Abstract
Leishmaniasis, caused by infection with the protozoan parasite Leishmania, affects millions of individuals worldwide, causing serious morbidity and mortality. This study directly determined the frequency of cells producing key immunoregulatory cytokines in response to the recombinant antigen Leishmania homolog of receptors for activated kinase C (LACK) and soluble leishmania antigen (SLA), and it determined relative contributions of these antigens to the overall cytokine profile in individuals infected for the first time with Leishmania braziliensis. All individuals presented with the cutaneous clinical form of leishmaniasis and were analyzed for proliferative responses to LACK antigen and SLA, frequency of lymphocyte subpopulations (analyzed ex vivo), and antigen-induced (LACK and SLA) cytokine production at the single-cell level (determined by flow cytometry). The following were determined. (i) The Th1-type response previously seen in patients with cutaneous leishmaniasis is due to gamma interferon (IFN-γ) production by several different sources, listed in order of contribution: CD4+ T lymphocytes, CD4−, CD8− lymphocytes, and CD8+ T lymphocytes. (ii) SLA induced a higher frequency of lymphocytes producing IFN-γ and tumor necrosis factor alpha (TNF-α) than did LACK. (iii) LACK induced an activation of monocyte populations as reflected by an increased percentage of CD14-positive cells. (iv) Neither SLA nor LACK induced detectable frequencies of cells producing interleukin-4 (IL-4) or IL-5. These data demonstrated a multifaceted immune response to SLA in human leishmaniasis involving Th1 CD4+ T lymphocytes (IFN-γ+ and IL-10−/IL-4−), Tc1 CD8+ T cells (IFN-γ+, and IL-10−/IL-4−), and a high frequency of TNF-α-producing lymphocytes. Moreover, it was determined that the recombinant antigen LACK acts as a weak inducer of Th1-type lymphocyte responses compared to SLA.
The clinical outcome of Leishmania infection in humans, ranging from relatively mild to severely life-threatening disease depends on several host- and parasite-related factors. One of these factors is the strain of Leishmania that is involved in the infection. However, it is clear that a single strain of Leishmania can give rise to more than one clinical form of the disease (9). Differences in the form of the disease are likely to be influenced by the individual's immune response. The most prevalent clinical forms include the cutaneous, mucocutaneous, and visceral clinical forms (9). The cutaneous clinical form is characterized by the presence of one or two skin lesions, predominantly on the extremities, and is caused by Leishmania braziliensis, as well as other species of Leishmania. In these patients, it has been determined that lymphocytes responding to soluble leishmania antigen (SLA) produce high levels of gamma interferon (IFN-γ) and low levels of interleukin-4 (IL-4) as measured by enzyme-linked immunosorbent assay (ELISA) and PCR (2, 3, 10). In individuals cured of cutaneous leishmaniasis, high levels of cells producing IFN-γ and tumor necrosis factor alpha (TNF-α) have been identified (6). Based on these findings it has been suggested that resolution of this clinical form is associated with a Th1-like immune response. Understanding what factors lead to the development and maintenance of the protective immune profile without associated pathology is critical for the design of new treatments and/or vaccines for this disease.
Murine models of leishmaniasis have been very important in the understanding of immune mechanisms that may lead to pathology and protection (8, 15). Moreover, the use of these models has led to the cloning of an immunodominant antigen from Leishmania termed Leishmania homolog of receptors for activated kinase C (LACK) (11). LACK acts as an effective vaccine in mouse models, both in DNA vaccination and in vaccinations with the protein associated with IL-12 (5, 11). This antigen induces strong, oligoclonal immune responses in animals with restricted T-cell receptor usage represented by T cells expressing preferentially Vβ4 Vα8 T-cell receptors (13). Interestingly, the T-cell response against LACK is involved in both the protective, Th1, immune response in C57BL/6 mice and in the pathogenic, Th2, immune response in BALB/c mice (7, 11, 14). Thus, the type of immune response directed against this antigen may be important in the development of both protective and pathogenic immune responses in leishmaniasis.
The appropriate design of immunological intervention in leishmaniasis and other diseases requires that the cellular sources of regulatory cytokines and the relative contributions of individual cell populations to the overall cytokine environment during immune responses be determined. Recombinant antigens, such as LACK, offer attractive vaccine candidates; however, the determination of the host immune response against recombinant antigens as well as to the whole pathogen is crucial before moving forward with vaccine trials.
Through a series of experiments, these studies have determined, at the cellular level, the relative contributions of lymphocyte populations to the overall cytokine profile directed against L. braziliensis SLA and LACK in a well-defined group of patients with cutaneous leishmaniasis, all of whom were infected for the first time and analyzed before treatment began. Thus, we were able to determine the naturally occurring immune response present up to the development of the first cutaneous lesions in these individuals with ulcerated lesions between 30 and 60 days of evolution. Finally, we have determined the intensity and type of immune response generated against the recombinant antigen LACK by these patients as well.
MATERIALS AND METHODS
Synthesis and purification of the antigens.
