Abstract
Aim
The sodium–glucose cotransporter 2 (SGLT2) inhibitor empagliflozin improved clinical outcomes in patients hospitalized for acute heart failure. In patients with chronic heart failure, SGLT2 inhibitors cause an early decline in estimated glomerular filtration rate (eGFR) followed by a slower eGFR decline over time than placebo. However, the effects of SGLT2 inhibitors on renal function during a hospital admission for acute heart failure remain largely unknown.
Methods and results
Between 1 and 5 days after a hospitalization for acute heart failure, 530 patients with an eGFR >20 ml/min/1.73 m2 were randomized to 10 mg of empagliflozin or placebo and treated for 90 days. Renal function and electrolytes were measured at baseline, and after 15, 30 and 90 days. We evaluated the effect of empagliflozin on eGFR over time and the impact of baseline eGFR on the primary hierarchical outcome of death, worsening heart failure events and quality of life. Mean baseline eGFR was 52.4 ml/min/1.73 m2 in the empagliflozin group and 55.7 ml/min/1.73 m2 in the placebo group. Empagliflozin caused an initial decline in eGFR (−2 ml/min/1.73 m2 at day 15 compared to placebo). At day 90, eGFR was similar between empagliflozin and placebo. Investigator‐reported acute renal failure occurred in 7.7% of empagliflozin versus 12.1% of placebo patients. The overall clinical benefit (hierarchical composite of all‐cause death, heart failure events and quality of life) of empagliflozin was unaffected by baseline eGFR.
Conclusion
In patients hospitalized for acute heart failure, empagliflozin caused an early modest decline in renal function which was no longer evident after 90 days. Acute renal events were similar in both groups. The clinical benefit of empagliflozin was consistent regardless of baseline renal function.
Keywords: Acute heart failure, SGLT2 inhibitor, Renal function, Chronic kidney disease
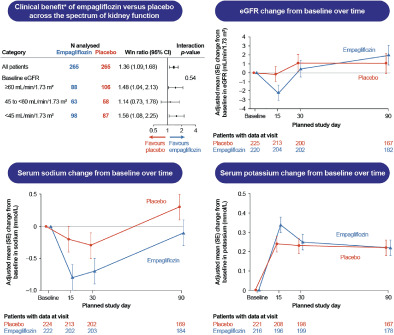
In patients hospitalized for acute heart failure (HF) in the EMPULSE trial, empagliflozin caused an early, modest decline in estimated glomerular filtration rate (eGFR) which was no longer evident after 90 days. Empagliflozin did not influence sodium, and potassium was consistent regardless of baseline renal function. *The hierarchy and components of the primary outcome measured by the win ratio were: (1) time to all‐cause deaths; (2) number of HF events; (3) time to first HF event; (4) ≥5 point difference in change from baseline in Kansas City Cardiomyopathy Questionnaire total symptom score after 90 days of treatment. CI, confidence interval; SE, standard error.
Introduction
Sodium–glucose cotransporter 2 (SGLT2) inhibitors improve clinical outcomes in patients with chronic heart failure, irrespective of left ventricular ejection fraction. 1 , 2 , 3 We recently demonstrated that initiation of the SGLT2 inhibitor empagliflozin in patients who were hospitalized for acute heart failure resulted in significant clinical benefit in the 90 days after starting treatment. 4
Randomized clinical trials with SGLT2 inhibitors in patients with diabetes, chronic kidney disease and chronic heart failure all demonstrate similar effects on renal function. 1 , 2 , 3 , 5 , 6 , 7 During the first weeks of administration, SGLT2 inhibitors cause an initial decline in estimated glomerular filtration rate (eGFR) of 2–4 ml/min/1.73 m2. This is followed by a slower decline in eGFR over time than placebo‐treated patients, eventually attenuating a decline in renal function. In addition, acute renal events occur less frequently in patients on SGLT2 inhibitors than those on placebo. 1 , 2 , 3 , 5 , 6 , 7
In patients with acute heart failure, worsening renal function frequently occurs. 8 Worsening renal function may occur during effective decongestion with loop diuretics which is not associated with worse outcomes. 9 In contrast, worsening renal function due to haemodynamic deterioration is associated with an increased risk of worsening heart failure, heart failure readmissions and (cardiovascular) death.
