Abstract
Background
Social disparities in cancer survival have been demonstrated in Australia despite a universal healthcare insurance system. Colorectal cancer is common, and reasons for survival disparities related to socioeconomic status need to be investigated and addressed. The aim is to evaluate the current Australian literature concerning the impact of socioeconomic status on colorectal cancer survival and stage at presentation.
Methods
A systematic search of PUBMED, EMBASE, SCOPUS and Clarivate Web of Science databases from January 2010 to March 2022 was performed. Studies investigating the impact of socioeconomic status on colorectal stage at presentation or survival in Australia were included. Data were extracted on author, year of publication, state or territory of origin, patient population, other exposure variables, outcomes and findings and adjustments made.
Results
Of the 14 articles included, the patient populations examined varied in size from 207 to 100 000+ cases. Evidence that socioeconomic disadvantage was associated with poorer survival was demonstrated in eight of 12 studies. Evidence of effect on late stage at presentation was demonstrated in two of seven studies. Area‐level measures were commonly used to assess socioeconomic status, with varying indices utilized.
Conclusion
There is limited evidence that socioeconomic status is associated with late‐stage at presentation. More studies provide evidence of an association between socioeconomic disadvantage and poorer survival, especially larger studies utilizing less clinically‐detailed cancer registry data. Further investigation is required to analyse why socioeconomic disadvantage may be associated with poorer survival.
Keywords: Australia, colorectal neoplasms, healthcare, outcome assessment, social class, socioeconomic factors
The aim of this scoping review is to evaluate the current Australian literature concerning the impact of socioeconomic status on colorectal cancer survival and stage at presentation. There is limited evidence that socioeconomic status is associated with late‐stage at presentation. More studies provide evidence of an association between socioeconomic disadvantage and poorer survival, especially larger studies utilizing less clinically‐detailed cancer registry data.
Introduction
Social inequalities in cancer are a global phenomenon with disparities existing within as well as between countries. In general, data suggest that a social gradient for cancer outcomes exists, with most disadvantaged communities having higher incidence and increased mortality for many types of cancer. 1 , 2 Further research into which components of socioeconomic inequalities might contribute to poorer outcomes is required in order to inform public health policy and to target areas that are likely to lead to improved cancer outcomes. Socioeconomic advantage and disadvantage is a complex concept that has multiple domains; in broad terms it is defined by the Australian Bureau of Statistics (ABS) as ‘people's access to material and social resources, and their ability to participate in society’. 3 An individual's position in society, occupation, education and income can influence healthcare status from early in life. 4
As of 2021 in Australia, colorectal cancer is the fourth most common cancer with an aged‐standardized rate of 49.7 cases per 100 000 persons. It is the second leading cause of cancer‐related deaths, and has the third highest cancer expenditure nationally. 5 , 6 Disparities in cancer survival between the least and most socially disadvantaged groups have been demonstrated for colorectal cancer, despite a universal health insurance scheme in Australia. 5 , 7 , 8 , 9 The disparity in cancer survival by socioeconomic status is likely to be multifactorial. Possible cancer‐specific factors could include increased tumour aggressiveness or decreased host response, which could be related to risk factor exposure, general health or lifestyle issues. Possible non‐cancer‐specific factors could include decreased uptake, availability and quality of investigations and treatments. 2
Cancer stage at diagnosis is an independent factor for survival; 5‐year relative survival is high (98.6%) for early‐stage colorectal cancer but decreases to 71% for locally advanced stage, and 13% for metastatic stage. 10 Evaluating if socioeconomic status has an effect on cancer stage at diagnosis, as one of the potential contributing factors in the association between lower socioeconomic status and colorectal cancer survival, has implications for health service provision. The evidence, however, of an association between socioeconomic status and cancer stage at diagnosis within Australia is inconsistent. 11 There is varying evidence among different countries on the contribution of cancer stage at diagnosis to the effect of socioeconomic disparities on cancer survival. 12 In Australia, analysis by Afshar et al. found that stage at diagnosis did not fully account for differences in survival by socioeconomic status. 12 One of the challenges of investigating the relationship between cancer stage at diagnosis and socioeconomic disadvantage on a population level is the relative lack of detailed staging information in population‐based cancer registries, which could explain why findings are inconsistent among different data sources. 13 , 14
Rather than information derived from individual patients, area‐level indices are frequently used to capture relative socioeconomic advantages or disadvantages. Within a certain geographic area, these indices are based on an aggregate of various measures, such as average household income, rate of unemployment, the highest level of education and number of unskilled workers. The Socioeconomic Indexes for Areas (SEIFA) scores are commonly used area‐level measures of socioeconomic status in Australia. There are four different SEIFA indices: IRSD (Index of relative socioeconomic disadvantage), IRSAD (Index of relative socioeconomic advantage and disadvantage), IER (Index of economic resources) and IEO (Index of education and occupation). Currently, the smallest geographic area unit measured consists of an average of 400 people. 3 SEIFA scores using larger geographical areas such as local government area (LGA), postal area (postcode) or suburb are aggregated from population‐weighted averages from the smallest base area unit. Previously, the smallest base area unit used was census collection district (CD), which was composed of about 200 dwellings and varied more in population size and character. 15
The objective of this scoping review was to map and summarize the literature on the topic of socioeconomic status and its impact on colorectal cancer in Australia, specifically its impact on cancer stage at presentation and cancer survival. A preliminary search for pre‐existing scoping reviews on this topic has been conducted on MEDLINE with no results returned.
