Abstract
In recent years, the practice of dentistry and periodontology has become complicated by several risk factors, including the treatment of an increasing number of patients with substance use disorder. This review presents an update in the current literature of the impact of illegal drug use on periodontal conditions and their possible effect as risk factors or indicators. The main illegal drugs that may have an impact on periodontal health and conditions are described, including their effect, medical manifestations, risks, and the overall effect on oral health and on the periodontium. Where available, data from epidemiologic studies are analyzed and summarized. The clinical management of periodontal patients using illegal drugs is reported in a comprehensive approach inclusive of the detection of illicit drug users, screening, interviewing and counseling, the referral to treatment, and the dental and periodontal management. With regard to the impact of illegal substance use on periodontal conditions, there is moderate evidence that regular long‐term use of cannabis is a risk factor for periodontal disease, manifesting as a loss of periodontal attachment, deep pockets, recessions, and gingival enlargements. Limited evidence also shows that the use of cocaine can cause a series of gingival conditions that mostly presents as chemical induced‐traumatic lesions (application of cocaine on the gingiva) or necrotizing ulcerative lesions. There is a scarcity of data regarding the impact of other drug use on periodontal health. There is evidence to suggest that regular long‐term use of cannabis is a risk factor for periodontal disease and that the use of cocaine can cause a series of periodontal conditions. The dental treatment of subjects that use illegal substances is becoming more common in the daily clinical practice of periodontists and other dental clinicians. When the clinicians encounter such patients, it is essential to manage their addiction properly taking into consideration the impact of it on comprehensive dental treatment. Further studies and clinical observations are required to obtain sound and definitive information.
Keywords: amphetamine, cannabis, cocaine, illegal drugs, oral health, periodontology
1. INTRODUCTION
This review aims to present an update in the current literature of the impact of illegal drug use on periodontal conditions and their possible effect as risk factors or indicators. It is estimated that one in 20 adults, or a quarter of a billion people between the ages of 15 and 64 years worldwide, used at least one illegal drug in 2014. 1 Cannabis remains the most commonly used drug at the global level, with an estimated 183 million users in 2014, while amphetamines are the second. With an estimated 33 million users, the use of opiates and prescription opioids is less common, but opioids potentiate major harm and health consequences. Precipitating the difficulties in studying the prevalence of illicit drug use, the pattern of usage can be occasional or regular; or, with a combination of both during different timelines, there are also multidrug users who utilize more than one substance concurrently or sequentially. More than 29 million drug users are estimated to experience drug‐use disorders, and, of those, 12 million use injected drugs. In users who obtain drugs via injection, 14.0% are HIV seropositive. The impact of drug use regarding health consequences is known to be strong, but the study of its impact is extremely difficult.
In 2014, there were an estimated 207 400 drug‐related deaths, corresponding to 43.5 deaths per million people aged 15‐64 years. This global number of drug‐related deaths has remained stable, albeit unacceptable and preventable. 1
Periodontal diseases are pathologic manifestations of the host response against the bacterial challenge from the dental biofilm at the tooth/gingival interface. 2 Many social, behavioral, genetic, systemic, and local factors have been identified as contributing risk factors for periodontitis. 3 The terminology associated with risk factors and indicators is not always clear in the scientific literature. We aim to ensure that the meaning of each term used in the current paper is clarified. A risk factor for periodontal disease is a characteristic aspect of behavior or an environmental exposure that is associated with periodontitis, 4 but it is important to stress that this does not necessarily define the causality in the association. It merely describes that when the factor is present, the likelihood of the health‐related condition or disease is increased; and the absence of the factor is directly correlated with a reduced likelihood of the health‐related condition or disease. 5 A risk indicator describes a potential risk factor identified as associated with a disease from case‐control or cross‐sectional studies, 3 but which has not been subjected to longitudinal observations. A risk factor is more appropriately reserved for those factors that have been verified as associated with the disease concerned through longitudinal studies.
In recent years, the practice of dentistry and periodontology has become complicated by several risk factors, including an increasing number of patients with substance use disorder.
Drug use is commonly associated with significant detrimental psychological, nutritional, and social changes, any of which can markedly affect the general and oral health of the individual user. 6
2. DEFINITIONS OF SUBSTANCE USE DISORDERS
2.1. Substance use
Substance use is a maladaptive pattern of chemical use, including illegal drugs, leading to clinically significant impairment or distress, and manifested by one (or more) of the following occurring within a 12‐month period 7 :
Recurrent chemical use resulting in a failure to fulfill major role obligations at work, school, or home.
Recurrent chemical use in situations in which it is physically hazardous.
Recurrent chemically related legal problems.
Continued chemical use despite having persistent or recurrent social or interpersonal problems caused by or exacerbated by the effects of chemical substances.
2.2. Addiction
Addiction is a primary chronic disease of brain reward, motivation, memory, and related circuitry. Dysfunction in these circuits leads to characteristic biological, psychological, social, and spiritual manifestations. This is reflected in an individual who pathologically pursues reward and/or relief by substance use and other behaviors. Addiction is characterized by the inability to consistently abstain, impairment in behavioral control, cravings, diminished recognition of significant problems with one's behaviors and interpersonal relationships, and a dysfunctional emotional response. Like other chronic diseases, addiction often involves cycles of remission and exacerbation. 8
2.3. Use
Use is a pattern of pathologic behavior associated with continued use of a drug or drugs despite persistent social, psychological, or physical problems caused by drug use. 9
2.4. Dependence
Dependence is defined as continued substance use caused by a physical or psychological need for a substance. Tolerance to the effects of the drug and development of characteristic withdrawal symptoms are required. It is considered a state in which an organism functions normally only in the presence of a drug. 10
2.5. Tolerance
Tolerance is defined as a need for markedly increased quantities of a drug to achieve the desired results or a condition in which a higher dose of the drug is required to achieve the same effect. 9
2.6. Withdrawal
Withdrawal is defined as psychological or physiological symptoms developed following discontinuation of the drug use. 9
3. CANNABIS AND PERIODONTAL COMPLICATIONS
3.1. Description of the drug and its effects
Cannabis is the most used illicit drug in developed countries, and it is currently legalized in certain nations (USA, Canada, Israel, Uruguay, and the Netherlands). Recently, additional countries have been considering cannabis legalization and these include New Zealand and Australia. 11 , 12
This drug originates from a mix of shredded flowers, stems seeds, and leaves of the hemp plant (Cannabis sativa or Cannabis indica). 13 It is usually smoked in the form of a rolled cigarette with or without tobacco or in a pipe. Nowadays, the availability and popularity of vaporizers and “vape shops” has mainstreamed a method of consumption previously reserved for a small percentage of marijuana users. Because vaporizers are marketed as a safer alternative to smoking tobacco, many subjects view their use as preferable to smoking cigarettes. Most commercially available vaporizers accept only concentrated resins, but some also vaporize plant matter. The easy access to these devices makes them appealing to young people. 14 Because they have become a growing trend, questions have been raised as to whether their use can represent a less harmful mode of intoxication. 15
The main active chemical of cannabis is delta‐9‐tetrahydrocannabinol. Cannabis exerts its effects on the body by interaction with specific endogenous receptors, CB1 and CB2. These receptors normally modulate neuronal activity by affecting the second messengers and the ion transport systems. CB1 receptors are found in the cerebral cortex, limbic areas, basal ganglia, cerebellum, and thalamic areas, explaining the mental health effects of cannabis. 16 , 17 Cannabis is therefore able to bind to brain receptors that regulate pleasure, memory, thoughts, concentration, sensory, time perception, and coordinate movements. It is also linked to CB2 receptors that are found in cells in the immune system, predominantly the macrophages. Moreover, other cannabinoids and a multitude of chemical compounds have been identified and, in fact, as many as 200 metabolites are produced in the body when cannabis is smoked, including numerous potential carcinogens.
Marijuana is the most common and least concentrated form of cannabis, followed by hashish, which is made by obtaining resin from the top of the plant (2%‐20% delta‐9‐tetrahydrocannabinol), and hash oil (15%‐50% delta‐9‐tetrahydrocannabinol), which is the most concentrated and potent form. 17 When cannabis is smoked, approximately 50% of the delta‐9‐tetrahydrocannabinol is absorbed through the lungs and enters the bloodstream, from where delta‐9‐tetrahydrocannabinol reaches the brain within seconds. 18 Delta‐9‐tetrahydrocannabinol psychotropic effects set in within minutes and its optimal effect is reached within 15‐30 minutes. The effects generally taper off in 2‐3 hours. 19 Within minutes of inhalation, a user may experience elevated heart rate, bloodshot eyes, and a slowed down respiration rate. Cannabis use can result in elevated blood pressure 20 while the user is sitting or supine, but may result in orthostatic hypotension and subsequent dizziness or fainting on standing. 21 Cardiac functions may be affected for several hours after cannabis use and bradycardia may be induced in some regular cannabis users. This further emphasizes the complex effects of delta‐9‐tetrahydrocannabinol on the body. 19
3.2. Illegal synthetic cannabinoids
Synthetic cannabinoids, frequently referred to as “synthetic marijuana, spice or K2”, are a group of compounds that produce an effect similar to the psychoactive ingredients in cannabis. In contrast to marijuana, synthetic cannabinoids are not derived from a plant; instead, the compounds are synthesized in a laboratory. Although the effects of these synthetic compounds may be similar to the natural delta‐9‐tetrahydrocannabinol compound in cannabis, they may be more potent and can result in additional adverse health effects not commonly seen with delta‐9‐tetrahydrocannabinol and may require hospitalization. 22
3.3. Medical manifestations and risks
The signs and symptoms of cannabis intoxications include euphoria, anxiety, paranoia, impaired judgment and motor coordination, irritated conjunctiva, and increased appetite. 23 Cardiovascular effects such as tachycardia, increased blood pressure, and lowered oxygen‐carrying capacity of blood are also among the adverse effects of this illicit drug. Furthermore, behavioral problems such as acute panic attacks and toxic psychosis have been reported. 24
The effects of cannabis use on the respiratory system are mainly associated with the long‐term smoking of marijuana. 25 The smoke from a cannabis cigarette contains the same contents as tobacco smoke, except for nicotine. Carbon monoxide, bronchial irritants, tar, and other carcinogens in cannabis smoke may be even higher in content than in tobacco smoke. Chronic smokers of cannabis usually have increased symptoms of bronchitis, including coughing, wheezing, sputum production, and emphysema. 25 , 26
The effects of heavy chronic cannabis use have been studied and an increased incidence of bronchial complaints is very similar to that found in tobacco smokers. The observed consequences include rhinopharyngitis, respiratory impairment, 27 and precancerous changes in the respiratory tract and the oral cavity. 28
Although cannabis is not a direct cause of death, the tobacco, which is usually mixed and smoked in adjunct with marijuana, can be, because it can triple the risk of lung cancer and is also related to some forms of oral cancer. 29
A predictable withdrawal pattern has been described for cannabis. It is usually exhibited with a series of symptoms involving behavioral changes, decreased appetite, weight loss, sleep difficulty, abdominal pain, tremor, fever, sweat, and headache. There are currently no approved medications to treat cannabis use disorder and the treatment generally consists of and is limited to engaging the patient in a psycho‐educational addiction treatment program. 23
3.4. Approved cannabinoids and medical use
Marijuana has been promoted for certain perceived health benefits, and in some countries its use is legalized.
