Abstract
Shigellae infect human intestine and cause intense inflammation and destruction of colonic and rectal mucosa. To model the interactions of shigella with human intestine in vivo, we have studied shigella infection in human intestinal xenografts in severe combined immunodeficient mice (SCID-HU-INT mice). Inoculation of shigella into human intestinal xenografts caused severe inflammation and mucosal damage, which was apparent as soon as 4 h following infection. Shigella infection was associated with human intestinal production of interleukin-1B (IL-1B) and IL-8 and a marked neutrophil influx into the graft. Depletion of neutrophils from SCID-HU-INT mice reduced inflammation in the human intestinal xenograft in response to shigella infection but failed to significantly alter tissue damage. However, the number of intracellular bacteria was more than 20-fold higher in the human intestinal xenografts from neutrophil-depleted SCID-HU-INT mice. Infection of human intestinal xenografts with an attenuated vaccine strain of shigella (CVD1203) induced lower levels of IL-1B and IL-8 than wild-type shigella and caused only moderate damage to the intestinal permeability barrier. Our studies establish the SCID-HU-INT mouse as a viable model for studying the interactions between shigella and human intestine and indicate that neutrophils are important for controlling the invasion of human intestine by shigella.
Shigella spp. cause bacillary dysentery by invading into human intestinal epithelial cells and stimulating a strong inflammatory reaction. Neutrophils are the predominant inflammatory cell seen in shigellosis and have been implicated in the pathogenesis of disease (8). Interleukin-8 (IL-8) produced by intestinal epithelial cells plays a key role in attracting neutrophils to intestinal tissue in a rabbit intestinal loop model of disease (14). Neutrophils may contribute to the tissue damage and diarrhea seen in shigella infection by their migration across the epithelial border and by the release of mediators. In vitro studies using intestinally derived cell lines and studies in the rabbit loop model of infection indicate that the migration of neutrophils across the epithelial border facilitates the invasion of the basolateral surface of intestinal epithelial cells by shigella (9, 10). However, recent studies in the rabbit model, as well as in vitro studies, suggest that neutrophils may also play a key role in controlling infection (5, 14).
Shigella spp. naturally cause diarrhea only in humans and nonhuman primates, and the ability to study the interactions between shigella and human intestine in vivo could potentially provide new insights into this disease. Here, we describe the successful establishment of Shigella flexneri infection in human intestinal xenografts in severe combined immunodeficient mice (SCID-HU-INT mice). We have used this model to demonstrate that shigellae rapidly induce human intestinal production of IL-1B and IL-8 in vivo and that this cytokine response is associated with a marked inflammatory response and tissue damage in the human intestine. Importantly, we find that neutrophils, which constitute the predominant cell in this inflammatory response, play a key role in controlling bacterial invasion into intestinal cells.
MATERIALS AND METHODS
Bacteria.
S. flexneri WT2457T and S. flexneri CVD1203 ΔaroA ΔvirG (provided by F. Noriega, Center for Vaccine Development, University of Maryland School of Medicine) were grown on tryptic soy broth (TSB) with aeration for 24 h prior to inoculation (6, 7). Escherichia coli laboratory strain DH5α was grown for 24 h in Luria-Bertani broth prior to inoculation.
SCID-HU-INT mice.
Human intestinal xenografts were placed into the subscapular region of 6-to 8-week-old SCID mice as previously described (17). Incisions were closed with Michel clips, and grafts were allowed to develop for at least 8 weeks before use.
Neutrophil depletion.
SCID mice received an intraperitoneal injection of 150 μg of monoclonal antibody RB6-8C5 24 h prior to infection and immediately before shigella infection (21). Control animals received the same dosage of the isotype-matched monoclonal antibody 148-D41 (18). Neutrophil depletion was measured by counting neutrophils in peripheral blood using fluorescence-activated cell sorter analysis as previously described (20).
Infection of human intestinal xenografts.
Bacteria grown for 24 h were centrifuged, washed once with saline, and resuspended in 1 ml of saline. Between 3 × 107 and 5 × 107 bacteria in a volume of 100 μl were injected directly into the lumen of the intestinal xenograft.
RT-PCR assay for human IL-1B and IL-8 transcripts.
For reverse transcriptase-mediated PCR (RT-PCR), 100 mg of human intestinal tissue was suspended in 1 ml of TRIZOL reagent (GibcoBRL, Gaithersburg, Md.) and then homogenized for 15 s with a Polytron. Samples underwent phase separation using chloroform, the aqueous layer was removed, and the RNA was precipitated with isopropyl alcohol. Following centrifugation at 12,000 × g for 15 min, the RNA pellet was washed in 70% ethanol, dried, resuspended in diethyl pyrocarbonate-treated water, and quantified by measuring absorbance at 260 nm. cDNA was synthesized from 2 μg of total RNA using 0.5 μg of oligo(dT) primer, 50 mM dithiothreitol, 10 μM each deoxynucleostide triphosphate, and 200 U of RNase H Moloney murine leukemia virus reverse transcriptase (GibcoBRL) in a final volume of 20 μl at 37°C for 1 h. PCR (using cDNA equivalent to 0.2 μg of starting RNA) was performed in a 100 μl volume containing 0.5 μM of the appropriate sense and antisense oligonucleotide primers, 200 mM each deoxynucleoside triphosphate, 5% dimethyl sulfoxide, 1 U of Taq polymerase (Boehringer Mannheim, Indianapolis, Ind.), and the supplied 10× buffer. PCR was performed using a program of 35 cycles of denaturing at 95°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1.5 min. Then 20 μl of the PCR product was subjected to electrophoresis in a 1.5% agarose gel and stained with ethidium bromide for visualization. Primers that specifically amplify transcripts for human actin, human IL-1β, and IL-8 in RT-PCRs have been previously described (17).
