Abstract
Marfan syndrome (MFS) is a connective tissue disorder affecting the cardiovascular, ocular, and skeletal system, which may be accompanied by psychological features. This study aimed to determine the prevalence of fatigue, anxiety, and symptoms of depression in MFS patients, and to assess the degree to which sociodemographic and clinical variables are associated with fatigue and psychological aspects. The prevalence of fatigue, anxiety, and symptoms of depression were assessed in two cohorts of MFS patients and compared with healthy controls. The checklist individual strength (CIS), and hospital anxiety and depression scale (HADS) questionnaires were utilized. Medical status was assessed (family history of MFS, aortic root dilatation >40 mm, previous aortic surgery, aortic dissection, chronic pain, skeletal involvement, and scoliosis). Severe fatigue was experienced by 37% of the total MFS cohort (n = 155). MFS patients scored significantly higher on the CIS questionnaire, concerning severe fatigue, as compared with the general Dutch population (p < 0.0001). There were no differences in HADS anxiety or depression scores. In older MFS patients, with a more severe cardiovascular phenotype, chronic pain, and a higher unemployment rate, significantly more symptoms of depression were observed, when compared with the general population (p = 0.027) or compared with younger MFS patients (p = 0.026). Multivariate analysis, showed that anxiety was associated with chronic pain (p = 0.022) and symptoms of depression with unemployment (p = 0.024). MFS patients report significantly more severe fatigue as compared with the general population. Since the cause of fatigue is unclear, more research may be needed. Psychological intervention, for example, cognitive behavioral therapy, may contribute to a reduction in psychological symptoms.
Keywords: anxiety, depression, fatigue, Marfan syndrome
Abbreviations
- CBT
cognitive behavioral therapy
- CI
confidence interval
- CIS
checklist individual strength
- FBN1
fibrillin‐1
- HADS
hospital anxiety and depression scale
- IRQ
interquartile range
- MFS
Marfan syndrome
- OR
odds ratio
- QOL
quality of life
1. INTRODUCTION
Marfan syndrome (MFS) is an inheritable connective tissue disorder, primarily caused by a mutation in the fibrillin‐1 gene (FBN1). Studies report a wide range of 1.5–17.2 per 100.000 MFS patients in the general population. 1 Patients with MFS predominantly suffer from cardiovascular, musculoskeletal, and ocular symptoms, which potentially influence their wellbeing. The cardiovascular manifestations largely determine patient mortality because of progressive aortic dilatation, leading to aortic dissection and fatal rupture, when no timely surgical intervention is undertaken. Apart from this life threating feature, patients with MFS also frequently show other debilitating features such as chest deformities, scoliosis, bone overgrowth, dural ectasia, ocular abnormalities, and arthritis. 2 , 3 , 4 , 5 Various studies indicate that patients across all age groups experience decreased mental health negatively related to their physical characteristics of MFS, 6 , 7 , 8 especially during the formative years. 9 , 10 Moreover, chronic pain is reported by a large group of patients with MFS, with prevalence ranging from 47% to 91.5%, which frequently remains undertreated. 6 , 11 , 12 As a result, reduced physical capacity and endurance may hamper a normal daily routine. Severe fatigue is reported more often in patients with MFS, as compared with the general population. 13 , 14 , 15
Fatigue is highly prevalent among individuals with chronic diseases, such as multiple sclerosis, rheumatic disease, and diabetes mellitus. 16 , 17 Multiple studies 13 , 14 found also that the prevalence of severe fatigue is higher in patients with MFS than in healthy controls. This can originate from the skeletal features of MFS. Joint‐ and back pain for instance can hinder sleep and lead to greater physical fatigue after exercise. Fatigue could also be a result of reduced physical activity, and thus reduced endurance. In addition, more severe fatigue is associated with lower participation in the labor market in patients with MFS. 18
Beside the physical aspects of the syndrome, MFS patients often simultaneously experience discomfort from psychological aspects. For example, the distinct appearance of many MFS patients who are often very tall, slender and have skeletal and ocular abnormalities due to the FBN1 mutation, leads to decreased self‐esteem. 7 Together with the constant fear of aortic complications and lifelong surveillance, MFS patients frequently experience psychological and social setback. 7 Anxiety may arise from the decreased life expectancy, many patients do not only fear these consequences for themselves, but also for their family members.
Recently, Andonian et al. showed that patients with MFS are at high risk for impaired quality of life (QOL), stating that mental health and psychological support should be part of a more integral cardiac care for these patients. 19 It is readily apparent that the interest on this topic is growing, and more research on the psychological aspects of MFS is performed. 20
Most published studies use subscales from QOL questionnaires, where MFS patients score lower than the healthy population in physical, as well as psychosocial domains. 21 Mental disorders, such as anxiety and depression, have been recognized as variables that have the strongest and most direct effects on QOL, and seemed to affect QOL significantly. 22 Nevertheless, research among patients with MFS on anxiety, and/or symptoms of depression determined by validated questionnaires is still scarce.
In the present study, we used the checklist individual strength (CIS) and hospital anxiety and depression scale (HADS) questionnaires (1) to establish the prevalence of severe fatigue, anxiety, and symptoms of depression in two cohorts of patients with MFS, in comparison to the general population, and (2) to assess the degree to which sociodemographic variables and especially clinical severity of the disease are associated with fatigue, anxiety, and symptoms of depression.
2. METHODS
2.1. Study design and participants
Two cohorts, where data were separately collected in two different studies of patients with MFS, were used for the current study. The first cohort consisted of Dutch speaking adult (≥18 years) patients, who were diagnosed with MFS by cardiologists according to the Ghent criteria of 2010. 23 All patients with MFS registered at the outpatient clinic Cardiology of the Amsterdam UMC—location AMC in 2017 were invited to participate. Invited patients received a letter per post including eight questionnaires, an informed consent form and a return envelope. Patients who responded provided their information on the basis of anonymity. From now on, we will refer to this cohort as the AMC MFS cohort.
