Abstract
Oxidative stress is one of the important mechanisms of inner ear cell damage, which can lead to age-related hearing loss (ARHL). LncRNA H19 is significantly downregulated in the cochlea of old mouse, however, the role of H19 in the development of ARHL remains unclear. In this study, we aim to investigate the expression and function of H19 in oxidative stress injury of cochlear hair cells induced by H 2O 2. RT-qPCR and western blot analysis confirms that HEI-OC1 cells stimulated with H 2O 2 decreases the expressions of H19 and SIRT1, but increases the expression of miR-653-5p. Overexpression of H19 could increase cell viability, ATP level and mitochondrial membrane potential, but reduce mitochondrial ROS generation and cell apoptosis ratio in H 2O 2-stimulated HEI-OC1 cells. MiR-653-5p is a target of H19, which can bind to the 3′-UTR of SIRT1. H19 is found to regulate the expression of SIRT1 through miR-653-5p. Further experiments demonstrates that H19 regulates HEI-OC1 cell viability, ATP level, mitochondrial membrane potential, mitochondrial ROS generation, and cell apoptosis ratio via the miR-653-5p/SIRT1 axis. In conclusion, lncRNA H19 inhibits oxidative stress injury of cochlear hair cells via the miR-653-5p/SIRT1 axis.
Keywords: cochlear hair cell, oxidative stress, lncRNA H19, SIRT1
Introduction
Age-related hearing loss (ARHL), also known as presbycusis, is one of the most common chronic diseases, characterized by gradual hearing decline with aging [1]. So far, hearing aid device is the most important method to prevent and treat ARHL. However, due to its high price, its application in the treatment of presbycusis is obviously limited. Therefore, understanding the pathogenesis of ARHL is an important way to prevent and treat ARHL. The pathological features of ARHL include hair cell loss, vascular atrophy, spiral ganglion neurons loss, and degeneration of central auditory pathway.
More and more studies have shown that oxidative stress plays an important role in the pathophysiology of ARHL [2]. In ARHL patients or animal models, the expressions of oxidative stress-related proteins or factors are increased, while the levels of antioxidant-related proteins or factors are significantly decreased, indicating that ARHL is closely related to oxidative stress [ 3– 5]. Under oxidative stress stimulation, cells produce a large amount of reactive oxygen species (ROS), and then ROS directly damage the cells and activate downstream caspase-3 by stimulating extracellular trans-membrane receptors, thus initiating cell apoptosis. Excessive accumulation of ROS can also damage the function of mitochondria by attacking the target mitochondria. The damage of mitochondrial function can increase the production of ROS, which cannot be removed in time, resulting in the formation of a vicious cycle that eventually leads to cell apoptosis [6]. Therefore, oxidative stress could damage cells by attacking the mitochondria.
LncRNAs have almost no ability to encode functional proteins, but are closely related to many human diseases. Using RNA-seq analysis, Su et al. [7] identified 134 abnormally expressed lncRNAs, with 88 upregulated and 46 downregulated, and Zhao et al. [8] found 738 differentially expressed lncRNAs in the cochleae of aged mice. In addition, Hao et al. [9] observed that lncRNA MIAT can inhibit oxidative stress-induced cochlear hair cell apoptosis through a ceRNA pattern by targeting SIRT1. Increasing evidence supports that lncRNA H19 is associated with some aging processes [10], and H19 is significantly low-expressed in cochlea of the aged mice [7], indicating that H19 overexpression may resist the pathological damage of ARHL.
MicroRNAs (miRNA) are a noncoding RNA subtype consisting of 19–25 nucleotides produced by a series of cleavage processes, which play an important role in regulating the expressions of protein-coding genes, mainly by binding to the 3′-untranslated region (3′-UTR) of target mRNA [ 11, 12]. The binding of miRNA to mRNA can lead to mRNA degradation and translation inhibition, which is the way that these molecules inhibit gene expression. Previous studies have shown that abnormally expressed miRNA may be involved in the pathophysiology of ARHL [ 13– 16]. MiR-653-5p is abnormally expressed in a variety of cancers and involved in the occurrence and development of cancers. At present, there is no report on the expression and role of miR-653-5p in ARHL.
In addition, some researchers reported that SIRT1 can inhibit oxidative stress, inhibit hair cell apoptosis, and improve age-related hearing loss [ 17, 18]. Bioinformatics analysis predicted that there is a binding site between H19 and miR-653-5p, and miR-653-5p could bind to SIRT1 3′-UTR. Therefore, we speculated that overexpression of H19 may promote SIRT1 expression by regulating miR-653-5p, thereby inhibiting oxidative stress-induced hair cell apoptosis and improving ARHL hearing loss.
Material and Methods
HEI-OC1 cell culture and H 2O 2 stimulation
HEI‑OC1 cells (MlBio, Shanghai, China) were cultured in DMEM medium (Gibco, Waltham, USA) supplemented with 10% FBS in an incubator at 33°C with 10% CO 2. HEI-OC1 cells were stimulated with 1 mM H 2O 2, and after incubation for 2 h, cells were collected for the subsequent experiments.
