Abstract
Chimeric antigen receptors (CAR)-modified T cells are an emerging therapeutic tool for chronic lymphocytic leukemia (CLL). However, in patients with CLL, well-known T-cell defects and the inhibitory properties of the tumor microenvironment (TME) hinder the efficacy of CAR T cells.
We explored a novel approach combining CARs with lenalidomide, an immunomodulatory drug that tempers the immunosuppressive activity of the CLL TME. T cells from patients with CLL were engineered to express a CAR specific for CD23, a promising target antigen. Lenalidomide preserves CD23.CAR T cells in vitro effector functions in terms of antigen-specific cytotoxicity, cytokine release and proliferation. Overall, lenalidomide preserved functional CAR T-CLL cell immune synapses. In a Rag2−/−γc−/−-based xenograft model of CLL, we demonstrated that, when combined with low-dose lenalidomide, CD23.CAR T cells efficiently migrated to leukemic sites and delayed disease progression when compared to CD23.CAR T cells given with rhIL-2. These observations underline the therapeutic potential of this novel CAR-based combination strategy in CLL.
Keywords: chronic lymphocytic leukemia, CAR T cells, CD23, lenalidomide, immunomodulation, immunotherapy
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is a chronic lymphoid malignancy characterized by immune dysfunction that particularly involves the T-cell compartment. Tumor-mediated immunosuppression is a key feature of CLL and is essentially due to malignant cells’ capacity to modify the surrounding microenvironment, including lymphoid and myeloid cells (1, 2). This immunosuppression likely contributes to disease progression and to limited effectiveness of current immunotherapeutic approaches (3–5). We recently reported that the targeting of the immunosuppressive myeloid and lymphoid cells of the TME has promising therapeutic value in CLL (2, 6).
Patients with CLL have low IgG levels associated with reduced survival. The respiratory tract represents a frequent site of infection (7, 8). This evidence highlights the unmet need of investigating alternative target antigens and immunotherapy strategies preserving normal CD19+ B cells and repairing immune dysfunction.
Chimeric antigen receptors (CARs) are emerging, powerful tools that redirect T-cell specificity against B-cell leukemias (9–11). CARs are artificial molecules comprising a single-chain variable fragment (scFv) of a monoclonal antibody fused with an intracellular signaling region, usually the zeta chain of the TCR/CD3 complex, that is triggered after antigen recognition. Therefore, CARs exploit both the non-MHC-restricted antigen binding properties of monoclonal antibodies and the typical T-cell mediated effector functions (12, 13). Significant response rates have been reported in patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL), diffuse large B-cell lymphomas (DLBCL), mantle cell lymphomas and follicular lymphomas (10, 14–17). In contrast, in CTL019 trials, only 26% of CLL patients had durable antitumor responses with dramatic mechanisms of resistance to anti-CD19.CAR T-cell therapy (18). Additional studies have demonstrated that CD8+ anti-CAR T cells from patients with CLL exhibit impaired metabolic function compared to CD8+ T cells from healthy donors and that these features are associated with T-cell exhaustion and a reduction in T-cell activation and degranulation (19).
Several T-cell defects have been observed in patients with CLL and are mainly related to impaired immune synapse (IS) formation, and these defects could ultimately impact on the CAR T-cell activation and expansion upon antigen encounter (1) (20) (21).
Thus, the purpose of this study was to explore the possibility of using lenalidomide, an immunomodulatory agent that has induced significant, long-lasting responses in CLL patients (22–25), to enhance the therapeutic efficacy of CD23.CAR-redirected T cells.
CD23 is typically overexpressed on leukemic B cells, but not on normal CD19+ B cells and CD19+ CD5– CD27+ antibody secreting cells (26) (27), therefore it represents a valuable alternative target antigen to consider in patients at risk of infections.
In vitro, lenalidomide repairs defects in the IS formation between T and CLL cells by interfering with several cytoskeletal molecules (1, 28, 29). The drug’s immunomodulatory effect is not only on T cells, but also on the myeloid tumor microenvironment (TME), mostly through the alteration of the protumor cytokine milieu. Lenalidomide specifically down-modulates in vitro the production of pro-CLL factors by nurse like cells (NLCs), identified as CLL-specific tumor-associated macrophages (30–32). Recent reports demonstrate the involvement of monocytes and macrophages in mechanisms of resistance and toxicity related to CAR-T cell therapy (33, 34).
In this study, we show that lenalidomide positively impacts in vitro activation of CD23.CAR T cells against CLL target cells. We have used a Rag2−/−γc−/−-based xenograft model of CLL (35) to demonstrate that lenalidomide can be efficiently associated to CD23.CAR-based immunotherapy. CD23.CAR+ T cells from patients with CLL combined with low-dose lenalidomide induced a potent anti-leukemic effect in lymphoid and non-lymphoid tissues and improved mice survival. CD23.CAR+ T cells exposed daily to lenalidomide in vivo efficiently migrated to leukemic sites and mounted a tumor-specific cytotoxic response ex vivo. These results warrant additional investigation of the clinical use of lenalidomide in patients with CLL to improve CAR T-cell therapeutic strategies.
MATERIALS AND METHODS
Generation of CD23.CAR T lymphocytes from primary CLL cells
Peripheral blood (PB) samples were obtained from RAI stage 0–1 patients with CLL, after informed consent as approved by the Institutional Ethical Committee (protocol VIVI-CLL) of the IRCCS San Raffaele Hospital in accordance with the Declaration of Helsinki. The CD23.CAR cloning, its expression in T lymphocytes and the cellular expansion were performed as previously described (27). Briefly, an optimized single chain antibody (scFv) was generated using a synthetic DNA technology (Assembly PCR Oligo Maker) starting from and already published monoclonal antibody sequence. The scFv was then cloned in frame with the human IgG1 endodomain, the CD28 co-stimulatory endodomain and the ζ chain of the TCR/CD3 complex into the SFG retroviral backbone to generate the CD23.CAR vector.
Activated T lymphocytes were then transduced with the retroviral supernatants using retronectin coated plates (Takara, Shuzo Co. Ltd, Shiga, Japan), as previously described (27).
Cell line and reagents
MEC1 cells are a CD5low/– CLL cell line established from a patient with CLL in prolymphocytoid transformation to B-cell prolymphocytic leukemia (B-PLL), obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ, Braunschweig, Germany).
Lenalidomide was provided by Celgene Corporation (Summit, NJ, USA) or purchased from Sigma-Aldrich. For MEC1 cell culture methods and lenalidomide powder dilution, see supplemental methods.
Immunophenotyping
For in vitro studies, human cells were stained with the following antibodies: FITC Anti-Human CD19, HIB19 clone; PE Anti-Human CD23, M-L233 clone; FITC Anti-Human CD45RO, UCHL1 clone, PE Anti-Human CD62L, DREG-56 clone; FITC Anti-Human CD8 from BD; PE Anti-Human CD4, SK3 clone. All the antibodies were obtained from BD Biosciences (San Jose, CA, USA). Anti-Fc-γCy5 antibody was obtained from Jackson ImmunoResearch (West Grove, PA, USA)(27).
Samples were acquired using the FACS Canto II flow cytometer (BD Biosciences) and data were analyzed using BD FACS DIVA software version 6.1.3 (BD Biosciences).
For detailed immunophenotyping methods, intracellular cytokine staining and proliferation assay, see supplemental methods.
FACS-based IS assay
To evaluate IS functionality on CD23.CAR+ T cells, we performed in vitro studies with lenalidomide. CellTracker FITC CD23.CAR+ T cells, both untreated control and those pretreated for 48 hours with 1 μM lenalidomide, were co-cultured with CellTracker PE MEC1 target cells (effector cell to target cell E:T ratio, 1:2). After 4 hours of co-culture, PE and FITC double-positive cells were quantified using flow cytometry.
Short-Term cytotoxicity assay
Non-transduced (NT) and CD23.CAR+ T cells, pretreated for 48 hours or not with 1μM lenalidomide, were co-cultured for 4-hours with MEC1 cells (previously labeled with CellTracker PE) at an E:T ratio of 5:1. Target cell killing was measured through apoptosis detection by flow cytometry as previously described (36).
For cytotoxicity assay in xenograft studies, see supplemental methods.
