Abstract
This report describes the development of a photochemical method for C(sp 2)−H pyridination that leverages the photoexcitation of electron donor–acceptor (EDA) complexes. Experimental and DFT studies show that black light (λ max≈350 nm) irradiation of solutions of protonated pyridines (acceptors) and aromatic C−H substrates (donors) results in single electron transfer to form aryl radical cation intermediates that can be trapped with pyridine nucleophiles under aerobic conditions. With some modification of the reaction conditions, this EDA activation mode is also effective for promoting the oxidatively triggered SNAr pyridination of aryl halides. Overall, this report represents an inexpensive and atom‐economical approach to photochemical pyridination reactions that eliminates the requirement of an exogenous photocatalyst.
Keywords: C−H Functionalization, N-Heterocycles, Photochemistry, Radical Intermediates, Reaction Mechanisms
We leverage the photoexcitation of arene–pyridinium electron donor–acceptor (EDA) complexes to achieve C(sp 2)−H pyridination. Black light irradiation of the simple combination of arene substrate, pyridine, acid (to generate a pyridinium acceptor in situ), and O2 (terminal oxidant) affords N‐arylpyridinium products. This transformation proceeds without an exogenous photocatalyst, complementing prior photochemical C(sp 2)−H amination methods.

The photochemical C(sp 2)−H amination of aromatic substrates offers an attractive route to arylamine products, which are of high value in pharmaceutical, agrochemical, and materials chemistry applications.[ 1 , 2 ] In 2015, Nicewicz and co‐workers developed a method for arene C(sp 2)−H amination via visible light photoredox catalysis. [3] Since then, there have been numerous reports of C(sp 2)−H amination utilizing a range of photocatalysts (PC) and nitrogen nucleophiles (Figure 1a).[ 4 , 5 ] A general mechanism for these sequences is shown in Figure 1a and involves: excitation of the photocatalyst (PC) to generate PC+*, single electron transfer (SET) between the PC+* and the arene substrate to form an arene radical cation (I), and capture of I by a nitrogen nucleophile under oxidative conditions to yield the aminated product.[ 2 , 3 , 6 ] In this report, we demonstrate that analogous C(sp 2)−H amination reactions can be achieved without the requirement for an exogenous photocatalyst. Instead, photoexcitation of an arene–pyridinium electron donor–acceptor (EDA) complex[ 7 , 8 , 9 , 10 ] is leveraged to access the key radical cation intermediate (I) that is then trapped with pyridine and an oxidant.
Figure 1.

a) Photocatalytic C−H amination of arenes, including common photocatalysts and nitrogen nucleophiles. b) Generation of radical ion pairs II and III via photoexcitation of EDA complex. [11] (c) Plausible mechanism for our approach. Anion omitted for clarity.
Our approach was inspired by early work of Bargon and Gardini, who studied the EDA complex between naphthalene (electron donor) and pyridinium‐1‐d acetate‐d 3 (electron acceptor).[ 11 , 12 , 13 , 14 ] As shown in Figure 1b, exposure of this EDA adduct to black light (λ=290–360 nm) resulted in change transfer from naphthalene to the pyridinium to generate first the radical ion pair II and then the diradical III, which was detected by CIDNP NMR spectroscopy. Notably, II bears strong resemblance to the radical cation intermediate formed during photocatalytic C−H amination (I, Figure 1a). However, under these conditions (which lacked a strong nucleophile and a terminal oxidant), no arene C−H functionalization products were detected. Instead, III decayed to the starting materials via back electron transfer and elimination of acetate‐d 3.
We noted that pyridine (the conjugate base of the electron acceptor in Figure 1b) is known to react with arene radical cations in the presence of oxidants.[ 5 , 15 , 16 ] As shown in Figure 1c, reversible protonation of pyridine could enable this reagent to serve a dual role in photochemical arene C−H pyridination: (1) as an electron acceptor (to form an EDA complex) and (2) as a nitrogen nucleophile (to functionalize the arene radical cation intermediate). We demonstrate herein that black light irradiation of the simple combination of arene substrate, pyridine, acid (to generate a pyridinium acceptor in situ), and O2 (to serve as a terminal oxidant) yields N‐arylpyridinium products. [17] These products are valuable synthetic intermediates that are readily converted to anilines (via reactions with amine bases)[ 15 , 17a , 17b ] and piperidine derivatives (via hydrogenation).[ 17a , 18 ] DFT calculations and UV/Vis studies are consistent with a mechanism involving photo‐induced charge transfer at an arene–pyridinium EDA complex in the parallel displaced geometry. Overall, this transformation represents a complementary approach to photochemical pyridination reactions that eliminates the need for an exogenous photocatalyst.