The recombinant antigen LACK from Leishmania major was produced in our laboratory. The plasmid pET3-A containing the cDNA for the protein LACK was obtained in collaboration with the University of California, San Francisco. The histidine-tagged recombinant protein was expressed in the Escherichia coli B21(DE3)pLysS (Novagen). It was then purified over an affinity column of nickel (His Trap; Pharmacia) through the interaction of the histidine tag. The purified fractions were confirmed using silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. LACK from L. major has 98% homology with that from L. braziliensis at the DNA level, and there are no amino acid changes in the predicted protein sequence. The SLA of L. braziliensis was provided by the Leishmaniasis Laboratory (ICB, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil) and is a freeze-thawed antigen preparation. Briefly, L. braziliensis braziliensis promastigotes (MHOM/BR/81/HJ9) were washed and adjusted to 108 promastigotes/ml in phosphate-buffered saline (PBS) followed by repeated freeze-thaw cycles and a final ultrasonication. All antigens were titrated using peripheral blood mononuclear cells (PBMC) from patients infected with L. braziliensis.
Patients.
The PBMC analyzed in this study were obtained from individuals from two different areas where leishmaniasis due-to-infection-with L. braziliensis is endemic. All individuals participated in the study through informed consent and received treatment whether they chose to participate in the study or not. One of the areas was near the city of Caratinga, Minas Gerais, Brazil, and the other was the village of Corte de Pedra, in the state of Bahia, Brazil. The diagnosis of leishmaniasis was based on dermatological findings, positive parasitological exams, and a positive skin test for leishmania antigens. The age range for the patients was 15 to 45 years (mean ± standard deviation, 24.7 ± 8.4 years). All patients presented with ulcerated lesions between 1 and 2 months of duration. None of the individuals had received previously treatment for leishmaniasis or had reported prior infections with Leishmania. In these areas approximately 90% of the patients are cured of the first cutaneous lesions following the treatment course. Blood was drawn immediately before treatment was initiated.
In vitro cultures.
All cultures were carried out using RPMI 1640 supplemented with 5% heat-inactivated AB Rh+ human serum (Sigma Chemical Co., St. Louis, Mo.), antibiotics (200 U of penicillin/ml), and 1 mM l-glutamine.
In vitro proliferation analysis.
In vitro proliferative responses of PBMC were performed according to the protocol described by Gazzinelli et al. (4). Briefly, PBMC from patients with cutaneous leishmaniasis or noninfected control individuals were obtained by separating whole blood over Ficoll and washing it three times with medium. Cells were counted and cultured in the presence or absence of different stimuli for 5 days at a concentration of 1.25 × 106 cells/ml in 96-well plates. Stimuli used in the cultures included LACK (at a 20-μg/ml final concentration), SLA (at a 10-μg/ml final concentration), and, as a positive control, the superantigen SEB (at a 15-ng/ml final concentration). After the incubation period, cultures were exposed to 0.5 μCi of 3[H]thymidine for 6 h and harvested, and the incorporated radioactivity was measured in an automatic scintillation counter. All cultures were performed in triplicate. The proliferative response was calculated using the mean of triplicate cultures with antigen minus the mean of triplicate cultures with medium alone for each individual patient (medium alone gave an average of 450 cpm). The above concentrations for the antigens were determined by performing titration experiments.
Ex vivo staining to determine lymphocyte profiles.
PBMC (2 × 105) from leishmaniasis patients were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled antibody solutions for 20 min at 4°C. After staining, preparations were washed with 0.1% sodium azide PBS, fixed with 200 μl of 2% formaldehyde in PBS and kept at 4°C until data were acquired using a fluorescence-activated cell sorter (FACS). The antibodies used for the staining were immunoglobulin FITC and PE controls, anti-CD4–PE, anti-CD8–FITC and -PE, anti-CD69–FITC (Pharmingen), anti-CD3–PE, anti-CD45RO–FITC, anti-CD19–PE, anti-CD14–FITC (Dako).
Single-cell cytoplasmic cytokine staining.
PBMC were analyzed for their intracellular cytokine expression pattern as described below and by Sornasse et al. (17). Briefly, 2.5 × 106 PBMC were cultured in 24-well plates in 1-ml cultures for 20 h with either medium alone, LACK (at a 20-μg/ml final concentration), SLA (at a 10-μg/ml final concentration), or a positive control of anti-CD3 and anti-CD28 (anti-CD3 of 2 ng/ml and anti-CD28 of 1 ng/ml). During the last 4 h of culture, brefeldin A (1 μg/ml), which impairs protein secretion by the Golgi complex, was added to the cultures. The cells were then harvested using ice-cold PBS plus azide, stained for surface markers, and fixed using 2% formaldehyde. The fixed cells were then permeabilized with a solution of saponin and stained, for 30 min at 4°C, using anticytokine monoclonal antibodies directly conjugated with either FITC (IFN-γ) or PE (IL-4, IL-5, TNF-α, IL-12, and IL-10). FITC- and PE-labeled immunoglobulin control antibodies and a control of unstimulated PBMC were included in all experiments. Preparations were then washed and fixed as described in the previous section and analyzed using a FACS Vantage or FACScan (Becton Dickinson), selecting the lymphocyte population. In several cases, the cytokine staining was associated with the staining of cell surface markers for studying together the production of cytokines and the phenotype of the cells that produced them. In all cases, 30,000 gated events were acquired for later analysis. This number was required due to the low frequency of positive events being analyzed. Controls of nonstimulated versus stimulated T-cell clones were used to standardize the antibodies used, as were medium alone and polyclonal stimulated (anti-CD3 and anti-CD28) PBMC, as positive controls.