Nanostring
NT and CD23.CAR+ T cells (5×106), either untreated or treated for 48 hours with 1μM lenalidomide were lysed with RLT Buffer (160 μL/106 cells) (Qiagen, Valencia, CA) and lysates were frozen at −80°C. RNA lysates were thawed and immediately analyzed for gene expression analysis by using standard nCounter®. For detailed methods/analysis, see supplemental methods.
The nanostring data have been deposited in NCBI’s Gene Expression omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/info/linking.html) public repository and are accessible through GEO Series accession number GSE178893.
In vivo studies
Female Rag2–/–γc–/– mice on BALB/c background were kindly provided by CIEA/Taconic (Kawasaki, Japan) or purchased from Taconic (Rensselaer, NY, USA). Mice were housed and bred in specific pathogen-free animal facilities at San Raffaele Scientific Institute and at the University of Texas MD Anderson Cancer Center. Depending on the experiments, mice were treated in accordance with the European Union guidelines and with the approval of the San Raffaele Scientific Institute Institutional Ethical Committee (protocol 601) or with the approval of the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center (protocol 00001627-RN00) and conducted in accordance with the Animal Welfare Act.
For detailed doses/schedules, murine cell preparation and related flow cytometry, see supplemental methods.
Statistical analysis
Statistical analyses were performed using the Student t-test. Data were expressed as the mean values (± standard deviation SD), and comparison of growth curves was considered statistically significant for P values less than 0.05. Survival curves were compared using the log-rank test. Nanostring data analysis is described in Supplementary Methods.
RESULTS
CD23.CAR+ T cells can be efficiently generated from samples obtained from patients with CLL
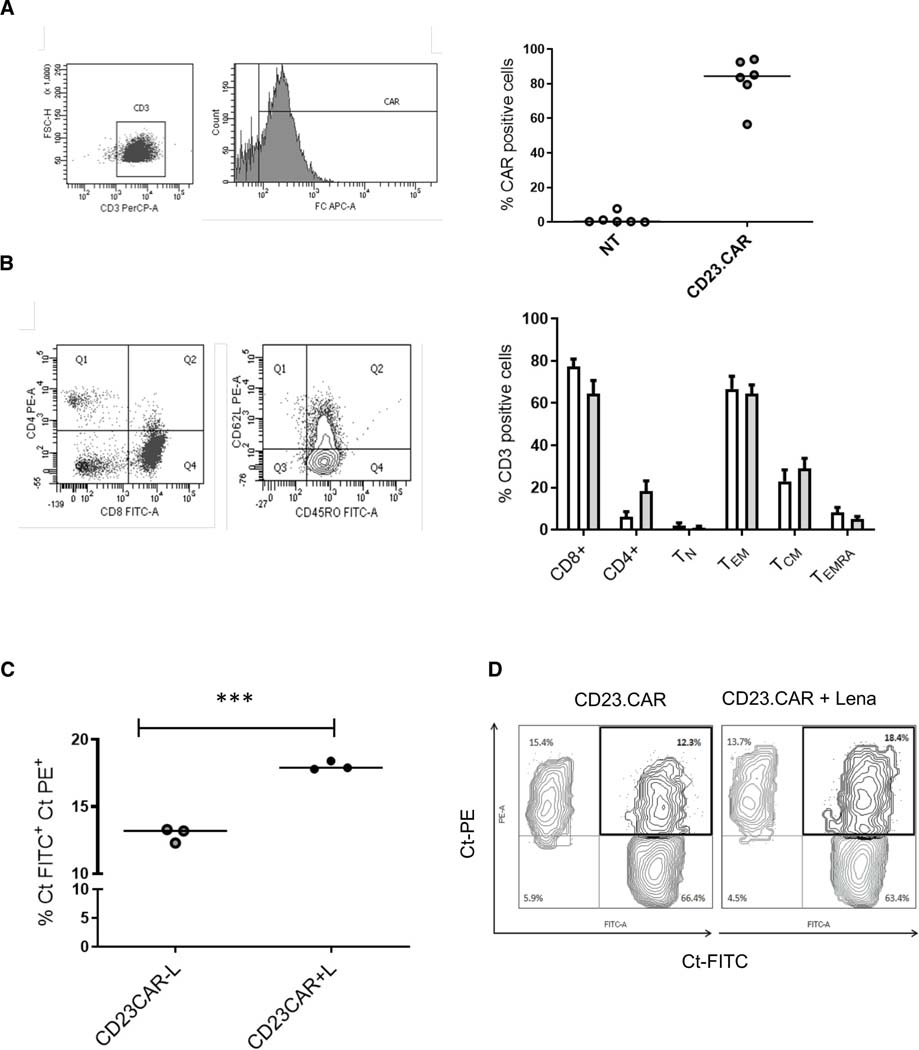
Starting with PB mononuclear cells (PBMCs) isolated from six patients with CLL, we successfully generated high numbers of CD23.CAR+ T lymphocytes from a residual percentage (3% −10%) of CD3+ cells (27). Non-transduced (NT) T cells were cultured as controls. The genetic manipulation produced a mean (± SD) CD23.CAR expression rate of 82% ± 5.5 (n=6, Figure 1A) in CAR.CD23+ T cells without alteration of the T-cell immunophenotype. In both NT T cells and CAR.CD23+ T lymphocytes, the polyclonal activation of T cells from all analyzed patients preferentially stimulated CD8+ cells on CD4+ cells, with high percentages of CD8+ TEM (Figure 1B).
Figure 1. Lenalidomide preserves IS formation on CD23.CAR+ T cells generated from CLL patient samples.
(A) Expression of CD23.CAR on the surface of T lymphocytes derived from a representative CLL patient evaluated by flow cytometry with APC-conjugated anti-human-Fc antibody (CAR) (left); CD23.CAR expression in CLL-derived T cells (gray) and control not-transduced (NT) T cells from 6 patients with CLL (white) (right). (B) Phenotype of NT (white bars), and CD23.CAR+ (gray bars) T lymphocytes generated from CLL samples. T cells have been identified as described in the Methods (left). The histogram data represents means (± SD) from six different differentiations (right). (C) For FACS-based synapse analysis, CD23.CAR+ T cells were treated with lenalidomide for 48h or left untreated. CellTracker (Ct) PE-labeled MEC1 cells were cocultured with CellTracker (Ct) FITC-labeled CD23.CAR+ T cells (E:T ratio, 1:2) for 4h hours, then PE and FITC double-positive cells were quantified by flow cytometry (n=3, ***, p<0.001). (D) Gating strategy of CellTracker (Ct) PE/ CellTracker (Ct) FITC double-positive cells in one representative experiment.
Lenalidomide preserves immune synapse formation and enhances T-cell function transcriptional signatures of CD23.CAR+ T cells
A critical immune defect induced by CLL cells is the disruption of the immune synapse (IS), the interface between T cells and tumor cells (1).
To evaluate IS functionality and the costimulatory effect of lenalidomide on CD23.CAR+ T cells, we performed 48 h in vitro studies with lenalidomide at 1 μM, a dose previously tested on BCMA CAR T cells (37). To assess IS formation, we cocultured CellTracker FITC-labeled CD23.CAR T cells (control cells and those pretreated with lenalidomide) with the CellTracker PE-labeled MEC1 CLL cells. After 4 h, we analyzed by flow cytometry the percentage of FITC-PE double-positive cells as a means of qualitatively measuring the interaction between the effector and target cells. Lenalidomide-based treatment increased the percentage of CD23.CAR T cell/ MEC1 cell pairs (Figure 1C–D). We also found that the addition of lenalidomide did not hamper the in vitro functional properties of CLL-derived T cells in terms of cytotoxicity, cytokine production, and proliferation in response to MEC1 target cells (Figure S1A–D). This confirms the beneficial effect of lenalidomide on IS formation.