Since changes in renal function are greater and occur more frequently in acute heart failure, drugs that influence renal function might have a distinct impact on changes in eGFR during a hospitalization for heart failure. Further, changes in eGFR may influence initiation and continuation of guideline‐directed medical therapies. However, the renal effects of SGLT2 inhibitors during an acute heart failure hospital admission have not been well established. We therefore aimed to investigate the effects of empagliflozin on renal function and renal events during hospital admission and early after discharge. Secondly, we aimed to explore the effects of empagliflozin on mortality, heart failure events and quality of life across the eGFR spectrum.
Methods
The design, baseline characteristics, and main results of EMPULSE (NCT04157751) have been published elsewhere. 10 In brief, EMPULSE was a randomized double‐blind, placebo‐controlled trial, including 530 patients with a primary diagnosis of acute de novo or decompensated chronic heart failure regardless of left ventricular ejection fraction. Patients were randomly assigned to receive empagliflozin 10 mg once daily or placebo. Patients were randomized in‐hospital when clinically stable (median time from hospital admission to randomization, 3 days) and were treated for up to 90 days. The primary outcome of the trial was clinical benefit, defined as a hierarchical composite of (i) time to death from any cause, (ii) number of heart failure events, (iii) time to first heart failure event, or (iv) a 5 point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ‐TSS) at 90 days. Clinical benefit was assessed using a win ratio. Deaths and heart failure events were not adjudicated. All patients provided written informed consent and the study protocol was approved by ethics committees and review boards at participating sites.
Laboratory measurements and definitions
Core laboratory serum creatinine, sodium, and potassium were measured at baseline, and after 15, 30 and 90 days of study medication. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation: eGFRcr = 142 × min (Scr/κ, 1) α × max (Scr/κ, 1) − 1.200 × 0.9938 Age × 1.012 [if female] where: Scr = serum creatinine in mg/dl, κ = 0.7 (females) or 0.9 (males), α = −0.241 (female) or −0.302 (male), min (Scr/κ, 1) is the minimum of Scr/κ or 1.0, max (Scr/κ, 1) is the maximum of Scr/κ or 1.0, Age (years).
Statistical analysis
In this post hoc analysis, patients were divided into three subgroups, based on baseline eGFR value: (i) <45, (ii) ≥45 and <60, (iii) ≥60 ml/min/1.73 m2. Baseline characteristics were summarized as means and standard deviations (SD), medians, and interquartile ranges, or percentages. Ordinal regression likelihood ratio tests were used to compare trends across eGFR subgroups at baseline.
To evaluate the effects of empagliflozin versus placebo on the primary hierarchical composite outcome of clinical benefit across the baseline eGFR subgroups, we conducted a post hoc analysis comparing patients randomized to empagliflozin with those randomized to placebo, within each baseline eGFR subgroup. Each comparison of two patients followed the hierarchy of comparing (i) time to death, (ii) number of heart failure events, (iii) time to heart failure event, or (iv) a 5 point or greater difference in change from baseline in KCCQ‐TSS at day 90, until conclusion of a win, loss or tie, as previously described. We calculated the win ratio as the number of wins in the empagliflozin group divided by the number of losses, within each stratum (subgroups of baseline eGFR). No further matching of patients at baseline was performed. The treatment by baseline eGFR subgroup interaction was tested using Cochran's Q statistic (inverse variance‐weighting approach). A multiple imputation approach, according to whether patients were on‐treatment or off‐treatment, was used to impute missing data for the KCCQ‐TSS, as previously described. 4
Time to cardiovascular death or heart failure event was analysed using the Cox proportional hazards model with terms for heart failure status, treatment group, baseline eGFR and treatment group by baseline eGFR interaction.
We analysed the differences between treatment groups in mean eGFR, sodium and potassium at day 15, 30 and 90 separately, using mixed effects models for repeated measures adjusted for heart failure status and baseline value by visit interaction.
The proportion of patients experiencing renal and urinary adverse events were compared between treatment groups using chi‐squared tests. Further comparisons were made by fitting logistic regression models with terms for treatment group, baseline eGFR and treatment group by baseline eGFR interaction.
All analyses were performed with SAS software, version 9.3 or higher (SAS Institute, Cary, NC, USA). A p‐value of 0.05 was considered statistically significant, and was not adjusted for multiple comparisons.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the study participants according to eGFR <45, 45 to <60, ≥60 ml/min/1.73 m2. Overall, mean ± SD age of the patients was 68.4 ± 13.3 years, 169 (33.8%) were female and mean ± SD baseline eGFR was 54.1 ± 20.3 ml/min/1.73 m2 (52.4 ± 19.8 ml/min/1.73 m2 in the empagliflozin group and 55.7 ± 20.7 ml/min/1.73 m2 in the placebo group). Patients with lower eGFR were older, more often female, with higher plasma N‐terminal‐prohormone brain natriuretic peptide concentrations, more frequent comorbidities, a higher left ventricular ejection fraction, and lower use of inhibitors of the renin–angiotensin–aldosterone system.
