Abstract
We have measured the humoral response to messenger RNA (mRNA) vaccines in COVID‐19 naïve and convalescent individuals. Third doses of mRNA COVID‐19 vaccines induced a significant increase in potency and breadth of neutralization against SARS‐CoV‐2 variants of concern (VoC) including Omicron subvariants BA.1, BA.2, and BA.2.12.1, that were cross‐neutralized at comparable levels and less for BA.4/5. This booster effect was especially important in naïve individuals that only after the third dose achieved a level that was comparable with that of vaccinated COVID‐19 convalescents except for BA.4/5. Avidity of RBD‐binding antibodies was also significantly increased in naïve individuals after the third dose, indicating an association between affinity maturation and cross neutralization of VoC. These results suggest that at least three antigenic stimuli by infection or vaccination with ancestral SARS‐CoV‐2 sequences are required to induce high avidity cross‐neutralizing antibodies. Nevertheless, the circulation of new subvariants such as BA.4/5 with partial resistance to neutralization will have to be closely monitored and eventually consider for future vaccine developments.
Keywords: avidity, COVID‐19, neutralizing antibodies, Omicron, SARS‐CoV‐2, vaccines, variants of concern
1. BACKGROUND
The emergence of the different SARS‐COV‐2 variants of concern (VoC) have represented an important challenge to achieve high levels of protection for COVID‐19 1 especially after the rapid spread of the Omicron VoC which combines an unprecedented number of mutations in its sequence clearly related with an increased transmission efficiency and neutralizing scape. 2 This was evident first for the Omicron BA.2 subvariant and now for the subvariants BA.2.12 and BA.5 that are currently dominating the pandemic transmission. 3 After the first prime‐boost administration of messenger RNA (mRNA) vaccines, a third dose has shown an important boost of potency and breadth of neutralizing response. 4 However, it is unclear to what extend this response can neutralize the new Omicron subvariants. Here we have investigated the neutralizing response after the third mRNA vaccine dose against SARS‐CoV‐2 VoC including Omicron BA.1, BA.2, BA.2.12.1, and BA.4/5 in a cohort of COVID‐19 convalescent patients in comparison with only‐vaccinated naïve individuals.
2. METHODS
We have included 21 COVID‐19 convalescent and 21 naïve healthcare workers from the Hospital Universitario 12 de Octubre in Madrid, Spain. Both groups were part of a follow‐up study (Solidarity II cohort, IRB approval ref CEIm 20/157) and were recruited after informed consent and randomly selected among those with serum samples available for the study period. Mean age was 49 and 48 years for the convalescent and naïve groups, respectively. All infections in convalescent individuals took place during the epidemic wave of COVID‐19 affecting Madrid during March–April 2020, and all had a mild clinical evolution. All participants were vaccinated in January–February 2021 with two doses of the Pfizer‐BNT162b2 vaccine 21 days apart. Blood samples were obtained at 61 days (range: 42–77) and 242 days (range: 238–252) after the first dose in the COVID‐19 convalescent group and at 67 days (range: 49–97) and 241 (range: 228–252) in the COVID‐19 naïve group. A third dose of 50 ug of the Moderna mRNA‐1273 vaccine was administered in December 2021 according with EMA recommendations. 5 Participants with documented SARS‐CoV‐2 infections by RT‐PCR or serology (anti‐N) were excluded. From the original group, 15 samples from COVID‐19 convalescent and 12 from the naïve group were collected 48 days after the third dose (range: 41–57). Detailed description of the cohort is summarized in Table S1.