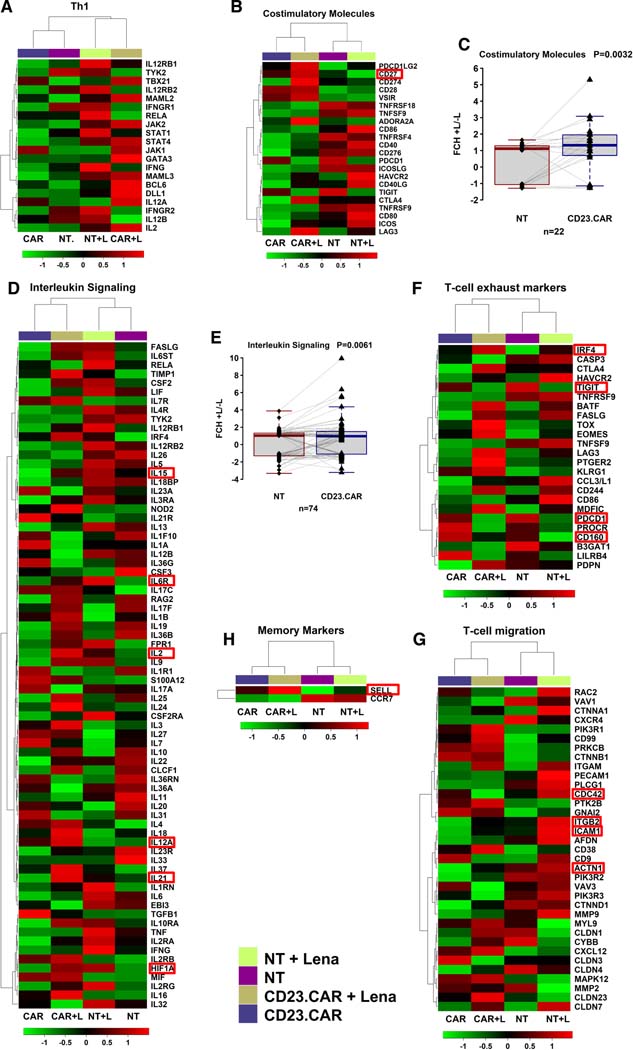
To evaluate whether lenalidomide is able to preserve CAR T-cell function machinery at transcriptional level, we performed Nanostring analysis of NT T cells and CD23.CAR T cells generated from CLL patient sample #1, with or without lenalidomide. A number of genes was modulated (fold change >1.5) between lenalidomide-treated and untreated samples (GEO, GSE178893). Gene signatures associated to Th1 T cell response, costimulation and interleukin signaling pathways were positively modulated by lenalidomide (Figure 2A–E), in line with the evidence generated in RNAseq studies of CAR T cells in multiple myeloma patients. IL-21 was found upregulated on CAR T cells exposed to lenalidomide (Figure 2D) and it is known to control gene expression and promote apoptosis of CLL cells (38, 39). Of note, the exposure of CD23.CAR T cells to lenalidomide downmodulated transcripts associated with T cells exhaustion, including PDC1, TIGIT and CD160 (Figure 2F), confirming previous results in multiple myeloma (40). As previously observed in CLL T cells (1, 28), CDC42, ACTN1, ICAM1 transcripts were found modulated by lenalidomide on CD23.CAR T cells (Figure 2G), thus confirming at transcriptional level the restoration of the IS machinery and T cell-adhesion/migration induced by lenalidomide. In general, the upregulation of some costimulatory/memory transcripts (e.g. CD27, IL6R, CD62L) previously observed in patients with CLL and MM who completely responded to CD19.CAR T cell therapy (Figure 2 B,D,H) (18, 41), was detected in CD23.CAR T cells treated with lenalidomide.
Figure 2. Lenalidomide enhances transcriptional signatures related to T-cell function.
The heat maps and dendrograms of unsupervised hierarchical clustering display differentially expressed genes between NT and CD23.CAR+ T lymphocytes obtained from CLL donor #1, treated in vitro with lenalidomide or left untreated. The gene expression differences among the samples were functionally classified as follows: (A) Th1; (B) costimulatory molecules; (D) interleukin signaling; (F) T-cell exhaustion; (G) T-cell migration; (H) memory markers. Fold changes between lenalidomide (+L) and untreated (-L) sample for costimulatory molecules (C) and interleukin signaling (E) are represented with box and whisker plots.
Conclusively, coupling the CD23.CAR redirection of T cells with lenalidomide-based immunomodulatory effect sustained all the functional properties of the T cells from patients with CLL, resulting in an efficient in vitro anti-leukemic activity.
Lenalidomide administered as a single agent reduces the growth of MEC1 cells transplanted into Rag2−/−γc−/− mice
We verified the in vivo antitumor activity of lenalidomide in our subcutaneously injected, MEC1-based xenograft model of CLL (35). Treatment with four escalating lenalidomide doses markedly reduced MEC1 tumor growth in mice receiving lenalidomide compared with control mice (starting at day 24 for all the doses, except for 0.214 mg/kg dose where the tumor reduction started significantly at day 33) and induced survival extension in mice administered with 0.357 mg/kg dose (Figure S2A–C). The drug did not modify the animal weights (data not shown), thereby excluding drug-related toxicity. This in vivo sensitivity to lenalidomide was confirmed in two different experiments in which 0.214 mg/kg lowest dose was the less efficient and did not induce significant improvement of the survival (Figure S2C). We selected 0.214 mg/kg lenalidomide dose for the studies in combination with CAR T cell therapy being the less efficient in terms of tumor burden reduction, as we aimed to exploit the immunomodulatory effect of the drug rather than its direct anti-leukemic effect.
Lenalidomide improves the therapeutic efficacy of CD23.CAR+ T lymphocytes in vivo
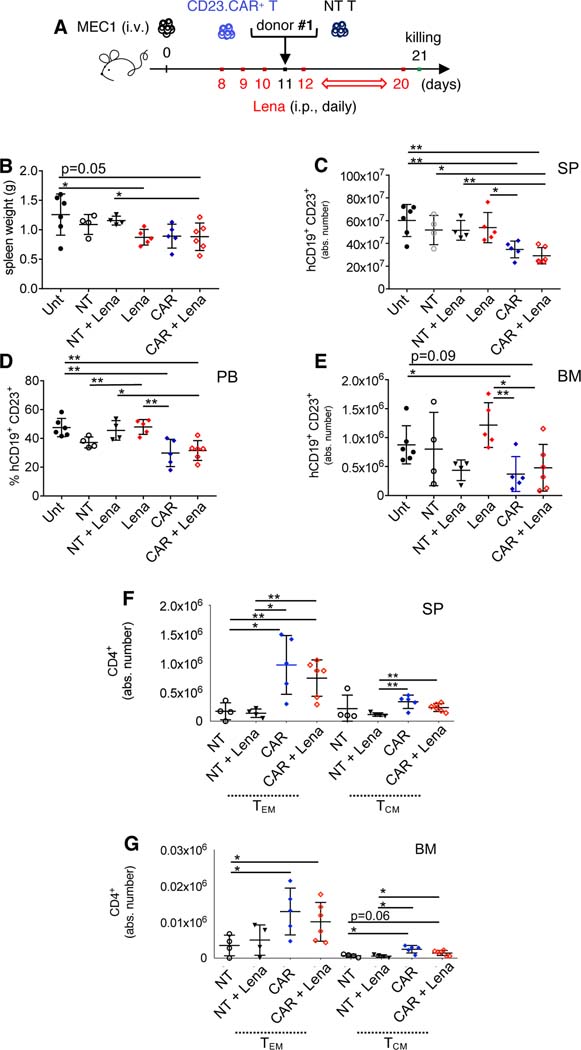
To assess the impact of CD23.CAR-T cells plus lenalidomide on the immune tumor microenvironment and on leukemic cells, Rag2−/−γc−/− mice transplanted i.v. with MEC1 cells (10×106) were adoptively transferred (day 11) with either NT T cells or CD23.CAR+ T cells from CLL patient #1, with or without lenalidomide. CD23.CAR T cells from CLL patient #1 have been previously analyzed at transcriptionl level (Fig 2A–H). Mice received 0.214 mg/kg lenalidomide i.p. daily starting at day 8, except for the day of the adoptive transfer (day 11) (Figure 3A). At day 21, reductions of the spleen weights have been detected (Figure 3B), and flow cytometry analysis showed reductions in the numbers of human CD19+ CD23+ MEC1 cells in the SP, PB, and BM of mice treated with CD23.CAR+ T cells, either in absence or presence of lenalidomide (Figures 3C–E). Human CD8+ and CD4+ T cells remained detectable in the lymphoid tissues but CD4+ CD23.CAR+ T cells with memory phenotypes significantly increased in the presence and absence of lenalidomide, when compared to NT controls (Figures 3F–G, S3A–B). An increase of TCM has been previously observed in myeloma patients that received lenalidomide in combination with a pneumococcal vaccine (42). Moreover, in an independent experiment, CAR T lymphocytes remained detectable in the BM of mice that had been given lenalidomide plus CD23.CAR+ T cells generated from CLL patient #2 and were sacrificed at day 30 (Figure S4A–B).
