Abstract
Non-alcoholic fatty liver disease (NAFLD) is currently the most prevalent metabolic disorder all over the world, and lipid metabolic disorders and inflammation are closely associated and contribute to the pathogenesis of NAFLD. Cholesterol 25-hydroxylase (Ch25h) and its product, 25-hydroxycholesterol (25-HC), play important roles in cholesterol homeostasis and inflammation, but whether Ch25h and 25-HC are involved in NAFLD remains uncertain. In this study, we use Ch25h knockout mice, hepatic cells and liver biopsies to explore the role of Ch25h and 25-HC in lipid metabolism and accumulation in liver, determine the molecular mechanism of lipid accumulation and inflammation influenced by Ch25h and 25-HC, and assess the regulatory effects of Ch25h and 25-HC on human NAFLD. Our results indicate that mice lacking Ch25h have normal cholesterol homeostasis with normal diet, but under the condition of high fat diet (HFD), the mice show higher total cholesterol and triglyceride in serum, and prone to hepatic steatosis. Ch25h deficiency reduces the cholesterol efflux regulated by liver X receptor α (LXRα), increases the synthesis of cholesterol mediated by sterol-regulatory element binding protein 2 (SREBP-2), and increases the activation of NLRP3 inflammasome, therefore promotes hepatic steatosis. Collectively, our data suggest that Ch25h and 25-HC play important roles in lipid metabolism and inflammation, thereby exerting anti-NAFLD functions.
Keywords: high fat diet, LXRα, NLRP3 inflammasome, Ch25h, 25-HC
Introduction
The metabolite 25-hydroxycholesterol (25-HC) is an oxysterol derived from cholesterol. The most potent enzyme that transforms cholesterol into 25-HC is cholesterol 25-hydroxylase (Ch25h) [1]. 25-HC is a physiological regulator of cholesterol homeostasis. It induces the binding of SREBP cleavage-activating protein to INSIG, thus blocks SREBP processing and downregulates HMG-CoA reductase [ 2, 3]. Moreover, 25-HC may accelerate the ubiquitination and degradation of HMG-CoA reductase protein. On the other hand, 25-HC is an endogenous ligand of the nuclear receptor LXRα [4], which modulates cholesterol metabolism.
In addition to their cholesterol regulatory role, 25-HC and Ch25h also play important roles in immune regulation and inflammation. Ch25h has been identified as an IFN-stimulated gene, and the expression of Ch25h is strongly induced by LPS [ 5, 6], type I interferon [7], viral infection [8], and TLR activation [9], leading to increased concentration of 25-HC [10]. Ch25h-knockout mice present marked changes in their inflammatory response, with increased frequencies of IL-17A + T cells and neutrophil count. Production of 25-HC induced by Ch25h suppresses IL-1β mRNA and protein expressions, as well as inflammasome activity via antagonizing SREBP, providing a negative feedback pathway for interferon-induced inflammation [ 3, 11]. Ch25h and 25-HC exert their broad antiviral functions via interfering with multiple steps in the lifecycle of viruses [ 8, 10, 12]. In addition, 25-HC also acts as an amplifier of inflammation by mediating the recruitment of AP-1 to the promoters of a subset of Toll-like receptor response genes, and by decreasing cytoplasmic IκBα level and further increasing TNFα-induced NF-κB activation [ 13, 14]. Taken together, Ch25h and 25-HC serve as potent regulators in the cross-talk between lipid metabolism and inflammatory response.
In mammals, liver plays critical roles in controlling metabolic homeostasis, and dysregulation of liver function leads to metabolic disorders. Non-alcoholic fatty liver disease (NAFLD) is currently the most prevalent metabolic disorder all over the world, which is not only associated with other metabolic diseases such as diabetes and atherosclerosis, but also invokes more severe liver diseases including non-alcoholic steatohepatitis (NASH), hepatic cirrhosis, and hepatocellular carcinoma (HCC) [ 15, 16]. Lipid metabolic disorders and inflammation are closely associated with and contribute to the pathogenesis of NAFLD [17], therefore understanding the cross-talk between lipid accumulation and inflammation is fundamental for the prevention and therapy of NAFLD.
Accumulating evidence demonstrates that LXRs and SREBPs are involved in the metabolism and inflammation in human NAFLD [ 18– 20], especially in the non-alcoholic steatohepatitis (NASH) phase, and 25-HC has been showed to regulate the activities of LXRs and SREBPs, so we hypothesize that Ch25h and 25-HC may be involved in the process of NAFLD. Preliminary data confirm our hypothesis: Ch25h-deficient mice are particularly prone to develop NAFLD under the condition of HFD. In this study, we further investigate the underline contribution of Ch25h and 25-HC to the development of NAFLD.
Materials and Methods
Animal experiments
ApoE –/–/ Ch25h –/– double knockout mice were obtained by crossbreeding ApoE –/ – mice (Vital River laboratory, Beijing, China) with Ch25h –/ – mice (Jackson Laboratory, Sacramento, USA) as previously described [21], and 6-week-old male mice were fed with a high fat, high cholesterol diet (HFD) containing 21% fat, and 1.5% cholesterol (D12079B; Research Diet, New Brunswick, USA). All animals were housed in colony cages (Animal Care Systems, Centennial, USA) with a 12-h light/12-h dark cycle. The mice body weights, teeth, fur, and behaviors were monitored on a daily base. According to our observation, no mice died during the experimental period. At the end of experiments, all animals were euthanized by CO 2, and tissues were collected for subsequent analyses. The animal experiments were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University (No. XJTULAC2015-648).