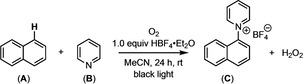
Initial studies targeted the reaction of naphthalene (A) and pyridine (B) to form pyridinium product C (Table 1). [11] With O2 as the terminal oxidant, a balanced equation for the proposed transformation is shown above Table 1. Using black light (UVA compact fluorescent lamp (CFL) with λ max≈350 nm, analogous to that used by Bargon and Gardini), a 1.05 : 1.0 : 1.0 molar mixture of pyridine : HBF4 : naphthalene in MeCN was irradiated for 24 h at room temperature under an atmosphere of O2. A slight excess of pyridine relative to HBF4 was used to maintain a reservoir of the nitrogen nucleophile to react with the putative radical cation intermediate. [19] Under these conditions, the C−H pyridination product C was formed in 5 % yield (Table 1, entry 1). Notably, using a 1 : 1 ratio of pyridine to HBF4 (such that no free pyridine is present in solution) resulted in <1 % yield under otherwise analogous conditions (entry 2).
Table 1.
C(sp 2)−H Pyridination of Naphthalene.
|
| ||||
|---|---|---|---|---|
|
Entry |
A [equiv] |
B [equiv] |
[HBF4⋅Et2O] |
C [%][a] |
|
1 |
1.0 |
1.05 |
0.1 M |
5 |
|
2 |
1.0 |
1.0 |
0.1 M |
<1 |
|
3 |
2.5 |
1.05 |
0.1 M |
6 |
|
4[b] |
2.5 |
1.05 |
0.1 M |
<1 |
|
5 |
2.5 |
1.05 |
0.05 M |
18 |
|
6[c] |
2.5 |
1.05 |
0.02 M |
23 |
|
7 |
1.0 |
2.0 |
0.02 M |
20 |
[a] Crude yields determined by 1H NMR spectroscopy. [b] Reaction was not sparged with an O2 balloon before light irradiation (conducted under ambient air). [c] Compound C was isolated in 17 % yield.
The reaction was further optimized with respect to concentration, equiv. of naphthalene and pyridine, and solvent (see Tables 1 and S2 for details). One key finding is that the reaction is sensitive to concentration, with higher yields at lower concentrations (compare entries 3, 5, and 6). Furthermore, the addition of O2 is important (compare entries 3 and 4), consistent with the oxidant driving the trapping and re‐aromatization sequence.[ 3 , 6c , 20 , 21 ] A peroxide strip test of the crude reaction mixture was positive, consistent with the formation of H2O2. Ultimately, under the best identified conditions (1.05 equiv pyridine, 1.0 equiv HBF4, 2.5 equiv naphthalene), product C was obtained in 23 % yield (entry 6). The mass balance was a complex mixture of products that appear to be derived from competing oxidation reactions of naphthalene (see Figure S3).[ 22 , 23 , 24 ]
Literature precedent suggests that the extended π‐system of naphthalene renders it susceptible to competing oxidation and other side reactions.[ 21 , 22 , 23 ] To circumvent this issue, we next conducted a computational screen to identify other aromatic substrates that could form EDA complexes with pyridinium salts and undergo photochemical excitation. Density functional theory (DFT) calculations were used to compare the energetics of the naphthalene–pyridinium EDA complex to that with other arenes. The calculations (using the B3LYP functional [25] and 6–311+G** basis set including empirical dispersion effects in an acetonitrile dielectric) indicate that EDA complexation with the pyridinium cation (PyH+) is energetically accessible for a variety of aromatic substrates, including biphenyl, anisole, tert‐butylbenzene, and benzene. As summarized in Figure 2 and Table S5, the binding energy (BE) for the EDA adduct in the parallel displaced (PD) geometry ranges from −9.1 kcal mol−1 (naphthalene) to −5.4 kcal mol−1 (benzene). Furthermore, in all cases DFT predicts a charge transfer band with λ max ranging from 399 nm (anisole) to 324 nm (benzene). Overall, biphenyl is predicted to have the most similar properties to naphthalene. Consistent with these calculations, experimental UV/Vis spectroscopic analysis of solutions of PyH+–naphthalene and PyH+–biphenyl show similar bathochromic shifts (Figures S7 and S8 in Supporting Information). Furthermore, a Job plot for the PyH+–biphenyl system shows a 1 : 1 stoichiometry between the donor and acceptor. [26]
Figure 2.