Figure 3. Lymphoid cell compartment characterization in CLL xenotransplanted mice treated with CD23.CAR+ T cells and lenalidomide.
(A-B-C-D-E-F-G) Rag2−/−γc−/− mice transplanted i.v. with MEC1 cells on day 11 of the leukemic challenge were left untreated (Unt, black circles), injected with lenalidomide (Lena) as monotherapy (red rhombi), or adoptively transferred with NT T cells (empty circles), NT T cells with lenalidomide (black triangles), CD23.CAR+ T cells (blue rhombi), CD23.CAR+ T cells with lenalidomide (empty red rhombi). Mice received 0.214 mg/kg of intraperitoneal lenalidomide daily starting at day 8, except for the day of the adoptive transfer. NT and CD23.CAR+ T lymphocytes were obtained from CLL donor #1. At day 23 after the transplantation, mice were evaluated by flow cytometry analysis for the presence of human CD19+ CD23+MEC1 cells in the lymphoid tissues. The graphs show: (B) spleen weight, (C) the mean value (± SD) of the relative contribution of hCD19+ CD23+cells (gated on CD19+ cells) in SP, (D) the mean value (± SD) of the percentage of hCD19+ CD23+cells (gated on CD19+ cells) in PB, (E) the mean value (± SD) of the relative contribution of hCD19+ CD23+cells (gated on CD19+ cells) in BM, (F-G) the mean value (± SD) of the relative contributions of hCD4+ TN, TEM, and TCM in SP and BM.*P < 0.05, **P < 0.01, Student’s t-test.
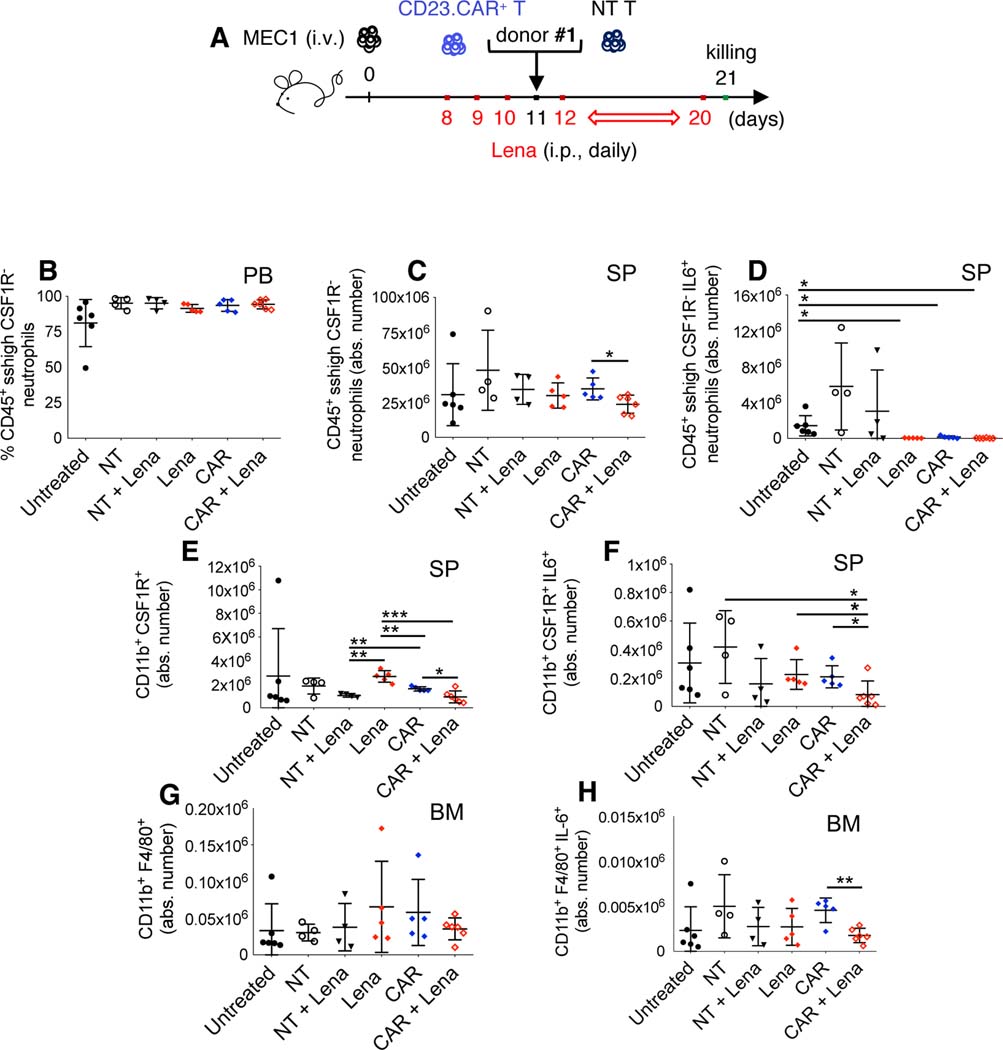
Low-dose lenalidomide did not induce neutropenia nor myelosuppression, but in combination with CD23.CAR+ T cells significantly reduced neutrophils, monocytes, and macrophages producing IL-6 (Figure 4A–H), a cytokine which promotes B-cell proliferation and correlates with poor prognosis in patients with CLL (43–45).
Figure 4. Lenalidomide impacts immune cells of the microenvironment.
(A-B-C-D-E-F-G-H) Rag2−/−γc−/− mice were transplanted with MEC1 cells and treated as described in Figure 4A. NT and CD23.CAR+ T lymphocytes were obtained from CLL donor #1. At day 23 after the transplantation, murine neutrophils, monocytes and macrophages were evaluated by flow cytometry analysis in the PB and lymphoid tissues. The graphs show: (B) the mean value (± SD) of the relative contribution of CD11b+ CSF1R– SSChigh neutrophils gated on CD45+ in PB, (C) the mean value (± SD) of the absolute number of CD11b+ CSF1R– SSChigh neutrophils gated on CD45+ in SP, (D) the mean value (± SD) of the absolute number of CD11b+ CSF1R– SSChigh IL-6+ neutrophils gated on CD45+ in SP, (E) the mean value (± SD) of the absolute number of CD11b+ CSF1R+ monocytes gated on CD45+ in SP, (F) the mean value (± SD) of the absolute number of CD11b+ CSF1R+ IL-6+ monocytes gated on CD45+ in SP, (G) the mean value (± SD) of the absolute number of CD11b+ F4/80+ macrophages gated on CD45+ in BM, (H) the mean value (± SD) of the absolute number of CD11b+ F4/80+ IL-6+ macrophages gated on CD45+ in BM. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test.
