Abstract
Aortic regurgitation (AR) is a common valvular pathology. Multimodality noninvasive cardiovascular imaging is routinely used to assess the mechanism of AR, degree, and its hemodynamic impact on the cardiovascular system. Collecting this information is crucial in establishing the prognosis and in guiding patient management and follow‐up. While echocardiography remains the primary test to assess AR, a comprehensive assessment of this valvulopathy can be obtained by combining the information from different techniques. This state‐of‐the‐art review is intended to provide an update ed overview of the applications, strengths, and limits of transthoracic echocardiography, cardiac magnetic resonance, and cardiac computed tomography in patients with AR.
Keywords: aortic regurgitation, cardiac magnetic resonance, echocardiography, computed tomography, multimodality imaging
Transthoracic echocardiography (TTE) remains the first line test to assess aortic regurgitation (AR). Transesophageal echocardiography and cardiac magnetic resonance (CMR) can better quantify AR if TTE is not conclusive. CMR offers also superb assessment of left ventricle (LV) remodeling in AR. CT is the best technique to study thoracic aorta anatomy.
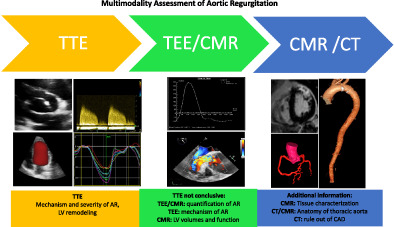
1. INTRODUCTION
Aortic regurgitation (AR) is defined as the presence of a regurgitant flow from the aorta to the left ventricle (LV) during diastole. Hemodynamic consequences are different in the acute or chronic setting. In severe acute AR, the volume overload on the LV results in an abrupt and prominent increase in LV filling pressures. As a consequence, cardiac output and systolic pressure are dramatically reduced. 1
In patients with chronic AR, volume and pressure overloads lead to progressive dilation and remodeling of the LV. Initially, these mechanisms of adaptation ensure preserved global systolic function. Diastolic pressure and cardiac output remain normal. In the earlier stages of the disease, patients could remain asymptomatic, and the hemodynamics are preserved. However, these compensation mechanisms may become inefficient in the long term, and LV dysfunction can occur with associated clinical symptoms. 2
AR is an insidious and asymptomatic disease, requiring close and accurate monitoring during long periods to anticipate myocardial damage. If not treated, AR can result in increased LV volume and causes heightened preload and afterload, which may be responsible for LV dilatation and dysfunction. Definitive treatment in case of severe AR is surgical intervention, which prevents heart failure and death and can significantly improve clinical outcome.
Management of patients with severe AR and related indications for surgery is based on clinical symptoms, left ventricular size, and aortic dimensions. 3
According to the European Society of Cardiology (ESC) guidelines, surgery is recommended in symptomatic patients with severe AR regardless of the LV systolic function and dimensions. In asymptomatic subjects, surgery is indicated in the presence of a LV ejection fraction (EF) ≤ 50% or LV end‐systolic diameter > 50 mm. 3 Furthermore left ventricular end‐systolic diameter should be indexed to the body surface area (BSA). Indeed, in subjects with low or large BSA who are not overweight, a value >25 mm/m2 is an indication for surgery. In addition, in selected asymptomatic cases who are at low risk for surgery, surgery may be considered in the presence of a LV end‐systolic diameter > 20 mm/m2 or LVEF between 50% and 55%. 3 In individuals with severe AR and an indication for coronary artery bypass grafting or surgery of the ascending aorta or another valve, intervention on the aortic valve is also recommended regardless of clinical symptoms. 3
Surgery in severe AR is mainly based on valve replacement. However, a repair could be considered in referral centers in selected patients with good expected durability based on the aortic valve morphological characteristics. Transcatheter aortic valve implantation (TAVI) may be evaluated in selected patients with AR and contraindication to valve replacement at experienced centers. 4 , 5
Close follow‐up is indicated in individuals with severe asymptomatic AR and normal LV systolic function, a relevant increase in LV diameters, or a reduction in LVEF. Intervention may be considered in these patients with a progressive reduction in LVEF or increased ventricular diameter during follow‐up and left ventricular end‐diastolic diameter (LVEDD) > 65 mm. 3
It appears clear that correctly grading AR and its hemodynamic repercussion is of crucial importance for patient's management since LV dysfunction caused by severe AR may be irreversible. This emphasizes the role of correct timing for surgical intervention. While echocardiography remains the first line test, other techniques can be used in order to solve specific diagnostic challenges. The role of each imaging modality is explained in the following sections.
2. TRANSTHORACIC ECHOCARDIOGRAPHY
2.1. Assessment of the AR mechanism
Transthoracic echocardiography (TTE) (Figure 1A,B,C) is the first‐line method for assessing the AR mechanism, severity, secondary degree of LV remodeling, and hemodynamic consequences.
FIGURE 1.
