Abstract
Background
Cancer‐associated venous thromboembolism (CAT) has detrimental impact on patients' clinical outcomes and quality of life. Data on CAT education, communication, and awareness among the general cancer population are scanty.
Methods
We present the preliminary results of an ongoing patient‐centered survey including 27 items covering major spheres of CAT. The survey, available in 14 languages, was promoted and disseminated online through social networks, email newsletters, websites, and media.
Results
As of September 20, 2022, 749 participants from 27 countries completed the survey. Overall, 61.8% (n = 460) of responders were not aware of their risk of CAT. Among those who received information on CAT, 26.2% (n = 56) were informed only at the time of CAT diagnosis. Over two thirds (69.1%, n = 501) of participants received no education on signs and symptoms of venous thromboembolism (VTE); among those who were educated about the possible clinical manifestations, 58.9% (n = 119) were given instructions to seek consultation in case of VTE suspicion. Two hundred twenty‐four respondents (30.9%) had a chance to discuss the potential use of primary thromboprophylaxis with health‐care providers. Just over half (58.7%, n = 309) were unaware of the risks of bleeding associated with anticoagulation, despite being involved in anticoagulant‐related discussions or exposed to anticoagulants. Most responders (85%, n = 612) valued receiving CAT education as highly relevant; however, 51.7% (n = 375) expressed concerns about insufficient time spent and clarity of education received.
Conclusions
This ongoing survey involving cancer patients with diverse ethnic, cultural, and geographical backgrounds highlights important patient knowledge gaps. These findings warrant urgent interventions to improve education and awareness, and reduce CAT burden.
Keywords: anticoagulants, neoplasms, patient outcome assessment, patient positioning, surveys and questionnaires, venous thromboembolism
Essentials.
Data on cancer‐associated venous thromboembolism (CAT) awareness among cancer patients are sparse.
An ongoing qualitative study is exploring patients' understanding and CAT education worldwide.
There is an urgent need to implement CAT education and communication programs globally.
Identification of patients' needs, barriers, and inequalities across different health‐care systems shall inform patient‐centered interventions to reduce CAT burden.
1. INTRODUCTION
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication in patients with cancer, who have an estimated 12‐ to 23‐fold increased risk to develop VTE compared to individuals without cancer. 1 Cancer‐associated venous thromboembolism (CAT) is expected to become more frequent due to improved cancer survival, which ultimately results in a larger and older cancer population that is longer exposed to CAT‐associated risk factors such as use of chemotherapy and immunotherapy. 2 Besides the substantial morbidity and mortality, CAT may also lead to delayed access to or withdrawal of cancer treatments, prolonged or repeated hospitalizations, thwarting optimal cancer management, and accounting for significant psychosocial distress for patients and their caregivers, reduced quality of life, and high health‐care system costs. In addition, CAT management is particularly challenging, as it is associated with relatively high risk of recurrent VTE and anticoagulation‐related bleeding. 3
Awareness, prevention, and prompt recognition of CAT are therefore fundamental to reduce its burden worldwide. This can be achieved through adequate CAT knowledge education and effective communication provided by cancer care professionals to cancer patients and their caregivers. In 2020, the American Society of Clinical Oncology (ASCO) emphasized the need for oncology team members to educate patients about CAT, highlighting once more the centrality of patient education and engagement around CAT during a cancer journey. 4 Despite its high incidence, detrimental impact on patient outcomes, and guidelines and recommendations, CAT might be underestimated, which may translate into limited efforts or effectiveness when informing and educating patients about CAT. 4 , 5 , 6 As a consequence, poor patient awareness, inadequate application of preventive measures, delayed recognition of CAT, and uncertainties and difficulties in seeking or receiving help from specialists may eventually result in missed opportunities to reduce CAT burden. While multiple studies have evaluated patient experience after being diagnosed with CAT, limited data exist on CAT education and awareness among the general cancer population. 7 , 8 , 9 , 10
We therefore launched the first global CAT survey to: (i) provide a snapshot of CAT education and awareness among a large, comprehensive, multiethnic, and multicultural population of individuals with cancer; (ii) explore the contents, sources, and timing of CAT education, and its psychological impact; and (iii) estimate patients' knowledge and perceptions about anticoagulation in CAT. On the occasion of the 2022 World Thrombosis Day, we report on the preliminary findings of this global patient‐centered initiative.