Primary mouse hepatocytes isolation and culture
Mouse hepatocytes were isolated by perfusing the liver with collagenase through the portal vein. Briefly, the liver was perfused with a Krebs buffer containing EGTA for 4 min, followed by perfusion with Krebs buffer containing 0.1% collagenase (ThermoFisher Scientific, Waltham, USA) for 6 min. The “softened” liver was transferred into DMEM and rinsed gently, and the hepatocytes were separated from the connective tissue by filtering through a macroporous filter. The hepatocytes were washed with DMEM twice, and the cell pellets were suspended in DMEM containing streptomycin (100 μg/mL), penicillin (100 U/mL), and fetal bovine serum (10%). The cells were cultured at 37°C under 5% CO 2 humidified air.
Lipid extraction and serum analysis
Mouse liver samples were homogenized for the extraction of lipid by chloroform/methanol method. Triglyceride (TG) and total cholesterol (TC) levels were determined using an automatic biochemical analyzer (AU5800; Beckman Coulter, Brea, USA). Blood samples were obtained from mouse retro-orbital veins and the serum was collected and used for the determination of serum TG, TC, AST and ALT levels using the automatic biochemical analyzer.
Immunohistochemical analysis
Frozen sections of liver (~5 μm thick) were stained with 0.5% Oil Red O for the visualization of hepatic fat and then counterstained with Hematoxylin or stained with hematoxylin and eosin. Frozen sections of liver were also processed for the immunohistochemical analyses of SREBP2, NLRP3, and LXRα, using anti-SREBP2 antibody (ab-30682; Abcam, Cambridge, USA), anti-NLRP3 antibody (ab4207; Abcam), and anti-LXRα antibody (sc-1202; Santa Cruz Biotechnology, Santa Cruz, USA), respectively. Histological analysis and image processing were carried out using a Leica DMRE microscope equipped with Spot digital image analysis software and camera (Leica, Wetzlar, Germany).
Preparation of culture media with free fatty acids
Palmitic acid and oleic acid (Sigma-Aldrich, St Louis, USA) were mixed at a molar ratio of 2:1. Then the mixture of oleic acid and palmitic acid was dissolved in pre-heated 100% ethanol and vortexed, and the mixture was heated at 60°C for a few minutes and vortex again until the fatty acids were dissolved completely. Then the solution was mixed with 10% fatty acid-free BSA in H 2O by stirring for 1 h at 37°C, and then diluted with culture media in order to adjust the final molar ratio of PA-OA/BSA to 5:1 and ethanol concentration at less than 0.33% ( v/v). Control media contained the same concentrations of ethanol and BSA, but without fatty acids.
Cell culture and RNA interference
LO2 cells (ATCC, Maryland, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and antibiotics at 37°C in a 5% CO 2/95% air incubator. Lipofectamine 2000 (Invitrogen, Carlsbad, USA) was used to transfect LO2 cells according to manufacturer’s protocols. The siRNA targeting Ch25h were obtained from GenePharma (Shanghai, China), and the sequences are as follows: 5′-CCUUCCACGUGGUCAACAUTT-3′ (sense) and 5′-AUGUUGACCACGUGGAAGGTT-3′ (antisense). The siRNA with scrambled sequence was used as negative control (NC siRNA: 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACAAGUUCGGAGAATT-3′ (antisense)).
Quantitative reverse transcriptase PCR
Total RNA was extracted from tissues and cells using TRIzol (Invitrogen, Carlsbad, USA). The isolated total RNA was converted into cDNA via reverse transcription using the iScript cDNA synthesis kit (Invitrogen). qRT-PCR were performed using iQ TMSYBRGreen PCR Supermix (Promega, Madison, USA) on the ABI 7500 real-time detection system (Applied Biosystems, Foster City, USA). Normalization was done by normalizing threshold cycles (C t value) to acidic ribosomal phosphoprotein P0 (Rplp0) within each sample to obtain sample specific ΔCt values (ΔCt=Ct gene of interest−Ct Rplp0). Fold expression levels were obtained by calculating the 2 −ΔΔCt level (ΔΔCt=ΔCt treatment−ΔCt control).
Western blot analysis
Tissues were homogenized in RIPA buffer (6.5 mM Tris, pH 7.4, 15 mM NaCl, 1 mM EDTA, 0.1% SDS, 0.25% sodium deoxycholate, 1% NP-40). The homogenate was cleared by centrifugation at 4°C for 20 min at 20,627 g, and the supernatant containing proteins were collected. Bicinchoninic Acid reagents (Thermo Fisher Scientific) were used to determine the protein concentration. Equal amounts of proteins were resolved by 10% SDS-PAGE, followed by transfer onto PVDF membranes. The membranes were blocked with 5% BSA in Tris-buffered saline containing 0.2% Tween-20 (TBS-T), and then incubated with primary antibodies at 4°C overnight. The blots were finally immunoreacted with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology) and visualized using an enhanced chemoluminescence (ECL) system (Vazyme, Nanjing, China). The primary antibodies include anti-HSC70, anti-caspase-1 p10 and anti-IL-1β antibodies were from Santa Cruz Biotechnology; anti-SREBP2, anti-NLRP3 and anti-LXRα antibodies were from Abcam.