Lentivirus infection and cell transfection
The lentivirus (LV-NC or LV-H19), miRNA mimic/inhibitor and siRNA interference sequences and their corresponding negative controls were purchased from Genechem (Shanghai, China). The sequences are shown in Table 1. For lentivirus infection, on the first day, 2 mL of HEI-OC1 cells were plated in 6-well plates (1×10 5 cells/well). On the second day, the virus particles (100 MOI) were pre-mixed with DMEM medium and added to each well of the 6-well plate. On the third day, the medium containing virus solution was replaced by normal medium. For cell transfection, when the confluence of HEI-OC1 cells reached 75%, the miRNA mimic/inhibitor and/or si-SIRT1 were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, USA) according to the manufacturer/s instructions.
Table1 The sequences used in study
|
Gene |
Sequence (5′→3′) |
|
Mimic NC |
UUCUCCGAACGUGUCACGUT (F) ACGUGACACGUUCGGAGAATT (R) |
|
MiR-653-5p mimic |
GUGUUGAAACAAUCUCUACUG (F) GUAGAGAUUGUUUCAACAGUA (R) |
|
Inhibitor NC |
CAGUACUUUUGUGUAGUACAA |
|
MiR-653-5p inhibitor |
CAGUAGAGAUUGUUUCAACAC |
|
Si-SIRT1 |
GGUUGUUAAUGAAGCUAUAGC (SS) UAUAGCUUCAUUAACAACCUC (AS) |
|
SiRNA |
UUCUCCGAACGUGUCACGUdTdT (SS) ACGUGACACGUUCGGAGAAdTdT (AS) |
|
Biotinylated NC |
Bio-CTGATAAGCTATAGCATATTT |
|
Biotinylated WT miR-653-5p |
Bio-GUGUUGAAACAAUCUCUACUG |
|
Biotinylated MUT miR-653-5p |
Bio-AGAGGAGGGCAAUCUCUACUG |
RT-qPCR analysis
After H 2O 2 stimulation or transfection, the cells were collected, and total RNA was extracted using Trizol reagent (Invitrogen). To detect H19 and SIRT1 expressions, total RNA was reverse-transcribed using a PrimeScriptTM II 1st Strand cDNA Synthesis kit (TaKaRa, Shiga, Japan), then quantified using the SYBR Green RT-PCR kit (TaKaRa). RT-qPCR was performed with SYBR Green dye (TaKaRa) on a 7500 Real-time PCR system (Applied Biosystems, Foster City, USA). The primers were designed and synthesized by RiboBio (Guangzhou, China) and the sequences are as follows: H19, 5′-GAACAGAAGCATTCTAGGCTGG-3′ (forward) and 5′-TTCTAAGTGAATTACGGTGGGTG-3′ (reverse); and SIRT1, 5′-TGATTGGCACCGATCCTCG-3′ (forward) and 5′-CCACAGCGTCATATCATCCAG-3′ (reverse); miR-653-5p, 5′-GTGTTGAAACAATCTCTACTG-3′ (forward) and 5′-GAACATGTCTGCGTATCTC-3′ (reverse); U6, 5’-GCTTCGGCAGCACATATACTAAAAT-3’ (forward) and CGCTTCACGAATTTGCGTGTCAT (reverse); β-actin, 5′-GTGACGTTGACATCCGTAAAGA-3′ (forward) and 5′-GCCGGACTCATCGTACTCC-3′ (reverse). For the detection of miR-653-5p, the reverse transcription was performed by using miRNA reverse transcriptase (TaKaRa). The expression levels were determined using the 2 −ΔΔCt method, and the results were normalized to the expression level of β-actin or U6 snRNA, respectively.
Western blot analysis
After H 2O 2 stimulation or transfection, the cells were collected, and total proteins were extracted using RIPA buffer and quantified using BCA protein quantitative kit (Beyotime, Haimen, China). The protein samples were separated by SDS-PAGE and transferred onto PVDF membrane (Millipore, Billerica, USA). The membrane was probed with primary antibodies against SIRT1 (ab189494; Abcam, Cambridge, USA), followed by incubation with the corresponding HRP-conjugated secondary antibody. The protein bands were finally visualized using Immobilon Western Chemiluminescent HRP substrate (Millipore), and quantified using Quantity One software (Bio-Rad Laboratories, Hercules, USA). β-Actin was used as the loading control.
CCK-8 assay
After transfection, HEI-OC1 cells were plated in 96-well plates (5×10 3 cells/well), with or without H 2O 2 stimulation. After 24 h of incubation, CCK8 reagent (10 μL; Biyuntian Biotechnology Research Institute, Nanjing, China ) was added to each well, and incubated for another 2 h. Finally, the absorbance of each well was measured with a microplate reader (Bio-Rad) at 450 nm.
Measurement of ATP level
After H 2O 2 stimulation or transfection, HEI-OC1 cells were homogenized with lysis buffer and centrifuged at 12,000 g for 5 min, and the supernatants were collected. The ATP detection reagent (5 μL; Thermo Fisher Scientific, Waltham, USA) was added to each well of a 96-well plate. The collected samples (200 μL) were then added to the wells and mixed. The absorbance of samples and standards were measured with a microplate reader at 560 nM. ATP levels were calculated according to the standard curve and normalized to the protein contents.
