Abstract
Temporin-GHa (GHa) was cloned from Hylarana guentheri, showing a weak antimicrobial activity. In order to improve its bactericidal efficacy, GHaR6R, GHaR7R, GHaR8R and GHaR9W were designed and synthesized. Compared to the parent peptide, the GHa-derived peptides show potent antimicrobial activities against methicillin-resistant Staphylococcus aureus (MRSA), which is the main pathogen with high morbidity and mortality that causes various infections in humans. These peptides exert bactericidal actions on MRSA by permeabilizing the cytoplasmic membranes and damaging membrane integrity. All of the four peptides exhibit excellent stability under harsh conditions, including extreme temperature and salts. Furthermore, they inhibit the formation of biofilm and eradicate mature biofilm of MRSA. The GHa-derived peptides decrease bacterial surface hydrophobicity, autoaggregation and polysaccharide intercellular adhesion synthesis in concentration-dependent manner. Real-time quantitative reverse transcription PCR analysis revealed that the peptides downregulate the expression of adhesion genes icaADBC involved in biofilm formation. Except for GHaR7R, the other three peptides have low hemolytic toxicity against human erythrocytes. In the presence of human erythrocytes, GHaR7R, GHaR8R and GHaR9W interact with MRSA preferentially. GHaR6R, GHaR8R and GHaR9W show less toxicity toward normal cells HL-7702 and hFOB1.19. These results suggest that the GHa-derived peptides may be promising antimicrobial candidates against MRSA infections.
Keywords: antimicrobial peptide, antibacterial activity, antibiofilm activity, methicillin-resistant Staphylococcus aureus
Introduction
Staphylococcus aureus is one of the main pathogens in hospitals and communities, which can cause a wide range of infectious diseases and associated complications, such as sepsis, endocarditis, pneumonia, and even death in the worst case [1]. S. aureus is an opportunistic pathogen, which is prone to acquire drug resistance against some antibiotics. Overuse and misuse of traditional antibiotics is a major contributor of drug resistance developed in bacteria. It was predicted that deaths caused by infections of drug resistant bacteria will be more than deaths caused by cancers by 2050 [ 2, 3]. Methicillin-Resistant S. aureus (MRSA) is defined as strains with an oxacillin minimum inhibitory concentration (MIC) more than 4 mg/L. The biofilms formation of MRSA is one of the important reasons for persistent infections and the development of multi-drug resistance [ 4, 5]. Biofilms are well-organized microbial communities, in which bacteria secrete a variety of virulence factors to ensure the survival in a malignant environment, and attach to the surface using extracellular matrix composed of polysaccharides, proteins, and extracellular DNA (eDNA). Currently, the glycopeptide antibiotic vancomycin remains the first-line treatment of MRSA infections. However, vancomycin-resistant Staphylococcus aureus (VRSA) were reported in 2002 [6]. Hence, the research and development of new candidates of antibiotics with different mechanism of action is urgently needed.
Antimicrobial peptides (AMPs) are widely found in various living organisms, and play important roles in the innate immune defense system. AMPs are amphiphilic small peptides consisting of 12–50 amino acid residues, showing diverse structures and biological functions, including antimicrobial, antifungal, and antitumor activities, etc. AMPs are usually positively charged by basic amino acids, and the hydrophobic amino acid residues consist of a hydrophobic surface contributing to the binding with bacterial membranes [ 7– 9]. In comparison with traditional antibiotics with a specific molecular target, most of the AMPs prefer to interact with bacterial cytoplasmic membranes and interfere with multiple biological function of pathogenic bacteria [ 10, 11]. This property hinders bacteria from developing a resistance strategy against AMPs. So, AMPs are considered as promising weapons in the fight against antibiotics resistance.
Temporin peptides, one group of the shortest naturally occurring AMPs consisting of 10–13 amino acid residues, are the excellent peptide templates for the design of novel AMPs with potent antimicrobial efficacy [12]. Temporin GHaR6R, GHaR7R, GHaR8R and GHaR9W are peptides derived from temporin-GHa (GHa) found in Hylarana guentheri. These four GHa-derived peptides have a broad spectrum of antimicrobial activity, including Gram-positive bacteria, Gram-negative bacteria and fungi [ 13, 14]. Here, we investigated their antimicrobial and antibiofilm activities and mechanism of actions against MRSA.
Materials and Methods
Bacterial strains and culture conditions
MRSA (ATCC 43300; ATCC, Manassas, USA) and clinically isolated strains (MRSA-1, MRSA-2, MRSA-3, MRSA-4 and MRSA-5; provided by Prof. Ke Yang, Guangxi University of Chinese Medicine, Nanning, China) were used in this study. The MRSA strains were cultivated aerobically at 37°C with shaking in Tryptic Soy Broth (TSB; HuanKai Microbial, Guangzhou, China).
Peptide synthesis
The peptides were synthesized by the Fmoc-based solid-phase synthesis (Jier Biochemical Co., Ltd., Shanghai, China). The purity of the peptides (>95%) was assessed by reversed phase high-performance liquid chromatography (RP-HPLC). Mass spectrometry was used to analyze the molecular mass of the synthesized peptides. The peptide sequences are as follows: GHaR6R (FLQRIRGALGRLF); GHaR7R (FLQRIIRALGRLF); GHaR8R (FLQRIIGRLGRLF); GHaR9W (FLQRIIGAWGRLF).
Antimicrobial assay
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the GHa-derived peptides were determined by two-fold broth microdilution method [15]. Briefly, the MRSA (1×10 6 CFU/mL) treated with or without the peptides (0.1–100 μM) were incubated in a 96-well micro-plate (Corning Co, Corning, USA) for 24 h at 37°C. The optical density (OD) of the culture was measured at 600 nm with a microplate reader (Multiskan Spectrum; BioTek, Winooski, USA). MIC is defined as the lowest concentration of the peptides that inhibits the growth of bacteria completely.
For the MBC determination, 10 μL of bacterial suspension from MIC measuring plate with the peptide concentration equal to and higher than MIC concentrations were pipetted and spread on TSB agars (TSA). MBC is defined as the concentration at which there is no bacterial growth after the TSA petri dishes are incubated at 37°C for 24 h.
Growth curve assay
Growth kinetics analysis was carried out to assess the influence of the peptides on the growth of MRSA within 24 h as previously reported [16]. In brief, MRSA (1×10 6 CFU/mL) treated with or without the peptides (0.4–3.1 μM) were incubated in a 96-well plate for 24 h at 37°C. OD was determined at 600 nm with a microplate reader (BioTek) every hour for 24 h.