2.1. VSV‐based pseudovirus neutralizing assays
VSV‐G pseudotyped rVSV‐luc recombinant viruses were produced according to previously published protocols. 6 , 7 The SARS‐CoV‐2 Spike mutant D614G was generated by site‐directed mutagenesis using a vector encoding SARS‐CoV‐2 Spike_614D protein (kindly provided by J. Garcia‐Arriaza, CNB‐CSIC). SARS‐CoV‐2 variant Alpha (B.1.1.7, GISAID: EPI_ISL_608430), SARS‐CoV‐2 variant Beta (B.1.351, GISAID: EPI_ISL_712096), SARS‐CoV‐2 variant Gamma (P.1, GISAID: EPI_ISL_833140), SARS‐CoV‐2 variant Delta (B.1.617.2, GISAID: EPI_ISL_1970335), SARS‐CoV‐2 variant Omicron BA.1 (B.1.1.529, GISAID: EPI_ISL_6640917), SARS‐CoV‐2 variant Omicron BA.2 (B.1.1.529, GISAID: EPI_ISL_6795834.2), SARS‐CoV‐2 variant Omicron BA.2.12.1 (B.1.1.529, GISAID: EPI_ISL_12304821) SARS‐CoV‐2 variant Omicron BA.4/5 (B.1.1.529, GISAID: EPI_ISL_12278971) were synthesized and cloned into pcDNA3.1 (Geneart). Neutralizing titer 50 (NT50) was calculated using a nonlinear regression model fit with settings for log agonist versus normalized response curve, in GraphPad Prism v8. Neutralization potency of serum samples was calibrated using World Health Organization (WHO) International Standard 20/136. 8
2.2. Avidity assay
The wild type RBD region of the Spike protein of SARS‐COV‐2 kindly provided by Florian Krammer was used as antigen for the avidity assay. 9 A modified ELISA protocol using urea 5 M as a chaotropic agent was performed to determine the avidity of the samples. 10 Micro‐well 96 plates were antigen coated for 24 h at 4°C with 50 μl of a 1 μg/ml phosphate‐buffered saline (PBS) solution. The next day, plates were washed 3 times with 200 μl of 0.1% PBST per well. The coating solution was removed and 100 μl of blocking solution (PBS with 0.1% Tween 20, PBST, and 3% nonfat milk) per well was added to the plates. Sera from each participant were diluted into PBST containing 1% nonfat‐milk at a concentration which would give an optical density (OD) reading close to 1. Blocking solution was removed and 120 μl of these dilutions were inoculated in duplicate per well and incubated for 2 h at room temperature. Following the incubation, the plates were washed as described in the previous step. One of the duplicated was then incubated for 15 min with PBS while the other duplicate was incubated with a 5 M solution of urea, which acted as a chaotropic agent. Another cycle of washing was performed and a 50 μl per well of 1:1.500 diluted goat anti‐human immunoglobulin G–horseradish peroxidase conjugated secondary antibody (Thermo Fisher Scientific) was added to each well. After 1 h, the plates were washed three times again. Next, 100 μl of SIGMAFAST OPD solution was added to each well. Following 10 min of incubation, the reaction was stopped by adding 50 μl per well of 3 M hydrochloric acid. The OD at 490 nm (OD490) was measured using a Thermo Scientific Multiskan FC plate reader. Avidity index (AI) was calculated for each sample simply by dividing the OD obtained in the urea‐incubated well by the PBS‐incubated one. Differences between groups were determined by a nonparametric Mann–Whitney test, using GraphPad Prism v8.
3. RESULTS
In COVID‐19 naïve individuals, neutralizing titers after prime‐boost vaccination with BNT‐162b2 were very low against all Omicron subvariants BA.1, BA.2, BA.12.1, and BA.4/5: 37, 53, 34, and 34 IU/ml, respectively. These levels of neutralization were significantly lower than those achieved in the convalescent group after the first two doses of mRNA vaccine (Figure 1). However, after the third dose neutralizing titers increased to 832, 742, 531, and 238 IU/ml, respectively (Figure 1), achieving now a comparable neutralizing potency as the convalescent group that was non statistically significative for all subvariants except BA.4/5 (607 vs. 328 IU/ml in the convalescent vs the naïve group [p < 0.05]) (Figure 1). Neutralizing levels for the same time points for SARS‐CoV‐2 VoC Alpha, Beta, Gamma, Delta followed a similar pattern as Omicron BA.1, BA.2, and BA.12.1 (Figure S1), significantly lower in naïve individuals after the first two doses (p < 0.001) and comparable to convalescents after the third dose. This pattern of response had a correlation with results obtained with RBD‐binding total antibodies at the same time points in both groups of participants, ie RBD‐binding Ig levels were basically the same between convalescents and naïve only after the third dose (Figure S2).
Figure 1.
