Abstract
There are increasing global data regarding the prevalence of food allergy and food‐induced anaphylaxis. However, knowledge in morbidity and mortality epidemiological data is still not optimal, and international comparable standards remain poorly accessed. This information could in turn support better clinical practice and possibly prevent future severe reactions and avoidable fatalities. The International Classification of Diseases (ICD) is the standard diagnostic tool used for epidemiology, health management, and clinical purposes supported by the World Health Organization (WHO). It is also used to determine health care payment and reimbursement of providers and health care services in hospitals. Thanks to the academic and technical efforts under the ALLERGY in ICD‐11 initiative the pioneer “Allergy and hypersensitivity conditions” section has been built under the “Disorders of the Immune System” chapter of the ICD‐11. The “Food hypersensitivity” (FH) subsection is classified under the “Complex allergic or hypersensitivity conditions” section and “Food‐induced anaphylaxis” is under the “Anaphylaxis” section. In order to inform the development of strategies to reduce preventable FHs/food allergies, the burden of disease in different healthcare settings and patient populations and their common etiologies need to be understood. Besides, greater specificity regarding clinical conditions and services delivered will provide payers, policy makers, and providers with better information to make major refinements to countries payment and reimbursement systems, including the design and implementation of pay‐for‐performance program.The new classification addressed to FHs will enable the collection of more accurate epidemiological data to support quality management of patients with FHs/food allergies, and better facilitate health care planning and decision‐making and public health measures to prevent and reduce their morbidity and mortality. The improved logic and standardized definitions through the ICD‐11 (and other WHO classifications) will also facilitate international comparisons of quality care and the sharing of best practice globally.
Keywords: anaphylaxis, epidemiology, food allergy, food hypersensitivity, international classification of diseases (ICD), prevention, public health
Abbreviations
- AAAAI
American Academy of Allergy Asthma and Immunology
- ACAAI
American College of Allergy Asthma and Immunology
- APAAACI
Asia Pacific Association of Allergy, Asthma and Clinical Immunology
- EAACI
European Academy of Allergy and Clinical Immunology
- EoE
eosinophilic esophagitis
- FH
food hypersensitivity
- ICD
International Classification of Diseases
- SLAAI
Latin American Society of Allergy, Asthma and Immunology
- WAO
World Allergy Organization
- WHO
World Health Organization
- WHO CC
WHO Collaborating Centre
Key Message.
There are increasing global data regarding the prevalence of food allergies (FA). However, knowledge in morbidity and mortality epidemiological data is still not optimal and international comparable standards remain poorly accessed. The new classification addressed to FA in the eleventh edition of the International Classification of Diseases will enable the collection of more accurate epidemiological data to support quality management of patients, and facilitate health care planning and decision‐making and public health measures to prevent and reduce their morbidity and mortality.
1. BACKGROUND
1.1. Food allergy epidemiological data is not optimal
A core focus of epidemiology is the systematic characterization of diseases trends and distribution in specific populations. 1 There are no doubts that epidemiological data provide insights that inform disease management and prevention, and tailor health policies and public health decision‐making.