MRSA killing assay
MRSA killing assay was performed as described previously with slight modifications [17]. MRSA (1×10 6 CFU/mL) was treated with the peptides at the final concentrations of 1/2×, 1×, 2×, 4× MIC in TSB in Eppendorf tubes, and incubated at 37°C. The suspensions were taken out after 0, 15, 30, 60, 90, 120 and 180 min of incubation and serially diluted, then 50 μL of the diluted suspensions were spread on TSA. Bacterial colonies were counted after incubation at 37°C for 24 h. Untreated MRSA served as a negative control.
Stability assay
The stability of the GHa-derived peptides was investigated under different temperature and salt conditions as previously reported [ 18, 19]. The peptide solutions (2 mM) were pretreated at different temperatures (40, 70 and 100°C) for 30 min individually. Medium was prepared containing different salt concentrations (150 mM NaCl, 4.5 mM KCl, 1 mM MgCl 2, and 2.5 mM CaCl 2). Then the MIC of peptides against MRSA was determined according to the MIC method as described above.
Membrane permeability assay
Fluorescence spectroscopy was used to assess the effect of the GHa-derived peptides on cell membrane permeability of MRSA as reported previously with proper modifications [20]. Briefly, 100 μL of serially two-fold diluted peptides (the concentrations ranging from 3.1 to 25 μM) were mixed with 92 μL of MRSA suspension (2×10 8 CFU/mL), and 8 μL of propidium iodide solution (PI, the final concentration of 20 μM) in each well on a black 96-well plate. Phosphate buffer saline (PBS) was used as a negative control. The plate was read with a microplate reader (Spark; Tecan, Männedorf, Switzerland) at an excitation wavelength of 560 nm and an emission wavelength of the 620 nm. The plate was shaken at 100 rpm every 5 min for 2 h at 37°C. Meanwhile, the growth of MRSA was monitored at 600 nm.
The morphological observation by using scanning electron microscopy
Scanning electron microscopy (SEM) was applied to investigate the effect of the GHa-derived peptides on morphology of MRSA as previously described [21]. MRSA (1×10 9 CFU/mL) in exponential-phase was incubated with the peptides (the final concentration of 12.5 μM) for 60 min at 37°C. Untreated MRSA was used as a negative control. The bacteria were collected, washed with PBS, fixed with 2.5% glutaraldehyde for 4 h at room temperature, followed by dehydration with graded series of ethanol (30%, 50%, 70% and 90%). MRSA was collected and resuspended in 100% anhydrous ethanol and freeze-dried overnight. The samples were coated with gold and observed under a scanning electron microscope (Verios G4 UC; Thermo Scientific, Waltham, USA).
Biofilm inhibition assay
Antibiofilm activity of the GHa-derived peptides was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [ 22, 23]. MRSA (1×10 6 CFU/mL) and the peptides (1.6–6.2 μM) were added to 96-well plates and incubated for 24 h at 37°C. TSB containing 1% glucose was used as a negative control. The plate was washed with PBS to remove the planktonic bacteria. The biofilms were stained with 100 μL MTT (250 μg/mL in PBS) for 3 h at 37°C. Subsequently, the stained biofilms were dissolved in 150 μL dimethyl sulfoxide (DMSO). OD was measured at 570 nm with a microplate reader (BioTek).
Biofilm eradication assay
Two hundred microliters of MRSA (1×10 6 CFU/mL) were added to each well of 96-well plates and incubated for 24 h at 37°C to establish mature biofilms. The supernatants were discarded and the wells were washed with PBS. Two hundred microliters of the serially diluted peptides (6.2–25 μM) were subsequently added to each well and incubated for 24 h at 37°C. Effect of the GHa-derived peptides on mature biofilms were quantified by using MTT method as described above.
Biofilm visualization by confocal laser scanning microscopy
The eradicative effect of the GHa-derived peptides on the matured biofilms of the MRSA was assessed by confocal laser scanning microscopy (CLSM), after the biofilms were stained with SYTO and propidium iodide (PI) [24]. Briefly, the established mature biofilms were exposed to the peptides at a final concentration of 25 μM for 24 h at 37°C, then washed with PBS, and subsequently stained with 300 μL premixed SYTO (10 μM) and PI (10 μM) for 15 min in the dark. The biofilms were monitored with a confocal laser scanning microscope (LEICA, Heidelberg, Germany).
Microbial adhesion to hydrocarbon assay
Microbial adhesion to hydrocarbon (MATH) assay was carried out to assess the effect of the GHa-derived peptides on the cell surface hydrophobicity (CSH) of the MRSA [25]. MRSA (1×10 6 CFU/mL) and the peptides (0.4–1.6 μM) were incubated for 24 h at 37°C. After incubation, the bacterial cells were washed twice and resuspended in PBS. The bacterial suspension was measured at 600 nm with a microplate reader (BioTek). The rest of the suspension was added to an equal volume of toluene and vortexed vigorously for 2 min. The suspension was incubated undisturbed at 4°C for 24 h, then the aqueous phase was collected and measured at 600 nm. The CSH was estimated as a hydrophobicity index (HI) and calculated by the formula: HI (%) = [1 – (OD 600 after vortex / OD 600 before vortex)] × 100%.
Autoaggregation assay
The MRSA (1×10 6 CFU/mL) was cultured in an Eppendorf tube in the presence or absence of the peptides (0.4–1.6 μM) for 24 h at 37°C. The cell pellets were washed three times with PBS and resuspended. The bacterial suspensions were incubated undisturbed for 24 h. One hundred microliters of supernatant were collected every 3 h up to 24 h, and the absorbance was measured at 600 nm. The bacterial autoaggregation was observed under a microscope and images were captured [26].
Polysaccharide intercellular adhesion analysis
Polysaccharide intercellular adhesion (PIA) biosynthesis of the MRSA with or without the GHa-derived peptides was measured by phenol-sulfuric acid method [27]. The MRSA (1×10 6 CFU/mL) was treated with the peptides (0.4–1.6 μM) for 24 h at 37°C. The suspension was centrifuged at 10,000 g for 15 min at 4°C. The bacterial pellets were washed with 4 mL of 0.1 M NaOH and centrifuged, and the supernatant was collected. This procedure was repeated twice, and the supernatant was combined with the previously prepared supernatant. Three times volume of chilled anhydrous ethanol was added and incubated overnight at 4°C to precipitate PIA. PIA was collected and freeze-dried, then dissolved in 0.1 M NaOH. Finally, 5% phenol, PIA solution and concentrated sulfuric acid were mixed at a molar ratio of 1:1:5, and incubated in the dark for 15 min. The absorbance of the mixture was measured at 570 nm with a microplate reader (BioTek).