The second cohort consisted of patients included in the RESVcue MFS study. In brief, the RESVcue MFS study included patients from four Dutch academic hospitals with a specialized MFS clinic (Amsterdam UMC—location AMC, Amsterdam; Radboud University Medical Center, Nijmegen; University Medical Center Groningen, Groningen; and Leiden University Medical Center, Leiden) between January 2019 and November 2020. Patients were eligible for inclusion if between 18 and 50 years old and with confirmed MFS (following the revised Ghent criteria 23 and with a known FBN1 mutation). The Medical Ethical Committee of Amsterdam UMC—location AMC, as well as all the institutional review boards of the participating centers approved the study. Written informed consent was obtained from all participants. From these patients data on clinical features were collected from electronic patient files. All patients filled out three questionnaires on fatigue, anxiety, and depression before and after 1 year of Resveratrol use. For the current study, we used the questionnaires at inclusion.
3. MEASURES
3.1. Sociodemographic variables and clinical outcomes
Sociodemographic variables, including sex, age, work participation (asked orally with the following yes or no question: have you permanently withdrawn from the labor market?), and marital status, were self‐reported by patients. Clinical variables, such as positive family history of MFS, aortic root dilatation, aortic surgery in history, aortic dissection in history, chronic pain, skeletal features, and scoliosis were partly self‐reported (AMC MFS cohort), and partly collected from electronic patient files by a research fellow (RESVcue MFS cohort). Aortic dilatation was defined as aortic root >40 mm, and aortic surgeries included all operations concerning the aorta (thoracic and abdominal). Scoliosis was defined as present, if it exceeded an angle of >20°. Data on medication use was extracted from electronic patient files, and verified with the patient by a research fellow.
3.2. Fatigue severity
Fatigue severity was assessed with the subscale fatigue severity of the Checklist Individual Strength ‐ 20 (CIS fatigue), a questionnaire that has excellent reliability and discriminative validity. 24 It consists of 20 questions, subdivided into the following four categories; Fatigue severity (eight items), Concentration (five items), Motivation (four items), and Activity (three items). The questions are scored on a 7‐point Likert scale. For the current study, we used the items concerning fatigue severity, resulting in a fatigue severity score ranging from 8 to 56. A cut‐off of ≥35 for fatigue severity was used, with a score of ≥35 meaning severe fatigue. This cut‐off has proven to have a sensitivity of 0.98 and a specificity of 0.83. 25 The general population norms of the CIS subscales was derived from 1923 people, randomly selected from a survey panel representing Dutch households, who reported no sick days in the past month, mean age 52 ± 17. 25
3.3. Anxiety and depression
The Dutch version of the HADS 26 was used to measure anxiety and symptoms of depression. This questionnaire consists of 14 statements, seven concerning anxiety and seven concerning depression. The scale ranges from 0 (no complaints) to 21 (maximum complaints). Either subscale has a score of ≥8 to indicate symptom severity of a possible psychiatric disorder (anxiety disorder and/or depression disorder), a score >11 indicates a probable psychiatric disorder in the same category. 27 The cut‐off score of 8 has been widely validated and detects anxiety and depression disorders at an early stage, 28 for this reason we choose to use this cut‐off. When used for research purpose this questionnaire is valid and reliable (α = 0.88). 26 The general population norm of the HADS was derived from 3492 respondents who attended general practices. A subgroup of the general population (n = 199) with a mean age of 40 ± 12 was used to compare means between the MFS cohort and general population. 26
3.4. Statistical analysis
Statistical analyses were performed using SPSS V.26 (IBM, Armonk, NY, USA). Descriptive statistics were used to assess the demographic and clinical characteristics. One‐sample t‐tests were used to compare mean fatigue, anxiety, and depression scores of MFS patients with the previously published general population norms. Independent sample t‐tests were used to compare mean fatigue, anxiety, and depression scores between the AMC MFS cohort and the RESVcue MFS cohort. Chi‐square tests were used to compare prevalence of clinical characteristics in both cohorts. A p‐value of <0.05 was considered significant.
We investigated whether the following sociodemographic and clinical characteristics were significantly associated with a CIS score ≥35 and HADS anxiety or depression score of ≥8 using univariate logistic regression analyses: age, sex, marital status (together vs. alone), employment (student/employment vs. unemployment), positive family history of MFS (yes vs. no), aortic dilatation (yes vs. no), aortic surgery in history (yes vs. no), aortic dissection in history (yes vs. no), chronic pain (yes vs. no), skeletal features (yes vs. no), and scoliosis (yes vs. no). Variables with p‐values <0.20 in the univariate analyses were subsequently included in a multivariate logistic regression analysis. Strengths of the associations were presented as odds ratios (OR) with 95% confidence intervals (CI). All multivariate tests were two‐sided and p‐values <0.05 were considered to indicate statistical significance.
4. RESULTS
4.1. Study population
For the AMC MFS cohort, 208 invitation letters were sent out of which six returned without reaching the patient, five patients were deceased, and one was diagnosed differently (no FBN1 mutation), resulting in 196 invited participants. In total, 109 patients responded (response rate of 55.6%) and filled out the questionnaires. In the RESVcue MFS study, 60 MFS patients were included, who all filled out the three questionnaires (response rate 100%). There was overlap between the two study populations of 14 patients, so these were excluded from the AMC MFS cohort, resulting in 95 patients in the AMC MFS cohort.
The characteristics of both cohorts of MFS patients are shown in Table 1. The AMC MFS cohort included significantly older patients (p < 0.0001) than the RESVcue MFS study, with more patients unemployed (p = 0.003). Moreover, patients in the AMC MFS cohort seemed to be more severely affected, concerning cardiovascular symptoms and chronic pain, than the patients included in the RESVcue MFS study.
TABLE 1.