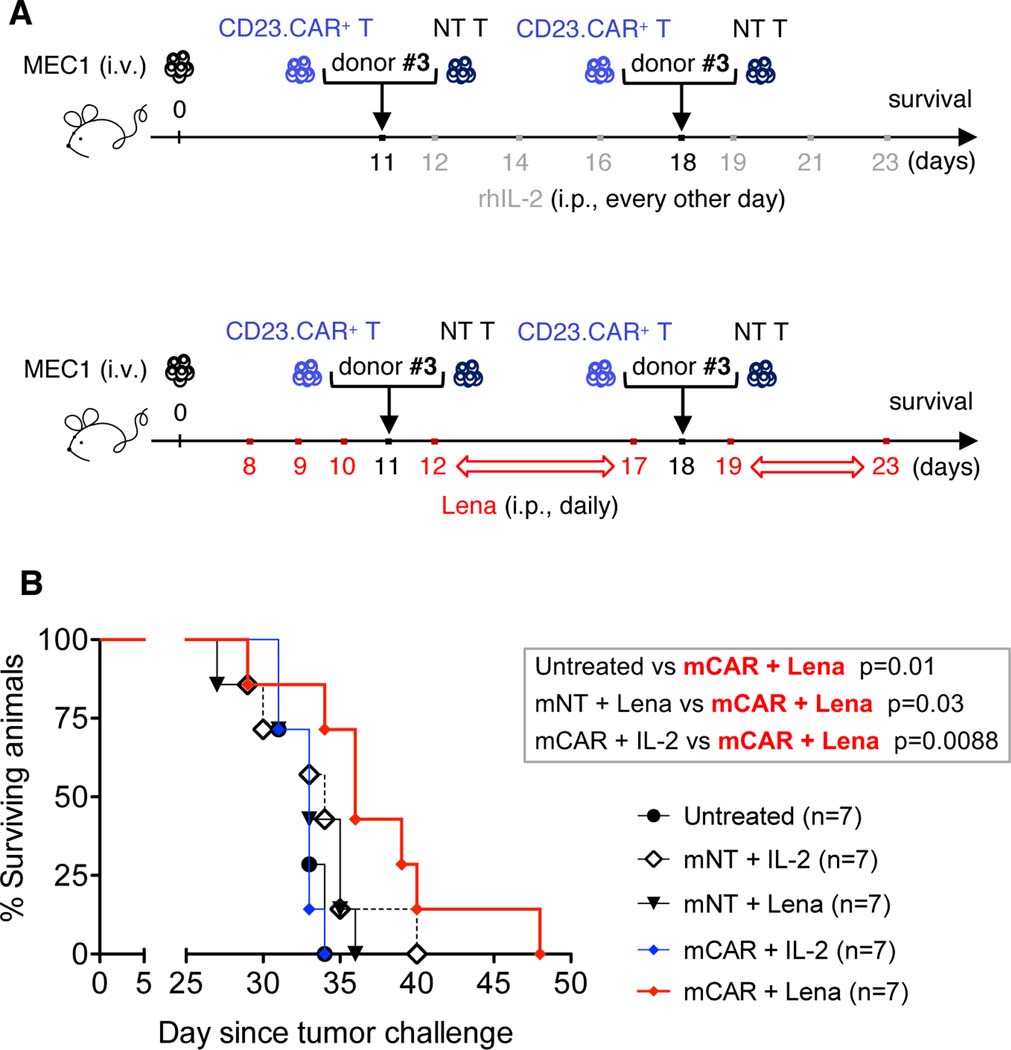
Knowing the delayed effect of immunotherapy and immunomodulatory agents in altering the biology of the microenvironment before inducing a decrease of the tumor burden, we evaluated the additive effect of lenalidomide and CD23.CAR in a survival experiment with an extended follow-up (46, 47).
We injected Rag2−/−γc−/−-engrafted mice with CD23.CAR+ T cells or NT T cells obtained from a different CLL patient (#3). Recombinant human interleukin-2 (rhIL-2) was included in the schedule, as previously described in CAR T cell preclinical studies (48).
Treatment with lenalidomide plus one or two adoptive transfers of CD23.CAR+ T lymphocytes from patient #3 significantly improved the survival of xenotransplanted mice more effectively than did the rhIL-2 combination used in preclinical studies (48) (Figure 5A–B, Figure S5).
Figure 5. Combination therapy with lenalidomide and CD23.CAR T cells impacts the survival of CLL xenotransplanted mice.
(A) Rag2−/−γc−/− mice who received MEC1 cells intravenously on day 0 were left untreated (black circles) or given NT T lymphocytes (days 11 and 18) with rhIL-2 every other day, starting at day 12 (six administrations, empty rhombi); NT T lymphocytes (days 11 and 18) with daily lenalidomide from day 8 (black triangles); CD23.CAR+ T lymphocytes (at days 11 and 18) with rhIL-2 every other day starting at day 12 (six administrations, full blue rhombi); or CD23.CAR+ T lymphocytes (at days 11 and 18) with daily lenalidomide from day 8 (full red rhombi) and monitored for survival. NT and CD23.CAR+ T lymphocytes were from CLL donor #3. mNT and mCAR refers to multiple adoptive transfer (days 11 and 18). (B) Kaplan-Meier survival curve is represented; statistical analysis was performed using Log-Rank test and is indicated in Figure.
Lenalidomide elicits a strong, tumor-specific cytotoxic activity of CD23.CAR+ T lymphocytes in the BM of xenotransplanted mice
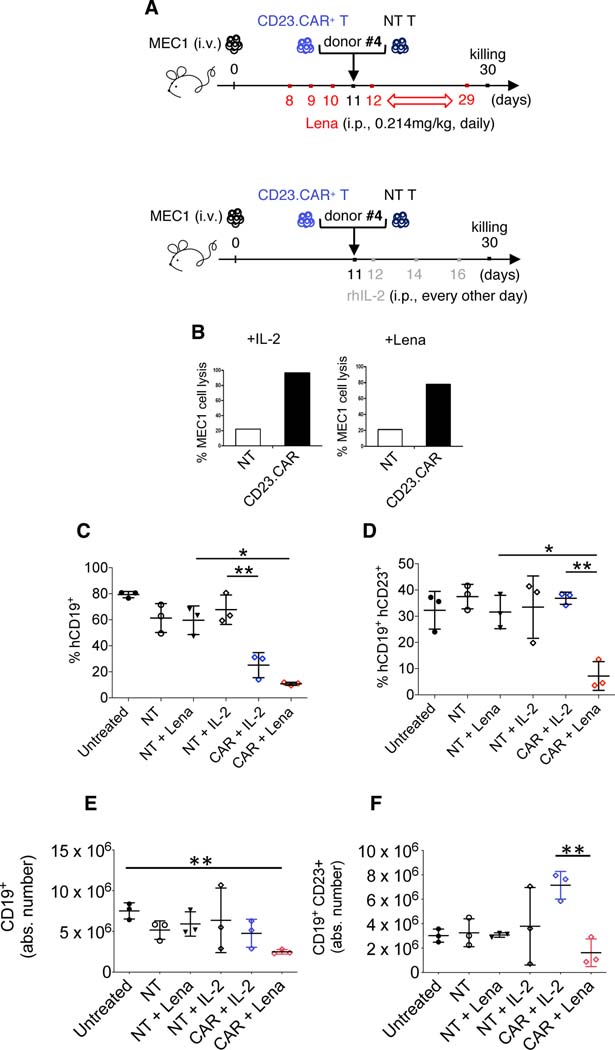
Finally, we investigated whether the therapeutic benefit obtained by combining lenalidomide with CD23.CAR+ T lymphocytes correlated with the induction of a productive tumor-specific immune response to CD23 antigen. To this end, we generated CD23.CAR+ T lymphocytes from the PB of a fourth CLL patient (#4).
Rag2−/−γc−/−-engrafted mice were randomized and adoptively transferred once with CD23.CAR T or NT cells in combination with lenalidomide or rhIL-2 (Figure 6A). After 30 days, we sacrificed the mice, we purified human CD3+ T cells from their BM and tested them in a cytotoxic assay. CD23.CAR+ T lymphocytes exposed to lenalidomide or rhIL-2 in vivo responded again to MEC1 cells in vitro (Figure 6B). Notably, we showed that lenalidomide combined with CD23.CAR T cells contributed to a CD23-specific in vivo anti-leukemic response.
Figure 6. Lenalidomide elicits a tumor-specific cytotoxic activity of CD23.CAR+ T lymphocytes in CLL xenotransplanted mice.
(A-B-C-D) Rag2−/−γc−/− mice who received MEC1 cells intravenously (day 0) were left untreated (black circles) or adoptively transferred with NT T lymphocytes (day 11, white circles), NT T lymphocytes (day 11, black triangles) with daily lenalidomide starting at day 8, NT T lymphocytes (day 11) with rhIL-2 every other day starting at day 12 (six administrations, empty rhombi), CD23.CAR+ T lymphocytes (day 11) with rhIL-2 every other day starting at day 12 (six administrations, blue rhombi), CD23.CAR+ T lymphocytes (day 11) with daily lenalidomide starting at day 8 (red rhombi). NT and CD23.CAR+ T lymphocytes were obtained from CLL donor #4. (B) BM cells were flushed from mice femurs and tibiae; using MACS-microbeads for human CD3 positive selection NT and CD23.CAR+ T cells were isolated via magnetic separation. After 12h of in vitro culture without restimulation, NT (white bar) and CD23.CAR+ (black bar) T cell cytotoxic activity (from IL-2- and lenalidomide-treated mice, respectively) was evaluated against CD23+ MEC1 target, in a 4-hour assay at an E:T ratio of 3:1. The graphs show (C) the mean value (± SD) of the relative contribution of hCD19+ cells in BM, (D) the mean value (± SD) of the relative contribution of hCD19+CD23+ cells in BM, (E) the mean value (± SD) of the absolute number of hCD19+ cells in the BM, and (F) the mean value (± SD) of the absolute number of hCD19+CD23+ cells in the BM. *P < 0.05, **P < 0.01, Student’s t-test.