Measurement of ROS levels
After H 2O 2 stimulation or transfection, HEI-OC1 cells were collected and washed with PBS. HEI-OC1 cells were incubated with DCFH-DA at 33°C for 40 min in the dark. The fluorescent images were observed and captured using an inverted microscope (Olympus, Tokyo, Japan). The average fluorescence intensity of DCFH-DA was calculated using Image-Pro Plus software 6.0 (Media Cybernetics Inc., Rockville, USA). The intracellular ROS level was expressed as the average fluorescence intensity.
Mitochondrial membrane potential assay
The mitochondrial membrane potential was measured using JC-1 kit (GeneCopoeia, Rockville, USA) according to the manufacturer’s instructions. After H 2O 2 stimulation or transfection, HEI-OC1 cells were collected and incubated with JC-1 solution (10 μM) at 33°C for 30 min. Then flow cytometry analysis was performed on FACSVerse flow cytometer (BD Biosciences, Franklin Lakes, USA) to determine the mitochondrial membrane potential [ ].
Cell apoptosis analysis
Annexin V-FITC/propidium iodide (PI) Apoptosis Detection kit (KeyGEN, Nanjing, China) was used to detect HEI-OC1 cell apoptosis according to the manufacturer’s protocol. Briefly, the harvested cells were thoroughly washed twice with binding buffer. The cells were resuspended in binding buffer and incubated with 5 μL FITC-conjugated Annexin V and 5 μL PI at room temperature in the dark for 5 min. Subsequently, cells were analyzed with a flow cytometer (BD Biosciences).
Luciferase reporter gene assay
The prediction of RNA-binding sites were performed by using starBase ( http://starbase.sysu.edu.cn/). The wild-type (WT)/mutant type (MUT, produced by mutant the miR-653-5p seed region) fragments of H19 containing putative miRNA binding sites were amplified and inserted into the Luciferase reporter plasmid (RiboBio), as well as the WT and mutant (MUT, produced by mutant the SIRT1 seed region) fragments of SIRT1 containing putative miRNA binding sites were amplified and inserted into the Luciferase reporter plasmid. 293T cells (ATCC, Manassas, USA) were seeded in a 96-well plate and then transfected with miR-653-5p mimics or inhibitors using Lipofectamine 2000. Finally, the luciferase activity was assessed using a dual-Luciferase Reporter Assay system (Promega, Madison, USA).
Pull-down assay
HEI-OC1 cells were transiently transfected with biotinylated WT/MUT miR-653-5p (Genechem, Shanghai, China) respectively, harvested 48 h after transfection, and then lysed. A total of 50 μL of the lysates were aliquoted for input, and the remaining lysates were incubated with Dynabeads M-280 Streptavidin (Invitrogen). The beads were washed and incubated with biotinylated probe. Subsequently, beads with immobilized miR-653-5p fragment were incubated with EDTA and 95% formamide. The bound RNA was purified using Trizol and then analyzed by qRT-PCR.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, USA). Results are expressed as the mean±standard deviation (SD). Statistic comparison between two groups were performed by the Student’s t test or one-way ANOVA followed by a post hoc test. Statistical significance was established at P<0.05.
Results
Overexpression of H19 inhibits cochlear hair cell apoptosis induced by H 2O 2
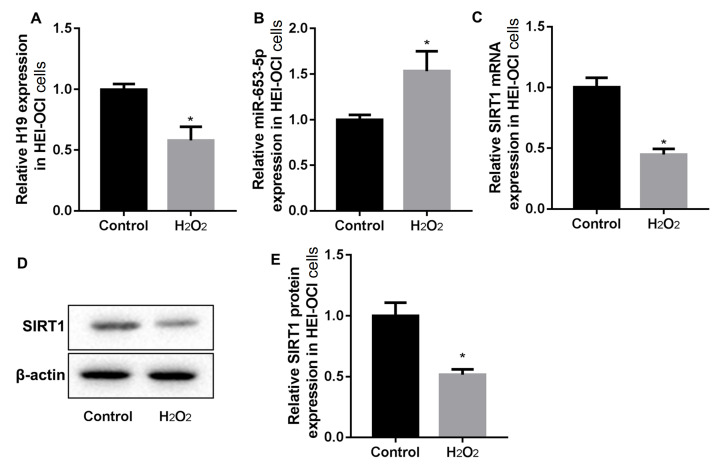
The mouse cochlear hair cells (HEI-OC1 cells) were stimulated with H 2O 2 to induce oxidative stress injury. As shown in Figure 1A,B, HEI-OC1 cells stimulated with H 2O 2 downregulated the expression of H19, but upregulated miR-653-5p. Meanwhile, RT-PCR and western blot analysis results revealed decreased mRNA and protein expressions of SIRT1 in H 2O 2-stimulated HEI-OC1 cells ( Figure 1C–E).
Figure1 .
H 2O 2 stimulation increased the expressions of H19 and SIRT1 but inhibited miR-653-5p in cochlear hair cells
HEI-OC1 cells were stimulated with 1 mM H2O2 for 2 h. (A) RT-PCR analysis of lncRNA H19. (B) RT-PCR analysis of miR-653-5p. (C–E) RT-PCR and western blot analysis of SIRT1. Data were shown as the mean±SD of three independent experiments. *P<0.05 vs control.