Real-time quantitative reverse transcription PCR
Real-time quantitative reverse transcription PCR (RT-qPCR) was carried out as previously reported with slight modifications [28]. Briefly, MRSA (1×10 6 CFU/mL) was cultured in the absence or presence of the GHa-derived peptides (the final concentration of 1.6 μM), and incubated at 37°C for 24 h. After incubation, total RNA was extracted using Trizol reagent (Sangon Biotech, Shanghai, China). The RNA was reversely transcribed into cDNA using Maxima Reverse Transcriptase (Thermo Scientific). Expression analysis was performed by using LightCycler480 II PCR (Roche, Rotkreuz, Switzerland). 16S rRNA was used as the reference gene. Candidate gene expressions were calculated using the 2 −ΔΔCt method. The primers used in this study were listed in Supplementary Table S1.
Hemolysis assay
The hemolytic toxicity of the peptides was tested based on previously described method [29]. Briefly , 4% fresh human red blood cells (hRBCs) were washed with PBS and centrifuged at 1000 g for 5 min until the supernatant was clear. The same volume of 4% hRBCs suspension and two-fold serially diluted peptides (the final concentration of 3.1–200 μM) were incubated for 1 h at 37°C. After incubation, the mixture was centrifuged at 1000 g for 5 min. The supernatant was collected and transferred to a new 96-well plate. The hemolytic toxicity of the peptides was measured by monitoring the absorbance at 540 nm, which reflect the release of hemoglobin. Triton X-100 (0.1%) and PBS were used as a positive control (100% hemolysis) and a negative control (zero hemolysis) respectively. In order to detect the selectivity of the peptides, the hemolytic toxicity of the GHa-derived peptides was assayed on hRBCs in the presence of MRSA (1×10 6 CFU/mL).
Cytotoxicity assay
The cytotoxicity of the peptides in human cells was determined by Cell Counting Kit-8 (CCK-8) assay [ 30, 31]. Human hepatocytes HL-7702 [L-02] were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, USA) and 1% penicillin and streptomycin (P/S). hFOB1.19, MCF-7 and SKOV3 were obtained from National Collection of Authenticated Cell Cultures (Shanghai, China), and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 1% P/S. Briefly, 100 μL of the cells were seeded into each well of 96-well plates (5×10 4 cells/mL) and incubated at 37°C for 24 h under 5% CO 2. After incubation, the medium was replaced by fresh medium (without FBS and 1% P/S) containing the peptides (the final concentrations ranging from 3.1 to 200 μM), and incubated for 24 h. Then, 10 μL of CCK-8 solution (Beyotime Institute of Biotechnology, Beijing, China) was added to each well and incubated for 2–4 h. Absorbance at 450 nm was measured with a microplate reader. Before the assay, we confirmed that the effect of the culture medium on the antibacterial activity of the peptides was negligible.
Statistical analysis
Statistical analysis was conducted by Student’s t-test and one-way analysis of variance with GraphPad Prism 6.02 (Software GraphPad, San Diego, USA). P<0.05 indicates a statistically significant difference. All the experiments were performed in triplicate independently.
Results
The anti-MRSA activity of GHa-derived peptides
The antibacterial activity of the GHa-derived peptides against MRSA and clinical isolates was determined in vitro. As shown in Table 1, compared with conventional antibiotics azithromycin, kanamycin, and daptomycin, the GHa-derived peptides showed impressive anti-MRSA efficacy. Vancomycin still exhibited the outstanding antibacterial activity on most tested MRSA stains, but the MIC of GHaR7R against MRSA-5 was less than that of vancomycin. The predicted structures of the GHa-derived peptides are shown in Supplementary Figure S1.
Table1 MIC and MBC of the GHa-derived peptides against the tested MRSA strains
|
MIC/MBC (μM) |
||||||
|
MRSA |
MRSA-1 |
MRSA-2 |
MRSA-3 |
MRSA-4 |
MRSA-5 |
|
|
Peptides | ||||||
|
GHaR6R |
6.2/12.5 |
12.5/25 |
12.5/25 |
6.2/12.5 |
12.5/12.5 |
12.5/25 |
|
GHaR7R |
3.1/6.2 |
6.2/12.5 |
3.1/6.2 |
6.2/6.2 |
3.1/3.1 |
6.2/12.5 |
|
GHaR8R |
3.1/3.1 |
6.2/12.5 |
3.1/6.2 |
6.2/12.5 |
3.1/6.2 |
12.5/12.5 |
|
GHaR9W |
6.2/12.5 |
12.5/25 |
12.5/12.5 |
6.2/12.5 |
6.2/6.2 |
12.5/12.5 |
|
Antibiotics | ||||||
|
Azithromycin |
>100/>100 |
>100/>100 |
>100/>100 |
3.1/6.2 |
6.2/6.2 |
3.1/6.2 |
|
Kanamycin |
>100/>100 |
50/100 |
>100/>100 |
3.1/3.1 |
>100/>100 |
3.1/3.1 |
|
Vancomycin |
0.4/1.6 |
0.8/1.6 |
0.8/1.6 |
0.8/1.6 |
0.8/1.6 |
12.5/12.5 |
|
Daptomycin |
25/50 |
25/25 |
3.1/3.1 |
25/25 |
12.5/12.5 |
6.2/6.2 |
MRSA-1-5: Methicillin-resistant Staphylococcus aureus (clinically isolated, No.1-5).
Bacterial growth inhibition and killing kinetics of the GHa-derived peptides
To evaluate the effect of the peptides on the growth of the MRSA at sub-MIC concentrations, we performed growth kinetics analysis ( Supplementary Figure S2A–D). At 3.1 μM, GHaR7R and GHaR8R completely inhibited the growth of MRSA, while GHaR6R and GHaR9W retarded the growth for 1–2 h. At the concentration of 1.6 μM, GHaR7R was able to suppress the growth of the MRSA up to 7 h, and GHaR8R inhibited the growth for 1–2 h. After exposure to the peptides at concentrations of 0.4–0.8 μM, the growth of bacteria was similar to that of the negative control group.