2. MATERIALS AND METHODS
The survey comprised 27 items dealing with common aspects of CAT as previously identified through discussions among CAT health‐care professionals including specialized physicians and nurses, patient representatives, education and communication experts, as well as industry stakeholders and policy makers. 11 The survey was designed in a simple structure, lay language, and ideally devoid of potential cultural‐ or language‐related barriers to allow wide dissemination, participation, and inclusiveness. A patient representative reviewed the survey draft to ensure comprehensibility and adequateness of the content and form of the survey.
The survey was then translated into 14 languages by native speaker experts and made available on browser‐based Research Electronic Data Capture (REDCap) software (Vanderbilt University) hosted on International Society on Thrombosis and Haemostasis servers. Survey promotion and dissemination occurred primarily online, through social networks, email newsletters, websites, and media addressed to cancer patients and their caregivers. The present study was considered exempt from ethics committee review as responders choose to complete an anonymous online survey, whose completion implies informed consent. The survey was launched on June 10, 2022, and will be available at https://redcap.isth.org/surveys/?s=APAPWWEWRA for approximately 6 months. Briefly, the survey explores: knowledge and awareness of CAT including type, source, and timing of education received by individuals with malignancy (either prior or active); awareness of the risk, predisposing factors, and possible clinical manifestations of CAT; patients' general knowledge and attitude toward anticoagulation and their engagement by health‐care providers in discussions and decisions regarding thromboprophylaxis options, anticoagulation management, and periodic reassessment of anticoagulant therapy in relation to thrombotic and bleeding risk. A section on cancer site, stage, treatments, eventual prior CAT diagnosis, and anticoagulant use and indication is included. Age, sex, ethnicity, education, and country of residence are also collected. We herein present the preliminary data as of September 20, 2022.
3. RESULTS AND DISCUSSION
Overall, 745 participants completed the survey of whom 68% (n = 509) identified as women, and 75.3% (n = 564) were aged over 50 years. Self‐reported ethnicity was White in 38% (n = 258), followed by Hispanic or Latino, Asian, and Black or African American in 23.3%, 16.3%, and 10.2%, respectively. Participants responded from 27 countries or territories located in Europe (32.7%, n = 208), Latin America (26.2%), Asia (17%), Middle East (12.5%), and Africa (10.2%). Of those surveyed, 33.8% (n = 250) were university graduates, while 16.7% received no or primary education only. The most frequent primary cancer sites were breast (24.8%, n = 196), hematopoietic and lymphoid tissues (16.1%), lung (14.4%), gastrointestinal (11.8%), and gynecological (9.6%). Approximately one third (36.9%, n = 267) of respondents reported having metastatic cancer, while 29.9% (n = 217) considered their disease stable and 20.2% progressive or recurrent. More than three quarters of respondents (81.1%, n = 603) were receiving or had received cancer treatments, with the most frequent being chemotherapy (68.3%, n = 446), surgery (29.7%), and radiotherapy (28.1%). Table 1 summarizes main patient characteristics.
TABLE 1.
Main patient characteristics
| Item (total N. of responses) | % (N) |
|---|---|
| Sex (749) | |
| Women | 68.0 (509) |