Methods
This methodology of this scoping review was based on the structure proposed by the Joanna Brigg's Institute (JBI) scoping review methodology group and developed according to the PRISMA‐Scr guidelines. 16 , 17 A review protocol was registered on the Open Sciences Framework (osf.io), registration DOI: 10.17605/OSF.IO/5F8AS.
Search strategy
A systematic search was conducted using the databases PUBMED, Elsevier EMBASE, Elsevier SCOPUS and Clarivate Web of Science to identify studies that examine the impact of socioeconomic disparities in cancer presentation, cancer outcomes and cancer survival in Australian adults. Different combinations of the following search terms, keywords and Medical Subject Headings (MeSH) were used in combination with Boolean operators: Socioeconomic status OR socioeconomic factors; Colorectal cancer OR colorectal neoplasms; Australia.
Studies published from 2010 to current were included, as studies conducted prior to this were felt to be less relevant given changes in measures of area‐level socioeconomic status over time, and also advances in screening and treatments for colorectal cancer in that time period. Reference lists from selected articles were reviewed to ensure additional relevant studies were identified and included. Eligible studies were peer‐reviewed English language articles that were conducted in Australia, with the main exposure variable being socioeconomic status or disparity, and main outcome being stage of cancer presentation and survival. Review articles were excluded. The date of the most recent search was 1 March 2022.
Selection of articles
All references identified through the database searches were exported to Endnote X9 citation software. After data extraction and removal of duplicates, screening of titles and abstracts was completed by two reviewers (N.T. and I.H.); full text of selected studies that met inclusion and exclusion criteria was read to assess for relevance to the aim of this scoping review. Any ambiguity or disagreement during the selection process was resolved through discussion or consultation with a third reviewer (A.S.).
Charting of data
Data extraction was conducted by the first author using a preliminary data charting table designed for this review. Information collated included author, year of publication, origin (state or territory), aims, study population, cancer streams investigated, methodology including type of socioeconomic measure used (area‐level or individual‐level measures, SEIFA index used), type of cancer staging used and key findings.
Results
Study selection
The initial literature search identified 187 articles after deduplication. After initial screening of title and abstracts, 144 studies were excluded. Of the remaining 43 studies, 14 were included in the final analysis. The Prisma‐Scr flow diagram is demonstrated in Figure 1.
Fig. 1.
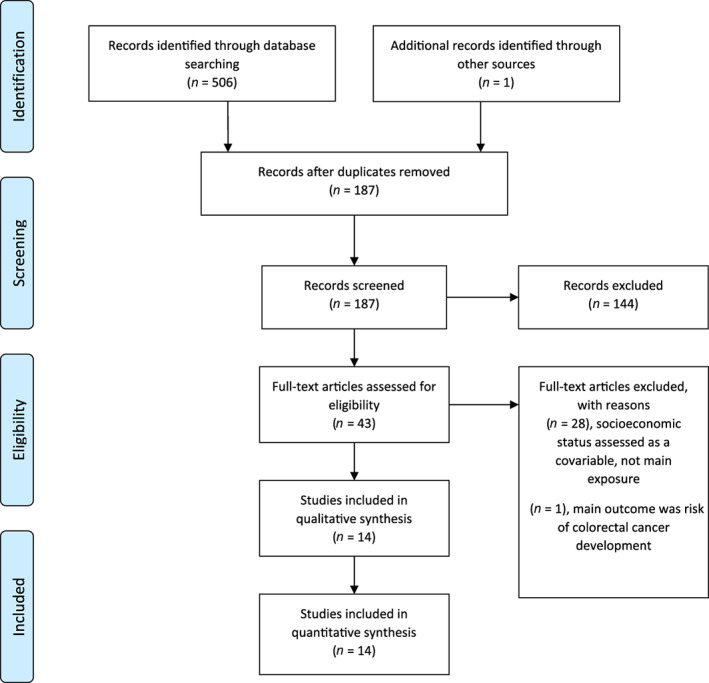
Prisma‐Scr flow diagram.
