CASE PRESENTATION
A previously healthy 15‐year‐old girl with obesity and type 2 diabetes developed painful, purple toes during hospitalization for critical COVID‐19 infection. A few weeks prior to admission, she developed upper respiratory symptoms with fever and chills; nasopharyngeal polymerase chain reaction (PCR) confirmed COVID‐19 infection. After 7 days of worsening fever and cough, she presented to the emergency department and was admitted to the pediatric intensive care unit with hypoxemic pneumonia. Treatment with remdesivir, dexamethasone, and ceftriaxone was initiated. The patient progressed to hypoxemic respiratory failure requiring BiPAP with maximum FiO2 100%. Baricitinib was added due to worsening cytokine storm with fever, shock, and laboratory abnormalities consistent with early macrophage activation syndrome. She improved slowly with treatment allowing for gradual weaning of respiratory support.
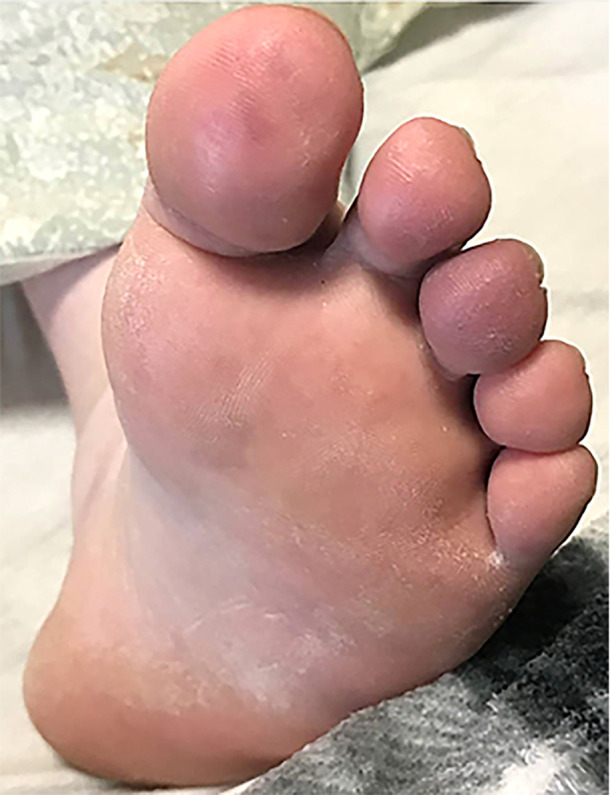
Approximately 3 weeks into her admission, her left foot was noted to be cold with edema up to her ankles. Doppler ultrasound demonstrated patent left lower extremity arterial vasculature with normal waveform. A painful purple nodule was noticed over her left first and third toes. Dermatology consult was requested to evaluate for pandemic‐associated pernio. Examination of the left lower extremity revealed edematous violaceous papules over the plantar first and third toes (Figure 1) and violaceous macules over the lateral ball of the foot. Livedo reticularis was also present over the dorsal hands. Punch biopsy of the left plantar first toe was obtained. Topical fluocinonide was applied to the rash twice daily with gradual improvement of her pain and swelling.
FIGURE 1.
WHAT IS THE DIAGNOSIS?
Diagnosis: Thrombogenic vasculopathy
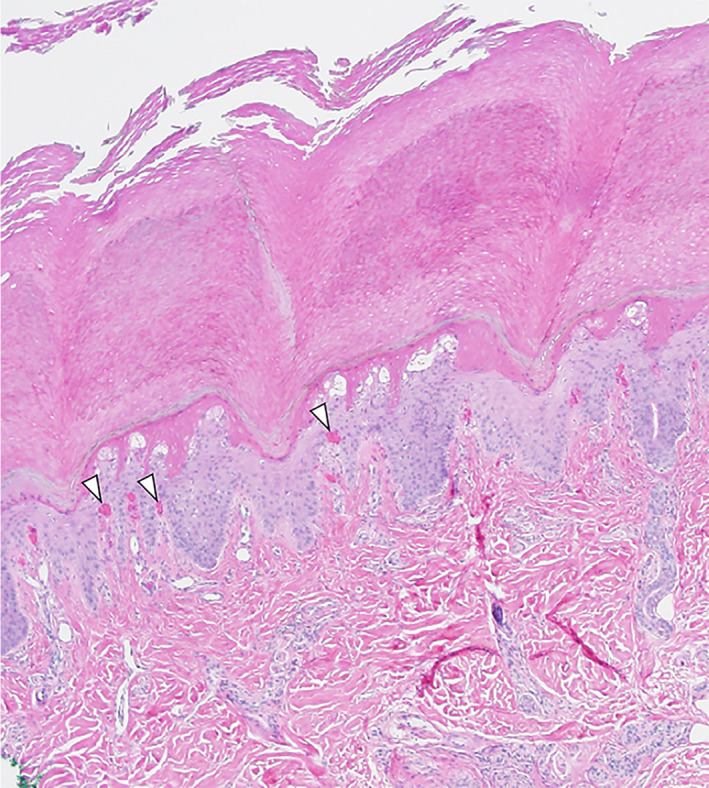
Histopathology revealed superficial epidermal necrosis and intradermal capillary microthrombi (arrowheads) with extravasated erythrocytes at 4.4× magnification (Figure 2, H&E stain). A pauci‐perivascular lymphocytic infiltrate was appreciated without vasculitis or papillary dermal edema. One week following onset of her skin findings, the patient developed persistent tachypnea, and subsequent CT angiography of the chest revealed a right lower lobe subsegmental pulmonary embolism which was treated with enoxaparin.