Comparison of the binding energy (BE) and excitation wavelength (λ max) of arene–pyridinium EDA complexes in the parallel‐displaced (PD) geometry. Values from DFT calculations (B3LYP functional and 6–311+G** basis set including empirical dispersion effects in an acetonitrile dielectric). As detailed in the Supporting Information (p. S12), this method captures trends, but overestimates binding energy and excitation wavelength.
Based on the computational and experimental UV/Vis results, we next explored biphenyl as a substrate. Under analogous conditions to Table 1, entry 6, biphenyl reacts with pyridine to form 1 in 72 % yield as determined by 1H NMR spectroscopy (Table 2, entry 1). Product 1 was isolated in 70 % yield under these conditions (entry 1). [19] Unlike the reaction of naphthalene, the C−H pyridination of biphenyl to form 1 proceeds cleanly, with unreacted starting material accounting for the mass balance (compare Figure S3 to S4). Control experiments demonstrate that the reaction requires both the light source (black light) and acid (HBF4⋅Et2O) to afford more than trace yield of 1 (entries 3 and 4). [27] Additionally, when the reaction mixture was not sparged with O2 the yield of 1 decreased to 14 % (entry 2). As discussed above, we propose that O2 serves as the terminal oxidant to promote rearomatization and generate the product.[ 3 , 6c , 20 , 21 ] Finally, the reaction afforded 1 in comparable (70 %) yield when biphenyl was used as the limiting reagent, along with 2 equiv. of pyridine (entry 7). Under these conditions, the mass balance was primarily the dipyridinated product (18 % yield) along with traces of unreacted starting material.
Table 2.
C(sp 2)−H Pyridination of Biphenyl.
|
| ||
|---|---|---|
|
Entry |
Modification |
Yield[a] |
|
1 |
none |
72 %, (70 %)[b] |
|
2 |
no O2 sparge (ambient air) |
14 % |
|
3 |
no light |
0 % |
|
4 |
no HBF4⋅Et2O |
1 % |
|
5 |
390 nm Kessil LED |
39 % |
|
6 |
440 nm Kessil LED |
0 % |
|
7 |
1 equiv biphenyl, 2 equiv pyridine |
70 %[c] |
[a] Crude yields determined by 1H NMR spectroscopy. [b] Isolated yield. [c] The 4,4′‐dipyridinated product was also formed in 18 % yield.
A variety of pyridine derivatives were next evaluated for the reaction with biphenyl. Importantly, the substituted pyridinium products serve as precursors to piperidines (via hydrogenation).[ 17a , 18 ] Since the pyridine is proposed to serve as both the nucleophile and the acceptor (as the conjugate acid), a key question was how sensitive this transformation would be to pyridine substitution. As summarized in Table 3, pyridines bearing electron donating methyl‐ and methoxy‐ substituents at the 3‐ and 4‐positions reacted to afford high yields (83–91 %) of pyridinium products 2–4. With 2‐methylpyridine, the pyridinium product 5 was also formed, albeit in significantly lower yield (39 %). Yields were also modest with pyridines bearing moderately electron‐withdrawing fluorine (7) or amide substituents (10) or when using less nucleophilic nitrogen heterocycles such as pyridazine (9). [28] Furthermore, pyridines bearing stronger electron withdrawing groups (e.g., 2‐ or 4‐CF3 or CN) afforded ≤5 % yield of the pyridinium product. These results indicate that the nucleophilicity of the pyridine derivative is a key driver in this transformation. In all cases, the para‐isomer was favored with good to excellent selectivity (presumably due to steric effects), but the minor regioisomer varied depending on the electronics of the pyridine. With electron rich pyridine nucleophiles the minor isomer was ortho‐, while with more electron deficient derivatives the minor isomer was meta‐substituted. [29]
Table 3.
Pyridine Substrate Scope for C(sp 2)−H Pyridination.[a]

[a] Reactions run under black light (λ max≈350 nm) for 24 h in MeCN. All products isolated as BF4 − salts. See Supporting Information for additional details. [b] 2 equiv of pyridine relative to 1 equiv of arene and HBF4⋅Et2O. [c] 3 equiv. of pyridine relative to 1 equiv of arene and HBF4⋅Et2O. [d] Ratio refers to para:meta:ortho (p:m:o) selectivity. Ratios represent ratio of crude products between major (pictured) and minor (*) isomers. All yields are isolated and represent mixtures of inseparable regioisomers unless otherwise noted. BF4 − counterion omitted for clarity.