Study characteristics
A summary of the articles analysed is charted in Table 1. Of the 14 articles, seven were based on data from New South Wales (NSW), three from Victoria, three from Queensland and one from South Australia. The impact of socioeconomic status on survival outcomes was investigated in 12 articles whereas stage at diagnosis was the primary outcome assessed in seven articles, with two further studies assessing stage as a secondary outcome. Individual‐level measures of socioeconomic status were only utilized in one study conducted by Yu et al. (highest level of education attainment), all others used various area‐level socioeconomic status measures. The most common SEIFA index used as an area‐level measure of socioeconomic status was the index of relative socioeconomic disadvantage (IRSD). In general, smaller, hospital‐based studies tended to use postcode as the geographic area unit for whichever SEIFA index was used, whereas studies using state‐based cancer registry data used smaller geographic area units for the SEIFA index employed.
Table 1.
Characteristics of included studies on impact of socioeconomic status on colorectal cancer in Australia, 2010–2022
| Author, year, state or territory | Cancer stream | Data sources | Population, year of diagnosis | Exposures | Outcomes | Adjustments |
|---|---|---|---|---|---|---|
|
Ngoo et al., 2020 18 QLD |
Rectal cancer Patients requiring neoadjuvant therapy and resection |
Single institution clinical database |
207 patients 2007–2017 |
Type of neoadjuvant therapy Area‐level socioeconomic status Geographical remoteness |
5‐year cancer specific survival and recurrence‐free survival Local recurrence |
Demographics Pathology |
|
Pasch et al., 2021 19 NSW |
Colorectal cancer Surgical resection patients |
Single institution clinical database linked to death index |
405 patients 2011–2019 |
Area‐level socioeconomic status Geographical remoteness |
5‐year overall survival Stage at presentation |
Demographics Pathology Stage |
|
Barclay et al., 2015 20 VIC |
Colorectal cancer | Single institution hospital records |
557 patients 2005–2010 |
Area‐level socioeconomic status Demographic factors |
Stage at presentation Overall survival |
Demographics Pathology Stage Type of surgery |
|
Macdermid et al., 2021 21 NSW |
Colorectal cancer Surgical resection patients |
CONCRETES database, 6 hospital clinical database of colorectal cancer resections linked with death index data |
1596 patients 2010–2016 |
Area‐level socioeconomic status |
Stage at presentation Cancer‐specific and all‐cause 5‐year survival |
Kaplan Meir analysis matched for age and birth country |
|
Yu et al., 2021 22 NSW |
Colorectal cancer | 45 and Up prospective survey study linked to NSW cancer registry and other admin. Databases |
1720 participants 2006–2009 |
Individual and area‐level socioeconomic status Patient, lifestyle, disease and treatment factors |
Cancer‐specific survival |
Demographics Patient factors Lifestyle factors Treatment factors |
|
Afshar et al., 2021 23 VIC |
Colon cancer | Victorian cancer registry linked to Victorian death index |
2203 patients 2008–2011 |
Area‐level socioeconomic status | 1‐and 5‐year net survival |
Demographics Comorbidities Stage Treatment |
|
Beckmann et al., 2016 14 SA |
Colorectal cancer | SA cancer registry linked to hospital admin. and clinical data and radiotherapy registry |
4641 patients 2003–2008 |
Area‐level socioeconomic status Geographical remoteness |
Colorectal cancer‐specific survival Stage at presentation |
Demographics Pathology Year of diagnosis Geographic remoteness Comorbidities Treatment |
|
Baade et al., 2011 24 QLD |
Colorectal cancer | QLD cancer registry |
18 561 patients 1997–2007 |
Area‐level socioeconomic status Geographic remoteness |
Advanced stage at presentation (Stage III‐IV) |
Demographics Year of diagnosis Geographic remoteness Cancer type |
|
Baade et al., 2013 9 QLD |
Colorectal cancer | QLD cancer registry |
22 727 patients 1997–2007 |
Area‐level socioeconomic status Geographic remoteness |
5‐year overall survival 5‐year colorectal cancer specific survival |
Demographics Pathology |
|
Tervonen et al., 2017 25 NSW |
Multiple cancer streams | NSW cancer registry |
264 236 cases 39 728 colorectal cancer cases 2000–2008 |
Area‐level socioeconomic status, different area units |
Distant summary stage at diagnosis Risk of cancer death (Sub‐hazard ratio) |
Demographics Geographic remoteness Stage Cancer site |
|
Afshar et al., 2020 2 VIC |
Multiple cancer streams | VIC cancer registry |
331 419 patients 44 483 colorectal cancer patients 2001–2015 |
Area‐level socioeconomic status |
5‐year net survival Excess risk of death from cancer diagnosis |
Demographics Year of diagnosis |
|
Stanbury et al., 2016 7 NSW |
Multiple cancer streams | NSW central cancer registry |
377 493 cases 72 486 colorectal cancer cases 1991–2008 |
Area‐level socioeconomic status | 5‐year relative (net) survival |
Demographics Year of follow up SEIFA quintile Stage |
|
Tervonen et al., 2016 11 NSW |
Multiple cancer streams | NSW central cancer registry |
699 382 cases 107 852 colorectal cases 1980–2009 |
Area‐level socioeconomic status Geographic remoteness Country of birth |
Distant summary stage at diagnosis |
Demographics Diagnostic time period Cancer site |
|
Stanbury et al., 2016 26 NSW |
Multiple cancer streams | NSW central cancer registry linked to death index |
176 322† cases 1999–2008 |
Area‐level socioeconomic status, size of geographic area unit | 5‐year relative (net) survival |
Demographics Stage SEIFA quintile |
Denotes number of cases in total from 10 different cancer streams, no breakdown of colorectal cancer numbers.