| Men | 32.0 (240) |
| Age (749) | |
| <50 years | 24.7 (185) |
| ≥50 years | 75.3 (564) |
| Ethnicity (679) | |
| Asian | 16.3 (111) |
| Black or African American | 10.2 (69) |
| Hispanic or Latino | 23.3 (158) |
| White | 38.0 (258) |
| Other | 12.2 (83) |
| Region of residence (637) | |
| Africa | 10.0 (64) |
| Europe | 32.7 (208) |
| Middle East | 13.2 (84) |
| Latin America | 26.2 (167) |
| Asia | 17.0 (108) |
| Educational status (740) | |
| Primary education | 11.4 (84) |
| Secondary education | 25.1 (186) |
| University degree | 33.8 (250) |
| Postgraduate degree or master's | 15.9 (118) |
| None | 5.3 (39) |
| Primary cancer site(s) (791) | |
| Breast | 24.8 (196) |
| Hematopoietic and lymphoid | 16.1 (127) |
| Gastrointestinal | 11.8 (93) |
| Genitourinary | 8.2 (65) |
| Gynecological | 9.6 (76) |
| Lung | 14.4 (114) |
| Metastatic cancer (723) | |
| Yes | 36.9 (267) |
| No | 49.7 (359) |
| Uncertain | 13.4 (97) |
| Cancer status (738) | |
| Cured | 26.3 (194) |
| Recently diagnosed | 17.6 (130) |
| Progressive or recurrent | 20.2 (149) |
| Stable | 29.4 (219) |
| Uncertain | 6.2 (46) |
| Cancer treatment (744) | |
| Ongoing | 51.9 (386) |
| Prior | 29.2 (217) |
| Scheduled | 6.5 (48) |
| None | 12.4 (93) |
| Types of cancer treatments (647) | |
| Anti‐hormonal agents | 16.2 (105) |
| Chemotherapy | 68.3 (446) |
| Immunotherapy | 10.2 (66) |
| Central catheter | 26.4 (171) |
| Radiotherapy | 28.1 (182) |
| Surgery | 29.7 (192) |
| Other | 12.5 (81) |
| Personal history of VTE (731) | |
| Yes | 23.9 (175) |
| Lower‐extremity DVT | 11.1 (81) |
| PE | 6.0 (44) |
| DVT in other sites | 5.3 (39) |
| SVT | 1.5 (11) |
| No | 70.5 (515) |
| Uncertain | 5.6 (41) |
| Prior or current anticoagulant exposure (721) | |
| Yes | 49.7 (358) |
| VTE treatment | 26.1 (188) |
| Other indication | 23.6 (170) |
| No | 50.3 (363) |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; PICC, peripherally inserted central catheter; SVT, superficial vein thrombosis; VTE, venous thromboembolism.
3.1. Education received about CAT, timing, and sources
Overall, 61.8% (n = 460) of cancer patients included in the survey stated they never received education regarding CAT and were not aware of the thrombotic risk associated with cancer and anti‐cancer treatments (Figure 1A). Among those who received information, 26% (n = 56) were informed only at the time of VTE diagnosis (Figure 1B). Oncologists (32.7%, n = 102), hematologists (23%), other specialty physicians (12.4%), surgeons (9.5%), and nurses (9.5%) were the most common sources of CAT education (Figure 1C). CAT understanding was rated as insufficient (≤5 on a 1–10 scale) by 30.4% (n = 66) of responders who received CAT education (median, 7; interquartile range, 5–9).
FIGURE 1.
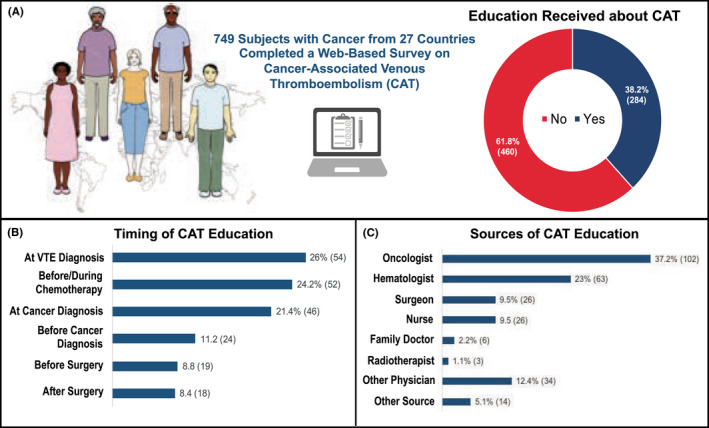
Education received about CAT (A), timing (B), and sources (C). CAT, cancer‐associated venous thromboembolism; VTE, venous thromboembolism.
3.2. Knowledge of CAT risk factors and clinical manifestations
Overall, 69.1% (n = 501) of the cancer patients surveyed were not provided any information regarding signs and symptoms of VTE (Figure 2A). Among those who were educated about possible VTE manifestations, only 58.9% (n = 119) received instructions on what to do or who to contact if one of those occurred (Figure 2B). Among a list of traditional risk factors for CAT, reduced level of physical activity (41.5%), previous VTE (25.6%), recent surgery (25.4%), and chemotherapy (21.4%) were those most frequently acknowledged, whereas 19.4% of responders could not identify any (Figure 2C).
FIGURE 2.