Over the last decade, the interest and knowledge in the field of food hypersensitivity (FH) has improved dramatically. There are increasing global data regarding prevalence of food allergy and food‐induced anaphylaxis. However, food allergy data are often based on self‐reported data, tertiary centres' cohorts or larger population sensitization surveys 2 , 3 , 4 , 5 and incidence on food‐induced anaphylaxis is derived from hospital admissions, emergency attendance or diagnostic coding data. 6
FH represents growing health problem worldwide, affecting more than 10% of the general population. 7 It is estimated that food allergy is likely to affect approximately 1 in 10 adults, 4 and 1 in 12 children. 2 Epidemiological studies have indicated increasing prevalence of FH, in particular IgE‐mediated conditions, impacting in incremental morbidity 8 and mortality. 9 , 10 , 11
FHs are considered public health issues by the allergy community and efforts have been made in order to gather more accurate epidemiological data, understand patterns and move specific policies such as labelling provisions with most frequent allergens 12 and providing auto‐injectable adrenaline/epinephrine in schools. 12 However, knowledge in FHs morbidity and mortality epidemiological data is still not optimal and the international comparable standards remain poorly accessed. As an example, the Food and Agriculture Organization of the United Nations and the World Health Organization (WHO) established the Codex Alimentarium, in which guidelines, food standards, and codes are provided to ensure safety of food supply. 13 Currently, there are protein in eight foods/food groups (and derived products), which should be disclosed in food provisions: cereals containing gluten, crustaceans, eggs, fish, milk, peanuts, soybeans, and tree nuts. 13 Although this list covers 90% of all cases of food‐induced allergies, other food triggers can be implicated depending on cultural consumption habits and regional or country consumption pattern and as an example the European Union has added another five with celery, lupin, mollusks, mustard, and sesame seeds in addition to sulfite‐containing food. 14
Overdiagnosis of FHs has known negative impact on clinical practice, labels out patients, preventing the ingestion of required nutrients and leading to malnutrition when the avoided food is considered essential. It also increases the economic burden of FHs related to unnecessary investigation. On the other hand, underdiagnosis and undernotification of these conditions are recognized as key factors to the potential increased number of avoidable reactions, treatment/hospitalization, and risk of death. Misclassification based on personal history of FHs alone impact on inaccurate epidemiological data, may limit nutrition options and can lead to the use of more expensive alternatives, such as formulas and eventually nutritional deficiencies.
Trends in international FHs morbidity and mortality rates would be able to provide a useful parameter of the burden of these conditions and the impact of changes in management and prevention. Administrative databases, electronic health records, and disease registries contain a plethora of health data that can be used to ascertain these health outcomes in clinical practice. 9 , 10 These data are generally accessible and have been collected without interfering in the delivery of care.
To further update the allergy community regarding the WHO International Classification of Diseases (ICD)‐11 implementation and surveillance processes 15 taking FHs/food allergies as a model, we report the representation and use of the pioneer “Food Hypersensitivity” subsection of the ICD‐11.
2. FOOD HYPERSENSITIVITY IN THE INTERNATIONAL CLASSIFICATION OF DISEASES
2.1. Representation of food hypersensitivity in the ICD‐10
The ICD is the standard diagnostic tool used for epidemiology, health management and clinical purposes supported by the WHO. It is also used to determine health care payment and reimbursement of providers and health care services in hospitals. Overall, the WHO indicates that the ICD is currently responsible for allocating about 70% of the world health expenditures, meaning by USD 2.3 trillions in 2013 and USD 2.6 trillions in 2014 according to the US National Center for Health Statistics. 16 All European Member States use the ICD, which has been translated in 43 languages. The ICD‐10 was endorsed by the forty‐third World Health Assembly in May 1990 and came into use in WHO Member States since 1994. 15 , 16
Institutional databases worldwide increasingly use the ICD system to classify diagnoses, health services utilization and death data. 15 The misclassification of disorders in the ICD system contributes to a lack of ascertainment and recognition of their importance for healthcare planning and resource allocation. It also hampers clinical practice and prevention actions. This results in poor understanding of their natural history and lack of knowledge of their epidemiology.
Currently, most of the countries are operating with the ICD‐10 (or national modifications) version. In general, the ICD‐10 coding system 17 enables coders to document food adverse effects in two ways: (1) by documenting diagnosis that may be caused by a food using “disease manifestation codes” (e.g.: T78.0 anaphylactic shock due to food reaction); (2) by documenting the food that caused the toxic effect using “injury, poisoning and certain other consequences of external causes” codes (e.g.: T61 toxic effect of noxious substances eaten as seafood). However, due to the multiple ways in which these conditions may be coded, methodologies need to be developed to avoid double counting. Besides, the ICD‐10 has been shown to be unable to capture accurate morbidity 18 and mortality 19 , 20 data of allergic and hypersensitivity conditions. The ICD‐10 framework has initially inherited a structure from previous ICD revisions in which topographic distribution frequently takes precedence, leading to misclassification of complex and systemic disorders such as FHs potentially affecting multiple organs. In fact, the ICD‐10 offers relatively few codes for FHs diseases, and because food allergens are not included in the framework, it is impossible to link the conditions with food triggers.