Statistical analysis
Statistical analysis was performed using the unpaired Student’s t-test between two groups or ANOVA. Data were expressed as the mean±SEM from at least 3 independent experiments. P<0.05 was considered statistically significant.
Results
Ch25h-knockout mice showed more lipid accumulation in livers under HFD
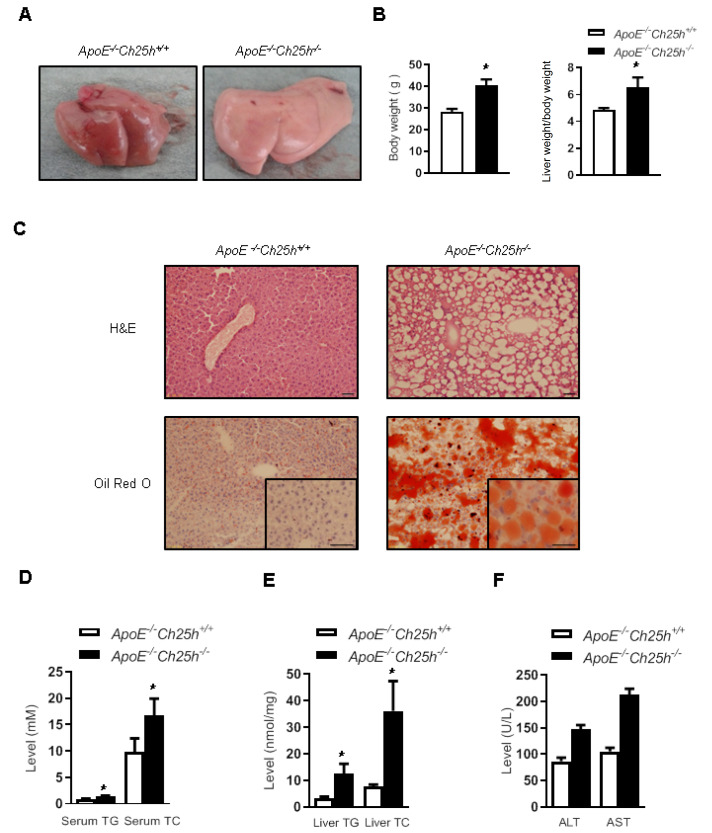
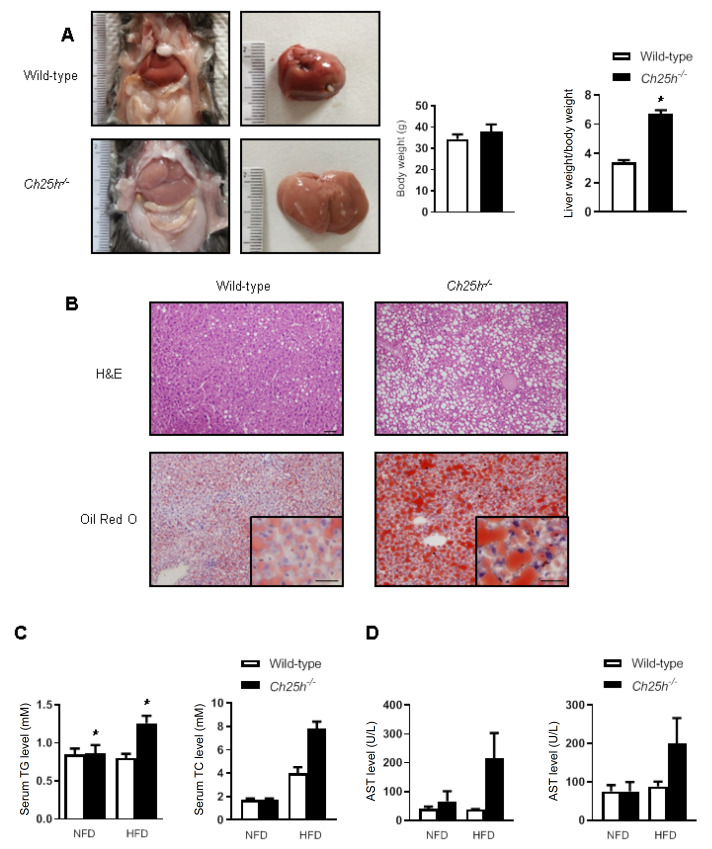
25-HC is a well-known negative feedback regulator of the sterol pathway, while the mice lacking Ch25h reportedly have normal cholesterol homeostasis. We established a strain of ApoE/ Ch25h double knockout mice by crossbreeding ApoE –/ – mice with Ch25h –/ – mice in our previous experiment [21]. Mice were fed for 12 weeks with HFD, and the livers of the ApoE –/ – / Ch25h –/ – mice were different from those of their ApoE –/ – / Ch25h +/ + littermates, i.e., their livers looked larger and paler, and the liver weight/body weight ratio was increased ( Figure 1A,B). H&E staining of liver tissue showed that knockout of Ch25h caused significant vacuolar degeneration of hepatocytes, and Oil Red O staining indicated lipid deposition within hepatocytes ( Figure 1C). In accordance with these histological findings, serum test showed that Ch25h deficiency caused increased levels of triglyceride (TG) and total cholesterol (TC) in serum under HFD ( Figure 1D), and lipid analysis of liver tissue also showed that the ApoE –/ – / Ch25h –/ – mice exhibited significantly increased TG and TC levels compared with the ApoE –/ – / Ch25h +/ + littermates ( Figure 1E). Furthermore, serum transaminase analysis showed that the ApoE –/ – / Ch25h –/ – mice exhibited higher ALT and AST concentrations under HFD than the ApoE –/ – / Ch25h +/ + littermates, but the difference was not statistically significant ( Figure 1F). In order to rule out the potential impact of ApoE knockout on the liver steatosis, the simple Ch25h –/– mice were also used to validate the above phenomenon. Consistently, the Ch25h –/– mice also showed significant hepatic steatosis after feeding with HFD for 15 weeks ( Figure 2A,B). Serum cholesterol concentration analysis showed that the mice lacking Ch25h with normal diet had normal cholesterol homeostasis, but the Ch25h-lacking mice fed with HFD showed higher TC and TG levels in serum ( Figure 2C). Serum ALT and AST determination showed that the mice lacking Ch25h with normal diet had normal serum ALT and AST levels, while serum ALT and AST levels seemed to be increased in mice fed with HFD, but the differences were not statistically significant ( Figure 2D). These results suggest that mice lacking Ch25h could basically maintain the whole body cholesterol homeostasis and normal liver function with normal diet, but could certainly dysregulate lipid metabolism and promote the development of hepatic steatosis with HFD.
