Abstract
Background
In recent years, many important advances have been seen in anticoagulation therapy. However, bleeding risk is still a major concern. Factor XI (FXI) inhibition has emerged as a potential advantageous target to minimize this risk.
Objectives
We conducted a systematic review and meta‐analysis of current evidence on FXI inhibitors for thromboprophylaxis in major orthopedic surgery.
Methods
We performed a systematic search of electronic databases (PubMed, CENTRAL, and Scopus) until May of 2022. Studies were considered eligible if they were randomized controlled trials (RCTs) evaluating FXI inhibitors in thromboprophylaxis versus low molecular weight heparin (LMWH). For analysis purposes, we considered efficacy (venous thromboembolism [VTE], symptomatic VTE) and safety (major and clinically relevant non‐major [CRNM] bleeding events, major bleeding events, blood transfusion necessities, adverse events, major adverse events) outcomes.
Results
Overall, four RCTs were included, with a total of 2269 patients, 372 VTE events, and 50 major or CRNM bleeding events. Regarding efficacy outcomes, FXI inhibitors were associated with a significant reduction in the incidence of VTE events (odds ratio [OR] 0.50; 95% confidence interval [CI: 0.36, 0.69]). Concerning safety outcomes, FXI inhibitors significantly reduced major or CRNM bleeding events (OR 0.41; 95% CI [0.22, 0.75]). It was also associated with a lower percentage of patients needing a blood transfusion, despite not meeting statistical significance (OR 0.69; 95% CI [0.32, 1.48]). Incidence of adverse events and major adverse events were similar between groups.
Conclusion
Factor XI inhibitors showed a significant reduction in the incidence of VTE and bleeding events among patients submitted to major orthopedic surgery.
Keywords: anticoagulants, factor XI, hemorrhage, low molecular weight heparin, meta‐analysis, venous thromboembolism
Essentials.
Bleeding risk is still a major concern when it comes to anticoagulation therapy.
Factor XI inhibition has been identified as a desirable target to minimize these risks.
Factor XI inhibitors reduced thromboembolic risk after major orthopedic surgery.
They were also associated with a lower incidence of clinically relevant bleeding events.
1. INTRODUCTION
The balance between the antithrombotic benefit of a drug compared to the associated bleeding risk is a dichotomy that extends across many aspects of medicine. Despite many significant advances in anticoagulation therapy over the last decades, bleeding risk is still an important concern. 1 , 2 , 3 This is apparent not only by the bleeding rates associated with atrial fibrillation or venous thromboembolism (VTE) but also by the rate of patients who are not prescribed this medication out of fear of this adverse event. 1 , 2 , 3
Prevention of VTE in the context of major orthopedic surgery is of paramount importance and its indication is unquestioned. 4 , 5 Not only is this a period of vulnerability for hemorrhagic events, but it also carries a significant risk for thromboembolic events. 6 Exposure of tissue factor (TF) at the surgical site initiates coagulation via the extrinsic pathway and triggers thrombin generation (Figure 1). This is thought to be one of the main drivers of postoperative VTE. 7 However, the importance of the intrinsic pathway in the pathogenesis of thrombosis is increasingly recognized and wide evidence suggests a limited role in normal hemostasis, making this pathway a potential therapeutic target. 7
FIGURE 1.