Cytokine analysis and MPO assay.
For cytokine analysis, tissue sections were homogenized at 50 mg/ml in phosphate-buffered saline (PBS) containing 1 μg each of leupeptin, aprotinin, and peptstatin A (Sigma Chemical Co., St. Louis, Mo.) per ml. Homogenized samples were centrifuged at 12,000 × g for 15 min, and supernatants were collected and processed for enzyme-linked immunosorbent assay (ELISA) to detect human IL-1B and IL-8 as instructed by the manufacturer (Endogen, Woburn, Mass.). For myeloperoxidase (MPO) assays, tissue was homogenized at 5 mg/ml in the same solution and spun at 12,000 × g, and the pellet was collected. The pellet was resuspended in the same volume of 80 mmol sodium phosphate per liter–1% hexadecyltrimethylammonium bromide (Sigma)–5 mmol of EDTA per liter (pH 5.4). Samples underwent three freeze-thaw cycles and were centrifuged at 2,000 × g for 15 min, and the supernatants were frozen until ready for analysis. A 25-μl aliquot of supernatant was mixed with 125 μl of 80 mmol of sodium phosphate per liter (pH 5.4) and 25 μl of 1.28 mmol of 3,3′,5,5′-tetramethylbenzidine dihydrochloride per liter in dimethyl sulfoxide (all from Sigma) as substrate. Twenty-five microliters of H2O2 in 80 mmol of sodium phosphate per liter was added immediately before analysis to yield a final concentration of 0.24 mmol/liter in a reaction volume of 200 μl. Conversion of the substrate was measured at 650 nm, and dilutions of known concentrations of purified MPO (Sigma) were used as standards to calculate MPO concentrations in the samples.
Measurement of intestinal permeability.
Fifty microliters of a 2.5-mg/ml solution of fluorescein isothiocyanate (FITC)-dextran (Sigma) was inoculated directly into the lumen of the human intestinal xenograft at the time of shigella or control infection. Under anesthesia, SCID mice were anesthesized, and the renal pedicle was tied off to prevent excretion of the fluorophore. At 0, 2, and 4 h following infection, animals were bled, and 20 μl of blood was diluted into 400 μl of 150 mmol of NaCl per liter–50 mmol of Tris per liter (pH 10.3) and centrifuged at 2,000 × g for 15 min. The supernatants were read on a Cytofluor 23000 fluorescent plate reader, with known dilutions of FITC-dextran used as standards.
Measurement of intracellular bacteria.
Human intestinal xenografts were treated with gentamicin (50 μg/ml) in 0.1 M PBS for 1 h, divided into 0.5- by 0.5-cm sections, washed two times with the gentamicin solution, and then incubated for an additional hour in fresh gentamicin-PBS (10). Following three additional washes in cold 0.1 M PBS, tissue sections were suspended in 1 ml of ice-cold PBS, homogenized, diluted 1/10 in TSB, and incubated at 37°C for 30 min. Samples were then serially diluted with TSB and plated overnight. The number of colonies obtained was counted, and data expressed as CFU per cubic centimeter.
RESULTS
Shigella infect and damage human intestinal xenografts.
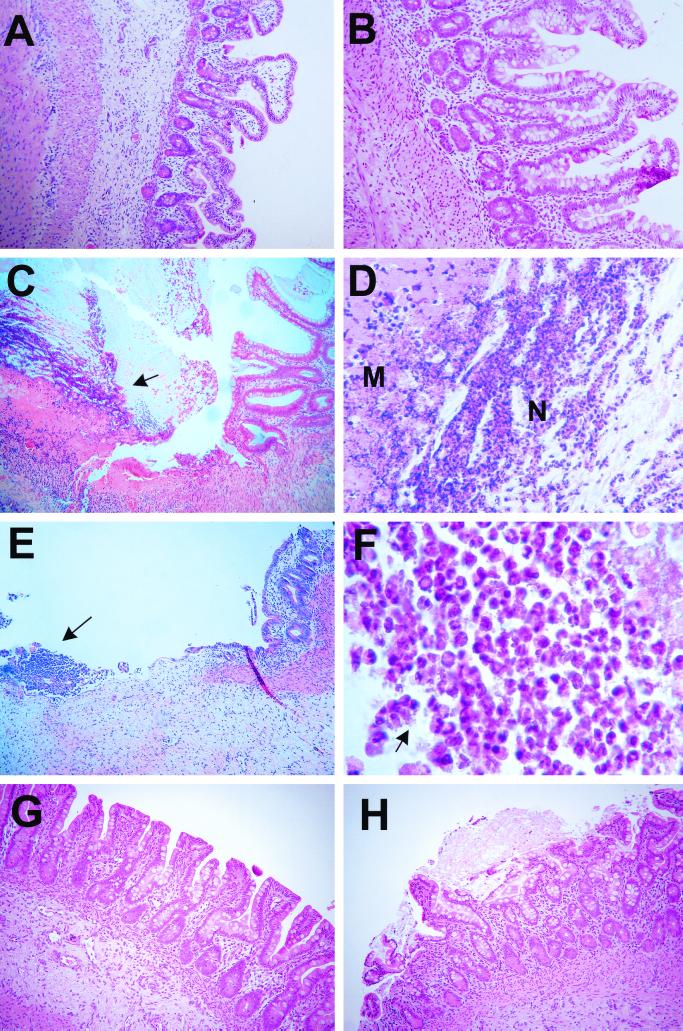
S. flexneri WT2457T or CVD1203 was directly inoculated into the lumen of human intestinal xenografts, while control xenografts received E. coli DH5α. Representative hematoxylin-and-eosin (H&E)-stained sections of uninfected human intestinal xenografts (Fig. 1A) and intestinal xenografts infected with E. coli DH5α (Fig. 1B) displayed normal villous and crypt architecture, lacked any inflammatory infiltrate, and showed no evidence for any mucosal damage. In contrast, inoculation of S. flexneri WT2457T into the human intestinal xenograft caused marked mucosal damage, with disruption of the mucosa, ulcer formation, and a marked neutrophil infiltrate into the lumen, mucosal, and submucosal layers, which was readily apparent by 8 h following bacterial inoculation (Fig. 1C and D). Complete loss of mucosa and frank ulceration with microabscess formation were apparent in other human intestinal xenografts 8 h after shigella inoculation (Fig. 1E). Neutrophils