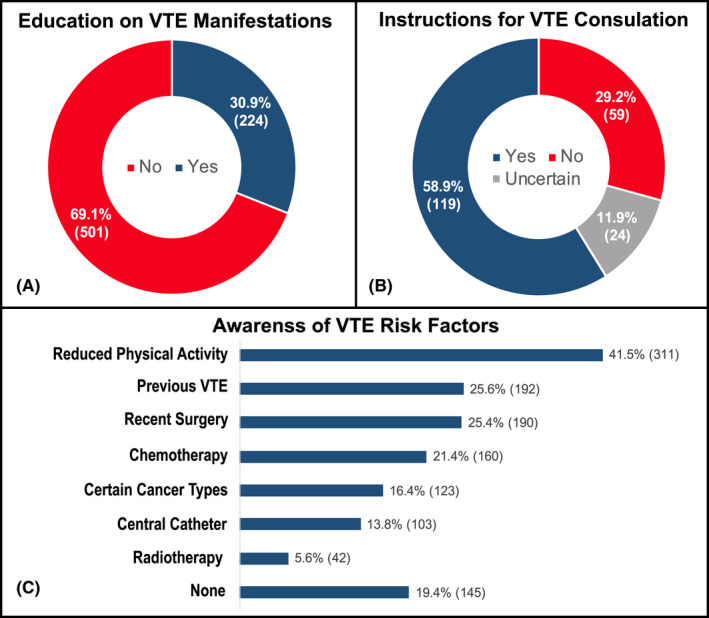
Education received on the signs and symptoms of VTE (A), instructions to seek clinical consultation or support (B), and awareness of VTE risk factors (C). VTE, venous thromboembolism.
3.3. CAT and patient involvement in anticoagulation‐related decisions
The majority (70.5%, n = 515) of participants were never diagnosed with VTE, whereas 11.1%, 6%, and 6.8% reported having experienced lower‐extremity DVT, PE, and DVT in other sites or superficial vein thrombosis, respectively (Table 1). VTE was diagnosed during chemotherapy in 31.1% (n = 52) of responders, at cancer diagnosis in 16.2%, and before cancer diagnosis in 23.4%. Approximately one quarter (26.1%, n = 188) of participants reported prior or current use of anticoagulants for the treatment of VTE, and 23.6% for indications other than VTE. Among the cancer patients who have been exposed to anticoagulants for any indication, the need and risks of anticoagulant therapy were not periodically reassessed in 56% (n = 234), while this was done approximately every 3–6 months in 39.5% of responders.
Overall, 69.1% (n = 502) of responders were never involved in a discussion with any of their treating physicians regarding the possibility of receivibg primary thromboprophylaxis during their cancer journey. Among those who were informed about the possibility of thromboprophylaxis and those who used anticoagulants for any indication, 58.6% (n = 309) indicated that they were not informed about the potential risk for bleeding complications associated with anticoagulant therapy.
3.4. Patients' experience with CAT education
The vast majority of cancer patients surveyed (85%, n = 612) considered CAT education absolutely essential or very important (Figure 3A). However, 51.7% (n = 375) of them stated that their health‐care providers did not spend sufficient time or were not clear enough in educating them about CAT (Figure 3B). Less than one quarter (22%, n = 118) of responders felt tense, anxious, depressed, or frustrated after receiving education about the risk of CAT and anticoagulation (Figure 3C).
FIGURE 3.
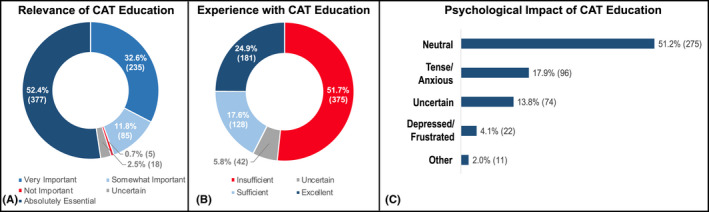
Relevance of CAT education through the cancer journey (A), patients' experience with CAT education (B), and its psychological impact (C). CAT, cancer‐associated venous thromboembolism.
