Abstract
Objectives
This study investigates patterns of bone functional adaptations in extant apes through comparing hindlimb to forelimb bone rigidity ratios in groups with varying levels of arboreality.
Materials and Methods
Using CT scans, bone rigidity (J) was calculated at three regions of interest (ROI) along femoral and humeral diaphyses in Homo, Pongo, Pan, and Gorilla with further comparisons made between species and subspecies divisions within Pan and Gorilla.
Results
Consistent with previous work on extant hominoids, species exhibited differences in midshaft femoral to humeral (F/H) rigidity ratios. Results of the present study confirm that these midshaft differences extend to 35% and 65% diaphyseal ROIs. Modern humans, exhibiting larger ratios, and orangutans, exhibiting smaller ratios, bracketed the intermediate African apes in comparisons. Within some African apes, limb rigidity ratios varied significantly between taxonomic groups. Eastern gorillas exhibited the highest mean ratios and chimpanzees the lowest at all three ROIs. In posthoc comparisons, chimpanzees and bonobos did not differ in relative limb rigidity ratios at any of the three ROIs. However, western gorillas were more similar to bonobos than eastern gorillas at 50% and 35% ROIs, but not at the 65% ROI.
Conclusion
Species, and to a lesser extent subspecies, can be distinguished by F/H limb rigidity ratios according to broad positional behavior patterns at multiple regions of interest along the diaphyses. Similarity of bonobos and western gorillas is in line with behavioral data of bonobos being the most terrestrial of Pan species, and western gorillas the most arboreal of the Gorilla groups.
Keywords: bonobo, chimpanzee, cross‐sectional geometry, gorilla, hominoid
Hominoid species can be distinguished by F/H limb rigidity ratios according to levels of arboreality at multiple regions of interest along the diaphyses.
1. INTRODUCTION
Long bone diaphyseal strength and robusticity proportions are useful indicators of relative limb involvement and loading during the locomotor repertoires of primates (Burr, Ruff, & Johnson, 1989; Demes & Jungers, 1993; Kimura, 2002; Ruff, Harper, Goldstein, Daegling, & McGraw, 2019; Schaffler, Burr, Jungers, & Ruff, 1985; Ruff, 2002; Carlson, 2005; Shaw & Ryan, 2012). This form‐function relationship has been formalized as bone functional adaptation (Ruff et al., 2006). According to the mechanostat proposed by Frost (1973, 1987, 1996, 2003), cortical bone modeling is responsible for the formation and redistribution of bone mass in a long bone diaphysis when mechanical usage exceeds a minimum loading threshold but falls below an upper threshold corresponding to overloading situations (Martin, Burr, Sharkey, & Fyhrie, 2015; Barak, 2020). Other factors besides mechanical usage, however, also clearly affect the modeling process (i.e., these factors can operate to adjust mechanostat thresholds), often complicating a straightforward interpretation of bone functional adaptations (Martin, Burr, Sharkey, & Fyhrie, 2015; Frost, 2003; Wergedal et al., 2005; Devlin et al., 2010; Devlin, 2011; Wallace et al., 2010, 2012).
Investigating patterns in the expression of bone functional adaptations in humeral and femoral diaphyses, especially in comparisons of great apes, has proven to be informative in differentiating their relative frequencies of arboreal versus terrestrial locomotor behavior. Pongo exhibits more arboreal locomotion than other great apes (Thorpe & Crompton, 2006). Arboreal Pongo also exhibits greater forelimb to hindlimb strength ratios compared to relatively more terrestrial African apes (Ruff, 2002; Shaw & Ryan, 2012; see Table 1) and even more so when compared to terrestrial Homo (Shaw & Ryan, 2012). Within African apes, Pan exhibits higher humeral to femoral ratios compared to those of Gorilla (Ruff, 2002). The vast majority of these efforts have focused on humeral and femoral evaluations in higher taxonomic levels of extant apes (e.g., interspecific comparisons), while finer scaled investigations (e.g., intraspecific comparisons) have been less numerous (Carlson, 2005; Carlson et al., 2006, 2011; Ruff et al., 2018). Additionally, attempts to coordinate structural patterns in bone functional adaptations with frequencies of specific positional modes, irrespective of substrate considerations, have been less successful at establishing clear behavior‐specific relationships (Runestad, 1997; Carlson, 2005; Carlson et al., 2011; Ruff et al., 2018; but see Runestad Connour, Glander, & Vincent, 2000 and Demes, Jungers, & Selpien, 1991).
TABLE 1.
Arboreal behavior percentages for different extant ape species and subspecies
| Arboreal locomotion | |||||
|---|---|---|---|---|---|
| Time Arb (%) | Loc Arb (%) | Suspend (%) | Climb (%) | Scramble (%) | |
| Taxon | F/M* | F/M | F/M | F/M | F/M |
| Pongo abelii | ∼100 a | 100 | 17–18 | 20–25 | 31–44 b |
| Pan troglodytes | |||||
| P. t. troglodytes | |||||
| P. t. schweinfurthii | 52/34 c | 12/8 d | 8/7 | 49/52 | 10/3 |
| P. t. verus | 65/49 c | 18/15 d | 7/6 | 51/59 | 8/8 |
| Pan paniscus | 47/41 e | 9 f | 2 | 40 | 33 |
| Gorilla gorilla | |||||
| G. g. gorilla | 9/2 d | 11/4 | 39/48 | 31/25 | |
| Gorilla beringei | |||||
| G. b. beringei | 7/2 g | 8/2 d | 3/0 | 33/27 | 3/9 |
| G. b. graueri | |||||
F = female, M = male; if only one number is provided, it refers to combined sexes.
Suaq Balimbing flanged males spend <0.25% of their time terrestrial and habituated females spend <0.05% terrestrial [personal communication with C. Schuppli referenced in Ashbury et al. (2015)], while others report approximately 0% terrestrial for Sumatran orangutans (personal communication with S.K.S. Thorpe).
Pongo data from Manduell, Harrison, & Thorpe (2012); data use 1‐min instantaneous focal sampling. Ranges are from two Sumatran study sites: Ketambe and Suaq Balimbing. Orangutans are highly arboreal, but the breakdown of their arboreal behavior depends on habitat structure to a high degree (Manduell et al., 2012; Manduell, 2013): suspend (includes brachiation and forelimb swing; torso pronograde suspensory behavior), quadrupedal climb (includes vertical climb and descent), and quadrupedal scramble (includes orthograde clamber and transfer, drop and leap, sway, ride, and bridge).
Data from Pan troglodytes and Gorilla from Carlson (2005): suspend (includes brachiate, arm swing, and drop), quadrupedal climb (on vertical or inclined substrates), and quadrupedal scramble (includes scramble, bridge, fireslide, tree sway, ride, and leap) within arboreal locomotor behaviors only. Carlson (2005) used original data from D. Doran and K. Hunt (Doran, 1989; Doran & Hunt, 1994; Hunt, 1989, 1992; Remis, 1994, 1995, 1998).
% time arboreal (Time Arb) and arboreal locomotion (Loc Arb) for bonobos from Lui Kotale taken from Ramos (2014: Tables 4.13 and 4.23).
Percentage of arboreal locomotion data for Pan paniscus reflects 69 arboreal locomotor bouts out of 789 total locomotor bouts (Ramos, personal communication).
From Doran (1997).
Limited upper limb involvement in locomotor behavior distinguishes modern humans from other extant hominoids, and has long been recognized as a feature that evolutionarily distinguishes the human lineage (Darwin, 1871; Huxley, 1863). While it is a widely held view that Homo erectus was an obligate biped by 1.5–1.7 mya (Ruff, 2008; Pontzer, 2012; Hatala et al., 2016), exactly when the shift in the human lineage from facultative to obligate bipedality occurred – along with the concomitant de‐emphasis of the upper limb in weight‐bearing locomotor activities – remains vigorously debated (Stern & Susman, 1983; Ward, 2002; Lovejoy, Suwa, Spurlock, Asfaw, & White, 2009; White et al., 2009; Churchill et al., 2013; Ruff, Burgess, Ketcham, & Kappelman, 2016; Kozma et al., 2018; Macchiarelli et al., 2020; Carlson et al., 2021). While extant great apes are the most informative modern analogs for framing utilization of the upper limbs in positional repertoires of hominins, it is increasingly clear that no living ape provides an exact replica of a hominin positional repertoire (see Almécija et al., 2021). Nonetheless, with the upper limb serving in some capacity as a weight‐bearing instrument during locomotor behavior over the evolutionary history of early hominins, as opposed to an absence of this capacity as in modern humans, relative humeral and femoral diaphyseal rigidities should differ from those of modern humans and approach those of other extant great apes to varying degrees. The aim of the present study is to quantify femoral‐humeral limb rigidities, along multiple diaphyseal locations, across a diverse sample of extant great apes to provide an interpretive framework for early hominin relative limb rigidities that may facilitate recognition of subtle pattern shifts in limb use. Specifically, we evaluate taxonomic and ecomorphological diversity within this framework by quantifying femoral‐humeral rigidity ratios in all commonly recognized African ape species and subspecies.
1.1. African ape taxonomy
Genetically derived phylogenetic relationships among the great apes are generally agreed upon with competing alternatives emphasizing differences in divergence times rather than differences in branching patterns. The family Hominidae consists of two subfamilies, Ponginae and Homininae, 1 that diverged more than 15 mya (Locke et al., 2011; Prado‐Martinez et al., 2013; Moorjani, Amorim, Arndt, & Przeworski, 2016; Almécija et al., 2021). Pongo is the only extant genus encompassed within Ponginae, and comprises two species, Pongo pygmaeus and Pongo abelii (Groves, 2001). The Homininae, on the other hand, include three extant genera: Gorilla, Pan, and Homo with Gorilla the first to diverge from stem hominines, and separate Homo and Pan lineages subsequently arising 6.5–9.3 mya (Moorjani, Amorim, Arndt, & Przeworski, 2016; Almécija et al., 2021). Gorilla consists of two species, G. beringei and G. gorilla (Groves, 2018; Xue et al., 2015), with both species further consisting of two subspecies (summarized in Groves, 2018). Gorilla beringei consists of G. b. graueri and G. b. beringei, while Gorilla gorilla includes the subspecies G. g. gorilla and G. g. diehli. The genus Pan is also split into two species, P. paniscus and P. troglodytes (Groves, 2018). While no subspecies have been designated within P. paniscus, we adhere to the consensus view that Pan troglodytes comprises four subspecies; P. t. verus, P. t. ellioti, P. t. troglodytes, and P. t. schweinfurthii [Das et al., 2014, although see Groves, 2018 for a fifth subspecies].
Among free‐ranging great apes, admixture has been documented between geographically adjacent taxa such as P. t. troglodytes and P. t. schweinfurthii (Hvilsom et al., 2013). Even modern ranges being geographically separated by 1000 km or more (e.g., those of Gorilla species) has not prevented small amounts of hypothesized admixture within the last 100,000 years (Scally et al., 2012; de Manuel et al., 2016). By comparison, hybridization within Pan or Gorilla species during captivity, such as in zoos, occurs to a substantially greater extent than has been documented in free‐ranging populations in natural habitats (Hvilsom et al., 2013). Admixture has practical implications in morphological studies such as the present one, for example, when trying to isolate unique features of specific taxa (e.g., cross‐sectional geometric properties of diaphyses) that may be under varying degrees of genetic control. While bone functional adaptations in limb diaphyses are genetically constrained to some extent, and likely are influenced by multiple other non‐behavioral factors such as diet, temperature, and hormonal fluctuations, there is a strong case to be made for activity patterns over the lifetime as being their primary determinant (Pearson & Lieberman, 2004; Ruff, Holt, & Trinkaus, 2006; Shaw & Stock, 2009; Carlson & Marchi, 2014; Saers et al., 2021). Thus, by prioritizing skeletal material from wild‐caught individuals in comparisons of diaphyseal structure, the potential signal in form‐function relationships will be less obscured by the possibility of taxonomic uncertainty due to admixture.