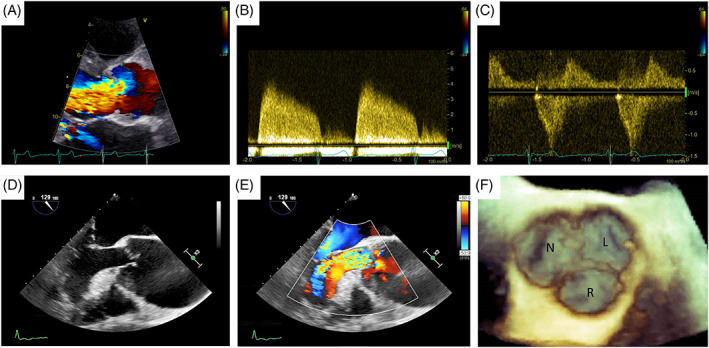
Echocardiographic assessment of aortic regurgitation: (A) TTE colorDoppler focused on left ventricle outflow tract showing a large aortic regurgitant jet, (B) TTE CW Doppler of the regurgitant jet, (C) TTE CW Doppler showing holodiastolic flow reversal in the descending aorta. (D,E) TEE assessment of a patient with severe aortic regurgitation due to a large coaptation defect secondary to aortic root dilatation. (F) 3D TEE assessment of the aortic valve 3D: three dimensional. CW, continuous wave; TTE, transthoracic echocardiography; TEE, transoesophageal echocardiography
The aortic valve is made up of three semilunar leaflets inserted superiorly to the aortic media and inferiorly to the myocardium of the left ventricular outflow tract (LVOT) and the anterior mitral leaflet. 6 These leaflets are normally symmetrical, with three commissures and free movement in the three aortic sinuses. 1
For the evaluation of AR, it is important to characterize both the etiology and the mechanism of AR. The etiology of AR is categorized as organic/primary when the regurgitation is mainly the result of structural leaflet abnormalities or as functional/secondary when regurgitation results from aortic root and/or ascending aorta dilatation in the presence of structural normal aortic valve leaflets. For primary AR, the most common causes of primary valve pathology in developed countries are degenerative AR and bicuspid aortic valve disease. 7 Other causes of AR are rheumatic disease, infective endocarditis, traumatic injuries, radiation‐induced valve disease, and inflammatory disorders. Functional AR is secondary to aortic dissection, annulo‐aortic ectasia, aortic aneurysm, Marfan syndrome, Ehlers–Danlos disease, and aortitis. 7
The mechanism of AR is categorized according to Carpentier's classification into type I, II, and III. 8 Type I is characterized by normal cusps movement and aortic root dilatation or leaflet perforation. Type II is defined by the presence of leaflet prolapse with excessive movement (IIa), or cusp with free edge fenestration (IIb), and consequent eccentric jet. Type III is characterized by leaflet restrictive motion. 8
2.2. Assessment of the severity of AR
The echocardiographic evaluation of the severity of AR is based on a multiparametric assessment which includes qualitative, semi‐quantitative, and quantitative parameters 9 (Table 1). The vena contracta and proximal isovelocity surface area (PISA) method are the most recommended parameters. Nonetheless, AR should always be evaluated in the context of other parameters corroborating the AR severity. This approach should be utilized to assess any AR, unless specific signs of severe regurgitation such as flail leaflet or a large coaptation defect are present. Assessment of AR using the extension of regurgitant jets color flow Doppler imaging alone is inadequate and dependent on LV compliance and diastolic pressure gradient.
TABLE 1.
AR severity subclasses according to the European Association of Cardiovascular Imaging 9
| AR severity classes | Mild | Moderate | Severe | |
| AR severity subclasses | Mild | Mild‐to‐moderate | Moderate‐to‐severe | Severe |
| Qualitative parameters | ||||
| Aortic valve morphology | Normal/abnormal | Normal/abnormal | Abnormal/prolapse/moderate coaptation defect | Abnormal/flail/large coaptation defect |
| Color flow AR jet width | Small in central jets | Intermediate | Large in central jet, variable in eccentric jets | Large in central jet, variable in eccentric jets |
| Color flow convergence | None or very small | Intermediate | Intermediate | Large |
| CW signal of AR jet | Incomplete/faint | Dense | Dense | Dense |
| Diastolic flow reversal in descending aorta | Brief, proto‐diastolic flow reversal | Intermediate | Holodiastolic flow reversal (end‐diastolic velocity 10 to <20 cm/s) | Holodiastolic flow reversal (end‐diastolic velocity ≥ 20 cm/s) |
| Diastolic flow reversal in abdominal aorta | Absent | Absent | Present | Present |
| Semi‐quantitative parameters | ||||
| VC width (mm) | <3 | 3–6 | 3–6 | >6 |
| Jet width/LVOT diameter (%) | <25 | 25–45 | 46–64 | ≥65 |
| Jet CSA/LVOT CSA (%) | <5 | 5–20 | 21–59 | ≥60 |
| Pressure half‐time (ms) | >500 | Intermediate, 500 to 200 | Intermediate, 500 to 200 | <200 |
| Quantitative parameters | ||||
| EROA (mm2) | <10 | 10–19 | 20–29 | ≥30 |
| R vol (mL) | <30 | 30–44 | 45–59 | ≥60 |
| RF (%) | <30 | 30–39 | 40–49 | ≥50 |
| CMR parameters | ||||
| RF (%) | <30 | 30–39 | 40–49 | ≥50 |
| Structural parameters | ||||
| LV size | Usually normal | Normal or dilatated | Usually dilatated | Usually dilatated |
Note: In bold: specific signs for severe AR.
Abbreviations: AR, aortic regurgitation; CSA, cross‐sectional area; CW, continuous wave; EROA, effective regurgitant orifice area; LA, left atrium; LV, left ventricle; RF, regurgitant fraction; R Vol, regurgitant volume; VC, vena contracta.
Color flow Doppler (Figure 1A,E) defines the AR jet in three components: flow convergence, vena contracta, and jet turbulence. The vena contracta (VC) method is based on the estimation of effective regurgitant orifice area (EROA) size. Parasternal long‐axis view is indicated for measuring the vena contracta width, immediately below the flow convergence zone. 10 The Nyquist limit for the diameter estimation is set to 50–60 cm/s with the optimization of the ratio between scale and color grade. In addition, a zoomed view of the aortic valve and maximization of frame rate is suggested. This method is independent of hemodynamic factors such as flow rate or diastolic pressure gradient. It is relatively independent of technical errors and can estimate eccentric jets.
VC width <3 mm is associated with mild AR, intermediate values of 3–6 mm with moderate AR, and values >6 mm indicate severe AR.
However, in case of AR with multiple jets, the value of VC is limited, and it is not advisable to add single widths. Another limitation of this method concerns the assumption of VC. Indeed, VC measurement is based on the circular shape of EROA, however, the orifice can be irregular (or triangular) and therefore change in diameter in different views.
PISA method allows the direct estimation of EROA and regurgitant volume. This approach is based on the study of flow convergence generated around the regurgitant orifice and the hemispheric concentric velocity shells related to the flow area. 11 Specifically, the parasternal long‐axis view is preferred for eccentric jets, while the apical five or three‐chamber view in the case of central jets. 12 A zoom view of AR color flow imaging is suggested, and the Nyquist limit is shifted in the direction of the jet to acquire a hemispheric proximal flow convergence area. Measurement of PISA radius is recommended using the first aliasing during diastole, removing the color Doppler to correctly visualize the regurgitating orifice. Consequently, the calculation of EROA and regurgitant volume is performed by the measurement of the peak velocity and velocity‐time integral (VTI) from the AR continuous‐wave Doppler signal.