2.2. Better representation of food hypersensitivity in the ICD‐11
The 11th ICD revision was officially launched by WHO in March 2007. Different from the ICD‐10, which was presented as just title headings, the proposed structure of ICD‐11 includes definitions for each entity and follows a content model. 15 The ICD‐11 is intended not only to rectify deficiencies in ICD‐10 and to incorporate changes driven by scientific advances, but also to take advantage of the revolution in electronic data handling since the publication of ICD‐10 a quarter of a century ago. The ICD‐11 framework has been designed to be a global standard for health data, clinical documentation, and statistical aggregation, with multiple uses, including primary care. It combined scientifically up‐to‐date information with the state‐of‐the‐art technology and multilingual design facilities for global use. Currently, the ICD‐11 counts with 17,000 categories, 80,000 concepts, 120,000 medical terms, and more than 1.6 million clinical terms. 15 The ICD‐11 was presented and adopted by the 72nd World Health Assembly in May 2019 and the implementation is ongoing worldwide. 15 , 19
2.3. The allergy in ICD‐11 initiative: Challenges and outcomes
In 2012, an analysis using the Brazilian Mortality Information System demonstrated undernotification of anaphylaxis deaths due to the difficulties of coding them using the current version of the International Classification of Diseases, ICD‐10. 17 , 20 This work triggered a cascade of strategic international actions supported by the Joint Allergy Academies and their ICD representatives at the World Health Organization (WHO) to update the classification of allergic disorders for the recently released eleventh revision (ICD‐11). Considering the ICD‐11 revision as a key window of opportunity, a detailed action plan was coordinated under the ALLERGY in ICD‐11 initiative (led by Luciana Kase Tanno and Pascal Demoly) with the aim of creating a more appropriate classification for allergic and hypersensitivity conditions in this new edition of ICD‐11. Subsequently, we have produced technical and scientific evidence demonstrating the need for classification and coding changes and we have participated in an ongoing dialog with the WHO ICD‐11 revision governance team. All these efforts have been documented in peer‐reviewed publications, 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 and are being acknowledged and supported by the Joint Allergy Academies comprising the American Academy of Allergy Asthma and Immunology (AAAAI), the European Academy of Allergy and Clinical Immunology (EAACI), the World Allergy Organization (WAO), the American College of Allergy Asthma and Immunology (ACAAI), the Asia Pacific Association of Allergy, Asthma and Clinical Immunology (APAAACI), and the Latin American Society of Allergy, Asthma and Immunology (SLAAI).
The main outcome of the process was the construction of the pioneer “Allergy and hypersensitivity conditions” section has been built under the “Disorders of the Immune System” chapter of the ICD‐11. 24 , 26 The “Food hypersensitivity” subsection is now classified under the “Complex allergic or hypersensitivity conditions” section (Figure 1) and “Food‐induced anaphylaxis” is under the “Anaphylaxis” section, currently counting with 18 conditions, which can be combined with characteristics available in the “Extension codes” chapter to provide additional specification (severity, temporality, topography, trigger, response to treatment). 24 The novelty of ICD‐11 as a digital instrument still permits some changes when proved to be necessary. Currently, the ICD‐11 presents general group of allergens, but no specifications of allergens are available. For this reason, work is ongoing to implement a more detailed classification of food allergens as extension codes. Another example is the new sub‐section addressed to anaphylaxis in the ICD‐11, in which more types of anaphylaxis are now available (Figure 2).
FIGURE 1.