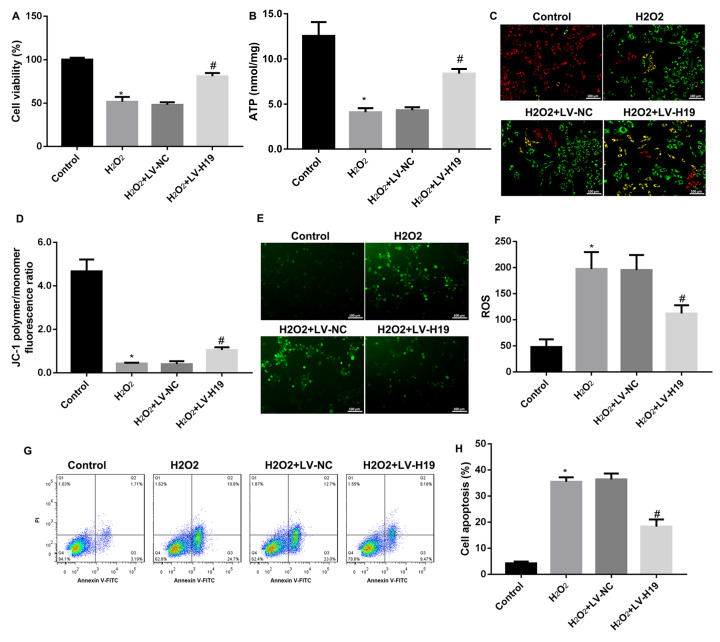
Next, to investigate the role of H19 in oxidative stress-induced cochlear hair cell apoptosis, HEI-OC1 cells were infected with lentivirus overexpressing H19, followed by H 2O 2 stimulation. The results showed high efficiency of lentivirus-mediated H19 overexpression ( Supplementary Figure S1). As shown in Figure 2A–D, H 2O 2 significantly reduced HEI-OC1 cell viability, ATP level, and mitochondrial membrane potential, and overexpression of H19 markedly improved these alterations. Moreover, the ROS level was increased by H 2O 2 stimulation, and overexpression of H19 reversed this increase ( Figure 2E,F). Finally, the effect of H19 on cell apoptosis was examined by flow cytometry. The results showed the same trend of cell apoptosis ratio as ROS level, showing that H 2O 2 stimulation induced HEI-OC1 cell apoptosis, while overexpression of H19 reversed it ( Figure 2G,H). Together, these data confirmed that H 2O 2 stimulation reduced H19 expression, and overexpression of H19 reversed the cochlear hair cells apoptosis induced by H 2O 2.
Figure2 .
Overexpression of H19 inhibited H 2O 2-induced cochlear hair cell apoptosis
(A) CCK8 assay for cell viability of HEI-OC1 cells infected with LV-H19 and LV-NC respectively. (B) ATP content in HEI-OC1 cells infected with LV-H19 and LV-NC respectively. (C,D) Representative JC-1 fluorescence micrographs in HEI-OC1 cells infected with LV-H19 and LV-NC respectively. (E,F) Representative ROS fluorescence micrographs in HEI-OC1 cells infected with LV-H19 and LV-NC respectively. (G,H). Annexin V/PI staining for cell apoptosis in HEI-OC1 cells infected with LV-H19 and LV-NC respectively. Data were shown as the mean±SD of three independent experiments. *P<0.05 vs control, and #P<0.05 vs LV-NC.
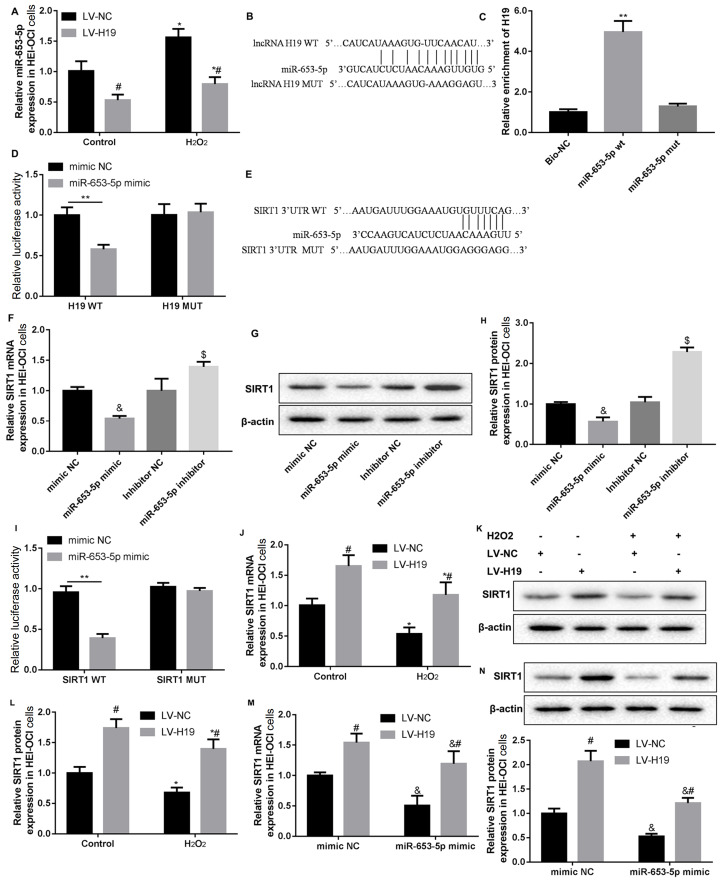
MiR-653-5p is a target of H19
It was found that overexpression of H19 suppressed miR-653-5p expression with or without H 2O 2 stimulation ( Figure 3A). Bioinformatics analysis showed that there is a binding site between H19 and miR-653-5p ( Figure 3B). RNA pull-down results showed that H19 was pulled down by WT miR-653-5p, but not by MUT miR-653-5p ( Figure 3C), proving the direct binding between H19 and miR-653-5p sequences. In addition, luciferase activity of cells co-transfected with miR-653-5p mimics and WT H19 was significantly reduced than that of cells co-transfected with NC and WT H19. At the same time, luciferase activity did not change significantly in cells co-transfected with miR-653-5p mimics or mimics NC and MUT H19 ( Figure 3D). These results indicated that miR-653-5p is a target of H19.