In conclusion, lenalidomide administration combined with CD23.CAR-T cells from patients #4 reduces disease progression, especially in the BM (Figure 6C–F).
DISCUSSION
In this study, we demonstrated that CD23.CAR+ T cells from patients with CLL exhibit efficient cytotoxic activity in vitro and in vivo preclinical settings. Furthermore, we showed that treatment with low-dose lenalidomide enhances CD23.CAR T-cell immunotherapy by targeting the pro-tumor immune cells in the TME.
Previous preclinical studies demonstrated the feasibility of lenalidomide plus CAR T- cell therapy for the treatment of multiple myeloma (18, 37, 49, 50). Two active clinical studies of patients with multiple myeloma (NCT03070327, NCT03651128) are using lenalidomide and CAR T cells targeting tumor-associated B-cell maturation (BCMA) antigen.
Our study is the first to investigate low-dose lenalidomide plus CD23.CAR T cell therapy for CLL. CD23 represents an attractive antigen in CLL because leukemic B cells typically overexpress CD23 compared to normal B cells (51) (52, 53). The majority of CAR T-cell strategies in CLL target CD19, however several reports on CART-19 therapy documented prolonged B-cell aplasia and tumor escape with loss of CD19 antigen. Furthermore, the features of severe immunosuppression of elderly patients with CLL raises the relevant concern of CD19+ cell-based targeting strategies. Therefore, the search of alternative target antigens is warranted (54, 55). We previously reported that healthy donor T cells engineered to express a CD23-specific CAR display in vitro cytotoxic activity against CD23+ tumor target cells and provide significant control of leukemia growth in vivo when infused into xenotransplanted mice (27). In the current study, we efficiently transduced T lymphocytes from CLL patient samples with CD23.CAR and showed relevant cytotoxic activity against CD23+ MEC1 cells. In short-term in vivo experiments, CD23.CAR+ T cells were able to control the tumor load. Despite their persistence in murine lymphoid tissues, CD23.CAR+ T cells as monotherapy were not able to efficiently improve survival in mice. Similar results were observed in a different CLL xenograft model when CTL019 CAR T-cells from CLL patients were administered as single agent (54). These data are in accordance with the response rates observed in CLL patients on different CAR T-cell platforms: only a minority of patients did respond to CAR T cells, and too few patients experienced complete remission (4, 18). It is conceivable that the limited activity of CAR T-cell therapy may be linked to the well-characterized immunosuppression and dysfunction of both adaptive and innate immune cells in patients with CLL. Along this line, it has been demonstrated that ibrutinib improves T cell function in CLL (56) and enhances CAR T-cell therapy in xenograft studies (54), therefore novel clinical studies combining ibrutinib and CAR T cells have been designed (NCT03960840, NCT02640209, and NCT03331198). Indeed, several patients with CLL are intolerant, fail to respond or develop resistant disease during ibrutinib treatment (57, 58). Recent studies demonstrated that patients with lymphoid malignancies receiving ibrutinib are at risk for serious infections, including lethal invasive fungal infections. Kinase inhibitors affect critical components of the immune system (including myeloid cells and neutrophils), thus increasing the infection burden in treated patients (59, 60) (61).
Therefore, in search of alternative strategies to improve CAR T cell therapy in CLL, we explored the combination of CD23.CAR+ T cells with an immunomodulatory drug targeting both the arms of the immune system such as lenalidomide. We first showed that, in vitro, lenalidomide did not interfere with CLL-derived CAR T-cell function, increased CD23.CAR T-cell cytotoxic activity and cytolytic IFNγ production, as well as CAR T-cell proliferation. It also had a positive effect on CAR T-cell ISs and preserved at transcriptional level the T-cell adhesion/migration machinery. These findings confirm those of previous reports on the activity of lenalidomide on ISs in the T cells of patients with CLL (1), in healthy donor CS1 or CD19 CAR T cells (40) and in BCMA CAR T cells generated from multiple myeloma patient samples (62). At transcriptional level lenalidomide induced regulation of Th1 T-cell response, costimulation and interleukin signaling pathways. We also confirmed the modulation of costimulatory/memory transcripts correlating with complete response of patients with CLL and MM treated with CD19.CAR T cell therapy (e.g. CD27, IL6R and CD62L) (18) (41). However, due to the lack of biological replicates in our study, a donor-dependent effect cannot be fully ruled out.
When we combined CD23.CAR+ T cells and lenalidomide in our Rag2−/−γc−/−-xenotransplanted CLL mouse model, lenalidomide enhanced the anti-leukemic function of CD23.CAR+ T cells and increased mice survival. In accordance with previous in vitro findings demonstrating that lenalidomide alters the CLL myeloid microenvironment (31), we observed a significant reduction in the number of IL-6-producing monocytes and macrophages in mice treated with the combination. It has been demonstrated in CTL019 clinical trials that CD19-directed T cells generated from patients with CLL in complete remission were characterized by IL-6/STAT3-pathway upregulation (18); however the increase of IL-6 plasma levels has been associated with poor prognosis in patients with CLL and its detrimental clinical activity is mediated by the microenvironment (45). Furthermore, recent findings highlight the role of macrophages (33) and monocyte-derived IL-6 in the development of Cytokine Release Syndrome (CRS) and neurotoxicity associated with CAR T-cell therapy (34). For this reason, our observation of a significant reduction in the number of CSF1R– neutrophils producing IL-6 in the spleens of xenotransplanted mice might be key not only to improve the efficacy of the treatment but also to reduce the possibility of CRS. These results are of particular interest because a pro-tumor activity of neutrophils in patients with CLL (63) together with a neutrophil-mediated suppression of T-cell activity in the TME of the lung (64) have been documented.
Overall, we demonstrated that the combination of lenalidomide and CAR T-cell therapy provides multiple effects, including anti-leukemia activity, enhancement of CAR T-cell function, and subversion of the immunosuppressive TME. This strategy may lead to a highly effective immunotherapy not only for patients with CLL but also for other types of cancer patients with immunosuppressive features.
Supplementary Material
ACKNOWLEDGMENTS
Rag2−/−γc−/− mice on BALB/c background were provided by CIEA (Kawasaki, Japan) and Taconic. The authors thank Laura Russell and the Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for reviewing the manuscript and providing constructive comments. Figures were produced using Servier Medical Art (https://smart.servier.com/).
FUNDING
This study was supported by Program Molecular Clinical Oncology-5 per mille numbers 9965 and 9962, Associazione Italiana per la Ricerca sul Cancro (AIRC, Italy), Celgene (to P. Ghia and M.T.S. Bertilaccio), CLL Global Research Foundation (to M.T.S. Bertilaccio); Italian Health Ministry project on CAR T RCR-2019–23669115), NCI P30CA016672 (to the South campus Flow Cytometry and Cell Sorting Core MD Anderson Cancer Center), and IG 2015 “Novel leukemia treatment by the use of chimeric antigen receptors (CARs)” grant 17248. S.T. was supported by a “Quelli che con Luca” fellowship.
Footnotes
DISCLOSURE STATEMENT: E.B. is currently an employee of BMS/Celgene, but with no conflicting interest with the current manuscript. All the remaining authors declare no competing financial interests.