Sample characteristics
| Total | AMC MFS cohort | RESVcue MFS cohort | p | |
|---|---|---|---|---|
| n = 155 | n = 95 | n = 60 | ||
| Sociodemographic characteristics | ||||
| Age at inclusion, years | 41 (31–51) | 48 (34–57) | 38 (30–46) | <0.0001* |
| Sex, male | 76 (49%) | 46 (48%) | 30 (50%) | 1.000 |
| Marital status, together | 102 (66%) | 62 (65%) | 40 (76%) | 0.722 |
| Employment, working | 113 (73%) | 61 (64%) | 52 (87%) | 0.003* |
| Clinical characteristics of MFS | ||||
| Positive family history | 79 (51%) | 57 (60%) | 22 (37%) | 0.003* |
| Aortic dilatation | 92 (59%) | 63 (66%) | 29 (48%) | 0.006* |
| Aortic surgery in history | 103 (67%) | 74 (78%) | 29 (48%) | <0.001* |
| Aortic dissection in history | 26 (17%) | 26 (27%) | 0 | <0.001* |
| Chronic pain | 37 (24%) | 33 (35%) | 4 (7%) | <0.001* |
| Skeletal involvement | 61 (39%) | 34 (38%) | 27 (45%) | 0.311 |
| Scoliosis | 29 (19%) | 14 (15%) | 15 (25%) | 0.139 |
p < 0.05 indicating a significant result.
4.2. Prevalence of fatigue, anxiety, and depression
Fatigue severity, anxiety, and symptoms of depression were assessed using the CIS and HADS questionnaires and compared between the AMC MFS cohort and patients included in the RESVcue MFS study. There were no significant differences between the prevalence of fatigue and anxiety between the two cohorts of MFS patients. However, patients in the RESVcue MFS study experienced significantly less symptoms of depression compared with patients in the AMC MFS cohort (p = 0.005) (Table 2).
TABLE 2.
Comparison of fatigue severity, anxiety, and depression scores (means) between the two MFS cohorts
| Total | AMC MFS cohort | RESVcue Marfan | t | p | Cohen's d | |
|---|---|---|---|---|---|---|
| n = 155 | n = 95 | n = 60 | ||||
| CIS fatigue severity | 31.9 ± 8.0 | 32.3 ± 5.0 | 30.95 ± 11.2 | 0.844 | 0.401 | 0.17 |
| HADS anxiety | 5.1 ± 3.6 | 5.4 ± 4.0 | 4.7 ± 3.2 | 1.133 | 0.259 | 0.20 |
| HADS depression | 3.7 ± 3.5 | 4.3 ± 3.9 | 2.8 ± 2.6 | 2.878 | 0.005 | 0.41 |
Note: Plus‐minus values are means ± SD.
Abbreviations: CIS, checklist individual strength; HADS, hospital anxiety and depression scale; MFS, Marfan syndrome.
When comparing the MFS patients to the general Dutch population, the two MFS cohorts combined did not report enhanced anxiety or symptoms of depression, while MFS patients did experience enhanced fatigue (t‐test; p < 0.0001) (Table 3). Of the patients in the AMC MFS cohort, 33 individuals (35%) reported severe enhanced fatigue, and in the RESVcue MFS cohort 24 individuals (40%). This extreme fatigue was also significant when comparing the cohorts separately to the general population. Interestingly, the mean age of the control cohort for the CIS was 52 ± 17 years, while the MFS population studied here is 43 ± 13.
TABLE 3.
Comparison of fatigue severity, anxiety, and depression scores (means) between the two cohorts and the general population
| MFS | General population | t | p | Cohen's d | |
|---|---|---|---|---|---|
| n = 155 | CIS n = 1923 | ||||
| HADS n = 199 | |||||
| CIS fatigue | 31.9 ± 8.0 | 23.0 ± 10.8 | 8.586 | <0.0001 | 0.92 |
| HADS anxiety | 5.1 ± 3.6 | 5.1 ± 3.6 | 0.000 | 1.000 | 0 |
| HADS depression | 3.7 ± 3.5 | 3.4 ± 3.3 | 0.826 | 0.288 | 0.09 |
| AMC cohort | General population | t | p | Cohen's d | |
|---|---|---|---|---|---|
| n = 95 | CIS n = 1923 | ||||
| HADS n = 199 | |||||
| CIS fatigue | 32.3 ± 5.0 | 23.0 ± 10.8 | 7.989 | <0.0001 | 1.00 |
| HADS anxiety | 5.4 ± 4.0 | 5.1 ± 3.6 | 0.644 | 0.467 | 0.08 |
| HADS depression | 4.3 ± 3.9 | 3.4 ± 3.3 | 2.059 | 0.027 | 0.26 |
| RESVcue cohort | General population | t | p | Cohen's d | |
|---|---|---|---|---|---|
| n = 60 | CIS n = 1923 | ||||
| HADS n = 199 | |||||
| CIS fatigue | 31.0 ± 11.2 | 23.0 ± 10.8 | 4.986 | <0.0001 | 0.73 |
| HADS anxiety | 4.7 ± 3.2 | 5.1 ± 3.6 | 0.773 | 0.337 | 0.11 |
| HADS depression | 2.8 ± 2.6 | 3.4 ± 3.3 | 1.292 | 0.079 | 0.19 |
Note: Plus‐minus values are means ± SD.
Abbreviations: CIS, checklist individual strength; HADS, hospital anxiety and depression scale; MFS, Marfan syndrome.
In the AMC MFS cohort, with older patients (mean age of 46 ± 14), the depression scores were significantly higher compared with the general population (HADS control cohort mean age is 40 ± 12). This was not observed in the RESVcue MFS cohort (mean age of 37 ± 9), which actually showed a trend towards lower depression scores compared with the general population.
4.3. Factors associated with fatigue severity
In 57 of the 155 patients with MFS (37%) a high score for fatigue severity (CIS ≥35) was observed. This severe fatigue, was significantly positively associated with female sex, and negatively associated with previous aortic surgery and chronic pain (Table 4). In the multivariate logistic regression analysis, female sex (OR = 3.085| 95% CI: 1.421–6.669|p = 0.004), and chronic pain (OR = 0.171| 95% CI: 0.058–0.506|p = 0.001) were significantly associated with severe fatigue.
TABLE 4.