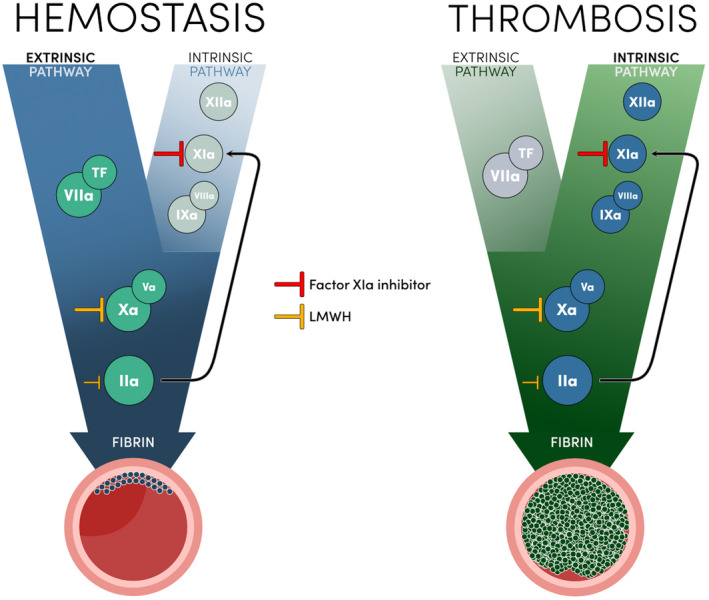
Schematic representation of hemostasis and thrombosis, and mechanism of action of studied drugs. IIa, activated factor II; IXa, activated factor IX; LMWH, low molecular weight heparin; TF, tissue factor; Va, activated factor V; VIIa, activated factor VII; VIIIa, activated factor VIII; Xa, activated factor X; XIa, activated factor XI; XIIa, activated factor XII
Epidemiological and animal studies have identified factor XI (FXI) as a desirable target. 8 First, congenital FXI deficiency is associated with a lower risk of thrombosis events, such as VTE and ischemic stroke. 7 Second, targeting this factor seems to be a safer option, because patients with FXI deficiency do not seem to be at increased risk for serious bleeding. 8 This supports the premise that targeting FXI, a key component of the intrinsic pathway, attenuates thrombosis with little disruption of hemostasis. 7
Novel agents directed against FXI are in development. They include biosynthesis inhibitors, antibodies, and small molecules, with a wide range of pharmacological properties. Until now, several phase II studies have been published with promising results. 9 , 10 , 11 , 12
With this background, and examining the publications regarding FXI inhibitors, we conducted a systematic review with meta‐analysis to compare the efficacy and safety profile of these new medications to low molecular weight heparin (LMWH) in patients undergoing major orthopedic surgery.
2. METHODS
2.1. Search of studies and data extraction
We conducted this meta‐analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) recommendations. 13 We performed a systematic search of three electronic databases (PubMed, Cochrane Central Register of Controlled Trials [CENTRAL], and Scopus) until May 31, 2022. No language restrictions were applied. Table S1 in supporting information shows the detailed search strategy. Two authors (JP and JF) independently assessed titles, abstracts, and full texts, when appropriate, for eligibility and data extraction. Disagreements were resolved by consensus. Studies were considered eligible if they were (1) randomized controlled trials (RCTs), (2) comparison of FXI inhibitors versus LMWH for prevention of venous thromboembolism, and (3) patients submitted to major orthopedic surgery. Studies were excluded if they were: non‐randomized studies, without full text published, or comparator other than LMWH. Data extraction was performed by both authors into a previously defined form. The protocol was registered in PROSPERO (CRD42022334604). The quality of reporting was independently assessed by two investigators (JP and JF) using the Cochrane Collaboration's tool.
2.2. Clinical outcomes
For analysis purposes, the primary efficacy outcome evaluated was the incidence of VTE, which was a composite of asymptomatic VTE (detected by mandatory venography of the lower limb undergoing surgical intervention) and symptomatic VTE. Safety outcomes analyzed were: a composite of major and clinically relevant non‐major (CRNM) bleeding events, incidence of adverse events, and necessity of blood transfusion. Subgroup analyses were done for symptomatic VTE, major bleeding events, and severe adverse events. For sensitivity analyses, we examined the performance of FXI inhibitor dosages that showed superior efficacy compared to LMWH. Outcome events were defined based on the definition used in each original study (Table S2). We performed two sensitivity analyses: one for the dosages that showed superior efficacy compared to LMWH, to perceive the magnitude of the superiority of these dosages in the reduction of thromboembolism and evaluate the associated bleeding risk and a second analysis for doses lower than the ones that showed superior efficacy to assess the extent of benefit in bleeding risk and evaluate the relative effect in the prevention of thromboembolism.