Analysis of FACS data.
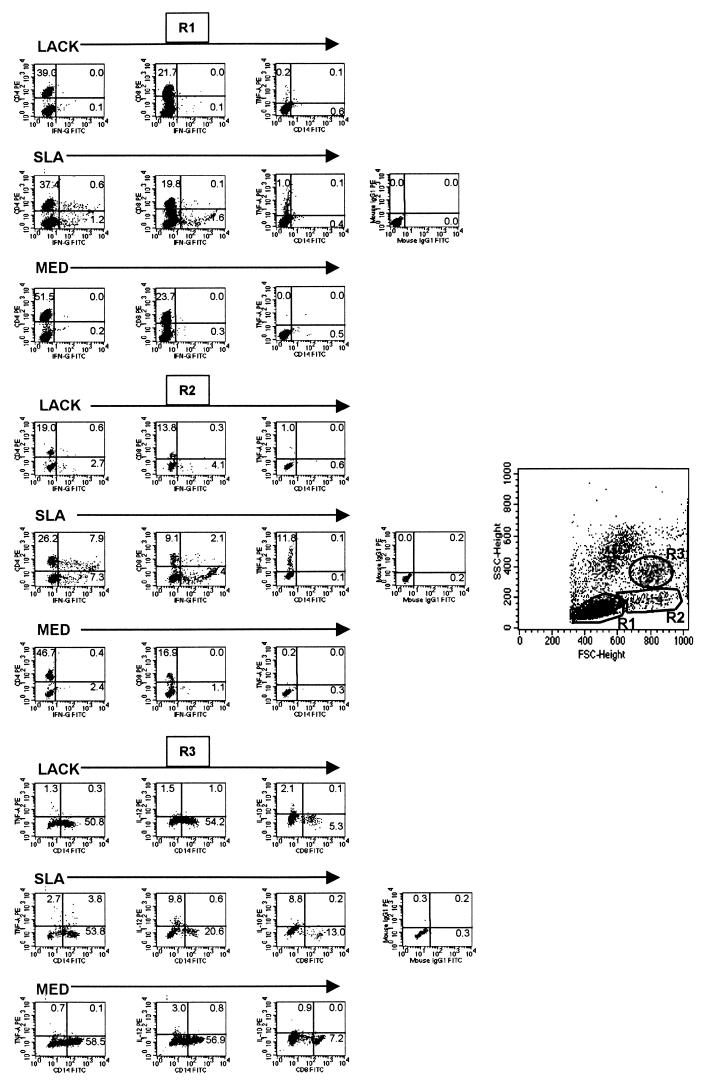
Lymphocytes were analyzed for their intracellular cytokine expression patterns and frequencies and for surface markers in a number of ways using the program Cell Quest. The frequency of positive cells was analyzed in three regions for each staining; region 1 (R1), lymphocyte gate; region 2 (R2), a large lymphocyte blast gate; and region 3 (R3), a macrophage gate (Fig. 1). Limits for the quadrant markers were always set based on negative populations and isotype controls. This approach to analysis allows for the frequency of populations to be determined in subregions of mononuclear cells, making use of known positioning of mononuclear cells based on size and granularity profiles. For analysis of CD4- and CD8-positive lymphocytes, quadrants were always set for CD4 and CD8 high populations so as not to include CD4 low-positive monocytes and macrophages and CD8 low-positive NK cells, respectively. Statistical analysis was performed using the all-pair, Student's t test contained in JMP, the statistical program from SAS.
FIG. 1.
Representative histograms from human patients with cutaneous leishmaniasis. Each group shows the frequency of cytokine-producing cells after 20 h of culture with LACK, SLA, or medium alone in R1, R2, or R3. The histograms demonstrate the frequencies of cells expressing the indicated molecules as detected using antibodies directly conjugated with either PE (y axis) or FITC (x axis) as described in Materials and Methods. The forward- and side-scatter histograms demonstrate the placement of R1 (small lymphocytes), R2 (blast lymphocytes), and R3 (monocytes/macrophages).
RESULTS
PBMC from patients with cutaneous leishmaniasis respond to recombinant LACK with an intensity of 14% of that seen against SLA.
Earlier studies with mice have shown that the LACK antigen is an important immunodominant antigen in infections with Leishmania. To characterize the response of patients with cutaneous leishmaniasis to the recombinant antigen LACK and to SLA, we performed in vitro proliferation assays as well as FACS analysis before and after antigenic stimulation. The response of PBMC from patients with cutaneous leishmaniasis to LACK was about 14% the intensity of that to SLA. The proliferative response to LACK (mean ± standard deviation) as determined by incorporation of [3H]-thymidine was 2,848 ± 558, while the response to SLA was 22,652 ± 2,610 (in counts per minute, after subtracting the spontaneous proliferation from medium alone, which averaged 450 cpm). Interestingly, the response to SLA was statistically equivalent to that of SEB (28,778 ± 4,347), even though the SEB superantigen stimulates T cells based solely on their Vβ region expression and is capable of stimulating about 20% of human T cells. The response of PBMC from noninfected individuals to LACK and SLA was not significantly different from that of medium alone (averaging 450 cpm).