Ordered by number of colorectal cancer cases, smallest to largest. NSW, New South Wales; QLD, Queensland; SA, South Australia; SEIFA, Socioeconomic Indexes for Areas; VIC, Victoria.
Five studies examined multiple cancer streams with sub‐group data for colorectal cancer, nine studies pertained only to colorectal cancer. In terms of data sources, the majority of studies used state‐based cancer registry data, three were based on single institution clinical data and one used patient survey data (45 and Up prospective survey study 22 ). The patient populations examined varied substantially and ranged from a cohort of 207 patients to over 100 000 cases. Three studies only investigated patients who had undergone surgical resection for colorectal cancer, and one study focused on patients with locally advanced rectal cancer who required neoadjuvant radiotherapy. 18 , 19 , 20 , 21
Study findings (impact of socioeconomic status on stage at presentation and survival)
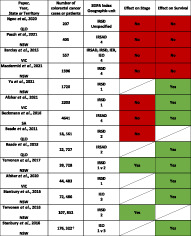
A summary of the results is presented in Figure 2. In general, larger studies utilizing cancer registry information and data linkage demonstrated an effect of socioeconomic deprivation on survival. Evidence that socioeconomic disadvantage was associated with poorer survival was demonstrated in eight studies. 2 , 7 , 9 , 11 , 14 , 22 , 23 , 25 , 26 Smaller, single institution studies did not demonstrate any effect of socioeconomic deprivation on stage or cancer survival. 18 , 19 , 20 Various methodologies were used to determine survival, which included overall survival, relative or net survival and cancer‐specific survival.
Fig. 2.
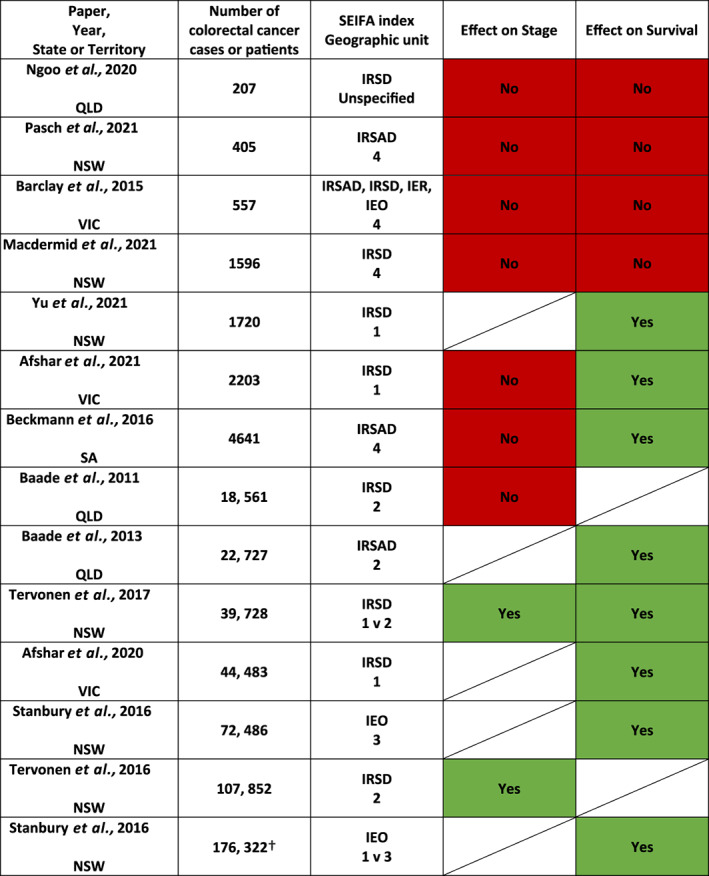
Heat map of evidence associated with socioeconomic deprivation on stage and survival. SEIFA (Socioeconomic indexes for areas) indices: IRSD (Index of relative socioeconomic disadvantage), IRSAD (Index of relative socioeconomic advantage and disadvantage), IEO (Index of education and occupation), IER (Index of economic resources). SEIFA geographic unit: 1–5 (smallest to largest geographic area unit). 1 = census collection district or statistical area level 1, 2 = statistical local area or statistical area level 2, 3 = local government area, 4 = postal area, 5 = suburb. Effect on stage or survival:  No effect
No effect  Effect
Effect  Not assessed. † Denotes number of cases in total from 10 different cancer streams, no breakdown of colorectal cancer numbers.
