥5% of patients within a treatment groupd by system organ class/preferred term‖ | |||
| Cardiac disorders | 8 (6.5) | 7 (3.8) | 5 (11.4) |
| Gastrointestinal disorders | 15 (12.2) | 25 (13.7) | 8 (18.2) |
| Abdominal pain | 5 (4.1) | 7 (3.8) | 3 (6.8) |
| General disorders and administration site conditions | 7 (5.7) | 16 (8.8) | 7 (15.9) |
| Multiple organ dysfunction syndrome | 6 (4.9) | 13 (7.1) | 7 (15.9) |
| Hepatobiliary disorders | 21 (17.1) | 37 (20.3) | 16 (36.4) |
| Chronic hepatic failure | 7 (5.7) | 12 (6.6) | 2 (4.5) |
| Hepatic failure | 6 (4.9) | 8 (4.4) | 7 (15.9) |
| Infections and infestations | 18 (14.6) | 21 (11.5) | 4 (9.1) |
| Sepsis | 5 (4.1) | 12 (6.6) | 1 (2.3) |
| Nervous system disorders | 11 (8.9) | 6 (3.3) | 1 (2.3) |
| Hepatic encephalopathy | 8 (6.5) | 1 (0.5) | 1 (2.3) |
| Renal and urinary disorders | 9 (7.3) | 12 (6.6) | 4 (9.1) |
| Respiratory, thoracic and mediastinal disorders | 21 (17.1) | 30 (16.5) | 6 (13.6) |
| Respiratory failure | 12 (9.8) | 15 (8.2) | 2 (4.5) |
| Vascular disorders | 8 (6.5) | 5 (2.7) | 2 (4.5) |
Pooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM.
Patients experiencing multiple adverse events are counted once; up to 7 days post-treatment.
For CONFIRM and REVERSE, permanent withdrawals due to an AE occurred when action taken was reported as permanently stopped. For OT-0401, permanent withdrawals due to an AE occurred when action taken with study drug was reported as discontinued permanently.
Up to 30 days post-treatment.
SAEs were classified by a prespecified MedDRA term within each system organ class.
Abbreviations: AE indicates adverse event; HRS-1, hepatorenal syndrome type 1; MedDRA, medical dictionary for regulatory activities; n, number of patients; SAE, serious adverse event.
DISCUSSION
In clinical studies, terlipressin has been used to treat patients with HRS-1 and facilitates HRS reversal by causing splanchnic vasoconstriction, thereby increasing blood flow to the kidneys.2 HRS reversal may ultimately result in an improved prognosis for patients, which may include a reduced hospital length of stay, and a reduced need for RRT before or after liver transplantation.14 This study, which represents the largest analysis of terlipressin-treated patients with HRS-1, demonstrated that those patients with lower baseline serum creatinine levels at the time of terlipressin initiation (i.e. <3 mg/dL) had higher HRS reversal, higher overall survival, and higher transplant-free survival at Day 90 than patients with higher baseline serum creatinine levels. Those patients receiving terlipressin at lower serum creatinine (<5 vs. >5 mg/dL) were also more likely to receive a liver transplant despite having lower MELD scores at the time of terlipressin administration. Moreover, lower baseline serum creatinine levels (i.e. <5 mg/dL) were significantly associated by both univariate and multivariate analyses to lead to a better prognosis for patients with HRS-1, consistent with a prior publication.14 Lastly, logistic regression modeling of the response to treatment with terlipressin demonstrated that as a patient’s baseline level of serum creatinine increased, their estimated probability of achieving HRS reversal decreased. For example, the probability of HRS reversal was 68.18% with a baseline serum creatinine level of 1.5 mg/dL compared with 31.00% at a baseline serum creatinine level of 3.5 mg/dL; further the probability drops to <10% for patients with a baseline serum creatinine level of ≥5.5 mg/dL.
While the 3 Phase III clinical studies used in this post hoc analysis administered terlipressin via a slow-push intravenous bolus over 2 minutes, it should be noted that Cavallin et al.24 demonstrated that terlipressin administered to patients with HRS-1 via a continuous intravenous infusion was associated with fewer AEs but with comparable efficacy to terlipressin administered by an intravenous bolus.