To further determine the degree and type of proliferative response observed, PBMC from these individuals were analyzed using flow cytometry both ex vivo and after a 5-day culture with LACK or SLA for a number of cell surface molecules related to lymphocyte subpopulations and cellular activation. All markers were analyzed using a total lymphocyte gate. The patients had normal percentages of CD4+ (41.1%) and CD8+ (20.0%) lymphocytes in the whole lymphocyte population and a mean of 61.8% CD3+ T cells (Table 1). Recombinant LACK and SLA both induced a statistically significant increase in the frequency of CD3+ T cells (78.8 and 76.8%, respectively) that was predominantly due to an increase in the frequency of CD4+ T cells, which increased to 52.4 and 54.1%, respectively. In addition, an increase in the frequency of the activation marker CD69 was seen in lymphocytes after culture with LACK (9.6%) and SLA (18.4%) compared to the ex vivo frequency (2.3%) (Table 1). In contrast, the ex vivo frequency of CD45RO+ CD4+ T cells, as an indicator of experienced T cells, was 21.2% and changed little after culture with LACK or SLA (Table 1).
TABLE 1.
Frequency of cells ex vivo and after culture with LACK and SLA
| Stimulus | Frequency (%)a of cell type after treatment
|
|||||
|---|---|---|---|---|---|---|
| CD4+ Tb | CD8+ T | CD3+ T | CD69+ T | CD45RO /CD4+ T | CD19+ B | |
| Ex vivo | 41.2 ± 1.6 | 20.0 ± 1.1 | 61.8 ± 2.5 | 2.3 ± 0.2 | 21.2 ± 0.8 | 9.6 ± 1.3 |
| LACKc | 52.4 ± 1.4d | 23.7 ± 1.3 | 78.8 ± 1.8d | 9.6 ± 0.4e | 20.4 ± 0.9 | 6.2 ± 1.0 |
| SLAc | 54.1 ± 2.6d | 21.7 ± 1.1 | 76.8 ± 6.0d | 18.4 ± 0.7f | 22.0 ± 1.8 | 10.2 ± 5.7 |
Mean ± standard error.
These values come from a total lymphocyte gate including small and large lymphocytes (R1 and R2) (Fig. 1).
Frequencies after 5 days of culture with the indicated antigen (LACK [20 μg/ml] and SLA [10 μg/ml]).
Significantly different from the frequency ex vivo using Student's t test and JMP with comparison of all pairs and a P value of <0.05.
Significantly different from the frequency ex vivo and with SLA using Student's t test and JMP with comparison of all pairs and a P value of <0.05.
Significantly different from the frequency ex vivo and with LACK using Student's t test and JMP with comparison of all pairs and a P value of <0.05.
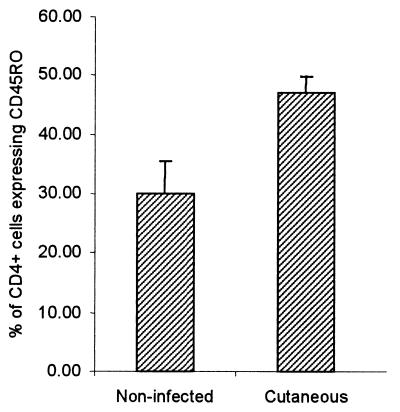
However, comparing the percentage of CD4+ cells expressing CD45RO within the CD4 T-cell population, a significantly higher percentage of the memory-activated phenotype was seen when comparing infected and noninfected individuals (Fig. 2). Thus, these results demonstrate a specific immune response against LACK by infected individuals, albeit significantly less intense than the response directed against SLA. They also demonstrate the involvement of CD4+-T-cell proliferation.
FIG. 2.
CD4+ T cells from patients with cutaneous leishmaniasis express a higher percentage of experienced T cells. The average frequency of CD4+ CD45RO+ T cells within the CD4+-T-cell population is represented for noninfected individuals (n = 6) and patients with cutaneous leishmaniasis (n = 13). The difference was significant using Student's t test with a P value of <0.05.
Several cell populations in addition to Th1 CD4+ T cells contribute to IFN-γ production in response to SLA, while LACK induced a weak Th1 response.
To investigate cellular sources and frequencies of IFN-γ-producing cells, the cytokine profile induced by SLA and the antigen LACK was determined following short-term stimulation. Twenty-hour cultures with either of the two leishmania-derived antigens, or medium alone (ex vivo endogenously produced cytokines without in vitro stimulation) were analyzed using single-cell cytoplasmic staining and flow cytometry. This early time point was chosen to allow for the analysis of cytokines that were being actively expressed in vivo (medium condition) and to reveal the cytokine profile of lymphocytes that were previously activated and differentiated in vivo in response to infection with L. braziliensis (SLA and LACK). Moreover, this short-term stimulation avoids problems of in vitro skewing of the cytokine profiles and consumption which can occur with longer culture times used with ELISA. Analysis was performed using different regions, R1 for lymphocytes, R2 for lymphocyte blasts, and R3 for monocytes macrophages (Fig. 1). Due to the limited amount of material (peripheral blood cells from infected individuals), certain combinations of antibodies (surface markers along with cytokine-specific antibodies) were chosen to cover the largest window of possibilities of cellular sources based on known positioning of leukocyte populations as well as known cytokine-producing cells.