formed the predominant cells in the ulcers, and extracellular bacteria could be seen (Fig. 1F). Despite this extensive mucosal damage, no bacteremia with shigella was detected in any SCID-HU-INT mice at time points extending to 24 h following infection. Human intestinal xenografts infected with the attenuated S. flexneri CVD1203 strain showed a range of pathology, from relatively normal appearing mucosa (Fig. 1G) to more marked mucosal damage including mucosal disruption with epithelial sloughing and excess mucin production (Fig. 1H), but did not exhibit the widespread mucosal destruction and ulceration seen with wild-type shigella infection.
FIG. 1.
Shigella infection of human intestinal xenografts. (A) H&E stain of a section of uninfected human intestinal xenograft 8 h following medium inoculation. No signs of villous or crypt destruction, cellular infiltration, or mucosal hemorrhage are seen. Magnification, ×86. (B) H&E stain of a section of human intestinal xenograft 8 h following inoculation with E. coli DH5α. No signs of villous or crypt destruction, cellular infiltration, or mucosal hemorrhage are seen. Magnification, ×86. (C) H&E stain of section of human intestinal xenograft infected 8 h with S. flexneri WT2457T. A region of normal mucosa can be seen on the right side of the field adjacent to a massive region of mucosal destruction. A cellular infiltrate of neutrophils and inflammatory cells (arrow) covered by fibrinous material sits over an acellular region of destroyed mucosa overlying an area with extensive cellular infiltration. Magnification, ×86. (D) Magnified view of the ulcer in panel C with the luminal neutrophil infiltrate (N), overlying the acellular hyaline-appearing region containing the remnants of the mucosal tissue (M). Magnification, ×172. (E) H&E stain of a section of a second human intestinal xenograft infected for 8 h with S. flexneri WT2457T. Loss of mucosal tissue, with an ulcer and microabscess (arrow) containing abundant polymorphonuclear cells is seen. Magnification, ×86. (F) Magnified view of the microabscess seen in panel E. Abundant neutrophils are present, and extracellular bacteria (arrow and throughout) can be seen adjacent to the polymorphonuclear cells. Magnification, ×860. (G) H&E stain of a section of a human intestinal xenograft infected for 8 h with S. flexneri CVD1203. No gross mucosal disruption or increased cellularity was seen. Magnification, ×86. (H) H&E stain of a section of a different human intestinal xenograft infected for 8 h with S. flexneri CVD1203. Disruption of the mucosa is present, with some epithelial cell slough. Excess mucin production is seen, with a mucinous plug overlying some of the damaged area. Magnification, ×86.
Introduction of shigella into human intestinal xenografts causes intestinal epithelial cells to produce human IL-1B and IL-8.
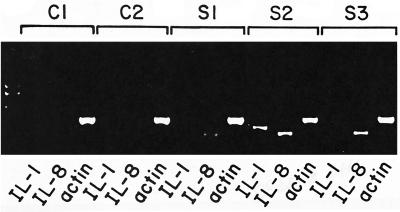
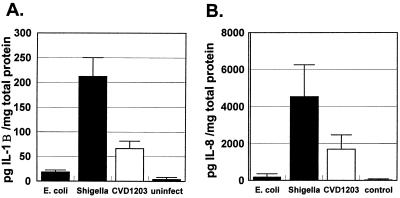
We found that human intestinal xenografts infected with S. flexneri WT2457T rapidly produced both human IL-1B and IL-8. Transcripts for human IL-1B and IL-8 mRNA were detected by RT-PCR as soon as 1 h following human intestinal infection with shigella (Fig. 2). No message for either IL-1B or IL-8 was detected in human intestinal xenografts 1 h following intestinal infection with E. coli DH5α. Human IL-1B and IL-8 could be detected by ELISA of human intestine as early as 4 h after shigella inoculation into the intestinal xenografts (Fig. 3). In contrast, only low levels of human IL-1B and IL-8 were seen in human intestinal xenografts infected with E. coli DH5α. Infection with S. flexneri CVD1203 also induced IL-1B and IL-8 in human intestinal xenografts. The values were significantly lower for IL-1B in CVD1203-infected human intestinal xenografts compared with those seen with wild-type S. flexneri WT2457T infection, but the difference in mean levels for IL-8 did not reach statistical significance.