FIGURE 2.
DISCUSSION
Pandemic‐associated pernio, commonly referred to as “COVID toes,” has drawn significant attention from the medical community and lay press. These lesions present as non‐specific red‐purple discoloration of the digits, making them difficult to discern from other acral pathologies. Histopathologically, pernio is characterized by a robust perivascular and peri‐eccrine dermal lymphocytic infiltrate without vasculitis or vasculopathy. Utilizing immunohistochemistry, abundant myxovirus resistance protein 1 (MxA), the downstream marker of type 1 interferon activation, can be visualized but is primarily performed for the purposes of research. 1 SARS‐CoV‐2 envelope and spike proteins are infrequently reported by immunohistochemistry, although recent reports have demonstrated poor specificity of SARS‐CoV‐2 targeted antibodies in tissue. 2 , 3 Pandemic‐associated pernio primarily affects young people who harbor few extracutaneous symptoms of viral respiratory infection. The majority lack evidence of previous COVID‐19 infection. 4 , 5
Interferon‐mediated innate immune responses are important early defenders against viral pathogens. Previous studies have confirmed a delayed, attenuated type I interferon (IFN‐I) response in individuals hospitalized with critical COVID‐19. Genetic variants in the IFN‐I pathway or neutralizing autoantibodies against IFN‐I underlie critical COVID in 15% of patients, rendering the pathway incapable of producing or responding to IFN‐I. 6 , 7 Some studies of pandemic‐associated pernio have reported an elevated local and systemic IFN‐I signature, supporting a hypothesis of a virus‐induced phenomenon that may be arguably protective against severe COVID‐19. 8 , 9 Additionally, IFN‐stimulated gene scores from whole blood at presentation of pandemic‐associated pernio have been reported, with a few patients reaching the level of interferon induction seen in the type I interferonopathies. This underscores the potential for a shared mechanism between pandemic‐associated pernio and pernio seen in the type 1 interferonopathies.
In contrast to pandemic‐associated pernio, thrombogenic vasculopathy is seen in critically ill patients with COVID‐19 infection. Thrombogenic vasculopathy can be a clinical mimic for pernio, as patients present with red‐purple discoloration of the toes, hemorrhagic blisters, and necrotic ulceration. In contrast, however, thrombogenic vasculopathy represents microvascular injury syndrome mediated by activation of complement pathways and an associated hypercoagulable state, as previous studies have demonstrated co‐localization of SARS‐CoV‐2 spike glycoproteins with C5b‐9 and C4d in in the cutaneous microvasculature. 10 Importantly, unlike pernio, thrombogenic vasculopathy is pauci‐cellular with vascular thrombosis and absent MxA expression on immunohistochemistry. 1
The underlying mechanism of thromboembolic events in COVID‐19 remains poorly understood. Complement activation in response to COVID‐19 infection may exacerbate viral‐induced systemic hyperinflammation and contribute to a cytokine storm‐like presentation. These inciting events can lead to vascular inflammation and endothelial cell dysfunction, triggering the release of several prothrombotic factors. Recent studies have demonstrated elevated inflammatory markers, including C‐reactive protein, ferritin, fibrinogen, procalcitonin, and interleukin‐6, in the setting of severe COVID‐19. 11 , 12
A robust interferon response in young, healthy individuals facilitates clearance of viral pathogens and correlates with a resilient COVID‐19 phenotype any may explain pandemic‐associated pernio. In critical COVID‐19, an attenuated IFN‐I response leads to persistent viral replication and extensive complement activation and deposition in skin and other organs, including the toes, leading to ensuing coagulopathy. Although pandemic‐associated pernio and thrombogenic vasculopathy can be clinical mimics, the patient populations are different, and histopathology may help discriminate between the diagnoses. The unusual presentation of critical COVID‐19 in this previously healthy adolescent could suggest an underlying IFN‐I deficiency, which could be investigated through genetic testing.
Ng AT, Miller A, Bodemer AA, Drolet BA, Arkin L. Purple toes following critical COVID‐19 infection. Pediatr Dermatol. 2022;39(5):815‐817. doi: 10.1111/pde.15075
REFERENCES
- 1. Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID‐19‐associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184(1):141‐150. doi: 10.1111/bjd.19415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS‐CoV‐2 infection. Proc Natl Acad Sci U S A. 2022;119(9):e2122090119. doi: 10.1073/pnas.2122090119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Massoth LR, Desai N, Szabolcs A, et al. Comparison of RNA in situ hybridization and immunohistochemistry techniques for the detection and localization of SARS‐CoV‐2 in human tissues. Am J Surg Pathol. 2021;45(1):14‐24. doi: 10.1097/PAS.0000000000001563 [DOI] [PubMed] [Google Scholar]
- 4. Freeman EE, McMahon DE, Lipoff JB, et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486‐492. doi: 10.1016/j.jaad.2020.05.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID‐19 outbreak occur in patients who are negative for COVID‐19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183(5):866‐874. doi: 10.1111/bjd.19377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hubiche T, Cardot‐Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon‐alpha response characteristics of patients with chilblains‐like lesions during the COVID‐19 pandemic. JAMA Dermatol. 2021;157(2):202‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frumholtz L, Bouaziz JD, Battistella M, et al. Type I interferon response and vascular alteration in chilblains‐like lesions during the COVID‐19 outbreak. Br J Dermatol. 2021;185(6):1176‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. doi: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]