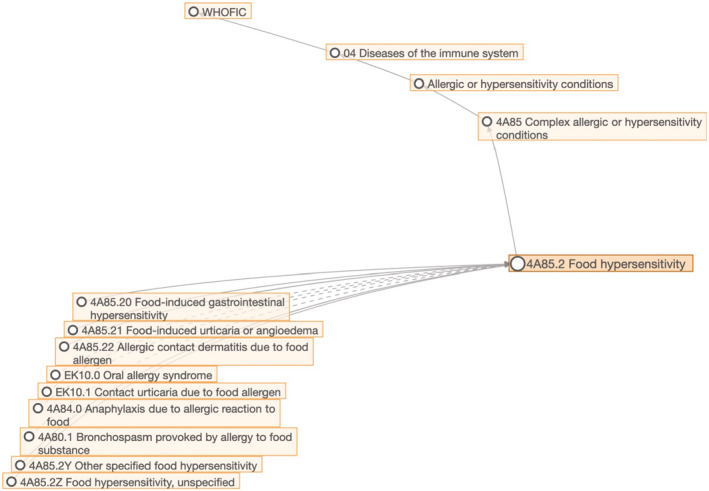
Generic framework demonstrating the classification of food hypersensitivity in the ICD‐11
FIGURE 2.
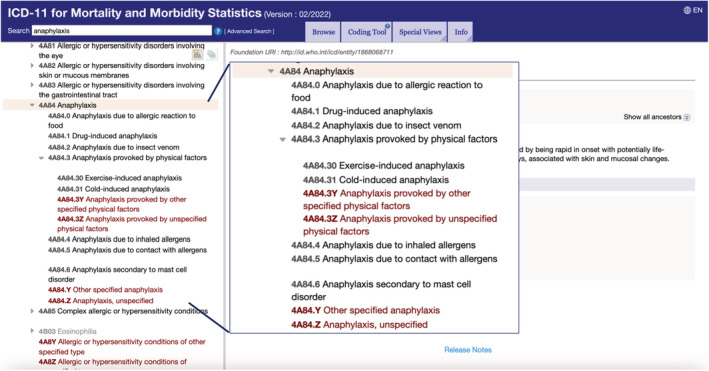
Anaphylaxis sub‐section in the International Classification of Diseases (ICD)‐11
In June 2018, the WHO Collaborating Center (WHO CC) for the Scientific Classification of Allergic and Hypersensitivity Diseases was established at the University Hospital of Montpellier, headed by Luciana Kase Tanno and Pascal Demoly. 42 This designation is the result of recognition by WHO of all the efforts of the ALLERGY in ICD‐11 initiative and is intended to provide academic, research, and scientific support to WHO in the implementation, refinement, and maintenance of the WHO‐FIC (Family of International Classifications) in our areas of expertize. WHO CCs are institutions designated by the Director‐General of the WHO and endorsed by the national minister of health to carry out activities in support of the WHO programs, such as communicable diseases, nutrition, mental health, occupational health among others. Currently, there are 25 WHO CCs responsible for the WHO‐FIC and the Montpellier WHO CC is the only one with expertize in allergy and clinical immunology.
2.4. ICD‐11 and food hypersensitivity management
The WHO ICD‐11 revision process and the construction of the new section addressed to these conditions open a window of opportunity for a better and harmonized classification, terminology, and definitions of FHs/food allergies enabling quality clinical practice and research. In addition, finer distinctions in the medical restricted data offer a more precise evaluation and management of patients.
By consolidating all allergic conditions into one ICD‐11 single section, as opposed to spreading them out over many ICD‐10 (and ICD‐10 adaptations) chapters (Table 1) and by allowing all the relevant codes to be used to represent specific conditions, our aim was to facilitate the use of such classification and codes by clinicians, epidemiologists, and statisticians, as well as all data custodians and other relevant personnel. In other words, aligning the clinical diagnosis to ICD classification and codes provides a realistic recognition of allergic and hypersensitivity conditions and allows a better management of allergic patients.
TABLE 1.