2.3. Statistical analysis
The extracted data were analyzed using the open‐source statistical software Review Manager (RevMan) V.5.4.1 (The Cochrane Collaboration) to aggregate the meta‐analysis results, and ProMeta 3 software. Odds ratio (OR) and 95% confidence intervals (95% CI) were used as summary statistics. Analysis for efficacy outcomes was performed for the modified intention‐to‐treat population. For safety outcomes, analysis was performed for the on‐treatment population. The pooled OR was estimated using the DerSimonian and Laird random effects model due to differences in pharmacologic profiles of FXI inhibitors and methodologies of studies. Additionally, a fixed effects model was used to explore the typical intervention effect from studies included. 14 Heterogeneity across studies was assessed by I2 using Cochran's Q test. We report prediction intervals for treatment effects on the primary efficacy outcome and the composite of major and CRNM bleeding events. Publication bias was not assessed as there were inadequate numbers of included trials to properly assess a funnel plot, Begg's rank test, or Egger's regression test. 14
3. RESULTS
3.1. Included studies and patient characteristics
A total of 52 studies were identified through database searching. After removing duplicates, 37 studies were screened. Following the application of inclusion and exclusion criteria, four RCTs were included, involving 2269 patients (1766 [77.8%] treated with FXI inhibitors; Figure 2). A list of characteristics from each study and study medication is presented in Table 1.
FIGURE 2.
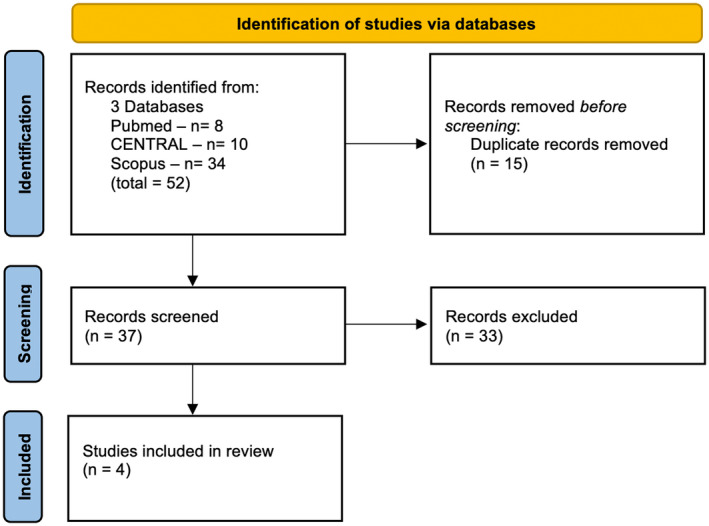
Flow diagram of the research strategy and study selection process
TABLE 1.