The scope of photochemical C−H pyridination was also evaluated with respect to the arene substrate (Table 4). As predicted by DFT (Figure 2), anisole showed high reactivity under the standard conditions, affording the pyridinium product 12 in 61 % isolated yield as a 5 : 1 mixture of the para and ortho isomers. The observed para selectivity is analogous with that reported by Yoshida [15] for electrochemical C(sp 2)−H pyridination of anisole via an analogous radical cation intermediate, and it is likely driven by sterics. [30] Under the same conditions tert‐butylbenzene reacted to form 11 in 83 % yield as a 1.4 : 1 mixture of the para and meta isomers. Other alkyl‐substituted aromatic substrates, including mesitylene, meta‐xylene, and ortho‐xylene, afforded comparable yields of the corresponding pyridinium products 13–15. Anisole derivatives bearing electron withdrawing cyano and ester substituents reacted to afford 16 and 17 in 66 % and 48 % yield, respectively, under the standard conditions. Benzene and trifluoromethoxy‐benzene gave <1 % yield of the corresponding C(sp 2)−H pyridination products upon irradiation with black light. However, switching to a higher energy light source (UVB CFL) with these less electron rich substrates led to the pyridinated products 19 and 20. These results are consistent with the DFT calculations showing that higher energy is required for charge transfer in the benzene–pyridinium EDA complex compared to that with more electron rich arenes (Figure 2).
Table 4.
Arene Substrate Scope for C(sp 2)−H Pyridination.[a]
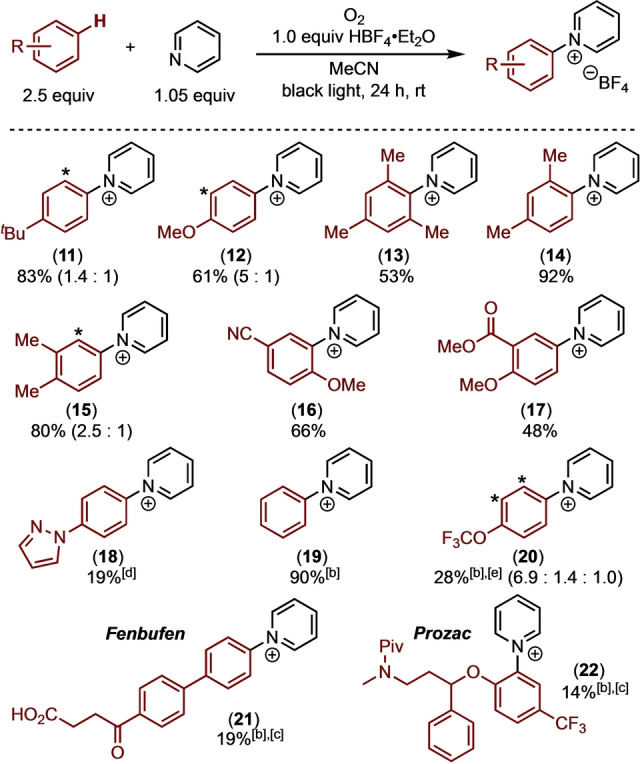
[a] Reactions run under black light (λ max≈350 nm) for 24 h in MeCN. All products isolated as BF4 − salts. See Supporting Information for additional details. [b] Reactions run using a UVB CFL (λ max≈300 nm). [c] 2 equiv of pyridine relative to 1 equiv of arene and HBF4⋅Et2O. [d] 3 equiv of pyridine relative to 1 equiv. of arene and HBF4⋅Et2O. [e] Ratio refers to para:ortho:meta (p:o:m) selectivity. Ratios represent ratio of crude products between major (pictured) and minor (*) isomers. All yields are isolated and represent mixtures of inseparable regioisomers unless otherwise noted. BF4 − counterion omitted for clarity.
While the reactions in Table 4 were conducted using a 2.5 : 1 ratio of arene to pyridine, the arene can also be used as the limiting reagent with minimal impact on the yield. For example, with anisole, mesitylene, and anisonitrile as the limiting reagent (along with 2 equiv. of pyridine), products 12, 13, and 16 were isolated in 56 %, 49 %, and 52 %, respectively. The mass balance in these reactions was primarily unreacted starting material.