Figure3 .
H19 increased SIRT1 expression via miR-653-5p
(A) RT-PCR analysis of miR-653-5p in HEI-OC1 cells infected with LV-H19 or LV-NC, and then stimulated with H2O2. (B) Schematic diagram of the interaction between H19 and miR-653-5p. (C,D) H19 was identified as a target of miR-653-5p by RNA pull-down assay and dual-luciferase reporter assay. (E) Schematic diagram of the interaction between SIRT1 and miR-653-5p. (F–H) HEI-OC1 cells were transfected with miR-653-5p mimic/inhibitor. The SIRT1 expression levels were detected by RT-PCR and western blot analysis. (I) SIRT1 was identified as a target of miR-653-5p by dual-luciferase reporter assay. (J–L) HEI-OC1 cells were infected with LV-H19 or LV-NC, and then stimulated with H2O2 or not. The SIRT1 expression levels were detected by RT-PCR and western blot analysis. (M,N) HEI-OC1 cells were co-transfected with miR-653-5p mimic and LV-H19, and the corresponding negative controls. The SIRT1 expression levels were detected by RT-PCR and western blot analysis. Data were shown as the mean±SD of three independent experiments. *P<0.05 vs NC, &P<0.05 vs mimic NC, $P<0.05 vs inhibitor NC, and #P<0.05 vs LV-NC.
H19 upregulates SIRT1 expression via miR-653-5p
Bioinformatics analysis also showed that miR-653-5p can bind with the 3′-UTR of SIRT1. As shown in Figure 3E, the possible binding sequence between SIRT1 3′-UTR and miR-653-5p was predicted by starbase 2.0. Then, we found that compared with mimic/inhibitor NC, the SIRT1 expression was decreased in the miR-653-5p mimic group and increased in the miR-653-5p inhibitor group ( Figure 3F–H). Furthermore, the luciferase activity of cells co-transfected with miR-653-5p mimics and WT SIRT1 3′-UTR was obviously decreased when compared with that of cells transfected with mimics NC. And there was no significant difference between cells co-transfected with MUT SIRT1 3′-UTR ( Figure 3I). These results suggested that miR-653-5p can bind to the 3′-UTR of SIRT1.
We also found that overexpression of H19 increased SIRT1 expression with or without H 2O 2 stimulation ( Figure 3J–L). Therefore, HEI-OC1 cells were co-transfected with miR-653-5p mimic and LV-H19 or their corresponding negative controls (mimic NC and LV-NC). The results showed that SIRT1 expression was increased by LV-H19, decreased by miR-653-5p mimic, which was consistent with previous results. However, miR-653-5p mimic could partly reverse the effect of LV-H19 on SIRT1 expression ( Figure 3M,N). These results proved that H19 can positively regulate the expression of SIRT1 via miR-653-5p.
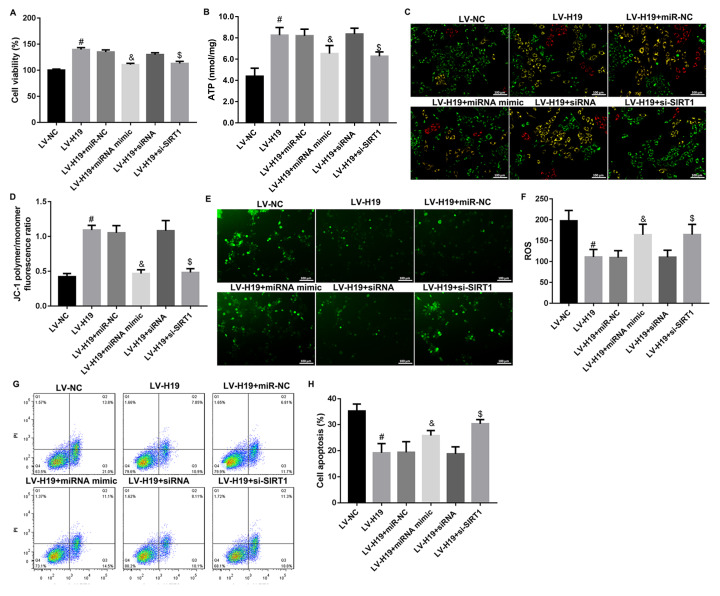
H19 inhibits cochlear hair cell apoptosis by regulating the miR-653-5p/SIRT1 axis
To explore the molecular mechanism of H19 in inhibiting cochlear hair cell apoptosis, HEI-OC1 cells were co-transfected with LV-H19 and miR-653-5p mimic or SIRT1 interference sequence (si-SIRT1) and then stimulated with H 2O 2. As shown in Figure 4A–D, under H 2O 2 stimulation, LV-H19 significantly increased HEI-OC1 cell viability, ATP level and mitochondrial membrane potential, while miR-653-5p mimic or si-SIRT1 reversed the effect of LV-19 on cell viability, ATP level and mitochondrial membrane potential. Meanwhile, the declined ROS level and cell apoptosis ratio in the LV-H19 group were reversed by miR-653-5p mimic or si-SIRT1 ( Figure 4E–H). These results suggested that under H 2O 2 stimulation, H19 inhibited cochlear hair cell apoptosis by regulating the miR-653-5p/SIRT1 axis.