The bacteria-killing kinetics was determined to further assess bactericidal efficiency of the peptides against MRSA ( Supplementary Figure S2E–H). All the GHa-derived peptides exerted bactericidal actions on MRSA in concentration-dependent and time-dependent manners. GHaR8R showed strong bactericidal potential that completely killed MRSA within 120 min and 180 min at concentrations of 4 × MIC and 2 × MIC, respectively. After exposure to GHaR7R and GHaR9W for 180 min, no live MRSA was observed at the concentrations of 4 × MIC. The decline in the number of bacteria (6 log reduction) after treatment with 4 × MIC GHaR6R was achieved compared with the untreated control. All peptides also had bactericidal potency against MRSA at sub-MIC.
The stability of the GHa-derived peptides against temperature and salts
The antimicrobial activity of the peptides was measured under harsh conditions, including extreme temperature and salt conditions. After being pretreated at different temperature and salt conditions, all of the peptides showed excellent stability with the MIC unchanged in comparison with the negative control ( Supplementary Table S2).
The GHa-derived peptides increase membrane permeability of MRSA
Membrane damage was assessed by propidium iodide (PI) uptake assay. PI is a nuclear dye that can penetrate damaged cell membranes only, and fluorescence was detected when it binds to DNA. Compared with the untreated bacteria, GHaR6R (1.6–12.5 μM) caused a slight increase in the fluorescence intensity, which was independent of GHaR6R concentration ( Supplementary Figure S3A). At 12.5 μM, the fluorescence intensity was increased dramatically, reaching maximum values at 5, 5 and 20 min after treatment with GHaR7R, GHaR8R and GHaR9W, respectively ( Supplementary Figure S3B–D). GHaR7R also showed stronger membrane permeability at the concentration of 6.2 μM. At lower concentrations, the fluorescence intensity was increased slowly during the treatment with all peptides ( Supplementary Figure S3A–D). At the same time, the growth of MRSA was also monitored, and there was no proliferation of MRSA within 2 h ( Supplementary Figure S3E–H).
The GHa-derived peptides targets the membrane of MRSA
Scanning electron microscopy (SEM) was used to observe the morphology of MRSA ( Figure 1). The untreated MRSA was intact, smooth and round, but the bacterial cells treated with the GHa-derived peptides were changed apparently. The MRSA cells were damaged severely after treatment with GHaR7R and GHaR8R at 12.5 μM. Some of the bacteria were destroyed completely, and cell debris attached together, leading to the leakage of intracellular contents and loss of the intact structure. GHaR6R and GHaR9W showed mild effects on the morphological changes, while the MRSA cells were distorted and wrinkled after exposure to them. In all peptide-treated groups, the membranes of most bacterial cells were fused to each other, contributing the unclear boundary between the bacteria cells.
Figure1 .
Morphological changes of MRSA exposed to GHa-derived peptides were examined by SEM
MRSA treated with PBS served as the negative control. SEM magnification, × 20,000. Scale bar=2 μm.
The GHa-derived peptides have antibiofilm activity
All of the tested peptides displayed the antibiofilm activity against MRSA in a concentration-dependent manner ( Figure 2). The four peptides could inhibit the formation of biofilm by more than 50% at 6.2 μM ( Figure 2A–D), especially GHaR7R with an inhibitory rate of 83.64% ( Figure 2B), comparable to that 6.2 μM of vancomycin ( Supplementary Figure S4A). At the low concentration of 1.6 μM, the peptides still inhibited the biomass of MRSA biofilms by 20%–40% ( Figure 2A–D). In comparison with other three peptides and vancomycin, GHaR7R also exerted higher efficiency to remove the mature biofilms ( Figure 2F). GHaR8R, GHaR9W, and vancomycin had similar ability to eradicate mature biofilms at various concentrations ( Figure 2G–H, Supplementary Figure S4B). After treatment with 25 μM the peptides, more than 50% of mature biofilms were eradicated ( Figure 2E–H).
Figure2 .
The antibiofilm formation activities of GHa-derived peptides
(A–D) The inhibition of biofilm formation and (E–H) the eradication of mature biofilms were analyzed. The biofilms were stained by MTT and the absorbance was monitored at 570 nm. Untreated-biofilms was used as the control. *P<0.05, **P<0.01. The dashed lines represent 10% and 50% of the biofilm biomass, respectively.
The biofilm eradication activity of the derived peptides
The eradication activity of the peptides against the mature biofilms of MRSA were observed by CLSM. Green fluorescence represents living bacteria (SYTO staining), while red fluorescence indicates dead bacteria (PI staining). As shown in Figure 3, the highly organized and aggregated live biofilm was observed in the negative control. Compared with the control, most of the mature biofilms were eradicated after exposure to the peptides. Only small and thin biofilm clusters were scatted, and some of them were killed. GHaR7R exerted an outstanding efficacy to eradicate more than 80% of the mature biofilms. GHaR6R, GHaR8R and GHaR9W showed similar ability to eradicate the mature biofilm.
Figure3 .
Eradicating potential of GHa-derived peptides to the mature biofilm of MRSA
The mature biofilms were treated with 25 μM of the peptides. Untreated-biofilms were used as the negative control. The Z axis in 3D images indicates the thickness of the biofilm.
The GHa-derived peptides decreases cell surface hydrophobicity of MRSA
MATH assay showed that the GHa-derived peptides decreased the hydrophobicity index of MRSA bacteria in a concentration-dependent manner. GHaR7R, GHaR8R and GHaR9W were found to reduce more than 50% of the hydrophobicity index at 1.6 μM ( Supplementary Figure S5).
The GHa-derived peptides reduces autoaggregation of MRSA
Autoaggregation is an important property for intercellular and surface adherence of MRSA [26]. After 24 h of culture, most of the MRSA bacteria autoaggregated in the negative control. While a significant reduction in MRSA autoaggregation was observed after treatment with the peptides, even at the low concentration of 0.4 μM ( Figure 4A–D). Autoaggregation was also detectable after incubating tubes for 24 h in static condition ( Figure 4E–H).
Figure4 .
Effect of GHa-derived peptides on auto-aggregation of MRSA
(A–D) The optical density of the bacteria culture was measured at 600 nm. (E–H) The images of MRSA were captured after 24 h of culture in static condition. Untreated bacteria served as the control.