Response rates to terlipressin may vary between patients, and appropriate biomarkers to predict responsiveness to treatment may aid in the decision to use this vasoconstrictor in the treatment of patients with decompensated cirrhosis and HRS-1.8 The present study demonstrates that an ACLF grade 3 was associated with lower rates of HRS reversal vs ACLF grades 0–2. These findings are similar to a retrospective analysis performed by Piano et al.25 that demonstrated an association between advancing grades of ACLF and lower rates of HRS reversal in terlipressin-treated patients (grade 1: 60%; grade 2: 48%; grade 3: 29%). Also similar to findings from the present study, Piano et al.25 identified baseline levels of serum creatinine to be independently associated with response to treatment. This North American-centric post hoc study highlights the need for early detection of HRS-1 to promote rapid treatment of patients, to facilitate a better prognosis, including a reduced need for RRT and to extend survival outcomes. Similar to the clinical studies retrospectively analyzed by Piano et al.25 the 3 clinical trials analyzed in this study used the older, more stringent definition of HRS-1 [i.e. requirement of a serum creatinine doubling to >2.5 mg/dL (OT-0401 and REVERSE) or >2.25 mg/dL (CONFIRM) in 14 d].2,9,10 Use of the new diagnostic parameters (i.e. the 2015 updated ICA diagnostic criteria) may allow earlier patient identification with HRS-AKI and may, therefore, facilitate treatment at an early stage of disease progression.6,7 Earlier detection is an integral part of improving prognosis in conditions such as HRS-1, where a patient’s rapidly deteriorating kidney function often leads to a need for RRT and liver transplantation sooner rather than later.7,13,14 In CONFIRM, patients in the terlipressin group had significant improvements in verified HRS reversal, and more importantly, clinically meaningful increases in initial survival for 10 days post-treatment.9 HRS reversal was sustainable with durable improvements for patients without the need for RRT through Day 30.9 Notably, while treatment with terlipressin may lead to improvements in renal blood flow and a clinically meaningful reversal in HRS-1, the underlying liver disease (i.e. decompensated cirrhosis) is a major driver for longer term survival and confounds the positive effects of terlipressin, making significant improvements in longer term survival less attainable.26 Liver transplantation may be the only curative treatment for patients with decompensated cirrhosis; however, liver transplantation may not be available in a timely manner, and therapeutic intervention with terlipressin may reduce mortality for patients on the transplant waiting list serving as a bridge to transplant.13 As noted in this study, initiation of terlipressin at a serum creatinine level of <5 mg/dL was associated with an increased rate of liver transplantation. Furthermore, HRS reversal pretransplant may reduce the need for RRT post-transplant.12,27
Collectively, the median total daily amount of concomitant albumin administered to the terlipressin-treated patients in the present analysis was higher than the daily amount recommended by ICA guidelines [i.e. the median (minimum, maximum) albumin exposure in the pooled terlipressin group was 45–50 g/d (12.5, 150.0); while ICA guidelines for daily albumin exposure recommend 1 g/kg on Day 1—up to a maximum of 100 g—followed by 20–40 g/day].4,5 However, the median exposures for both prior albumin and concomitant daily albumin administered were similar across serum creatinine subgroups. Excess albumin exposure is associated with an increased risk of volume overload and, potentially, a respiratory event.28 Future clinical studies of terlipressin should aim to refine and personalize concomitant albumin dosing strategies for patients with HRS-1. Further, additional studies could consider the use of point-of-care ultrasound assessments or other noninvasive technologies to monitor intravascular volume during treatment.28
CONCLUSIONS
In summary, data from the pooled post hoc analyses of the OT-0401, REVERSE, and CONFIRM studies demonstrate that serum creatinine levels correlated with the efficacy of terlipressin treatment in patients with HRS-1. Across efficacy outcomes, patients with lower serum creatinine levels derived more benefit from treatment with terlipressin. This study outlines the significant need to identify and treat patients with HRS-1 as soon as clinically possible.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Medical writing and editorial support conducted in accordance with Good Publication Practice 2022 Update (GPP 2022) and the International Committee of Medical Journal Editors (ICMJE) guidelines were provided by Tarah M. Connolly, PhD, and Corey A. Rynders, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, USA, and funded by Mallinckrodt Pharmaceuticals.
CONFLICTS OF INTEREST
M.P.C. reports consultant fees and grants from Mallinckrodt Pharmaceuticals during the conduct of the studies, and grants from Gilead and Sonic Incytes, outside the submitted work. H.E.V. reports consultation fees and grants received from Mallinckrodt during the conduct of this study. He received grants from Ocelot and Sequana. A.S.B. reports grants received from Mallinckrodt for the conduct of these clinical studies and Exact Sciences for the conduct of a clinical trial. N.T.P. reports grants received from Mallinckrodt for the conduct of the study. He also report grants from Grifols, intercept, cytosorbents, durect, and Salix. V.R.P. reports receiving no grants or consultation fees for the conduct of these clinical studies. K.J. holds intellectual property rights with Mallinckrodt Pharmaceuticals.
DATA AVAILABILITY STATEMENT
Discussion of statistical endpoints and analysis are included in the manuscript. Summary aggregate (basic) results (including adverse event information) and the study protocols will be available on clinicaltrials.gov (CONFIRM, NCT02770716; OT-0401, NCT00089570; REVERSE, NCT01143246) when required by regulation. Individual de-identified patient data will not be disclosed. Requests for additional information should be directed to the sponsor of the study at medinfo@mnk.com.
Footnotes
Funding information This analysis was funded by Mallinckrodt Pharmaceuticals. The clinical study reports were developed by the sponsor, Mallinckrodt Pharmaceuticals. Data were collected by trial investigators and analyzed by the sponsor.