Figure1 .
Knockout of Ch25h in ApoE –/ – mice induced hepatic steatosis with HFD
ApoE–/–Ch25h+/+ and ApoE–/–Ch25h–/– mice were fed with HFD for 12 weeks. (A) Liver photographs of the mice. (B) The body weight and liver weight/body weight ratio. Results are presented as the mean±SEM (n=5). *P<0.05 versus ApoE–/–Ch25h+/+ mice. (C) Liver sections stained with H&E and Oil Red O. Scale bar=50 μm. (D) Concentrations of triglyceride (TG) and total cholesterol (TC) in the serum of ApoE–/–Ch25h+/+ and ApoE–/–Ch25h–/– mice. (E) Concentrations of TG and TC in the liver of ApoE–/–Ch25h+/+ and ApoE–/–Ch25h–/– mice. (F) Concentrations of ALT and AST in the serum of ApoE–/–Ch25h+/+ and ApoE–/–Ch25h–/– mice.
Figure2 .
Knockout of Ch25h in wild-type mice induced hepatic steatosis under HFD but not under normal diet
(A) Liver photographs of Ch25h–/– and wild-type mice. The body weight and liver weight/body weight ratio of Ch25h–/– and wild-type mice. Results are presented as the mean±SEM (n=5). *P<0.05 versus wild-type mice. (B) Liver sections stained with H&E and Oil Red O. Scale bar=50 μm. (C) Serum concentrations of TG and TC of Ch25h–/– and wild-type mice. (D) Serum concentrations of ALT and AST of Ch25h–/– and wild-type mice.
Ch25h deficiency impaired the cholesterol efflux regulated by LXRα and activated NLRP3 inflammasome in liver
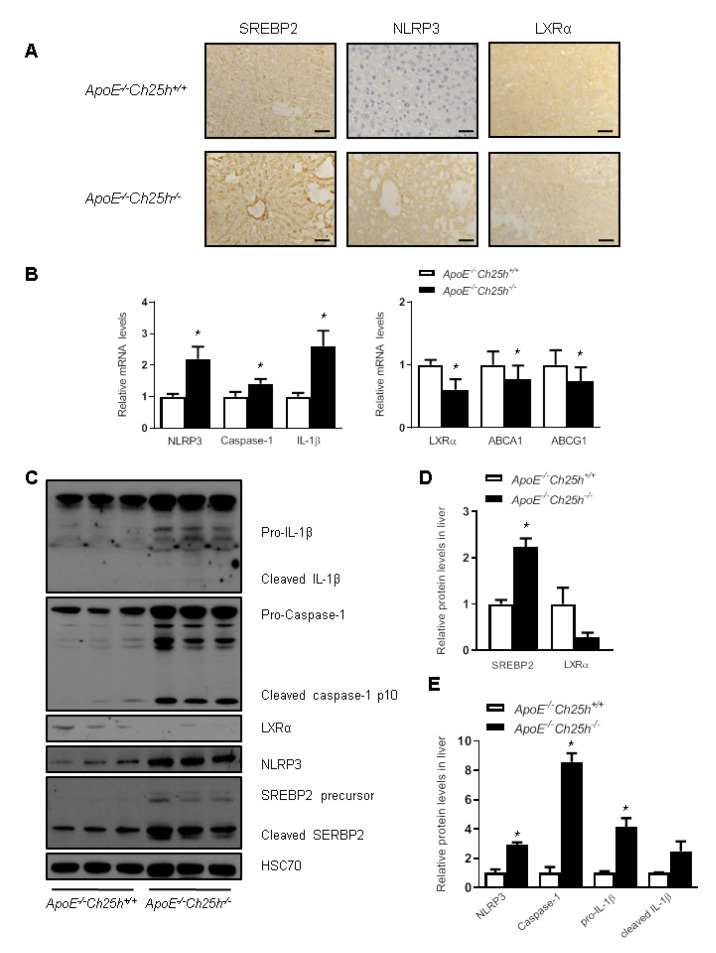
LXRs and SREBPs are involved in lipid metabolism and inflammation in NAFLD, while 25-HC has been shown to regulate LXRs and SREBPs activity as well as inflammasome [ 18– 21]. We firstly analyzed the expressions of SREBP2, NLRP3 and LXRα in the liver by immunohistochemistry staining. As shown in Figure 3A, SREBP2 expression was increased in ApoE –/ – Ch25h –/ – mice, so does the NLRP3 expression. As expected, the expression of LXRα was decreased. We further tested the mRNA levels of the relevant genes in the liver extract, and the results showed that NLRP3 and its associated genes caspase-1 and Il-1β were increased in the livers of ApoE –/ – Ch25h –/ – mice, while the mRNA levels of LXRα and its target genes Abca1 and Abcg1 were decreased ( Figure 3B). Correspondingly, western blot analysis showed that the protein levels of NLRP3 and SREBP-2 were upregulated in ApoE –/ – Ch25h –/ – mice, and cleaved Caspase-1 and IL-1β were also increased, while the protein level of LXRα was decreased ( Figure 3C–E). Taken together, these results suggest that Ch25h deficiency reduces the cholesterol efflux regulated by LXRα, increases the synthesis of cholesterol mediated by SREBP-2, and increases the expression of NLRP3 inflammasome proteins, and therefore promotes hepatic steatosis.