Characteristics of each study and study medication
| Characteristics | FXI‐ASO TKA, 2015 9 | FOXTROT, 2020 10 | ANT‐005 TKA, 2021 11 | AXIOMATIC‐TKR, 2021 12 |
|---|---|---|---|---|
| Study medication–n | FXI‐ASO–221 patients | Osocimab–450 patients | Abelacimab–299 patients | Milvexian–796 patients |
| Type of agent | Second generation antisense oligonucleotide | Monoclonal antibody (fully human) | Monoclonal antibody (fully human) | Small molecule |
| Mode of action | Decreases FXI synthesis | FXia inhibition | Dual FXI/XIa inhibition | FXia inhibition |
| Administration route | Subcutaneous | Intravenous | Intravenous | Oral |
| Dose | 200/300 mg | 0.3/0.6/1.2/1.8 mg/kg | 30/75/150 mg | 25/50/100/200 mg |
| Administration frequency | First administration was 36 days before surgery (day 1); then on days 3, 5, 8, 15, 22, 29, 36 (6 h after surgery), and 39. | Single administration the day before/after surgery | Single administration 4–8 h after surgery | Once/twice a day for 10 to 14 days–first dose 12/24 h after surgery |
| Comparator–n | Enoxaparin 40 mg s.c.–72 patients‐first dose in the evening before or 6 h post‐op | Enoxaparin 40 mg s.c.–77 patients‐first dose in the evening before or 6‐8 h post‐op | Enoxaparin 40 mg s.c.–101 patients‐first dose in the evening before or 12 h post‐op | Enoxaparin 40 mg s.c.–252 patients‐first dose the day before surgery |
| Duration of comparator treatment–median | 10 days [10; 10] | 13 days [10; 16] | 9 days [6; 12] | 12 days [1; 15] |
| Trial design | Randomized, parallel‐group, adaptive design, open‐label with blinded outcome adjudication | Randomized, parallel‐group, adaptive design, open‐label with blinded outcome adjudication | Randomized, parallel‐group, open‐label with blinded outcome adjudication | Randomized, parallel‐group, adaptive design, open‐label with blinded outcome adjudication |
| Age | 63–64 years | 66.5 ± 8.2 years | 67–68 years | 68–69 years |
| Female–% | 81.2% | 74.2% | 81.8% | 69.4% |
| Venogram timing—days after surgery | 8–12 | 10–13 | 8–12 | 10–14 |
| Time analysis for safety outcomes | 136 days | 150 days | 30 days | On‐treatment period +2 days |
| Total VTE events—n (%) | 61 (21.7%) | 105 (19.9%) | 44 (11.0%) | 162 (15.5%) |
| Study medication | 39 (18.6%) | 85 (18.9%) | 22 (7.4%) | 108 (13.6%) |
| Enoxaparin | 31.0%) | 20 (26.0%) | 22 (21.8%) | 54 (21.4%) |
| Total major or clinically relevant non‐major bleeding events—n (%) | 12 (4.1%) | 22 (3.2%) | 4 (1.0%) | 12 (1.0%) |
| Study medication | 6 (2.7%) | 15 (2.6%) | 4 (1.3%) | 7 (0.8%) |
| Enoxaparin | 6 (8.3%) | 7 (6.9%) | 0 (0%) | 5 (1.7%) |
Abbreviations: FXI, factor XI; VTE, venous thromboembolism.
3.2. Efficacy outcomes
Regarding efficacy outcomes, FXI inhibitors were associated with a significantly lower incidence of total VTE (14.5% vs. 23.6%, OR [95% CI = 0.50 [0.36, 0.69]; p = <.001) compared to LMWH (Figure 3). Heterogeneity for this outcome was non‐significant (I2 = 36%).
FIGURE 3.

Forest plot comparing factor XI inhibitors versus low molecular weight heparin regarding the incidence of venous thromboembolism
On subgroup analysis, FXI inhibitors were not associated with statistically significant lower incidence of symptomatic VTE (0.7% vs. 0.8%, OR [95% CI] = 0.78 [0.24, 2.57]; p = .680; Figure S2).
3.3. Safety outcomes
Among patients receiving FXI inhibitors, the incidence of major and CRNM bleeding events was significantly lower (1.6% vs. 3.2%, OR [95% CI] = 0.41 [0.22, 0.75]; p = .003), with non‐significant heterogeneity (I2 = 0%; Figure 4).
FIGURE 4.