The medical usefulness of the cannabis plant is regarded to arise from its cannabinoid compounds. 30 The four most common cannabinoid categories that have therapeutic potential for medical treatment are phytocannabinoids (the raw marijuana plant), synthetic cannabinoids (dronabinol, nabilone), purified cannabinoids (nabiximols, cannabidiol), and endogenous cannabinoids. 31
These legal drugs are approved in different countries for the treatment of anorexia in HIV seropositive patients with weight loss, as well as cancer chemotherapy‐associated nausea and vomiting that has failed standard therapies and strategies for pain management and spasticity in certain types of patients. 30
3.5. Effects of cannabis on overall oral health
The combined consumption of cannabis and tobacco, which is common among users, poses challenges for researchers who are interested in identifying the effects of cannabis alone. 15
An important side effect of cannabis is xerostomia; chronic use of cannabis may consequently increase the risk of carries. 32 , 33 , 34 In addition, infection with Candida albicans, nicotine stomatitis, higher incidence of periodontal disease, oral leukoedema, occasional hyperkeratosis, 35 , 36 and oral cancer 28 , 37 have also been reported. Darling and Arendorf 32 and Hashibe et al 38 discovered that cannabis smoke is associated with dysplastic changes within the epithelium of the buccal mucosa and the subsequent oral premalignant lesions, including leukoplakia and erythroplakia. However, the concurrent intake of alcohol, tobacco, and possibly other social drugs makes it difficult to be certain if cannabis alone is a risk factor for oral cancer should there be confounding and even synergistic effects. In order to reach a firm conclusion, rigorous clinical trials with robust methods would be required. 15 Uvulitis has also been reported in cannabis smokers. 27 , 39 , 40 In 2008, a systematic review confirmed that cannabis usage has a significant impact on increased xerostomia, leukoedema, and Candida albicans infection; the significance was illustrated even when the users were compared with tobacco smokers. 41 However, no definitive evidence of an independent association was found between cannabis usage and oral cancer or gingivitis. 15 , 39 , 42 Others have reported that cannabis users brushed their teeth less frequently than a control group (tobacco smokers only) and visited their dentist less regularly. 43
3.6. Cannabis use and periodontal disease
The deeper inhalation and prolonged contact and absorption time associated with cannabis smoking suggests that it may contribute to the etiology of periodontal disease (Figures 1 and 2).
FIGURE 1.
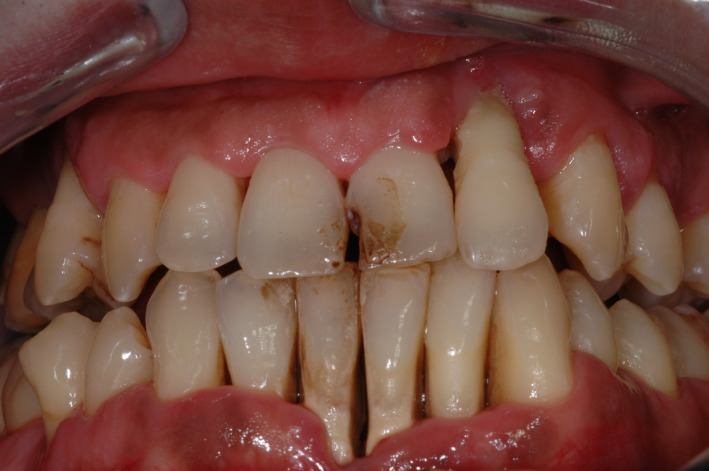
Clinical presentation of a 25‐year‐old Caucasian male patient (cannabis user) with generalized Stage IV, Grade C periodontitis
FIGURE 2.
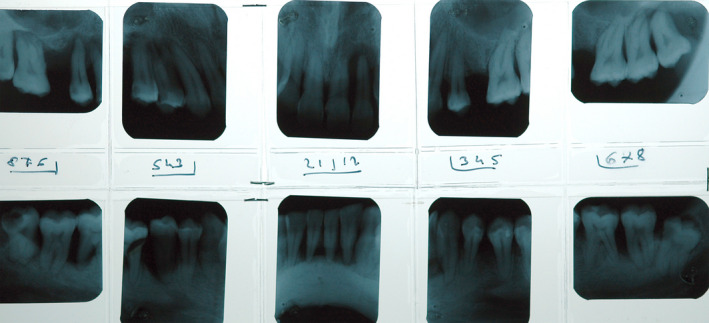
Radiographic full status of the patient in Figure 1 showing severe bone loss with multiple angular intrabony defects
As already mentioned, there are many deleterious constituents in cannabis like those of tobacco. The negative impact on periodontal tissues is likely to be related to the combustion products resulting from the burning of these substances rather than from the main active ingredients of the cannabis itself. 44 In addition, cannabinoids, as the main active cannabis components, may suppress important biological pathways related to inflammation. 45 , 46 Unfortunately, investigating such associations and mechanisms is challenging because of the confounding potential of concurrent tobacco smoking. 47
Despite the fact that a potential genetic susceptibility was discussed, in a recent study no genetic liability for lifetime cannabis use or cannabis use disorder with periodontitis was observed. 48 Although there is a scarcity of epidemiologic data on the impact of regular use of cannabis on periodontal tissues, five epidemiologic studies in adult participants of different ages and populations have indicated an independent detrimental role of cannabis use on periodontal conditions in a similar way to tobacco smoking (Table 1). 44 , 49 , 50 , 51 , 52 , 53 On the contrary, one cross‐sectional study has suggested that there is no significant association between cannabis smoking and signs of periodontitis. 44
TABLE 1.
Association between cannabis exposure and periodontal conditions
| Study | Design | Intervention/comparison | Clinical parameters | Results | Conclusions | Notes |
|---|---|---|---|---|---|---|
| Shariff 2017 51 | Cross‐sectional epidemiologic study. Survey on 1939 subjects from the NHANES 2011‐2012 | FRC vs non‐FRC users and periodontitis (also assessed after controlling for tobacco use) | PD; AL | FRC users had a significantly higher mean number of sites with PD ≥ 4, ≥ 6, and ≥ 8 mm higher mean number of sites with AL ≥ 3, ≥ 5, and ≥ 8 mm compared with non‐FRC users | FRC users exhibit deeper PDs, higher AL score and higher odds of having severe periodontitis than non‐FRC users | This is the only epidemiologic study that assessed the potential relationship between cannabis use and periodontitis in adults up to the age of 59 y |
| Thomson 2008 52 | Cross‐sectional cohort epidemiologic study. Clinical and medical data from 1037 participants of a large multi‐disciplinary study at ages 18, 21, 26, 32 y | Cannabis use (no exposure; some exposure; high exposure) and periodontitis (also assessed after controlling for tobacco use) |
Assessment of CAL: PD + REC collected in 2 quadrants at 26 and 32 y. Plaque and full mouth bleeding scores were also recorded |
Cannabis use associated with periodontitis (greatest differences in the ≥ 5 mm CAL case category) Incidence of periodontitis cases at 32 y, respectively, 19.3% and 10.4% for the ≥ 4 mm and the ≥ 5 mm CAL categories | Exposure to cannabis was strongly associated with the prevalence and incidence of periodontal attachment loss by age 32 y | All regression analyses were repeated using the top quartile and the highest 10% for cannabis use. Cannabis exposure remained a highly significant predictor of having 1 or more sites with 4 mm or greater CAL |
| Zeng 2014 53 | Cross‐sectional cohort epidemiologic study Participants form the same population of Thomson 2008 at ages 26, 32, and 38 y with a GLLMM | Cannabis use (no exposure; some exposure; high exposure) and periodontitis (also after controlling for tobacco use) |
Assessment of CAL: PD + REC collected in 2 quadrants at 26, 32, and 38 y. Plaque and full mouth bleeding scores were also recorded |
Smoking cannabis weekly or daily was associated with higher AL from 26 to 32 and 32 to 38 y | Smoking cannabis weekly or daily was associated with higher AL | Reexamination of the periodontal effects of smoking with a multilevel modeling (GLLMM) |
| Meier 2016 50 | Cross‐sectional cohort epidemiologic study. Same population of Thomson 2008 with analysis of medical and periodontal health from 18 to 38 y | Cumulative cannabis (joint)‐years and tobacco pack years and their effect on periodontal health (also after controlling for tobacco use) | Assessment of CAL PD+ gingival recession REC) collected in 2 quadrants at 26 y and full mouth at 18, 32, 38 y Plaque score and BoP | Cannabis use was associated with increase of AL even after accounting for tobacco packs‐year | Cannabis use from ages 26‐38 y was associated with decline of periodontal health | Analyses limited to 947 study members with laboratory health data at age 38 y |
| Jamieson 2010 49 | Cross‐sectional investigation study involving part (441 out of 686) of the ABC members | Cannabis use: (1) “never or only tried it once”; (2) “used to smoke”; and (3) “still smoke” and periodontal conditions (no adjustment for cofactors was performed) | PD and REC were recorded on 2 sites (mesiobuccal and buccal) per tooth and CAL obtained as sum of the 2 parameters | 26% of the participants had moderate or severe periodontitis. Without adjusting for other factors there was an elevated risk of periodontitis in cannabis users |
Cannabis, tobacco smoking, and petrol use associated with periodontal disease. Poly‐drug use does not allow an assessment of the impact of cannabis as independent risk factor |
Same periodontal classification used by Shariff 2017 (CDC/AAP classification) |
| Lopez 2009 44 | Screening epidemiologic study involving 9163 high school students (age 12‐21 y) with dental examination and questionnaires regarding socioeconomic factors and use of drugs. | Cannabis exposure (ever use of and regular use) and periodontal conditions (also assessed after controlling for tobacco use) | Two periodontal outcome variables: presence of CAL ≥ 3 mm (yes/no), and presence of NUG (yes/no) | No association between “Ever use of cannabis” or “Regular cannabis use” and CAL ≥ 3 mm. Association observed between use of cannabis and presence of NUG among nonsmokers | The use of cannabis is not associated with periodontal diseases with the exception of NUG in cannabis smokers (nontobacco smokers) |
The study was conducted on an adolescent/young adult population. No attempt of assessing the length of the exposure or the amount of cannabis used. |
Abbreviations: ABC, Aboriginal Birth Cohort; AL, attachment loss; BoP, bleeding on probing; CAL, combined attachment loss; CDC/AAP, Centers for Disease Control and Prevention/American Academy of Periodontology; FRC, frequent recreational cannabis; GLLMM, generalized statistical linear mixed model; NHANES, National Health and Nutrition Examination Survey; NUG, necrotizing ulcerative gingivitis; PD, probing depth; REC, Recession.