FIG. 2.
mRNA transcripts for human IL-1B and IL-8 are induced in human intestinal xenografts 1 h following shigella infection of the xenograft. Results of RT-PCR assay for human IL-1B, IL-8, and actin from samples of two human intestinal xenografts infected with E. coli DH5α (C1 and C2) and three different human intestinal xenografts infected with S. flexneri (S1 to S3) are shown. Transcripts of the predicted size for human IL-1B are visible in S1 and S2 and seen faintly in S3. Transcripts of the predicted size for human IL-8 are visible in all three shigella-infected human intestinal xenografts. The equivalent intensity of the human actin control suggests that equivalent quantities of cDNA were present in the samples. The far-left lane contains the DNA size standards.
FIG. 3.
S. flexneri infection induces human intestinal xenografts to produce IL-1B and IL-8. (A) Mean levels of human IL-1B found in lysates of human intestinal xenografts 4 h following infection with E. coli DH5α (n = 7), S. flexneri WT2457T (n = 8) or S. flexneri CVD1203 (n = 5) or in uninfected intestinal xenografts (n = 7). Error bars show standard errors of the means. The difference between the mean IL-1B levels of lysates obtained from S. flexneri WT2457T-infected human intestinal xenografts or S. flexneri CVD1203-infected intestinal xenografts against either E. coli-infected or uninfected human intestinal xenografts was significant at P < 0.01 (two-tailed t test). The difference between the mean IL-1B levels of lysates obtained from S. flexneri WT2457T-infected human intestinal xenografts and S. flexneri CVD1203-infected intestinal xenografts was significant at P < 0.05 (two-tailed t test). (B) Mean levels of human IL-8 found in lysates of human intestinal xenografts 4 h following infection with E. coli DH5α (n = 7), S. flexneri WT2457T (n = 8) or S. flexneri CVD1203 (n = 5) or in uninfected intestinal xenografts (n = 7). Error bars show standard errors of the means. The difference between the mean IL-8 levels of lysates obtained from S. flexneri WT2457- or S. flexneri CVD1203-infected human intestinal xenografts and either E. coli-infected or uninfected human intestinal xenografts was significant at P < 0.01 (two-tailed t test). The difference between the mean IL-8 levels of lysates obtained from S. flexneri WT2457-infected and S. flexneri CVD1203-infected human intestinal xenografts was not significant (P = 0.2, two-tailed t test).
Neutrophils migrate into human intestine in response to shigella infection.
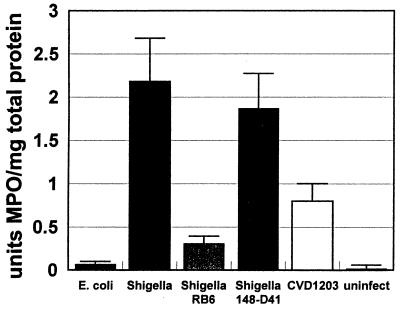
A marked inflammatory response could be seen in human intestinal xenografts infected with S. flexneri WT2457T (Fig. 1C to F). To quantify this response, we measured MPO levels in human intestines infected with S. flexneri or E. coli DH5α. MPO is produced almost exclusively by neutrophils and some monocyte populations, and its presence in tissue can be used to measure neutrophil influx. Human intestinal xenografts infected with S. flexneri WT2457T showed a marked elevation in MPO activity as soon as 4 h after the inoculation of shigella into the intestine (Fig. 4). Human intestinal xenografts infected with S. flexneri CVD1203 also displayed an elevation in MPO activity, but to an extent significantly lower than that seen with wild-type S. flexneri. No significant elevation in MPO activity was seen in human intestinal xenografts infected with E. coli DH5α.
FIG. 4.
S. flexneri infection causes neutrophil influx into human intestinal xenografts, with resultant elevation of intestinal tissue MPO levels. Mean levels of MPO were assayed in human intestinal xenografts infected with E. coli DH5α (E. coli; n = 7), S. flexneri WT2457 (Shigella; n = 8), S. flexneri WT2457T in SCID-HU-INT mice treated with monoclonal antibody RB6-8C5 for neutrophil depletion (Shigella RB6; n = 7), S. flexneri WT2457 in SCID-HU-INT mice treated with monoclonal antibody 148-D41 (Shigella 148-D41; n = 7), and S. flexneri CVD1203 (CVD1203; n = 5) and in uninfected human intestinal xenografts (n = 7). MPO levels in S. flexneri WT2457- or S. flexneri CVD1203-infected human intestinal xenografts were significantly higher (P < 0.05) than those detected in E. coli DH5α-infected or uninfected human intestinal xenografts. MPO levels in shigella-infected human intestinal xenografts obtained from SCID-HU-INT mice treated with RB6-8C5 were significantly lower than those seen in SCID-HU-INT mice treated with monoclonal antibody 148-D41 (P < 0.05). MPO levels in S. flexneri WT2457T-infected xenografts were significantly higher (P < 0.05) than those seen in S. flexneri CVD1203-infected human intestinal xenografts.
Shigella infection rapidly damages the intestinal permeability barrier in human intestinal xenografts.