The drug molecules Fenbufen and Prozac underwent C(sp 2)−H pyridination to form 21 and 22 in modest yields utilizing a UVB CFL. With these substrates the arene was used as the limiting reagent, and the mass balance was unreacted starting material. [31] Notably, the reaction of Prozac was conducted with the HBF4 salt of the amine, and the product was subsequently converted to the pivolyl amide to facilitate isolation. [32] Prozac presents two aromatic rings that could undergo C−H pyridination, and the reaction is selective for the ring bearing ether and CF3 substituents. These examples demonstrate compatibility with ketones, carboxylic acids, and protonated amines.
Finally, we sought to expand this EDA activation mode to aromatic functionalization reactions beyond just C−H pyridination. We initially targeted S N Ar pyridination based on a preliminary result showing that 4‐bromobiphenyl reacts under the standard conditions to afford traces (≈5 %) of the S N Ar product 24 along with the expected C(sp 2 )−H pyridination product 23 (36 %; Scheme 1A). [33] Optimization of the SNAr reaction, including switching to an N2 atmosphere with DCE : TFE (1 : 1) as the solvent[ 5 , 34 ] and using a Kessil LED (390 nm) light source, resulted in enhanced yield and selectivity for 24 (19 % yield; >20 : 1 selectivity; Scheme 1B). [35] Furthermore, changing the leaving group from bromide to chloride under otherwise identical conditions, led to a 67 % isolated yield of 24, along with <1 % of the C−H pyridination product. [36] Similar results were obtained with 4‐chloroanisole and Clofibrate as substrates, affording exclusively S N Ar products 25 and 26 in 71 % and 54 % isolated yield, respectively.
Scheme 1.

A) Mixture of C(sp 2)−H pyridination and S N Ar pyridination with 4‐bromobiphenyl. B) Selective SNAr pyridination. [a] Crude yield determined by 1H NMR spectroscopy.
In conclusion, this paper demonstrates the photochemical C(sp 2 )−H pyridination of electron rich and ‐neutral arenes using pyridine as a nucleophile and protonated pyridine as an acceptor for EDA complex formation and photoactivation. These transformations use O2 as the terminal oxidant and proceed without an exogenous photocatalyst. DFT studies of the proposed EDA intermediates guided the selection of arene substrates. This activation mode enables C(sp 2 )−H pyridination of both electron rich and electron‐neutral arenes, which complements the scope of analogous transformations using visible light and acridinium photocatalysts. [5] Furthermore, with substrates bearing aryl halide functional groups, this activation mode also enables S N Ar reactivity. Ongoing work is focused on detailed studies of the scope of arene functionalization reactions enabled by this approach.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
We gratefully acknowledge funding from Dow through the University Partnership Initiative. We also thank the Szymczak Lab, at the University of Michigan, for allowing us to utilize their UV/Vis spectrophotometer.
M. R. Lasky, T. K. Salvador, S. Mukhopadhyay, M. S. Remy, T. P. Vaid, M. S. Sanford, Angew. Chem. Int. Ed. 2022, 61, e202208741; Angew. Chem. 2022, 134, e202208741.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
References
- 1.
- 1a. Holmberg-Douglas N., Nicewicz D. A., Chem. Rev. 2022, 122, 1925–2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1b. Candish L., Collins K. D., Cook G. C., Douglas J. J., Gómez-Suárez A., Jolit A., Keess S., Chem. Rev. 2022, 122, 2907–2980; [DOI] [PubMed] [Google Scholar]
- 1c. Guillemard L., Kaplaneris N., Ackermann L., Johansson M. J., Nat. Chem. Rev. 2021, 5, 522–545; [DOI] [PubMed] [Google Scholar]
- 1d. Bariwal J., Van der Eycken E., Chem. Soc. Rev. 2013, 42, 9283; [DOI] [PubMed] [Google Scholar]