Recently, Shariff et al 51 published the results of the relationship between frequent recreational cannabis use and periodontitis prevalence among a sample of 1939 adults (aged 30‐59 years) in the United States. This is the only epidemiologic study that has assessed this potential relationship in adult subjects up to the age of 59 years. The authors analyzed the available data from the 2011‐2012 National Health and Nutrition Examination Survey. The National Health and Nutrition Examination Survey uses a complex stratified multistage probability sampling design to select noninstitutionalized civilians to nationally represent the Unites States population of all ages. 54
Groups based on cannabis use were constructed using two items from the questionnaire section of the 2011‐2012 National Health and Nutrition Examination Survey: (1) “Did you ever use marijuana or hashish?” and (2) “Did you use marijuana or hashish every month for a year?” Respondents who used marijuana or hashish once or more than once per month for the last 12 months were categorized as frequent recreational cannabis users, and those who did not use marijuana and hashish or reported to use marijuana or hashish fewer than once per month in the past year were categorized as nonfrequent recreational cannabis users. Out of 1939 subjects involved in this study, 60% of participants reported using cannabis at some point in their lifetime, whereas 27% reported cannabis use at least once per month over the last year.
Clinical parameters such as measurements of probing depth and clinical attachment loss were obtained from the examination section of the 2011‐2012 National Health and Nutrition Examination Survey database. All these measurements were recorded at six sites per tooth (mesio‐, mid‐, and disto‐buccal; mesio‐, mid‐, and disto‐lingual) for all teeth, excluding third molars. Periodontitis was examined using continuous and categorical measures. The primary outcome (periodontitis) was defined using the Centers for Disease Control and Prevention/American Academy of Periodontology classification for the surveillance of periodontitis. Probing depth and attachment level data were used to classify participants into one of four groups as follows: (1) “severe” periodontitis: two or more interproximal sites with attachment loss ≥ 6 mm (not on the same tooth) and one or more interproximal sites with probing depth ≥ 5 mm; (2) “moderate” periodontitis: two or more interproximal sites with attachment loss ≥ 4 mm (not on the same tooth) or two or more interproximal sites with probing depth ≥ 5 mm (not on the same tooth); (3) “mild” periodontitis: two or more interproximal sites with attachment loss ≥ 3 mm and two or more interproximal sites with probing depth ≥ 4 mm (not on the same tooth) or one site with probing depth ≥ 5 mm; and (4) “no” periodontitis: those that did not qualify as mild, moderate, or severe. The study analysis included the mean number of sites per participant with probing depth ≥ 4, ≥ 6, and ≥ 8 mm; and the mean number of sites per participant with attachment loss ≥ 3, ≥ 5, and ≥ 8 mm.
Several variables available in the National Health and Nutrition Examination Survey database with evidence of association with periodontitis were included as covariates (age category in groups of 10‐year intervals; sex; race, and ethnicity), and additional risk factors were factored and analyzed (diabetes mellitus; smoking; alcohol use). Because of its potentially large impact as a confounding factor, smoking status was particularly categorized on the basis of the response to two items from the questionnaire section: (1) “Have you smoked at least 100 cigarettes in your entire life?”; and (2) “Do you now smoke cigarettes?” Respondents who reported smoking every day or some days and had smoked > 100 cigarettes were categorized as “current smokers”; respondents who reported currently not smoking but having smoked > 100 cigarettes in the past were categorized as former smokers; and respondents who reported having smoked < 100 cigarettes ever were categorized as nonsmokers. Of all frequent recreational cannabis users, 40% reported that they currently smoked tobacco.
The results from the overall sample (including tobacco smokers) showed that frequent recreational cannabis users had a significantly higher mean number of sites with probing depth ≥ 4, ≥ 6, and ≥ 8 mm (mean difference ranged from six to seven sites) and significantly higher mean number of sites with attachment loss ≥ 3, ≥ 5, and ≥ 8 mm (mean difference ranged from six to 13 sites) compared with nonfrequent recreational cannabis users. In addition, the mean attachment loss was higher among frequent recreational cannabis users (1.8 mm) than among nonfrequent recreational cannabis users (1.6 mm; P = .004) and this observation was valid in both the anterior and posterior sextants.
The use of dedicated statistical software revealed that tobacco smoking was the only identified confounder among all other covariates. To eliminate the effect of tobacco smoking on the relationship between frequent recreational cannabis use and severe periodontitis, a second analysis model was created, which investigated only participants who had never smoked tobacco in their lifetime (n = 1118). The bivariate analysis of this sample revealed an odds ratio of 2.0 (95% confidence interval: 1.2‐3.5; P = .01) for severe periodontitis among frequent recreational cannabis users.
On the contrary, no significant associations were observed between cannabis use and severe periodontitis in bivariate models that included exclusively former smokers (odds ratio: 0.9, 95% confidence interval: 0.4‐1.8; P = .77) or current smokers (odds ratio: 0.9, 95% confidence interval: 0.4‐1.8; P = .68).
The results of this study revealed that frequent recreational cannabis users exhibit significantly deeper probing depths, higher attachment loss score, and higher odds of having severe periodontitis than nonfrequent recreational cannabis users. Moreover, frequent recreational cannabis use in the absence of tobacco smoking appeared to have equally adverse effects on periodontal tissues.
A large cohort epidemiologic study was conducted by Thomson et al 50 , 52 , 53 and resulted in the publication of three scientific papers that described the possible effects of cannabis smoking as a risk factor for periodontal disease in young adults. This is the only epidemiologic study with longitudinal periodontal data followed through participants aged in their 20s and 30s. 50 , 52 , 53
The authors assessed the clinical and medical data of 1037 participants of the Dunedin Multidisciplinary Health and Development Study at ages 18, 21, 26, 32, 52 , 53 and 38 years. 50 The Dunedin Multidisciplinary Health and Development Study is a longitudinal study of a cohort of children born in Dunedin, New Zealand, from 1 April 1972 to 31 March 1973.
Dental examinations were conducted at age 26, 32, and 38 years and included periodontal measurements collected in two quadrants. Three sites (mesio‐buccal, buccal, and disto‐lingual) per tooth were examined, and gingival recession and probing depths were recorded. Combined attachment loss for each site was calculated by summing up the probing depth and the gingival recession (third molars were not included). At 32 years, the clinical procedures were repeated as a full‐mouth examination. Dental plaque accumulation was measured at age 32 years using the Simplified Oral Hygiene Index. 55
Participants were assigned to one of three cannabis exposure groups according to this rate: “no exposure” group (those who reported no occasions of cannabis use); “some exposure” group (a mean of 1‐40 occasions of cannabis use during the previous year); and “high exposure” group (those with a mean of 41 or more occasions of cannabis use during the previous year).
Membership of the cannabis exposure groups was recorded as follows: there were 293 (32.3%) in the “no exposure” group, 428 (47.4%) in the “some exposure” group, and 182 (20.2%) in the “high exposure” group (41 or more occasions). However, frequent cannabis smokers were also more likely to smoke tobacco as well.
Tobacco smoking was measured and its effect as a confounding factor was considered. The number of pack‐years exposure (number of packs of cigarettes smoked per day multiplied by the number of years smoked at that rate) was computed at different time intervals. Half of the cohort (451, or 49.9%) had never smoked tobacco; one‐third were smoking at age 32 years (298, or 33.0%); and the remaining 154 (17.1%) were ex‐smokers. Other measures included in the study were adult socioeconomic status collected at age 32 years. At age 32 years, periodontal examination data and cannabis smoking history information from at least two assessments between the ages of 18 and 32 years were available for 903 participants.
The association between cannabis exposure and the prevalence of periodontitis was assessed using the following two case definitions: an individual with one or more sites experiencing ≥ 4 mm combined attachment loss and a more severe case definition considered for those individuals showing ≥ 5 mm combined attachment loss. Cannabis use was strongly associated with periodontitis prevalence, and the greatest relative differences were seen with the ≥ 5 mm combined attachment loss case definition, with the prevalence among the high exposure group almost seven times that of the no exposure group.
Incidence of periodontal disease in the cohort was measured by assessing the number of new cases with an increase in combined attachment loss between the ages of 26 and 32 years while controlling for tobacco, smoking, sex, socioeconomic status, dental service use, and self‐plaque control. Among the cannabis consumers, the incidence of new cases affected by periodontitis at age 32 years was 19.3% and 10.4% for the ≥ 4 mm combined attachment loss and the ≥ 5 mm combined attachment loss categories, respectively.
When regression analysis was used to control for the confounding factors (tobacco smoking exposure, sex, socioeconomic status, irregular dental service use, and the amount of plaque present), the relative risk of having one or more sites with ≥ 4 mm combined attachment loss for those who were in the high exposure group was still high, with a relevant risk of 1.61 (95% confidence interval, 1.16‐2.24).
As a validity check, all regression analyses were repeated using the top quartile and then the highest 10% for cannabis use (with all other variables remaining unchanged), and cannabis exposure remained a highly significant predictor for having one or more sites with ≥ 4 mm combined attachment loss.
It was evident that, after controlling for tobacco smoking and other possible confounders, regular exposure to cannabis smoke was strongly associated with the prevalence and incidence of periodontal attachment loss by the age of 32 years. In 2014, the same authors reexamined the association between cannabis and periodontal disease using statistical hierarchical modeling to: (1) overcome the limitations of the statistical approach used in the previous study (trajectory analysis); and (2) determine the robustness of the earlier inferences. 53 This study corroborated that frequent cannabis use was associated with greater periodontal attachment loss (higher attachment loss) and was considered by the authors as a risk factor for periodontal disease. These results were confirmed by analyzing the clinical and systemic health data of the same cohort at age 38 years with additional confirmation that long cannabis use (up to 20 years) is associated with periodontal disease and with individual decline in periodontal health from the age of 26‐38 years. 50
Jamieson et al 49 provided additional insight into cannabis use and periodontal conditions. A cross‐sectional investigation was performed within long‐standing prospective longitudinal research in the Aboriginal communities in Australia. Members of the Aboriginal Birth Cohort study who were born between January 1987 and March 1990 at the Royal Darwin Hospital, Northern Territory, Australia, were included in the study group. Data regarding drug use and periodontal disease were collected from the cohort when the mean age of participants was 18 years. Dental examinations for periodontal assessment were conducted and two sites (mesio‐buccal and buccal) per tooth, excluding the third molars, were examined. Probing depth and gingival recession were recorded obtaining the combined attachment loss for each site. The same definitions used by Shariff et al 51 (Centers for Disease Control and Prevention/American Academy of Periodontology classification) to describe moderate and severe periodontitis were adopted in this study.
The study subjects were also interviewed about petrol sniffing, marijuana, tobacco, and alcohol use. Specifically, participants were asked “How much marijuana do you smoke?” and “How much tobacco do you smoke?”; the response options were: (1) “Never or only tried it once”; (2) “Used to smoke, but not anymore”; or (3) “Still smoke sometimes”. The following additional covariates were collected: age, sex, education, occupation, and location (regional or rural). Four hundred and forty‐two participants agreed to be dentally examined and provided complete information in the self‐report dental questionnaire, which was 95% of the total number of participants examined at a mean age of 18 years. Substance use information was available for 425 (96%) of those individuals, and all subsequent analyses were limited to those 425 participants. The authors concluded that their results supported previous research indicating the negative impact of the use of marijuana and other substances on periodontal health.