1.2. African ape form‐function diversity
Behavioral observation studies of African apes have documented variability in positional behavior attributable to subspecies or population (Table 1; Hunt, 1992; Doran & Hunt, 1994; Doran, 1996), age (Doran, 1997; Sarringhaus et al., 2014), sex (Doran, 1993b; White et al., 2020), and body size (Hunt, 1994). Some have broadly characterized chimpanzees as more arboreal (climbing) than gorillas in comparative investigations of hominoid long bone diaphyseal structure (Marchi, 2005, 2007, 2015a; Ruff, 2002), as well as when inferring form‐function relationships in homininan long bones (Marchi, 2015b; Marchi et al., 2019). While there are no behavioral observation data on percentage of time spent arboreal vs. terrestrial for western gorillas, they are thought to be more arboreal than the better studied eastern gorillas (especially mountain gorillas), based off of seasonal arboreal feeding and nesting behavior (Brugiere & Sakom, 2001; Doran, 1997; Masi, 2004; Masi, Cipolletta & Robbins, 2009; Mehlman & Doran, 2002; Ostrofsky & Robbins, 2020; Remis, 1999; Tutin, Parnell, White & Fernandez, 1995). Additionally, lower elevation eastern gorilla groups have been recently shown to utilize arboreal behaviors more than previously thought (Neufuss et al., 2017, 2018). Bonobos were long thought to be more arboreal than chimpanzees (Susman, 1984; Doran, 1993a; Crompton et al., 2010), however, lack of full habituation made accurate estimates of rates of overall levels of both arboreality and arboreal locomotion impossible (Doran, 1993a). More recent positional behavior work from a habituated community, Lui Katole, suggests that bonobos are not more arboreal than chimpanzees, calling into question this long‐held belief (Lomako: Doran, 1989, 1993a; Lui Kotale: Ramos, 2014; Table 1). Re‐evaluation of relative rigidities in African ape humeri and femora would be timely in light of these behavioral studies. Ultimately, we encourage caution when broadly characterizing behavioral repertoires across great ape genera to avoid obscuring potentially informative form‐function links in their postcranial skeleton, especially in features that are demonstrably plastic (e.g., long bone diaphyseal cross‐sectional geometric properties).
Within extant ape species, limited subspecific comparisons of (and between) forelimb and hindlimb strengths have mirrored relative differences observed in apparent limb involvement in positional repertoires. More arboreal western gorillas (Gorilla gorilla) exhibited greater forelimb to hindlimb strength proportions compared to more terrestrial eastern gorillas (Gorilla beringei; Ruff, 2002; Ruff, Burgess, Bromage, Mudakikwa, & McFarlin, 2013; Ruff et al., 2018). In an examination of ratios of cross‐sectional diaphyseal properties of gorilla species and subspecies, Ruff et al. (2018) observed an altitudinal trend among groups whereby high elevation G. b. graueri were more similar to G. b. beringei than G. g. gorilla in both cross‐sectional geometric properties and the likely degree of arboreality, while low elevation G. b. graueri were more similar to G. g. gorilla in these respects.
Investigating free‐ranging populations in archetypal habitats where locomotor patterns have been documented at the individual level through observation studies could maximize the likelihood of uncovering form‐function signals associated with representative positional behavior patterns. Carlson et al. (2008, 2011) documented humeral diaphyseal differences in shape ratios (Imax/Imin) in more arboreal Taï chimpanzees (P. t. verus) compared to several habituated chimpanzee populations (P. t. schweinfurthii) occupying more open habitats with more variable vertical relief in the terrain. This previous work attributed the unique aspects of the Taï chimpanzee humeri to potentially more multidirectional loading of their upper limb during more frequent arboreal behavior (Carlson et al., 2008, 2011). Unfortunately, Carlson et al. (2008, 2011) did not conduct comparisons between upper versus lower limb diaphyses in these groups.
1.3. Study objectives
In this study, we aim to compare a large and geographically diverse extant ape sample at generic and species levels using free‐ranging individuals. We also aim to assess subspecies level patterns within both Pan and Gorilla. We test two hypotheses using a series of predictions. First, we investigate whether extant great ape species exhibit similar rigidity ratios in their femoral and humeral diaphyses (Hypothesis 1). We predict that orangutans and humans will bracket African apes, whereby orangutans will exhibit the lowest femoral‐humeral ratios given their propensity for greater frequencies of forelimb‐dominated arboreal and suspensory positional behaviors and modern humans will exhibit the highest femoral‐humeral ratios given their obligate bipedalism. Additionally, we predict that Pan and Gorilla species will be intermediate given greater overlap in their limb use during documented positional repertoires. Among the African apes, we further predict that the relationship between limb ratios will inversely rank according to degree of arboreality and arboreal locomotion recorded in behavioral observation studies (i.e., the highest femoral‐humeral ratios are expected in eastern gorillas followed by western gorillas, bonobos, and then the lowest femoral‐humeral ratios are expected in chimpanzees).
Secondly, we investigate whether African ape species and subspecies within Pan and Gorilla exhibit similar rigidity ratios of femoral and humeral diaphyses (Hypothesis 2). We predict that P. t. verus will exhibit the lowest limb ratios of any chimpanzee subspecies because of a greater arboreal component in its positional repertoire (i.e., greater forelimb involvement in arboreal weight‐bearing activities; Table 1). We also predict that bonobos will exhibit a similar ratio to chimpanzee subspecies because of the recent behavioral evidence suggesting less emphasis in their positional behavior on arboreal locomotion than long thought (Table 1). Finally, we evaluate the prediction that G. b. graueri will exhibit intermediate limb ratios to those of G. g. gorilla and G. b. beringei, similar to what has been previously reported by Ruff et al. (2018) because of the presumed intermediate (in frequency) arboreal component in the overall positional repertoire of this group.
2. MATERIALS AND METHODS
2.1. Samples
Humeri and femora from 254 free‐ranging, adult great apes were investigated (Table 2). Cumulatively, these samples incorporate a majority of the currently recognized taxonomic diversity in Pan and Gorilla (Table 2). We excluded P. t. ellioti and G. g. diehli as discrete groups in this study, however, due to unavailable or small samples of specimens from both taxa. Data from non‐human African apes in this sample have been published previously (Sumner, Morbeck, & Lobick, 1989; Carlson, 2005; Carlson et al., 2006; Carlson et al., 2008; Carlson et al., 2011; Sarringhaus et al., 2016). The chimpanzee sample also includes known individuals from free‐ranging habituated groups (Carlson et al., 2006; Carlson et al., 2011). The orangutan sample consists of six adult Pongo abelii individuals from the collection of the National Museum of Natural History (NMNH), Washington DC, USA. The modern human sample consists of adult Homo sapiens individuals from the Raymond A. Dart Collection of Modern Human Skeletons in the School of Anatomical Sciences of the University of the Witwatersrand, Johannesburg, South Africa (Dayal et al., 2009). Adult status of each individual was assessed according to epiphyseal fusion and, where possible, eruption of third molars. Sex determination was based on information obtained from collection records. When sex information was not recorded, individuals were designated as ‘unknown’ sex. Only humeri and femora of individuals with no apparent trauma or movement‐altering pathologies were used in this study. In addition to the targeted humeri and femora, we inspected other bones in each skeleton for trauma or pathology. Minor evidence of a degenerative joint condition was not considered an exclusionary criterion for the purposes of this study. Preference was given to using the ipsilateral humerus and femur, but in a minority of cases it was necessary to mix sides within an individual.
TABLE 2.
Taxonomic distribution of samples (N = 254)
| Sex* | |||||
|---|---|---|---|---|---|
| Taxon | F | M | U | T | Museum or location |
| Pongo abelii | 1 | 5 | 0 | 6 | NMNH |
| Pan troglodytes | 50 | 43 | 37 | 130 | AIMUZ, AMNH, BMNH, CMNH, LHU, MNHU, NMNH, PCM, RMCA, Gombe, Kibale |
| P. t. troglodytes a | 22 | 16 | 18 | 56 | |
| P. t. schweinfurthii | 15 | 22 | 17 | 54 | |
| P. t. verus | 13 | 5 | 2 | 20 | |
| Pan paniscus | 8 | 4 | 0 | 12 | RMCA |
| Gorilla gorilla b | 19 | 27 | 7 | 53 | AIMUZ, AMNH, BMNH, MNHU, NMNH, PCM, RMCA, |
| Gorilla beringei c | 18 | 19 | 4 | 41 | AIMUZ, AMNH, BMNH, MNHU, NMNH, RMCA |
| G. b. beringei | 11 | 11 | 0 | 22 | |
| G. b. graueri | 7 | 8 | 4 | 19 | |
| Homo sapiens | 5 | 7 | 0 | 12 | RADUW |
Abbreviations: AIMUZ, Anthropolisches Institut und Museum der Universität Zürich‐Irchel, Zürich, Switzerland; AMNH, American Museum of Natural History, New York, NY, USA; BMNH, British Museum of Natural History, London, UK; CMNH, Cleveland Museum of Natural History, Cleveland, OH, USA; LHU, Laboratory of Human Evolution, Kyoto University, Kyoto, Japan; MNHU, Museum für Naturkunde der Humboldt Universität, Berlin, Germany; NMNH, National Museum of Natural History, Washington, DC, USA; PCM, Powell‐Cotton Museum, Birchington, Kent, UK; RADUW, Raymond A. Dart Collection, University of the Witwatersrand, Johannesburg, SA; RMCA, Musée Royal de l’ Afrique Centrale, Tervuren, Belgium.
M, male; F, female; U, undetermined; T, total number.
Including two individuals (50% ROI only) who may be P. t. ellioti based on their port of origin provided in collection records. These two individuals were included in the P. t. troglodytes group as results did not differ with the inclusion or exclusion of both specimens.
Gorilla gorilla is comprised of 52 G. g. gorilla specimens and one G. g. diehli specimen, which was included as results did not vary with the inclusion or exclusion of this specimen.
2.2. Data acquisition
Single slice computed tomography (CT) images were collected from three specific regions of interest (ROI) along diaphyses corresponding to 35%, 50% and 65% femoral mechanical length and humeral maximum length (Figure 1), where the distal ends of femora and humeri represent 0% length (see Carlson, 2005 for additional protocol details on specimen positioning and leveling). For the majority of African apes, this resulted in three CT slices being obtained from each diaphysis. While there is some indication that adjacent diaphyseal regions may show broad similarities with these targeted ROIs (Davies & Stock, 2014; Mongle et al. 2015a, 2015b), we chose these three specific locations to create representative comparisons with published data and also incomplete bones, such as fossils, that may not preserve entire diaphyses.
FIGURE 1.

Locations of femoral (left) and humeral (right) diaphyseal regions of interest (35%, 50%, and 65% length) analyzed in the present study.
Other samples were represented by serial image data sets of entire bones. Orangutan cross‐sectional data were collected following the same protocol as African apes, but with modifications made for digitally positioning and extracting cross sections of interest from three‐dimensional image data sets of whole bones rather than physically positioning bones on a scanner bed prior to the image acquisitions and collecting single slice CT scans of leveled bones (see Carlson et al., 2008 for protocol details). Modern human data were also collected from serial image acquisitions of entire bones. The human image data were processed according to a published protocol for conducting structural analyses of hominoid metatarsals (Dowdeswell et al., 2017; Jashashvili et al., 2015) that was adapted for use with humeri and femora in the present study.