Factors associated with high levels of fatigue severity (CIS fatigue severity subscale ≥35), indicative for severe fatigue
| Severe fatigue | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Total n = 155 | 57 (37%) | OR | CI (95%) | p | OR | CI (95%) | p |
| Sociodemographic characteristics | |||||||
| Age at inclusion, years | 44 (35–51) | 1.006 | 0.981–1.033 | 0.627 | 1.023 | 0.994–1.055 | 0.143** |
| Sex, male | 19 (33%) | 0.357 | 0.180–0.708 | 0.003* | 0.324 | 0.149–0.704 | 0.004* |
| Marital status, together | 38 (67%) | 1.287 | 0.618–2.680 | 0.501 | 1.516 | 0.689–3.504 | 0.286 |
| Employment, working | 44 (77%) | 1.394 | 0.652–2.978 | 0.392 | 0.945 | 0.381–2.343 | 0.902 |
| Clinical characteristics of MFS | |||||||
| Positive family history | 26 (46%) | 0.717 | 0.369–1.390 | 0.324 | 0.834 | 0.406–1.715 | 0.622 |
| Aortic dilatation | 34 (60%) | 1.023 | 0.515–2.030 | 0.949 | 1.014 | 0.469–2.193 | 0.972 |
| Aortic operation in history | 31 (54%) | 0.437 | 0.218–0.876 | 0.020* | 0.779 | 0.354–1.711 | 0.534 |
| Aortic dissection in history | 9 (16%) | 0.957 | 0.392–2.337 | 0.924 | 1.730 | 0.601–4.981 | 0.310 |
| Chronic pain | 5 (9%) | 0.206 | 0.075–0.566 | 0.002* | 0.171 | 0.058–0.506 | 0.001* |
| Skeletal involvement | 20 (35%) | 0.738 | 0.375–1.453 | 0.380 | 0.582 | 0.249–1.358 | 0.210 |
| Scoliosis | 10 (18%) | 0.873 | 0.374–2.037 | 0.754 | 1.094 | 0.414–2.893 | 0.857 |
Abbreviations: CI (95%), 95% confidence interval; OR, odds ratio.
p < 0.05 according to the logistic regression analyses, indicating a significant result.
p < 0.2 according to the logistic regression analyses, indicating a possible trend.
Since we had more data available from the RESVcue MFS cohort, we performed an analysis for additional MFS features and medication use (Table 5). MFS patients in the RESVcue MFS study either used no medication (23%), beta‐blockers (57%), losartan (63%), or both beta‐blockers and losartan (42%). Interestingly, there was a trend that the patients using losartan complained less of severe fatigue (p = 0.084). Of note, use of beta‐blockers was not associated with severe fatigue.
TABLE 5.
Factors associated with high levels of fatigue (CIS fatigue subscale ≥35), indicative for severe fatigue in the RESVcue MFS cohort
| Severe fatigue | Univariate | |||
|---|---|---|---|---|
| Total n = 60 | 24 (40%) | OR | CI (95%) | p |
| Sociodemographic characteristics | ||||
| Age at inclusion, years | 40 (33–47) | 1.060 | 1.00–1.13 | 0.065 |
| Sex, male | 7 (29%) | 0.233 | 0.08–0.71 | 0.010* |
| Marital status, together | 15 (63%) | 0.733 | 0.25–2.18 | 0.577 |
| Employment, working | 18 (75%) | 0.176 | 0.32–0.97 | 0.045* |
| Clinical characteristics of MFS | ||||
| Positive family history | 5 (21%) | 0.294 | 0.09–0.96 | 0.043* |
| Aortic dilatation | 14 (58%) | 1.960 | 0.69–5.59 | 0.208 |
| Aortic operation in history | 11 (46%) | 0.846 | 0.30–2.38 | 0.752 |
| Chronic pain | 3 (13%) | 5.000 | 0.49–51.23 | 0.175 |
| Skeletal involvement | 15 (63%) | 3.333 | 1.34–9.80 | 0.029* |
| Scoliosis | 8 (33%) | 2.071 | 0.63–6.77 | 0.228 |
| Ectopia lentis | 9 (38%) | 1.800 | 0.59–5.51 | 0.303 |
| Spontaneous pneumothorax | 4 (17%) | 1.240 | 0.30–5.18 | 0.768 |
| β‐blocker use | 15 (63%) | 1.491 | 0.52–4.28 | 0.457 |
| Losartan use | 12 (50%) | 0.385 | 0.13–1.14 | 0.084** |
| Heart rate | 63 ± 12 | 1.023 | 0.97–1.08 | 0.388 |
| Aortic valve insufficiency | 7 (29%) | 0.554 | 0.18–1.69 | 0.300 |
| Mitral valve insufficiency | 15 (63%) | 1.196 | 0.55–2.60 | 0.652 |
Abbreviations: CI (95%), 95% confidence interval; OR, odds ratio.
p < 0.05 according to the logistic regression analyses, indicating a significant result.
p < 0.2 according to the logistic regression analyses, indicating a possible trend.
4.4. Factors associated with anxiety and depression
A high score for anxiety (HADS anxiety subscale ≥8) was observed in 30 of the 155 included patients (19%), and significantly associated with chronic pain (Table 6). Furthermore, trends signified a possible association between anxiety and skeletal involvement and scoliosis. In the multivariate logistic regression analysis, chronic pain (OR = 3.059 |95% CI: 1.175–7.965 |p = 0.022) was significantly associated with high anxiety scores.
TABLE 6.
Factors associated with high levels of anxiety (HADS anxiety subscale ≥8), indicative of an anxiety disorder
| Anxiety ≥8 | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Total n = 155 | 30 (19%) | OR | CI (95%) | p | OR | CI (95%) | p |
| Sociodemographic characteristics | |||||||
| Age at inclusion, years | 38 (31–50) | 0.994 | 0.960–1.029 | 0.717 | 0.987 | 0.950–1.025 | 0.485 |
| Gender, male | 12 (40%) | 1.503 | 0.625–3.467 | 0.339 | 0.806 | 0.312–2.086 | 0.657 |
| Marital status, together | 19 (63%) | 1.072 | 0.437–2.628 | 0.880 | 1.275 | 0.485–3.350 | 0.622 |
| Employment, working | 20 (67%) | 0.532 | 0.216–1.306 | 0.168 | 0.908 | 0.320–2.575 | 0.856 |
| Clinical characteristics of MFS | |||||||
| Positive family history | 16 (53%) | 1.477 | 0.643–3.394 | 0.358 | 1.688 | 0.693–4.113 | 0.249 |
| Aortic dilatation | 17 (57%) | 0.953 | 0.410–2.213 | 0.910 | 1.272 | 0.510–3.171 | 0.606 |
| Aortic operation in history | 22 (73%) | 2.095 | 0.819–5.363 | 0.123 | 1.957 | 0.712–5.383 | 0.193 |
| Aortic dissection in history | 4 (13%) | 0.840 | 0.257–2.742 | 0.773 | 0.467 | 0.124–1.768 | 0.262 |
| Chronic pain | 12 (40%) | 3.706 | 1.489–9.226 | 0.005* | 3.059 | 1.175–7.965 | 0.022* |
| Skeletal involvement | 16 (53%) | 2.063 | 0.904–4.708 | 0.085** | 1.490 | 0.589–3.767 | 0.399 |
| Scoliosis | 9 (30%) | 2.277 | 0.884–5.864 | 0.088** | 1.714 | 0.609–4.890 | 0.305 |
Abbreviations: CI (95%), 95% confidence interval; OR, odds ratio.