However, among nonusers of tobacco, there were only 13 marijuana users, none of whom had periodontal disease, and no statistical assessment was possible. In addition, although tobacco, marijuana, and petrol use were strongly associated with the prevalence of periodontal disease, it was not possible to assess the impact of poly‐drug addiction and tobacco smoking on the periodontal conditions of the participants. These circumstances do not allow a clear assessment of the impact of cannabis as an independent risk factor for periodontal disease. Overall, this study showed weak evidence that medium‐term exposure to cannabis, tobacco, and petrol sniffing have a detrimental effect on the periodontal conditions.
The results from the National Health and Nutrition Examination Survey analysis in the USA, the Dunedin Study in New Zealand, and the limited data from the Aboriginal Birth Cohort study in Australia, are in contradiction to the findings from Lopez and Baelum. 44
In this study, data from a population‐screening examination carried out among Chilean high school students from the Province of Santiago were used to determine whether there was an association between the use of cannabis and signs of periodontal diseases as defined by (1) the presence of necrotizing ulcerative gingivitis lesions or (2) the presence of clinical attachment loss ≥ 3 mm. A total of 9163 high school students (age 12‐21 years) underwent dental examination and a questionnaire regarding information on socioeconomic factors 56 and the use of drugs including cannabis 57 was answered by the participants.
Regarding the periodontal analysis, combined attachment loss was defined as the distance from the cemento‐enamel junction to the base of the clinical pocket, and direct recordings of combined attachment loss were obtained at six sites (mesio‐buccal, mid‐buccal, disto‐buccal, mesio‐lingual/mesio‐palatal, mid‐lingual/mid‐palatal, and disto‐lingual/disto‐palatal) of each of the incisors and all first and second molars. The presence of necrotizing ulcerative gingivitis lesions was considered positive if at least one interproximal papilla presented with necrotic ulcerated lesions (described as a “punched‐out” appearance and loss of surface tissue). Therefore, two periodontal disease outcome variables were defined, one being the presence of combined attachment loss ≥ 3 mm (yes/no), and the other being the presence of necrotizing ulcerative gingivitis (yes/no). Using multiple logistic regression analysis, the associations between either of the two outcome variables and cannabis use were explored for each of three tobacco smoking strata, the nonsmokers (n = 4885), the occasional smokers (n = 1997), and the daily smokers (n = 2281). Two cannabis exposure variables were considered, one being “Ever use of cannabis” (yes/no) and the other being “Regular use of cannabis”. No attempts were made to assess the length of the exposure to cannabis, or the amount of cannabis used.
The statistical logistic regression analyses were adjusted for age, gender, paternal income, paternal education, frequency of toothbrushing, and time since last dental visit. The results showed no association between “Ever use of cannabis” and combined attachment loss ≥ 3 mm where nonsmokers (odds ratio = 0.95), occasional smokers (odds ratio = 1.15), or daily tobacco smokers (odds ratio = 0.98) were concerned. Similarly, there was no evidence for any association between “regular cannabis use” and combined attachment loss ≥ 3 mm irrespective of the tobacco smoking category. When analyses were adjusted for the effects of all the covariates, all but one odds ratio estimate indicated a negative association between cannabis use and the presence of necrotizing ulcerative gingivitis. An inverse association was in fact observed between the use of cannabis and the presence of necrotizing ulcerative gingivitis among nontobacco smokers (odds ratio = 0.47).
The authors concluded that there was no evidence to suggest that the use of cannabis is positively associated with periodontal diseases in an adolescent/young adult population except for necrotizing ulcerative gingivitis in cannabis smokers who are not tobacco smokers.
It is important to note that the studies from Thomson et al 50 , 52 , 53 obtained clinical, exposure, and disease data in a prospective way, while in the study conducted by Lopez 44 the relationship between exposure and disease was collected simultaneously and is considered less reliable or accurate. Another important difference to be considered is the age ranges that were covered in the studies: Lopez (12‐21 years), 44 New Zealand studies (26‐38 years), 50 , 52 , 53 and the USA studies (30‐59 years). 51 This difference has significant implications, as the duration of exposure is likely to have been longer in the studies of older age groups.
Another frequent, interesting clinical observation is the association between chronic use of cannabis and gingival enlargement (Figure 3). Several authors 35 , 40 , 58 have reported cases of marijuana‐associated gingival enlargements. It appears that marijuana‐associated gingival enlargement is a condition seen primarily in young adult males who have had 2 or more years of continuous marijuana consumption. 40 The gingival enlargements are mostly papillary and marginal, comparable with the gingival enlargement that occurred with phenytoin (dilantin) therapy, where the areas primarily affected are the interdental papillae and the marginal gingiva; sometimes they appear to be nodular. 59 , 60 There may be a biochemical basis for the clinical similarities between marijuana‐associated and phenytoin‐induced gingival enlargement. Cannabidiol is a major nonpsychotropic constituent of cannabis. As indicated above, attention has been focused on its pharmacologic aspects over the past few years because of its anticonvulsive, anxiolytic, antipsychotic, antiemetic, and antiarthritic properties. 61 Considering the common anticonvulsant properties and the similarity in structure between cannabidiol and phenytoin, it may be hypothesized that the enlargement seen in marijuana users is caused by pathogenetic mechanisms similar to those implicated in phenytoin‐induced gingival enlargement. These include an increase of gingival fibroblast growth and connective tissue matrix production, inflammation, and altered effects on calcium metabolism in a complex epigenetic interactive environment. 62
FIGURE 3.
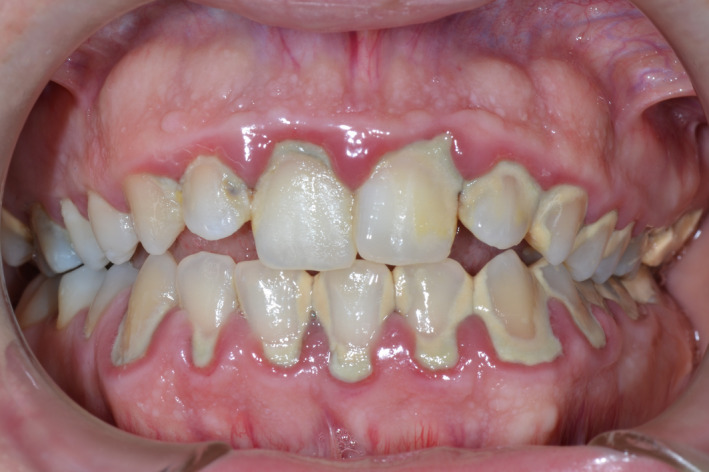
Clinical presentation of a 20‐year‐old Caucasian male patient with plaque‐induced gingivitis and cannabis‐induced gingival enlargement
3.7. Conclusions on the effect of cannabis and periodontal disease
Overall, despite the variations of case definitions of cannabis exposure and periodontal disease, and the clinical parameters analyzed, data from epidemiologic studies on different populations show that medium‐ to long‐term cannabis smoking in adult subjects can be a risk factor for periodontal disease independent of the use of tobacco (Tables 1 and 4). 44 , 49 , 50 , 51 , 52 , 53 The main clinical periodontal manifestations observed in cannabis smokers are the increase of attachment loss and probing depth; cannabis can also cause gingival enlargements and influence necrotizing ulcerative gingivitis. 35 , 40 , 44
TABLE 4.
Dental and periodontal conditions and treatment considerations in illegal drug users (modified from O'Neil Wiley Blackwell 2015 (81)
| Used illegal drug | Psychiatric signs and symptoms | Physical examination findings | Dental conditions | Periodontal conditions | Dental treatment considerations |
|---|---|---|---|---|---|
| Cannabis |
Euphoria Hyperactivity Dissociation Tachycardia Paranoia Delusions Hallucinations |
Poor coordination Irritated conjunctiva |
Xerostomia Increased risk of caries Leukoplakia |
FRC users exhibit deeper PDs, higher AL score and higher odds of having severe periodontitis than non‐FRC users. Gingivitis; gingival enlargement and possible ulcerative conditions |
Avoid local anesthetic with epinephrine Xerostomia treatment products Topical fluoride treatment Avoid treatment at least 24 h from last drug use |
| Cocaine and crack |
Euphoria Insomnia Paranoia Dissociation Aggressive behavior Delirium Restlessness |
Nasal septal and palatal necrosis and perforation Powder in nares Weigh loss Dilated pupils Tachycardia Skin abscess Slurred speech Vomiting Increased heart rate Wide QRS complex and QT/QTc prolongation |
Xerostomia Occlusal wear Generalized caries Bruxism Orofacial pain Graft failure Alterations of taste perception Keratinization of oral mucosa Discoloration of saliva Involuntary facial movements |
NUG Gingival ulceration and erythema Painful gingival retraction Periodontitis No alterations in microbiota composition and counts |
Avoid local anesthetic with epinephrine Occlusive guards Topical fluoride treatment Avoid treatment at least 24 h from last drug use |
| Methamptemins; ecstasy and MDMA |
Euphoria Dissociation Panic reactions Impulsive behavior Depression Psychotic episodes |
Black rotting teeth Nasal septal necrosis Poweder in nares Mucus discharge from the naris Jaw clenching Jaw soreness Xerostomia Occlusal wear Generalized caries Bruxism |
Dehydration Dilated pupils Excessive sweating Tachycardia Skin abscess Weigh loss Burnt fingers Track marks |
Abbreviations: AL, attachment loss; FRC, frequent recreational cannabis; MDMA, 3,4‐Methylenedioxy‐Methamphetamine; NUG, necrotizing ulcerative gingivitis; PD, probing depth.
4. STIMULANTS
Stimulants (or psychostimulants) are a class of psychoactive drugs that induce temporary improvements in mental or physical functions. Psychologically, the euphoriant effect staves off fatigue; and physically, it has a slimming effect 29 by enhancing the activity of the central and peripheral nervous systems via the stimulation of both alpha‐ and beta‐adrenergic receptors. Stimulants exert their effects by enhancing norepinephrine (noradrenaline) and/or dopamine brain activity. Psychostimulants of common use include amphetamine (or methamphetamine), 3,4‐methylenedioxymethamphetamine (ecstasy), and cocaine. 63
5. COCAINE/CRACK AND PERIODONTAL COMPLICATIONS
5.1. Description of the drug and its effects
Cocaine (benzoylmethylecgonine) is a strong central nervous system stimulant that produces a profound immediate effect by potentiating catecholamines and interfering with the reuptake process of dopamine, a chemical messenger associated with pleasure and movement. It is available in powder or crystal form. 64 Extracted from coca leaves, cocaine is an alkaloid, originally developed as a painkiller. Crack cocaine is the crystal, hydrochloride form of cocaine. It comes in solid blocks or crystals varying in color from yellow to pale rose or white and it is usually processed with ammonia or sodium bicarbonate. 29 , 65 Crack is heated and smoked. It is named so because it makes a cracking or popping sound when heated. It is the most potent form in which cocaine appears and the riskiest to health. It ranges from 75% to 100% in purity, much stronger and more potent than regular cocaine. Cocaine is most often sniffed, with the powder absorbed into the bloodstream via the nasal tissues. It can also be ingested or rubbed into the gums. The cocaine powder is usually mixed with other substances such as corn starch, talcum powder, and/or sugar or other drugs such as procaine (a local anesthetic) or amphetamines. To promote more rapid absorption of the drug into the body, some users inject it, but this substantially increases the risk of overdose. Inhaling it as smoke or vapor speeds absorption without the health risks as severe as injection.