Scan parameters for use with medical CT scanners [e.g., a range of pixel dimensions (0.352–0.500 mm) and slice thicknesses (1.0–2.0 mm)] and high resolution CT scanners (e.g., voxel dimensions of 45 or 93 μm) during the acquisition of non‐human African ape data have been reported elsewhere (i.e., medical CT scanners: Sumner, Morbeck, & Lobick, 1989; Carlson, 2002; Carlson, 2005; Yamanaka, Gunji, & Ishida, 2005; Carlson, Doran‐Sheehy, Hunt, Nishida, Yamanaka, & Boesch, 2006; Carlson, Sumner, Morbeck, Nishida, Yamanka, & Boesch, 2008; Carlson, Wrangham, Muller, Sumner, Morbeck, Nishida, Yamanaka, & Boesch, 2011; high resolution CT scanners: Sarringhaus, MacLatchy, & Mitani, 2016). Orangutan femora and humeri were scanned using a Siemens Emotion 6 medical CT scanner in the Department of Anthropology at the National Museum of Natural History, Washington D.C. (Smithsonian). Scan parameters included: tube voltage = 130 kVp, tube current = 98 mA, exposure time = 600 ms, slice thickness = 0.63 mm, pixel resolutions between 0.199–0.371 mm, a 512 × 512 matrix, and an inner ear ‘bone’ reconstruction kernel (H90s). From scan data, we generated 16‐bit DICOM images. Modern human femora and humeri were scanned using the Nikon Metrology XTH 225/320 LC dual source high resolution CT scanner in the Evolutionary Studies Institute of the University of the Witwatersrand (see https://wits.ac.za/microct).
2.3. Measurements
Once slices corresponding to 35%, 50%, and 65% ROIs were identified in non‐human ape diaphyses, they were processed in the freeware program, Scion Image (release Beta 4.0.2; see Carlson, 2005 for additional details), with a customized macro modeled after the SLICE program (Nagurka & Hayes, 1980; see Carlson, 2005 for additional details). Modern human cross sections were analyzed in BoneJ (Doube et al., 2010) using the Slice Geometry macro. We use the polar moment of area, J (mm4), to assess (twice average) bending rigidities, or torsional rigidity. It is equivalent to the sum of any two perpendicular second moments of area in a cross section (e.g., Imax + Imin or Ix + Iy). The polar moment of area has been argued to be the single best estimator of bone loading, better than any second moment of area, when direct diaphyseal loading data are unavailable (Lieberman, Polk, & Demes, 2004). Since relative limb rigidity measures are of primary interest, we do not standardize bone‐specific structural properties (i.e., J) by bone length nor by the product of bone length and body mass since these would effectively cancel out in ratios calculated from the same individuals. The range of reported body sizes for great ape taxa in our study is within an order of magnitude (Smith & Jungers, 1997), thus log‐transforming structural properties was deemed unnecessary for the purposes of this investigation.
2.4. Analyses
In order to conduct group comparisons at species and subspecies levels with subsample sizes that permitted statistical rigor, we followed others who pooled males and females (Shaw & Ryan, 2012; Patel, Ruff, Simons, & Organ, 2013; Ruff et al., 2018). Prior to performing statistical testing, we explored variables of interest using several approaches. We assessed normality of distributions using Kolmogorov–Smirnov (K‐S) tests. In practically all cases, data distributions did not depart significantly from normal distributions (Gorilla gorilla K‐S test, p = 0.05; all other group K‐S tests, p > 0.05). Thus, transforming data (e.g., log‐transformation) was deemed unnecessary. Following these assessment methods, we used a one‐way ANOVA to evaluate statistical differences in femoral‐humeral J ratios between groups of species or subspecies (i.e., to test Hypotheses 1 & 2, respectively; Sokal & Rohlf, 1995). All pairwise comparisons evaluating explicit predictions were assessed using the Tamhane post hoc test due to unequal variances at species (i.e., 35% ROI: Levene Statistic = 23.124, df = 5, p < 0.001; 50% ROI: Levene Statistic = 25.663, df = 5, p < 0.001; and 65% ROI: Levene Statistic = 20.944, df = 5, p < 0.001) and subspecies levels (i.e., 35% ROI: Levene Statistic = 4.112, df = 6, p = 0.001; 50% ROI: Levene Statistic = 3.101, df = 6, p = 0.006; and 65% ROI: Levene Statistic = 3.902, df = 6, p = 0.001).
3. RESULTS
3.1. Hominoid interspecific differences (Hypothesis 1)
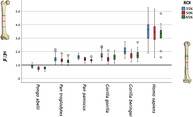
Hominoid species exhibit significant differences in femoral‐humeral (F/H) J ratios at all three ROIs (35%: ANOVA F = 105.800, df = 5, p < 0.001; 50%: ANOVA F = 128.766, df = 5, p < 0.001; 65%: ANOVA F = 97.937, df = 5, p < 0.001; Tables 3 & 4, Figure 2). Tamhane post hoc analyses consistently reveal that P. abelii exhibits significantly lower F/H J ratios than those of all other great ape species at each of the three ROIs (i.e., for all 15 pairwise comparisons, p ≤ 0.005; Table 4, Figure 2). Humans, on the other hand, consistently exhibit significantly larger F/H J ratios compared to those of all other great ape species at each of the three ROIs (i.e., for all 15 pairwise comparisons, p ≤ 0.011; Tables 3 & 4, Figure 2). Among the other African ape species, P. troglodytes exhibits significantly lower F/H J ratios compared to those of both Gorilla species: G. gorilla and G. beringei (Tables 3 & 4, Figure 2). Interestingly, P. paniscus exhibits F/H J ratios that are intermediate between those of P. troglodytes and Gorilla species (Figure 2), although only the differences from Gorilla species are statistically significant (Table 4). Specifically, P. paniscus exhibits F/H J ratios that are lower than those of both Gorilla species at the 65% ROI, but only lower than G. beringei at 35% and 50% ROIs (Table 4).
TABLE 3.
Species mean RAW JF/JH
| 35% | 50% | 65% | |
|---|---|---|---|
| Pongo abelii | 0.930 | 0.733 | 0.755 |
| (0.162) | (0.145) | (0.129) | |
| 0.740‐1.202 | 0.524‐0.924 | 0.532‐0.883 | |
| Pan troglodytes | 1.441 | 1.351 b | 1.289 |
| (0.196) | (0.160) | (0.172) | |
| 0.995‐2.161 | 0.991‐1.861 | 0.944‐1.740 | |
| P. t. troglodytes | 1.460 | 1.356 | 1.316 |
| (0.212) | (0.153) | (0.172) | |
| 1.050‐2.161 | 1.069‐1.861 | 0.969‐1.740 | |
| P. t. schweinfurthii | 1.445 | 1.354 | 1.261 |
| (0.202) | (0.173) | (0.172) | |
| 0.995‐1.822 | 0.991‐1.716 | 0.944‐1.646 | |
| P. t. verus | 1.382 | 1.328 | 1.303 |
| (0.118) | (0.148) | (0.167) | |
| 1.104‐1.601 | 1.041‐1.578 | 0.973‐1.647 | |
| Pan paniscus | 1.611 | 1.429 | 1.370 |
| (0.171) | (0.148) | (0.129) | |
| 1.382‐1.896 | 1.105‐1.603 | 1.141‐1.641 | |
| Gorilla gorilla | 1.734 | 1.455 | 1.597 |
| (0.242) | (0.224) | (0.251) | |
| 1.340‐2.385 | 1.073‐2.077 | 1.105‐2.119 | |
| Gorilla beringei | 2.044 | 1.705 | 1.748 |
| (0.321) | (0.244) | (0.293) | |
| 1.471‐2.750 | 1.220‐2.187 | 1.243‐2.692 | |
| G. b. graueri | 1.917 | 1.658 | 1.589 |
| (0.298) | (0.237) | (0.204) | |
| 1.471‐2.485 | 1.220‐2.168 | 1.243‐1.961 | |
| G. b. beringei | 2.153 | 1.744 | 1.886 |
| (0.305) | (0.247) | (0.291) | |
| 1.550‐2.750 | 1.304‐2.187 | 1.503‐2.692 | |
| Homo sapiens a | 3.767 | 3.400 b | 3.537 |
| (1.291) | (1.005) | (1.327) | |
| 1.871‐6.565 | 1.546‐5.181 | 1.574‐6.781 |
Note: Cells report mean values, standard deviations in parentheses, and minimum to maximum ranges for each region of interest.
Human ratios have a large range, which may be due in part to the sample being comprised of individuals from different ages (20–90 years) and ethnic groups. The mean for the 50% ROI, however, is similar to published values (Shaw & Ryan, 2012).
Comparative 50% ROI values from Shaw & Ryan (2012) for Pan troglodytes (N = 17, mean = 1.34, SD = 0.15) and Homo sapiens (N = 20, mean = 3.47, SD = 0.80) are similar to values reported in the present study.
TABLE 4.
Species ANOVA post hoc results for RAW JF/JH at each ROI
| P. abelii | P. troglodytes | P. paniscus | G. gorilla | G. beringei | H. sapiens a | |
|---|---|---|---|---|---|---|
| J 35% | ||||||
| P. abelii | X | 0.005 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| P. troglodytes | X | 0.086 | <0.001 * | <0.001 * | 0.001 * | |
| P. paniscus | X | 0.537 | <0.001 * | 0.002 * | ||
| G. gorilla | X | <0.001 * | 0.003 * | |||
| G. beringei | X | 0.011 * | ||||
| J 50% | ||||||
| P. abelii | X | 0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| P. troglodytes | X | 0.811 | 0.042 * | <0.001 * | <0.001 * | |
| P. paniscus | X | 1.000 | 0.001 * | <0.001 * | ||
| G. gorilla | X | <0.001 * | <0.001 * | |||
| G. beringei | X | 0.002 * | ||||
| J 65% | ||||||
| P. abelii | X | 0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| P. troglodytes | X | 0.631 | <0.001 * | <0.001 * | 0.002 * | |
| P. paniscus | X | 0.001 * | <0.001 * | 0.002 * | ||
| G. gorilla | X | 0.136 | 0.005 * | |||
| G. beringei | X | 0.010 * |
Note: Species ANOVA results for RAW data, while Tamhane used for post hoc analysis as Levene's test was significant: 35% ANOVA F = 105.800, df = 5, p < 0.001; 50% ANOVA F = 128.766, df = 5, p < 0.001; and 65% ANOVA F = 97.937, df = 5, p < 0.001.
Bold and asterisk denote a significant result. Italicized denotes a borderline non‐significant result (0.05–0.099). Regular font denotes non‐significant results.
Results between the other species remained the same when humans were removed at all ROIs, i.e., the same post hoc comparisons were significant/non‐significant.
FIGURE 2.

Species boxplot of femoral J to humeral J ratios for each region of interest. Reference line = 1.0 in which the femur and humerus are equivalent in value. Two human outliers were removed for easier visualization of data but the original figure has been included in supplementary figures for the sake of transparency.
3.2. Hominoid intraspecific differences (Hypothesis 2)
Intraspecific comparisons within Pan and Gorilla also reveal significant differences in F/H J ratios at all three ROIs (35%: ANOVA F = 40.119, df = 6, p < 0.001; 50%: ANOVA F = 17.997, df = 6, p < 0.001; 65%: ANOVA F = 34.730, df = 6, p < 0.001; Table 5, Figure 3). Subspecies of Pan troglodytes do not differ significantly from one another at any of the three ROIs (i.e., for all 6 pairwise comparisons, p > 0.735; Table 5, Figure 3). Notably, only P. t. verus among the chimpanzee subspecies exhibits a significantly lower F/H J ratio than that of P. paniscus, and only at the 35% ROI (p = 0.016; Table 5, Figure 3). All F/H J ratios of G. b. graueri do not differ significantly from the lower ratios of G. g. gorilla, while all F/H J ratios of G. b. beringei are significantly greater than those of G. g. gorilla (Table 5, Figure 3). The F/H J ratios of G. b. beringei also consistently exceed those of G. b. graueri, although this difference is significant only at the 65% ROI (Table 5; Figure 3).
TABLE 5.