- 1e. Yi H., Zhang G., Wang H., Huang Z., Wang J., Singh A. K., Lei A., Chem. Rev. 2017, 117, 9016–9085; [DOI] [PubMed] [Google Scholar]
- 1f. Park Y., Kim Y., Chang S., Chem. Rev. 2017, 117, 9247–9301. [DOI] [PubMed] [Google Scholar]
- 2. Romero N. A., Nicewicz D. A., Chem. Rev. 2016, 116, 10075–10166. [DOI] [PubMed] [Google Scholar]
- 3. Romero N. A., Margrey K. A., Tay N. E., Nicewicz D. A., Science 2015, 349, 1326–1330. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Zheng Y.-W., Chen B., Ye P., Feng K., Wang W., Meng Q.-Y., Wu L.-Z., Tung C.-H., J. Am. Chem. Soc. 2016, 138, 10080–10083; [DOI] [PubMed] [Google Scholar]
- 4b. Margrey K. A., Levens A., Nicewicz D. A., Angew. Chem. Int. Ed. 2017, 56, 15644–15648; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 15850–15854; [Google Scholar]
- 4c. Morofuji T., Ikarashi G., Kano N., Org. Lett. 2020, 22, 2822–2827; [DOI] [PubMed] [Google Scholar]
- 4d. Das S., Natarajan P., König B., Chem. Eur. J. 2017, 23, 18161–18165; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4e. Ruffoni A., Juliá F., Svejstrup T. D., McMillan A. J., Douglas J. J., Leonori D., Nat. Chem. 2019, 11, 426–433; [DOI] [PubMed] [Google Scholar]
- 4f. Targos K., Williams O. P., Wickens Z. K., J. Am. Chem. Soc. 2021, 143, 4125–4132. [DOI] [PubMed] [Google Scholar]
- 5. Mantell M. A., Lasky M. R., Lee M., Remy M., Sanford M. S., Org. Lett. 2021, 23, 5213–5217. [DOI] [PubMed] [Google Scholar]
- 6.For additional reports utilizing non-amine nucleophiles under similar reaction conditions, see:
- 6a. Chen W., Huang Z., Tay N. E. S., Giglio B., Wang M., Wang H., Wu Z., Nicewicz D. A., Li Z., Science 2019, 364, 1170–1174; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Holmberg-Douglas N., Onuska N. P. R., Nicewicz D. A., Angew. Chem. Int. Ed. 2020, 59, 7425–7429; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 7495–7499; [Google Scholar]
- 6c. McManus J. B., Nicewicz D. A., J. Am. Chem. Soc. 2017, 139, 2880–2883; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6d. Ohkubo K., Kobayashi T., Fukuzumi S., Angew. Chem. Int. Ed. 2011, 50, 8652–8655; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 8811–8814; [Google Scholar]
- 6e. Ohkubo K., Fujimoto A., Fukuzumi S., J. Phys. Chem. A 2013, 117, 10719–10725; [DOI] [PubMed] [Google Scholar]
- 6f. Tlili A., Lakhdar S., Angew. Chem. 2021, 133, 19678–19701; [Google Scholar]
- 6g. Zheng Y.-W., Ye P., Chen B., Meng Q.-Y., Feng K., Wang W., Wu L.-Z., Tung C.-H., Org. Lett. 2017, 19, 2206–2209; [DOI] [PubMed] [Google Scholar]
- 6h. Meyer A. U., Berger A. L., König B., Chem. Commun. 2016, 52, 10918–10921. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Mulliken R. S., J. Am. Chem. Soc. 1952, 74, 811–824; [Google Scholar]
- 7b. Foster R., J. Phys. Chem. 1980, 84, 2135–2141; [Google Scholar]
- 7c. Yasuda M., Pac C., Sakurai H., J. Chem. Soc. Perkin Trans. 1 1981, 746–750; [Google Scholar]
- 7d. Nagakura S., Mol. Cryst. Liq. Cryst. 1985, 126, 9–18; [Google Scholar]
- 7e. Singh J. O., Anunziata J. D., Silber J. J., Can. J. Chem. 1985, 63, 903–907; [Google Scholar]
- 7f. Pandey G., Krishna A., Rao J. M., Tetrahedron Lett. 1986, 27, 4075–4076; [Google Scholar]
- 7g. Pandey C., Sridhar M., Bhalerao U. T., Tetrahedron Lett. 1990, 31, 5373–7376. [Google Scholar]
- 8. Rosokha S. V., Kochi J. K., Acc. Chem. Res. 2008, 41, 641–653. [DOI] [PubMed] [Google Scholar]
- 9. Lima C. G. S., de M. Lima T., Duarte M., Jurberg I. D., Paixão M. W., ACS Catal. 2016, 6, 1389–1407. [Google Scholar]
- 10.For recent examples employing EDA complexes in alternative photochemical transformations, see:
- 10a. Arceo E., Jurberg I. D., Álvarez-Fernández A., Melchiorre P., Nat. Chem. 2013, 5, 750–756; [DOI] [PubMed] [Google Scholar]