5.2. Medical manifestations and risks
Because of the lipophilic features of the noionized form, cocaine diffuses across the neurons' membranes and returns to the active cationic form in the axoplasm, where it can bind to the sodium‐gated channels, acting as a reversible anesthetic. Euphoria, hyperstimulation, reduced fatigue, heightened mental clarity, and arousal are the consequences of the blocking of presynaptic reuptake of serotonine and norepinephrine as the levels of these neurotransmitters increase. 29 These effects appear in < 5 minutes and the duration of the stimulant effects usually last up to 30 minutes and can vary depending on its route of administration. Other symptoms include dizziness, blurred vision, light‐headedness, tinnitus, disorientation, paranoia, hallucinations, restlessness, aggressive behavior, delirium, vomiting, tremors, shivering, insomnia, dilated pupils, hyperthermia, hypertension, tachycardia, and an increased rate of respiration. 66
With increasing doses of cocaine, these initial signs of central nervous system excitation are rapidly followed by a generalized state of central nervous system depression, a craving for sleep, and frequently result in a decreased respiratory rate with periods of apnea. 67 Withdrawal from cocaine is typically not life threatening and rarely requires medical intervention, although it can be quite distressing. 23 Alcohol and cocaine are commonly used together; between 50% and 90% of cocaine users also concurrently ingest ethanol during their binges. Cocaine users frequently report that the use of ethanol and cocaine together prolongs the effect. 68 In this case, the liver manufactures cocaethylen, which intensifies the euphoric effects and raises the risk of sudden death. 29 Cocaine's effects can increase the risk of cardiovascular damage related to the sympathomimetic effects of cocaine. It exhibits “slow” on–off kinetics at the sodium channels. Ventricular arrhythmias and electrocardiogram alterations can occur subsequently. Cocaine is also known to cause vasoconstriction, which can result in hypertension, heart arrest, cardiac ischemia, and end organ and/or tissue infarcts. 69 , 70
5.3. Effects on overall oral health
Oral health is compromised in several ways by the snorting, smoking or oral use of cocaine. Oral use of cocaine temporarily numbs the lips and tongue and can cause gingival or mucosa erosions, dry mouth, bruxism and/or dental erosions. 29 , 71 , 72 , 73
Several cases of palatal perforations have also been described in the scientific literature. Most of the patients with cocaine‐induced palatal necrosis are female (72%), despite the fact that more men use cocaine than women. 66 Patients with a palatal perforation experience serious speech impairment. Speech becomes hypernasal and articulation may decrease the effectiveness of their communication. In addition, eating and drinking are difficult because of the oronasal reflux of both solids and liquids. 71 , 74 , 75
Friedlander et al 71 analyzed the dental management of cocaine‐addicted subjects. They focused on the hard tissue damage and described that cocaine users are affected by bruxism with involvement of the temporomandibular joint and painful symptoms of the masticatory muscles. 71
In a review on oral health of cocaine effects, Brand et al 76 described a series of orofacial manifestations compatible with cluster headache. Cocaine triggering pain in the premolar zone of the maxilla, followed by spread to the periorbital zone on the same side, has also been reported. 77
Also, the risks of cervical abrasions and caries were found to be higher in cocaine users with a stronger brushing activity. 71 A study on rats demonstrated a nonfunctional masticatory activity and an increased dental attrition rate. 77 Parry et al 74 reported similar effects on humans describing a mild attrition on canines, first premolars, and upper incisors. The same authors found cervical caries of incisors and canines in a young patient who rubbed cocaine on the frontal gingivae. 78 A reduction of pH has been observed in cocaine powder users when the substance is dissolved in saliva. The consequence of dissolving hydroxyapatite increases the risk of enamel loss, which gave the tooth a glassy appearance. 79 , 80 A decrease in salivary pH has also been described in crack smokers and may be responsible for the rapid tarnishing of gold crowns. 81
Some authors reported that the failure of bone graft caused by the rubbing mode of consumption and the vasoconstrictive effect of cocaine may have caused the graft exposure 3 months after the surgery. 82 Other effects can be related to the joint medication use of the addicted patients. Levodopa and lithium, which are often mixed with cocaine, may alter taste perception, induce a red discoloration of saliva, or induce involuntary facial movements. 83
Several authors assessed the effects of crack and cocaine on oral mucosa. These studies revealed that crack cocaine smoke increases the rate of cellular proliferation in cells of normal buccal mucosa, inducing clastogenic effects. Higher degrees of keratinization in the floor of the mouth were observed. Because illicit drug use is normally associated with other risk factors identified for oral cancer (eg, tobacco and alcohol), crack cocaine users should have frequent preventive oral examinations to allow early diagnosis and treatment. 64 , 84 , 85
5.4. Cocaine use and periodontal disease
Multiple studies reported the general impact of crack and cocaine on periodontal conditions (Table 2). 33 , 86 , 87 , 88 , 89 , 90
TABLE 2.
Association between cocaine exposure and periodontal conditions
| Study | Design | Intervention comparison | Clinical parameters | Results | Conclusions | Notes |
|---|---|---|---|---|---|---|
| Antoniazzi 2016 86 | Cross‐sectional study that evaluated 106 individuals exposed to crack cocaine and 106 never exposed, matched for age, sex, and tobacco use | Periodontal status between crack cocaine users and crack cocaine nonusers. Investigation of the association between crack cocaine and periodontitis after adjustment for confounding variables. | VPI, MBI, supragingival dental calculus, PD, CAL, and BoP |
Prevalence of periodontitis among crack nonusers and crack users was 20.8% and 43.4%, respectively. Crack users had greater VPI, BoP, PD ≥ 3 mm, and CAL ≥ 4 mm than crack nonusers. Periodontitis was associated with age > 24 y, schooling £8 y, smoking, moderate/heavy alcohol use, and plaque rate ≥ 41%. Crack users had an approximately 3‐fold greater chance (odds ratio: 3.44; 95% confidence interval: 1.51 to 7.86) of periodontitis than nonusers. |
Occurrence of periodontitis, visible plaque, and gingival bleeding was significantly higher among crack users, and crack use was associated with occurrence of periodontitis. | |
| Yukna 1991 89 | Twenty case reports with usual and nonusual oral manifestations selected and ordered by increasing severity | No intervention comparisons were performed | Periodonatal tissue damage | The same pysiologic effects of vasocostriction, epithelial sloughinf, ischemic necrosis and local anesthesia. Oral hygiene trauma may explain the observed lesions |
Cocaine users may be trading one problem for another when they change the location of drug administration from the nasal site to the gingiva |
Most of those interviewed were aware of the damage that could be done to the nasal septum but felt that nothing would happen to the gingiva. |
| Ramos Cury 2017 88 | cross‐sectional cohort study on 160 patients | Periodontal parameters in crack cocaine addicted and not addicted patients. | PD, CAL, BoP, and plaque index | PD was significantly greater in crack/cocaine addicted individuals compared with nonaddicted individuals. After adjusting for covariates, periodontitis was not significantly associated with crack/cocaine use, which was only associated with age ≥ 35 y and higher dental plaque index. | Although PD was greater in crack/cocaine addicted individuals, destructive periodontal disease was not associated with crack and cocaine addiction. Periodontal disease was associated with age and dental plaque | The population of the present study, in general, was quite young to have moderate to severe periodontal disease. |
| Casarin 2017 87 | Cross‐sectional study was conducted involving 74 crack cocaine users and 81 nonusers matched for age, gender, and tobacco use. | Periodontal pathogens in crack users and nonusers | Subgingival bacterial samples collected from 4 sites with the greatest PDs and analyzed using RT PCR | No significant difference was found in the prevalence of total counts for each bacterial species analyzed between groups. | Although some crack users had higher (> 75th percentile) bacterial counts for Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum, total counts did not differ between crack users and nonusers, leading to the hypothesis that the higher occurrence of periodontitis on crack users may be related to other nonbacterial factors. | Crack users had a greater probability of having the higher for Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum, respectively. |
Abbreviations: BoP, bleeding on probing; CAL, combined attachment loss; MBI, marginal bleeding index; PD, probing depth; RT‐PCR, real‐time polymerase chain reaction; VPI, visible plaque index.
A recent study compared periodontal status between crack cocaine users and nonusers and investigated the association between crack cocaine and periodontitis after adjustments for confounding variables. Prevalence of periodontitis was significantly higher among users than controls, and crack cocaine use was associated with the occurrence of periodontitis after adjustments for confounding variables. 86 This study was designed in a cross‐sectional fashion and was conducted with a convenience sample of individuals exposed to crack cocaine, and a control group never exposed to the drug. There were 212 participants (158 males and 54 females aged 13‐46 years; mean age: 25.4 years). Inclusion criteria were a diagnosis of chemical dependency on the drug, having used the drug for at least 1 year, and absence of cognitive impairment. Individuals who had never used crack cocaine were selected for the control group and matched for sex, age, and smoking habit. Use of psychoactive substances was determined using a closed‐answer questionnaire that has been tested and adapted to the Brazilian population. All erupted teeth (except third molars) were evaluated with six sites probing for the determination of probing depth, clinical attachment level, and bleeding on probing. Visible plaque index, marginal bleeding index, and supragingival dental calculus (presence/absence) were determined at four sites per tooth. Individuals exposed to crack cocaine had a greater prevalence and severity of periodontitis as well as greater degrees of dental plaque and bleeding on probing. After adjustments for other variables, crack cocaine use remained significantly and positively associated with periodontitis. Crack users had greater visible plaque index and bleeding on probing scores, number of sites with probing depth ≥ 3 mm, and/or combined attachment loss ≥ 4 mm than crack nonusers. Periodontitis was associated with age > 24 years, schooling ≤ 8 years, smoking, moderate/heavy alcohol use, and plaque rate ≥ 41%. Crack users had an approximately three‐fold greater chance (odds ratio: 3.44; 95% confidence interval: 1.51‐7.86) of periodontitis than nonusers.
Contrasting results were obtained by Cury et al. 88 Although this study revealed a higher probing depth in crack/cocaine‐dependent men, destructive periodontal disease, clinical attachment level, and bleeding on probing were not found to be associated with crack/cocaine dependence. This cross‐sectional study included 160 men consisting of 120 nonusers of illicit drugs and 40 crack/cocaine‐addicted individuals, consecutively seen in the School of Dentistry, Federal University of Bahia (Salvador, Bahia, Brazil). Addiction to both crack and cocaine was the exposure, and destructive periodontal disease was the outcome in their study. The route of cocaine administration was intranasal (snorting) and that of crack was oral (smoking). Males aged older than 19 years of age in good general health, with a minimum of six teeth, were included in this study. The exclusion criteria were previous subgingival periodontal therapy, systemic diseases that could affect the progression of periodontal disease (eg, diabetes and immunological disorders), alcohol, and other illicit drug dependence, long‐term administration of anti‐inflammatory medication, need for antibiotic coverage for routine dental therapy, and antibiotic therapy in the previous 6 months. Eventually, 40 out of 120 exposed individuals that were invited to participate were eligible and enrolled. All permanent, fully erupted teeth, excluding the third molars, were probed at six points; combined attachment loss and bleeding on probing were recorded. Mean probing depth value was significantly greater in crack/cocaine‐addicted individuals (2.84 ± 0.76 mm) compared with nonaddicted individuals (2.55 ± 0.73 mm, P = .04). Although the probing depth was greater in crack/cocaine‐dependent individuals, destructive periodontal disease was not associated with the use of crack and cocaine in this population, but was associated with higher dental plaque index and older age.