Figure3 .
Loss of Ch25h impaired the cholesterol efflux regulated by LXRα and activated NLRP3 inflammasome in liver
ApoE–/–Ch25h+/+ and ApoE–/–Ch25h–/– mice were fed with the HFD for 12 weeks. (A) Immunohistochemistry staining of SREBP2, NLRP3 and LXRα in the livers of ApoE–/–Ch25h+/+ and ApoE–/–Ch25h–/– mice. Scale bar=50 μm. (B) mRNA level of NLRP3 inflammasome and cholesterol efflux genes in the livers of ApoE–/–Ch25h+/+ and ApoE–/–Ch25h–/– mice. Results are presented as the mean±SEM (n=5). *P<0.05 versus ApoE–/–Ch25h+/+mice. (C–E) Western blots (C) and quantification (D,E) of SREBP2, NLRP3 inflammasome and LXRα in the livers of ApoE–/–Ch25h+/+ and ApoE–/–Ch25h–/– mice. Results are presented as the mean±SEM (n=5). *P<0.05 versus ApoE–/–Ch25h+/+ mice.
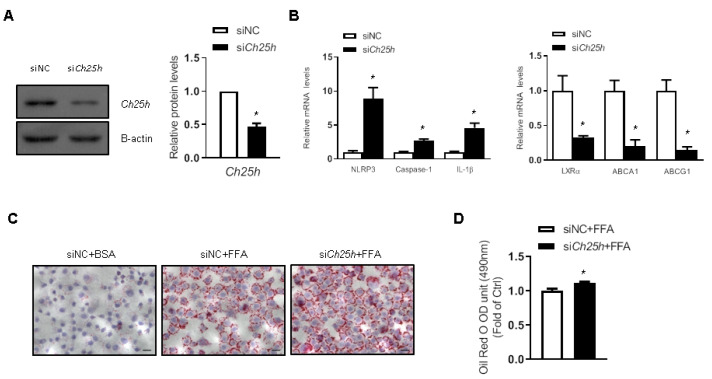
Ch25h deficiency promoted lipid droplet accumulation in LO2 cells
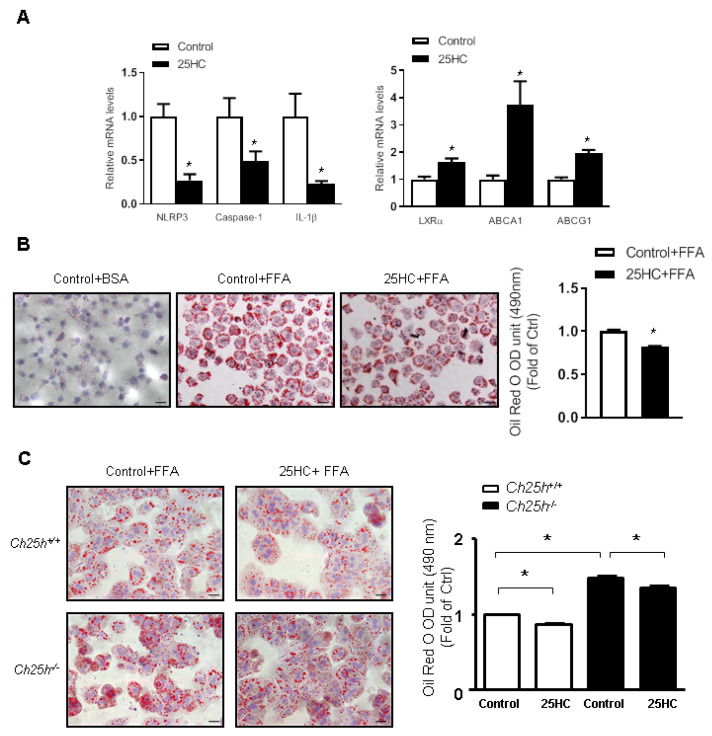
The in vivo data described above urged us to further investigate the role of Ch25h in regulating lipid metabolism and inflammasome in vitro. Transfection of Ch25h siRNA effectively decreased the expression of Ch25h in LO2 cells ( Figure 4A). Knockdown of Ch25h induced the expression of NLRP3 inflammasome proteins and the suppression of expressions of LXRα and its target genes ( Figure 4B). Furthermore, Oil Red O staining showed that Ch25h siRNA-transfected LO2 cells accumulated more lipid droplets when treated with free fat acid (FFA) ( Figure 4C). The colored lipid droplets within LO2 cells were extracted and quantitatively analyzed by measuring the absorbance at 490 nm, and the results further confirmed the above-mentioned results ( Figure 4D). These data indicated that knockdown of Ch25h suppressed LXR-mediated lipid efflux and activated inflammasome.