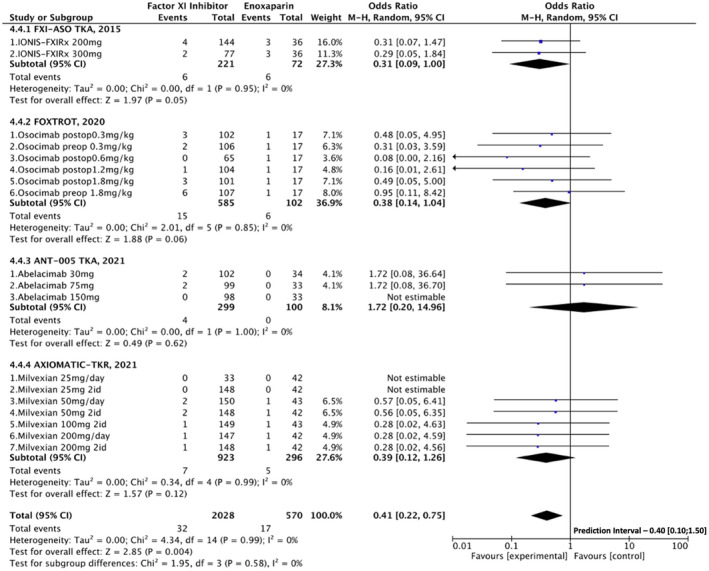
Forest plot comparing factor XI inhibitors versus low molecular weight heparin regarding the incidence of major or clinically relevant non‐major bleeding events
Major bleeding events were very rare for both FXI inhibitors and LMWH—n = 4 (0.2%) and n = 1 (0.2%), respectively. For this reason, no analysis was done.
Statistical significance was not met for lower need for blood transfusion in patients treated with FXI inhibitors (11.5% vs. 15.3%, OR [95% CI] = 0.69 [0.32, 1.48]; p = .350; Figure S3). Significant heterogeneity was identified in this analysis (I2 = 57%).
Incidence of adverse events (50.0% vs. 44.0%, OR [95% CI] = 1.17 [0.85, 1.62]; p = .340) and severe adverse events (3.7% vs. 4.0%, OR [95% CI] = 0.69 [0.42, 1.12]; p = .130) were not significantly different between groups (Figures S4 and S5). No significant heterogeneity was found for both outcomes (I2 = 47% and 0%, respectively).
3.4. Sensitivity analysis
First, we performed an analysis including grouped FXI inhibitor dosages that showed superior efficacy compared to LMWH. These included the following doses: 75 mg and 150 mg of abelacimab; 300 mg of FXI‐ASO; 50 mg twice daily, 100 mg twice daily, 200 mg daily, and 200 mg twice daily of milvexian; and 1.8 mg/kg of osocimab preoperative administration. The analysis showed consistent results for the primary efficacy outcome, with lower VTE risk (7.6% vs. 23.6%, OR [95% CI] = 0.25 [0.15, 0.42]; p = <.001; Figure 5). Conversely, the analysis for incidence of major or CRNM bleeding events compared to LMWH did not meet statistical significance for lower bleeding risk, despite the lower absolute incidence of these events (1.4% vs. 3.2%, OR [95% CI] = 0.60 [0.29, 1.24]; p = .160; Figure 6).
FIGURE 5.
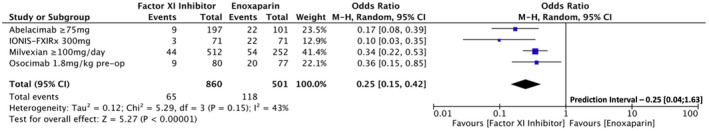
Forest plot for the sensitivity analysis comparing factor XI inhibitors dosages that showed superior efficacy to low molecular weight heparin regarding the incidence of venous thromboembolism
FIGURE 6.