Subspecies ANOVA post hoc results for RAW JF/JH at each ROI
| P.t. s. | P. t. v. | P. p. | G. g. g. | G. b. g. | G. b. b. | |
|---|---|---|---|---|---|---|
| J35% | ||||||
| P. t. t. | 1.000 | 0.735 | 0.311 | <0.001 * | <0.001 * | <0.001 * |
| P. t. s. | X | 0.909 | 0.168 | <0.001 * | <0.001 * | <0.001 * |
| P. t. v. | X | 0.016 * | <0.001 * | <0.001 * | <0.001 * | |
| P. p. | X | 0.659 | 0.022 * | <0.001 * | ||
| G. g. g. | X | 0.388 | <0.001 * | |||
| G. b. g. | X | 0.305 | ||||
| J50% | ||||||
| P. t. t. | 1.000 | 1.000 | 0.959 | 0.161 | 0.001 * | <0.001 * |
| P. t. s. | X | 1.000 | 0.957 | 0.191 | 0.001 * | <0.001 * |
| P. t. v. | X | 0.821 | 0.169 | <0.001 * | <0.001 * | |
| P. p. | X | 1.000 | 0.051 | 0.001 * | ||
| G. g. g. | X | 0.059 | 0.001 * | |||
| G. b. g. | X | 0.998 | ||||
| J65% | ||||||
| P. t. t. | 0.993 | 1.000 | 0.997 | <0.001 * | <0.001 * | <0.001 * |
| P. t. s. | X | 1.000 | 0.385 | <0.001 * | <0.001 * | <0.001 * |
| P. t. v. | X | 0.994 | <0.001 * | 0.001 * | <0.001 * | |
| P. p. | X | 0.002 * | 0.022 * | <0.001 * | ||
| G. g. g. | X | 1.000 | 0.005 * | |||
| G. b. g. | X | 0.010 * |
Bold and asterisk denote a significant result. Italicized denotes a borderline non‐significant result (0.05–0.099). Regular font denotes non‐significant results. Species ANOVA results for RAW data, while Tamhane used for post hoc analysis as Levene's test was significant: 35% ANOVA F = 40.119, df = 6, p < 0.001; 50% ANOVA F = 17.997, df = 6, p < 0.001; and 65% ANOVA F = 34.730, df = 6, p < 0.001.
FIGURE 3.

Subspecies boxplot of femoral J to humeral J ratios for each region of interest.
4. DISCUSSION
4.1. Species level diversity in interlimb rigidity ratios
Femoral‐humeral J ratios varied significantly between great ape species with post hoc tests confirming that Pongo and Homo bracket the African apes, with arboreal Pongo exhibiting lower F/H J ratios and terrestrial bipedal Homo exhibiting higher F/H J ratios compared to all other groups in the study (Table 4). These results were consistent across the three ROIs and support broad comparability of our results with those from previous investigations of diaphyseal rigidity at hominoid femoral and humeral midshafts (Ruff, 2002; Shaw & Ryan, 2012). Among great apes, the relative ranking of mean F/H J ratios was as predicted, with chimpanzees < bonobos < western gorillas < eastern gorillas for all ROIs (Table 3). Diaphyseal rigidity ratios of chimpanzees and bonobos differed significantly from western gorillas, including at some non‐midshaft ROIs. Specifically, chimpanzees exhibited significantly lower F/H J ratios than western gorillas at all three ROIs, while bonobos exhibited significantly lower F/H J ratios than western gorillas only at the 65% ROI, which is the only ROI where the two gorilla species did not differ significantly (Figure 2). In the present study, non‐midshaft ROIs ultimately expanded the diaphyseal signal that differentiates groups.
While mean F/H J ratios were indeed higher in bonobos than chimpanzees (Table 3), as predicted based on the behavioral data from Lui Kotale (Ramos, 2014), it must be noted that post hoc tests did not support this prediction with statistical significance (Table 4). Compared to chimpanzees, bonobos from Lui Kotale spend less overall time arboreally and less locomotor time arboreally (Table 1). Morphological results of the present study appear to confirm what the behavioral data from Lui Kotale may lead one to infer (Ramos, 2014): the absence of significant differences in F/H J ratios of bonobos and chimpanzees suggests that long‐held beliefs about arborealism in bonobos must be revised (Susman, 1984, Doran, 1993a, Crompton et al., 2010). The general behavioral similarity between bonobos and western gorillas may partially explain why F/H J ratios did not differ significantly at either the 35% or 50% ROIs between these two groups, even though chimpanzees did exhibit lower F/H J ratios than western gorillas (Table 4). Further behavioral observation studies of ape taxa (e.g., bonobos and western gorillas) would be helpful in corroborating these results, as may targeted comparisons between bonobos and groups of chimpanzees with known contextual information on habitat and behavioral repertoires.
To the extent that F/H J ratios may reflect ape body plans, the bonobo results offer additional insights. Bonobos have been previously suggested to represent the subadult form of chimpanzees in both morphology and behavior (i.e., paedomorphism and greater arboreality, Doran, 1992). Results of the present study, however, do not support this idea since bonobo F/H J ratios were not indicative of a significantly more arboreal body plan in the former compared to chimpanzees. Bonobos have also been argued to exhibit a more basal body plan with regards to the human‐chimpanzee last common ancestor (HCLCA: Diogo, Molnar, and Wood, 2017; Hunt, 2020). Their apparent intermediate degree of arboreality between that of chimpanzees and gorillas, supported by their somewhat intermediate F/H J ratios in this study, are consistent with the notion that arboreal locomotor behavior in the HCLCA may have been more bonobo‐like than chimpanzee‐like in frequency. Additional behavioral observation studies of bonobos would offer opportunities to more robustly contextualize the Lui Kotale results.
Intrageneric differences between gorilla species were observed at the midshaft ROI in the present study, which is consistent with the overall pattern reported in studies by Ruff et al. (2013, 2018). These authors observed that western gorillas exhibited lower femoral/humeral Zp ratios compared to eastern gorillas in comparisons of 50% femur to 40% humerus. In the present study, we have extended the range of significant differences between gorilla species to include non‐midshaft ROIs. Femoral‐humeral J ratios differed between gorilla species at both 35% and 50% ROIs, but no significant differences were observed at the 65% ROI (Table 4). This suggests that interlimb structural differences between gorilla groups are highest in the midshaft or mid‐distal diaphysis compared to more proximal diaphyseal locations (we address variation later in the discussion).
In addition to calling for more observational studies of habituated apes, as in any interspecific analysis, we point to the possibility that phylogeny influences patterns of F/H J ratios. One way to incorporate phylogeny into these analyses would be to conduct a phylogenetic ANOVA on F/H J ratios and the degree of arboreality. The small sample size (e.g., n = 6 species) and an incomplete behavioral record for degree of arboreality in all our subgroups (e.g., P. t. troglodytes), however, limits the utility of this approach in the current study. Specifically, estimates of phylogenetic signal are unreliable when phylogenies include fewer than 20 taxa (Freckleton et al., 2002; Münkemüller et al., 2012), often resulting in large confidence intervals on phylogenetic signal estimates such as Pagel's lambda.
4.2. Intraspecific diversity in interlimb rigidity ratios
Counter to our predictions of intraspecific differences (Hypothesis 2), P. t. verus did not exhibit lower F/H J ratios compared to the two other P. troglodytes subspecies investigated in the present study. Notably, P. t. verus did exhibit a significantly lower F/H J ratio at the 35% ROI compared to P. paniscus, but analogous F/H J ratios of P. t. schweinfurthii and P. t. troglodytes were not significantly lower than that of P. paniscus. Since the P. t. verus sample largely consisted of individuals from the Taï Forest population (Carlson et al., 2006, 2008), which has a relatively high component of closed forest in their home range, a more substantial difference in F/H J ratios was expected due to their higher levels of arboreality. It may be the case that comparisons undertaken at a subspecies level, such as in the present study, inadvertently conflate localized differences in habitat conditions. For example, we would predict that chimpanzees from the opposite end of the habitat spectrum to the Taï Forest population (e.g., groups occupying dry, open habitats, such as those at Fongoli, Mt. Assirik, or Semliki) may show a more dramatic difference in F/H J ratios. Such a comparison would more effectively dichotomize documented variation in chimpanzee habitat conditions and probably behavioral repertoires too. The existence of a potentially distinct signal in F/H J ratios of dry habitat chimpanzees is especially intriguing given recent reporting of other ecomorphological skeletal features in such groups, for example, more human‐like bicondylar angles characterize femora of Semliki chimpanzees compared to femora from forest‐dwelling chimpanzees (Hunt, Dunevant, Yohler, & Carlson, 2021).
Our prediction that G. b. graueri would exhibit intermediate F/H J ratios to those of G. g. gorilla and G. b. beringei was partially supported in all three ROIs. G. g. gorilla exhibited significantly lower F/H J ratios than did G. b. beringei, but the former did not differ significantly from G. b. graueri at any of the three diaphyseal locations (Table 5). Moreover, G. b. graueri exhibited lower F/H J ratios at the 65% ROI, but not at the 35% and 50% ROIs compared to G. b. beringei. These results are consistent with the findings of Ruff et al. (2018) who compared 50% femur Zp to 40% humerus Zp, observing that G. b. graueri was intermediate between G. b. beringei and G. g. gorilla Ruff et al. (2018) further divided G. b. graueri into lowland and highland groups, noting that lowland G. b. graueri was more similar to G. g. gorilla than G. b. beringei, with highland individuals being intermediate between the groups. Our results extend this intermediate designation of G. b. graueri between G. g. gorilla and G. b. beringei to additional areas of the diaphysis beyond the midshaft.
Across species groups, the F/H J ratios at 35% ROIs were consistently higher compared to those at 50% and 65% ROIs (Figures 2 and 3). Changes in ratios along the shaft may be a reflection of an increase in humeral values more than a decrease in femoral values between the 35% ROI on the one hand and the 50% and 65% ROIs on the other hand (e.g., see trends in Figures S1‐S3). One potential explanation for the observed pattern along diaphyses may be the presence of the deltoid insertion, which spans parts of the proximal and lateral surface of the great ape humerus (see Gómez et al., 2020). The most prominent area of the diaphyseal attachment site is typically the most distal portion of the attachment, which often coincides with the midshaft region in great ape humeri (Swindler & Wood, 1973). For example, Larson (1998) reported that the distal extent of the deltoid insertion in great apes (Pongo 52%, Pan 45%, and Gorilla 46% length) narrowly varied in its distance from the distal end of the humerus. There is precedence in the literature for avoiding the deltoid tuberosity when measuring humeral cross‐sectional properties (e.g., see Marchi, Ruff, Capobianco, Rafferty, Habib, & Patel, 2016; Patel, Ruff, Simons, & Organ, 2013), as it has been suggested to unduly complicate diaphyseal loading of the shaft as estimated by cross‐sectional properties (Ruff, 2002). Here, we deliberately chose to sample the midshaft to intentionally incorporate this complexity, as other ROIs of the diaphysis also are not excluded due to consideration of local muscle attachments.
Based on comparisons of cross‐sectional geometric properties from the three ROIs in the present study, the 35% ROIs appear to be more successful in differentiating groups than midshafts or 65% ROIs. This again underscores the value of diaphyseal ROIs in addition to midshaft when differentiating internal structure in hominoid femoral and humeral diaphyses. Interestingly, Mongle, Wallace, & Grine (2015a) observed that Pan exhibited a change in J values along the humeral shaft when comparing other ROIs to midshaft while Gorilla did not display the same trend. In the present study, this difference between the African ape genera was supported by F/H J ratios in Pan being smaller at 65% ROIs and greater at 35% ROIs when comparing them to midshaft F/H J ratios, while Gorilla did not exhibit the same trends across the three ROIs (i.e., midshaft ROIs tended to exhibit the lowest F/H J ratios in gorillas rather than intermediate ratios).