- 10b. Tobisu M., Furukawa T., Chatani N., Chem. Lett. 2013, 42, 1203–1205; [Google Scholar]
- 10c. Liu B., Lim C.-H., Miyake G. M., J. Am. Chem. Soc. 2017, 139, 13616–13619; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10d. Fu M.-C., Shang R., Zhao B., Wang B., Fu Y., Science 2019, 363, 1429–1434; [DOI] [PubMed] [Google Scholar]
- 10e. Bosque I., Bach T., ACS Catal. 2019, 9, 9103–9109; [Google Scholar]
- 10f. Xie S., Li D., Huang H., Zhang F., Chen Y., J. Am. Chem. Soc. 2019, 141, 16237–16242; [DOI] [PubMed] [Google Scholar]
- 10g. de Pedro Beato E., Spinnato D., Zhou W., Melchiorre P., J. Am. Chem. Soc. 2021, 143, 12304–12314; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10h. Sakakibara Y., Murakami K., Itami K., Org. Lett. 2022, 24, 602–607; [DOI] [PubMed] [Google Scholar]
- 10i. Escolano M., Cabrera-Afonso M. J., Ribagorda M., Badir S. O., Molander G. A., J. Org. Chem. 2022, 87, 4981–4990; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10j. Wang H., Wu J., Noble A., Aggarwal V. K., Angew. Chem. Int. Ed. 2022, 61, e202202061; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2022, 134, e202202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bargon J., Gardini G. P., Tetrahedron Lett. 1976, 17, 2993–2996. [Google Scholar]
- 12.
- 12a. Hilinski E. F., Masnovi J. M., Amatore C., Kochi J. K., Rentzepis P. M., J. Am. Chem. Soc. 1983, 105, 6167–6168; [Google Scholar]
- 12b. Lee K. Y., Kochi J. K., J. Chem. Soc. Perkin Trans. 2 1992, 1011; [Google Scholar]
- 12c. Kim E. K., Bockman T. M., Kochi J. K., J. Am. Chem. Soc. 1993, 115, 3091–3104. [Google Scholar]
- 13. Crisenza G. E. M., Mazzarella D., Melchiorre P., J. Am. Chem. Soc. 2020, 142, 5461–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McClain E. J., Monos T. M., Mori M., Beatty J. W., Stephenson C. R. J., ACS Catal. 2020, 10, 12636–12641. [Google Scholar]
- 15. Morofuji T., Shimizu A., Yoshida J., J. Am. Chem. Soc. 2013, 135, 5000–5003. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Herold S., Möhle S., Zirbes M., Richter F., Nefzger H., Waldvogel S. R., Eur. J. Org. Chem. 2016, 1274–1278; [Google Scholar]
- 16b. Möhle S., Herold S., Richter F., Nefzger H., Waldvogel S. R., ChemElectroChem 2017, 4, 2196–2210; [Google Scholar]
- 16c. Wesenberg L. J., Diehl E., Zähringer T. J. B., Dörr C., Schollmeyer D., Shimizu A., Yoshida J., Hellmich U. A., Waldvogel S. R., Chem. Eur. J. 2020, 26, 17574–17580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alternative photochemical methods to form N-arylpyridinium products:
- 17a. Rössler S. L., Jelier B. J., Tripet P. F., Shemet A., Jeschke G., Togni A., Carreira E. M., Angew. Chem. Int. Ed. 2019, 58, 526–531; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 536–541; [Google Scholar]
- 17b. Ham W. S., Hillenbrand J., Jacq J., Genicot C., Ritter T., Angew. Chem. Int. Ed. 2019, 58, 532–536; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 542–546; [Google Scholar]
- 17c. Hillenbrand J., Ham W. S., Ritter T., Org. Lett. 2019, 21, 5363–5367. [DOI] [PubMed] [Google Scholar]
- 18.
- 18a. Hamilton T. S., Adams R., J. Am. Chem. Soc. 1928, 50, 2260–2263; [Google Scholar]
- 18b. Sowmiah S., Esperança J. M. S. S., Rebelo L. P. N., Afonso C. A. M., Org. Chem. Front. 2018, 5, 453–493; [Google Scholar]
- 18c. Nairoukh Z., Wollenburg M., Schlepphorst C., Bergander K., Glorius F., Nat. Chem. 2019, 11, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The yields in Tables 1–4 are calculated with respect to HBF4⋅Et2O as the limiting reagent under our standard reaction conditions (1 equiv HBF4⋅Et2O, 1.05 equiv pyridine, 2.5 equiv arene), because one equivalent of the BF4 anion is required to balance the charge of the pyridinium product. In cases were 1 equiv of the arene is utilized, yields are calculated with respect to the arene.