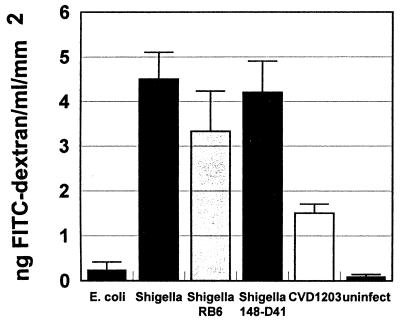
Human intestinal xenografts normally possess a permeability barrier to the flow of macromolecules from the lumen to the circulation of the SCID-HU-INT mouse. Infection and inflammation can damage that barrier, allowing macromolecules such as FITC-dextran to reach the systemic circulation (18). This correlates with histologic evidence for mucosal damage. We found that within 4 h of S. flexneri WT2457T inoculation into human intestinal xenografts, intestinal permeability to FITC-dextran was markedly increased, with FITC-dextran detectable in sera of SCID-HU-INT mice (Fig. 5). There was also an increase in intestinal permeability seen after S. flexneri CVD1203 infection, but this was significantly lower than that seen with wild-type shigella. E. coli DH5α did not damage the intestinal permeability barrier in human intestinal xenografts.
FIG. 5.
Infection with shigella increases human intestinal permeability to FITC-dextran at 4 h following infection. Mean serum levels of FITC-dextran were obtained from SCID-HU-INT mice with human intestinal xenografts infected with E. coli DH5α, (E. coli; n = 7), S. flexneri WT2457T (Shigella; n = 11), S. flexneri WT2457T in SCID-HU-INT mice treated with monoclonal antibody RB6-8C5 for neutrophil depletion (Shigella RB6; n = 7), S. flexneri WT2457T in SCID-HU-INT mice treated with monoclonal antibody 148-D41 (Shigella 148-D41; n = 7), and S. flexneri CVD1203 (CVD1203; n = 5) and uninfected human intestinal xenografts (n = 7). Serum levels of FITC-dextran were significantly higher (P < 0.01) in SCID-HU-INT mice with S. flexneri WT2457T-infected or S. flexneri CVD1203-infected human intestinal xenografts compared to E. coli DH5α-infected xenografts and uninfected human intestinal xenografts. The difference between the mean serum levels of FITC-dextran in S. flexneri WT2457T-infected and S. flexneri CVD1203-infected SCID-HU-INT mice was significant at P < 0.02. Serum levels of FITC-dextran were also significantly higher (P < 0.01) in RB6-8C5-treated SCID-HU-INT mice with intestinal xenografts infected with S. flexneri WT2457T or 148-D41-treated SCID-HU-INT mice with S. flexneri WT2457T-infected human intestinal xenografts than in SCID-HU-INT mice with E. coli DH-5α-infected or uninfected human intestinal xenografts. The difference between the mean serum levels of FITC-dextran in RB6-8C5-treated SCID-HU-INT mice with shigella-infected intestinal xenografts infected with or 148-D41-treated SCID-HU-INT mice with shigella-infected human intestinal xenografts was not significant (P = 0.18).
Effect of neutrophil depletion on gut inflammation and tissue damage in shigella infection of human intestine.
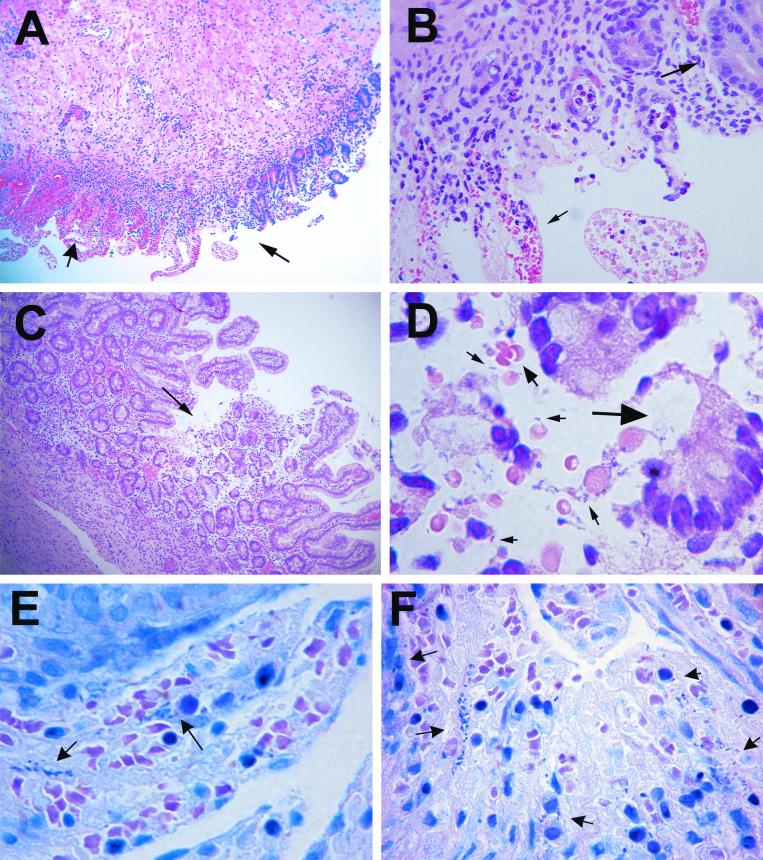
To study the function of neutrophils in the host response to intestinal infection with wild-type shigella, we depleted SCID-HU-INT mice of neutrophils with monoclonal antibody RB6-8C5 prior to S. flexneri WT2457T infection of the human intestinal xenograft. Control SCID-HU-INT mice were treated with the isotype-matched control monoclonal antibody 148-D41 prior to S. flexneri WT2457T challenge of the human intestinal xenograft. Significant damage to the intestinal mucosa could be seen in human intestinal xenografts from neutrophil-depleted SCID-HU-INT mice at 8 h following inoculation, but this was associated with a reduced inflammatory response compared with intestinal xenografts from control SCID-HU-INT mice (Fig. 6A). Marked mucosal hemorrhage (Fig. 6A and B) with disruption of the villi was prominent in many of these xenografts. Even in areas with more focal mucosal damage (Fig. 6C), mucosal hemorrhage was present, and extracellular bacteria could be seen in association with regions of mucosal hemorrhage and damage (Fig. 6D). On Giemsa-stained sections, bacteria were visible both within cells and in extracellular locations in human intestinal xenografts from both control SCID-HU-INT mice (Fig. 6E) and neutrophil-depleted SCID-HU-INT mice (Fig. 6F). As would be expected, the neutrophil-depleted SCID-HU-INT mice showed significantly lower MPO levels in S. flexneri WT2457T-infected human intestinal xenografts than in S. flexneri WT2457T-infected human intestinal xenografts from control SCID-HU-INT mice (Fig. 4). Infection with S. flexneri WT2457T caused increased permeability to FITC-dextran in human intestinal xenografts at 4 h after infection in both neutrophil-depleted and control SCID-HU-INT mice (Fig. 5). There was a greater increase in intestinal permeability in human intestinal xenografts infected with shigella in control SCID mice than in human intestinal xenografts from neutrophil-depleted SCID-HU-INT mice, but the difference was not statistically significant between the two groups.