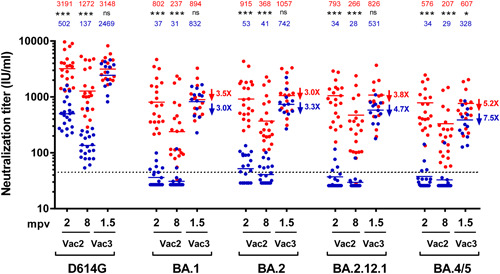
Neutralizing activity against SARS‐CoV‐2 Omicron subvariants: COVID‐19 convalescent vaccinated (n = 21) individuals are presented as scatter red dot plots and COVID‐19 naïve vaccinated (n = 21) are presented as scatter blue dot plots. Both groups were tested at 2 and 8 months post‐two doses of BNT162b2 vaccination (mpv) indicated on the graph as Vac2. Additionally, 15/21 of COVID‐19 convalescent vaccinated and 12/21 of COVID‐19 naïve vaccinated were tested at 1.5 months post‐Moderna mRNA‐1273 vaccination indicated on the graph as Vac3. Neutralization potency of serum samples were calibrated using WHO International Standard 20/136 and are presented on the graph as International Units per ml (IU/ml). Solid red lines and numbers correspond to geometric mean in vaccinated COVID‐19 convalescent individuals. Solid blue lines and numbers correspond to geometric mean in vaccinated COVID‐19 naïve individuals. Dashed line marks the cut‐off titer for neutralization assay (45 IU/ml). NT50 was calculated from individual results obtained by duplicates/triplicates using a nonlinear regression model fit with settings for log inhibitor versus normalized response curves by GraphPad Prism v8. Calibrated NT50 in IU/ml were calculated as the observed NT50 titers multiplied by the calibration factor, which is estimated as 813 IU/ml divided by NT50 of the WHO International Standard 20/136 tested in parallel in each assay. NT50 decrease fold of each Omicron variant as compared to D614G in Vac3 group is indicated by arrow and number next to corresponding geometric mean line. NT50 decrease fold in COVID‐19 convalescent vaccinated individuals are presented in red and NT50 decrease fold in COVID‐19 naïve vaccinated are presented in blue. ns: not significant; *p < 0.05; **p < 0.01; ***p < 0.001. IU/ml, international units per ml; mpv, months postvaccination; mRNA, messenger RNA; Vac2, 2 doses of BNT162b2 vaccination; Vac3, third dose of Moderna mRNA‐1273 vaccination; WHO, World Health Organization.
In the avidity assay, naïve individuals showed a statistically significant lower AI than convalescent individuals after 2 vaccine doses with a median AI of 0.50 vs. 0.86, respectively (p < 0.0001). After the third dose, avidity greatly increased in the naïve group, from a median AI of 0.50–0.88 (p < 0.0001). No statistically significant differences in the AI were found after 2 and 3 doses in the convalescent group (0.86 vs. 0.92, p = 0.0607) (Figure 2). Noticeably, all 12 naïve individuals had an increase in the AI in the 2 measurements (Figure 2).
Figure 2.
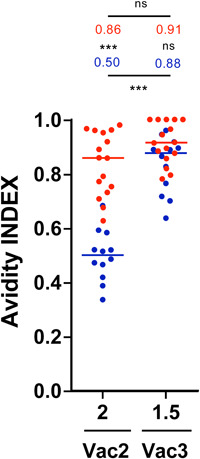
Avidity index (AI): AI in both naive and convalescent individuals 2 months after 2 vaccine doses and 1.5 months after a third dose. Individual and median results from the naive group shown in blue and from the convalescent group in red. ***p < 0.001. ns, not statistically significant.