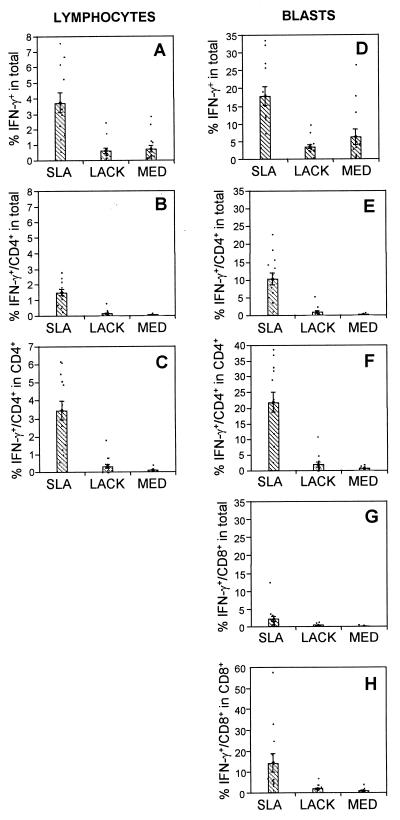
IFN-γ is a key cytokine involved in cellular immune responses and is also a direct indicator of Th1 cells when present in CD4+ T cells in the absence of IL-4 production. Moreover, it is essential for inducing the leishmanicidal activity of macrophages. Analyzing lymphocytes in R1, it was seen that SLA induced a high frequency of IFN-γ-producing cells (3.8%) compared to medium (0.8%) or stimulation with LACK (0.7%) (Fig. 3A). Analysis of the cellular sources of this IFN-γ production induced by SLA revealed that 42% came from CD4+ T cells (Fig. 3B). Finally, the frequency of CD4+ IFN-γ+ T cells within the CD4+ population was 3.5% (Fig. 3C). The frequency of CD8+ T cells producing IFN-γ was less than 0.3% with SLA stimulation in R1 (data not shown).
FIG. 3.
Multiple sources of IFN-γ-producing cells in response to SLA, with little LACK-induced IFN-γ. Using single-cell cytoplasmic staining and analysis by flow cytometry, the frequencies of lymphocyte subpopulations producing IFN-γ were determined. The first column represents data obtained from the small lymphocyte gate, R1 (Fig. 1). (A) Percentage of IFN-γ-producing cells in the total lymphocyte population; (B) percentage of CD4+ cells producing IFN-γ in the whole population; (C) percentage of cells producing IFN-γ within the CD4+-T-cell population. The second column represents data obtained from the lymphocyte blast gate, R2 (as shown in fig. 1). (D) Percentage of IFN-γ+-producing cells in the whole gated population; (E) percentage of CD4+ IFN-γ-producing T cellsin the whole gated population; (F) the percentage of IFN-γ-producing T cells within the CD4+ population; (G) the percentage of CD8+ IFN-γ-producing T cells in the whole gated population; (H) percentage of IFN-γ-producing T cells within the CD8+ population. Data are the means ± standard errors for 13 individual patients with cutaneous leishmaniasis. The means were compared using the statistical program JMP and Student's t test; comparison of all pairs was made with a P value of <0.05.
In the lymphocyte blast gate, R2, a striking increase in the frequency of IFN-γ-producing cells was revealed, with an average of 18% of the cells producing IFN-γ after stimulation with SLA (Fig. 3D). Moreover, as seen in Fig. 3E, the majority of this IFN-γ production, 58%, was derived from Th1 CD4+ T cells (10.4% from CD4+ T cells [Fig. 3e] divided by 18% of total IFN-γ-producing cells [Fig. 3D]). An average of 22% of the CD4+-T-cell population responded against SLA by secreting IFN-γ as seen in Fig. 3F. In contrast, LACK induced a frequency of CD4+ IFN-γ+ T cells of 0.8% and medium alone induced a frequency of 0.3% (Fig. 3E), and looking within the CD4+-T-cell compartment, only 1.8% of the T cells responded to LACK by making IFN-γ (Fig. 3F).
Two other important sources of IFN-γ-producing cells were identified in the blast lymphocyte gate, R2. The frequency of CD8+ T cells producing IFN-γ was 2.5% from SLA-stimulated cultures, accounting for about 16% of the overall cytokine-producing blasts (Fig. 3G and D). When analyzing the frequency of cells producing IFN-γ within the CD8+ population, it was seen that an average of 14.5% of the CD8+ T cells produced IFN-γ in response to SLA (Fig. 3H). Lastly, there was a significant contribution (an average of 30% of the overall IFN-γ-producing cells) from CD4−/ CD8− large lymphocytes. This is clearly seen by comparing the frequency of each subpopulation, CD4+ (10.4%) and CD8+ (2.5%), to the total IFN-γ production (18%), leaving 5.5% coming from CD4− CD8− lymphocytes.