An EROA <10 mm2 or regurgitant volume < 30 mL is classified as mild AR. An EROA of 10–19 mm2 or regurgitant volume of 30–44 mL is subclassified in mild‐to‐moderate AR, while in moderate‐to‐severe AR an EROA of 20–29 mm2 or regurgitant volume of 45–59 mL is present. Finally, an EROA ≥30 mm2 or regurgitant volume ≥ 60 ml indicates severe AR.
However, this method is affected by several limitations. 13 , 14 The presence of valve calcifications reduces the feasibility. It is not also reliable in the presence of multiple jets, and alteration of the PISA assumption determines a severity under‐or overestimation (e.g., noncircular orifice, adjacent contrasting flows, or nonplanar flow convergence).
Several other parameters should be evaluated to confirm the AR severity. The ratio between jet width and LVOT diameter indicates a severe AR when >65%. 15 The pressure half time (PHT) from the continuous wave (CW) regurgitant Doppler trace depends from the LV and aortic compliance, and it is associated with severe AR if <200 ms. The regurgitant fraction is calculated from the regurgitation volume divided by the LVOT stroke volume and indicates severe AR when ≥50%.
The strongest additional method for assessing AR is diastolic flow reversal in aorta (Figure 1C). 16 Specifically, holodiastolic flow reversal in the descending aorta, with end‐diastolic velocity ≥ 20 cm/s at peak R wave, is associated with severe AR. Pulsed wave Doppler sample is placed in the upper descending aorta, just below the left subclavian artery, with a Doppler filter <10 cm/s.
The presence of diastolic reversal flow in the abdominal aorta is a parameter with a high specificity for severe AR.
Three‐dimensional (3D) echocardiography provides incremental information about aortic valve anatomy and AR hemodynamic consequences on cardiac chambers geometry and function. Indeed, this method allows a better evaluation of volumes and LVEF. 16 In addition, 3D color flow Doppler enables direct planimetry assessment of the VC area, revealing a circular or more elliptical shape of the jet, by using multiplanar reconstructions parallel to the axis of the AR flow. 17
Transesophageal echocardiography (TEE) (Figure 1D,E) is recommended in case of indeterminate AR severity due to an inconclusive TTE or the need for further diagnostic definition in case of discrepancy between AR severity and clinical status. 9 In addition, TEE improves the accuracy of TTE in specific situations such as endocarditis, and isolated aortic root dilation. 18 , 19 , 20 Furthermore, 3D TEE (Figure 1F) is useful for evaluating the aortic valve morphology and the AR mechanism (in particular valuable in selecting patients for aortic valve repair), while the multiplanar color Doppler allows to calculate the 3D vena contracta. 16
Exercise echocardiography helps assess symptomatic status in patients with severe and asymptomatic AR. Furthermore, this method can provide additional parameters such as LV contractile reserve 21 Exercise‐induced change in LVEF is determined by several components such as systolic contractile function, degree of volume overload variation in preload, and resistance during effort. 9
2.3. Left ventricle remodeling assessment
Hemodynamic changes on the LV alter its dimensions and systolic function. Generally, LV dilatation is present in chronic AR of moderate‐to‐severe grade. Instead, LV size is preserved in acute severe or chronic mild AR. LV is identified as dilated in presence of end‐diastolic diameter > 56 mm, end‐diastolic volume > 82 ml/m2, end‐systolic diameter > 40 mm, or end‐systolic volume > 30 ml/m2. 9 In addition, chronic AR generates a progressive, irreversible damage to the LV with a reduction in EF. This reduction in LVEF to a value <50% might represent a late stage of myocardial overload and irreversible damage could be prevented by better cut‐off values. Indeed, some reports have already suggested that the cut‐off level of LVEF should probably be raised to 55%. 22
Perhaps a better method to quantify myocardial function is by means of myocardial deformation imaging. Global longitudinal strain (GLS) is a simple and very robust parameter derived from bidimensional (2D) echocardiographic images. Several studies have shown that in asymptomatic patients with more than moderate chronic AR and preserved LVEF, worsening LV‐GLS was associated with longer term mortality, providing incremental prognostic value and improved reclassification. 23 In addition, in patients with a preserved LVEF but with a reduced GLS, long term outcome is worse than those with a preserved GLS after AVR, indicating that this early systolic dysfunction is already associated with adverse remodeling impacting outcome. 24
3. ROLE OF CARDIAC MAGNETIC RESONANCE IN AORTIC REGURGITATION
Cardiac magnetic resonance (CMR) (Figure 2) has an emerging role in the assessment of the patient with AR for several reasons. First, it represents the current reference standard for evaluating cardiac volumes, mass, and systolic function. 25 Second, it characterizes the myocardial tissue, providing additional prognostic information. 26 Third, CMR allows anatomical and functional assessment of the aortic valve and the entire thoracic aorta. Finally, CMR provides accurate quantification of AR regurgitant volume and fraction. CMR is recommended by both ESC and American Heart Association/American College of Cardiology (AHA/ACC) as a valuable complementary modality to quantify AR where echocardiography results are inconclusive. 27
FIGURE 2.