2
,
7
,
9
,
11
,
14
,
18
,
19
,
20
,
21
,
22
,
23
,
24
,
25
,
26
Not assessed. † Denotes number of cases in total from 10 different cancer streams, no breakdown of colorectal cancer numbers.
2
,
7
,
9
,
11
,
14
,
18
,
19
,
20
,
21
,
22
,
23
,
24
,
25
,
26
The impact of socioeconomic status on stage at presentation was inconsistent, and only two large studies from NSW demonstrated that socioeconomic disadvantage was associated with an increased odds of distant summary staging at diagnosis (where distant stage is defined as tumours that have ‘extended to distant lymph nodes or sites’) (Fig. 2). 11 , 25 Both studies showing an effect on stage used the SEIFA IRSD index and statistical local area (SLA) as the geographic area unit. One study compared the smallest geographic area unit (CD) to SLA and found that socioeconomic disadvantage was associated with higher odds of distant stage at diagnosis, but the effect was greater when the smaller, less heterogenous area unit was used (OR 1.27 (95% CI 1.16–1.38) vs. OR 1.24 (95% CI 1.14–1.36)). 25 Another large study of 18, 561 patients based in Queensland did not show an effect of area‐level socioeconomic disadvantage on advanced stage at presentation, however, had a different definition of advanced stage which included locally advanced and metastatic stage together (advanced stage defined as TNM group Stage III–IV, where early stage was defined as TNM group Stage I–II). 24
Discussion
This scoping review of the impact of socioeconomic status on colorectal cancer in Australia has identified studies mostly focused on assessing outcomes of advanced stage at presentation or cancer survival. In Australia, there is more evidence supporting an association between lower socioeconomic status and poorer cancer survival for colorectal cancer. Only two studies in NSW demonstrated an effect of socioeconomic deprivation on advanced stage at presentation where more socioeconomically deprived patients had an increased likelihood of presenting with metastatic disease. These two studies had large populations (39 000 and 107 000 cases), and used a small geographic area unit in their measurement of area‐level socioeconomic status. Smaller, single institution studies, did not demonstrate an effect of socioeconomic deprivation on stage at presentation or survival, despite more detailed clinical data.