Table 1.
Baseline characteristics according to baseline estimated glomerular filtration rate of patients who were hospitalized for acute heart failure
| Baseline eGFR (ml/min/1.73 m2) | |||||
|---|---|---|---|---|---|
| <45 (n = 185) | 45 to <60 (n = 121) | ≥60 (n = 194) | Total (n = 500) | p‐value for trend | |
| Age, years, mean ± SD | 73.8 ± 10.1 | 70.0 ± 11.8 | 62.2 ± 14.4 | 68.4 ± 13.3 | <0.0001 |
| Sex, n (%) | |||||
| Men | 114 (61.6) | 74 (61.2) | 143 (73.7) | 331 (66.2) | 0.0125 |
| Women | 71 (38.4) | 47 (38.8) | 51 (26.3) | 169 (33.8) | |
| Race or ethnic group, n (%) | |||||
| White | 149 (80.5) | 100 (82.6) | 140 (72.2) | 389 (77.8) | 0.1061 |
| Black | 14 (7.6) | 8 (6.6) | 27 (13.9) | 49 (9.8) | |
| Asian | 20 (10.8) | 11 (9.1) | 26 (13.4) | 57 (11.4) | |
| Other/mixed race | 2 (1.1) | 1 (0.8) | 1 (0.5) | 4 (0.8) | |
| Missing | 0 | 1 (0.8) | 0 | 1 (0.2) | |
| Geographic region, n (%) | |||||
| Europe | 121 (65.4) | 86 (71.1) | 116 (59.8) | 323 (64.6) | 0.4930 |
| North America | 44 (23.8) | 24 (19.8) | 53 (27.3) | 121 (24.2) | |
| Asia | 20 (10.8) | 11 (9.1) | 25 (12.9) | 56 (11.2) | |
| NYHA class, n (%) | |||||
| I | 6 (3.2) | 3 (2.5) | 5 (2.6) | 14 (2.8) | 0.2738 |
| II | 52 (28.1) | 52 (43.0) | 72 (37.1) | 176 (35.2) | |
| III | 109 (58.9) | 56 (46.3) | 96 (49.5) | 261 (52.2) | |
| IV | 18 (9.7) | 10 (8.3) | 21 (10.8) | 49 (9.8) | |
| KCCQ‐TSS, mean ± SD | 39.35 ± 23.34 | 42.71 ± 24.20 | 40.92 ± 24.30 | 40.78 ± 23.91 | 0.5383 |
| NT‐proBNP, pg/ml, median [IQR] | 4666 [2436–9362] (n = 184) | 3287 [1817–6223] (n = 120) | 2378 [1288–3832] (n = 190) | 3240 [1725–6104] (n = 494) | <0.0001 a |
| Blood pressure, mmHg, mean ± SD | |||||
| Systolic | 124.5 ± 18.2 | 124.7 ± 18.1 | 122.7 ± 17.7 | 123.8 ± 18.0 | 0.3312 |
| Diastolic | 70.4 ± 11.9 | 74.5 ± 12.8 | 74.9 ± 11.8 | 73.2 ± 12.2 | 0.0004 |
| Body mass index, kg/m2, mean ± SD | 29.45 ± 6.30 (n = 183) | 29.80 ± 8.22 (n = 120) | 30.64 ± 9.17 (n = 194) | 30.00 ± 7.98 (n = 497) | 0.1488 |
| Left ventricular ejection fraction, n (%) | |||||
| ≤40% | 106 (57.3) | 78 (64.5) | 152 (78.4) | 336 (67.2) | <0.0001 |
| >40% | 77 (41.6) | 40 (33.1) | 41 (21.1) | 158 (31.6) | |
| Missing | 2 (1.1) | 3 (2.5) | 1 (0.5) | 6 (1.2) | |
| Haemoglobin, g/dl, mean ± SD | 12.41 ± 1.96 (n = 172) | 13.63 ± 1.94 (n = 115) | 13.89 ± 1.98 (n = 187) | 13.29 ± 2.07 (n = 474) | <0.0001 |
| Medical history, n (%) | |||||
| Diabetes | 103 (55.7) | 49 (40.5) | 73 (37.6) | 225 (45.0) | 0.0004 |
| Hypertension | 159 (85.9) | 102 (84.3) | 138 (71.1) | 399 (79.8) | 0.0002 |
| Myocardial infarction | 61 (33.0) | 29 (24.0) | 31 (16.0) | 121 (24.2) | 0.0001 |
| Atrial fibrillation | 114 (61.6) | 58 (47.9) | 71 (36.6) | 243 (48.6) | <0.0001 |
| CABG or PCI | 75 (40.5) | 32 (26.4) | 40 (20.6) | 147 (29.4) | <0.0001 |