More fully documenting variability in F/H J ratios within African apes, including extending investigations to non‐midshaft locations, offers a more rigorous framework for evaluating F/H J ratios in stem homininans. In the present study, African ape subspecies displayed a range of F/H J ratios where P. t. verus generally exhibited the lowest ratios (e.g., mean F/H J ratios in ROIs ranging between 1.30–1.38), while G. b. beringei generally exhibited the highest ratios (e.g., mean F/H J ratios of ROIs between 1.74–2.15). Obtaining finer resolution within these ranges may be possible by grouping individuals according to habitat conditions rather than taxonomic status. In particular, adding F/H J ratios from a sample of dry habitat chimpanzees could be especially illuminating for developing insights into relative limb use by stem homininans. Ruff (2008) reported F/H J ratios in two H. erectus partial skeletons as 3.04 (KNM‐WT 15000) and 5.31 (KNM‐ER 1808). Values for both of these partial skeletons fall comfortably within the range of values obtained from the small representative sample of H. sapiens in the present study, and well above ranges of values observed in any other ape group. Midshaft F/H J ratios from early stem homininans, such as Homo habilis at 1.70 (OH 62: Ruff, 2009) and Australopithecus afarensis at 2.16 (A.L. 288–1: Ruff et al., 2016), on the other hand, are more like those of the extant African ape groups in the present study, even overlapping with the ranges of some of these taxa. Discoveries of additional partial skeletons (e.g., Berger et al., 2010; Clarke, 2019; Heaton et al., 2019) offer critical and exciting opportunities to more fully explore variation in relative limb use among early stem homininans.
As noted earlier, small sample sizes acted as a limitation in this study, including during the analysis of intraspecific variation. In some cases, this is unavoidable given the rarity of postcranial material of wild‐caught, adult bonobos and orangutans curated in museum collections. The substantial variation in F/H J ratios observed in our small modern human sample (i.e., 10 right humeri, 2 left humeri) may be partly attributable to humeral bilateral asymmetry. Humeral bilateral asymmetry is typically attributed to handedness in studies of human upper limb activity (Sparacello et al., 2017; Wei et al., 2020), but it is not similarly expressed at the population level in other apes (Marchant & McGrew, 2013). Exploring sources of variation in the modern human sample, such as handedness, however, is beyond the scope of the present study. Finally, while it is commonly believed that western gorillas are more arboreal than eastern gorillas (Doran, 1997; Remis, 1994, 1995, 1998; Ruff et al., 2018), another limitation in this study is the lack of available positional behavior data on fully habituated western gorilla individuals. Future observational studies providing more positional behavior data on habituated G. g. gorilla, G. b. graueri, and P. t. troglodytes individuals would be helpful in this regard.
5. CONCLUSIONS
Differences in F/H J ratios according to positional behavior patterns were easily discernible between ape groups at the species level, strengthening the call to use these ratios for interpreting the limb use and behavior of fossil specimens where femoral and humeral remains are present. This study expands on midshaft usage to demonstrate that both 35% and 65% length from the distal ends of diaphyses can be informative areas for exploring species differences in relative limb rigidity (J) ratios.
Behavioral data suggest that bonobos may be intermediate in degree of arboreality between chimpanzees and western gorillas. Diaphyseal femoral to humeral J ratios documented in the present study, in part, corroborate this aspect of their reassessed behavioral repertoire since they are also somewhat intermediate between chimpanzees and western gorillas, further indicating that diaphyseal J ratios can be useful for interpreting relative limb involvement in broad behavioral repertoires of fossil taxa. Comparisons of chimpanzee subspecies J ratios were less informative for insights into behavioral differences, likely due to the smaller shifts in behavioral repertoires that occur at this level. Evaluating chimpanzees according to habitat differences, however, may provide additional, new information. Subspecies differences in relative J ratios at one ROI (65%) were observed in eastern gorillas (Gorilla beringei), possibly due to the behavioral differences between these subspecies being more drastic.
AUTHOR CONTRIBUTIONS
Lauren Sarringhaus: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Kristi L. Lewton: Formal analysis (supporting); writing – review and editing (equal). Safiyyah Iqbal: Data curation (equal); writing – review and editing (equal). Kristian J. Carlson: Conceptualization (equal); data curation (equal); funding acquisition (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary Figure 1 Scatterplot of Femoral and Humeral J Values for Each Species at 35% Region of Interest.
Supplementary Figure 2 Scatterplot of Femoral and Humeral J Values for Each Species at 50% Region of Interest.
Supplementary Figure 3 Scatterplot of Femoral and Humeral J Values for Each Species at 65% Region of Interest.
Supplementary Figure 4 Species Boxplot of Femoral J to Humeral J Ratios for Each Region of Interest. The two human outliers excluded in Figure 2 are included here.
Appendix S1 Excel data set. The Mahale, Gombe, and Kibale chimpanzees have been excluded from this file.
ACKNOWLEDGMENTS
We acknowledge the extraordinary cooperation of several museums, hospitals, and their staffs that made this research possible. For granting access to their collections and arranging specimen loans for CT scanning, we express gratitude to the American Museum of Natural History, New York, NY; the Musee Royal de l'Afrique Centrale, Tervuren, Belgium; das Anthropologisches Institut und Museum der Universitat Zurich‐Irchel, Zurich, Switzerland; the National Museum of Natural History, Washington, DC; the Powell‐Cotton Museum, Birchington, Kent, UK; the Natural History Museum, London, UK; das Museum fur Naturkunde der Humboldt Universitat, Berlin, Germany; the Cleveland Museum of Natural History, Cleveland, OH; and the Field Museum of Natural History, Chicago, IL. We acknowledge and thank the cooperation of the government of Uganda, the Uganda National Council for Science and Technology, the Uganda Wildlife Authority, and Makerere University. We also thank Christoph Boesch, Lawrence Heaney, Adam Ferguson, John Kasenene, Zhe‐Xi Luo, M.E. Morbeck, Martin N. Muller, Toshisada Nishida, Bruce Patterson, Bill Stanley, D. Rick Sumner, Richard W. Wrangham, and Atsushi Yamanaka for enabling access to facilities and specimens. For the use of their CT facilities, we acknowledge Hammersmith Hospital, London, UK; QEQM Hospital, Margate, Kent, UK; Charite’ Hospital, Berlin, Germany; Mount Sinai Hospital, New York, NY; Universitaire Ziekenhuizen, Leuven, Belgium; Kantonsspital, Institut fur Radiologie, Winterthur, Switzerland; the Department of Anthropology, Smithsonian Institution, Washington, DC; the Cleveland Clinic, Cleveland, OH; Rush University Medical Center, Department of Radiology, Chicago, IL; the Kampala Imaging Centre, Kampala, Uganda; and the PaleoCT lab at the University of Chicago, Chicago, IL. For their personal cooperation, we offer our sincerest gratitude to the numerous people who assisted us with CT data collection: Stuart Daws, CT Superintendent; Anja Boldt, M.T.A.R.; Justine DePonte, B.Sc. (Hons.), Diagnostic Radiography; Sandra Jones Dillard, R.T., CT; Marc Verburgh; Isuf Hoxha; Chris Archer; Edward Jones; Craig Wambura; and April Neander. Without their cooperation, this research would not have been possible. Kristian J. Carlson acknowledges funding support from the National Science Foundation (DDIG BCS‐0002686; BCS‐1719140), the L.S.B. Leakey Foundation, and the Department of Anthropology and University Graduate School of Indiana University. The National Research Foundation (NRF) and the Department of Science and Innovation (DSI), South Africa, along with the Evolutionary Studies Institute at the University of the Witwatesrand, also provided financial support for this research. Lauren Sarringhaus acknowledges funding support from the National Science Foundation (BCS‐0850951) and the L.S.B. Leakey Foundation.
Sarringhaus, L. , Lewton, K. L. , Iqbal, S. , & Carlson, K. J. (2022). Ape femoral‐humeral rigidities and arboreal locomotion. American Journal of Biological Anthropology, 179(4), 624–639. 10.1002/ajpa.24632
Funding information L.S.B. Leakey Foundation, and the Department of Anthropology and University Graduate School of Indiana University; National Research Foundation (NRF) and the Department of Science and Innovation (DSI); Evolutionary Studies Institute at the University of the Witwatesrand; National Science Foundation, Grant/Award Numbers: BCS‐0850951, BCS‐1719140, DDIG BCS‐0002686
Endnote
DATA AVAILABILITY STATEMENT
Data are included in an excel data in the appendix.
REFERENCES
- Almécija, S. , Hammond, A. S. , Thompson, N. E. , Pugh, K. D. , Moya‐Sola, S. , & Alba, D. M. (2021). Fossil apes and human evolution. Science, 372, eabb4363. 10.1126/science.abb4363 [DOI] [PubMed] [Google Scholar]
- Ashbury, A. M. , Posa, M. R. C. , Dunkel, L. P. , Spillmann, B. , Atmoko, S. S. U. , van Schaik, C. P. , & van Noordwiik, M. A. (2015). Why do orangutans leave the trees? Terrestrial behavior among wild Bornean orangutans (Pongo pygmaeus wurmbii) at Tuanan, Central Kalimantan. American Journal of Physical Anthropology, 77, 1216–1122. 10.1002/ajp.22460 [DOI] [PubMed] [Google Scholar]
- Ax, P. (1985). SEM species and the stem lineage concept. Cladistics, 1, 279–287. [DOI] [PubMed] [Google Scholar]
- Barak, M. M. (2020). Bone modeling or bone remodeling: That is the question. American Journal of Physical Anthropology, 172, 153–155. [DOI] [PubMed] [Google Scholar]
- Berger, L. R. , de Ruiter, D. J. , Churchill, S. E. , Schmid, P. , Carlson, K. J. , Dirks, P. H. G. M. , & Kibii, J. M. (2010). Australopithecus sediba: A new species of homo‐like australopith from South Africa. Science, 328, 195–204. [DOI] [PubMed] [Google Scholar]
- Brugiere, D. , & Sakom, D. (2001). Population density and nesting behavior of lowland gorillas (Gorilla gorilla gorilla) in the Ngotto forest, Central African Republic. Journal of Zoology London, 255, 251–259. [Google Scholar]
- Burr, D. B. , Ruff, C. B. , & Johnson, C. (1989). Structural adaptations of the femur and humerus to arboreal and terrestrial environments in three species of macaque. American Journal of Physical Anthropology, 79, 357–367. [DOI] [PubMed] [Google Scholar]
- Carlson, K. J. (2002). Shape and material properties of African pongid femora and humeri: their relationship to observed positional behaviors. (Publication No. 3075975). [Doctoral dissertation, Indiana University]. UMI Publications.