FIG. 6.
(A) H&E stain of a section of human intestinal xenograft obtained from a neutrophil-depleted SCID-HU-INT mouse 8 h following S. flexneri WT2457T inoculation. A region with some preserved mucosal architecture (right of the thin arrow) is adjacent to a region with disruption of both villi and crypts and with massive mucosal hemorrhage (wider arrow). Magnification, ×80. (B) Magnified view of the section in panel A, showing retention of some normal mucosal structures (long arrow) adjacent to areas that show epithelial cell loss, disruption of mucosal architecture, and marked mucosal hemorrhage (small arrow). Note the absence of a marked inflammatory cell infiltration in this region. Magnification, ×360. (C) H&E stain of a second human intestinal xenograft obtained from a neutrophil-depleted SCID-HU-INT mouse 8 h following S. flexneri WT2457T inoculation. A focal area of mucosal destruction (arrow) is seen, without marked neutrophil infiltration. Magnification, ×80. (D) Magnified view of the region seen in panel C. There is epithelial cell loss (large arrow) and hemorrhage (medium arrow), and extracellular bacteria are visible (small arrows). Magnification, ×800. (E) Giemsa stain of a section from a human intestinal xenograft 8 h following infection with S. flexneri WT2457T. Mucosal destruction and hemorrhage are present, and invading bacteria (arrows) are visible. Magnification, ×800. (F) Giemsa stain of a section from a human intestinal xenograft from a neutrophil-depleted SCID-HU-INT mouse 8 h following infection with S. flexneri WT2457T. Mucosal destruction and hemorrhage are present, and invading bacteria (arrows) are visible. Magnification, ×800.
Neutrophils control shigella invasion into intestinal cells.
To look at the role of neutrophils in limiting the spread of wild-type shigella infection, we measured intracellular bacteria concentrations in S. flexneri WT2457T-infected human intestinal xenografts from neutrophil-depleted and control SCID-HU-INT mice. We found that 4 h after shigella inoculation, intestinal xenografts obtained from neutrophil-depleted SCID-HU-INT mice (n = 6) contained 1.03 × 106 ± 5 × 105 CFU/cm2, compared to 4.93 × 104 ± 2.92 × 104 CFU/cm2 in shigella-infected human intestinal xenografts from control SCID-HU-INT mice (n = 7). The difference between the values was highly significant (P < 0.001). In separate studies, we also measured intracellular bacteria concentrations in CVD1203-infected human intestinal xenografts (n = 5) and found mean levels of 3.2 × 103 ± 8 × 102 CFU/cm2, a value significantly different (P < 0.01) from that obtained for S. flexneri WT2457T infection.
DISCUSSION
Animal models for pathogens that normally infect only humans may fail to mimic key aspects of the infectious process (16). We approached this problem by using SCID-HU-INT mice, an established model for enteric infections, to study the interactions between shigella and human intestine (1, 3, 17–19). We found that direct inoculation of wild-type S. flexneri WT2457T into the human intestinal xenografts of SCID-HU-INT mice resulted in infection of human intestinal epithelial cells and a marked intestinal inflammatory response. This inflammation was not due simply to the introduction of gram-negative bacteria into the gut, as a nonpathogenic, noninvasive E. coli strain failed to induce a significant inflammatory response. The ability of shigella to establish infection in the human intestinal xenograft was not necessarily predictable, as studies have indicated that shigellae initiate human intestinal infection by entering into the specialized M cells overlying Peyer's patches (12, 22). The human intestinal xenografts lack both Peyer's patches and M cells (15). It is possible that a high bacterial inoculum and the lack of peristalsis and movement of intestinal contents in the graft may make it especially susceptible to shigella infection, but our findings indicate that shigellae do not require entry through Peyer's patches to establish infection in this system.