Figure4 .
H19 inhibited H 2O 2--induced cochlear hair cell apoptosis via regulating miR-653-5p/SIRT1 axis
HEI-OC1 cells were co-transfected with LV-H19 and miR-653-5p mimic or SIRT1 interference sequence, and then stimulated with H2O2. (A) CCK8 assay for HEI-OC1 cell viability. (B) ATP content in HEI-OC1 cells co-transfected with LV-H19, miR-653-5p mimic and si-SIRT1. (C,D) Representative JC-1 fluorescence micrographs of HEI-OC1 cells co-transfected with LV-H19, miR-653-5p mimic and si-SIRT1. (E,F) Representative ROS fluorescence micrographs of HEI-OC1 cells co-transfected with LV-H19, miR-653-5p mimic and si-SIRT1. (G,H) Annexin V/PI staining for cell apoptosis in HEI-OC1 cells co-transfected with LV-H19, miR-653-5p mimic and si-SIRT1. Data were shown as the mean±SD of three independent experiments. #P<0.05 vs LV-NC, &P<0.05 vs mimic NC, and $P<0.05 vs siRNA.
Discussion
Oxidative stress is one of the important mechanisms of inner ear cell damage, which can lead to hearing loss in severe cases [19]. ARHL is related to mitochondrial dysfunction induced by oxidative stress, which leads to aging and death of auditory hair cells [20]. Here, we used H 2O 2 stimulation to induce oxidative stress injury of cochlear hair cells. Our results showed that H 2O 2 significantly reduced cell viability, ATP level and mitochondrial membrane potential, and increased ROS level and cell apoptosis ratio, which is consistent with previous studies [7].
More and more evidence shows that lncRNAs play a key role in aging-related diseases [21]. H19 is a paternally imprinted and maternally expressed lncRNA. Su et al. [7] found that the expression of H19 is decreased in the cochlea of aged mice. Another study showed that H 2O 2 treatment can significantly inhibit the expression of H19 in cardiomyocytes (H9c2 cells), and the expression of H19 is deceased with the increase of H 2O 2 concentration [22]. In our study, H19 was found to be significantly down-regulated in mouse cochlear hair cells (HEI-OC1) stimulated with H 2O 2, which is consistent with the results in the literature [22]. Functional experiments showed that overexpression of H19 could reverse the effect of H 2O 2 on cell viability, ATP level, mitochondrial membrane potential, and the increased ROS level and cell apoptosis, which proved that H19 might be a potential method to treat oxidative stress injury of cochlear hair cells.
Bioinformatics analysis showed that miR-653-5p is a direct target of H19. In this study, we identified increased expression of miR-653-5p in H 2O 2-stimulated cells. RNA pull-down assays and dual-luciferase reporter assays demonstrated that H19 is a target of miR-653-5p. miR-653-5p mimic could reverse the effect of H19 overexpression on cell viability, ATP level, mitochondrial membrane potential, and the increased ROS level and cell apoptosis, which indicated that the miR-653-5p participates in the oxidative stress injury of cochlear hair cells. By binding to the 3′-UTR of target genes, miRNAs result in the degradation of target mRNAs and reduction of target protein translation.
Bioinformatics analysis also showed that miR-653-5p can bind to SIRT1 3′-UTR. Here, dual-luciferase reporter assay verified that miR-653-5p is able to bind to SIRT1 3′-UTR. It has been widely reported that H19 acts as a ceRNA to bind to miRNA via a “sponge” to modulate mRNA expression [ 23– 25]. Here, rescue experiments confirmed that H19 acts as a ceRNA to bind to miR-653-5p via a “sponge” to modulate SIRT1 expression. Many researchers have reported that SIRT1 can inhibit oxidative stress and hair cell apoptosis, and further improve age-related hearing loss [ 9, 17, 18]. In the present study, we found that SIRT1 was significantly reduced in H 2O 2-stimulated cells, which is consistent with previous findings [ 26– 28]. Moreover, knockdown of SIRT1 could reverse the effect of H19 overexpression on cell viability, ATP level, mitochondrial membrane potential, and increased ROS level and cell apoptosis. These data supported that H19 inhibits oxidative stress injury of cochlear hair cells via the miR-653-5p/SIRT1 pathway.