Thus, it was demonstrated that the high IFN-γ profile previously seen in leishmaniasis patients is accounted for by more than one cellular source. The cell type with the highest frequency was CD4+ T cells, followed by CD4− CD8− lymphocytes and lastly CD8+ T cells. This profile is strongly induced by SLA, with little induction by the recombinant antigen LACK. Under all conditions, medium alone, SLA, or LACK, no IL-4- or IL-5-producing cells were detected, indicating a true Th1 phenotype.
TNF-α production was induced by SLA stimulation, but weakly by LACK.
TNF-α, similar to IFN-γ, has important stimulatory effects on macrophage killing activity. To determine whether TNF-α production was induced by the LACK antigen, and to determine which cells are responsible for producing this cytokine in response to LACK and SLA, 20-h cultures were established using PBMC under the conditions mentioned above.
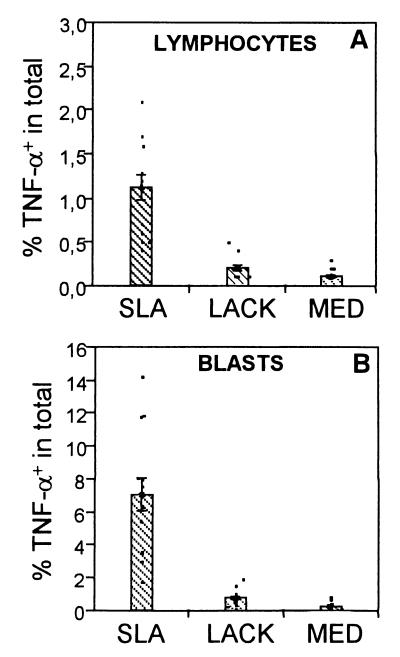
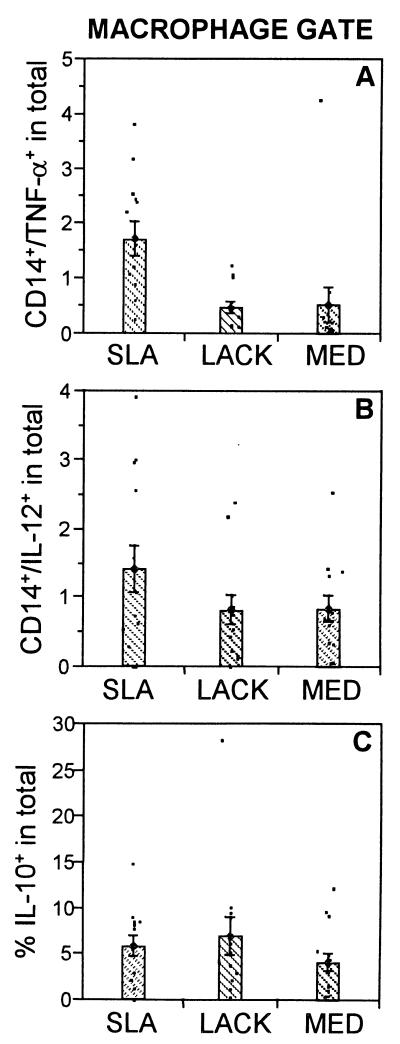
As seen in Fig. 4A, stimulation with SLA induced the highest frequency of TNF-α-producing lymphocytes in R1 (1.1%), followed by LACK (0.3%) and medium (0.1%). The production of TNF-α by lymphocyte blasts in R2 was also induced by SLA (Fig. 4B). The contribution of TNF-α by macrophages (1.7%), as shown in Fig. 6A, was once again induced most efficiently by SLA. Thus, TNF-α production comes primarily from SLA-stimulated lymphocytes as well as CD14+ monocytes, with little induction by LACK.
FIG. 4.
SLA induces a higher frequency of TNF-α-producing lymphocytes than LACK. (A) Percentage of TNF-α-producing cells in R1; (B) percentage of TNF-α-producing cells in the large lymphocyte region, R2. In all cases the frequency of cells after stimulation with SLA was significantly greater than after stimulation with LACK or medium alone. (MED). Data are the means ± standard errors for 13 individual patients with cutaneous leishmaniasis. The frequency of positive cells was determined using intracellular cytoplasmic staining of cytokines in conjunction with cell surface markers and flow cytometry analysis. The means were compared using the statistical program JMP and Student's t test; comparison of all pairs was made with a P value of <0.05.
FIG. 6.
Frequency of cytokine-producing cells in the macrophage gate, R3. (A) Percentage of CD14+ TNF-α-producing monocytes in the whole gated population; (B) percentage of CD14+ IL-12-producing monocytes in the whole gated population; and (C) percentage of IL-10-producing monocytes. The means were compared using the statistical program JMP and Student's t test; comparison of all pairs was made with a P value of <0.05. In all cases, there were no significant differences using the 95% confidence interval.
SLA is a poor inducer of IL-10-producing cells.