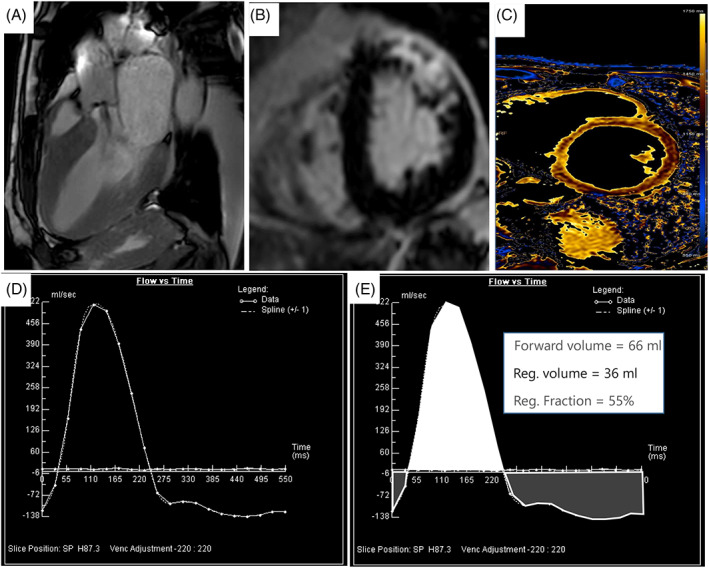
Cardiac magnetic resonance assessment of aortic regurgitation: (A) Visualization of a “jet” (hypointense signal due to phase loss of proton spin due to turbulent flow) of aortic insufficiency with a cine‐SSFP sequence; (B) late gadolinium enhancement sequence showing an intramyocardial enhancement of the anterior and antero‐lateral wall of the left ventricle. (C) Native T1 mapping to assess the presence of interstitial fibrosis, (D) flow/time curve phase contrast derived: positive values refer to forward flow while negative values indicate backward (regurgitant) flow, (E) example of measurement of regurgitant volume (Reg. volume) and regurgitant fraction (Reg. fraction) using flow/time curve phase contrast derived. SSFP, steady state free precession
3.1. Assessment of aortic valve
Morphology and function of the aortic valve is assessed with steady‐state free precession (SSFP) pulse sequences (Figure 2A).
A stack of cines SSFP covering the aortic valve is used to assess the planimetry of the aortic valve which provides the size of the aortic valve area. 28
However, the spatial resolution of CMR is often not enough to visualize small vegetations in infective endocarditis and small valvular masses especially if high mobile. 28
Moreover, cine SSFP imaging is affected by arrhythmias.
3.2. Assessment of thoracic aorta
The assessment of the aortic root is crucial for the correct identification of the AR etiology (e.g., hypertension, aortic dissection, and Marfan syndrome) as well as for the surgical planning of aortic root repair/replacement. 29 Furthermore, the CMR is accurate for evaluating the thoracic aorta. 29 , 30 The use of SSFP sequences has become prevalent because they broadly provide high contrast between liquid compartments and surrounding tissue, making it suitable for vessel imaging. 31 Moreover, the 3‐dimensional contrast‐enhancement magnetic resonance angiography (the so called “3D Whole Heart”) is helpful to visualize and assess the thoracic aorta and for the surgical plan and follow‐up after the intervention. 32 Eventually, four dimensional (4D) flow MRI is emerging as a tool that can be used to study both thoracic aorta anatomy and aortic flow dynamics. This new sequences allow quantification of blood flow volume and velocity and study of velocity‐encoded 3D path lines. 33 , 34
3.3. Left ventricular remodeling
SSFP pulse sequences are validated for evaluating LV volume, mass, and function. 35 Ventricular volumes are calculated from a short‐axis stack of 6–8 mm thick slices with an interslice gap of 4 mm. CMR can assess regional fibrosis using late gadolinium enhancement (LGE) imaging (Figure 2B), which represents the noninvasive reference standard to quantify myocardial fibrosis. 28 A recent study found that in a third of patients with AR was present LGE and that it was independently associated with a 2.5‐fold increase in mortality in patients with moderate or severe AR. Moreover, in patients with LGE, aortic valve surgery was associated with a better prognosis as compared to medical therapy. 36 In addition to LGE assessment, T1 mapping techniques are useful in quantifying the extracellular matrix expansion, which can be inferred to represent diffuse interstitial fibrosis in several cardiac disorders, including those caused by chronic hemodynamic (volume and/or pressure) overload in valvular heart disease (Figure 2C). 37 In the most recent literature, two surrogate CMR biomarkers for diffuse interstitial fibrosis have emerged: global extracellular volume fraction (ECV), which measures the proportion of LV myocardium that is extracellular matrix; and indexed ECV (iECV),the amount of extracellular matrix correlated to LV mass. 38 Senapati et al. demonstrated that iECV is more strongly associated with AR severity and adverse clinical outcomes than ECV or replacement fibrosis. Because iECV represents the total LV fibrosis burden, it better characterizes the remodeling changes occurring in progressive AR that lead to cellular and extracellular expansion. Future multicenter randomized studies are needed to validate using iECV as a CMR prognostic marker for clinical outcomes.
3.4. Aortic regurgitation quantification
Phase‐contrast sequences (Figure 2D,E) are used to assess the AR severity. Specifically, phase contrast velocity mapping planned at the sino‐tubular junction level can quantify regurgitant volumes (RV) and fractions (RF). 25 RV and RF are prognostic independent predictors in patients with AR. Indeed, A RF of >33% and a RV > 42 ml predict the likelihood of intervention. 28 In another study, all patients with RF <26% had no need for surgery. 39 In Table 1 are reported the cut off of RF to establish AR severity in CMR according to the recent recommendation of the European Association of Cardiovascular Imaging. 9
However, the RV and RF measurements are reproducible and reliable only when the flow is laminar or noncomplex. When there are pathologies that determinate complex flow, such as a bicuspid valve or ascending aortic dilatation, the measurements may be biased and no longer reliable, as has been shown by Frida Truedsson et al. 40
Finally, 4D flow CMR has been studied as an alternative method for flow measurement. According to the literature, values obtained by 4D flow CMR are more accurate than the 2D Phase Contrast in the presence of turbulent flows. 41 , 42 However, 4D flow is still not commonly used in clinical routines to quantify AR.
4. ROLE OF CT IN AORTIC REGURGITATION
Computed tomography (CT) is frequently used preoperatively in patients with AR as it gives accurate information about the aorta size and morphology of the valve. It can also rule out the presence of associated coronary artery disease. The scan protocol includes the use of iodinated contrast agents and electrocardiographic (ECG) gating, with retrospective reconstruction or prospective ECG triggering. Retrospective ECG gating offers a bigger acquisition of data by scanning through multiple cardiac cycles. This allows the imager to postprocess different cardiac phases but gives the patient a higher amount of radiation. Prospective ECG gating allows the acquirement of images once triggered by the R peak on the ECG tracing leading to a lower exposition for the patient. 43 , 44
4.1. Assessment of the aorta
Imaging and measuring the aortic size is fundamental for stratification and surgical planning of patients with AR. CT can provide a detailed anatomic assessment of the thoracic aorta. Maximum diameters are measured specifically at four levels: annulus, sinus of Valsalva, sino‐tubular junction, and tubular ascending aorta (Figure 3B,C). 3 One of the major causes of acute AR is aortic dissection (Figure 3A). Contrast‐enhanced, ECG‐gated CT angiography, thanks to its wide availability, short scan time, a sensibility of 100%, and a specificity of 98% is commonly used to rule out aortic dissections. 45 , 46 Furthermore, CT leads to an optimal visualization of the aortic root and the valvular plan (Figure 3D).