Our findings demonstrated the mechanism of H19/miR-653-5p/SIRT1 in H 2O 2 stimulation-induced oxidative stress injury of cochlear hair cells, which may provide a new target for the treatment of ARHL. To further investigate the H19/miR-653-5p/SIRT1 axis, specimens from patients with hearing loss should be used and studied, which will further validate the functions of H19. On the other hand, different therapies or medicines can be used to treat HEI-OC1 cells to help estimate the performance by detecting the expression of H19 and the related regulation cascade. Prospective detection of H19/miR-653-5p/SIRT1 expression and targeted intervention of H19/miR-653-5p/SIRT1 may be a new strategy for the diagnosis and treatment of ARHL.
In summary, this study demonstrates that H19/miR-653-5p/SIRT1 is a new signaling axis which may modulate the biogenesis of mitochondria to maintain the function of mitochondria and inhibit oxidative stress injury to reduce cell apoptosis ( Figure 5). Our results reveal the mechanism of H19 in H 2O 2 stimulation-induced oxidative stress injury of cochlear hair cells, which may provide a new target for the treatment of ARHL.
Figure5 .
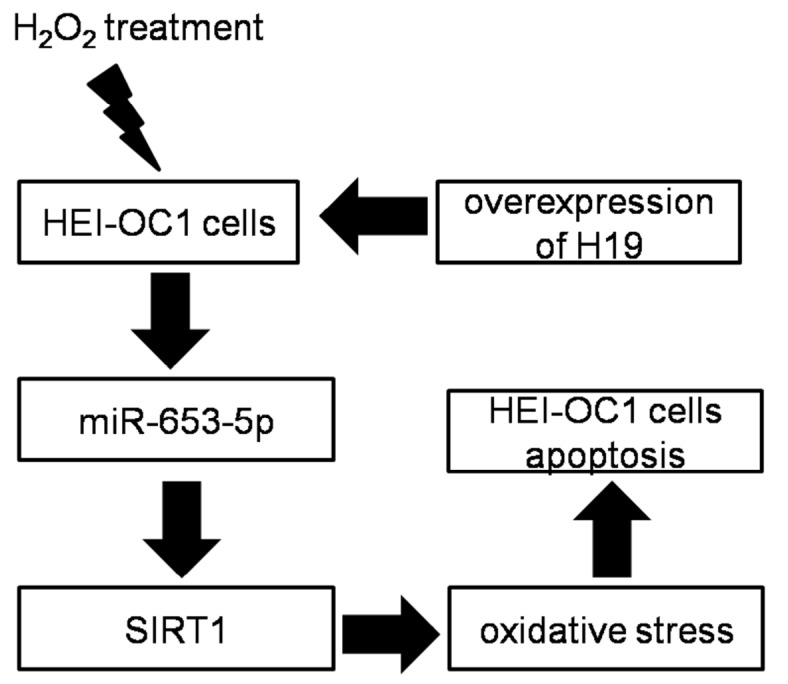
lncRNA H19 inhibits oxidative stress injury of cochlear hair cells via the miR-653-5p/SIRT1 axis
Supplementary Data
Supplementary data is available at Acta Biochimica et Biophysica Sinica online.
Supporting information
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grant from the Science and Technology Department of Jiangxi Province (No. 20202BBGL73017).
References
- 1.Bowl MR, Dawson SJ. Age-related hearing loss. Cold Spring Harb Perspect Med. . 2019;9:a033217. doi: 10.1101/cshperspect.a033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto C, Yamasoba T. Oxidative stresses and mitochondrial dysfunction in age-related hearing loss. Oxid Med Cell Longev. . 2014;2014:1–6. doi: 10.1155/2014/582849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lasisi TJ, Lasisi AO. Evaluation of serum antioxidants in age-related hearing loss. Aging Clin Exp Res. . 2015;27:265–269. doi: 10.1007/s40520-014-0282-3. [DOI] [PubMed] [Google Scholar]
- 4.Qi F, Zhang R, Chen J, Zhao F, Sun Y, Du Z, Bing D, et al. Down-regulation of Cav1.3 in auditory pathway promotes age-related hearing loss by enhancing calcium-mediated oxidative stress in male mice. Aging. . 2019;11:6490–6502. doi: 10.18632/aging.102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong H, Chen S, Lai L, Yang H, Xu Y, Pang J, Su Z, et al. Modulation of miR-34a/SIRT1 signaling protects cochlear hair cells against oxidative stress and delays age-related hearing loss through coordinated regulation of mitophagy and mitochondrial biogenesis. Neurobiol Aging. . 2019;79:30–42. doi: 10.1016/j.neurobiolaging.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Giorgi C, Marchi S, Simoes ICM, Ren Z, Morciano G, Perrone M, Patalas-Krawczyk P, et al. Mitochondria and reactive oxygen species in aging and age-related diseases. 2018, 340: 209–344 . [DOI] [PMC free article] [PubMed]
- 7.Su Z, Xiong H, Pang J, Lin H, Lai L, Zhang H, Zhang W, et al. LncRNA AW112010 promotes mitochondrial biogenesis and hair cell survival: implications for age-related hearing loss. Oxid Med Cell Longev. . 2019;2019:1–13. doi: 10.1155/2019/6150148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao T, Liu X, Sun Z, Zhang J, Zhang X, Wang C, Geng R, et al. RNA-seq analysis of potential lncRNAs for age-related hearing loss in a mouse model. Aging. . 2020;12:7491–7510. doi: 10.18632/aging.103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao S, Wang L, Zhao K, Zhu X, Ye F. Rs1894720 polymorphism in MIAT increased susceptibility to age‐related hearing loss by modulating the activation of miR‐29b/SIRT1/PGC‐1α signaling. J Cell Biochem. . 2019;120:4975–4986. doi: 10.1002/jcb.27773. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Suen CW, Ma H, Wang Y, Kong L, Qin D, Lee YW, et al. The roles of h19 in regulating inflammation and aging. Front Immunol. . 2020;11:579687. doi: 10.3389/fimmu.2020.579687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. . 