REFERENCES
- 1.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427–37. doi: 10.1172/JCI35017. PubMed PMID: 18551193; PubMed Central PMCID: PMC2423865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galletti G, Caligaris-Cappio F, Bertilaccio MT. B cells and macrophages pursue a common path toward the development and progression of chronic lymphocytic leukemia. Leukemia. 2016;30(12):2293–301. doi: 10.1038/leu.2016.261. PubMed PMID: 27677742. [DOI] [PubMed] [Google Scholar]
- 3.Riches JC, Gribben JG. Immunomodulation and immune reconstitution in chronic lymphocytic leukemia. Semin Hematol. 2014;51(3):228–34. doi: 10.1053/j.seminhematol.2014.05.006. PubMed PMID: 25048786. [DOI] [PubMed] [Google Scholar]
- 4.Bair SM, Porter DL. Accelerating chimeric antigen receptor therapy in chronic lymphocytic leukemia: The development and challenges of chimeric antigen receptor T-cell therapy for chronic lymphocytic leukemia. Am J Hematol. 2019;94(S1):S10–S7. doi: 10.1002/ajh.25457. PubMed PMID: 30861173. [DOI] [PubMed] [Google Scholar]
- 5.Maffei R, Maccaferri M, Arletti L, Fiorcari S, Benatti S, Potenza L, et al. Immunomodulatory effect of ibrutinib: Reducing the barrier against fungal infections. Blood Rev. 2019:100635. Epub 2019/11/09. doi: 10.1016/j.blre.2019.100635. PubMed PMID: 31699465. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee P, Zhang R, Ivan C, Galletti G, Clise-Dwyer K, Barbaglio F, et al. Trabectedin Reveals a Strategy of Immunomodulation in Chronic Lymphocytic Leukemia. Cancer Immunol Res. 2019;7(12):2036–51. Epub 2019/09/19. doi: 10.1158/2326-6066.CIR-19-0152. PubMed PMID: 31530560; PubMed Central PMCID: PMC6891195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilal T, Gea-Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: Linking mechanisms with infections. Blood Rev. 2018;32(5):387–99. Epub 2018/03/25. doi: 10.1016/j.blre.2018.03.004. PubMed PMID: 29571669. [DOI] [PubMed] [Google Scholar]
- 8.Lasica M, Tam CS. Management of Ibrutinib Toxicities: a Practical Guide. Curr Hematol Malig Rep. 2020. Epub 2020/05/18. doi: 10.1007/s11899-020-00576-3. PubMed PMID: 32415406. [DOI] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. PubMed PMID: 21830940; PubMed Central PMCID: PMC3387277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. PubMed PMID: 23527958; PubMed Central PMCID: PMC4058440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheadle EJ, Gornall H, Baldan V, Hanson V, Hawkins RE, Gilham DE. CAR T cells: driving the road from the laboratory to the clinic. Immunol Rev. 2014;257(1):91–106. doi: 10.1111/imr.12126. PubMed PMID: 24329792. [DOI] [PubMed] [Google Scholar]
- 12.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123(17):2625–35. doi: 10.1182/blood-2013-11-492231. PubMed PMID: 7; PubMed Central PMCID: PMC3999751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257(1):107–26. doi: 10.1111/imr.12131. PubMed PMID: 24329793; PubMed Central PMCID: PMC3874724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. PubMed PMID: 24553386; PubMed Central PMCID: PMC4684949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–9. doi: 10.1200/JCO.2014.56.2025. PubMed PMID: 25154820; PubMed Central PMCID: PMC4322257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna BS, McClanahan F, Yazdanparast H, Zaborsky N, Kalter V, Rossner PM, et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2016;30(3):570–9. doi: 10.1038/leu.2015.305. PubMed PMID: 26522085. [DOI] [PubMed] [Google Scholar]
- 17.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. PubMed PMID: 25317870; PubMed Central PMCID: PMC4267531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71. doi: 10.1038/s41591-018-0010-1. PubMed PMID: 29713085; PubMed Central PMCID: PMC6117613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Bruggen JAC, Martens AWJ, Fraietta JA, Hofland T, Tonino SH, Eldering E, et al. Chronic lymphocytic leukemia cells impair mitochondrial fitness in CD8(+) T cells and impede CAR T-cell efficacy. Blood. 2019;134(1):44–58. Epub 2019/05/12. doi: 10.1182/blood.2018885863. PubMed PMID: 31076448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612–21. Epub 2012/12/19. doi: 10.1182/blood-2012-09-457531. PubMed PMID: 23247726; PubMed Central PMCID: PMC3587324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115(7):1797–805. Epub 2005/06/21. doi: 10.1172/JCI24176. PubMed PMID: 15965501; PubMed Central PMCID: PMC1150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol. 2011;29(9):1175–81. doi: 10.1200/JCO.2010.29.8133. PubMed PMID: 21189385; PubMed Central PMCID: PMC4874217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badoux XC, Keating MJ, Wen S, Wierda WG, O’Brien SM, Faderl S, et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013;31(5):584–91. doi: 10.1200/JCO.2012.42.8623. PubMed PMID: 23270003; PubMed Central PMCID: PMC4878047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borgoni S, Iannello A, Cutrupi S, Allavena P, D’Incalci M, Novelli F, et al. Depletion of tumor-associated macrophages switches the epigenetic profile of pancreatic cancer infiltrating T cells and restores their anti-tumor phenotype. Oncoimmunology. 2018;7(2):e1393596. doi: 10.1080/2162402X.2017.1393596. PubMed PMID: 29308326; PubMed Central PMCID: PMC5749621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CI, Paul H, Snitzler S, Kakar S, Le LW, Wei EN, et al. A phase 2 study of lenalidomide and dexamethasone in previously untreated patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma. 2019;60(4):980–9. Epub 2018/10/03. doi: 10.1080/10428194.2018.1508669. PubMed PMID: 30277089. [DOI] [PubMed] [Google Scholar]
- 26.Darwiche W, Gubler B, Marolleau JP, Ghamlouch H. Chronic Lymphocytic Leukemia B-Cell Normal Cellular Counterpart: Clues From a Functional Perspective. Front Immunol. 2018;9:683. Epub 2018/04/20. doi: 10.3389/fimmu.2018.00683. PubMed PMID: 29670635; PubMed Central PMCID: PMC5893869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giordano Attianese GM, Marin V, Hoyos V, Savoldo B, Pizzitola I, Tettamanti S, et al. In vitro and in vivo model of a novel immunotherapy approach for chronic lymphocytic leukemia by anti-CD23 chimeric antigen receptor. Blood. 2011;117(18):4736–45. doi: 10.1182/blood-2010-10-311845. PubMed PMID: 21406718; PubMed Central PMCID: PMC3100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsay AG, Evans R, Kiaii S, Svensson L, Hogg N, Gribben JG. Chronic lymphocytic leukemia cells induce defective LFA-1-directed T-cell motility by altering Rho GTPase signaling that is reversible with lenalidomide. Blood. 2013;121(14):2704–14. Epub 2013/01/18. doi: 10.1182/blood-2012-08-448332. PubMed PMID: 23325833; PubMed Central PMCID: PMC3617635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120(7):1412–21. Epub 2012/05/02. doi: 10.1182/blood-2012-02-411678. PubMed PMID: 22547582; PubMed Central PMCID: PMC3423779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kater AP, Tonino SH, Egle A, Ramsay AG. How does lenalidomide target the chronic lymphocytic leukemia microenvironment? Blood. 2014;124(14):2184–9. doi: 10.1182/blood-2014-05-578286. PubMed PMID: 25161268. [DOI] [PubMed] [Google Scholar]
- 31.Schulz A, Durr C, Zenz T, Dohner H, Stilgenbauer S, Lichter P, et al. Lenalidomide reduces survival of chronic lymphocytic leukemia cells in primary cocultures by altering the myeloid microenvironment. Blood. 2013;121(13):2503–11. Epub 2013/01/26. doi: 10.1182/blood-2012-08-447664. PubMed PMID: 23349394. [DOI] [PubMed] [Google Scholar]
- 32.Fiorcari S, Martinelli S, Bulgarelli J, Audrito V, Zucchini P, Colaci E, et al. Lenalidomide interferes with tumor-promoting properties of nurse-like cells in chronic lymphocytic leukemia. Haematologica. 2015;100(2):253–62. Epub 2014/11/16. doi: 10.3324/haematol.2014.113217. PubMed PMID: 25398834; PubMed Central PMCID: PMC4803140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–8. doi: 10.1038/s41591-018-0041-7. PubMed PMID: 29808005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–48. doi: 10.1038/s41591-018-0036-4. PubMed PMID: 29808007. [DOI] [PubMed] [Google Scholar]