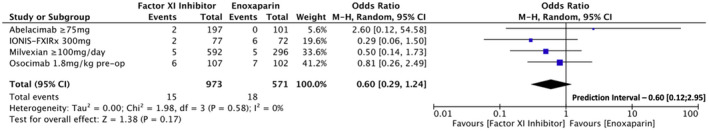
Forest plot for the sensitivity analysis comparing factor XI inhibitors dosages that showed superior efficacy to low molecular weight heparin regarding the incidence of major or clinically relevant non‐major bleeding events
Second, we performed an analysis including lower FXI inhibitor dosages (grouped). These included: 30 mg of abelacimab; 200 mg of FXI‐ASO; 25 mg once daily, 25 mg twice daily, and 50 mg once daily of milvexian; 0.3 mg/kg pre‐op, 0.3 mg/kg post‐op, 0.6 mg/kg post‐op, and 1.2 mg/kg post‐op of osocimab. The incidence of VTE was not significantly different between groups (21.5% vs. 23.6%, OR [95% CI] = 0.85 [0.65, 1.12]; Figure S6). Regarding safety outcomes, these dosages showed a significantly lower risk for bleeding events (1.5% vs. 3.2%, OR [95% CI] = 0.34 [0.15, 0.80]; Figure S7).
3.5. Fixed effects analyses
Fixed effects analyses are available in the Table S3 in supporting information. Results are roughly similar to random effects model with the exception of the borderline lower incidence of necessity of blood transfusion in patients receiving FXI inhibitors (OR [95% CI] = 0.64 [0.41, 0.99]).
3.6. Quality assessment and risk of bias
The risk of bias evaluation is reported in Figure S1 in supporting information. Overall studies were evaluated as “some concerns” due to the open‐label design for study treatment assignment to participants and investigators. However, this potential source of bias was attenuated by the blinded evaluation of outcomes. The small number of studies included did not allow any reliable analysis of publication bias.
4. DISCUSSION
To our knowledge, this is the first meta‐analysis of RCTs comparing FXI inhibition versus LMWH for thromboprophylaxis in patients submitted to major orthopedic surgery. FXI inhibitors were associated with a significant reduction both in VTE and major or CRNM bleeding events.
Inhibition of FXI was associated with a relative risk reduction of about 50% in total (asymptomatic and symptomatic) VTE events. This result is very significant because it arises from phase II studies, which naturally included subtherapeutic dosages of FXI inhibitors. The magnitude of relative risk reduction of VTE events increased to almost 75% in the sensitivity analysis including optimized dosages of FXI inhibitors. The results are consistent among the studies included in the meta‐analysis, as evidenced by the non‐significant heterogeneities. Absolute rates of VTE for LMWH observed in this meta‐analysis are roughly in line with contemporary landmark clinical trials of thromboprophylaxis in patients submitted to total knee replacement. The incidence of VTE in patients receiving LMWH was 23.6% in our meta‐analysis, 18.9% in the Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism (RECORD) 3 study, and 27.8% in the Pentasaccharide in Major Knee Surgery Study. 15 , 16
In addition to lowering the risk of thromboembolism, FXI inhibitors exhibited a significant reduction in the risk of major or CRNM bleeding compared to LMWH. The magnitude of relative risk reduction was approximately 55% and was mainly driven by a reduction in CRNM bleeding events. In the sensitivity analysis comparing the relative benefit of more effective dosages of FXI to LMWH, the reduction in major or CRNM bleeding events was 39% but did not reach statistical significance. Notwithstanding this fact, no significant heterogeneity was found in these safety analyses. Again, absolute rates of major or CRNM bleeding events observed in this meta‐analysis were superimposed with those reported in previous similar clinical trials. The incidence of major or CRNM bleeding in patients receiving LMWH was 3.2% in our meta‐analysis, 2.7% in the RECORD 3 study, and 4.3% in the Apixaban Dose Orally Versus Anticoagulation with Enoxaparin (ADVANCE) trial. 3
On further subgroup evaluation comparing FXI inhibitors to LMWH, despite showing a lower absolute rate of necessity of blood transfusion the difference did not meet statistical significance. This result and heterogeneity present are probably justified by the low number of events and studies included in this analysis. Moreover, the incidence of adverse events and major adverse events did not differ between groups, with both analyses showing non‐significant heterogeneity. All in all, no sign of harm associated with the use of FXI inhibitors was raised by this meta‐analysis.