There are many underlying factors that potentially contribute to why socioeconomic deprivation can be associated with poorer survival or later‐stage presentation in colorectal cancer. These factors should be examined and identified to seek areas for intervention, and to ensure that cancer interventions reduce disparities in outcomes. 1 Broadly speaking these can be classified into patient factors, disease factors and treatment factors. Once colorectal cancer has already developed, patient factors that could be related to lower socioeconomic status such as comorbidities, overall health and life‐style related risk factors, host response to cancer as well as health‐seeking behaviours and health literacy may impact outcomes. Disease factors such as tumour stage have not been demonstrated to contribute to the socioeconomic disparity in survival in Australia in studies which have adjusted for this variable, though it may be a partially contributing factor in studies from England and New Zealand. 23 , 27 , 28 Differences in tumour biology and aggressiveness relating to socioeconomic deprivation may be difficult to directly assess. Variation in uptake, availability and quality of investigations and treatments could also be an underlying factor in outcome differences. For example, patients from lower socioeconomic groups are more likely to have a longer wait to starting adjuvant chemotherapy. 29 The influence of geographic remoteness on the same mediating factors also needs to be taken into account independent of and related to socioeconomic status, highlighting the complex interplay of individual and social determinants of health. 9 , 30 , 31 , 32
There is evidence for lower uptake of colorectal cancer screening in patient populations with a lower education level, lower income, and non‐English speaking backgrounds. 33 Ananda et al. investigated the initial impact of the NBCSP in 2009, 3 years after its implementation, and highlighted that those from more disadvantaged areas were less likely to participate. 34 The National Bowel Cancer Screening Program (NBCSP) commenced in 2006 with a staged roll‐out (completed in 2020) inviting all eligible participants aged 50–74 for biennial faecal occult blood test (FOBT) screening. 33 The screening participation rate for people from least disadvantaged areas was 45.9% compared with 40.5% for patients from most disadvantaged areas, with an overall screening participation rate of 43.5% in the 2018–2019 time period. 33 , 35 Half of the papers included in this review have studied patient populations in the time period that predate the NBCSP. None of the papers in this review were able to measure screening participation rates in the patient populations studied. As a potential mediating or influencing variable on the impact of socioeconomic status on stage at diagnosis or cancer survival, FOBT screening and colonoscopic participation would be difficult to directly assess as it is not mandatory to report NBCSP colonoscopies. 33
The differences in findings in the various studies could be due to several reasons. The varying definitions and measurement of socioeconomic status present a challenge. Individual‐level measures such as financial resources, education and occupation are difficult to attain on a large scale (this scoping review has only identified one such study using these). Area‐level indices such as SEIFA scores have several limitations. Importantly, an individual's socioeconomic status may not be represented by the aggregate data about the area they reside in, and assumptions of outcomes based on these area‐level measures may lead to ecological fallacy. Additionally, the larger the geographic area unit used (such as postcode or LGA), the more heterogeneity within the study population; studies comparing the geographic area unit used in area‐level socioeconomic indices have demonstrated misclassification of individuals using a larger area unit to measure socioeconomic status, with diminished effect on outcomes. 25 , 26 A confounding issue in the studies identified is that the smaller, more clinically‐detailed studies used larger geographic area units (most commonly residential postcode which is easier to obtain), while paradoxically the larger studies with less clinical detail used a SEIFA index with a smaller geographic area unit.
While SEIFA scores are one way to measure socioeconomic status, they do not include an assessment of access to infrastructure or remoteness of an area which can also impact health outcomes. 9 The four indices reflect different measures of socioeconomic status; the choice of index used in analysis of cancer care outcomes could potentially yield differing results. For example, the IRSD focuses on relative social disadvantage only, while the IRSAD looks at indicators of both relative social advantage and disadvantage. The same geographical area with a mix of disadvantaged and advantaged people may score low on the IRSD but moderate on the IRSAD due to attenuating social advantage measures. 3 The various studies identified in this scoping review most commonly use IRSD, which measures relative socioeconomic disadvantage. Four studies used IRSAD (advantage and disadvantage) either alone or in addition to another index. 9 , 14 , 19 , 20 The use of different indices may be another reason for varying outcomes.
This review has several limitations. The studies included were restricted to studies that had socioeconomic status as a main exposure variable rather than as a co‐variable that was adjusted for, as well as specific outcomes of stage at presentation and/or survival. This could potentially have excluded studies that had secondary findings related to how socioeconomic status impacts colorectal cancer outcomes. A meta‐analysis of the data was unable to be performed due to heterogeneity of study methodology and outcomes.
Conclusions
This scoping review summarizes the evidence of the impact of socioeconomic disadvantage on colorectal cancer stage at presentation and survival. There is limited evidence that socioeconomic status is associated with late stage at presentation. More studies suggest evidence of an association between socioeconomic disadvantage and poorer survival, especially larger studies utilizing less clinically‐detailed cancer registry data. Further investigation is required to analyse why socioeconomic disadvantage may be associated with poorer survival.
Author contributions
Nicole Li Tham: Conceptualization; data curation; formal analysis; methodology; writing – original draft; writing – review and editing. Anita Skandarajah: Formal analysis; methodology; supervision; writing – review and editing. Ian Paul Hayes: Conceptualization; data curation; formal analysis; methodology; supervision; writing – original draft; writing – review and editing.
Conflict of interest
None declared.