Representation of food hypersensitivities in the International Classification of Diseases (ICD)‐10 and − 11
| Hypersensitivity condition | ICD‐10 Corresponding chapter (S) (Tabular list, access November 2021) | ICD‐11 for Mortality and Morbidity statistics (access November 2021) | |
|---|---|---|---|
| Main FOOD HYPERSENSITIVITY headings in the “ALLERGIC AND HYPERSENSITIVITY CONDITIONS” section | “EXTENSION CODES” CHAPTER | ||
| Food hypersensitivity | Chapter XIX: Injury, poisoning and certain other consequences of external causes (T78.0, T78.1) |
‐ Food‐induced anaphylaxis ‐ Bronchospasm provoked by allergy to food substance ‐ Food –induced eosinophilic gastroenteritis ‐ Food‐induced eosinophilic oesophagitis ‐ Food‐induced gastrointestinal hypersensitivity ‐ Food‐induced urticaria or angioedema ‐ Allergic contact dermatitis due to food allergen ‐ Other specified food hypersensitivity ‐ Food hypersensitivity, unspecified |
Severity scale value ‐ Mild Moderate Severe scale ‐ Grading scale |
| Chapter XII: Diseases of the skin and subcutaneous tissue (L23.6) |
Temporality Course of the condition |
||
|
Chapter XI: Diseases of the digestive system (K52.2, K52.3, K52.8, K52.9) |
Etiology Allergens |
||
| Chapter XXI: Factors influencing health status and contact with health services (Z71.3) |
Diagnosis code descriptors Diagnosis timing Diagnosis method of confirmation Diagnosis certainty Discharge diagnosis types |
||
Standardized criteria are critical for facilitating adequate and accurate patient recruitment into clinical trials and translational research. The built “Food hypersensitivity” subsection also intends to support personalized translational medicine through providing tools of reaching standard food allergy phenotypes recognized by health professionals worldwide by supporting the stratification of subjects for multicentric clinical and/or research studies.
As the ICD‐11 allows descriptions regarding the diagnosis (Table 1), the new framework will support the correct patients' FHs/food allergy labeling in administrative databases.
The ICD‐11 is currently being implemented globally and the velocity of adopting it in the national level depends on the complexity of health systems in use in each country. Some low‐incoming countries, in which ICD has never been used, are moving faster and directly to the ICD‐11 instead of adopting ICD‐10 first, being then considered “early adopters”. 43 In order to support countries and regions to implement the ICD‐11, the WHO together with the CCs prepared many tools, such as the ICD‐11 Implementation and Transition Guide, the ICD‐11 reference guide and training materials. 44 With the implementation of the ICD‐11, two immediate consequences are expected: (1) the reported number of FH/FA will likely increase due to more appropriate coding and (2) the cross‐sectional and longitudinal morbidity data generated will ultimately lead to better understanding of FH/FA trends and patterns and improved health policies directed at reducing anaphylaxis‐related morbidity and mortality. With the use of ICD‐11 globally, it is expected to gather more accurate harmonized comparable official statistics, which can be the starting point to trigger public health actions and tailor investments in health care and prevention.
2.5. Clinical vignette: From ICD‐10 and ICD‐11 perspectives
A 25‐year‐old male has been diagnosed with eosinophilic esophagitis by a gastroenterologist (EoE) and referred to the allergist to investigate possible FH. A week before, he was presented to the emergency department with a 3‐week history of progressive dysphagia to solids more than liquids, localized to the mid‐sternum. Manifested severe odynophagia, chest pain, and marked weight loss (5.5 kg or 7% of baseline body weight) and was under treatment to progressive symptoms of esophageal reflux for the last seven years. Esophagogastroduodenoscopy demonstrated: features suggestive of EoE with longitudinal furrowing, concentric ridged folds, and micro‐abscesses. Esophageal biopsies showed spongiotic squamous epithelium and >25 eosinophils per high power field. Allergy testing was positive to wheat. The final diagnosis is Food‐induced eosinophilic esophagitis.
By looking at this condition in the ICD‐10 platform, 17 we are addressed to the “K52.8 Other specified non‐infective gastroenteritis or colitis”, with no possibility to combine with food allergy or food allergen. On the other hand, the ICD‐11 24 allows us to gather the “4A83.1 Food‐induced eosinophilic oesophagitis” and provide more details such as etiology “XM3XH8 Grains and flours” scattered under the “Allergens” subsection.