In this survey that included 749 cancer patients with diverse ethnic, cultural, and geographical backgrounds, almost two in three were not aware of the thrombotic risk associated with cancer and anti‐cancer therapies, with a large proportion of the remainder becoming aware at the time of VTE diagnosis only. More than two thirds of responders were not educated to recognize the signs and symptoms of VTE. Among those who were educated about possible VTE manifestations, more than 40% were not provided with basic instructions for seeking support or clinical consultation. These findings, although preliminary, provide a contemporary snapshot of the contents and quality of patients' education and awareness regarding CAT at a global scale. In agreement with another large survey conducted in 2018 by the European Cancer Patient Coalition across six countries in Europe, they also confirm and extend to non‐European health‐care systems an urgent need for a quantitative and qualitative implementation of CAT educational efforts, awareness, and communication programs worldwide. 11 These shall encompass multiple structured interventions that actively and cooperatively engage patients and their caregiver as well as cancer patients' associations, cancer care professionals, scientific societies, industry, and policy makers. The validity of a mixed‐methods, patient‐centered strategy for CAT education has been suggested by a Welsh experience in which an information video was developed and delivered to patients receiving systemic anti‐cancer therapy leading to shorter mean time to presentation with VTE symptoms (from 8.9 to 2.9 days), possibly reflecting greater CAT awareness resulting in earlier recognition and clinical consultation. 12
Less than one third of the cancer patients surveyed were engaged in discussions with their physicians regarding primary thromboprophylaxis, currently recommended by international guidelines in high‐risk subgroups. 4 , 5 , 6 , 13 While a large proportion of responders might have been at low or intermediate risk for VTE and thus not eligible for prophylactic anticoagulation at the time of survey completion, informing patients with general notions about anticoagulation and other strategies to abate CAT risk should be an integral part of CAT education and might remedy, at least in part, some of the patients' concerns regarding CAT. Almost one in four participants had a history of VTE, and about half of the overall population included reported anticoagulant use during their lifetime. Despite a relatively high exposure to anticoagulants in the surveyed population, the majority of responders were unaware of the potential risk for bleeding complications, suggesting that greater efforts should be made to educate patients about the risks and benefits associated with these medications, which might increase patients' engagement and compliance and contribute to reduce adverse events.
CAT education was valued as highly relevant by most cancer patients surveyed. Nevertheless, more than half of them believed that the time spent by their health‐care providers in educating them about CAT and the clarity of CAT education and communication were insufficient. Health‐care professionals should therefore be aware that cancer patients may require additional education regarding CAT, and that this information may generate psychological distress in patients and their caregivers. Failure to adequately intercept and fulfill these needs may result in greater distress, misunderstanding, and reduced compliance.
Altogether, the data herein presented may also underlie an increasing need to build or implement (when already in place) thrombo‐oncology care pathways, shared by oncology and thrombosis specialists, patients, and their caregivers, encompassing CAT education and communication programs; routine assessment of CAT risk; standardized algorithms for the preventive, diagnostic, and therapeutic management of CAT; as well as adequate psychological support.
The present findings should, however, be interpreted cautiously because the population surveyed so far, although relatively large and diverse, might not be fully representative of the general cancer population, and responders could have been potentially subject to recall bias. In addition, due to the browser‐based nature of the survey, subjects with limited access to or familiarity with online platforms and digital devices could have been underrepresented.
Once completed, this ongoing survey can contribute to identifying unmet gaps in CAT education and communication, and inform on the needs, barriers, and inequalities across different health‐care systems, which might be used for tailoring and prioritizing patient‐centered interventions to reduce the burden of CAT worldwide.
AUTHOR CONTRIBUTIONS
Study conception and design: NP, SB, MDN; data acquisition and interpretation: all authors; drafting of the manuscript: NP, SB, MDN; critical revision of the manuscript for important intellectual content: all authors; final approval of the manuscript: all authors.
FUNDING INFORMATION
There was no funding source for this study.