| Valvular heart disease | 124 (67.0) | 79 (65.3) | 115 (59.3) | 318 (63.6) | 0.1143 |
| Heart failure status, n (%) | |||||
| Decompensated CHF | 144 (77.8) | 80 (66.1) | 111 (57.2) | 335 (67.0) | <0.0001 |
| Acute de novo | 41 (22.2) | 41 (33.9) | 83 (42.8) | 165 (33.0) | |
| Medication, n (%) | |||||
| ACE inhibitor and/or ARB and/or ARNi | 110 (59.5) | 91 (75.2) | 150 (77.3) | 351 (70.2) | 0.0001 |
| ACE inhibitor | 49 (26.5) | 39 (32.2) | 77 (39.7) | 165 (33.0) | 0.0061 |
| ARB | 44 (23.8) | 34 (28.1) | 32 (16.5) | 110 (22.0) | 0.0862 |
| ARNi | 18 (9.7) | 18 (14.9) | 43 (22.2) | 79 (15.8) | 0.0008 |
| MRA | 75 (40.5) | 67 (55.4) | 120 (61.9) | 262 (52.4) | <0.0001 |
| Beta‐blocker | 146 (78.9) | 98 (81.0) | 153 (78.9) | 397 (79.4) | 0.9823 |
| Loop diuretic | 159 (85.9) | 101 (83.5) | 154 (79.4) | 414 (82.8) | 0.0885 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; CABG, coronary artery bypass graft; CHF, chronic heart failure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal‐prohormone brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SD, standard deviation.
Note: 30 patients were excluded due to missing baseline eGFR results.
Based on log‐transformed results.
Effects of empagliflozin on the trajectories of renal function
The change in mean eGFR over time in the empagliflozin and placebo group is depicted in Figure 1 . Empagliflozin caused an initial decline in eGFR, reaching a difference of 2 ml/min/1.73 m2 at day 15 compared with placebo (p = 0.0822). At day 90, eGFR was similar between empagliflozin and placebo groups (mean difference 0.9 ml/min/1.73 m2; p = 0.5714). The absolute eGFR values for both treatment groups at each time point are presented in online supplementary Figure S1 .
Figure 1.
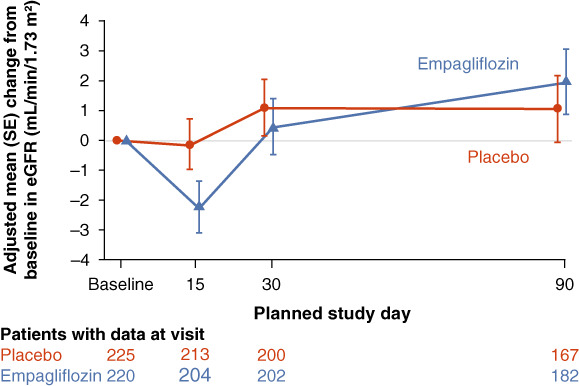
Estimated glomerular filtration rate (eGFR) change from baseline over time. Note: 30 patients were excluded due to missing baseline eGFR results. SE, standard error.
Effects of empagliflozin on trajectories of serum sodium and potassium
Baseline mean ± SD serum sodium concentration was 140.5 ± 2.7 mmol/L in the placebo group and 140.0 ± 3.3 mmol/L in the empagliflozin group. Baseline mean ± SD serum potassium concentration was 4.17 ± 0.43 mmol/L in the placebo group and 4.16 ± 0.45 mmol/L in the empagliflozin group. Figure 2 shows the changes in sodium and potassium. Differences between baseline sodium at day 15 (p = 0.0503), day 30 (p = 0.1534) and day 90 (p = 0.1314) were small and not statistically significant between groups. Similarly, differences between baseline potassium at day 15 (p = 0.0723), day 30 (p = 0.7347) and day 90 (p = 0.9618) were also not significantly different between the placebo and empagliflozin groups.
Figure 2.