FIGURE 3.
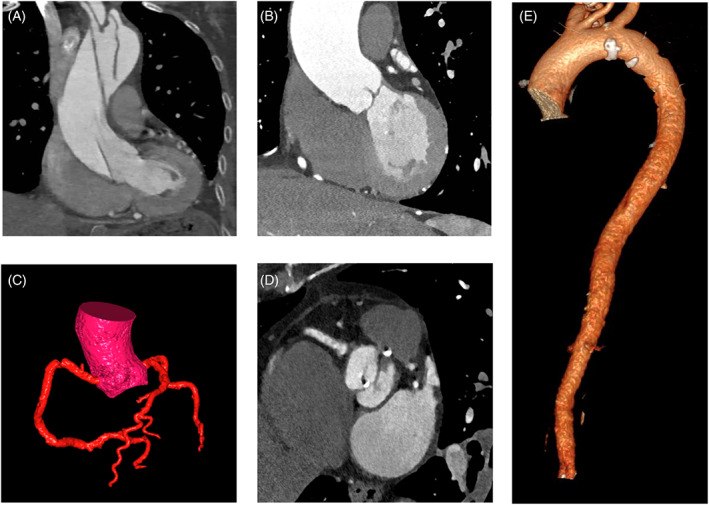
Computed tomography assessment of aortic regurgitation. (A) type A aortic dissection in a patient with AR, (B) aorta ascendens dilatation in a patient with moderate AR (C), volume rendering of the coronary arteries, (D) Bicuspid aortic valve with degeneration of the valve leaflets, (E) volume rendering of the thoracoabdominal aorta. AR, aortic RegurgitationFlow
Frazao et al. compared the agreement of TTE, CT, and CMR in sizing the aortic root. While CT and CMR did not show significant differences, TTE longitudinal measurement was significantly smaller than the measurements from CCT and CMR. Moreover, since the aortic root is not symmetric (especially in bicuspid aortic valve), a one‐plane echocardiography measurement can lead to an underestimation of the real size of the aortic valve. 47 , 48 , 49 Various methods have been proposed to assess the aortic root in CT. The latest evidence suggests calculating diameters using the inner‐inner edge technique by taking sinus‐to‐sinus diameters rather than sinus‐to‐commissure as the sinus‐to‐commissure method can underestimate the real enlargement. 3 , 47 , 49 , 50
CT can be useful to rule out coronary artery disease (CAD) in patients who must undergo an intervention (Figure 3E). 51 2021 ESC/European Association Cardiovascular Thoracic Surgery (EACTS) guidelines for the management of valvular heart disease propose the use of CT as an alternative to coronary angiography to rule out CAD in patients with low atherosclerosis risk seeing the high negative predictive value of the CT. 3
4.2. Aortic valve anatomy and regurgitation
CT can help to evaluate aortic valve morphology and pathology (e.g., calcifications, prolapse, infective process, rheumatic diseases). Koo et al. demonstrated that the functional classification of AR is useful for TAVI planning and that CT can show cusp abnormalities that can lead to tailored intervention. 52 Moreover, a precise understanding of the aortic root and valve via CT is helpful in predicting surgical reparability and can spare the patient unnecessary aortic root replacement. 52
Endocarditis is one of the major causes of AR. In the last few years, the importance of a deep understanding of the pathological findings before the surgery is increased, and so has the importance of CT. 53 , 54 Retrospective electrocardiographic (ECG) gated CT is the best modality to visualize and localize abscess and calcification. 53 Latest literature remarks on the superiority of TEE for small vegetations but underlines the better comprehension of the CT for the periannular complications, the presence of intracoronary embolism, and prosthetic valve infection. 53 , 55 , 56
The importance of CT is also emerging for assessing valve functionality and complications. Several studies has been showing how CT can be helpful in evaluating aortic prosthetic valve dysfunctions by assessing opening and closing angles with a strong correlation with the fluoroscopy results. Moreover, CT is useful for highlighting the presence of a fibrous pannus or thrombi. 57 , 58 , 59
4.3. LV sizing and myocardial fibrosis
CMR remains the gold standard for LV volume, function and mass measurement. Nevertheless, in patients with contraindication to CMR, ECG‐gated CT offers a useful tool to define LV size and systolic function. 60 Moreover, Klein et al. found a correlation between LV mass/BSA measurement, sizing the LV with CT and adverse cardiac major events. 61
As iodinated‐contrast agents and gadolinium‐based materials have similar pharmacokinetics, cardiac CT can be used as an alternative to CMR in assessing focal myocardial fibrosis. 62
Moreover, extracellular volume can be calculated with cardiac CT in order to assess diffuse fibrosis. 63 However, no studies have explored so far the role of CT tissue characterization in patients with AR.
5. CONCLUSIONS
Multimodality imaging provides great complementary data that can be tailored to the single individual and to the specific clinical case (Figure 4).
FIGURE 4.
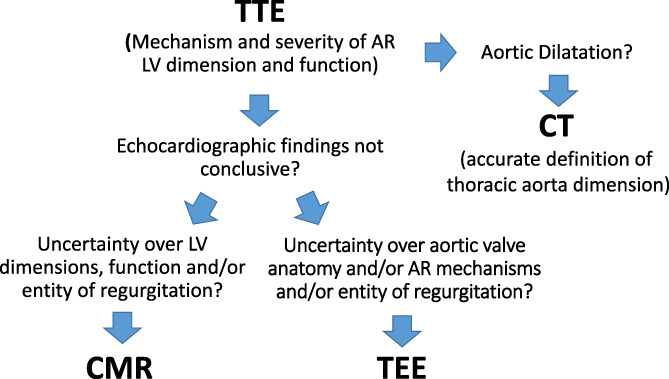
How to choose the right imaging modality in patients with aortic regurgitation. CMR, cardiac magnetic resonance; CT, computed tomography; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography
Echocardiography remains the first technique to be used in patients with AR. The assessment starts with TTE in order to precise the mechanism and severity of regurgitation. Then, TTE defines the hemodynamic impact of valvulopathy. In particular, LV remodeling is assessed by measuring volumes and myocardial function. The latter, classically identified with LVEF can be more precisely measured using deformation parameters, in particular GLS.