Effect of the GHa-derived peptides on PIA biosynthesis and gene expression of MRSA
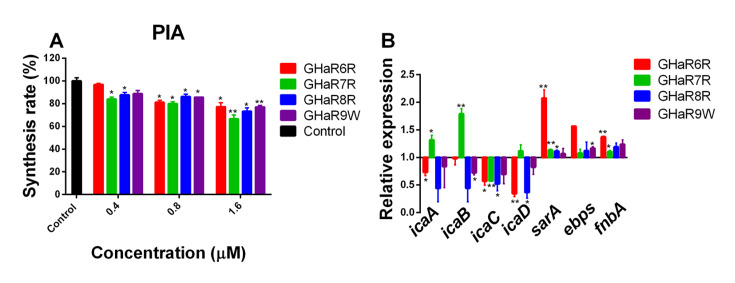
PIA constitutes the exopolysaccharide for many staphylococcal strains, contributing to bacterial aggregation and adhesion on the surface [32]. As shown in Figure 5A, the inhibition rates of the peptides for PIA ranged from 3.2% to 33.5% at 0.4–1.6 μM. The inhibitory effect of GHaR7R on PIA biosynthesis of MRSA was higher than those of GHaR6R, GHaR8R and GHaR9W. As shown in Figure 5B, the expressions of three genes ( sarA, ebps, fnbA) were upregulated upon GHaR6R treatment, with sarA being the most upregulated one, while the expressions of four ica genes ( icaA, icaD, icaB, and icaC) were downregulated. GHaR7R enhanced the expressions of icaA and icaB, whereas the expressions of icaD, sarA, ebps and fnbA remained unaltered. On the contrary, the expression of icaC was downregulated. GHaR8R had no impact on the expressions of sarA, ebps and fnbA, while the expression of four ica genes ( icaA, icaD, icaB, and icaC) were downregulated. The expressions of the three genes ( sarA, ebps, and fnbA) almost unchanged after exposure to GHaR9W, whereas the expressions of icaA, icaD, icaB, and icaC were downregulated.
Figure5 .
Effect of GHa-derived peptides on PIA biosynthesis and gene expression of MRSA
(A) The inhibition of PIA production in MRSA by the peptides at 0.4-1.6 μM. Untreated bacteria served as the negative control. (B) The effect of GHa-derived peptides on gene expressions in MRSA. Changes in gene expression are shown as normalized mean fold change. Data are presented as the mean±SD (n=3). *P<0.05, **P<0.01.
Hemolytic toxicity of the GHa-derived peptides
Hemolysis of the GHa-derived peptides was evaluated in human erythrocytes (hRBCs) with or without MRSA ( Supplementary Figure S6). Hemolytic toxicity of GHaR7R and GHaR9W were decreased in the presence of MRSA, indicating that the peptides bound to MRSA preferentially. GHaR6R did not show any hemolytic toxicity even at 200 μM. GHaR8R and GHaR9W showed slight hemolytic effect. Comparatively, GHaR7R disrupted hRBCs with 50% hemolysis rate at 25 μM. The cell selectivity index (CSI) of the peptides were listed in Supplementary Table S3.
Cytotoxicity of the GHa-derived peptides to human cells
The cytotoxicity of the peptides against normal (HL-7702 and hFOB1.19) and tumor (MCF-7 and SKOV3) cells were assessed ( Figure 6). GHaR6R and GHaR9W showed slight cytotoxicity to all tested cells with the 50% inhibiting concentration (IC 50) ranging from 68.8 to >200 μM ( Supplementary Table S4). GHaR7R exhibited high cytotoxicity, and reduced the viability of all the cells by 50% at 25 μM. On tumor cells MCF-7 and SKOV3, GHaR8R showed the same toxicity as GHaR7R, but on normal cells HL-7702 and hFOB1.19, its cytotoxicity was less than that of GHaR7R.
Figure6 .
Cytotoxicity of GHa-derived peptides against human normal and tumor cells
Untreated cells served as the negative control. *P<0.05, **P<0.01.
Discussion
Temporins are antimicrobial peptides containing 13 amino acids initially found in amphibians. Temporin peptides have a board spectrum bioactivity against bacteria, fungi, viruses, protozoa and cancer cells [33]. Most members of the temporin have a common motif FLP- at the N-terminal and leucine at the second position of the C-terminal. The basic amino acids, arginine and lysine are frequently found in temporins, but rarely for histidine. The positively charged amino acids contribute temporins to positive charges between 0 to +3 [22]. Temporin peptides share a common α-helical conformation in hydrophobic environment [ 12, 34]. Temporin-GHa (GHa) was cloned from Hylarana guentheri, with histidine as the basic amino acid [13]. Our previous studies showed that temporin peptides with histidine showed weaker antimicrobial activity than those with arginine and lysine [22]. In order to improve the antibacterial efficacy of GHa, GHaR6R, GHaR7R, GHaR8R and GHaR9W were designed [14].
MRSA infection is one of the significant life-threatening diseases to human. Here, we investigated the antibacterial activity of GHaR6R, GHaR7R, GHaR8R and GHaR9W against MRSA. GHaR7R and GHaR8R have similar high antibacterial activity, whereas the MICs of GHaR6R and GHaR9W are comparable. The structure predictions showed that all the four GHa-derived peptides have an α-helix structure. Both GHaR7R and GHaR8R have 3 arginines to form a hydrophilic surface, with the hydrophobic amino acids constituting a hydrophobic surface. Compared with these two peptides, GHaR6R also contains the same number of positively charged amino acids, but one of the arginine separates from the hydrophilic surface and is close to the hydrophobic surface, leading to the reduction of its antimicrobial activity. GHaR9W has the same hydrophobic surface with GHaR7R and GHaR8R. These three peptides have similar bactericidal efficiency according to the bacteria-killing kinetics. The results confirmed that reducing the non-polar surface of the α-helical amphipathic peptide might decrease the antibacterial efficacy of AMPs. It is consistent with the previous research that the hydrophobic motif is essential for antimicrobial activity of Pep19-4LF [35]. Generally, the increase in hydrophobicity of the hydrophobic surface of α-helical AMPs would enhance their antibacterial activities [36].