Recently, some authors quantified, through real‐time PCR, the presence and counts of Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Porphyromonas gingivalis, and Fusobacterium nucleatum in crack users and nonusers. 87 A cross‐sectional study was conducted involving 74 crack cocaine users and 81 nonusers matched for age, gender, and tobacco use. Demographic and clinical variables were analyzed. Subgingival bacterial samples were collected from four sites with the greatest probing depths and analyzed using real‐time PCR. No significant difference was found in the prevalence of total counts for each bacterial species analyzed between groups. However, crack users had a greater probability of having higher counts for A. actinomycetemcomitans, P. gingivalis, P. intermedia, and F. nucleatum. Because the total counts did not differ between crack users and nonusers, the authors hypothesized that the higher occurrence of periodontitis in crack users may be related to other nonbacterial factors.
In an interesting case series, it has been shown that cocaine users have diverse manifestations of gingival and alveolar bone destruction; in particular, abnormal gingival conditions and acute necrotizing ulcerative gingivitis‐type lesions resulted from local application of the illegal drug. 89 The gingival effects of cocaine are mostly related to the practice of rubbing in users (Figure 4). The direct vasoconstrictive effect of cocaine at the sites of application causes a white slough, which can be easily removed, and that shows underlying ulcerations and erythema. Painful and marginal gingival recessions are reported by patients (Figure 5). Gingival recession can be exacerbated by aggressive and overzealous brushing. Within 2 weeks to 18 months of reestablishment of correct oral hygiene procedures, gingival lesions were reported to disappear spontaneously. 91 , 92
FIGURE 4.

Cocaine‐induced keratosis of the attached gingiva resulting from repeated gingival rubbing of the illegal substance
FIGURE 5.

Clinical presentation of a patient with deep narrow recession and bone dehiscence on the lower left central incisor induced by cocaine use
Overall, the association between crack cocaine and periodontitis can be explained by both systemic and local factors. The systemic biologic mechanism seems to be the most plausible explanation as the effect of exposure was maintained after adjustments for clinical variables. In periodontitis, cytokines and growth factors produced by cells in inflamed periodontal tissue can influence osteoclast differentiation and function, providing a link between inflammation and the process of bone destruction. The potential mechanisms may involve the upregulation of pro‐inflammatory and downregulation of anti‐inflammatory cytokines profile, which eventually favors the periodontal bone loss clinically observed. 76
5.5. Conclusions on the effect of cocaine/crack on periodontal disease
The main clinical periodontal conditions observed in cocaine/crack users are gingival lesions subjected to chemical trauma, owing to the local applications of the substances, and are usually manifested as recessions or necrotizing ulcerative gingivitis‐type lesions (Tables 2 and 4; Figures 6 and 7).
FIGURE 6.

Chemical traumatic lesion localized at the level of the mucogingival junction in a crack user
FIGURE 7.

Chemical traumatic lesion of the lip caused by crack use
6. ILLEGAL SYNTHETIC AMPHETAMINE DERIVATIVES AND PERIODONTAL COMPLICATIONS
6.1. Description of the drugs and their effects
Amphetamine is a central nervous system stimulant that can also be used medically to treat attention deficit hyperactivity disorder, narcolepsy, and obesity in people who failed to lose weight with diets or alternative treatments. 93 Legally produced amphetamines, such as methylphenidate and phenmetrazine, are sometimes diverted to recreational use; illegally produced members of the amphetamine class of drugs include dextroamphetamine, methcathinone, and methamphetamine. 94
3,4‐Methylenedioxy‐Methamphetamine is an illegal synthetic n‐methyl homologue of amphetamine, 95 more commonly referred to as “ecstasy” or XTC, patented in 1914 by the German pharmaceutical company Merck. 96 It is known by a variety of street names, including Blue meth, Chicken feed, Cinnamon, Crink, Crystal meth, Desocsins, Geep, Granulated orange, Hot ice, Ice, Kaksonjae, LA glass, Lemon drop, Meth, OZs, Peanut butter, Sketch, Spoosh, Stove top, Super ice, Tick tick, Trash, Wash, Working man's cocaine, Yellow barn, and Yellow powder. 97 It is currently the third most widely used illegal drug: > 40 million use amphetamine‐based drugs each year worldwide. Specifically, 3,4‐Methylenedioxy‐Methamphetamine causes a massive synaptic release of serotonin (5‐hydroxytryptamine) and, to a lesser extent, of dopamine and norepinephrine. Because 3,4‐Methylenedioxy‐Methamphetamine also inhibits the reuptake transporters of the synapse, there is an acute increase in the intrasynaptic concentration of these neurotransmitters, followed by a period of depletion. 98 When an ecstasy tablet is orally ingested, its effect begins after 20‐60 minutes and lasts for 4‐6 hours, with a peak after 2 hours. The half‐life of 3,4‐Methylenedioxy‐Methamphetamine in plasma is 6‐9 hours. About 80% of 3,4‐Methylenedioxy‐Methamphetamine is cleared metabolically in the liver, catalyzed by the cytochrome P450 isoenzyme CYP2D6. The remaining 20% of the dose is excreted unaltered in urine, 99 where it can still be detected 2 to 3 days after use. Ecstasy is also excreted in other body fluids such as tears, saliva, sweat, and breast milk. 99 , 100 3,4‐Methylenedioxy‐Methamphetamine is a class II‐controlled stimulant with limited medical use and a high potential for use. This potent psychomotor stimulant is synthesized in a single, straightforward process through the reduction of ephedrine or pseudoepherine. The product is a white, odorless, bitter crystalline powder that can be taken intravenously, intranasally, orally, or smoked. Methamphetamine is alluring because it is cheap, widely available, and produces many desirable effects. Both amphetamine and 3,4‐Methylenedioxy‐Methamphetamine are highly addictive and have a high potential for use; however, 3,4‐Methylenedioxy‐Methamphetamine's effects in the central nervous system are longer lasting and the systemic effects are more deleterious. 97
Ecstasy is normally sold as tablets, which have different colors, shapes, and logos. Tablets sold as ecstasy contain varying amounts of 3,4‐Methylenedioxy‐Methamphetamine (typically 30‐150 mg, on average 77 mg) or none at all. 101 These tablets may also contain other substances, such as methylenedioxyethylamphetamine, methylenedioxyamphetamine, methamphetamine, ketamine, caffeine, and/or salicylic acid, and they may contain additives used as fillers or binders, and occasionally other psychoactive compounds. 102 Ecstasy is frequently used in combination with alcohol or other types of drugs, which can result in unpredictable effects. 96 It is very popular with users because of its relatively low cost and its long “high” period. This “high” period consists of enhanced well‐being, increased energy, heightened libido, and appetite suppression. 103 , 104
The pattern of drug use varies, ranging from infrequent use as a socializing action termed “recreational use” to continuous chronic use characteristic of drug addiction. 105 The addiction risk of ecstasy seems to be limited. Animal studies, however, suggest that long‐term use of ecstasy is toxic to neurons. 106 Recent evidence suggests that serotonin‐neurotoxicity may also occur with repeated 3,4‐Methylenedioxy‐Methamphetamine use in humans, which explains the progressive memory deficits after prolonged consumption. 107 , 108
6.2. Medical manifestations and risks
Gantos et al 109 divided the medical general effects of methamphetamine use into two main groups: behavioral/psychological changes and poor nutrition. The behavioral/physiologic effects of MA are well known and they are related to sympathomimetic manifestations, in turn caused by the stimulation of the nervous system via the adrenal glands, which increases heart rate and tachypnea via vasoconstriction and bronchodilation, fatal kidney disease, and hyperthermia. 110 The short‐term behavioral effects include intensified emotions, euphoria, aggression, talkativeness, increased alertness, insomnia, hyperactivity, decreased appetite, increased respiration and hyperthermia, increased sensory perception, and sense of closeness to other people. 96 The long‐term effects include psychological (but not physical) addiction and dependence, sleeplessness, restlessness, hyperactivity, loss of appetite and weight, tremor, and repetitive movements 112 ; paranoia is a long‐term effect, which needs years after quitting to be controlled, and it can be worsened by auditory and visual hallucinations. 97 Moreover, chronic methamphetamine use increases aggression and impulsivity and impairs executive functions, causing interpersonal difficulties and leading to a disorganized lifestyle. 110 , 112 Patients frequently fail to show up for appointments and may be irritable, restless, or anxious during medical treatments. The greatest adverse effect is its impact on cognition and learning, caused by depletion of monoamines in the brain, 113 and on mood disturbances, which can last for months after cessation of the drug intake 114 , 115 , 116 ; major depressive disorder, psychosis, and concurrent drug and alcohol‐use disorders have been reported. 98 , 106 , 117 There is also an increased risk of mortality because of suicide and overdose 118 ; indeed, mood disturbances may precipitate and delusions (eg, formication, the sensation of insects creeping on the skin) may contribute to homicidal or suicidal thoughts and actions. 70
Poor nutrition is related to skipping meals and appetite suppression to the point where users are often unhealthily thin and undernourished, with brittle bones, or anorexic and more vulnerable to infections (Figure 8). Methamphetamines disrupt metabolic and neuroendocrine regulation, leading to improper calorie consumption and impaired nutrient processing. The users tend to “snack” and consume huge amounts of sugar for a drug‐induced need of high‐calorie carbonated beverages. 119 Because low weight and eating disorders may be of concern, encouraging and educating patients on proper nutrition and helping them achieve a healthy body mass index is important. Indeed, detoxification programs commonly lead to weight gain, as addicts turn to food instead of their drugs of choice.
FIGURE 8.