CONFLICTS OF INTEREST
NP received a training fellowship from the International Society on Thrombosis and Haemostasis. SB received consultancy and speaker fees from Bayer, Concept Medical, Boston Scientific, and Inari, outside of the submitted work. IM reports research funding from Leo Pharma and BMS‐Pfizer outside of the submitted work, and advisory board/honoraria from Bayer, BMS Pfizer, Leo, and Sanofi. GCM reports personal fees from Bayer and BMS‐Pfizer outside the submitted work. AL reports research funding from Pfizer, Bayer, Sanofi, Leo Pharma, and Novartis outside the submitted work. HCO received a training fellowship from the International Society on Thrombosis and Haemostasis. EO reports funding from Imara outside the submitted work, and Pfizer advisory board/honoraria. CA reports personal fees from Bayer, BMS/Pfizer, Daiichi‐Sankyo, and Sanofi, outside the submitted work. MC reports research funding from BMS, Pfizer, and Leo Pharma outside of the submitted work, and advisory board/honoraria from Leo Pharma, Bayer, Sanofi, BMS, Servier, and Valeo. JMC has received personal fees for scientific advisory boards and consulting from Abbott, Anthos, Alnylam, Bristol Myers Squibb, Five Prime Therapeutics, Pfizer, Takeda, and research funding from CSL Behring, outside of the submitted work. LHL reports speaker, advisory board/honoraria from Pfizer, Bayer, and Sanofi. FNA reports grant funding (IIS paid to university) from Daiichi Sankyo, Leo Pharma, Sanofi, and Actelion, and consultancy for Boston Scientific. GG reports lecture/consultant fees from Bayer HealthCare, Pfizer, and LeoPharma. SG reports advisory fee from Jansen, ANTOS, and AMGEN outside of the submitted work, and quality fee from the American Heart Association as the Editor for Circulation. SG is a Steering Committee Member for Clinical Trials sponsored by Boehringer Ingelheim. MCGE reports support for attending meetings from Nolver Uruguay, outside the submitted work. LJP reports personal fees from Bayer Hispania, Actelion, Rovi, Pfizer, Menarini, and Leo Pharma, outside the submitted work. NVE has received, outside of the submitted work, advisory board honoraria from Daiichi‐Sankyo, Bayer, and LEO Pharma, which were transferred to his institute. TFW reports research funding from Leo Pharma outside of the submitted work, and advisory board/honoraria from Servier and Valeo. MDN reports personal fees from Bayer, Daiichi Sankyo, BMS‐Pfizer, Leo Pharma, and Viatris, outside the submitted work. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to acknowledge Mr. Cary Clark and the International Society on Thrombosis and Haemostasis for their invaluable support with the development, promotion, and dissemination of the survey, and the World Thrombosis Day Steering Committee for endorsing this initiative. Open Access Funding provided by Universita degli Studi Gabriele d'Annunzio Chieti Pescara within the CRUI‐CARE Agreement. [Correction added on 29 Nov 2022, after first online publication: CAUL funding statement has been added.]
Potere N, Barco S, Mahé I, et al. Awareness of venous thromboembolism among patients with cancer: Preliminary findings from a global initiative for World Thrombosis Day. J Thromb Haemost. 2022;20:2964‐2971. doi: 10.1111/jth.15902
Nicola Potere and Stefano Barco equally contributed to this work.
REFERENCES
- 1. Mulder FI, Horváth‐Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2021;137(14):1959‐1969. [DOI] [PubMed] [Google Scholar]
- 2. Grilz E, Posch F, Nopp S, et al. Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer‐a nationwide analysis. Eur Heart J. 2021;42(23):2299‐2307. [DOI] [PubMed] [Google Scholar]
- 3. Sanfilippo KM, Moik F, Candeloro M, Ay C, Di Nisio M, Lee AYY. Unanswered questions in cancer‐associated thrombosis. Br J Haematol. 2022;198(5):812‐825. [DOI] [PubMed] [Google Scholar]
- 4. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496‐520. [DOI] [PubMed] [Google Scholar]
- 5. Farge D, Frere C, Connors JM, et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID‐19. Lancet Oncol. 2022;23(7):e334‐e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Font C, Nelson A, Garcia‐Fernandez T, Prout H, Gee P, Noble S. Patients' experience of living with cancer‐associated thrombosis in Spain (PELICANOS). Support Care Cancer. 2018;26(9):3233‐3239. [DOI] [PubMed] [Google Scholar]
- 8. Noble S, Nelson A, Scott J, et al. Patient experience of living with cancer‐associated thrombosis in Canada (PELICANADA). Res Pract Thromb Haemost. 2020;4(1):154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noble S, Prout H, Nelson A. Patients' experiences of LIving with CANcer‐associated thrombosis: the PELICAN study. Patient Prefer Adherence. 2015;9:337‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahé I, Chidiac J, Pinson M, et al. Patients experience of living with cancer associated thrombosis in France (Le PELICAN). Thromb Res. 2020;194:66‐71. [DOI] [PubMed] [Google Scholar]
- 11. Falanga A, Girvalaki C, Monreal M, Easaw JC, Young A. How well do European patients understand cancer‐associated thrombosis? A patient survey. Cancer Treat Res Commun. 2022;31:100557. [DOI] [PubMed] [Google Scholar]
- 12. Baddeley E, Torrens‐Burton A, Newman A, et al. A mixed‐methods study to evaluate a patient‐designed tool to reduce harm from cancer‐associated thrombosis: the EMPOWER study. Res Pract Thromb Haemost. 2021;5(5):e12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17(10):1772‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]