Figure4 .
Lacking of Ch25h in LO2 cells increased lipid droplet accumulation
LO2 cells were transfected with Ch25h siRNA for 6 h. (A) Western blots and quantification of Ch25h protein in LO2 cells. (B) mRNA levels of NLRP3 inflammasome and cholesterol efflux related genes in LO2 cells. (C) Oil Red O staining of LO2 cells treated with FFA for 24 h. Scale bar=50 μm. (D) Quantification of Oil Red O staining by measuring the optical densities at 490 nm. Results are presented the mean±SEM (n=3). *P<0.05 versus siNC.
Conversely, treatment with 25-HC induced the expressions of LXRα and its target genes, and suppressed the activation of NLRP3 inflammasome in LO2 cells ( Figure 5A). Moreover, in the presence of FFA, 25-HC treatment decreased lipid droplets accumulation within LO2 cells ( Figure 5B). To further validate the experimental results from LO2 cell line, primary hepatocytes were also isolated from the livers of Ch25h –/– mice and their littermates. These primary hepatocytes were also treated with FFA, and similar results were obtained. Knockout of Ch25h led to increased FFA accumulation within the cytoplasm of hepatocytes, while 25-HC treatment decreased lipid droplets accumulation in hepatocytes and at least partially restored its FFA efflux function ( Figure 5C). These data further proved that Ch25h and 25-HC play important roles in regulating lipid metabolism and inflammation.
Figure5 .
25-HC treatment decreased lipid droplets in LO2 cells and primary mouse hepatocytes
LO2 cells were treated with 25-HC for 6 h. (A) mRNA levels of NLRP3 inflammasome and cholesterol efflux-related genes in LO2 cells. (B) Oil Red O staining of LO2 cells treated with FFA for 24 h (Scale bar=50 μm), and quantification of Oil Red O staining by measuring the optical densities at 490 nm. Results are presented as the mean±SEM (n=3). *P<0.05 versus control. (C) Oil Red O staining of primary mouse hepatocytes treated with 25-HC for 6 h and then with FFA for 24 h, and quantification of Oil Red O staining by measuring the optical densities at 490 nm. Scale bar=50 μm. Results are presented as the mean±SEM (n=3). *P<0.05 versus control.
Ch25h expression was decreased in human NAFLD liver tissues
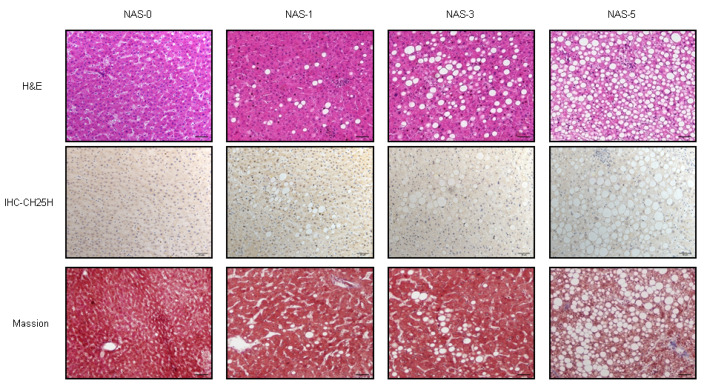
The above in vivo and in vitro data showed that Ch25h is involved in the process of hepatic steatosis in mice, we therefore further tested the roles of Ch25h in human NAFLD. We collected paraffin-embedded blocks of 30 liver tissues with NAFLD and 10 without NAFLD from the Department of Pathology of the First Affiliated Hospital of Xi’an Jiaotong University. Immunohistochemistry staining was used to detect the expression of Ch25h in these human liver tissues. Expectedly, Ch25h expression was detected in the cytoplasm of hepatocytes, and the expression level was higher in normal group than in the NAFLD group. Meanwhile, the expression level of Ch25h was proportionally decreased to a certain extent with the severity of NAFLD ( Figure 6). These results indicated that Ch25h is also involved in the development of human NAFLD.
Figure6 .
Ch25h expression is decreased in humans NAFLD
Upper: representative H&E-stained liver sections from normal and three NAFLD groups: NAS1, NAS3 and NAS5. Middle: immunohistochemistry staining of Ch25h. Bottom: Masson staining of the liver tissues. Scale bar=50 μm.