4. DISCUSSION
In the current context of the evolution of the COVID‐19 pandemics it is particularly important to understand the level of protection against new SARS‐CoV‐2 variants conferred by both vaccination and infection. In the last months different subvariants of the VoC Omicron have been competing for the transmission globally and most recently it seems that the subvariant BA.5 is dominating the dynamics in most regions probably due to advantages in transmission and evasion of immune response. 3 , 11
In our study we have aimed to understand the evolution of the neutralizing response and its cross reactivity after repeated immune stimuli by infection and/or vaccination. To measure neutralization to the different SARS‐CoV‐2 variants we have used a VSV‐based pseudovirus that is fully validated for this purpose 12 and results have been normalized with the WHO International Standard 20/136. 8
A third dose of an mRNA vaccine resulted as expected in an increase of the neutralizing response whose potency and breadth that was especially significant in naïve individuals as compared with COVID‐19 convalescent patients. This later group, as described, 13 experienced after the first two doses a great neutralizing response with a wide coverage against SARS‐CoV‐2 Omicron subvariants, although less potent against BA.4/5. A third dose of an mRNA vaccine restored neutralizing levels in convalescents after a waning process developed during the subsequent months. However, the effect of a third dose in naïve individuals induced a significant increase of neutralizing titers against the reference SARS‐CoV‐2 sequence that exceeded the levels achieved after the first two doses (fivefold, p < 0.0001) and in a similar range of titers achieved by convalescent (GMT 2469 and 3148 IU/ml, respectively). Neutralizing titers against VoC Alpha, Beta, Gamma, and Delta exhibited the same pattern of a significant increase in the naïve group as compared with convalescents (p < 0.001). In a similar way, the neutralizing activity against Omicron experienced an important boost after the third dose achieving comparable level as those of convalescents for BA.1, BA.2, BA.2.12.1 but not for BA.4/5. Neutralization against the two Omicron subvariants BA.1 and BA.2 was comparable suggesting that the initial prevalence of BA.2 subvariant over BA.1 was not related to a higher resistance to neutralization but most probably due to increased transmission properties. Our results highlight the neutralizing resistance of the subvariants BA.4/5 whose Spike sequences are identical. It is in fact for this highly mutated Omicron subvariants that protection by neutralizing antibodies induced by ancestral SARS‐CoV‐2 is less potent and also a difference between the antibodies of the vaccinated convalescents and the only vaccinated group is noticed. This most probably indicates a different quality of the so‐called hybrid immunity in terms of cross‐neutralization of the most antigenically distant variants such as BA.4/5. This is supported by the avidity data that indicate an important increased of the AI in the naïve group only after the third dose achieving comparable levels of those exhibited by vaccinated convalescents after the first two doses. These results also support the fact that repeated events of immune stimulation either by natural infection and/or vaccination are required to achieved maximum levels of neutralizing response with a wide breadth against SARS‐CoV‐2. The number of stimulation events is likely limited to three, since a fourth dose of an mRNA vaccine did not significantly increase the neutralizing levels achieved after three doses, as it has been recently observed. 14 In the naïve group the third dose of mRNA vaccine induced a clear boosting of neutralizing response in terms of potency and breadth that was comparable for the first time to the levels of convalescents vaccinated. Affinity maturation of antibodies against SARS‐CoV‐2 Spike protein induced by repeated exposures to infection by pre‐VoC SARS‐CoV‐2 and/or mRNA vaccines based in the ancestral sequence appears to play a role in the increased breadth of neutralization demonstrated in both groups against Omicron subvariants. 15 COVID‐19 vaccines and specially those based in mRNA confer durable protection against severe disease and death upon SARS‐CoV‐2 infection. This is also achieved by the stimulation of cell‐mediated immunity that seems to be well preserved against the circulating VoC. 16 However, at the upper respiratory tract mucosal level, neutralization mediated by antibodies is the main response able to block infection and the level of neutralizing antibodies is considered the best correlate of protection for COVID‐19 vaccines. 17 This response can be outsmarted by the great capability of SARS‐CoV‐2 to accommodate changes in critical neutralizing epitopes within the spike protein and particularly the RBD region. Three doses of mRNA COVID‐19 vaccines, being the third stimulus the most potent, achieved high levels of neutralizing antibodies with a reasonable coverage of VoC. Nevertheless, new subvariants of the highly mutated Omicron VoC with further antigenic changes such as BA.4/5 can partially scape of this elaborate defensive output. Based in the results obtained in convalescent patients undergoing the same vaccination schedule, it does not appear that subsequent vaccine doses will achieve further potency apart from restoration of previous levels. These results have implication in the development of long‐term vaccination strategies.
AUTHOR CONTRIBUTIONS
Joanna Luczkowiak, Gonzalo Rivas, Nuria Labiod and Fátima Lasala: performed experimental work. Marta Rolo, Jaime Lora‐Tamayo, Mikel Mancheno‐Losa, David Rial‐Crestelo, Alfredo Pérez‐Rivilla and María Dolores Folgueira: were responsible of clinical cohort, sampling and database management. Rafael Delgado: designed the study and wrote the first version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary information.