Clinical presentation of a patient (MDMA user) with cheilitis related to poor nutrition. MDMA, 3,4‐Methylenedioxy‐Methamphetamine
A series of other systemic adverse effects have been described and include cardiac arrhythmias, hyperthermia, increased heart rate, hypertension, stroke, anxiety, nausea, tremor, serotonin (5‐hydroxytryptamine) syndrome, liver complications, dilated pupils, seizures, coma and, in rare cases, death. 120 Mild doses are characterized by hyperreflexia, hypertension, irritability, headaches, and dizziness, while toxic doses may cause palpitations, hallucinations, convulsions, and comas. 121 Neurotoxicity and neurodegeneration are associated with long‐term methamphetamine use. Sometimes, body coordination may become difficult. The induced neuromuscular stimulation results in muscle rigidity and breakdown of muscle fibers (rhabdomyolysis), which in turn may raise the body temperature. In combination with prolonged vigorous dancing in hot and crowded clubs, this could lead to fulminant hyperthermia with body temperatures as high as 44°C. 107 , 122 Fulminant hyperthermia has a poor prognosis, as it might lead to further rhabdomyolysis, acute renal and liver failure, and disseminated intravascular coagulation. 101 , 107 , 111 , 122 , 123 Therefore, it is important that individuals experiencing the symptoms of ecstasy intoxication are cooled down as soon as possible. The combination of hyperthermia and the warm environment of dance clubs often results in an excessive water intake. However, ecstasy also stimulates the secretion of antidiuretic hormone. This increased water intake with impaired renal excretion will dilute body fluids, causing hyponatremia and cerebral edema with insults and coma. Therefore, consumption of isotonic fluids (such as sport drinks) instead of water is recommended, as isotonic fluids will help to restore minerals and reduce the risk of developing hyponatremia. 124
6.3. Effects on overall oral health
Amphetamines and methamphetamine have a variety of effects on oral health. These include broken or missing teeth, 125 bruxism, 126 xerostomia or dry mouth, 127 increased risk of dental erosion, 96 tooth surface loss, 128 tooth‐wear, 96 and caries 119 , 129 , 130 (Figure 9). 3,4‐Methylenedioxy‐Methamphetamine users often show severe xerostomia from the use of antidepressant and antipsychotic medications. In addition, patients taking amphetamines have an increased risk of gingival enlargement, 131 periodontitis, and mucosal ulceration. 132
FIGURE 9.

Clinical presentation of a patient (MDMA user) with generalized gingival recessions and abrasions. MDMA, 3,4‐Methylenedioxy‐Methamphetamine
Ecstasy users have reported that jaw tension, trismus, 132 jaw pain, and tooth grinding were common side effects. 133 Some authors observed that ecstasy users also reported a habit of biting their cheeks, tongue, or lips during and after using drugs because of the numbness of their mouth and reduced teeth sensitivity. 133 Oral mutilation 134 as well as lip paresthesia 135 were reported. In literature, it has recently been proposed that the diagnosis of “meth mouth” should include the devastating dental and oral effects of methamphetamine use (Figures 10 and 11). 136 , 137 , 138 , 139
FIGURE 10.

Clinical presentation of “meth mouth” in a MDMA user (right side). MDMA, 3,4‐Methylenedioxy‐Methamphetamine
FIGURE 11.

Clinical presentation of “meth mouth” in a MDMA user (left side). MDMA, 3,4‐Methylenedioxy‐Methamphetamine
The average duration of action of methamphetamines is 8‐12 hours. 94 However, it can be found in the saliva up to 24‐48 hours after use. 140 Therefore, methamphetamine patients on “high” episodes should not be subjected to any dental treatment for at least 8 hours or more after the last administration of drug. 95
6.4. Illegal synthetic amphetamine derivatives and periodontal disease
There is very limited scientific evidence on the correlation of amphetamines/methamphetamines use with periodontal conditions (Table 3). One retrospective study revealed that 94% of methamphetamine users had visible plaque on their teeth compared with 24% of nonusers; users were also more likely to have never brushed their teeth. 119 The authors compared the retrospective dietary patterns, oral hygiene behaviors, and current oral health status of methamphetamine users and nonusers. Eighteen adults with a history of the drug (methamphetamine) use and 18 age‐ and sex‐matched control subjects (nonusers) completed retrospective questionnaires concerning meal patterns, food group intakes, beverage habits, oral hygiene behaviors, smoking behaviors, and drug use. Oral examinations were performed to identify the number of remaining teeth, the number of teeth with obvious decay, and the presence of visible plaque. Marginal dietary and oral hygiene behaviors associated with methamphetamine use were likely to increase the caries risk. Methamphetamine users, specifically those who obtained the drug via injections, have a higher level of addiction than those who smoke or inhale methamphetamine, and thus were less likely to practice oral hygiene. 125
TABLE 3.
Association between amphetamine use and periodontal conditions
| Study | Design | Intervention comparison | Clinical parameters | Results | Conclusions | Notes |
|---|---|---|---|---|---|---|
| Hasan and Ciancio 2004 131 | Case‐control cross‐sectional study evaluated 20 subjects taking amphetamines (Adderall) to treat ADHD (test), and 20 healthy subjects not taking any medications (control). | The comparison was based on: age; clinical evaluation of gingival enlargement; MGI; PI according to Silness and Löe (1964) 143 ; comparison of intra‐oral photographs to visually evaluate gingival enlargement. |
PI, MGI according ‐ a periodontal probe was used to measure the gingival enlargement from the cementoenamel junction: 0 = no enlargement 1 = mild (1 mm or less); 2 = moderate (2‐3 mm); 3 = severe enlargement (> 3 mm). |
There was a statistically significant association between the amphetamine group and gingival enlargement. In the test group there was a significant correlation between medication dosage and the gingival index in patients who had gingival enlargement; moreover, gingival enlargement was statistically significantly associated with the gingival index. | Patients taking amphetamines have an increased risk of gingival enlargement. |
The study was conducted on children (age 6‐14 y) in treatment with amphetamines for ADHD. Validity of the data for adult amphetamines users should be assessed in depth. Moreover, a double‐blind approach should be considered for further studies. |
Abbreviations: ADHD, attention deficit hyperactivity disorder; MGI, modified gingival index; PI, plaque index.
Methamphetamine users can also exhibit significant inflammation and destruction of the soft and hard tissues of the mouth. 141 In periodontitis, monocyte/macrophages stimulated by bacterial lipopolysaccharide produce interleukin‐1β, resulting in bone and soft tissue degradation. Our knowledge on the effects of 3,4‐Methylenedioxy‐Methamphetamine on monocyte/macrophages and its role in periodontitis is limited. However, in an in vitro study, 3,4‐Methylenedioxy‐Methamphetamine cytotoxicity and its impact on lipopolysaccharide‐stimulated interleukin‐1β production in THP‐1 human monocytes were evaluated. 3,4‐Methylenedioxy‐Methamphetamine significantly reduced cell viability, assessed by the activity of a mitochondrial enzyme, by 20%‐40% after 24 hours, with recovery taking longer periods. Generally, 3,4‐Methylenedioxy‐Methamphetamine was found to increase the lipopolysaccharide‐stimulated interleukin‐1β levels. This study suggests that 3,4‐Methylenedioxy‐Methamphetamine potentiation of periodontopathogens' lipopolysaccharide stimulation of interleukin‐1β in monocytes could contribute to periodontitis in 3,4‐Methylenedioxy‐Methamphetamine users, consistent with other studies suggesting a role for increased interleukin‐1β in the deleterious effects of 3,4‐Methylenedioxy‐Methamphetamine. Therefore, methamphetamine might promote gingival inflammation and destruction via increased monocyte/macrophage production of interleukin‐1β in the presence of bacterial lipopolysaccharide in plaque. 142 Breivik et al 120 demonstrated, in an animal model, that the 3,4‐Methylenedioxy‐Methamphetamine treatment might increase the susceptibility to periodontal disease in terms of enhanced bone loss and periodontal fiber loss, because of disturbances in brain immune‐regulatory systems induced by the drug and alteration of the immune response.
Hasan and Ciancio 131 evaluated the relationship between gingival enlargement and amphetamine ingestion. Forty subjects were included and divided into two groups. The first group consisted of 20 subjects not taking medications, which could promote gingival enlargement (cyclosporine, sodium channel blockers), and taking amphetamines. Patients with cardiovascular or hormonal disorders were excluded from the study. Data about the time when the patient started taking the medication, how often the patient took the medication per day, and the medication's dosage were collected. Gingival and plaque indices were also measured to assess gingival health in accordance with Silness and Löe. 143 A second group of 20 healthy subjects not taking any medications was used as a control group. Gingival enlargement was evaluated clinically and on intra‐oral photographs. The results demonstrated a relationship between amphetamine usage and increased risk of gingival enlargement. A stringent effort to minimize gingival inflammation should be instituted in dental and periodontal practice, and patients should be monitored closely with more follow‐up appointments than nonmedicated patients.
Henkel et al 144 demonstrated the incorporation of illicit and medicinal drugs into nonmineralized dental biofilm (plaque) (amphetamine, 3,4‐Methylenedioxy‐Methamphetamine, cocaine, benzoylecgonine, morphine, and codeine) in a postmortem human model. Half of the drug findings in plaque were not detected in femoral blood. These results suggest that plaque offers a prolonged window of detection in comparison with blood and oral fluid, and is a medium for drug retention. 144 A case report regarding a young boy with a 2‐year history of substance use including cocaine, ecstasy, speed, heroin, alcohol, and tobacco reported necrotizing gingivitis, with erythema and ulceration of the gingiva adjacent to the upper labial vestibule, which was the site of drug (more frequently cocaine and amphetamines) applications. Even although necrosis induced by the ischemic vasoconstricting action of cocaine has already been demonstrated, 3,4‐Methylenedioxy‐Methamphetamine could also play a crucial role in the induction of periodontal injuries. 78 , 145
6.5. Oral mucosa
In an interview with 466 regular ecstasy users, 2.3% reported that they got oral ulcers 24 hours later and 8.2% 24‐48 hours later. These ulcers can manifest as mucosal fenestrations of the attached gingiva 146 ; one case report that describes a diagnosis termed “necrotising gingivitis related to the use of ecstasy” is available in the literature. 132 Ahmed et al 102 reported a case of rapidly developed (within 2 hours) and widespread edema (involving perioral, intra‐oral, and oropharyngeal spaces) after one ecstasy tablet ingestion. The extensive edema involved both the upper and lower labial mucosa, bilateral buccal mucosa, dorsum of the tongue, and the bilateral tonsillar regions. The appearance was grayish white without evidence of ulceration or exudation. The patient was treated with corticosteroids, antibiotics, and chlorhexidine mouthwash. After 10 days, the mucosal reactions were able to be completely resolved. 102 The literature also documents a case of extensive tissue loss (3 cm area) from lower lip caused by involuntary chewing after 3,4‐Methylenedioxy‐Methamphetamine consumption, 134 as well as ulcerations resulting from cheek biting. 147
6.6. Conclusions on the effect of Illegal synthetic amphetamine derivatives and periodontal complications
According to a recent study by Hegazi et al, 148 overall, 7.8% of US adults aged ≥ 30 years had used 3,4‐Methylenedioxy‐Methamphetamine. Although only very few studies discussed the periodontal manifestations of amphetamine derivatives, it can be concluded that long‐term exposure to 3,4‐Methylenedioxy‐Methamphetamine may increase one's susceptibility to periodontal disease by dysregulating the serotonergic and dopaminergic transport systems and thus alter the reactivity of brain‐controlled immunoregulatory systems. Indeed, compared with controls, 3,4‐Methylenedioxy‐Methamphetamine‐treated rats developed significantly more periodontitis 120 and patients taking amphetamines have an increased risk of gingival enlargement, gingivitis, 131 and unusual periodontal conditions. 132 Therefore, more follow‐up appointments and an effort to minimize gingival inflammation, by establishing an appropriate oral hygiene regimen, should be instituted in the treatment plan to minimize the effects of illegal synthetic amphetamine derivatives on the periodontium (Tables 3 and 4).