Acknowledgement
Dr. Nicole Tham is a recipient of the Research Training Program Scholarship as a source of support awarded by the Australian Commonwealth Government and the University of Melbourne. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
N. L. Tham MBBS, FRACS; A. Skandarajah MD, FRACS; I. P. Hayes MEpi, FRACS.
References
- 1. Vaccarella S, Lortet‐Tieulent J, Saracci R et al. Reducing social inequalities in cancer: setting priorities for research. CA Cancer J. Clin. 2018; 68: 324–6. [DOI] [PubMed] [Google Scholar]
- 2. Afshar N, English DR, Blakely T et al. Differences in cancer survival by area‐level socio‐economic disadvantage: a population‐based study using cancer registry data. PLoS One 2020; 15: e0228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Australian Bureau of Statistics . 2033.0.55.001 Socio‐Economic Indexes for Areas (SEIFA) 2016 Technical Paper [PDF on Internet]. Canberra: Commonwealth of Australia, 2018. [Cited 13 Jul 2022.]. [Google Scholar]
- 4. Quaglia A, Lillini R, Mamo C, Ivaldi E, Vercelli M. Socio‐economic inequalities: a review of methodological issues and the relationships with cancer survival. Crit. Rev. Oncol. Hematol. 2013; 85: 266–77. [DOI] [PubMed] [Google Scholar]
- 5. Australian Institute of Health and Welfare . Cancer Data in Australia: Web Report. Canberra: Australian Government, 2021. [Cited 13 Jul 2022.] Available from URL: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-rankings-data-visualisation. [Google Scholar]
- 6. Australian Institute of Health and Welfare . Health System Expenditure on Cancer and Other Neoplasms in Australia, 2015–16. Cancer series no. 131 [PDF on Internet]. Canberra: Australian Government, 2021. [Cited 13 Jul 2022.] Available from URL: https://www.aihw.gov.au/reports/cancer/health‐system‐expenditure‐cancer‐other‐neoplasms/summary. [Google Scholar]
- 7. Stanbury JF, Baade PD, Yu Y, Yu XQ. Cancer survival in New South Wales, Australia: socioeconomic disparities remain despite overall improvements. BMC Cancer 2016; 16: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Booth CM, Li G, Zhang‐Salomons J, Mackillop WJ. The impact of socioeconomic status on stage of cancer at diagnosis and survival. Cancer 2010; 116: 4160–7. [DOI] [PubMed] [Google Scholar]
- 9. Baade PD, Dasgupta P, Aitken JF, Turrell G. Geographic remoteness, area‐level socioeconomic disadvantage and inequalities in colorectal cancer survival in Queensland: a multilevel analysis. BMC Cancer 2013; 13: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cancer Australia. National Cancer Control Indicators: Relative Survival by Stage at Diagnosis (Colorectal Cancer). Canberra: Australian Government, 2019. [Cited 13 Jul 2022.] Available from URL: https://ncci.canceraustralia.gov.au/outcomes/relative‐survival‐rate/relative‐survival‐stage‐diagnosis‐colorectal‐cancer. [Google Scholar]
- 11. Tervonen HE, Walton R, Roder D et al. Socio‐demographic disadvantage and distant summary stage of cancer at diagnosis‐a population‐based study in New South Wales. Cancer Epidemiol. 2016; 40: 87–94. [DOI] [PubMed] [Google Scholar]
- 12. Afshar N, English DR, Milne RL. Factors explaining socio‐economic inequalities in cancer survival: a systematic review. Cancer Control 2021; 28: 107327482110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tham N, Skandarajah A, Hayes IP. Colorectal cancer databases and registries in Australia: what data is available? ANZ J. Surg. 2022; 92: 27–33. [DOI] [PubMed] [Google Scholar]
- 14. Beckmann KR, Bennett A, Young GP et al. Sociodemographic disparities in survival from colorectal cancer in South Australia: a population‐wide data linkage study. BMC Health Serv. Res. 2016; 16: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Australian Bureau of Statistics . 2039.0 An Introduction to Socio‐Economic Indexes for Areas (SEIFA) 2006. [PDF on Internet]. Canberra: Commonwealth of Australia, 2006. [Cited 13 Jul 2022.] Available from URL: https://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/2039.0Main+Features12006?OpenDocument. [Google Scholar]
- 16. Peters MDJ, Marnie C, Tricco AC et al. Updated methodological guidance for the conduct of scoping reviews. JBI evidence. Synthesis 2020; 18: 2119–26. [DOI] [PubMed] [Google Scholar]
- 17. Tricco AC, Lillie E, Zarin W et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann. Intern. Med. 2018; 169: 467–73. [DOI] [PubMed] [Google Scholar]