Studies have shown that most cationic AMPs can bind to negative charges of bacterial cytoplasmic membrane and insert into the membrane to form channels which interfere with the membrane potential, causing leakage of bacterial contents and cell death [ 37, 38]. It is worth noting that the antimicrobial activity of cationic AMPs can be affected by divalent cations (Mg 2+ and Ca 2+) and monovalent cations (Na + and K +) [39]. Therefore, we determined the activity of the GHa-derived peptides against MRSA in physiological salt environments. The tested cations showed no effect on the antimicrobial activity. GHaR7R, GHaR8R, and GHaR9W could quickly increase the permeability of bacterial membranes, while GHaR6R showed mild effect. The cells treated with GHaR7R and GHaR8R were damaged seriously, with the cell membrane interrupted and irregularly wrinkled, and leakage of intracellular contents. Comparatively, GHaR6R and GHaR9W had slight effect on bacterial morphology, reflected by their lower MIC values. The results indicated that the four GHa-derived peptides may target bacterial cytoplasmic membranes. Arginine-rich peptides prefer to exert direct penetration effect on the cell membranes [40], which explains why the GHa-derived peptides exhibit stronger antimicrobial potential than the parent peptide containing histidines. Optimizing AMPs by designing GHa-derived variant peptides has been one of the important ways to develop novel bioactive peptides [41]. Many of these peptides exhibit multiple functions, such as antibiofilm and immunomodulatory activity, accompanied by their antimicrobial functions [ 42, 43]. AMPs with antibiofilm formation activity at concentrations of lower than their minimum inhibitory concentrations (MICs) are considered to be antibiofilm peptides [44]. It has been reported that inhibition of biofilm formation of antibiofilm peptides is related to their effective bactericidal activity against the planktonic bacteria [ 45, 46]. As the temporin-derived peptides showed higher antibacterial effect than the mother peptides, the antibiofilm formation activity assays were performed.
Many MRSA strains are prone to form biofilms and produce a variety of virulence factors, such as PIA and eDNA, which are critical elements in the pathogenesis of acute and chronic infections [47]. All of the GHa-derived peptides inhibited biofilm formation at the concentrations of sub-MIC and eradicated mature biofilms of MRSA, especially GHaR7R and GHaR8R. In addition, GHaR7R had stronger effect on the eradication of mature biofilms than vancomycin. Vancomycin can kill planktonic bacteria and the bacteria on the surface of biofilm, but it is difficult to penetrate into biofilm to kill the protected bacteria [48]. The GHa-derived peptides, especially GHaR7R can penetrate into the MRSA biofilm, and exerts bactericidal activity, causing the death of bacteria in deep biofilm. Although vancomycin shows excellent inhibitory efficacy on biofilm formation, it has high toxicity [49], therefore the GHa-derived peptides have great potential in the treatment of MRSA infections. The regulation pathways of biofilm formation are very complex, which are strictly controlled by multiple regulatory systems, such as accessory gene regulator ( Agr), ica operon, SarA, SaeRS two-component and quorum sensing (QS) [ 50, 51]. PIA is the important extracellular matrix to provide the biofilm attaching architecture, contributing MRSA cells to embedding in PIA fiber meshes [52]. Moreover, the hydrophobicity of cell surface and auto-aggregation enhances the adhesion of bacteria to hydrophobic surfaces [53]. The GHa-derived peptides reduce the surface hydrophobicity and auto-aggregation, leading to decreased biofilm formation of MRSA. PIA biosynthesis of MRSA also is diminished after the bacteria are exposed to the GHa-derived peptides. PIA is synthesized mainly by the enzymes encoded by the icaADBC genes [54]. GHaR6R, GHaR8R and GHaR9W downregulate the expressions of icaADBC genes. It has been reported that co-expression of icaA and icaD renders the optimal PIA synthesis in staphylococci [55]. GHaR7R attenuates the expression of icaC, whereas the expressions of icaA and icaB are upregulated and the expression of icaD remains constant. We deduced that GHaR7R may interrupt the co-expression of icaA and icaD, leading to the low synthesis of PIA. The sar locus encodes a DNA-binding protein staphylococcal accessory regulator A ( SarA), which is a known master controller for biofilm formation [56]. The expressions of sarA and sarA-controlled adhesion proteins Fibronectin-binding protein-A ( fnbA) are enhanced upon GHaR6R treatment. GHaR7R, GHaR8R and GHaR9W have no negative effect on the expressions of sarA and fnbA. In previous studies, a similar expression pattern was observed, in which magnolol induced the expression of sarA in S. aureus. Elastin-binding proteins gene ( ebps) product is an integral membrane protein of S. aureus that binds to elastin. And this interaction makes bacteria colonization and pathogenesis much easier in wound-infected tissues [57]. The expression of ebps remians unchanged upon treatment with GHaR7R, GHaR8R and GHaR9W. The GHa-derived peptides show multifactorial impact on the expressions of the biofilm-related genes, but the main contributor to the antibiofilm formation activity is the downregulation of icaADBC genes.
Cytotoxicity is one of the critical factors to evaluate the applicability of drug candidates in clinic. Thus, we assessed the toxicity of the GHa-derived peptides on human erythrocyte, normal cells and tumor cells. Compared with the other three peptides, GHaR6R did not display any hemolytic toxicity even at the concentrations of up to 200 μM. In the presence of human erythrocytes, the reduced hemolytic toxicities of GHaR7R, GHaR8R and GHaR9W demonstrated that the GHa-derived peptides preferred to act on MRSA. GHaR6R and GHaR9W showed less cytotoxicity on the tested cells than GHaR7R and GHaR8R. Interestingly, GHaR8R inhibited the growth of MCF-7 and SKOV3 cells by 60%–80% at 25 μM, but had slight cytotoxicity on normal cells. The high selectivity to tumor cells of GHaR8R makes it a potential anticancer drug candidate. According to the antimicrobial and toxicity studies, we confirmed that with less hydrophobic surface in AMPs, their cytotoxicity was reduced, but their antibacterial activity was also decreased. It is difficult to completely overcome toxicity of AMPs and retain a strong antimicrobial activity at the same time. Hence, we need to seek a balance point between high efficacy and low toxicity in the design of AMPs.
In summary, temporin peptides are outstanding templates for the development of novel antibacterial and antibiofilm reagents. The temporin-GHa-derived peptides may be promising candidates against MRSA infections.
Supplementary Data
Supplementary data is available at Acta Biochimica et Biophysica Sinica online.
Supporting information
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 32060130 and 31560593), and the Education Department of Hainan Province (No. Hnjg2021-26).