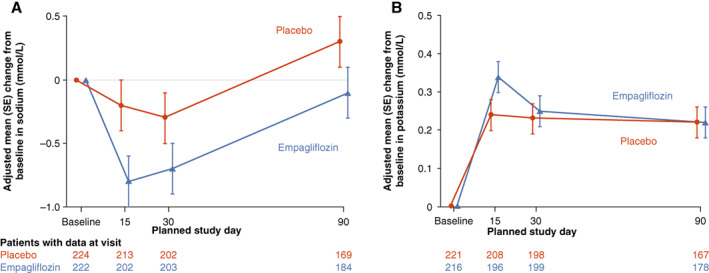
Changes in serum sodium and potassium over time in the empagliflozin versus placebo group. SE, standard error.
Primary endpoint according to baseline estimated glomerular filtration rate
There was no evidence of effect modification of baseline eGFR on the primary outcome of EMPULSE. The win ratio (and 95% confidence interval) for the patients with an eGFR <45, 45 to <60 and ≥ 60 ml/min/1.73 m2 was 1.56 (1.08–2.25), 1.14 (0.73–1.78) and 1.48 (1.04–2.13), respectively (p‐value for interaction = 0.5400) (Figure 3 ).
Figure 3.
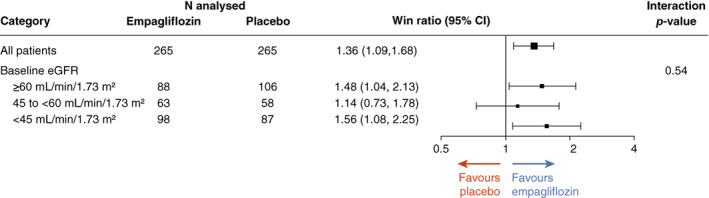
Clinical benefit of empagliflozin versus placebo across the spectrum of kidney function. Note: 30 patients were excluded due to missing baseline estimated glomerular filtration rate (eGFR) results. CI, confidence interval.
Similarly, baseline eGFR did not modify the effect of empagliflozin on the composite secondary outcome of cardiovascular death or a heart failure event: hazard ratio (95% confidence interval) 0.54 (0.28–1.04) for eGFR <45, 0.91 (0.35–2.35) for eGFR 45 to <60, and 0.82 (0.35–1.91) for eGFR ≥60 ml/min/1.73 m2 (p‐value for trend = 0.4079; Table 2 ).
Table 2.
Cox regression for time to first occurrence of cardiovascular death or heart failure event until end of trial visit by baseline estimated glomerular filtration rate (<45, 45 to <60, ≥60 ml/min/1.73 m2) a
| eGFR subgroup (ml/min/1.73 m2) | Number of patients with event | Hazard ratio (95% confidence interval) | p‐value for trend | |
|---|---|---|---|---|
| Empagliflozin | Placebo | |||
| ≥60 | 9 (10.2%) | 13 (12.3%) | 0.82 (0.35–1.91) | 0.4079 |
| 45 to <60 | 8 (12.7%) | 9 (15.5%) | 0.91 (0.35–2.35) | |
| <45 | 15 (15.3%) | 23 (26.4%) | 0.54 (0.28–1.04) | |
CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate.
Note: 30 patients were excluded due to missing baseline eGFR results.
Based on a Cox regression model with terms for heart failure status (p = 0.0251), treatment (p = 0.2113), baseline eGFR (CKD‐EPI) (p = 0.1605) and treatment by baseline eGFR (CKD‐EPI) interaction (p = 0.6070).
Renal adverse events
Renal adverse events by treatment group and baseline eGFR (<45, 45 to <60, ≥60 ml/min/1.73 m2) are presented in Table 3 . Renal disorders occurred more frequently in patients with a baseline eGFR <45 ml/min/1.73 m2 compared to patients with a baseline eGFR 45 to <60 and ≥60 ml/min/1.73 m2, but there was no significant treatment group by baseline eGFR interaction (p‐value for trend = 0.6620). Overall renal disorders occurred in 17.0% of the placebo group compared to 11.2% in the empagliflozin group (p = 0.0528). Investigators more often reported acute kidney injury events in patients with a baseline eGFR <45 ml/min/1.73 m2 compared to patients with a baseline eGFR 45 to <60 and ≥60 ml/min/1.73 m2, but there were insufficient events to fit the appropriate model. Investigator‐reported acute kidney injury occurred in 7.2% of patients in the placebo group compared to 3.8% in the empagliflozin group (p = 0.0935) during the 90‐day treatment period.
Table 3.