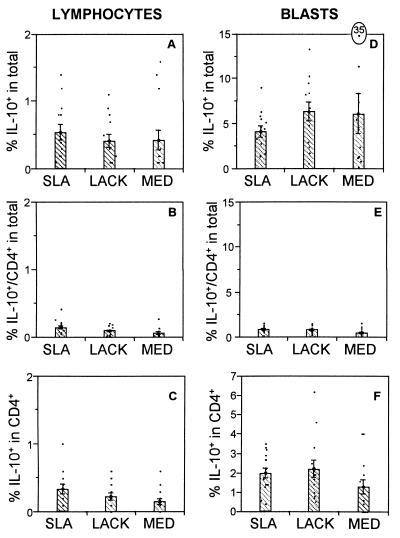
The immunoregulatory cytokine IL-10 plays an important role in down modulation of immune responses and inhibition of macrophage activity. Hence, PBMC from leishmaniasis patients were analyzed for expression of this cytokine. Analysis of R1 revealed that the frequency of IL-10-producing lymphocytes was statistically equivalent to the frequency of cells induced by SLA (0.5%) and LACK (0.4%) stimulation (Fig. 5A). CD4+-T-cell production of IL-10 accounted for an average of about 55% of this production (Fig. 5B). In contrast to IFN-γ (Fig. 3C), the frequency of IL-10+ T cells was averaged 0.4% (Fig. 5C), with no statistically significant differences between the stimuli. IL-10 production by CD8+ T cells, independent of the stimulus used, was below the limits of reliability (data not shown).
FIG. 5.
The frequencies of IL-10-producing cells are equivalent among the various stimuli. The first column represents data obtained from the small lymphocyte gate, R1 (Fig. 1). (A) Percentage of lymphocytes producing IL-10 in the whole population; (B) percentage of CD4+ IL-10+-producing T cells in the whole population; (C) percentage of IL-10+-producing cells within the CD4+ population. The second column represents data obtained from the lymphocyte blast gate, R2 (as shown in figure 1). (D) Percentage of lymphocytes producing IL-10 in the whole population; (E) percentage of CD4+ IL-10-producing T cells in the whole population; (F) percentage of IL-10-producing cells within the CD4+population. Data are the means ± standard errors for 13 individual patients with cutaneous leishmaniasis. The frequency of positive cells was determined using intracellular cytoplasmic staining of cytokines in conjunction with cell surface markers followed by using flow cytometry analysis. The antibodies used were anti-CD4–FITC and anti-IL-10–PE. The means were compared using the statistical program JMP, and Student's t test; comparison of all pairs was made with a P value of <0.05. In all cases, there were no significant differences among the stimuli.
Further analysis was performed focusing on large lymphocytes (R2) and monocytes (R3) as important possible sources of IL-10. Again, the frequency of IL-10-producing cells induced by SLA, LACK, or medium did not differ significantly using a P value of 0.05 (Fig. 5D). Analysis of CD4+ T cells as the source of this cytokine gave no significant differences between the groups (Fig. 5E). Moreover, the frequency of IL-10+ CD4+ T cells was approximately 2%, independent of the stimulus used (Fig. 5F). IL-10 production by CD8+ T cells, independent of the stimulus used, was below the limits of reliability (data not shown).
DISCUSSION
The cellular immune response of individuals presenting with cutaneous leishmaniasis for the first time and with lesions less than 60 days old was analyzed. In these studies we determined directly the cellular sources and frequencies of cytokine-producing populations ex vivo and after stimulation with SLA and recombinant LACK. The response of these individuals against LACK was approximately 14% of that seen with SLA. When one considers that it reflects the proliferative response of a genetically heterogeneous population of individuals to a single recombinant antigen, this response seems reasonably intense. Furthermore, it has recently been demonstrated that LACK makes up only about 0.03% of the total protein present in Leishmania (12). The response to LACK was significantly higher than that seen without stimuli in vitro while weaker than that seen with SLA, as determined by a number of criteria including proliferation and expression of activation markers (Table 1). In addition to the difference in intensity of the response, a clear difference in the nature of the response was also determined.
The finding that LACK induced a response fundamentally different from that induced by SLA, in that SLA induces a severalfold-higher frequency of IFN-γ- and TNF-α-producing cells (Fig. 3, 4, and 6A), with equivalent, or a tendency toward fewer IL-10-producing cells (Fig. 5 and 6C), suggests a possible role for the response in human leishmaniasis against the LACK antigen in immunoregulation. Moreover, it was seen that the frequency of CD14+ macrophages increased following stimulation with LACK (57.6% ± 4.5%) compared to medium (41.5% ± 3.9%) and SLA (36.1% ± 5.9%).This profile has implications for immunoregulation of cellular responses depending on the effects that LACK stimulation may have on the macrophage population. Through IL-10 production, antigen presentation could be inhibited (1), while an increase of IFN-γ by SLA-responding T cells could counteract the inhibitory effects of LACK-responsive cells. These results characterizing the cellular response directed against LACK during infection in humans are in contrast to that defined for the recombinant antigen LeIF (16), which is associated with a Th1-type profile in human leishmaniasis. Because of this profile, one must question the adequacy of LACK antigen as a vaccine candidate in human leishmaniasis. However, as with any vaccine, LACK could presumably be used in conjunction with an adjuvant that in noninfected individuals could induce a more favorable Th1-type response.