References
- 1.Malanovic N, Lohner K. Antimicrobial peptides targeting Gram-positive bacteria. Pharmaceuticals. . 2016;9:59. doi: 10.3390/ph9030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus . Science. . 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 3.United Nations meeting on antimicrobial resistance. Bull World Health Organ. 2016, 94: 638-639 . [DOI] [PMC free article] [PubMed]
- 4.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu Rev Microbiol. . 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 5.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. . 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 6.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene . N Engl J Med. . 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 7.Hancock RE. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis. . 2001;1:156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 8.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. . 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 9.Raheem N, Straus SK. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front Microbiol. . 2019;10:2866. doi: 10.3389/fmicb.2019.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. . 2010;1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 11.Ciandrini E, Morroni G, Arzeni D, Kamysz W, Neubauer D, Kamysz E, Cirioni O, et al. Antimicrobial activity of different antimicrobial peptides (AMPs) against clinical methicillin-resistant Staphylococcus aureus (MRSA) . Curr Top Med Chem. . 2018;18:2116–2126. doi: 10.2174/1568026618666181022140348. [DOI] [PubMed] [Google Scholar]
- 12.Simmaco M, Mignogna G, Canofeni S, Miele R, Mangoni ML, Barra D. Temporins, antimicrobial peptides from the European red frog Rana temporaria . Eur J Biochem. . 1996;242:788–792. doi: 10.1111/j.1432-1033.1996.0788r.x. [DOI] [PubMed] [Google Scholar]
- 13.Dong Z, Luo W, Zhong H, Wang M, Song Y, Deng S, Zhang Y. Molecular cloning and characterization of antimicrobial peptides from skin of Hylarana guentheri . Acta Biochim Biophys Sin. . 2017;49:450–457. doi: 10.1093/abbs/gmx023. [DOI] [PubMed] [Google Scholar]
- 14.Wei H, Xie Z, Tan X, Guo R, Song Y, Xie X, Wang R, et al. Temporin-Like peptides show antimicrobial and anti-biofilm activities against Streptococcusmutans with reduced hemolysis . Molecules. . 2020;25:5724. doi: 10.3390/molecules25235724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram‐positive bacterial infections. Clin Infect Dis. . 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 16.Marquardt P, Seide R, Vissiennon C, Schubert A, Birkemeyer C, Ahyi V, Fester K. Phytochemical characterization and in vitro anti-inflammatory, antioxidant and antimicrobial activity of Combretum collinumfresen leaves extracts from benin . Molecules. . 2020;25:288. doi: 10.3390/molecules25020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang J, Liu L, Wu X, Sun Y, Liu Z. Effect of thyme essential oil against Bacillus cereusplanktonic growth and biofilm formation . Appl Microbiol Biotechnol. . 2018;102:10209–10218. doi: 10.1007/s00253-018-9401-y. [DOI] [PubMed] [Google Scholar]
- 18.Rajasekaran G, Dinesh Kumar S, Nam J, Jeon D, Kim Y, Lee CW, Park IS, et al. Antimicrobial and anti-inflammatory activities of chemokine CXCL14-derived antimicrobial peptide and its analogs. Biochim Biophys Acta - Biomembr. . 2019;1861:256–267. doi: 10.1016/j.bbamem.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Ji S, Li W, Zhang L, Zhang Y, Cao B. Cecropin A–melittin mutant with improved proteolytic stability and enhanced antimicrobial activity against bacteria and fungi associated with gastroenteritis in vitro. Biochem Biophys Res Commun. . 2014;451:650–655. doi: 10.1016/j.bbrc.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Ko SJ, Kang NH, Kim MK, Park J, Park E, Park GH, Kang TW, et al. Antibacterial and anti-biofilm activity, and mechanism of action of pleurocidin against drug resistant Staphylococcus aureus . Microb Pathog. . 2019;127:70–78. doi: 10.1016/j.micpath.2018.11.052. [DOI] [PubMed] [Google Scholar]
- 21.Kang S, Kong F, Liang X, Li M, Yang N, Cao X, Yang M, et al. Label-Free quantitative proteomics reveals the multitargeted antibacterial mechanisms of lactobionic acid against methicillin-resistant Staphylococcus aureus (MRSA) using SWATH-MS technology . J Agric Food Chem. . 2019;67:12322–12332. doi: 10.1021/acs.jafc.9b06364. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Wei H, Meng J, Cheng T, Song Y, Wang M, Zhang Y. The analogs of temporin-GHa exhibit a broader spectrum of antimicrobial activity and a stronger antibiofilm potential against Staphylococcus aureus . Molecules. . 2019;24:4173. doi: 10.3390/molecules24224173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reis SV, Couto NM, Brust FR, Trentin DS, Silva JK, Arruda MS, Gnoatto SC, et al. Remarkable capacity of brosimine b to disrupt methicillin-resistant Staphylococcus aureus (MRSA) preformed biofilms . Microb Pathog. . 2020;140:103967. doi: 10.1016/j.micpath.2020.103967. [DOI] [PubMed] [Google Scholar]
- 24.Xie J, Li Y, Guo X, Rao J, Yan T, Mou L, Wu X, et al. CPF-C1 analog with effective antimicrobial and antibiofilm activities against Staphylococcus aureus including MRSA . Biochimie. . 2020;176:1–11. doi: 10.1016/j.biochi.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Selvaraj A, Valliammai A, Premika M, Priya A, Bhaskar JP, Krishnan V, Pandian SK. Sapindus mukorossi Gaertn. and its bioactive metabolite oleic acid impedes methicillin-resistant Staphylococcus aureus biofilm formation by down regulating adhesion genes expression . Microbiol Res. . 2021;242:126601. doi: 10.1016/j.micres.2020.126601. [DOI] [PubMed] [Google Scholar]