Abbreviations: ACLF, acute-on-chronic liver failure; AE, adverse event; AKI, acute kidney injury; HRS-1, hepatorenal syndrome type 1; ICA, International Club of Ascites; ITT, intent-to-treat; MAP, mean arterial pressure; MELD, model for end-stage liver disease; RRT, renal replacement therapy; SAE, serious adverse event.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Michael P. Curry, Email: mcurry@bidmc.harvard.edu.
Hugo E. Vargas, Email: vargas.hugo@mayo.edu.
Alex S. Befeler, Email: befelera@slu.edu.
Nikolaos T. Pyrsopoulos, Email: pyrsopni@njms.rutgers.edu.
Vilas R. Patwardhan, Email: vpatward@bidmc.harvard.edu.
Khurram Jamil, Email: khurram.jamil@mnk.com.
REFERENCES
- 1.Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–22. [DOI] [PubMed] [Google Scholar]
- 2.Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O’Leary JG, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology. 2016;150:1579–89. e2. [DOI] [PubMed] [Google Scholar]
- 3.Tariq R, Singal AK. Management of hepatorenal syndrome: a review. J Clin Transl Hepatol. 2020;8:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968–74. [DOI] [PubMed] [Google Scholar]
- 6.Acevedo JG, Cramp ME. Hepatorenal syndrome: update on diagnosis and therapy. World J Hepatol. 2017;9:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baraldi O, Valentini C, Donati G, Comai G, Cuna V, Capelli I, et al. Hepatorenal syndrome: update on diagnosis and treatment. World J Nephrol. 2015;4:511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384:818–28. [DOI] [PubMed] [Google Scholar]
- 10.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore K, Jamil K, Verleger K, Luo L, Kebede N, Heisen M, et al. Real-world treatment patterns and outcomes using terlipressin in 203 patients with the hepatorenal syndrome. Aliment Pharmacol Ther. 2020;52:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piano S, Gambino C, Vettore E, Calvino V, Tonon M, Boccagni P, et al. Response to terlipressin and albumin is associated with improved liver transplant outcomes in patients with hepatorenal syndrome. Hepatology. 2021;73:1909–19. [DOI] [PubMed] [Google Scholar]
- 13.Chaney A. A review for the practicing clinician: hepatorenal syndrome, a form of acute kidney injury, in patients with cirrhosis. Clin Exp Gastroenterol. 2021;14:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dundar HZ, Yilmazlar T. Management of hepatorenal syndrome. World J Nephrol. 2015;4:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576–84. [DOI] [PubMed] [Google Scholar]
- 16.Jamil K, Pappas SC, Devarakonda KR. In vitro binding and receptor-mediated activity of terlipressin at vasopressin receptors V1 and V2.J Exp Pharmacol. 2018;10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60. [DOI] [PubMed] [Google Scholar]
- 18.Biggins SW, Angeli P, Garcia-Tsao G, Gines P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–48. [DOI] [PubMed] [Google Scholar]
- 19.TERLIVAZ® (terlipressin) Full Prescribing Information. Bedminster, NJ: Mallinckrodt Pharmaceuticals; 2022. [Google Scholar]
- 20.Clinicaltrials.gov. A placebo-controlled, double-blind study to confirm the reversal of hepatorenal syndrome type 1 with terlipressin. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT01143246. Accessed October 20, 2021.
- 21.Clinicaltrials.gov. Study of terlipressin versus placebo to treat hepatorenal syndrome type 1. 2021. Available at: https://www.clinicaltrials.gov/ct2/show/NCT00089570. Accessed October 20, 2021.
- 22.Clinicaltrials.gov. Study to confirm efficacy and safety of terlipressin in hepatorenal syndrome (HRS) type 1. 2021. Available at: https://www.clinicaltrials.gov/ct2/show/NCT02770716. Accessed October 20, 2021.
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24.Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016;63:983–92. [DOI] [PubMed] [Google Scholar]
- 25.Piano S, Schmidt HH, Ariza X, Amoros A, Romano A, Husing-Kabar A, et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol. 2018;16:1792–800. e3. [DOI] [PubMed] [Google Scholar]
- 26.Mindikoglu AL, Pappas SC. New developments in hepatorenal syndrome. Clin Gastroenterol Hepatol. 2018;16:162–77. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong F, Leung W, Al Beshir M, Marquez M, Renner EL. Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl. 2015;21:300–7. [DOI] [PubMed] [Google Scholar]
- 28.Allegretti AS, Subramanian RM, Francoz C, Olson JC, Cárdenas A. Respiratory events with terlipressin and albumin in hepatorenal syndrome: a review and clinical guidance. Liver Int. 2022;42:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Discussion of statistical endpoints and analysis are included in the manuscript. Summary aggregate (basic) results (including adverse event information) and the study protocols will be available on clinicaltrials.gov (CONFIRM, NCT02770716; OT-0401, NCT00089570; REVERSE, NCT01143246) when required by regulation. Individual de-identified patient data will not be disclosed. Requests for additional information should be directed to the sponsor of the study at medinfo@mnk.com.