- 18. Ngoo AG, Tan AHM, Mushaya CD, Ho YH. Short and long course neoadjuvant therapy compared for management of locally advanced rectal cancer: 11 years' experience at a regional centre. ANZ J. Surg. 2020; 90: 812–20. [DOI] [PubMed] [Google Scholar]
- 19. Pasch JA, MacDermid E, Velovski S. Effect of rurality and socioeconomic deprivation on presentation stage and long‐term outcomes in patients undergoing surgery for colorectal cancer. ANZ J. Surg. 2021; 91: 1569–74. [DOI] [PubMed] [Google Scholar]
- 20. Barclay KL, Goh PJ, Jackson TJ. Socio‐economic disadvantage and demographics as factors in stage of colorectal cancer presentation and survival. ANZ J. Surg. 2015; 85: 135–9. [DOI] [PubMed] [Google Scholar]
- 21. MacDermid E, Pasch J, Fok KY et al. The effect of socioeconomic deprivation on presentation stage and long‐term outcomes in patients undergoing colorectal cancer resection in Western Sydney. ANZ J. Surg. 2021; 91: 1563–8. [DOI] [PubMed] [Google Scholar]
- 22. Yu XQ, Goldsbury D, Feletto E, Koh CE, Canfell K, O'Connell DL. Socioeconomic disparities in colorectal cancer survival: contributions of prognostic factors in a large Australian cohort. J. Cancer Res. Clin. Oncol. 2021; 148: 2971–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afshar N, Dashti SG, Te Marvelde L et al. Factors explaining socio‐economic inequalities in survival from colon cancer: a causal mediation analysis. Cancer Epidemiol. Biomark. Prev. 2021; 30: 1807–15. [DOI] [PubMed] [Google Scholar]
- 24. Baade PD, Dasgupta P, Aitken J, Turrell G. Geographic remoteness and risk of advanced colorectal cancer at diagnosis in Queensland: a multilevel study. Br. J. Cancer 2011; 105: 1039–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tervonen HE, Morrell S, Aranda S et al. The impact of geographic unit of analysis on socioeconomic inequalities in cancer survival and distant summary stage – a population‐based study. Aust. N. Z. J. Public Health 2017; 41: 130–6. [DOI] [PubMed] [Google Scholar]
- 26. Stanbury JF, Baade PD, Yu Y, Yu XQ. Impact of geographic area level on measuring socioeconomic disparities in cancer survival in New South Wales, Australia: a period analysis. Cancer Epidemiol. 2016; 43: 56–62. [DOI] [PubMed] [Google Scholar]
- 27. Jeffreys M, Sarfati D, Stevanovic V et al. Socioeconomic inequalities in cancer survival in New Zealand: the role of extent of disease at diagnosis. Cancer Epidemiol. Biomark. Prev. 2009; 18: 915–21. [DOI] [PubMed] [Google Scholar]
- 28. Lejeune C, Sassi F, Ellis L et al. Socio‐economic disparities in access to treatment and their impact on colorectal cancer survival. Int. J. Epidemiol. 2010; 39: 710–7. [DOI] [PubMed] [Google Scholar]
- 29. Bergin RJ, Thomas RJS, Whitfield K, White V. Concordance between optimal care pathways and colorectal cancer care: identifying opportunities to improve quality and reduce disparities. J. Eval. Clin. Pract. 2020; 26: 918–26. [DOI] [PubMed] [Google Scholar]
- 30. Baade PD, Dasgupta P, Aitken JF, Turrell G. Distance to the closest radiotherapy facility and survival after a diagnosis of rectal cancer in Queensland. Med. J. Aust. 2011; 195: 350–4. [DOI] [PubMed] [Google Scholar]
- 31. Afshar N, English DR, Chamberlain JA et al. Differences in cancer survival by remoteness of residence: an analysis of data from a population‐based cancer registry. Cancer Causes Control 2020; 31: 617–29. [DOI] [PubMed] [Google Scholar]
- 32. Afshar N, English DR, Milne RL. Rural‐urban residence and cancer survival in high‐income countries: a systematic review. Cancer 2019; 125: 2172–84. [DOI] [PubMed] [Google Scholar]
- 33. Australian Institute of Health and Welfare . National Bowel Cancer Screening Program: Monitoring Report 2021. Cancer Series No. 132 [PDF on Internet]. Canberra: AIHW, 2021. [Cited 15 Sep 2022.] Available from URL: https://www.aihw.gov.au/reports/cancer-screening/nbcsp-monitoring-report-2021/summary. [Google Scholar]
- 34. Ananda SS, McLaughlin SJ, Chen F et al. Initial impact of Australia's National Bowel Cancer Screening Program. Med. J. Aust. 2009; 191: 378–81. [DOI] [PubMed] [Google Scholar]
- 35. Australian Institute of Health and Welfare . National Bowel Cancer Screening Program: Monitoring Report 2020. Cancer Series No. 128 [PDF on Internet]. Canberra: AIHW, 2020. [Cited 15 Sep 2022.] Available from URL: https://www.aihw.gov.au/reports/cancer-screening/national-bowel-cancer-screening-monitoring-2020/summary. [Google Scholar]