Inflammatory mediators, which can be produced by intestinal epithelial cells, play a crucial role in initiating and regulating host inflammatory responses to enteric pathogens (2). Both the potent neutrophil chemoattractant IL-8 and the pleiotropic inflammatory cytokine IL-1B have been implicated in the pathogenesis of shigellosis. Experiments in the rabbit loop model have shown that IL-8 is crucial in attracting neutrophils to the intestinal mucosa (14), and IL-8 can be detected in intestinal epithelial cells in rectal biopsies from humans with shigellosis (11). IL-1 is produced in response to shigella infection in animal models and in humans, and blockade of IL-1 through the action of the IL-1 receptor antagonist inhibits tissue damage and inflammation in experimental models of shigella (11, 13). Mononuclear inflammatory cells serve as sources of IL-1 in shigella-infected intestine, but a clear role for intestinal epithelial cell-produced IL-1 has not been shown (11). We found that human intestinal xenografts infected with shigella produced both human IL-1B and IL-8. Transcripts for the mRNA of each cytokine could be detected by primers designed to amplify only the human message (as opposed to the murine mRNA) 1 h following luminal inoculation of shigella. The protein products were detected by ELISAs that specifically recognize human IL-1B and IL-8. The SCID-HU-INT mouse is a chimera, with the human intestinal xenograft serving as the sole source for human cytokines. Thus, our data indicate that intestinally derived cells are a potential source for both IL-8 and IL-1B in shigella-infected human intestine. Whether intestinal cell production of IL-1B is physiologically important in the pathogenesis of shigella infection was not addressed by these studies.
A striking finding from this model of shigellosis was the rapidity of the inflammatory response and associated mucosal injury. Ulceration of the intestinal mucosa, as well as regions of intestine with complete loss of mucosal tissue, were predominant findings in human intestinal xenografts following 8 h of shigella infection. The histologic findings were confirmed by studies of the intestinal permeability barrier, which demonstrated that shigella-infected human intestinal xenografts lost their permeability barrier within 4 h of infection. The speed of this response can be compared to those seen in Entamoeba histolytica infection of human intestinal xenografts, where significant changes in the permeability barrier were not seen until 24 h following amoebic inoculation (18). Whether this finding reflects a dosage phenomenon or differences in the virulence or pathogenetic mechanisms between the two major causes of dysentery is unclear.
Neutrophils are the predominant component of the host immune/inflammatory response in patients with bacterial dysentery caused by shigella. Shigella infection in human intestinal xenografts was associated with a marked influx of neutrophils into the mucosa and lumen of the human intestine, detectable both histologically and by the measurement of MPO levels in shigella-infected human xenografts. The role of neutrophils in the pathogenesis of shigella infection is complex. Mediators released by neutrophils may contribute to diarrhea and to tissue damage (4). Neutrophils facilitate the invasion of the basilar surface of intestinal epithelial cell lines by shigella in vitro, and studies in the rabbit loop model of shigella infection demonstrated that blocking IL-8, a potent chemoattractant and activator of neutophils, could reduce damage to the intestinal mucosa (9, 14). However, neutrophils effectively kill shigellae in vitro, and the inhibition of IL-8 in the rabbit loop model of disease was associated with greater dissemination of shigella infection (5, 14).
We found that neutrophil depletion of SCID-HU-INT mice did not obviously exacerbate or ameliorate disease in shigella-infected human intestinal xenografts. Reduced numbers of inflammatory cells were measured by the MPO assay in infected human intestinal xenografts from neutrophil-depleted mice, but mucosal hemorrhage and loss of mucosal tissue were still prominent in those grafts. While there was a trend toward less extensive damage to the intestinal permeability barrier in shigella-infected human intestinal xenografts from neutrophil-depleted SCID-HU-INT mice, it did not reach statistical significance. One possible explanation for a failure to detect a clear role for neutrophils in exacerbating or ameliorating intestinal tissue damage from shigellosis could be the fact that neutrophil depletion is not complete in this system, as about 2% of the neutrophil population remains after antibody depletion. However, this seems unlikely, as in similar experiments, E. histolytica-infected human intestinal xenografts from neutrophil-depleted SCID-HU-INT mice showed significantly less damage to the intestinal permeability barrier, implicating neutrophils, and the inflammatory response, in the tissue damage seen early in intestinal amebiasis (18). In the case of shigella infection, the benefits of reducing the amount of inflammatory damage to intestinal tissue by neutrophil depletion may be outweighed by increased dissemination of shigellae within the human intestinal xenograft in neutrophil-depleted SCID-HU-INT mice.
We detected a significant difference in the number of shigellae found within intestinal cells between human intestinal xenografts from neutrophil-depleted or control SCID-HU-INT mice. The quantity of intracellular bacteria was more than 20-fold higher in shigella-infected human intestinal xenografts from neutrophil-depleted SCID-HU-INT mice. These data provide direct evidence that neutrophils play a critical role in containing and controlling the spread of shigella within human intestine. Our findings provide further support for the concept that the host inflammatory response to shigella contributes to tissue damage but is necessary for the control of bacterial spread (14).
We extended our studies by looking at human intestinal xenograft infection with an attenuated vaccine strain of shigella, CVD1203, which is derived from S. flexneri WT2457T and contains selective deletions of the chromosomal gene aroA and the plasmid gene virG (icsA). This strain can invade into epithelial cells but undergoes minimal intracellular proliferation and cell-to-cell spread (6). CVD1203 exhibited reduced virulence in the Serenyi test and markedly reduced reactogenicity in human volunteers compared to the parent strain (6, 7). However, at high oral doses (108 to 109 CFU), it induced fever, diarrhea, or dysentery in 18% (108 dosage) or 72% (109 dosage) of recipients (7). We found at 4 h following infection that intracellular concentrations of CVD1203 were about 10-fold lower than those seen with wild-type shigella and that CVD1203 was significantly less virulent than the parent strain in most measured parameters—induction of IL-1B, neutrophil influx, and intestinal permeability. S. flexneri CVD1203 did cause induction of IL-8 and IL-1B, and small increases in MPO and intestinal permeability that were significantly greater than those seen with infection by the noninvasive E. coli strain, but CVD1203 infection did not cause the widespread mucosal destruction seen with the parent wild-type S. flexneri WT2457T. The induction of IL-1B and IL-8 by CVD1203 was not unexpected because of its ability to invade into epithelial cells and is consistent with the finding of cytokine production in human volunteers receiving the CVD1203 vaccine (6, 7). Our findings suggest the SCID-HU-INT model may be useful for assessing the virulence of attenuated shigellae. It is possible that more dramatic differences in virulence would have been seen if lower doses of S. flexneri WT2457T and CVD1203 had been compared in this study.