Discussion
Cholesterol metabolic dysregulation is closely associate with and contribute to the pathogenesis of NAFLD [ 22– 24]. 25-HC has been shown to have a potent ability to mediate feedback regulation of cholesterol biosynthesis [ 2, 25]. However, the role of Ch25h and 25-HC in the development of NAFLD has rarely been reported. There are even reports showing that mice lacking Ch25h have normal cholesterol homeostasis [1]. In this study, we for the first time showed that Ch25h-deficient mice maintained cholesterol metabolism only under the condition of normal diet, but had an abnormal cholesterol metabolism and were prone to NAFLD under HFD.
25-HC is a physiological regulator of cellular lipid homeostasis. It downregulates HMG-CoA reductase, a rate-limiting enzyme in the cholesterol biosynthetic pathway, by blocking the processing of SREBPs [2]. Meanwhile, 25-HC is also an endogenous ligand for the nuclear receptor liver X receptor (LXRα) [4], which regulates various metabolic pathways, including cholesterol [26], bile acids, FAs, and glucose. Our in vivo data confirmed that mice lacking Ch25h expressed high level of SREBP2 and low levels of LXRα and LXRα target genes, Abca1 and Abcg1. In vitro data further demonstrated that Ch25h knockdown in LO2 cells led to increased SREBP2 expression and decreased LXRα expression, while 25-HC treatment led to the opposite results. LXRα expression was decreased, lipid efflux was inhibited, and lipid droplets within cytoplasm were increased. These results indicated that mice lacking Ch25h were prone to NAFLD at least partially through SREBP2 and LXRα-mediated lipid metabolic pathways, including: (1) relieving the feedback inhibition of cholesterol biosynthesis; (2) inhibiting the cholesterol to bile acid conversion; and (3) inhibiting the transportation of intracellular cholesterol.
Studies showed that the accumulation of cholesterol in hepatocytes accelerates the transition from steatosis to steatohepatitis [22], aggravates liver inflammation [23], and simultaneously causes the inflammation of extrahepatic tissue [24]. Diet rich in cholesterol could obviously promote liver steatosis in mice, activate the inflammasome, and accumulate cholesterol crystals in hepatocytes. The expression of Ch25h is very strongly induced by lipopolysaccharide, by type I interferons and by viral infections, leading to increased concentration of 25-HC which mediates the inhibition of inflammasome. In this study, the in vivo data showed that mice lacking Ch25h led to the accumulation of cholesterol and the activation of NLRP3 inflammasome in the liver. In vitro data proved that knockdown of Ch25h in LO2 cells also resulted in NLRP3 inflammasome activation. Accumulated evidence showed that inflammatory stress disrupts hepatic SREBP2-mediated LDLR and HMG-CoA reductase feedback regulation, resulting in exacerbated cholesterol accumulation in hepatocytes [27]. Our data further demonstrated that the accumulation of cholesterol and inflammatory activation in hepatocytes are reciprocal causation, resulting in the development of NALFD.
A recent paper reported that Ch25h is not essential for HFD-induced NASH in Ch25h –/– mice [28], but our study showed that both Ch25h –/– mice and ApoE –/–/ Ch25h –/– mice were prone to steatosis with HFD. The only difference between these two experiments is the cholesterol content of the HFD, i.e., 0.21% in the reported study versus 1.5% in our experiment, indicating that cholesterol may play important roles in the process of NASH development. The in vitro cell experiment also showed that Ch25h deficiency led to LXRα inhibition, SREBP2 activation, NLRP3 inflammasome activation and FFA accumulation. All these data further support that mice lacking Ch25h are prone to NAFLD. In our previous study, we reported that HFD did not cause any changes in serum lipids of ApoE/ Ch25h double knockout mice [21], however, in this study, we found that serum lipids were increased in the double knockout mice compared with their littermates, when fed with the same diet. This difference may be caused by different sensitivities of the assay methods. In the previous study, a kit from Nanjing Jiancheng Bioengineering Institution was used, while in this study we used an Automatic Biochemical Analyzer which is much more sensitive. In addition, the double knockout mice are prone to atherosclerosis and hepatic steatosis with HFD, indicating that the double knockout mice have dysregulated lipid metabolism.
NAFLD is also characterized by triglyceride accumulation [29], while the relationship between Ch25h and triglyceride has not been completely elucidated, and the existing data are contradictory. One report showed that addition of 25-HC to primary rat hepatocytes increased nuclear LXR and SREBP-1 protein levels [28], up-regulated the expressions of their target genes, including acetyl CoA carboxylase 1 ( ACC1) and fatty acid synthase ( FAS) which encode the key enzymes involved in fatty acid biosynthesis. However, other studies showed that overexpression of Insig inhibited the activation of SREBP-1c [30], and 25-HC could effectively activate Insig [31]. In our study, we found that mice lacking Ch25h showed triglyceride increase in serum and accumulation in liver under HFD. Treatment of LO2 cells and primary hepatocytes with 25-HC reduced fatty acid accumulation within the cytoplasm, while knockdown of Ch25h increased fatty acid accumulation in LO2 cells and primary hepatocytes. These data convincingly indicated that Ch25h and 25-HC are involved in the activation of SREBP-1c and in the regulation of fatty acid and triglyceride synthesis, but the exact mechanism needs to be further elucidated.
In summary, our in vivo and in vitro data demonstrated that Ch25h and 25-HC play protective roles against HFD-induced hepatic steatosis, and are involved in the process of human NAFLD and NASH. Ch25h and 25-HC maintain the homeostasis of cholesterol and triglyceride metabolism, reduce their accumulation within the liver, and inhibit the inflammation induced by cholesterol accumulation. Since NAFLD has become a life-threatening problem all over the world, this study maybe helpful to the preventive treatment of human NAFLD.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grant from the National Science Foundation of China (No. 31070146).