Although international classification systems, such as the ICD, aims to be used by a broad audience besides allergists, efforts to reach standard classification, coding, and definitions for anaphylaxis and FHs through the ICD‐11 revision are aligned to the developments of precision medicine and the allergy community is strategically positioned to provide the diagnosis and management tools for these patients and educate colleagues from different specialties, health professionals, and health care providers.
3. CONCLUSIONS
3.1. Preventing food hypersensitivity through the ICD‐11
The WHO advocates for global food supplies as fundamental for obtaining universal health coverage. 45 However, all foods can induce FHs. Better understanding of risk factors and real incidence for individual populations and/or for individual food‐triggers is a key step to prevent FH‐harm.
Although the allergy community has been moving efforts to improve knowledge in the field of FHs/food allergies, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 there is still restrict data regarding their epidemiology, limiting the identification of potential risk factors, and retarding the development of diagnostic procedures and predictive tests. In this regard, the development of tools, such as recognized international coding systems, is essential to capture realistic and comparable health information to allow a common understanding between international networks. Therefore, the ICD‐11 enhancement and utilization are expected to constitute a significant advancement in global health.
In order to inform the development of strategies to reduce preventable FHs/food allergies, the burden of disease in different healthcare settings and patient populations and their common etiologies need to be understood. Besides, greater specificity regarding clinical conditions and services delivered will provide payers, policy makers, and providers with better information to make major refinements to countries payment and reimbursement systems, including the design and implementation of pay‐for‐performance program.
The new classification addressed to FHs will enable the collection of more accurate epidemiological data to support quality management of patients with food allergies, and better facilitate health care planning and decision‐making and public health measures to prevent and reduce the morbidity and mortality attributable to FHs. The improved logic and standardized definitions through the ICD‐11 (and other WHO classifications) will also facilitate international comparisons of quality care and the sharing of the best practice globally.
AUTHOR CONTRIBUTIONS
The first and the last authors contributed to the construction of the document (designed the study, including the questionnaire, analyzed and interpreted the data, and wrote the manuscript).
FUNDING INFORMATION
Pascal Demoly and Luciana Kase Tanno received an unrestricted ANS grants through CHUM administration. LKT received a research AllerGOS grant.
CONFLICT OF INTERESTS
The authors declare that they do not have conflict of interests related to the contents of this article.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13882.
CONSENT FOR PUBLICATION
The manuscript does not contain data from any individual person.
Tanno LK, Demoly P. Food allergy in the World Health Organization's International Classification of Diseases (ICD)‐11. Pediatr Allergy Immunol. 2022;33:e13882. doi: 10.1111/pai.13882
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Warren CM, Turner PJ, Chinthrajah RS, Gupta RS. Advancing food Allergy through epidemiology: understanding and addressing disparities in food Allergy management and outcomes. J Allergy Clin Immunol Pract. 2021. Jan;9(1):110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent‐reported childhood food allergies in the United States. Pediatrics. 2018;142(6):e20181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prescott SL, Pawankar R, Allen KJ, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta RS, Warren CM, Smith BM, et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2(1):e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nwaru BI, Hickstein L, Panesar SS, et al. The epidemiology of food allergy in Europe: a systematic review and meta‐analysis. Allergy. 2014;69(1):62‐75. [DOI] [PubMed] [Google Scholar]
- 6. Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011;22(2):155‐160. [DOI] [PubMed] [Google Scholar]
- 7. Tanno LK, Clark E, Mamodaly M, et al. Food‐induced anaphylaxis morbidity: emergency department and hospitalization data support preventive strategies. Pediatr Allergy Immunol. 2021;32(8):1730‐1742. [DOI] [PubMed] [Google Scholar]
- 8. Baseggio Conrado A, Patel N, Turner PJ. Global patterns in anaphylaxis due to specific foods: a systematic review. J Allergy Clin Immunol. 2021;148(6):1515‐1525.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pouessel G, Turner PJ, Worm M, et al. Food‐induced fatal anaphylaxis: from epidemiological data to general prevention strategies. Clin Exp Allergy. 2018;48(12):1584‐1593. [DOI] [PubMed] [Google Scholar]
- 10. Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5(5):1169‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umasunthar T, Leonardi‐Bee J, Hodes M, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta‐analysis. Clin Exp Allergy. 2013;43(12):1333‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. UK Department of Health . Guidance on the use of adrenaline auto‐injectors in school. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/645476/Adrenaline_auto_injectors_in_schools.pdf Accessed October 2022.