p < 0.05 according to the logistic regression analyses, indicating a significant result.
p < 0.2 according to the logistic regression analyses, indicating a possible trend.
Employment status, aortic dissection in the medical history, and chronic pain were significantly associated with a high score for symptoms of depression, indicative of a depression disorder (HADS depression subscale ≥8) (Table 7). Moreover, trends suggested a possible association between depression and older age, and skeletal involvement. After multivariate logistic regression analysis, only employment status remained significantly associated (OR = 0.302| 95% CI: 0.107–0.853 |p = 0.024) with a high score of symptoms of depression.
TABLE 7.
Factors associated with high levels of depression (HADS depression subscale ≥8), indicative of a depression disorder
| Depression ≥8 | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Total n = 155 | 23 (15%) | OR | CI (95%) | p | OR | CI (95%) | p |
| Sociodemographic characteristics | |||||||
| Age at inclusion, years | 48 (35–59) | 1.033 | 0.997–1.071 | 0.073** | 1.014 | 0.975–1.054 | 0.489 |
| Gender, male | 9 (39%) | 0.661 | 0.264–1.654 | 0.376 | 0.980 | 0.326–2.946 | 0.972 |
| Marital status, together | 17 (74%) | 1.619 | 0.558–4.701 | 0.376 | 1.591 | 0.457–5.539 | 0.466 |
| Employment, working | 10 (44%) | 0.206 | 0.081–0.520 | 0.001* | 0.302 | 0.107–0.853 | 0.024* |
| Clinical characteristics of MFS | |||||||
| Positive family history | 13 (57%) | 1.422 | 0.568–3.562 | 0.453 | 1.321 | 0.455–3.841 | 0.609 |
| Aortic dilatation | 13 (57%) | 0.979 | 0.378–2.537 | 0.965 | 1.027 | 0.342–3.082 | 0.865 |
| Aortic operation in history | 16 (70%) | 1.397 | 0.510–3.822 | 0.515 | 0.852 | 0.338–3.709 | 0.848 |
| Aortic dissection in history | 8 (35%) | 3.731 | 1.362–10.219 | 0.010* | 2.521 | 0.836–7.599 | 0.101 |
| Chronic pain | 10 (44%) | 3.269 | 1.273–8.396 | 0.014* | 1.397 | 0.459–4.256 | 0.556 |
| Skeletal involvement | 13 (57%) | 2.268 | 0.923–5.573 | 0.074** | 1.950 | 0.690–5.510 | 0.208 |
| Scoliosis | 6 (26%) | 1.717 | 0.608–4.845 | 0.307 | 1.239 | 0.372–4.128 | 0.727 |
Abbreviations: CI (95%), 95% confidence interval; OR, odds ratio.
p < 0.05 according to the logistic regression analyses, indicating a significant result.
p < 0.2 according to the logistic regression analyses, indicating a possible trend.
5. DISCUSSION
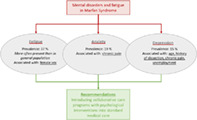
The current study investigated fatigue severity, anxiety, and symptoms of depression in patients with MFS by using the well‐standardized CIS and HADS questionnaires. The results showed that, MFS patients appear to experience more often severe fatigue as compared with a control cohort representing the general population. 25 Fatigue was more prominent in female MFS patients and negatively associated with chronic pain. Other variables that may affect fatigue severity showed no significant associations, providing no clear‐cut cause to explain severe fatigue in MFS. Anxiety and symptoms of depression was overall not more prevalent in patients with MFS compared with the general population.
5.1. Fatigue severity
Severe fatigue has been reported previously in adult MFS patients, 13 , 14 , 15 although not in children between the age of 4–18 with MFS. 29 It remains challenging to establish what causes this severe fatigue in patients with MFS, but the following few aspects of the disease might explain the increased prevalence.
First, interestingly, a trend was observed in the RESVcue MFS cohort that use of the blood pressure regulating drug losartan was negatively associated with fatigue. What is known about blood pressure medication in general is that a common side effect is fatigue, in particular in case of β‐blockers. β‐blockers could give chronotropic incompetence, and are associated with decreased exercise capacity. However, previous findings regarding blood pressure lowering medication and their association with fatigue are contradicting in MFS patients. Peters et al. found that fatigue was positively associated with medication use such as β‐blocker and calcium channel blockers, 30 while two other studies found no significant relationship between fatigue and β‐blocker use in MFS patients. 14 , 31 van Dijk et al. did note a significant relationship between fatigue and orthostatic intolerance in MFS patients, which is a possible side effect of blood pressure lowering medication. Replacement of β‐blockers for a different medication type with a similar effect on aortic pathology in MFS patients, for example an angiotensin‐II receptor blocker (ARB) such as losartan, may reduce complaints of severe fatigue. Different ARBs were proven to be effective against aorta pathology in several MFS studies, 32 , 33 , 34 thus these drugs seem a valuable alternative to β‐blockers.