The results of FXI inhibitors compared to LMWH in patients undergoing total knee replacement challenge the paradigm of anticoagulant therapy. Higher efficacy in the reduction of thrombotic events is typically obtained at the expense of increased risk of bleeding. This paradigm has even been compared to navigating between Scylla and Charybdis from Greek mythology, a metaphor for thrombotic and hemorrhagic risks using anticoagulant therapy. 17 This model results from conventional anticoagulant therapy that blocks the common pathway represented by factors X and II. All anticoagulants available in clinical practice, administered orally or parenterally, act in the common pathway of the coagulation cascade. 18 This pharmacodynamic effect ensures the inhibition of fibrin generation, essential for the formation of venous, arterial, or intracardiac pathological thrombus, but blocks hemostasis in cases of vascular injury associated with hemorrhage. The initiation of hemostasis is mainly driven by the extrinsic pathway with the exposure of subendothelial TF and activation of circulating factor VII that triggers the common pathway (Figure 1). Thrombin (factor IIa) can activate the intrinsic pathway through FXI to amplify the common pathway in cases of insufficient hemostasis. 7 This role of the intrinsic pathway in hemostasis is described by some authors as minor, but of paramount importance in joints and skeletal muscle that express low levels of TF. 7 , 19 On the other hand, animal studies of gene‐specific knockout of coagulation factors denote that the intrinsic pathway plays a major role in thrombosis. 20 Mice deficient for factor XII, the initiating link of the intrinsic pathway, and FXI demonstrated reduced thrombus formation in thrombosis models. Epidemiological evidence also supports the crucial role of the intrinsic pathway in thrombosis. Indeed, patients with congenital FXI deficiency have a reduced risk of VTE, and elevated plasma FXI levels are associated with an increased risk of VTE. 20 In this context, inhibition of the intrinsic pathway through reduced FXI activity can decrease the risk of thrombosis without compromising hemostasis through maintenance of the extrinsic pathway.
Our results highlight the importance of the intrinsic pathway in thrombus stabilization and growth, predicting important research in other thrombosis fields such as treatment and secondary prevention of VTE, stroke prevention in atrial fibrillation, intracardiac thrombus, atherothrombosis, and possibly in several unmet needs of thromboprophylaxis like mechanical heart valves, ventricular assist devices, and end‐stage renal disease.
4.1. Limitations
Our study has some limitations that should be acknowledged. First, the small number of published trials regarding this subject is a limitation. All included trials were open label concerning assignment to drug treatment. Nevertheless, to minimize bias, outcomes were adjudicated by a blinded core committee in every trial. Also, no significant differences between modified intention‐to‐treat and per‐protocol analysis were found in any of the included studies.
The strength of our conclusion regarding safety outcomes is limited by the low incidence of clinically relevant bleeding events, especially major bleeding ones. Another limitation is the fact that this meta‐analysis includes different types of agents, with distinct mechanisms, administration routes, dosages, and half‐lives. Although the end pathophysiologic result seems to be roughly the same, these findings may not be generalizable. This may explain some heterogeneity between studies, which was addressed by the use of the random effects method. Also, due to the low number of included studies, heterogeneity analysis should be interpreted with caution. 14 In fact, the prediction intervals for the primary efficacy outcome and the composite of major and CRNM bleeding events raise the possibility of a lower treatment effect in future phase III studies. Furthermore, because these were phase II studies, the most effective and safe dose for each drug is still under evaluation. The small number of studies also precluded the possibility to assess publication bias.
5. CONCLUSION
Factor XI inhibitors reduce the risk of VTE among patients submitted to major orthopedic surgery while also reducing the risk of bleeding events compared to LMWH.