- 26.Selvaraj A, Jayasree T, Valliammai A, Pandian SK. Myrtenol attenuates MRSA biofilm and virulence by suppressing sarA expression dynamism . Front Microbiol. . 2019;10:2027. doi: 10.3389/fmicb.2019.02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong H, Xie Z, Wei H, Zhang S, Song Y, Wang M, Zhang Y. Antibacterial and antibiofilm activity of temporin-GHc and temporin-GHd against cariogenic bacteria, Streptococcus mutans . Front Microbiol. . 2019;10:2854. doi: 10.3389/fmicb.2019.02854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atshan SS, Shamsudin MN, Karunanidhi A, van Belkum A, Lung LTT, Sekawi Z, Nathan JJ, et al. Quantitative PCR analysis of genes expressed during biofilm development of methicillin resistant Staphylococcus aureus(MRSA) . Infect Genet Evol. . 2013;18:106–112. doi: 10.1016/j.meegid.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Mishra B, Wang X, Lushnikova T, Zhang Y, Golla RM, Narayana JL, Wang C, et al. Antibacterial, antifungal, anticancer activities and structural bioinformatics analysis of six naturally occurring temporins. Peptides. . 2018;106:9–20. doi: 10.1016/j.peptides.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao X, Lu Y, Xing Y, Ma Y, Lu J, Bao W, Wang Y, et al. A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiol Res. . 2012;167:616–622. doi: 10.1016/j.micres.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Oh D, Sun J, Nasrolahi Shirazi A, LaPlante KL, Rowley DC, Parang K. Antibacterial activities of amphiphilic cyclic cell-penetrating peptides against multidrug-resistant pathogens. Mol Pharm. . 2014;11:3528–3536. doi: 10.1021/mp5003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen HTT, Nguyen TH, Otto M. The staphylococcal exopolysaccharide PIA – Biosynthesis and role in biofilm formation, colonization, and infection. Comput Struct Biotechnol J. . 2020;18:3324–3334. doi: 10.1016/j.csbj.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero SM, Cardillo AB, Martínez Ceron MC, Camperi SA, Giudicessi SL. Temporins: an approach of potential pharmaceutic candidates. Surg Infects. . 2020;21:309–322. doi: 10.1089/sur.2019.266. [DOI] [PubMed] [Google Scholar]
- 34.Sang M, Wu Q, Xi X, Ma C, Wang L, Zhou M, Burrows JF, et al. Identification and target-modifications of temporin-PE: A novel antimicrobial peptide in the defensive skin secretions of the edible frog, Pelophylaxkl. esculentus . Biochem Biophys Res Commun. . 2018;495:2539–2546. doi: 10.1016/j.bbrc.2017.11.173. [DOI] [PubMed] [Google Scholar]
- 35.Storck P, Umstätter F, Wohlfart S, Domhan C, Kleist C, Werner J, Brandenburg K, et al. Fatty acid conjugation leads to length-dependent antimicrobial activity of a synthetic antibacterial peptide (Pep19-4LF) Antibiotics. . 2020;9:844. doi: 10.3390/antibiotics9120844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dathe M, Wieprecht T, Nikolenko H, Handel L, Maloy WL, MacDonald DL, Beyermann M, et al. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. . 1997;403:208–212. doi: 10.1016/S0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 37.Yin LM, Edwards MA, Li J, Yip CM, Deber CM. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J Biol Chem. . 2012;287:7738–7745. doi: 10.1074/jbc.M111.303602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hancock REW. Peptide antibiotics. Lancet. . 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 39.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. . 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 40.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. . 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 41.Haney EF, Mansour SC, Hilchie AL, de la Fuente-Núñez C, Hancock REW. High throughput screening methods for assessing antibiofilm and immunomodulatory activities of synthetic peptides. Peptides. . 2015;71:276–285. doi: 10.1016/j.peptides.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pletzer D, Hancock REW. Antibiofilm peptides: potential as broad-spectrum agents. J Bacteriol. . 2016;198:2572–2578. doi: 10.1128/JB.00017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haney EF, Hancock REW. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers. . 2013;100:572–583. doi: 10.1002/bip.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Zheng Z, Kim W, Burgwyn Fuchs B, Mylonakis E. Influence of subinhibitory concentrations of NH125 on biofilm formation & virulence factors of Staphylococcus aureus . Future Medicinal Chem. . 2018;10:1319–1331. doi: 10.4155/fmc-2017-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinto SN, Dias SA, Cruz AF, Mil-Homens D, Fernandes F, Valle J, Andreu D, et al. The mechanism of action of pepR, a viral-derived peptide, against Staphylococcus aureusbiofilms . J Antimicrob Chemother. . 2019;74:2617–2625. doi: 10.1093/jac/dkz223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strempel N, Strehmel J, Overhage J. Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr Pharm Des. . 2014;21:67–84. doi: 10.2174/1381612820666140905124312. [DOI] [PubMed] [Google Scholar]
- 47.Lakshmi SA, Bhaskar JP, Krishnan V, Sethupathy S, Pandipriya S, Aruni W, Pandian SK. Inhibition of biofilm and biofilm-associated virulence factor production in methicillin-resistant Staphylococcus aureus by docosanol . J Biotechnol. . 2020;317:59–69. doi: 10.1016/j.jbiotec.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Yao Z, Lai J, Chen X, Gao X, Liu Y. Effect of D-tyrosine combined with vancomycin on elimination of in vitro methicillin-resistant Staphylococcus aureus and its biofilms. Chin J Nosocomiol 2017, 27: 5045–5048
- 49.Wilhelm MP. Vancomycin. Mayo Clin Proc 1991, 66: 1165–1170 . [DOI] [PubMed]
- 50.Schilcher K, Horswill AR. Staphylococcal biofilm development: structure, regulation, and treatment strategies . Microbiol Mol Biol Rev. . 2020;84:e00026-19. doi: 10.1128/MMBR.00026-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenul C, Horswill AR. Regulation of Staphylococcus aureus virulence . Microbiol Spectr. . 2019;7:1. doi: 10.1128/microbiolspec.GPP3-0031-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Formosa-Dague C, Feuillie C, Beaussart A, Derclaye S, Kucharíková S, Lasa I, Van Dijck P, et al. Sticky Matrix: adhesion mechanism of the staphylococcal polysaccharide intercellular adhesin. ACS Nano. . 2016;10:3443–3452. doi: 10.1021/acsnano.5b07515. [DOI] [PubMed] [Google Scholar]
- 53.Collado MC, Meriluoto J, Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol. . 2008;226:1065–1073. doi: 10.1007/s00217-007-0632-x. [DOI] [Google Scholar]
- 54.Chen Q, Xie S, Lou X, Cheng S, Liu X, Zheng W, Zheng Z, et al. Biofilm formation and prevalence of adhesion genes among Staphylococcus aureus isolates from different food sources . MicrobiologyOpen. . 2020;9:e00946. doi: 10.1002/mbo3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cue D, Lei MG, Lee CY. Genetic regulation of the intercellular adhesion locus in staphylococci. Front Cell Inf Microbio. . 2012;2:38. doi: 10.3389/fcimb.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arya R, Ravikumar R, Santhosh RS, Princy SA. SarA based novel therapeutic candidate against Staphylococcus aureus associated with vascular graft infections . Front Microbiol. . 2015;6:416. doi: 10.3389/fmicb.2015.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Downer R, Roche F, Park PW, Mecham RP, Foster TJ. The elastin-binding protein of Staphylococcus aureus (EbpS) is expressed at the cell surface as an integral membrane protein and not as a cell wall-associated protein . J Biol Chem. . 2002;277:243–250. doi: 10.1074/jbc.M107621200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.