Another potential cause for fatigue in patients with MFS is the psychological distress they experience, because of their chronic disease with many different symptoms. Rand‐Hendriksen et al. 14 found a significant association between psychological distress and fatigue in their female MFS population. This is in line with our results that female MFS patients were indeed significantly affected by fatigue. Male and female differences in MFS have been thoroughly studied by Thijssen et al., who showed that female MFS patients reported significantly reduced physical functioning, when compared with men or the general (female) population. 35 To assess psychological distress further, mental health care, and psychiatric medicine utilization could be assessed.
A third possible explanation for the fatigue is mitochondrial dysfunction in MFS patient cells and in Marfan mouse aortic smooth muscle cells. 36 , 37 , 38 , 39 Mitochondria are cellular organelles delivering most of our energy in the form of ATP, so defective mitochondria in different tissues may result in lack of energy, with consequent enhanced fatigue. This should be further investigated.
Finally, in multiple studies, chronic pain in patients with MFS has been associated with fatigue, and registered as a major complaint. 13 , 30 , 40 , 41 We could not reproduce these findings. A number of factors may underlie this. In our study, chronic pain was not assessed by a validated questionnaire, but rated by a simple question if they experienced chronic pain. Speed et al. 42 showed that 89% of their respondents with MFS reported having chronic pain, while only 28% of individuals reported pain as their most notable symptom of MFS. This discrepancy in reporting pain suggests that patients with MFS are used to living with pain, and may have developed coping mechanisms, thereby reporting pain differently depending on how these questions are asked (by questionnaire or as yes/no). Furthermore, we did not established where the pain was located, and how often this pain occurred. Different sorts of pain (skeletal pain, pain from scar tissue) could also be associated with fatigue, but we were not able to distinguish between this.
Fatigue in patients with MFS can arise from many different causes, which can all contribute to the severity. This might be the reason we were not able to find a clear association between fatigue and one of the causes. However, considering fatigue is proven to be a major problem in patients with MFS, it is necessary to address these symptoms with care and treat patients accordingly. Existing psychological treatment strategies, such as cognitive behavioral therapy (CBT) for symptoms of fatigue, 43 , 44 in our opinion, could be contributing to the integral care of patients with MFS.
5.2. Anxiety and depression
MFS patients in our combined cohort did not report more anxiety or symptoms of depression compared with the general population. This has been observed previously by Tongerloo et al., when using the Beck Depression Inventory and the State and Trait Anxiety Inventory in a group of 17 young MFS patients (16–35 years old). 9 Findings on depression and anxiety levels in patients with MFS are however conflicting. Schneider et al. 7 similarly showed no higher level of anxiety or depression in their MFS cohort, while Benke et al. showed enhanced anxiety by the Spielberger's anxiety (STAI) test, which was related to acute aortic surgery. 45 Peters et al. 46 found that approximately 44% of patients with MFS experienced a depression, which was significantly higher than the general population. 46 The studies that showed increased anxiety or depression scores, were however studies that included patients who were in fairly extreme circumstances, namely just after undergoing surgery or patients who felt stigmatized. This makes it difficult to compare these results, and to distract a conclusion from it.
While MFS patients did not report more anxiety than the normal population, their anxiety was associated with chronic pain. As previously mentioned, chronic pain is common in MFS. 6 , 11 , 12 , 29 Complaints of chronic pain in our cohort are most likely caused by skeletal involvement (back pain) and scoliosis, since these two features showed a trend in association with anxiety in the univariate analysis. Patients with MFS are less satisfied with their “chronic pain care” compared with other pain populations. 11 In this light, more attention could be given to pain management.
In our AMC MFS cohort, depression scores were significantly higher than in the general population, which may be explained by the older age in these MFS patients, their more severe cardiovascular phenotype, enhanced chronic pain and increased unemployment rate when compared with the RESVcue MFS cohort. Indeed, unemployment was the only feature significantly associated with symptoms of depression, which has been observed previously by Thijssen et al., where QOL was related to employment state in MFS. 35 Interestingly, our results show no relation between cardiovascular disease severity or skeletal features and symptoms of depression. This is in accordance with previous findings, were no correlations among psychological distress, symptoms of depression and the medical severity of the diagnosis were found. 46 , 47 Moreover, psychological distress is determined by the subjective perception of the severity of the disease and less by the objective physical severity of the disease. 47 Unemployment may be caused by the history in aortic dissection and experiencing chronic pain, since these features were significantly associated with symptoms of depression in the univariate analysis. This, again, shows the importance of an optimal treatment strategy for pain in patients with MFS.
5.3. Perspectives and future recommendations
Routine patient care often only concerns the cardiovascular manifestations in MFS, especially in adult patients. Collaborative care programs that combine improving mental and physical state, and at the same time address social, work‐related, and coping skills could be beneficial for patients with MFS. Focusing on improving these factors will possibly reduce fatigue severity, and might help for relieve of anxiety and symptoms of depression after major events (e.g., aortic surgery) to enhance QOL. Recommendations of Nielsen et al., 20 with as main goal to create programs that introduce coping mechanisms for psychological stress, and add these to routine care for patients with MFS, seem valid and in line with our results. Evidenced‐based protocolled CBT treatment strategies for severe fatigue symptoms, as well as for anxiety and symptoms of depression, exist, and could be introduced into integral care of patients with MFS. 43 , 48 , 49 In addition, neuropsychiatric interventions (i.e., psychotropic medication for pain, anxiety, and depression) might also be beneficial in these patients. To optimize this program, an overview of psychological distress, mental health care and psychiatric medicine utilization in MFS could be beneficial. Further research on psychiatric medicine use, and frequency of visiting psychiatrists or psychologists could give insight in the extent of the problem in this population and where additional treatment is needed. Concerning the high prevalence of fatigue in the MFS population, attention could be paid to the chronic use of β‐blockers in a large part of this population. Considerations on switching to a different medication type, with the same effect, could be made for these patients. Furthermore, future research should use validated chronic pain questionnaires to explore the interesting and conflicting relations between chronic pain and fatigue.