- Carlson, K. J. (2005). Investigating the form‐function interface in African apes—Relationships between principal moments of area and positional behaviors in femoral and humeral diaphyses. American Journal of Physical Anthropology, 127, 312–334. 10.1002/ajpa.20124 [DOI] [PubMed] [Google Scholar]
- Carlson, K. J. , Doran‐Sheehy, D. M. , Hunt, K. D. , Nishida, T. , Yamanaka, A. , & Boesch, C. (2006). Locomotor behavior and long bone morphology in individual free‐ranging chimpanzees. Journal of Human Evolution, 50, 394–404. 10.1016/j.jhevol.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Carlson, K. J. , Green, D. J. , Jashashvili, T. , Pickering, T. R. , Heaton, J. L. , Beaudet, A. , Stratford, D. , Crompton, R. , Kuman, K. , Bruxelles, L. , & Clarke, R. J. (2021). The pectoral girdle of StW 573 (‘little foot’) and its implications for shoulder evolution in the Hominina. Journal of Human Evolution, 158, 102983. 10.1016/j.jhevol.2021.102983 [DOI] [PubMed] [Google Scholar]
- Carlson, K. J. , & Marchi, D. (Eds). (2014). Reconstructing mobility: Environmental, behavioral, and morphological determinants. Springer Press. 10.1007/978-1-4899-7460-0 [DOI] [Google Scholar]
- Carlson, K. J. , Sumner, D. R. , Morbeck, M. E. , Nishida, T. , Yamanaka, A. , & Boesch, C. (2008). The role of non‐behavioral factors in adjusting long bone diaphyseal structure in free‐ranging chimpanzees. International Journal of Primatology, 29, 1401–1420. 10.1007/s10764-008-9297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, K. J. , Wrangham, R. W. , Muller, M. N. , Sumner, D. R. , Morbeck, M. E. , Nishida, T. , Yamanaka, A. , & Boesch, C. (2011). Comparison of limb structural properties in free‐ranging chimpanzees from Kibale, Gombe, Mahale, and Taï communities. In D'Août K. & Vereecke E. (Eds.), Primate locomotion: Linking field and laboratory research. Developments in primatology: Progress and prospects (pp. 155–182). Springer Press. 10.1007/978-1-4419-1420-0 [DOI] [Google Scholar]
- Churchill, S. E. , Holliday, T. W. , Carlson, K. J. , Jashashvili, T. , Macias, M. E. , Mathews, S. , Sparling, T. L. , Schmid, P. , de Ruiter, D. J. , & Berger, L. R. (2013). The upper limb of Australopithecus sediba . Science, 340, 1233477. [DOI] [PubMed] [Google Scholar]
- Clarke, R. J. (2019). Excavation, reconstruction and taphonomy of the StW 573 Australopithecus prometheus skeleton from Sterkfontein cavies, South Africa. Journal of Human Evolution, 127, 41–53. [DOI] [PubMed] [Google Scholar]
- Crompton, R. H. , Sellers, W. I. , & Thorpe, S. K. S. (2010). Arboreality, territoriality, and bipedalism. Philosophical Transactions of the Royal Society B, 365, 3301–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin, C. (1871). The descent of man and selection in relation to sex (p. 688). John Murray. [Google Scholar]
- Das, R. , Hergenrother, S. D. , Soto‐Calderon, I. D. , Dew, J. L. , Anthony, N. M. , & Jensen‐Seaman, M. I. (2014). Complete mitochondrial genome sequence of the eastern gorilla (gorilla beringei) and implications for African ape biogeography. Journal of Heredity, 105, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, T. G. , & Stock, J. T. (2014). Human variation in the periosteal geometry of the lower limb: Signatures of behavior among human Holocene populations. In Carlson K. J. & Marchi D. (Eds.), Reconstructing mobility: Environmental, behavioral, and morphological determinants (pp. 67–90). Springer Press. 10.1007/978-1-4899-7460-0_5 [DOI] [Google Scholar]
- Dayal, M. R. , Kegley, A. D. T. , Štrkalj, G. , Bidmos, M. A. , & Kuykendall, K. L. (2009). The history and composition of the Raymond a. dart collection of human skeletons at the University of the Witwatersrand, Johannesburg, South Africa. American Journal of Physical Anthropology, 140, 324–335. 10.1002/ajpa.21072 [DOI] [PubMed] [Google Scholar]
- De Manuel, M. , Kuhlwilm, M. , Frandsen, P. , Sousa, V. C. , Desai, T. , Prado‐Martinez, J. , Hernandez‐Rodriguez, J. , Dupanloup, I. , Lao, O. , Hallast, P. , Schmidt, J. M. , Heredia‐Genestar, J. M. , Benazzo, A. , Barbujani, G. , Peter, B. M. , Kuderna, L. F. K. , Casals, F. , Angedakin, S. , Arandjelovic, M. , … Marques‐Bonet, T. (2016). Chimpanzee genomic diversity reveals ancient admixture with bonobos. Science, 354(6311), 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demes, B. , & Jungers, W. L. (1993). Long bone cross‐sectional dimensions, locomotor adaptations and body size in prosimian primates. Journal of Human Evolution, 25, 57–74. [Google Scholar]
- Demes, B. , Jungers, W. L. , & Selpien, K. (1991). Body size, locomotion, and long bone cross‐sectional geometry in indriid primates. American Journal of Physical Anthropology, 86, 537–547. [DOI] [PubMed] [Google Scholar]
- Devlin, M. J. (2011). Estrogen, exercise, and the skeleton. Evolutionary Anthropology, 20, 54–61. [DOI] [PubMed] [Google Scholar]
- Devlin, M. J. , Cloutier, A. M. , Thomas, N. A. , Panus, D. A. , Lotinun, S. , Pinz, I. , Baron, R. , Rosen, C. J. , & Bouxsein, M. L. (2010). Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. Journal of Bone and Mineral Research, 25, 2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo, R. , Molnar, J. L. , & Wood, B. (2017). Bonobo anatomy reveals stasis and mosaicism in chimpanzee evolution, and supports bonobos as the most appropriate extant model for the common ancestor of chimpanzees and humans. Scientific Reports, 7, 608. 10.1038/s41598-017-00548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran, D. M. (1989). Chimpanzee and pygmy chimpanzee positional behavior: The influence of environment, body size, morphology, and ontogeny on locomotion and posture [unpublished doctoral dissertation]. State University of New York. [Google Scholar]
- Doran, D. M. (1992). The ontogeny of chimpanzee and pygmy chimpanzee locomotor behavior: A case study of paedomorphism and its behavioral correlates. Journal of Human Evolution, 23, 139–157. [Google Scholar]
- Doran, D. M. (1993a). Comparative locomotor behavior of chimpanzees and bonobos: The influence of morphology on locomotion. American Journal of Physical Anthropology, 91, 83–89. [DOI] [PubMed] [Google Scholar]
- Doran, D. M. (1993b). Sex differences in adult chimpanzee positional behavior: The influence of body size on locomotion and posture. American Journal of Physical Anthropology, 91, 99–115. [DOI] [PubMed] [Google Scholar]
- Doran, D. M. (1996). Comparative positional behavior of the African apes. In Great ape societies (pp. 213–224). Cambridge University Press. [Google Scholar]
- Doran, D. M. (1997). Ontogeny of locomotion in mountain gorillas and chimpanzees. Journal of Human Evolution, 32(4), 323–344. 10.1006/jhev.1996.0095 [DOI] [PubMed] [Google Scholar]
- Doran, D. M. , & Hunt, K. D. (1994). Comparative locomotor behavior of chimpanzees and bonobos. In Wrangham R. W., McGrew W. C., de Waal F. B. M., & Heltne P. G. (Eds.), Chimpanzee cultures (pp. 93–108). Harvard University. [Google Scholar]
- Doube, M. , Kłosowski, M. M. , Arganda‐Carreras, I. , Cordeliéres, F. , Dougherty, R. P. , Jackson, J. , Schmid, B. , Hutchinson, J. R. , & Shefelbine, S. J. (2010). BoneJ: Free and extensible bone image analysis in ImageJ. Bone, 47, 1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdeswell, M. R. , Jashashvili, T. , Patel, B. A. , Lebrun, R. , Susman, R. L. , Lordkipanidze, D. , & Carlson, K. J. (2017). Adaptation to bipedal gait and fifth metatarsal structural properties in Australopithecus, Paranthropus, and Homo. Comptes Rendus Palevol, 16, 585–599. 10.1016/j.crpv.2016.10.003 [DOI] [Google Scholar]
- Freckleton, R. P., Harvey, P. H., & Pagel, M. (2002). Phylogenetic Analysis and Comparative Data: A Test and Review of Evidence. The American Naturalist, 160(6), 712–726. 10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Frost, H. M. (1973). Bone modeling and skeletal modeling errors. Thomas. [Google Scholar]
- Frost, H. M. (1987). The mechanostat: A proposed pathogenic mechanism of osteoporosis and the bone mass effects of mechanical and non‐mechanical agents. Bone and Mineral, 2, 73–85. [PubMed] [Google Scholar]
- Frost, H. M. (1996). Perspectives: A proposed general model for the mechanostat (suggestions from a new paradigm). The Anatomical Record, 240, 447–455. [DOI] [PubMed] [Google Scholar]
- Frost, H. M. (2003). Bone's mechanostat: A 2003 update. The Anatomical Record, 275A, 1081–1101. [DOI] [PubMed] [Google Scholar]
- Gómez, M. , Casado, A. , De Diego, M. , Arias‐Martrell, J. , Pastor, J. F. , & Potau, J. M. (2020). Quantitative shape analysis of the deltoid tuberosity of modern humans (Homo sapiens) and common chimpanzees (pan troglodytes). Annals of Anatomy, 230, 151505. 10.1006/j.aanat.2020.151505 [DOI] [PubMed] [Google Scholar]
- Groves, C. P. (2018). The latest thinking about the taxonomy of great apes. International Zoo Yearbook, 52(1), 16–24. [Google Scholar]
- Hatala, K. G. , Roach, N. T. , Ostrofsky, K. R. , Wunderlich, R. E. , Dingwall, H. L. , Villmoare, B. A. , Green, D. J. , Harris, J. W. K. , Braun, D. R. , & Richmond, B. G. (2016). Footprints reveal direct evidence of group behavior and locomotion in Homo erectus. Scientific Reports, 6, 28766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, J. L. , Pickering, T. R. , Carlson, K. J. , Crompton, R. H. , Jashashvili, T. , Beaudet, A. , Bruxelles, L. , Kuman, K. , Heile, A. J. , Stratford, D. , & Clarke, R. J. (2019). The long limb bones of the StW 573 Australopithecus skeleton from Sterkfontein member 2: Descriptions and proportions. Journal of Human Evolution, 133, 167–197. [DOI] [PubMed] [Google Scholar]
- Hunt, K. D. (1989). Positional behavior in pan troglodytes at the Mahale Mountains and the Gombe stream National Parks, Tanzania [unpublished doctoral dissertation]. University of Michigan. [DOI] [PubMed] [Google Scholar]
- Hunt, K. D. (1992). Positional behavior of pan troglodytes in the Mahale mountains and Gombe stream national parks. Tanzania. American Journal of Physical Anthropology, 87(1), 83–105. 10.1002/ajpa.1330870108 [DOI] [PubMed] [Google Scholar]
- Hunt, K. D. (1994). Body size effects on vertical climbing among chimpanzees. International Journal of Primatology, 115, 855–865. [Google Scholar]
- Hunt, K. D. (2020). Chimpanzee: Lessons from our sister species. Cambridge University Press. [Google Scholar]
- Hunt, K. D., Dunevant, S. E., Yohler, R. M., & Carlson, K. J. (2021). Femoral bicondylar angles among dry‐habitat chimpanzees (Pan troglodytes schweinfurthii) resemble those of humans: Implications for knee function, australopith sexual dimorphism, and the evolution of bipedalism. Journal of Anthropological Research, 77, 303–337. [Google Scholar]
- Huxley, T. H. (1863). Evidence as to Man's place in nature (p. 159). Williams and Norgate. [Google Scholar]