References
- 1.Cyster JG, Dang EV, Reboldi A, Yi T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol. . 2014;14:731–743. doi: 10.1038/nri3755. [DOI] [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer′s bottle to Scap′s MELADL. J Lipid Res. . 2009;50:S15–S27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. 25-Hydroxycholesterol suppresses interleukin-1–driven inflammation downstream of type I interferon. Science. . 2014;345:679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. . 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 5.Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc Natl Acad Sci USA. . 2009;106:16764–16769. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diczfalusy U, Olofsson KE, Carlsson AM, Gong M, Golenbock DT, Rooyackers O, Fläring U, et al. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J Lipid Res. . 2009;50:2258–2264. doi: 10.1194/jlr.M900107-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park K, Scott AL. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J Leukocyte Biol. . 2010;88:1081–1087. doi: 10.1189/jlb.0610318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc M, Hsieh WY, Robertson KA, Kropp KA, Forster T, Shui G, Lacaze P, et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. . 2013;38:106–118. doi: 10.1016/j.immuni.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bah SY, Dickinson P, Forster T, Kampmann B, Ghazal P. Immune oxysterols: role in mycobacterial infection and inflammation. J Steroid Biochem Mol Biol. . 2017;169:152–163. doi: 10.1016/j.jsbmb.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Liu SY, Aliyari R, Chikere K, Li G, Marsden MD, Smith JK, Pernet O, et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. . 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. . 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arita M, Kojima H, Nagano T, Okabe T, Wakita T, Shimizu H. Oxysterol-binding protein family I is the target of minor enviroxime-like compounds. J Virol. . 2013;87:4252–4260. doi: 10.1128/JVI.03546-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold ES, Diercks AH, Podolsky I, Podyminogin RL, Askovich PS, Treuting PM, Aderem A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc Natl Acad Sci USA. . 2014;111:10666–10671. doi: 10.1073/pnas.1404271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koarai A, Yanagisawa S, Sugiura H, Ichikawa T, Kikuchi T, Furukawa K, Akamatsu K, et al. 25-Hydroxycholesterol enhances cytokine release and Toll-like receptor 3 response in airway epithelial cells. Respir Res. . 2012;13:63. doi: 10.1186/1465-9921-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Digestive Liver Dis. . 2015;47:181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Day CP. Non-alcoholic fatty liver disease: a massive problem. Clin Med. . 2011;11:176–178. doi: 10.7861/clinmedicine.11-2-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. Canadian Med Assoc J. . 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Investigation. . 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo G, Iracheta-Vellve A. Inflammasome activation in the liver: focus on alcoholic and non-alcoholic steatohepatitis. Clin Res Hepatol Gastroenterol. . 2015;39:S18–S23. doi: 10.1016/j.clinre.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. . 2012;57:642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Martin M, Zhang J, Huang HY, Bai L, Zhang J, Kang J, et al. Krüppel-like factor 4 regulation of cholesterol-25-hydroxylase and liver X receptor mitigates atherosclerosis susceptibility. Circulation. . 2017;136:1315–1330. doi: 10.1161/CIRCULATIONAHA.117.027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. . 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Zeng XY, Zhou X, Wang H, Jo E, Robinson SR, Xu A, et al. Dietary cholesterol induces hepatic inflammation and blunts mitochondrial function in the liver of high-fat-fed mice. J Nutral Biochem. . 2016;27:96–103. doi: 10.1016/j.jnutbio.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw Iii A, Kirk EA, et al. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor–deficient mice. Arterioscler Thromb Vasc Biol. . 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandutsch AA, Chen HW. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J Biol Chem. . 1974;249:6057–6061. doi: 10.1016/S0021-9258(19)42218-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Breevoort SR, Angdisen J, Fu M, Schmidt DR, Holmstrom SR, Kliewer SA, et al. Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J Clin Invest. . 2012;122:1688–1699. doi: 10.1172/JCI59817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Chen Y, Tang R, Chen Y, Li Q, Gong J, Huang A, et al. Inflammatory stress exacerbates hepatic cholesterol accumulation via increasing cholesterol uptake and de novo synthesis. J Gastroenterol Hepatol. . 2011;26:875–883. doi: 10.1111/j.1440-1746.2010.06560.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu L, Bai Q, Rodriguez-Agudo D, Hylemon PB, Heuman DM, Pandak WM, Ren S. Regulation of hepatocyte lipid metabolism and inflammatory response by 25-hydroxycholesterol and 25-hydroxycholesterol-3-sulfate. Lipids. . 2010;45:821–832. doi: 10.1007/s11745-010-3451-y. [DOI] [PubMed] [Google Scholar]
- 29.Sanyal AJ. Insulin resistance and nonalcoholic steatohepatitis: fat or fiction? Am J Gastroenterol. . 2001;96:274–276. doi: 10.1111/j.1572-0241.2001.03548.x. [DOI] [PubMed] [Google Scholar]
- 30.Engelking LJ, Kuriyama H, Hammer RE, Horton JD, Brown MS, Goldstein JL, Liang G. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J Clin Invest. . 2004;113:1168–1175. doi: 10.1172/JCI20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem. . 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]