In presence of an inadequate acoustic window and when the parameters used to grade the regurgitation are discordant, TEE and CMR can be valuable additional techniques in patients with AR. In particular, CMR not only represents the gold standard for the assessment of volumes and EF but is used to accurately determine the regurgitant volume and fraction of AR providing in doubtful cases diriment information for surgical indication. Furthermore, CMR tissue characterization with LGE and T1 mapping can be used to better stratify patient's prognosis.
Finally, in patients candidate to surgery, CT provides accurate measurement of thoracic aorta and can rule out coronary artery disease. Moreover, CT can be particularly useful to study patients with aortic prosthesis dysfunction and to identify periannular complication in patients with endocarditis of the aortic valve.
The study of flow patterns with 4D‐flow CMR or with particle image velocimetry or vector flow mapping in echocardiography are still in the realm of research. However, we expect that in the future they will provide other precious piece of information to further improve AR patient's care.
List of abbreviations
- 2D
bi‐dimensional
- 3D
three‐dimensional
- 4D
four‐dimensional
- AR
Aortic regurgitation
- BSA
Body surface area
- CAD
Coronary artery disease
- CMR
Cardiac magnetic resonance
- CT
Computed tomography
- ECG
Electrocardiographic
- ECV
Extracellular volume
- iECV
Indexed extracellular volume
- EDD
End‐diastolic diameter
- EF
Ejection fraction
- EROA
Effective regurgitant orifice area
- CW
Continuous wave
- GLS
Global longitudinal strain
- LGE
Late gadolinium enhancement
- LVOT
Left ventricular outflow ttract
- LV
Left ventricle
- PISA
Proximal isovelocity surface area
- PW
Pulsed wave
- PHT
Pressure half time
- RF
Regurgitant fraction
- RV
Regurgitant volume
- SSFP
Steady state free precession
- TAVI
Transcatheter aortic valve implantation
- TTE
Transthoracic echocardiography
- TEE
Transesophageal echocardiography
- VC
Vena contracta
CONFLICT OF INTEREST
The authors declare no relationship with industry and financial associations within the past two years that pose a conflict of interest in the submitted article.
Siani A, Perone F, Costantini P, et al. Aortic regurgitation: A multimodality approach. J Clin Ultrasound. 2022;50(8):1041‐1050. doi: 10.1002/jcu.23299
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native Valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303‐371. [DOI] [PubMed] [Google Scholar]
- 2. Maurer G. Aortic regurgitation. Heart. 2006;92(7):994‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Rev Esp Cardiol. 2022;75(6):524. [DOI] [PubMed] [Google Scholar]
- 4. Sawaya FJ, Deutsch MA, Seiffert M, et al. Safety and efficacy of Transcatheter aortic valve replacement in the treatment of pure aortic regurgitation in native valves and failing surgical bioprostheses: results from an international registry study. JACC Cardiovasc Interv. 2017;10(10):1048‐1056. [DOI] [PubMed] [Google Scholar]
- 5. Yoon SH, Schmidt T, Bleiziffer S, et al. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J Am Coll Cardiol. 2017;70(22):2752‐2763. [DOI] [PubMed] [Google Scholar]
- 6. Muraru D, Badano LP, Vannan M, Iliceto S. Assessment of aortic valve complex by three‐dimensional echocardiography: a framework for its effective application in clinical practice. Eur Heart J Cardiovasc Imaging. 2012;13(7):541‐555. [DOI] [PubMed] [Google Scholar]
- 7. Iung B, Delgado V, Rosenhek R, et al. Contemporary presentation and Management of Valvular Heart Disease: the EURObservational research Programme Valvular heart disease II survey. Circulation. 2019;140(14):1156‐1169. [DOI] [PubMed] [Google Scholar]
- 8. El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol. 2005;20(2):115‐121. [DOI] [PubMed] [Google Scholar]
- 9. Lancellotti P, Pibarot P, Chambers J, et al. Multi‐modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. 2022;23(5):e171‐e232. [DOI] [PubMed] [Google Scholar]
- 10. Eren M, Eksik A, Gorgulu S, et al. Determination of vena contracta and its value in evaluating severity of aortic regurgitation. J Heart Valve Dis. 2002;11(4):567‐575. [PubMed] [Google Scholar]
- 11. Tribouilloy CM, Enriquez‐Sarano M, Fett SL, Bailey KR, Seward JB, Tajik AJ. Application of the proximal flow convergence method to calculate the effective regurgitant orifice area in aortic regurgitation. J Am Coll Cardiol. 1998;32(4):1032‐1039. [DOI] [PubMed] [Google Scholar]
- 12. Pouleur AC, le Polain de Waroux JB, Goffinet C, et al. Accuracy of the flow convergence method for quantification of aortic regurgitation in patients with central versus eccentric jets. Am J Cardiol. 2008;102(4):475‐480. [DOI] [PubMed] [Google Scholar]
- 13. Simpson IA, Shiota T, Gharib M, Sahn DJ. Current status of flow convergence for clinical applications: is it a leaning tower of "PISA"? J Am Coll Cardiol. 1996;27(2):504‐509. [DOI] [PubMed] [Google Scholar]
- 14. Yosefy C, Levine RA, Solis J, Vaturi M, Handschumacher MD, Hung J. Proximal flow convergence region as assessed by real‐time 3‐dimensional echocardiography: challenging the hemispheric assumption. J Am Soc Echocardiogr. 2007;20(4):389‐396. [DOI] [PubMed] [Google Scholar]
- 15. Perry GJ, Helmcke F, Nanda NC, Byard C, Soto B. Evaluation of aortic insufficiency by Doppler color flow mapping. J Am Coll Cardiol. 1987;9(4):952‐959. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233‐270. [DOI] [PubMed] [Google Scholar]
- 17. Fang L, Hsiung MC, Miller AP, et al. Assessment of aortic regurgitation by live three‐dimensional transthoracic echocardiographic measurements of vena contracta area: usefulness and validation. Echocardiography. 2005;22(9):775‐781. [DOI] [PubMed] [Google Scholar]