The determination of cellular sources of IFN-γ and TNF-α in response to SLA was also somewhat surprising in some aspects. When analysis was made in R1 and R2, there was a clear difference seen in the severalfold-increased frequency of CD4+ T cells producing IFN-γ (Fig. 3 and 4) compared to those obtained with the other stimuli. While the CD4+ Th1-produced IFN-γ accounted for the majority of IFN-γ-producing cells, there was another significant source of IFN-γ-producing cells present in R1 and R2 identified as CD4− CD8− lymphocytes (Fig. 3 and 4). Additionally, the CD8+-T-cell population contributed to the IFN-γ production in response to exogenously added SLA. It is possible that peptides from the SLA preparation bound directly to major histocompatibility complex class I and/or that activation was due to indirect activation of previously activated CD8+ T cells by Th1 cells. Thus, the overall cytokine profile represented by high levels of IFN-γ seen in previous studies using ELISA and PCR is likely due to a mixture of different cell populations.
Moreover, the data give us an idea as to the level of commitment of the T-cell compartment to leishmania antigens present in the SLA preparation. As shown in Fig. 3F, an average of 22% of the CD4+ T-cell blasts produced IFN-γ in response to SLA. This level of commitment from blood lymphocytes indicates the systemic nature of infection with Leishmania and the extent to which circulating lymphocytes are committed to the response against Leishmania. How this profile will be reflected at the lesion site and in draining lymph nodes is currently being studied to determine if differential recruitment is seen.
These findings have several implications concerning the immune response during human cutaneous leishmaniasis. First, the SLA-induced IFN-γ production by CD4+ T cells in the absence of IL-4 or IL-5 production indicates the existence of true Th1 CD4+ T cells in this disease. Second, the response to LACK made up of a combination of the low frequency of IFN-γ- or TNF-α-producing cells, along with the induction of CD14+ cells, suggests that the response against LACK could be important for immunoregulation of the response against Leishmania. Lastly, the finding that there were very low frequencies of macrophages producing IL-12 may suggest that at this stage of the disease, high levels of IL-12 are no longer needed for the maintenance of the activated Th1-type response. These studies demonstrate an intricate level of control over the cytokine environment in the human disease, and depending on what compartment of the immune system is being stimulated by a given antigen, different types of responses may be encountered. Moreover, they aid in our understanding of the maintenance of the immune phenotype seen in these individuals and will be useful in studies designed around immunomodulatory therapy and vaccine development.
ACKNOWLEDGMENTS
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), PRONEX (Brazilian Research Financing Agency), and CNPq (Brazilian Research Financing Agency supplying student fellowships). Also, significant support was given by DNAX Research Institute, Palo Alto, Calif.
REFERENCES
- 1.Carvalho E M, Bacellar O, Brownell C, Regis T, Coffman R L, Reed S G. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–5956. [PubMed] [Google Scholar]
- 2.Carvalho E M, Correia F D, Bacellar O, Almeida R P, Lessa H, Rocha H. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg. 1995;53:273–277. doi: 10.4269/ajtmh.1995.53.273. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho S G, Oliveira M P, Da-Cruz A M, et al. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–155. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 4.Gazzinelli G, Katz N, Rocha R S, Colley D G. Immune responses during human Schistosomiasis mansoni. VIII. Differential in vitro cellular responsiveness to adult worm and schistosomular tegumental preparations. Am J Trop Med Hyg. 1983;32:326–333. [PubMed] [Google Scholar]
- 5.Gurunathan S, Sacks D L, Brown D R, Reiner S L, Charest H, Glaichenhaus N, Seder R A. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp K, Theander T G, Hviid L, Garfar A, Kharazmi A, Kemp M. Interferon-gamma- and tumour necrosis factor-alpha-producing cells in humans who are immune to cutaneous leishmaniasis. Scand J Immunol. 1999;49:655–659. doi: 10.1046/j.1365-3083.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- 7.Launois P, Maillard I, Pingel S, Swihart K G, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley R M, MacDonald H R, Louis J A. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 8.Liew F Y. Induction, regulation and function of T-cell subsets in leishmaniasis. Chem Immunol. 1992;54:117–135. [PubMed] [Google Scholar]
- 9.Liew F Y, O'Donnell C A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 10.Melby P C, Andrade-Narvaez F J, Darnell B J, Valencia-Pacheco G, Tryon V V, Palomo-Cetina A. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect Immun. 1994;62:837–842. doi: 10.1128/iai.62.3.837-842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z E, Waldmann R, Locksley R M, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 12.Pingel S, Launois P, Fowell D J, et al. Altered ligands reveal limited plasticity in the T cell response to a pathogenic epitope. J Exp Med. 1999;189:1111–1120. doi: 10.1084/jem.189.7.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiner S L, Fowell D J, Moskowitz N H, et al. Control of Leishmania major by a monoclonal alpha beta T cell repertoire. J Immunol. 1998;160:884–889. [PubMed] [Google Scholar]
- 14.Reiner S L, Wang Z E, Hatam F, Scott P, Locksley R M. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993;259:1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- 15.Scott P. T-cell subsets and T-cell antigens in protective immunity against experimental leishmaniasis. Curr Top Microbiol Immunol. 1990;155:35–52. doi: 10.1007/978-3-642-74983-4_3. [DOI] [PubMed] [Google Scholar]
- 16.Skeiky Y A, Guderian J A, Benson D R, et al. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sornasse T, Larenas P V, Davis K A, de Vries J E, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–483. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]