In summary, our studies establish the SCID-HU-INT mouse as a viable model for studying the interactions between shigella and human intestine. We found that shigellae can invade human intestinal cells in the absence of Peyer's patches, induce inflammatory cytokine production from intestinal cells, and cause severe damage to the intestinal mucosa. Neutrophils, and their products, may contribute to the tissue damage but are important for controlling the dissemination of shigellae.
ACKNOWLEDGMENTS
We thank F. Noriega for providing the Shigella strains used in this study. We extend special thanks to Paul Swanson for reviewing the photomicrographs presented in this study.
This work was supported by NIH grant A130084 to S.L.S., NIH training grant 5T32AI-07172 to K.B.S. NIH grant DK52574 for the Washington University Digestive Diseases Research Core Center, and NIH grant HD 00836 to the Birth Defects Research Laboratory at the University of Washington, Seattle. S.L.S. is a Burroughs Wellcome Scholar in Molecular Parasitology.
REFERENCES
- 1.Huang G T-J, Eckmann L, Savidge T C, Kagnoff M F. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM-1) expression and neutrophil adhesion. J Clin Investig. 1996;98:572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Investig. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent F, Eckmann L, Savidge T C, Morgan G, Theodos C, Naciri M, Kagnoff M F. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madara J L, Parkos C A, Colgan S P, MacLeod R J, Nash S, Matthews J, Delp C, Lencer W. Cl- secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J Clin Investig. 1992;89:1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandic-Mulec I, Weiss J, Zychlinsky A. Shigella flexneri is trapped in polymorphonuclear leukocyte vacuoles and efficiently killed. Infect Immun. 1997;65:110–115. doi: 10.1128/iai.65.1.110-115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noriega F R, Wang J Y, Losonsky G, Maneval D R, Hone D M, Levine M M. Construction and characterization of attenuated ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203, a prototype live oral vaccine. Infect Immun. 1994;62:5168–5172. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotloff K L, Noriega F, Losonsky G, Sztein M B, Wasserman S S, Nataro J P, Levine M M. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect Immun. 1996;64:4542–4548. doi: 10.1128/iai.64.11.4542-4548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsot C, Sansonetti P J. Invasion and the pathogenesis of Shigella infections. Curr Top Microbiol Immunol. 1996;209:25–42. doi: 10.1007/978-3-642-85216-9_2. [DOI] [PubMed] [Google Scholar]
- 9.Perdomo J J, Gounon P, Sansonetti P J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Investig. 1994;93:633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perdomo O J, Cavaillon J M, Huerre M, Ohayon H, Gounon P, Sansonetti P J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raqib R, Lindberg A A, Wretlind B, Bardhan P K, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sansonetti P J, Arondel J, Cantey J R, Prevost M C, Huerre M. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sansonetti P J, Arondel J, Cavaillon J M, Huerre M. Role of interleukin-1 in the pathogenesis of experimental shigellosis. J Clin Investig. 1995;96:884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sansonetti P J, Arondel J, Huerre M, Harada A, Matsushima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun. 1999;67:1471–1480. doi: 10.1128/iai.67.3.1471-1480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savidge T C, Morey A L, Ferguson D J, Fleming K A, Shmakov A N, Phillips A D. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation. 1995;58:361–371. doi: 10.1046/j.1432-0436.1995.5850361.x. [DOI] [PubMed] [Google Scholar]
- 16.Scaramuzzino D A, McNiff J M, Bessen D E. Humanized In vivo model for streptococcal impetigo. Infect Immun. 2000;68:2880–2887. doi: 10.1128/iai.68.5.2880-2887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seydel K B, Li E, Swanson P E, Stanley S L., Jr Human intestinal epithelial cells produce pro-inflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seydel K B, Li E, Zhang Z, Stanley S L., Jr Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology. 1998;115:1446–1453. doi: 10.1016/s0016-5085(98)70023-x. [DOI] [PubMed] [Google Scholar]
- 19.Seydel K B, Zhang T, Champion G A, Fichtenbaum C, Swanson P E, Tzipori S, Griffiths J K, Stanley S L., Jr Cryptosporidium parvum infection of human intestinal xenografts in SCID mice induces production of human tumor necrosis factor alpha and interleukin-8. Infect Immun. 1998;66:2379–2382. doi: 10.1128/iai.66.5.2379-2382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seydel K B, Zhang T, Stanley S L., Jr Neutrophils play a critical role in early resistance to amebic liver abscesses in SCID mice. Infect Immun. 1997;65:3951–3953. doi: 10.1128/iai.65.9.3951-3953.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tepper R I, Coffman R L, Leder P. An eosinophil-dependent mechanism for the anti-tumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 22.Wassef J S, Keren D F, Mailloux J L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989;57:858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]