AUTHOR CONTRIBUTIONS
Conception and design (JP, JF, RR, MM), data collection (JP, JF), data analysis (JP, JF), drafting (JP, JF, RR, MM), revising (JP, JF, RR, MM). All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
Dr. Ferreira reports consultancy fees for Astra Zeneca and Boehringer‐Ingelheim. None of the other authors declare any conflicts of interest.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
Special thanks to Ricardo Verdugo and Laura Thomas for drawing an enlightening illustration.
Presume J, Ferreira J, Ribeiras R, Mendes M. Achieving higher efficacy without compromising safety with factor XI inhibitors versus low molecular weight heparin for the prevention of venous thromboembolism in major orthopedic surgery—Systematic review and meta‐analysis. J Thromb Haemost. 2022;20:2930‐2938. doi: 10.1111/jth.15890
Manuscript handled by: Sigrid Braekkan
Final decision: Sigrid Braekkan, 19 September 2022
REFERENCES
- 1. Kakkar A, Weitz JI, Büller HR, Connors JM. Factor XI inhibition: the holy grail of of thrombosis: six decades of progress. Eur Med J Rev. 2021;6(4):12‐20. [Google Scholar]
- 2. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE‐2): a randomised double‐blind trial. Lancet [Internet]. 2010;375(9717):807‐815. doi: 10.1016/S0140-6736(09)62125-5 [DOI] [PubMed] [Google Scholar]
- 3. Lassen MR, Raskob GE, Gallus AS, Pineo GF, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594‐604. [DOI] [PubMed] [Google Scholar]
- 4. Falck‐Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 SUPPL):e278S‐e325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson DR, Morgano GP, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3(23):3898‐3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fuji T, Fujita S, Tachibana S, Kawai Y. A dose‐ranging study evaluating the oral factor Xa inhibitor edoxaban for the prevention of venous thromboembolism in patients undergoing total knee arthroplasty. J Thromb Haemost. 2010;8(11):2458‐2468. [DOI] [PubMed] [Google Scholar]
- 7. Hsu C, Hutt E, Gailani D, Weitz JI. Factor XI inhibition to uncouple. JACC. 2021;78(6):625‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fredenburgh JC, Weitz JI. Factor XI as a target for new anticoagulants. Hamostaseologie. 2021;41:104‐110. [DOI] [PubMed] [Google Scholar]
- 9. Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weitz JI, Bauersachs R, Becker B, et al. Effect of Osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty the FOXTROT randomized clinical trial. JAMA. 2020;323(2):130‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verhamme P, Yi BA, Segers A, et al. Abelacimab for prevention of venous thromboembolism. N Engl J Med. 2021;385:609‐617. [DOI] [PubMed] [Google Scholar]
- 12. Weitz JI, Strony J, Ageno W, et al. Milvexian for the prevention of venous thromboembolism. N Engl J Med. 2021;385:2161‐2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ [Internet]. 2009;339(7716):332‐336. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). [Internet]. 6.0. 2019. www.training.cochrane.org/handbook.Title [DOI] [PMC free article] [PubMed]
- 15. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty Michael. N Engl J Med. 2008;358:2776‐2786. [DOI] [PubMed] [Google Scholar]
- 16. Bauer KA, Eriksson BI, Lassen MR, Turpie AGG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip‐fracture surgery. N Engl J Med. 2001;345(18):1298‐1304. [DOI] [PubMed] [Google Scholar]
- 17. Rosendaal FR. The Scylla and Charybdis of oral anticoagulant treatment. N Engl J Med [Internet]. 1996;335(8):587‐589. doi: 10.1056/NEJM199608223350810 [DOI] [PubMed] [Google Scholar]
- 18. Weitz JI, Harenberg J. New developments in anticoagulants: past, present and future. Thromb Haemost. 2017;117(7):1283‐1288. [DOI] [PubMed] [Google Scholar]
- 19. Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24(6):1015‐1022. [DOI] [PubMed] [Google Scholar]
- 20. Grover SP, Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(3):331‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