- 18. Thorsgard ME, Morrissette GJ, Sun B, et al. Impact of intraoperative transesophageal echocardiography on acute type‐a aortic dissection. J Cardiothorac Vasc Anesth. 2014;28(5):1203‐1207. [DOI] [PubMed] [Google Scholar]
- 19. Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr. 2003;16(1):67‐70. [DOI] [PubMed] [Google Scholar]
- 20. la Canna G, Maisano F, De Michele L, et al. Determinants of the degree of functional aortic regurgitation in patients with anatomically normal aortic valve and ascending thoracic aorta aneurysm. Transoesophageal Doppler echocardiography study. Heart. 2009;95(2):130‐136. [DOI] [PubMed] [Google Scholar]
- 21. Lancellotti P, Pellikka PA, Budts W, et al. The clinical use of stress echocardiography in non‐ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17(11):1191‐1229. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Q, Zhang B, Ye Y, et al. Prognostic impact of left ventricular ejection fraction in patients with moderate aortic regurgitation: potential implications for treatment decision‐making. Front Cardiovasc Med. 2021;8:800961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alashi A, Mentias A, Abdallah A, et al. Incremental prognostic utility of left ventricular global longitudinal strain in asymptomatic patients with significant chronic aortic regurgitation and preserved left ventricular ejection fraction. JACC Cardiovasc Imaging. 2018;11(5):673‐682. [DOI] [PubMed] [Google Scholar]
- 24. Alashi A, Khullar T, Mentias A, et al. Long‐term outcomes after aortic valve surgery in patients with asymptomatic chronic aortic regurgitation and preserved LVEF: impact of baseline and follow‐up global longitudinal strain. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):12‐21. [DOI] [PubMed] [Google Scholar]
- 25. Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two‐dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 26. Guglielmo M, Pontone G. Risk stratification in cardiomyopathies (dilated, hypertrophic, and arrhythmogenic cardiomyopathy) by cardiac magnetic resonance imaging. Eur Heart J Suppl. 2021;23(Suppl E):E118‐E122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reid A, Blanke P, Bax JJ, Leipsic J. Multimodality imaging in valvular heart disease: how to use state‐of‐the‐art technology in daily practice. Eur Heart J. 2021;42(19):1912‐1925. [DOI] [PubMed] [Google Scholar]
- 28. Guglielmo M, Rovera C, Rabbat MG, Pontone G. The role of cardiac magnetic resonance in aortic stenosis and regurgitation. J Cardiovasc Dev Dis. 2022;9(4):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Knobelsdorff‐Brenkenhoff F, Trauzeddel RF, Schulz‐Menger J. Cardiovascular magnetic resonance in adults with previous cardiovascular surgery. Eur Heart J Cardiovasc Imaging. 2014;15(3):235‐248. [DOI] [PubMed] [Google Scholar]
- 30. Sundstrom E, Jonnagiri R, Gutmark‐Little I, et al. Hemodynamics and tissue biomechanics of the thoracic aorta with a trileaflet aortic valve at different phases of valve opening. Int J Numer Method Biomed Eng. 2020;36(7):e3345. [DOI] [PubMed] [Google Scholar]
- 31. Groth M, Henes FO, Mullerleile K, Bannas P, Adam G, Regier M. Accuracy of thoracic aortic measurements assessed by contrast enhanced and unenhanced magnetic resonance imaging. Eur J Radiol. 2012;81(4):762‐766. [DOI] [PubMed] [Google Scholar]
- 32. Srichai MB, Kim S, Axel L, Babb J, Hecht EM. Non‐gadolinium‐enhanced 3‐dimensional magnetic resonance angiography for the evaluation of thoracic aortic disease: a preliminary experience. Tex Heart Inst J. 2010;37(1):58‐65. [PMC free article] [PubMed] [Google Scholar]
- 33. Gil‐Sala D, Guala A, Garcia Reyes ME, et al. Geometric, biomechanic and Haemodynamic aortic abnormalities assessed by 4D flow cardiovascular magnetic resonance in patients treated by TEVAR following blunt traumatic thoracic aortic injury. Eur J Vasc Endovasc Surg. 2021;62(5):797‐807. [DOI] [PubMed] [Google Scholar]
- 34. Takahashi K, Sekine T, Ando T, Ishii Y, Kumita S. Utility of 4D flow MRI in thoracic aortic diseases: a literature review of clinical applications and current evidence. Magn Reson Med Sci. 2022;21(2):327‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller CA, Jordan P, Borg A, et al. Quantification of left ventricular indices from SSFP cine imaging: impact of real‐world variability in analysis methodology and utility of geometric modeling. J Magn Reson Imaging. 2013;37(5):1213‐1222. [DOI] [PubMed] [Google Scholar]
- 36. Malahfji M, Senapati A, Tayal B, et al. Myocardial scar and mortality in chronic aortic regurgitation. J Am Heart Assoc. 2020;9(23):e018731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kammerlander AA, Marzluf BA, Zotter‐Tufaro C, et al. T1 mapping by CMR imaging: from histological validation to clinical implication. JACC Cardiovasc Imaging. 2016;9(1):14‐23. [DOI] [PubMed] [Google Scholar]
- 38. Senapati A, Malahfji M, Debs D, et al. Regional replacement and diffuse interstitial fibrosis in aortic regurgitation: prognostic implications from cardiac magnetic resonance. JACC Cardiovasc Imaging. 2021;14(11):2170‐2182. [DOI] [PubMed] [Google Scholar]
- 39. Myerson SG, d'Arcy J, Mohiaddin R, et al. Aortic regurgitation quantification using cardiovascular magnetic resonance: association with clinical outcome. Circulation. 2012;126(12):1452‐1460. [DOI] [PubMed] [Google Scholar]