Supplementary information.
Supplementary information.
ACKNOWLEDGMENTS
We are greatly thankful to all participants in the Solidarity II cohort study. This work was supported by grants from the Instituto de Investigación Carlos III, ISCIII, (FIS PI1801007 and PI2100989), by the European Commission Horizon 2020 Framework Programme: Project VIRUSCAN FETPROACT‐2016: 731868, Horizon Europe Framework programme: Project EPIC‐CROWN‐2 ID: 101046084 and by Fundación Caixa‐Health Research (Project StopEbola HR18‐00469) to RD. M.M‐L holds a clinical research contract Río Hortega (CM19/00226) from the Instituto de Salud Carlos III, ISCIII (Spanish Ministry of Science, Innovation and Universities).
Luczkowiak J, Rivas G, Labiod N, et al. Cross neutralization of SARS‐CoV‐2 omicron subvariants after repeated doses of COVID‐19 mRNA vaccines. J Med Virol. 2022;95:e28268. 10.1002/jmv.28268
DATA AVAILABILITY STATEMENT
Raw data of this work is available in: https://www.dropbox.com/s/966kniph4010xgr/Supplementary%20Data_JMV-22-16944.pzfx?dl=0.
REFERENCES
- 1. Goldberg Y, Mandel M, Bar‐On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS‐CoV‐2 omicron variant. N Engl J Med. 2022;386(6):599‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS‐CoV‐2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387:86‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wratil PR, Stern M, Priller A, et al. Three exposures to the spike protein of SARS‐CoV‐2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nature Med. 2022;28:496‐503. 10.1038/s41591-022-01715-4 [DOI] [PubMed] [Google Scholar]
- 5. Spikevax: EMA . EMA recommendation on booster. Oct 25, 2021. Available from https://www.ema.europa.eu/en/news/spikevax-ema-recommendation-booster
- 6. Whitt MA. Generation of VSV pseudotypes using recombinant ΔG‐VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J Virol Methods. 2010;169(2):365‐374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luczkowiak J, Labiod N, Rivas G, et al. Neutralizing response against SARS‐CoV‐2 variants 8 months after BNT162b2 vaccination in naive and COVID‐19–Convalescent individuals. J Infect Dis. 2021;225:1905‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu F, Althaus T, Tan CW, et al. WHO international standard for SARS‐CoV‐2 antibodies to determine markers of protection. The Lancet Microbe. 2022;3(2):e81‐e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stadlbauer D, Amanat F, Chromikova V, et al. SARS‐CoV‐2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Current Protocols in Microbiology. 2020;57(1):e100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis CW, Jackson KJL, McElroy AK, et al. Longitudinal analysis of the human B cell response to ebola virus infection. Cell. 2019;177(6):1566‐1582.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tegally H, Moir M, Everatt J, et al. Emergence of SARS‐CoV‐2 Omicron lineages BA.4 and BA.5 in South Africa. Nature Med. 2022;28:1785‐1790. 10.1038/s41591-022-01911-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luczkowiak J, Radreau P, Nguyen L, et al. Potent neutralizing activity of polyclonal equine antibodies against severe acute respiratory syndrome coronavirus 2 variants of concern. J Infect Dis. 2022:jiac331. 10.1093/infdis/jiac331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stamatatos L, Czartoski J, Wan Y‐H, et al. mRNA vaccination boosts cross‐variant neutralizing antibodies elicited by SARS‐CoV‐2 infection. Science. 2021;372:1413‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Regev‐Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of Covid‐19 mRNA vaccine against Omicron. N Engl J Med. 2022;386:1377‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muecksch F, Wang Z, Cho A, et al. Increased memory B cell potency and breadth after a SARS‐CoV‐2 mRNA boost. Nature. 2022;607:128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS‐CoV‐2 spike cross‐recognize Omicron. Nature. 2022;603:488‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nature Med. 2021;27(7):1205‐1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary information.
Supplementary information.
Data Availability Statement
Raw data of this work is available in: https://www.dropbox.com/s/966kniph4010xgr/Supplementary%20Data_JMV-22-16944.pzfx?dl=0.