- 35.Bertilaccio MT, Scielzo C, Simonetti G, Ponzoni M, Apollonio B, Fazi C, et al. A novel Rag2−/−gammac−/−-xenograft model of human CLL. Blood. 2010;115(8):1605–9. doi: 10.1182/blood-2009-05-223586. PubMed PMID: 20018917. [DOI] [PubMed] [Google Scholar]
- 36.Arcangeli S, Rotiroti MC, Bardelli M, Simonelli L, Magnani CF, Biondi A, et al. Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia. Mol Ther. 2017;25(8):1933–45. Epub 2017/05/10. doi: 10.1016/j.ymthe.2017.04.017. PubMed PMID: 28479045; PubMed Central PMCID: PMC5542631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain N, Keating M, Thompson P, Ferrajoli A, Burger J, Borthakur G, et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N Engl J Med. 2019;380(22):2095–103. Epub 2019/05/30. doi: 10.1056/NEJMoa1900574. PubMed PMID: 31141631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Totero D, Meazza R, Capaia M, Fabbi M, Azzarone B, Balleari E, et al. The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. Blood. 2008;111(2):517–24. Epub 2007/10/17. doi: 10.1182/blood-2007-04-087882. PubMed PMID: 17938255. [DOI] [PubMed] [Google Scholar]
- 39.De Cecco L, Capaia M, Zupo S, Cutrona G, Matis S, Brizzolara A, et al. Interleukin 21 Controls mRNA and MicroRNA Expression in CD40-Activated Chronic Lymphocytic Leukemia Cells. PLoS One. 2015;10(8):e0134706. Epub 2015/08/26. doi: 10.1371/journal.pone.0134706. PubMed PMID: 26305332; PubMed Central PMCID: PMC4549109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Walter M, Urak R, Weng L, Huynh C, Lim L, et al. Lenalidomide Enhances the Function of CS1 Chimeric Antigen Receptor-Redirected T Cells Against Multiple Myeloma. Clin Cancer Res. 2018;24(1):106–19. Epub 2017/10/25. doi: 10.1158/1078-0432.CCR-17-0344. PubMed PMID: 29061640; PubMed Central PMCID: PMC5991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng Q, Han G, Puebla-Osorio N, Ma MCJ, Strati P, Chasen B, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med. 2020;26(12):1878–87. Epub 2020/10/07. doi: 10.1038/s41591-020-1061-7. PubMed PMID: 33020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noonan K, Rudraraju L, Ferguson A, Emerling A, Pasetti MF, Huff CA, et al. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res. 2012;18(5):1426–34. Epub 2012/01/14. doi: 10.1158/1078-0432.CCR-11-1221. PubMed PMID: 22241792; PubMed Central PMCID: PMC3640365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17(6):395–412. Epub 2018/05/05. doi: 10.1038/nrd.2018.45. PubMed PMID: 29725131. [DOI] [PubMed] [Google Scholar]
- 44.Galletti G, Scielzo C, Barbaglio F, Rodriguez TV, Riba M, Lazarevic D, et al. Targeting Macrophages Sensitizes Chronic Lymphocytic Leukemia to Apoptosis and Inhibits Disease Progression. Cell Rep. 2016;14(7):1748–60. doi: 10.1016/j.celrep.2016.01.042. PubMed PMID: 26876171. [DOI] [PubMed] [Google Scholar]
- 45.Yoon JY, Lafarge S, Dawe D, Lakhi S, Kumar R, Morales C, et al. Association of interleukin-6 and interleukin-8 with poor prognosis in elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(9):1735–42. Epub 2012/04/06. doi: 10.3109/10428194.2012.666662. PubMed PMID: 22475215. [DOI] [PubMed] [Google Scholar]
- 46.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11(11):805–12. Epub 2011/10/25. doi: 10.1038/nrc3153. PubMed PMID: 22020206; PubMed Central PMCID: PMC3426440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dromain C, Beigelman C, Pozzessere C, Duran R, Digklia A. Imaging of tumour response to immunotherapy. Eur Radiol Exp. 2020;4(1):2. Epub 2020/01/05. doi: 10.1186/s41747-019-0134-1. PubMed PMID: 31900689; PubMed Central PMCID: PMC6942076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110(7):2620–30. Epub 2007/05/18. doi: 10.1182/blood-2006-11-059139. PubMed PMID: 17507664; PubMed Central PMCID: PMC1988944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuramitsu S, Ohno M, Ohka F, Shiina S, Yamamichi A, Kato A, et al. Lenalidomide enhances the function of chimeric antigen receptor T cells against the epidermal growth factor receptor variant III by enhancing immune synapses. Cancer Gene Ther. 2015;22(10):487–95. Epub 2015/10/10. doi: 10.1038/cgt.2015.47. PubMed PMID: 26450624. [DOI] [PubMed] [Google Scholar]
- 50.Otahal P, Prukova D, Kral V, Fabry M, Vockova P, Lateckova L, et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology. 2016;5(4):e1115940. Epub 2016/05/04. doi: 10.1080/2162402X.2015.1115940. PubMed PMID: 27141398; PubMed Central PMCID: PMC4839314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–60. Epub 2018/03/16. doi: 10.1182/blood-2017-09-806398. PubMed PMID: 29540348. [DOI] [PubMed] [Google Scholar]
- 52.Fournier S, Tran ID, Suter U, Biron G, Delespesse G, Sarfati M. The in vivo expression of type B CD23 mRNA in B-chronic lymphocytic leukemic cells is associated with an abnormally low CD23 upregulation by IL-4: comparison with their normal cellular counterparts. Leuk Res. 1991;15(7):609–18. Epub 1991/01/01. doi: 10.1016/0145-2126(91)90030-w. PubMed PMID: 1830631. [DOI] [PubMed] [Google Scholar]
- 53.Fournier S, Yang LP, Delespesse G, Rubio M, Biron G, Sarfati M. The two CD23 isoforms display differential regulation in chronic lymphocytic leukaemia. Br J Haematol. 1995;89(2):373–9. Epub 1995/02/01. doi: 10.1111/j.1365-2141.1995.tb03314.x. PubMed PMID: 7873388. [DOI] [PubMed] [Google Scholar]
- 54.Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117–27. Epub 2016/01/28. doi: 10.1182/blood-2015-11-679134. PubMed PMID: 26813675; PubMed Central PMCID: PMC4778162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5(12):1282–95. Epub 2015/11/01. doi: 10.1158/2159-8290.CD-15-1020. PubMed PMID: 26516065; PubMed Central PMCID: PMC4670800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052–64. Epub 2017/07/18. doi: 10.1172/JCI89756. PubMed PMID: 28714866; PubMed Central PMCID: PMC5531425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahn IE, Underbayev C, Albitar A, Herman SE, Tian X, Maric I, et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood. 2017;129(11):1469–79. doi: 10.1182/blood-2016-06-719294. PubMed PMID: 28049639; PubMed Central PMCID: PMC5356450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furman RR, Cheng S, Lu P, Setty M, Perez AR, Guo A, et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med. 2014;370(24):2352–4. doi: 10.1056/NEJMc1402716. PubMed PMID: 24869597; PubMed Central PMCID: PMC4512173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin Infect Dis. 2018;67(5):687–92. Epub 2018/03/07. doi: 10.1093/cid/ciy175. PubMed PMID: 29509845; PubMed Central PMCID: PMC6093991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell. 2017;31(6):833–43 e5. Epub 2017/05/30. doi: 10.1016/j.ccell.2017.04.012. PubMed PMID: 28552327; PubMed Central PMCID: PMC5571650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blez D, Blaize M, Soussain C, Boissonnas A, Meghraoui-Kheddar A, Menezes N, et al. Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica. 2019. Epub 2019/06/07. doi: 10.3324/haematol.2019.219220. PubMed PMID: 31171644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Works M, Soni N, Hauskins C, Sierra C, Baturevych A, Jones JC, et al. Anti-B-cell maturation antigen chimeric antigen receptor T cell function against multiple myeloma is enhanced in the presence of lenalidomide. Mol Cancer Ther. 2019. Epub 2019/08/10. doi: 10.1158/1535-7163.MCT-18-1146. PubMed PMID: 31395689. [DOI] [PubMed] [Google Scholar]
- 63.Podaza E, Sabbione F, Risnik D, Borge M, Almejun MB, Colado A, et al. Neutrophils from chronic lymphocytic leukemia patients exhibit an increased capacity to release extracellular traps (NETs). Cancer Immunol Immunother. 2017;66(1):77–89. Epub 2016/11/01. doi: 10.1007/s00262-016-1921-7. PubMed PMID: 27796477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res. 2016;76(5):999–1008. Epub 2016/02/03. doi: 10.1158/0008-5472.CAN-15-1439. PubMed PMID: 26833127; PubMed Central PMCID: PMC4775354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.