- Hvilsom, C. , Frandsen, P. , Børsting, C. , Carlsen, F. , Sallé, B. , Simonsen, B. T. , & Siegismund, H. R. (2013). Understanding geographic origins and history of admixture among chimpanzees in European zoos, with implications for future breeding programs. Heredity, 110, 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jashashvili, T. , Dowdeswell, M. R. , Lebrun, R. , & Carlson, K. J. (2015). Cortical structure of hallucal metatarsals and locomotor adaptations in hominoids. PLoS One, 10(1), e0117905. 10.1371/journal.pone.0117905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, T. (2002). Primate limb bones and locomotor types in arboreal or terrestrial environments. Zeitschrift fur Morphologie und Anthropologie, 83, 201–219. [PubMed] [Google Scholar]
- Kozma, E. E. , Webb, N. M. , Harcourt‐Smith, W. E. H. , Raichlen, D. A. , D'Aoút, K. , Brown, M. H. , Finestone, E. M. , Ross, S. R. , Aerts, P. , & Pontzer, H. (2018). Hip extensor mechanics and the evolution of walking and climbing capabilities in humans, apes, and fossil hominins. Proceedings of the National Academy of Sciences USA, 115, 4134–4139. 10.1073/pnas.1715120115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langergraber, K. E. , Prüfer, K. , Rowney, C. , Boesch, C. , Crockford, C. , Fawcett, K. , Inoue, E. , Inoue‐Muruyama, M. , Mitani, J. C. , Muller, M. N. , Robbins, M. M. , Schubert, G. , Stoinski, T. S. , Viola, B. , Watts, D. , Wittig, R. M. , Wrangham, R. W. , Zuberbühler, K. , Pääbo, S. , & Vigilant, L. (2012). Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proceedings of the National Academy of Sciences USA, 109, 15716–15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, S. G. (1998). Parallel evolution in the hominoid trunk and forelimb. Evolutionary Anthropology, 6(3), 87–99. [Google Scholar]
- Lefcheck, J. S. (2016). piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods in Ecology and Evolution, 7(5), 573–579. 10.1111/2041-210X.12512 [DOI] [Google Scholar]
- Lieberman, D. E. , Polk, J. D. , & Demes, B. (2004). Predicting long bone loading from cross‐sectional geometry. American Journal of Physical Anthropology, 123, 156–171. 10.1002/ajpa.10316 [DOI] [PubMed] [Google Scholar]
- Locke, D. , Hillier, L. , Warren, W. , et al. (2011). Comparative and demographic analysis of orang‐utan genomes. Nature, 469, 529–533. 10.1038/nature09687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy, C. O. , Suwa, G. , Spurlock, L. , Asfaw, B. , & White, T. D. (2009). Ardipithecus ramidus: The emergence of upright walking. Science, 326, 71e1–71e6. [PubMed] [Google Scholar]
- Macchiarelli, R. , Bergeret‐Medina, A. , Marchi, D. , & Wood, B. (2020). Nature and relationships of Sahelanthropus tchadensis . Journal of Human Evolution, 149, 201898. 10.1016/j.jhevol.2020.102898. [DOI] [PubMed] [Google Scholar]
- Manduell, K. L. (2013). Habitat variation and its influence on the locomotor ecology of wild orangutans [doctoral dissertation, University of Birmingham]. University of Birmingham UBIRA E THESES https://etheses.bham.ac.uk/id/eprint/4700/
- Manduell, K. L. , Harrison, M. E. , & Thorpe, S. K. (2012). Forest structure and support availability influence orangutan locomotion in Sumatra and Borneo. American Journal of Primatology, 74(12), 1128–1142. 10.1002/ajp.22072 [DOI] [PubMed] [Google Scholar]
- Marchant, L. M. , & McGrew, W. C. (2013). Handedness is more than laterality: Lessons from chimpanzees. Annals of the New York Academy of Science, 1288, 1–8. [DOI] [PubMed] [Google Scholar]
- Marchi, D. (2005). The cross‐sectional geometry of the hand and foot bones of the Hominoidea and its relationship to locomotor behavior. Journal of Human Evolution, 49, 743–761. [DOI] [PubMed] [Google Scholar]
- Marchi, D. (2007). Relative strength of the tibia and fibula and locomotor behavior in hominoids. Journal of Human Evolution, 53, 647–655. [DOI] [PubMed] [Google Scholar]
- Marchi, D. (2015a). Variation in tibia and fibula diaphyseal strength and its relationship with arboreal and terrestrial locomotion: Extending the investigation to non‐hominoid primates. Journal of Anthropological Sciences, 93, 153–156. [DOI] [PubMed] [Google Scholar]
- Marchi, D. (2015b). Using the morphology of the hominoid distal fibula to interpret arboreality in Australopithecus afarensis . Journal of Human Evolution, 85, 136–148. [DOI] [PubMed] [Google Scholar]
- Marchi, D. , Harper, C. M. , Chirchir, H. , & Ruff, C. B. (2019). Relative fibular strength and locomotor behavior in KNM‐WT 15000 and OH 35. Journal of Human Evolution, 131, 48–60. [DOI] [PubMed] [Google Scholar]
- Marchi, D. , Ruff, C. B. , Capobianco, A. , Rafferty, K. L. , Habib, M. B. , & Patel, B. A. (2016). The locomotion of Babakotia radofilai inferred from epiphyseal and diaphyseal morphology of the humerus and femur. Journal of Morphology, 277, 1199–1218. 10.1002/jmor.20569 [DOI] [PubMed] [Google Scholar]
- Martin, R. B. , Burr, D. B. , Sharkey, N. A. , & Fyhrie, D. P. (2015). Skeletal tissue mechanics (2nd ed.). Springer. [Google Scholar]
- Masi, S. (2004). Tree use by a western gorilla group (Gorilla gorilla gorilla) in a Dzanga‐Ndoki National Park. Central African Republic. Folia Primatologica, 75, 393. [Google Scholar]
- Masi, S. , Cipolletta, C. , & Robbins, M. M. (2009). Western lowland gorillas (Gorilla gorilla gorilla) change their activity patterns in response to frugivory. American Journal of Primatology, 71, 91–100. [DOI] [PubMed] [Google Scholar]
- Mehlman, P. T. , & Doran, D. M. (2002). Influencing western gorilla nest construction at Mondika research center. International Journal of Primatology, 23, 1257–1258. [Google Scholar]
- Mongle, C. S. , Wallace, I. J. , & Grine, F. E. (2015a). Cross‐sectional structural variation relative to midshaft along hominine diaphyses. I. the forelimb. American Journal of Physical Anthropology, 158, 386–397. [DOI] [PubMed] [Google Scholar]
- Mongle, C. S. , Wallace, I. J. , & Grime, F. E. (2015b). Cross‐sectional structural variation relative to midshaft along hominine diaphyses. II. The hind limb. American Journal of Physical Anthropology, 158, 398–407. [DOI] [PubMed] [Google Scholar]
- Moorjani, P. , Amorim, C. E. G. , Arndt, P. F. , & Przeworski, M. (2016). Variation in the molecular clock of primates. Proceedings of the National Academy of Science, 113, 10607–10612. 10.1073/pnas.1600374113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münkemüller, T., Lavergne, S., Bzeznik, B., Dray, S., Jombart, T., Schiffers, K., & Thuiller, W. (2012). How to measure and test phylogenetic signal. Methods in Ecology and Evolution, 3(4), 743–756. Portico. 10.1111/j.2041-210x.2012.00196.x [DOI] [Google Scholar]
- Nagurka, M. L. , & Hayes, W. C. (1980). Technical notes: An interactive graphics package for calculating cross‐sectional properties of complex shapes. Journal of Biomechanics, 13, 59–64. [DOI] [PubMed] [Google Scholar]
- Nater, A. , Mattle‐Greminger, M. P. , Nurcahyo, A. , Nowak, M. G. , de Manuel, M. , Desai, T. , Groves, C. , Pybus, M. , Sonay, T. B. , Roos, C. , Lameira, A. R. , Wich, S. A. , Askew, J. , Davila‐Ross, M. , Fredriksson, G. , de Valles, G. , Casals, F. , Prado‐Martinez, J. , Goossens, B. , … Krützen, M. (2017). Morphometric, behavioral, and genomic evidence for a new orangutan species. Current Biology, 27, 3487–3498. 10.1016/j.cub.2017.09.047 [DOI] [PubMed] [Google Scholar]
- Neufuss, J. , Robbins, M. M. , Baeumer, J. , Humle, T. , & Kivell, T. L. (2017). Comparison of hand use and forelimb posture during vertical climbing in mountain gorillas (Gorilla gorilla beringei) and chimpanzees (pan troglodytes). American Journal of Physical Anthropology, 164, 651–664. [DOI] [PubMed] [Google Scholar]
- Neufuss, J. , Robbins, M. M. , Baeumer, J. , Humle, T. , & Kivell, T. L. (2018). Gait characteristics of vertical climbing in mountain gorillas and chimpanzees. Journal of Zoology, 306, 129–138. [Google Scholar]
- Ostrofsky, K. R. , & Robbins, M. M. (2020). Fruit‐feeding and activity patterns of mountain gorillas (gorilla beringei beringei) in Bwindi impenetrable National Park, Uganda. American Journal of Physical Anthropology, 173, 3–20. 10.1002/ajpa.24056 [DOI] [PubMed] [Google Scholar]
- Patel, B. A. , Ruff, C. B. , Simons, E. L. R. , & Organ, J. M. (2013). Humeral cross‐sectional shape in suspensory primates and sloths. Anatomical Record, 296, 545–556. 10.1002/ar.22669 [DOI] [PubMed] [Google Scholar]
- Pearson, O. M. , & Lieberman, D. E. (2004). The aging of “Wolff's law”: Ontogeny and responses to mechanical loading in cortical bone. Yearbook of Physical Anthropology, 125(39), 63–99. [DOI] [PubMed] [Google Scholar]
- Plumptre, A. J. , Nixon, S. , Kujirakwinja, D. K. , Vieilledent, G. , Critchlow, R. , Williamson, E. A. , … Hall, J. S. (2016). Catastrophic decline of world's largest primate: 80% loss of Grauer's gorilla (gorilla beringei graueri) population justifies critically endangered status. PLoS One, 11(10), e0162697. 10.1371/journal.pone.0162697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk, J. D. , Demes, B. , Jungers, W. L. , Biknevicius, A. R. , Heinrich, R. E. , & Runestad, J. A. (2000). A comparison of primate, carnivoran and rodent limb bone cross‐sectional properties: Are primates really unique? Journal of Human Evolution, 39, 297–325. 10.1006/jhev.2000.0420 [DOI] [PubMed] [Google Scholar]
- Pontzer, H. (2012). Ecological energetics in early homo. Current Anthropology, 53, S346–S358. [Google Scholar]
- Prado‐Martinez, J. , Sudmant, P. H. , Kidd, J. M. , Li, H. , Kelley, J. L. , Lorente‐Galdos, B. , Veeramah, K. R. , Woerner, A. E. , O'Connor, T. D. , Santpere, G. , Cagan, A. , Theunert, C. , Casals, F. , Laayouni, H. , Munch, K. , Hobolth, A. , Halager, A. E. , Malig, M. , Hernandez‐Rodriguez, J. , … Marques‐Bonet, T. (2013). Great ape genetic diversity and population history. Nature, 499(7459), 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos III, G. L. (2014). Positional behavior of Pan paniscus at LuiKotale, Democratic Republic of Congo Publication No. 2014. 3645601 [Doctoral dissertation, Indiana University]. PQDT Open https://search.proquest.com/openview/ccec7081f0cce42e2b142beb5f4f8595/1?pq-origsite=gscholar&cbl=18750&diss=y
- Remis, M. (1994). Feeding ecology and positional behavior of western lowland gorillas (Gorilla gorilla gorilla) in the Central African Republic. [Unpublished doctoral dissertation]. Yale University. [Google Scholar]
- Remis, M. (1995). Effects of body size and social context on the arboreal activities of lowland gorillas in the Central African Republic. American Journal of Physical Anthropology, 97(4), 413–433. 10.1002/ajpa.1330970408 [DOI] [PubMed] [Google Scholar]
- Remis, M. J. (1998). The gorilla paradox. In Strasser E., Fleagle J., Rosenberger A., & McHenry H. M. (Eds.), Primate locomotion (pp. 95–106). Plenum Press. [Google Scholar]
- Remis, M. J. (1999). Tree structure and sex differences in arboreality among western lowland gorillas (Gorilla gorilla gorilla) at Bai Jokou, Central African Republic. Primates, 40, 383–396. [Google Scholar]