Renal and urinary adverse events by treatment group and baseline estimated glomerular filtration rate (<45, 45 to <60, ≥60 ml/min/1.73 m2)
| Baseline eGFR (ml/min/1.73 m2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 | 45 to <60 | ≥60 | ||||||||||
| Placebo | Empa | Placebo | Empa | Placebo | Empa | |||||||
| n (%) | Rate/100 pt‐yrs | n (%) | Rate/100 pt‐yrs | n (%) | Rate/100 pt‐yrs | n (%) | Rate/100 pt‐yrs | n (%) | Rate/100 pt‐yrs | n (%) | Rate/100 pt‐yrs | |
| Number of patients | 87 (100) | 98 (100) | 58 (100) | 62 (100) | 105 (100) | 87 (100) | ||||||
| Renal and urinary disorders | 24 (27.6) | 157.58 | 17 (17.3) | 86.99 | 9 (15.5) | 72.31 | 5 (8.1) | 36.69 | 9 (8.6) | 37.12 | 6 (6.9) | 29.19 |
| Acute kidney injury | 9 (10.3) | 50.97 | 6 (6.1) | 28.79 | 3 (5.2) | 22.87 | 1 (1.6) | 7.16 | 5 (4.8) | 20.05 | 2 (2.3) | 9.35 |
| Renal impairment | 6 (6.9) | 34.32 | 6 (6.1) | 29.44 | 3 (5.2) | 22.39 | 2 (3.2) | 14.37 | 2 (1.9) | 7.95 | 1 (1.1) | 4.71 |
| Chronic kidney disease | 4 (4.6) | 22.71 | 1 (1.0) | 4.69 | ||||||||
| Urinary retention | 3 (3.4) | 16.50 | 1 (1.0) | 4.69 | 2 (1.9) | 7.96 | 0 | 0 | ||||
| Dysuria | 1 (1.1) | 5.48 | 1 (1.0) | 4.69 | 1 (1.7) | 7.59 | 1 (1.6) | 7.10 | 0 | 0 | 1 (1.1) | 4.66 |
| Renal failure | 2 (2.3) | 11.11 | 1 (1.0) | 4.71 | 0 | 0 | 1 (1.6) | 7.07 | ||||
| Bladder spasm | 1 (1.1) | 5.48 | 0 | 0 | ||||||||
| Haematuria | 1 (1.1) | 5.50 | 0 | 0 | 0 | 0 | 1 (1.6) | 7.10 | 2 (1.9) | 7.94 | 0 | 0 |
| Hydronephrosis | 1 (1.1) | 5.54 | 0 | 0 | ||||||||
| Leucocyturia | 1 (1.1) | 5.53 | 0 | 0 | ||||||||
| Nephrotic syndrome | 1 (1.1) | 5.53 | 0 | 0 | ||||||||
| Nocturia | 1 (1.1) | 5.53 | 0 | 0 | ||||||||
| Urinary tract obstruction | 1 (1.1) | 5.50 | 0 | 0 | ||||||||
| Proteinuria | 0 | 0 | 1 (1.0) | 4.73 | 1 (1.0) | 3.97 | 0 | 0 | ||||
| Renal artery stenosis | 0 | 0 | 1 (1.0) | 4.72 | ||||||||
| Nephropathy | 1 (1.7) | 7.51 | 0 | 0 | ||||||||
| Pollakiuria | 1 (1.7) | 7.58 | 0 | 0 | ||||||||
| Haemorrhage urinary tract | 0 | 0 | 1 (1.6) | 7.18 | ||||||||
| Glycosuria | 0 | 0 | 1 (1.1) | 4.70 | ||||||||
| Urinary hesitation | 0 | 0 | 1 (1.1) | 4.71 | ||||||||
| Urinary incontinence | 0 | 0 | 1 (1.1) | 4.70 | ||||||||
eGFR, estimated glomerular filtration rate; Empa, empagliflozin; pt‐yrs, patient‐years.
Note: 30 patients were excluded due to missing baseline eGFR results.
Discussion
In EMPULSE, patients hospitalized for acute heart failure with an eGFR as low as 20 ml/min/1.73 m2 were randomized to empagliflozin or placebo and treated for 90 days. In this post hoc analysis we found the clinical benefit seen in the overall primary comparison 4 was consistent regardless of baseline eGFR. 4 Patients treated with empagliflozin had a modest early decline in eGFR, but the difference with placebo was no longer apparent after 90 days. Renal adverse events were comparable between patients treated with empagliflozin or placebo. Finally, empagliflozin did not influence serum sodium and potassium concentrations throughout 90 days of treatment (Graphical Abstract).