- 13. Codex alimentarius Website https://www.fao.org/fao‐who‐codexalimentarius/sh‐proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B80‐2020%252FCXC_080e.pdf Accessed November 2021.
- 14. European Union law Website (available: https://eur‐lex.europa.eu/legal‐content/EN/TXT/?uri=CELEX:52017XC1213[01] accessed November 2021).
- 15. World Health Organization, International Classification of Diseases Website. (cited, available: https://icd.who.int/en accessed November 2021).
- 16. Tanno LK, Sublett JL, Meadows JA, et al. Perspectives of the international classification of diseases (ICD)‐11 in Allergy clinical practice in the united Staes of America. Ann Allergy Asthma Immunol. 2017. Feb;118(2):127‐132. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization , ICD‐10 version 2019. (cited, available: https://icd.who.int/browse10/2019/en#/ accessed November 2021).
- 18. Tanno LK, Calderon MA, Goldberg BJ, Akdis CA, Papadopoulos NG, Demoly P. Categorization of allergic disorders in the New World health organization international classification of diseases. Clin Transl Allergy. 2014;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanno LK, Chalmers R, Bierrenbach AL, Simons FER, Molinari N, et al. Changing the history of anaphylaxis mortality statistics througth the World Health Organization's international classification of diseases (ICD)‐11. J Allergy Clin Immunol. 2019;144(3):627‐633. [DOI] [PubMed] [Google Scholar]
- 20. Tanno LK, Ganem F, Demoly P, Toscano CM, Bierrenbach AL. Undernotification of anaphylaxis deaths in Brazil due to difficult coding under the ICD‐10. Allergy. 2012;67:783‐789. [DOI] [PubMed] [Google Scholar]
- 21. Demoly P, Tanno LK, Akdis CA, et al. Global classification and coding of hypersensitivity diseases – an EAACI – WAO survey, strategic paper and review. Allergy. 2014;69:559‐570. [DOI] [PubMed] [Google Scholar]
- 22. Tanno LK, Simons FER, Sanchez‐Borges M, et al. Applying prevention concepts to anaphylaxis: a call for worldwide availability of adrenaline auto‐injectors. Clin Exp Allergy. 2017;47(9):1108‐1114. [DOI] [PubMed] [Google Scholar]
- 23. Tanno LK, Demoly P, Joint Allergy Academies . Action plan to ensure global availability of adrenaline autoinjectors. J Investig Allergol Clin Immunol. 2020;30(2):77‐85. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization , ICD‐11. (cited, available: https://icd.who.int/browse11/l‐m/en accessed July 2021.)