5.4. Limitations and strengths
First, limitations of the study include its retrospective design and the fact that information about medical history in the AMC MFS cohort was obtained from the patients and not the electronic patient files or treating physician, which resulted in less granular information about medical features. Second, no validated questionnaires on chronic pain or work participation were used. Finally, two different cohorts, which were combined at a later stage, were used for this study to create a larger population and a broader understanding of the psychological problems of Dutch Marfan population. Less data was available in the AMC MFS cohort, which made more extensive analyses impossible. In our opinion, in this study it was interesting and relevant to use both cohorts also in the context of generalizability of the data. The first strength of this study is the relatively large sample size, combing the two cohorts of MFS patients. Second, patients were stringently diagnosed by their cardiologist using the revised Ghent criteria of 2010, and in the RESVcue MFS cohort all patients had a known FBN1 mutation.
6. CONCLUSION
MFS patients experience more often severe fatigue as compared with a control cohort representing the general population. Fatigue was more prominent in female MFS patients. Signs of anxiety were significantly associated with chronic pain. Symptoms of depression appeared more frequent in older MFS patients, with a more severe cardiovascular phenotype, chronic pain and an increased unemployment rate. For these patients it is of great importance that screening for these symptoms is performed by their treating physician, and that they are referred for an appropriate treatment strategy, for example, CBT, if needed.
CONFLICT OF INTEREST
V. de Waard obtained grants from the AMC Foundation, supporting M.M. van Andel during the conduct of the study. The other authors have nothing to disclose.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/cge.14211.
ETHICS STATEMENT
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
ACKNOWLEDGMENT
Not applicable.
van Andel MM, Graaumans K, Groenink M, et al. A cross‐sectional study on fatigue, anxiety, and symptoms of depression and their relation with medical status in adult patients with Marfan syndrome. Psychological consequences in Marfan syndrome. Clinical Genetics. 2022;102(5):404‐413. doi: 10.1111/cge.14211
Funding information AMC Foundation
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study will become available from the corresponding author on reasonable non‐commercial request.
REFERENCES
- 1. von Kodolitsch Y, De Backer J, Schüler H, et al. Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome. Appl Clin Genet. 2015;8:137‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grahame R, Pyeritz RE. The Marfan syndrome: joint and skin manifestations are prevalent and correlated. Br J Rheumatol. 1995;34(2):126‐131. [DOI] [PubMed] [Google Scholar]
- 3. Foran JR, Pyeritz RE, Dietz HC, Sponseller PD. Characterization of the symptoms associated with dural ectasia in the Marfan patient. Am J Med Genet. 2005;134A(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 4. Dean JC. Marfan syndrome: clinical diagnosis and management. Eur J Hum Genet. 2007;15(7):724‐733. [DOI] [PubMed] [Google Scholar]
- 5. Hasan A, Poloniecki J, Child A. Ageing in Marfan syndrome. Int J Clin Pract. 2007;61(8):1308‐1320. [DOI] [PubMed] [Google Scholar]
- 6. Peters KF, Kong F, Horne R, Francomano CA, Biesecker BB. Living with Marfan syndrome I. Percept Cond Clin Genet. 2001;60(4):273‐282. [DOI] [PubMed] [Google Scholar]
- 7. Schneider MB, Davis JG, Boxer RA, Fisher M, Friedman SB. Marfan syndrome in adolescents and young adults: psychosocial functioning and knowledge. J Dev Behav Pediat. 1990;11(3):122‐127. [PubMed] [Google Scholar]
- 8. Ratiu I, Virden TB, Baylow H, Flint M, Esfandiarei M. Executive function and quality of life in individuals with Marfan syndrome. Qual Life Res. 2018;27(8):2057‐2065. [DOI] [PubMed] [Google Scholar]
- 9. Van Tongerloo A, De Paepe A. Psychosocial adaptation in adolescents and young adults with Marfan syndrome: an exploratory study. J Med Genet. 1998;35(5):405‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fusar‐Poli P, Klersy C, Stramesi F, Callegari A, Arbustini E, Politi P. Determinants of quality of life in Marfan syndrome. Psychosomatics. 2008;49(3):243‐248. [DOI] [PubMed] [Google Scholar]
- 11. Nelson AM, Walega DR, McCarthy RJ. The incidence and severity of physical pain symptoms in Marfan syndrome: a survey of 993 patients. Clin J Pain. 2015;31(12):1080‐1086. [DOI] [PubMed] [Google Scholar]
- 12. Velvin G, Bathen T, Rand‐Hendriksen S, Geirdal A. Systematic review of chronic pain in persons with Marfan syndrome. Clin Genet. 2016;89(6):647‐658. [DOI] [PubMed] [Google Scholar]
- 13. Bathen T, Velvin G, Rand‐Hendriksen S, Robinson HS. Fatigue in adults with Marfan syndrome, occurrence and associations to pain and other factors. Am J Med Genet A. 2014;164a(8):1931‐1939. [DOI] [PubMed] [Google Scholar]
- 14. Rand‐Hendriksen S, Sørensen I, Holmström H, Andersson S, Finset A. Fatigue, cognitive functioning and psychological distress in Marfan syndrome, a pilot study. Psychol Health Med. 2007;12(3):305‐313. [DOI] [PubMed] [Google Scholar]
- 15. Velvin G, Bathen T, Rand‐Hendriksen S, Geirdal A. Satisfaction with life in adults with Marfan syndrome (MFS): associations with health‐related consequences of MFS, pain, fatigue, and demographic factors. Qual Life Res. 2016;25(7):1779‐1790. [DOI] [PubMed] [Google Scholar]
- 16. Goërtz YMJ, Braamse AMJ, Spruit MA, et al. Fatigue in patients with chronic disease: results from the population‐based lifelines cohort study. Sci Rep. 2021;11(1):20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menting J, Tack CJ, Bleijenberg G, et al. Is fatigue a disease‐specific or generic symptom in chronic medical conditions? Health Psychol. 2018;37(6):530‐543. [DOI] [PubMed] [Google Scholar]
- 18. Velvin G, Bathen T, Rand‐Hendriksen S, Geirdal A. Work participation in adults with Marfan syndrome: demographic characteristics, MFS related health symptoms, chronic pain, and fatigue. Am J Med Genet A. 2015;167a(12):3082‐3090. [DOI] [PubMed] [Google Scholar]