- Roy, J. , Arandjelovic, M. , Bradley, B. J. , Guschanski, K. , Stephens, C. R. , Bucknell, D. , Cirhuza, H. , Kusamba, C. , Kyungu, J. C. , Smith, V. , Robbins, M. M. , & Vigilant, L. (2014). Recent divergences and size decreases of eastern gorilla populations. Biology Letters, 10, 20140811. 10.1098/rsbl.2014.0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, C. (2008). Femoral/humeral strength in early African Homo erectus . Journal of Human Evolution, 54, 383–390. [DOI] [PubMed] [Google Scholar]
- Ruff, C. (2009). Relative limb strength and locomotion in Homo habilis. American Journal of Physical Anthropology, 138, 90–100. [DOI] [PubMed] [Google Scholar]
- Ruff, C. B. (2002). Long bone articular and diaphyseal structure in old world monkeys and apes. 1: Locomotor effects. American Journal of Physical Anthropology, 119, 305–342. 10.1002/ajpa.10117 [DOI] [PubMed] [Google Scholar]
- Ruff, C. B. , Burgess, M. L. , Bromage, T. G. , Mudakikwa, A. , & McFarlin, S. C. (2013). Ontogenetic changes ontogenetic changes in limb bone structural proportions in mountain gorillas (gorilla beringei beringei). Journal of Human Evolution, 65, 693–703. 10.1016/j.jhevol.2013.06.008 [DOI] [PubMed] [Google Scholar]
- Ruff, C. B. , Burgess, M. L. , Junno, J.‐A. , Mudakikwa, A. , Zollikofer, C. P. E. , Ponce de León, M. S. , & McFarlin, S. C. (2018). Phylogenetic and environmental effects on limb bone structure in gorillas. American Journal of Physical Anthropology, 166, 353–372. 10.1002/ajpa.23437 [DOI] [PubMed] [Google Scholar]
- Ruff, C. B. , Burgess, M. L. , Ketcham, R. A. , & Kappelman, J. (2016). Limb bone structural proportions and locomotor behavior in a.L. 288‐1 (“Lucy”). PLoS One, 11, e0166095. 10.1371/journal.pone.0166095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, C. B. , Harper, C. M. , Goldstein, D. M. , Daegling, D. J. , & McGraw, W. S. (2019). Long bone structural proportions and locomotor behavior in Cercopithecidae. Journal of Human Evolution, 132, 47–60. 10.1016/j.jhevol.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Ruff, C. B. , Holt, B. , & Trinkaus, E. (2006). Who's afraid of the big bad Wolff?: “Wolff's law” and bone functional adaptation. American Journal of Physical Anthropology, 129, 484–498. [DOI] [PubMed] [Google Scholar]
- Runestad Connour, J. , Glander, K. , & Vincent, F. (2000). Postcranial adaptations for leaping in primates. Journal of Zoology, 251, 79–103. 10.1111/j.1469-7998.2000.tb00595.x [DOI] [Google Scholar]
- Runestad, J. A. (1997). Postcranial adaptations for climbing in Loridae (primates). Journal Zoology London, 242, 261–290. [Google Scholar]
- Saers, J. P. P. , DeMars, L. J. , Stephens, N. B. , Jashashvili, T. , Carlson, K. J. , Gordon, A. D. , Shaw, C. N. , Ryan, T. M. , & Stock, J. T. (2021). Combinations of trabecular and cortical bone properties distinguish various loading modalities between athletes and controls. American Journal of Physical Anthropology, 174, 434–450. [DOI] [PubMed] [Google Scholar]
- Sarringhaus, L. A. , MacLatchy, L. M. , & Mitani, J. C. (2014). Locomotor and postural development of wild chimpanzees. Journal of Human Evolution, 66, 29–38. [DOI] [PubMed] [Google Scholar]
- Sarringhaus, L. A. , MacLatchy, L. M. , & Mitani, J. C. (2016). Long bone cross‐sectional properties reflect changes in locomotor behavior in developing chimpanzees. American Journal of Physical Anthropology, 160, 16–29. 10.1002/ajpa.22930 [DOI] [PubMed] [Google Scholar]
- Scally, A. , Dutheil, J. Y. , Hillier, L. W. , Jordan, G. E. , Goodhead, I. , Herrero, J. , Hoboloth, A. , Lappalainen, T. , Mailund, T. , Marques‐Bonet, T. , McCarthy, S. , Montgomery, S. H. , Schwalie, P. C. , Tang, Y. A. , Ward, M. C. , Xue, Y. , Yngvadottir, B. , Alkan, C. , Andersen, L. N. , … Durbin, R. (2012). Insights into hominid evolution from the gorilla genome sequence. Nature, 483, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler, M. B. , Burr, D. B. , Jungers, W. L. , & Ruff, C. B. (1985). Structural and mechanical indicators of limb specialization in primates. Folia Primatologica, 45, 61–75. 10.1159/000156218 [DOI] [PubMed] [Google Scholar]
- Shaw, C. N. , & Ryan, T. M. (2012). Does skeletal anatomy reflect adaptation to locomotor patterns? Cortical and trabecular architecture in human and nonhuman anthropoids. American Journal of Physical Anthropology, 147, 187–200. 10.1002/ajpa.21635 [DOI] [PubMed] [Google Scholar]
- Shaw, C. N. , & Stock, J. T. (2009). Intensity, repetitiveness, and directionality of habitual adolescent mobility patterns influence the tibial diaphysis morphology of athletes. American Journal of Physical Anthropology, 140, 149–159. [DOI] [PubMed] [Google Scholar]
- Smith, R. J., & Jungers, W. L. (1997). Body mass in comparative primatology. Journal of Human Evolution, 32(6), 523–559. 10.1006/jhev.1996.0122 [DOI] [PubMed] [Google Scholar]
- Sokal, R. R. , & Rohlf, F. J. (1995). Biometry. Freeman and Company. [Google Scholar]
- Sparacello, V. S. , Villotte, S. , Shackelford, L. L. , & Trinkaus, E. (2017). Patterns of humeral asymmetry among late Pleistocene humans. Comptes Rendus Palevol, 16, 680–689. [Google Scholar]
- Stern, J. T., Jr. , & Susman, R. L. (1983). The locomotor anatomy of Australopithecus afarensis. American Journal of Physical Anthropology, 60, 279–317. [DOI] [PubMed] [Google Scholar]
- Strait, D. S. (2013). Human systematics. In Begun D. R. (Ed.), A companion to paleoanthropology (pp. 37–54). Wiley‐Blackwell. [Google Scholar]
- Sumner, D. R. , Morbeck, M. E. , & Lobick, J. J. (1989). Apparent age‐related bone loss among adult female Gombe chimpanzees. American Journal of Physical Anthropology, 79, 225–234. 10.1002/ajpa.1330790210 [DOI] [PubMed] [Google Scholar]
- Susman, L. (1984). The locomotor behavior of Pan paniscus in the Lomako forest. In The pygmy chimpanzee (pp. 369–393). Springer. [Google Scholar]
- Swindler, D. R. , & Wood, C. D. (1973). An atlas of primate gross anatomy: Baboon, chimpanzee, and man. University of Washington Press. [Google Scholar]
- Thalmann, O. , Wegmann, D. , Spitzner, M. , Arandjelovic, M. , Guschanski, K. , Leuenberger, C. , Bergl, R. A. , & Vigilant, L. (2011). Historical sampling reveals dramatic demographic changes in western gorilla populations. BMC Evolutionary Biology, 11, 85. 10.1186/1471-2148-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe, S. K. S., & Crompton, R. H. (2006). Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea. American Journal of Physical Anthropology, 131(3), 384–401. 10.1002/ajpa.20422 [DOI] [PubMed] [Google Scholar]
- Tocheri, M. W. , Dommain, R. , McFarlin, S. C. , Burnett, S. E. , Troy Case, D. , Orr, C. M. , Roach, N. T. , Villmoare, B. , Eriksen, A. , Kalthoff, D. , Senck, S. , Assefa, Z. , Groves, C. P. , & Jungers, W. L. (2016). The evolutionary origin and population history of the grauer gorilla. American Journal of Physical Anthropology, 159, 4–18. 10.1002/ajpa.22900 [DOI] [PubMed] [Google Scholar]
- Tutin, C. E. G. , Parnell, R. J. , White, L. J. T. , & Fernandez, M. (1995). Nest building by lowland gorillas in Lopé reserve, Gabon: Environmental influences and implications for censusing. International Journal of Primatology, 16, 53–76. [Google Scholar]
- Tuttle, R. H. , & Watts, D. P. (1985). The positional behavior and adaptive complexes of pan gorilla. In Kondo S. (Ed.), Primate morphophysiology, locomotor analyzes, and human bipedalism (pp. 261–288). University of Tokyo Press. [Google Scholar]
- van der Valk, T. , Sandoval‐Castellanos, E. , Caillaud, D. , Ngobobo, U. , Binyinyi, E. , Nishuli, R. , Stoinski, T. , Gilissen, E. , Sonet, G. , Semal, P. , Kalthoff, D. C. , Dalén, L. , & Guschanski, K. (2018). Significant loss of mitochondrial diversity within the last century due to extinction of peripheral populations in eastern gorillas. Scientific Reports, 8(1), 1–10. 10.1038/s41598-018-24497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, I. J. , Middleton, K. M. , Lublinsky, S. , Kelly, S. A. , Judex, S. , Garland, T., Jr. , & Demes, B. (2010). Functional significance of genetic variation underlying limb bone diaphyseal structure. American Journal of Physical Anthropology, 143, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, I. J. , Tommasini, S. M. , Judex, S. , Garland, T., Jr. , & Demes, B. (2012). Genetic variations and physical activity as determinants of limb bone morphology: An experimental approach using a mouse model. American Journal of Physical Anthropology, 148, 24–35. [DOI] [PubMed] [Google Scholar]
- Ward, C. V. (2002). Interpreting the posture and locomotion of Australopithecus afarensis: Where do we stand? Yearbook of Physical Anthropology, 119(S35), 185–215. [DOI] [PubMed] [Google Scholar]
- Wei, P. , Lu, H. , Carlson, K. J. , Zhang, H. , Hui, J. , Zhu, M. , He, K. , Jashashvili, T. , Zhang, X. , Yuan, H. , & Xing, S. (2020). The upper limb skeleton and behavioral lateralization of modern humans from Zhaoguo cave, southwestern China. American Journal of Physical Anthropology, 173, 671–696. [DOI] [PubMed] [Google Scholar]
- Wergedal, J. E. , Sheng, M. H. C. , Ackert‐Bicknell, C. L. , Beamer, W. G. , & Baylink, D. J. (2005). Genetic variation in femur extrinsic strength in 29 different inbred strains of mice is dependent on variations in femur cross‐sectional geometry and bone density. Bone, 36, 111–122. [DOI] [PubMed] [Google Scholar]
- White, F. J. , Brand, C. M. , Hickmott, A. J. , & Minton, I. R. (2020). Sex differences in bonobo (pan paniscus) terrestriality: Implications for human evolution. Journal of Anthropological Sciences, 98, 5–14. [DOI] [PubMed] [Google Scholar]
- White, T. D. , Asfaw, B. , Beyene, Y. , Haile‐Selassie, Y. , Lovejoy, C. O. , Suwa, G. , & WoldeGabriel, G. (2009). Ardipithecus ramidus and the paleobiology of early hominids. Science, 326, 64–86. [PubMed] [Google Scholar]
- Xue, Y. , Prado‐Martinez, J. , Sudmant, P. H. , Narasimhan, V. , Ayub, Q. , Szpak, M. , Frandsen, P. , Chen, Y. , Yngvadottir, B. , Cooper, D. N. , de Manuel, M. , Hernandez‐Rodriguez, J. , Lobon, I. , Siegismund, H. R. , Pagani, L. , Quail, M. A. , Hvilsom, C. , Mudakikwa, A. , Eichler, E. E. , … Scally, A. (2015). Mountain gorilla genomes reveal the impact of long‐term population decline and inbreeding. Science, 348(6231), 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka, A. , Gunji, H. , & Ishida, H. (2005). Curvature, length, and cross‐sectional geometry of the femur and humerus in anthropoid primates. American Journal of Physical Anthropology, 127, 46–57. 10.1002/ajpa.10439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Scatterplot of Femoral and Humeral J Values for Each Species at 35% Region of Interest.
Supplementary Figure 2 Scatterplot of Femoral and Humeral J Values for Each Species at 50% Region of Interest.
Supplementary Figure 3 Scatterplot of Femoral and Humeral J Values for Each Species at 65% Region of Interest.
Supplementary Figure 4 Species Boxplot of Femoral J to Humeral J Ratios for Each Region of Interest. The two human outliers excluded in Figure 2 are included here.
Appendix S1 Excel data set. The Mahale, Gombe, and Kibale chimpanzees have been excluded from this file.
Data Availability Statement
Data are included in an excel data in the appendix.