The finding that the beneficial clinical effects of empagliflozin remained present in patients with all severities of chronic kidney disease is important since the effects of SGLT2 inhibitors are primarily related to sodium–glucose exchange in the proximal tubule of the kidney. Its tubular effects might theoretically be influenced by lower glomerular filtration rate, which did not appear in the present analysis. This is particularly relevant in acute heart failure, where chronic kidney disease and worsening renal function are very common.
An early decline in eGFR of 2–4 ml/min/1.73 m2 in patients treated with SGLT2 inhibitors is a very consistent and common finding, which has been previously demonstrated in patients with diabetes and high cardiovascular risk, chronic kidney disease and chronic heart failure. 1 , 2 , 3 , 5 , 6 , 7 The present paper extends these findings and those from EMPA‐RESPONSE‐AHF, where patients with acute heart failure were randomized to empagliflozin or placebo within 24 h of admission. 11 In that pilot study, the effect on eGFR with SGLT2 inhibitors was seen as early as 24 h after start and was larger (8 ml/min/1.73 m2) than in the present analysis. Consistent with findings from the current analysis from EMPULSE, the difference between empagliflozin and placebo was attenuated already 30 days after admission.
The early decline observed with SGLT2 inhibitors is most likely caused by the so‐called tubuloglomerular feedback mechanism where an increase in tubular sodium and chloride excretion is sensed by the macula densa, leading to afferent vasoconstriction, resulting in a reduction in renal blood flow and thereby glomerular filtration. 5 , 6 Similar to loop diuretics, this early decline in renal function together with adequate diuresis is likely related to a lower risk of worsening heart failure, (cardiovascular) death and heart failure rehospitalizations. Previous studies in chronic heart failure showed that the early decline in eGFR after initiation of SGLT2 inhibitors is followed by a slower rate of long‐term decline in renal function, indicating long‐term renal protective effects. These effects might be related to improved haemodynamics, leading to better renal perfusion. There is however one remarkable difference between the present study in patients with acute heart failure and studies in patients with diabetes, chronic kidney disease and chronic heart failure. 1 , 2 , 3 , 5 , 6 , 7 In the latter groups, the change in eGFR typically cross at 52–76 weeks of randomized treatment. At this point, the slower rate of decline in renal function has compensated the early decline in renal function. In the present study, the lines already cross at 90 days, which is much earlier than in the chronic studies. This might be related to greater changes in renal function and haemodynamics in patients with acute heart failure. However, it is remarkable that the initial decline in renal function was only 2 ml/min/1.73 m2, compared to 2.5–4.5 ml/min/1.73 m2 in the chronic patients.
Finally, renal adverse events were comparable between patients treated with empagliflozin and placebo. In patients with diabetes, chronic kidney disease and chronic heart failure, renal adverse events were less often observed in patients treated with SGLT2 inhibitors. 1 , 2 , 3 , 5 , 6 , 7 For example, in patients with chronic kidney disease, dapagliflozin reduced the primary outcome of a composite of a sustained decline in the eGFR of at least 50%, end‐stage kidney disease, or death from renal or cardiovascular causes by 44%. 7 Similarly, in patients with diabetes, empagliflozin reduced the incidence of doubling of serum creatinine by 44%. 5 And in patients with chronic heart failure, empagliflozin reduced the composite renal outcome of chronic dialysis or renal transplantation or a profound, sustained reduction in the eGFR by 50%. 2
This study has several limitations. First, we did not collect urine samples to determine urinary albumin excretion. Second, the number of patients in the present study (n = 530) was lower than the randomized clinical trials with SGLT2 inhibitors in patients with diabetes, chronic kidney disease and chronic heart failure. Nonetheless, the current findings in patients with acute heart failure were very consistent with these larger studies in chronic patients.
In conclusion, the present study shows that the beneficial clinical effects of empagliflozin in patients who are hospitalized for acute heart failure were consistent regardless of baseline eGFR. Empagliflozin caused an early modest decline in renal function, but already after 90 days, the difference in renal function had disappeared. Overall renal adverse events, and investigator‐reported acute kidney injury were similar between placebo and empagliflozin treated patients.
Funding
The trial was funded by the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance.
Supporting information
Appendix S1. Supporting information.
Acknowledgments
Figure support was provided by Michael Trim at 7.4 Limited, Bollington, Cheshire, UK, and supported financially by BI. General administrative support in relation to development of the final submission package, supported financially by BI, was provided by Paul Lidbury, Scientific Solutions, Horsham, West Sussex, UK.