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. . 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Liu H, McGee JA, Walsh EJ, Soukup GA, He DZZ. Identifying microRNAs involved in degeneration of the organ of corti during age-related hearing loss. PLoS ONE. . 2013;8:e62786. doi: 10.1371/journal.pone.0062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmoudian-Sani MR, Mehri-Ghahfarrokhi A, Ahmadinejad F, Hashemzadeh-Chaleshtori M, Saidijam M, Jami MS. MicroRNAs: effective elements in ear-related diseases and hearing loss. Eur Arch Otorhinolaryngol. . 2017;274:2373–2380. doi: 10.1007/s00405-017-4470-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen HHR, Wijesinghe P, Nunez DA. MicroRNAs in acquired sensorineural hearing loss. J Laryngol Otol. . 2019;133:650–657. doi: 10.1017/S0022215119001439. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Wang N, Xu A. Cmah deficiency may lead to age-related hearing loss by influencing miRNA-PPAR mediated signaling pathway. Peer J. . 2019;7:e6856. doi: 10.7717/peerj.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong H, Dai M, Ou Y, Pang J, Yang H, Huang Q, Chen S, et al. SIRT1 expression in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Exp Gerontol. . 2014;51:8–14. doi: 10.1016/j.exger.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Pang J, Xiong H, Ou Y, Yang H, Xu Y, Chen S, Lai L, et al. SIRT1 protects cochlear hair cell and delays age-related hearing loss via autophagy. Neurobiol Aging. . 2019;80:127–137. doi: 10.1016/j.neurobiolaging.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Pak JH, Kim Y, Yi J, Chung JW. Antioxidant therapy against oxidative damage of the inner ear: protection and preconditioning. Antioxidants. . 2020;9:1076. doi: 10.3390/antiox9111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park DJ, Ha S, Choi JS, Lee SH, Park JE, Seo YJ. Induced short-term hearing loss due to stimulation of age-related factors by intermittent hypoxia, high-fat diet, and galactose injection. Int J Mol Sci. . 2020;21:7068. doi: 10.3390/ijms21197068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. . 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 22.Su Y, Xu R, Zhang R, Qu Y, Zuo W, Ji Z, Geng H, et al. N6-methyladenosine methyltransferase plays a role in hypoxic preconditioning partially through the interaction with lncRNA H19. Acta Biochim Biophys Sin. . 2020;52:1306–1315. doi: 10.1093/abbs/gmaa130. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Yan X, Liu K, Wu M, Li Z, Wang Y, Zhong X, et al. lncRNA H19 acts as a ceRNA to regulate the expression of CTGF by targeting miR-19b in polycystic ovary syndrome. Braz J Med Biol Res. . 2020;53:e9266. doi: 10.1590/1414-431x20209266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan L, Yang S, Yue CX, Wei XY, Peng W, Dong ZY, Xu HN, et al. Long noncoding RNA H19 acts as a miR-340-3p sponge to promote epithelial-mesenchymal transition by regulating YWHAZ expression in paclitaxel-resistant breast cancer cells. Environ Toxicol. . 2020;35:1015–1028. doi: 10.1002/tox.22938. [DOI] [PubMed] [Google Scholar]
- 25.Gao N, Tang H, Gao L, Tu GL, Luo H, Xia Y. LncRNA H19 aggravates cerebral ischemia/reperfusion injury by functioning as a ceRNA for miR-19a-3p to target PTEN. Neuroscience. . 2020;437:117–129. doi: 10.1016/j.neuroscience.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Jing Z, Wang C, Wen S, Jin Y, Meng Q, Liu Q, Wu J, et al. Phosphocreatine promotes osteoblastic activities in H 2O 2-induced MC3T3-E1 cells by regulating SIRT1/FOXO1/PGC-1α signaling pathway . Curr Pharm Biotechnol. . 2021;22:609–621. doi: 10.2174/1389201021999201116160247. [DOI] [PubMed] [Google Scholar]
- 27.Gao T, Che X, Wang R, Xiao C, Jia Y. Protective effect of overexpression of PrxII on H2O2-induced cardiomyocyte injury. 2020, 24: 9055–9062. [DOI] [PubMed]
- 28.Park JH, Park SA, Lee YJ, Joo NR, Shin J, Oh SM. TOPK inhibition accelerates oxidative stress‑induced granulosa cell apoptosis via the p53/SIRT1 axis. Int J Mol Med. . 2020;46:1923–1937. doi: 10.3892/ijmm.2020.4712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.