- 40. Truedsson F, Polte CL, Gao SA, Johnsson AA, Bech‐Hanssen O, Lagerstrand KM. Importance of complex blood flow in the assessment of aortic regurgitation severity using phase contrast magnetic resonance imaging. Int J Cardiovasc Imaging. 2021;37(12):3561‐3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alvarez A, Martinez V, Pizarro G, Recio M, Cabrera JA. Clinical use of 4D flow MRI for quantification of aortic regurgitation. Open Heart. 2020;7(1):e001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lenz A, Petersen J, Riedel C, et al. 4D flow cardiovascular magnetic resonance for monitoring of aortic valve repair in bicuspid aortic valve disease. J Cardiovasc Magn Reson. 2020;22(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murphy DJ, Aghayev A, Steigner ML. Vascular CT and MRI: a practical guide to imaging protocols. Insights Imaging. 2018;9(2):215‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalisz K, Buethe J, Saboo SS, Abbara S, Halliburton S, Rajiah P. Artifacts at cardiac CT: physics and solutions. Radiographics. 2016;36(7):2064‐2083. [DOI] [PubMed] [Google Scholar]
- 45. Vargo PR, Reich H, Roselli EE. Computed tomography imaging of aortic dissections with endovascular treatment considerations. Curr Cardiol Rep. 2021;23(9):113. [DOI] [PubMed] [Google Scholar]
- 46. Carroll BJ, Schermerhorn ML, Manning WJ. Imaging for acute aortic syndromes. Heart. 2020;106(3):182‐189. [DOI] [PubMed] [Google Scholar]
- 47. Frazao C, Tavoosi A, Wintersperger BJ, et al. Multimodality assessment of thoracic aortic dimensions: comparison of computed tomography angiography, magnetic resonance imaging, and echocardiography measurements. J Thorac Imaging. 2020;35(6):399‐406. [DOI] [PubMed] [Google Scholar]
- 48. Neumann N, Petersen J, Sinning C, et al. Focus on the annuloplasty in aortic valve repair: implications from a quantitative multislice computed tomography analysis. Quant Imaging Med Surg. 2020;10(4):853‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buzzatti N, Maisano F, Latib A, et al. Computed tomography‐based evaluation of aortic annulus, prosthesis size and impact on early residual aortic regurgitation after transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2013;43(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 50. Freeman LA, Young PM, Foley TA, Williamson EE, Bruce CJ, Greason KL. CT and MRI assessment of the aortic root and ascending aorta. AJR Am J Roentgenol. 2013;200(6):W581‐W592. [DOI] [PubMed] [Google Scholar]
- 51. Gohmann RF, Seitz P, Pawelka K, et al. Combined coronary CT‐angiography and TAVI planning: utility of CT‐FFR in patients with morphologically ruled‐out obstructive coronary artery disease. J Clin Med. 2022;11(5):1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koo HJ, Kang JW, Kim JA, et al. Functional classification of aortic regurgitation using cardiac computed tomography: comparison with surgical inspection. Int J Cardiovasc Imaging. 2018;34(8):1295‐1303. [DOI] [PubMed] [Google Scholar]
- 53. Khalique OK, Veillet‐Chowdhury M, Choi AD, Feuchtner G, Lopez‐Mattei J. Cardiac computed tomography in the contemporary evaluation of infective endocarditis. J Cardiovasc Comput Tomogr. 2021;15(4):304‐312. [DOI] [PubMed] [Google Scholar]
- 54. Khalique OK, Pulerwitz TC, Halliburton SS, et al. Practical considerations for optimizing cardiac computed tomography protocols for comprehensive acquisition prior to transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2016;10(5):364‐374. [DOI] [PubMed] [Google Scholar]
- 55. Sims JR, Anavekar NS, Chandrasekaran K, et al. Utility of cardiac computed tomography scanning in the diagnosis and pre‐operative evaluation of patients with infective endocarditis. Int J Cardiovasc Imaging. 2018;34(7):1155‐1163. [DOI] [PubMed] [Google Scholar]
- 56. Kim IC, Chang S, Hong GR, et al. Comparison of cardiac computed tomography with transesophageal echocardiography for identifying vegetation and Intracardiac complications in patients with infective endocarditis in the era of 3‐dimensional images. Circ Cardiovasc Imaging. 2018;11(3):e006986. [DOI] [PubMed] [Google Scholar]
- 57. LaBounty TM, Agarwal PP, Chughtai A, Bach DS, Wizauer E, Kazerooni EA. Evaluation of mechanical heart valve size and function with ECG‐gated 64‐MDCT. AJR Am J Roentgenol. 2009;193(5):W389‐W396. [DOI] [PubMed] [Google Scholar]
- 58. Sucha D, Symersky P, Vonken EJ, Provoost E, Chamuleau SA, Budde RP. Multidetector‐row computed tomography allows accurate measurement of mechanical prosthetic heart valve leaflet closing angles compared with fluoroscopy. J Comput Assist Tomogr. 2014;38(3):451‐456. [DOI] [PubMed] [Google Scholar]
- 59. Teshima H, Hayashida N, Fukunaga S, et al. Usefulness of a multidetector‐row computed tomography scanner for detecting pannus formation. Ann Thorac Surg. 2004;77(2):523‐526. [DOI] [PubMed] [Google Scholar]
- 60. Ko SM, Hwang SH, Lee HJ. Role of cardiac computed tomography in the diagnosis of left ventricular myocardial diseases. J Cardiovasc Imaging. 2019;27(2):73‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klein R, Ametepe ES, Yam Y, Dwivedi G, Chow BJ. Cardiac CT assessment of left ventricular mass in mid‐diastasis and its prognostic value. Eur Heart J Cardiovasc Imaging. 2017;18(1):95‐102. [DOI] [PubMed] [Google Scholar]
- 62. Emoto T, Oda S, Kidoh M, et al. Myocardial extracellular volume quantification using cardiac computed tomography: a comparison of the dual‐energy iodine method and the standard subtraction method. Acad Radiol. 2021;28(5):e119‐e126. [DOI] [PubMed] [Google Scholar]
- 63. Scully PR, Bastarrika G, Moon JC, Treibel TA. Myocardial extracellular volume quantification by cardiovascular magnetic resonance and computed tomography. Curr Cardiol Rep. 2018;20(3):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