Data availability statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer‐reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Conflict of interest: A.A.V. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. K.D. has received consultancy fees from Abbott. J.R.T. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics. C.E.A. has received research support from and/or has been a consultant for Abbott, Boehringer Ingelheim, Medtronic, Novartis, ResMed, Thermo Fisher, Vifor and German Federal Ministry of Education and Research. S.P.C. is a consultant for Aiphia, Siemens, Bristol Myers Squibb, Boehringer Ingelheim and Vixiar and receives research support from the NIH, PCORI, AstraZeneca and Beckman Coulter. M.K. has received research grants from AstraZeneca and Boehringer Ingelheim, and has served as a consultant for Alnylam, AstraZeneca, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Esperion Therapeutics, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Sanofi and Vifor. J.P.F. is a consultant for Boehringer Ingelheim and receives research support from AstraZeneca. M.E.N. has received speaking honoraria from Abbott, and is a consultant for Vifor, Roche and Amgen. J.T. is supported by the National University of Singapore Start‐up grant, the tier 1 grant from the ministry of education and the CS‐IRG New Investigator Grant from the National Medical Research Council; has received consulting or speaker fees from Daiichi‐Sankyo, Boehringer Ingelheim, Roche diagnostics and Us2.ai, owns patent US‐10702247‐B2 unrelated to the present work. J.P.B. is an employee of Elderbrook Solutions. P.P. reports personal fees from Boehringer Ingelheim, AstraZeneca, Servier, Bristol Myers Squibb, Amgen, Novartis, Merck, Pfizer, Berlin Chemie, and grants and personal fees from Vifor Pharma. M.B. and A.S. are employees of Boehringer Ingelheim. J.B. and M.A.P. have nothing to disclose.
References
- 1. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 2. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- 3. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al.; EMPEROR‐Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61. [DOI] [PubMed] [Google Scholar]
- 4. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al.; EMPA‐REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. [DOI] [PubMed] [Google Scholar]
- 6. Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704. [DOI] [PubMed] [Google Scholar]
- 7. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, et al.; DAPA‐CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. [DOI] [PubMed] [Google Scholar]
- 8. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, et al. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:584–603. [DOI] [PubMed] [Google Scholar]
- 9. Emmens JE, Ter Maaten JM, Matsue Y, Figarska SM, Sama IE, Cotter G, et al. Worsening renal function in acute heart failure in the context of diuretic response. Eur J Heart Fail. 2022;24:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tromp J, Ponikowski P, Salsali A, Angermann CE, Biegus J, Blatchford J, et al. Sodium‐glucose co‐transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial. Eur J Heart Fail. 2021;23:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, et al. Randomized, double‐blind, placebo‐controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA‐RESPONSE‐AHF). Eur J Heart Fail. 2020;22:713–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer‐reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Conflict of interest: A.A.V. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. K.D. has received consultancy fees from Abbott. J.R.T. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics. C.E.A. has received research support from and/or has been a consultant for Abbott, Boehringer Ingelheim, Medtronic, Novartis, ResMed, Thermo Fisher, Vifor and German Federal Ministry of Education and Research. S.P.C. is a consultant for Aiphia, Siemens, Bristol Myers Squibb, Boehringer Ingelheim and Vixiar and receives research support from the NIH, PCORI, AstraZeneca and Beckman Coulter. M.K. has received research grants from AstraZeneca and Boehringer Ingelheim, and has served as a consultant for Alnylam, AstraZeneca, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Esperion Therapeutics, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Sanofi and Vifor. J.P.F. is a consultant for Boehringer Ingelheim and receives research support from AstraZeneca. M.E.N. has received speaking honoraria from Abbott, and is a consultant for Vifor, Roche and Amgen. J.T. is supported by the National University of Singapore Start‐up grant, the tier 1 grant from the ministry of education and the CS‐IRG New Investigator Grant from the National Medical Research Council; has received consulting or speaker fees from Daiichi‐Sankyo, Boehringer Ingelheim, Roche diagnostics and Us2.ai, owns patent US‐10702247‐B2 unrelated to the present work. J.P.B. is an employee of Elderbrook Solutions. P.P. reports personal fees from Boehringer Ingelheim, AstraZeneca, Servier, Bristol Myers Squibb, Amgen, Novartis, Merck, Pfizer, Berlin Chemie, and grants and personal fees from Vifor Pharma. M.B. and A.S. are employees of Boehringer Ingelheim. J.B. and M.A.P. have nothing to disclose.