- 19. Andonian C, Freilinger S, Achenbach S, et al. Quality of life in patients with Marfan syndrome: a cross‐sectional study of 102 adult patients. Cardiovasc Diagn Ther. 2021;11(2):602‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nielsen C, Ratiu I, Esfandiarei M, Chen A, Selamet Tierney ES. A review of psychosocial factors of Marfan syndrome: adolescents, adults, families, and providers. J Pediatr Genet. 2019;8(3):109‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldfinger JZ, Preiss LR, Devereux RB, et al. Marfan syndrome and quality of life in the GenTAC registry. J Am Coll Cardiol. 2017;69(23):2821‐2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moon JR, Cho YA, Huh J, Kang IS, Kim DK. Structural equation modeling of the quality of life for patients with marfan syndrome. Health Qual Life Outcomes. 2016;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476‐485. [DOI] [PubMed] [Google Scholar]
- 24. Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38(5):383‐392. [DOI] [PubMed] [Google Scholar]
- 25. Worm‐Smeitink M, Gielissen M, Bloot L, et al. The assessment of fatigue: psychometric qualities and norms for the checklist individual strength. J Psychosom Res. 2017;98:40‐46. [DOI] [PubMed] [Google Scholar]
- 26. Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363‐370. [DOI] [PubMed] [Google Scholar]
- 27. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52(2):69‐77. [DOI] [PubMed] [Google Scholar]
- 28. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361‐370. [DOI] [PubMed] [Google Scholar]
- 29. Warnink‐Kavelaars J, de Koning LE, Rombaut L, et al. Heritable connective tissue disorders in childhood: increased fatigue, pain, disability and decreased general health. Genes (Basel). 2021;12(6):831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peters KF, Horne R, Kong F, Francomano CA, Biesecker BB. Living with Marfan syndrome II. Medication adherence and physical activity modification. Clin Genet. 2001;60(4):283‐292. [DOI] [PubMed] [Google Scholar]
- 31. van Dijk N, Boer MC, Mulder BJ, van Montfrans GA, Wieling W. Is fatigue in Marfan syndrome related to orthostatic intolerance? Clin Auton Res. 2008;18(4):187‐193. [DOI] [PubMed] [Google Scholar]
- 32. Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J. 2013;34(45):3491‐3500. [DOI] [PubMed] [Google Scholar]
- 33. van Andel MM, Indrakusuma R, Jalalzadeh H, et al. Long‐term clinical outcomes of losartan in patients with Marfan syndrome: follow‐up of the multicentre randomized controlled COMPARE trial. Eur Heart J. 2020;41(43):4181‐4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mullen M, Jin XY, Child A, et al. Irbesartan in Marfan syndrome (AIMS): a double‐blind, placebo‐controlled randomised trial. Lancet (London, England). 2020;394(10216):2263‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thijssen CGE, Doze DE, Gökalp AL, et al. Male‐female differences in quality of life and coping style in patients with Marfan syndrome and hereditary thoracic aortic diseases. J Genet Couns. 2020;29(6):1259‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Pluijm I, Burger J, van Heijningen PM, et al. Decreased mitochondrial respiration in aneurysmal aortas of Fibulin‐4 mutant mice is linked to PGC1A regulation. Cardiovasc Res. 2018;114(13):1776‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He H, Yu B, Liu Z, et al. Vascular progenitor cell senescence in patients with Marfan syndrome. J Cell Mol Med. 2019;23(6):4139‐4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. You W, Hong Y, He H, et al. TGF‐β mediates aortic smooth muscle cell senescence in Marfan syndrome. Aging (Albany NY). 2019;11(11):3574‐3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oller J, Gabandé‐Rodríguez E, Ruiz‐Rodríguez MJ, et al. Extracellular tuning of mitochondrial respiration leads to aortic aneurysm. Circulation. 2021;143(21):2091‐2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rao SS, Venuti KD, Dietz HC 3rd, Sponseller PD. Quantifying health status and function in Marfan syndrome. J Surg Orthop Adv. 2016;25(1):34‐40. [PubMed] [Google Scholar]
- 41. Percheron G, Fayet G, Ningler T, et al. Muscle strength and body composition in adult women with Marfan syndrome. Rheumatology (Oxford). 2007;46(6):957‐962. [DOI] [PubMed] [Google Scholar]
- 42. Speed TJ, Mathur VA, Hand M, et al. Characterization of pain, disability, and psychological burden in Marfan syndrome. Am J Med Genet A. 2017;173(2):315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knoop H, Stulemeijer M, de Jong LW, Fiselier TJ, Bleijenberg G. Efficacy of cognitive behavioral therapy for adolescents with chronic fatigue syndrome: long‐term follow‐up of a randomized, controlled trial. Pediatrics. 2008;121(3):e619‐e625. [DOI] [PubMed] [Google Scholar]
- 44. Poort H, Peters M, van der Graaf WTA, et al. Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: a randomized controlled trial. Ann Oncol. 2020;31(1):115‐122. [DOI] [PubMed] [Google Scholar]
- 45. Benke K, Ágg B, Pólos M, et al. The effects of acute and elective cardiac surgery on the anxiety traits of patients with Marfan syndrome. BMC Psychiatry. 2017;17(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peters K, Apse K, Blackford A, McHugh B, Michalic D, Biesecker B. Living with Marfan syndrome: coping with stigma. Clin Genet. 2005;68(1):6‐14. [DOI] [PubMed] [Google Scholar]
- 47. De Bie S, De Paepe A, Delvaux I, Davies S, Hennekam RC. Marfan syndrome in Europe. Community Genet. 2004;7(4):216‐225. [DOI] [PubMed] [Google Scholar]
- 48. Twomey C, O'Reilly G, Byrne M. Effectiveness of cognitive behavioural therapy for anxiety and depression in primary care: a meta‐analysis. Fam Pract. 2015;32(1):3‐15. [DOI] [PubMed] [Google Scholar]
- 49. Santoft F, Axelsson E, Öst LG, Hedman‐Lagerlöf M, Fust J, Hedman‐Lagerlöf E. Cognitive behaviour therapy for depression in primary care: systematic review and meta‐analysis. Psychol Med. 2019;49(8):1266‐1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study will become available from the corresponding author on reasonable non‐commercial request.