- 25. Tanno LK, Casale T, Papadopoulos NG, et al. A call to arms of specialty societies to review the WHO international classification of diseases, eleventh revision terms appropriate for the diseases they manage: the example of the Joint Allergy Academies. Allergy Asthma Proc. 2017;38(4):54‐55. [DOI] [PubMed] [Google Scholar]
- 26. Tanno LK, Calderon MA, Demoly P, on behalf the Joint Allergy Academies . New allergic and hypersensitivity conditions section in the international classification of Diseases‐11. Allergy asthma. Immunol Res. 2016;8:383‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanno LK, Bierrenbach AL, Simons FER, et al. Critical view of anaphylaxis epidemiology: open questions and new perspectives. Allergy Asthma Clin Immunol. 2018;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanno LK, Calderon MA, Goldberg BJ, et al. Constructing a classification of hypersensitivity/allergic diseases for ICD‐11 by crowdsourcing the allergist community. Allergy. 2015;70:609‐615. [DOI] [PubMed] [Google Scholar]
- 29. Tanno LK, Simons FER, Annesi‐Maesano I, Calderon M, Aymé S, Demoly P. Fatal anaphylaxis registries data support changes In the WHO anaphylaxis mortality coding rules. Orphanet J Rare Dis. 2017;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanno LK, Calderon M, Papadopoulos NG, Demoly P. Mapping hypersensitivity/allergic diseases in the international classification of diseases (ICD)‐11: cross‐linking terms and unmet needs. Clin Transl Allergy. 2015;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanno LK, Calderon MA, Demoly P, on behalf the Joint Allergy Academies . Making allergic and hypersensitivity conditions visible in the international classification of Diseases‐11. Asian Pac Allergy. 2015;5:193‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanno LK, Calderon MA, Demoly P, on behalf the Joint Allergy Academies . Optimization and simplification of the allergic and hypersensitivity conditions classification for the ICD‐11. Allergy. 2016;71:671‐676. [DOI] [PubMed] [Google Scholar]
- 33. Tanno LK, Calderon MA, Papadopoulos NG, et al. Revisiting desensitization and allergen immunotherapy concepts for the international classification of diseases (ICD)‐11. J Allergy Clin Immunol Pract. 2016;4:643‐649. [DOI] [PubMed] [Google Scholar]
- 34. Tanno LK, Calderon MA, Li J, Casale T, Demoly P. Updating Allergy/hypersensitivity diagnostic procedures in the WHO ICD‐11 revision. J Allergy Clin Immunol Pract. 2016;4:650‐657. [DOI] [PubMed] [Google Scholar]
- 35. Tanno LK, Calderon MA, Papadopoulos NG, et al. Surveying the new allergic and hypersensitivity conditions chapter of the international classification of diseases (ICD)‐11. Allergy. 2016;71:1235‐1240. [DOI] [PubMed] [Google Scholar]
- 36. Tanno LK, Calderon M, Demoly P, Joint Allergy Academies . Supporting the validation of the new allergic and hypersensitivity conditions section of the World Health Organization international classification of Diseases‐11. Asia Pac Allergy. 2016;6:149‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanno LK, Calderon M, Sublett JL, Casale T, Demoly P, Joint Allergy Academies . Smoothing the transition from international classification of diseases, tenth revision, clinical modification to international classification of diseases, Eleventh Revision. J Allergy Clin Immunol Pract. 2016;4:1265‐1267. [DOI] [PubMed] [Google Scholar]
- 38. Tanno LK, Calderon MA, Smith HE, et al. Dissemination of definitions and concepts of allergic and hypersensitivity conditions. World Allergy Organ J. 2016;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanno LK, Bierrenbach AL, Calderon MA, et al. Decreasing the undernotification of anaphylaxis deaths in Brazil through the international classification of diseases (ICD)‐11 revision. Allergy. 2017;72:120‐125. [DOI] [PubMed] [Google Scholar]
- 40. Tanno LK, Ansotegui I, Demoly P. Globalization and anaphylaxis. Curr Opin Allergy Clin Immunol. 2018;18(5):365‐369. [DOI] [PubMed] [Google Scholar]
- 41. Tanno LK, Molinari N, Bruel S, et al. Field‐testing the new anaphylaxis' classification for the WHO international classification of Diseases‐11 revision. Allergy. 2017;72(5):820‐826. [DOI] [PubMed] [Google Scholar]
- 42. World health Organization . Collaborating Centres (available: https://www.who.int/about/partnerships/collaborating‐centres accessed July 2021).
- 43. World health Organization International Statistical Classification of Diseases and Related Health Problems (ICD) (available: https://www.who.int/standards/classifications/classification‐of‐diseases accessed October 2022).
- 44. World health Organization , ICD‐11 implementation. (available: https://icd.who.int/en accessed October 2022).
- 45. World Health Organization , Universal Health Coverage (available: https://www.who.int/health‐topics/universal‐health‐coverage#tab=tab_1, accessed November 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