- 20. Ohkubo K., Mizushima K., Iwata R., Fukuzumi S., Chem. Sci. 2011, 2, 715–722. [Google Scholar]
- 21. Reed N. L., Yoon T. P., Chem. Soc. Rev. 2021, 50, 2954–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.
- 22a. Aubry J.-M., Mandard-Cazin B., Rougee M., Bensasson R. V., J. Am. Chem. Soc. 1995, 117, 9159–9164; [Google Scholar]
- 22b. Pierlot C., Aubry J.-M., Chem. Commun. 1997, 2289–2290; [Google Scholar]
- 22c. Martinez G. R., Ravanat J.-L., Medeiros M. H. G., Cadet J., Di Mascio P., J. Am. Chem. Soc. 2000, 122, 10212–10213. [Google Scholar]
- 23. Fukuzumi S., Ohkubo K., Org. Biomol. Chem. 2014, 12, 6059–6071. [DOI] [PubMed] [Google Scholar]
- 24.A control experiment was conducted to confirm that naphthyl-pyridinium product C is stable under photochemical conditions. Complete details can be found in the Supporting Information (p. S19–20).
- 25.
- 25a. Azizi A., Ebrahimi A., J. Mol. Liq. 2019, 276, 170–178; [Google Scholar]
- 25b. Hohenstein E. G., Sherrill C. D., J. Phys. Chem. A 2009, 113, 878–886; [DOI] [PubMed] [Google Scholar]
- 25c. Sherrill C. D., Takatani T., Hohenstein E. G., J. Phys. Chem. A 2009, 113, 10146–10159. [DOI] [PubMed] [Google Scholar]
- 26.See Figure S10 for the Job plot and Figure S12 for NMR spectra of biphenyl, pyridinium tetrafluoroborate and a 1 : 1 mixture of these compounds.
- 27.The time course for the formation of 1 was nearly identical under the standard conditions and upon the addition of 25 mol % of 3 at the outset of the reaction (Figure S13). This result provides evidence against an autocatalysis scenario in which the N-arylpyridinium products accelerate the C(sp2)−H pyridination reaction.
- 28.With electron deficient pyridines, higher yields were obtained using biphenyl as the limiting reagent in the presence of an excess of the pyridine, potentially due to slow capture of the radical cation intermediate with these nucleophiles.
- 29.We hypothesize that the ortho selectivity observed with stronger pyridine nucleophiles may reflect a kinetic preference, while the meta selectivity with weaker nucleophiles may be a thermodynamic effect. Ongoing work is focused on gaining further mechanistic insights.
- 30.We are actively investigating the unusual ortho-selectivity observed in our previous report of acridinium-catalyzed C(sp2)−H pyridination, ref. [5].
- 31.The crude yield of 21 was 52 %, and 40 % unreacted Fenbufen starting material remained. Similarly, the crude yield of 22 was 20 %, and 70 % unreacted Prozac remained.
- 32. Lee M., Sanford M. S., J. Am. Chem. Soc. 2015, 137, 12796–12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SNAr reactions are overall redox neutral and are thus most typically performed under an atmosphere of nitrogen. Nonetheless, there is precedent for oxidatively triggered S N Ar reactions proceeding under net oxidizing conditions. See for example,
- 33a. Ohkubo K., Fujimoto A., Fukuzumi S., J. Am. Chem. Soc. 2013, 135, 5368–5371; [DOI] [PubMed] [Google Scholar]
- 33b. Sheridan T., Yayla H. G., Lian Y., Genovino J., Monck N., Burton J. W., Eur. J. Org. Chem. 2020, 2766–2770. [Google Scholar]
- 34.These conditions (DCE/TFE solvent mixture, N2 atmosphere) are similar to those used in other photochemical SNAr reactions. See ref. [5] and Tay N. E. S., Nicewicz D. A., J. Am. Chem. Soc. 2017, 139, 16100–16104. [DOI] [PubMed] [Google Scholar]
- 35.Lower yield of 24 (10 %) was observed using black light under otherwise identical conditions. See Table S8 for comparison of the two light sources with each substrate.
- 36.UV/Vis spectroscopic analysis of a 1 : 1 mixture of PyH+ and 4-chlorobiphenyl shows a bathochromic shift that is very similar to that observed in the analogous experiment with PyH+ and biphenyl. In both cases there is significant absorbance at 365 and 390 nm, consistent with both black light and 390 nm Kessil lamps being effective for these transformations (Tables 2, S7, and S8).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.