7. CLINICAL MANAGEMENT OF ILLICIT DRUG USERS
Historically, substance use disorders were treated almost exclusively from a tertiary care perspective, as manifestations present clinically, often among only the most acutely and chronically ill. Contemporarily, in response to the emergence of clinical research, an innovative focus on evidence‐based practices, and recommendations from professional organizations and governmental agencies, an increased emphasis has been placed on prevention, screening, and early interventions. With attention to this gradual clinical reorientation, primary care clinicians (including physicians, physician assistants, and nursing practitioners) are in a prime position to contribute and optimize the improved health of patients, families, and communities. 149
Dental practitioners are at the center of a very complex, demanding profession that requires, as a minimum, significant skills in dental and surgical procedures, knowledge of medical diagnosis, recognition of concurrent medical and psychiatric disorders, advanced communication and interview skills and advanced knowledge in pharmacology, pharmacotherapy, pain management, drug diversion, and substance use disorder. 150 Therefore, as part of a comprehensive dental treatment plan, both general and specialist dental practitioners must be able to identify risk factors/indicators and consult the patients' options of strategies to reduce or eliminate these risk factors. 13 It has been clearly demonstrated that the use and abuse of illicit drugs are definite medical and dental risk factors. A multidisciplinary approach would be the most appropriate management in caring for these patients and collaboration should be in alignment across disciplines to enhance ultimate outcomes.
7.1. Detection of illicit drug use and use
It has been suggested that Screening, Brief Intervention and Referral for Treatment should be integrated into dental practice. 151 , 152 Screening, Brief Intervention and Referral for Treatment is a comprehensive, integrated public health approach for the delivery of early intervention and treatment services to people with substance‐use disorders as well as those who are at risk of developing such disorders. Primary care centers, office‐based practices, and other community settings provide opportunities for early intervention of at‐risk substance users before more serious consequences occur. Screening, Brief Intervention and Referral for Treatment can be carried out through 152 :
Screening that quickly assesses the severity of substance use and identifies the appropriate level of treatment.
Brief intervention that focuses on increasing insight and awareness regarding substance use and motivation towards behavioral changes.
Referral for treatment that provides those identified as needing more extensive treatment access to specialty care.
7.2. Screening
Quick screening questions can be included in the medical history that is obtained from dental patients. Ideally, open‐ended questions that cannot be answered with a definite yes or no, are encouraged, especially at the initiation of the interview. The utilization of open‐ended questions prevents a patient from effectively shutting down the interviewing process. 153 The National Institute of Drug Use screening tool is another interactive web‐based tool that offers a single question to identify patients with recent substance use. 31 Alternatively, the CAGE (Cut; Annoyed; Guilty; Eye Opener)–AID questionnaire is a screening tool that can be used for addiction assessment. Patients are asked about their feelings and awareness of the need to cut down drug use, checking their sense of guilt and acknowledgment/needs for medication to help stop drug withdrawal. 154 A positive response to these quick questionnaires prompts a more detailed screening and interview such as a drug use screening test. When the dental practitioner suspects a patient uses illicit drugs, they should express their concerns and offer initial counseling and referral for treatment.
7.3. Brief intervention (interviewing and counseling)
The provider's ethical and moral obligations is to treat the dental needs of the patient; because of the illustrated dental impact from substance use, the clinician should also offer counseling to assist the patient, as well as provide referrals for a variety of services if requested. 153 It is important for the dental professionals to acknowledge (in a non‐judgmental manner) that a patient has disclosed sensitive information about their life regarding the use of an illegal substance. Patients should be made aware that all information provided and discussed will remain confidential and that any information will only be shared with the patient's informed consent. 15
Once the patient has disclosed the use of an illegal drug, different behavioral changes counseling techniques can be applied. Motivational interviewing is considered one of the best methods 155 ; it is defined as a client‐centered, “directive method for enhancing intrinsic motivation to change by exploring and resolving ambivalence”. It is short‐term counseling to help explore and resolve a patient's conflicts involving health decisions. This approach is respectful to the individual patient's preferences, needs, and values, and ensures that the patient's values guide all clinical decisions. 156
Motivational interviewing is based on an assumption that knowledge is insufficient to bring about behavioral changes. It is much more likely to happen when the need to change is connected to something the individual values. It consists of two phases. During phase one, intrinsic motivation for change is enhanced, whereas in phase two, commitment to change is strengthened. 156 The goal of motivational interviewing is to strengthen the importance of change from the patient's perspective, 157 using four basic principles to enhance motivation: (a) expression of empathy, (b) development of discrepancy, (c) rolling with resistance, and (d) the support of self‐efficacy. 156
The tone of the motivational interviewing encounter should be nonjudgmental, empathetic, and encouraging. As a counselor, the clinician must establish a nonconfrontational and supportive climate in which patients feel comfortable expressing both the positive and negative aspects of their current behavior. It can be helpful to ask the patient to help set the agenda for the encounter to ensure that they are active and willing participants in the process. This may include deciding what behavior(s) to talk about (including drug use and use) and what goals they have for the session (or the intervention in general) to be achieved. 155
7.4. Referral to treatment
Patients identified as needing more help than brief interventions can be referred for specialty treatment. In preparation for this eventuality, primary care clinicians are encouraged to identify, establish, and maintain collaborative relationships with clinicians and facilities that specialize in the treatment of substance‐use disorders. This approach may include consulting with colleagues or contacting and visiting local treatment centers. 149 A multidisciplinary approach aligns with the concept of individualized patient medicine and the collaboration should include colleagues working in rehabilitation facilities of substance use.
7.5. Dental and periodontal management of illicit drug users
Dentists should familiarize themselves with the signs and symptoms of illegal drug intoxication and develop an understanding of the potential effects of these drugs on the patient's overall health. 14 Common signs include a change in the individuals' physical appearance, behavior, personality, or attitude. Physical signs or symptoms of substance use include unusual laziness, changes in appetite, unusual body odors, needle marks, or deterioration in the individual's general appearance and cleanliness. 150
Usually, elective dental care can be carried out 24 hours after the use of a stimulant drug (cocaine, crack, amphetamine) or cannabis. 111 Dental management issues in addicts may include, but are not limited to (Table 4):
Acute anxiety, dysphonia, and paranoid thoughts.
Behavioral problems.
Poor compliance with treatment.
Immune response defects.
Liver damage.
Malnourishment.
Viral hepatitis, HIV, or other infections.
The periodontal management of the cocaine‐addicted patient has been comprehensively described by Yukna. 89 The main aspect that the author focused on was the anesthesiologic risk. Many cocaine users may premedicate themselves before dental appointments to help relieve anxiety associated with the anticipated dental therapy. Because the physiological effects of cocaine are sympathomimetic in nature, administration of peripheral vasoconstrictors (that increase blood pressure and heart rate, and/or increased rate but decreased depth of respiration), epinephrine‐containing local anesthetics or nitrous oxide analgesia may be contraindicated. Also, risk may arise from the use of retraction cords impregnated with epinephrine that may place an individual that has recently used cocaine at an increased cardiovascular risk during dental treatment. The potentiation of cardiovascular effects of epinephrine observed with cocaine is also valid with other stimulants (methampthetamines) and cannabis.
It can be concluded that most drug users show a higher tolerance to local anesthetics and conscious sedation may require greater quantities of anesthetic agent to achieve pain‐free dental treatment. 6 However, the vasoconstrictor in the local anesthetic could place the patient at an increased risk for myocardial infarction, hypertension, cardiac dysrhythmias, and cerebrovascular accidents. 95 , 158 , 159 , 160 Therefore, a local anesthetic without vasoconstrictor should be used in such patients if local anesthetic is indicated. 95 , 121 , 159 , 160 Furthermore, caution should be alerted for the administration of nitrous oxide 136 and a consultation with the patient's physician preceding analgesics prescription is recommended.
If it is confirmed the patient is indeed an ecstasy user, the dental team should educate the patient (and family members in case of minors) about the effects of methamphetamine use on overall and oral health. Because meth mouth is a condition with devastating effects on oral conditions, thoughtful considerations about the patient's dental management are necessary. The key to successful dental treatment starts with the cessation of ecstasy. 109 Dentists should first comprehensively evaluate to what extent the patient can actively participate in the dental treatment. In general, the treatment plans made specifically for addicted patients should be less elaborated than those for nonaddicted patients. 161 All dental treatments should be postponed to after 24 hours of the last drug usage. 60 Informed consent about the implication of methamphetamine use in dental restorations should be obtained, in both verbal and written matters. 162 Dry mouth and its consequences can be prevented and treated with the use of topical fluorides, remineralization products, and chlorhexidine applications. The use of salivary stimulants should also be considered rather than saliva substitutes. Patients should be advised to consume an adequate amount of water or artificially sugared beverages instead of sugar‐containing beverages or soft drinks (sweet and carbonated sodas). Sugar‐free gums can also be used to promote salivation. 163
Intravenous sedation with a benzodiazepine or general sedation should be avoided. Pain control of patients “high” on ecstasy can be accomplished with adequate postoperative doses of acetaminophen or ibuprofen, because nonsteroidal anti‐inflammatory medications are not contraindicated. 95 , 164
In the work carried out by Gantos et al, 109 some additional treatment considerations were proposed, notably the multidisciplinary approach. Destructive oral and psychological changes must be identified and controlled. A thorough risk assessment, caries control, and preventative plan should be established before initiating prosthodontic treatment. Patient motivation, support, and a timely recall schedule are integral for dental health longevity. When the patient is emotionally stable and motivated to proceed with dental treatment, the dental provider should complete a risk assessment, develop a prevention plan, and arrest the active diseases. Scheduling dental appointments for these patients should accommodate their special characteristics. Because of their heightened anxiety, there is often a need for shorter appointments with multiple breaks during the dental appointments. Providing several reminders to ensure attendance, being lenient in rescheduling, and requesting family members to accompany could enhance attendance and provide some stress relief for the patient. A regular reevaluation of the disease control in combination with patient compliance should be the key to developing the final restorative treatment plan. Caries management is a fundamental aspect of disease control, risk assessment, and prognosis of the dental treatment. Diet counseling would include avoiding carbohydrate‐rich foods and drinks, minimizing snacking between meals, and incorporating xylitol use. In patients with several teeth extracted resulting in different degrees of edentulism, fixed and/or removable prostheses should be considered to improve esthetics and function. Fixed dental prostheses present higher patient satisfaction but are more expensive and require maintenance that is more demanding. A full or partial removable dental prosthesis may be a valid treatment option because it provides both esthetics and function without a challenging management. Dental implant is a helpful option, but it must be emphasized that a careful individual evaluation precedes treatment. 165
Pain, one of the most common reasons patients seek medical care, is often undertreated. As for pain management in active or recovering illicit drug users, it is challenging for several reasons. Some of the challenges faced by practitioners include distinguishing between those seeking pain relief and seeking drugs for the euphoric effects; and identifying predictable neuroadaptations such as tolerance and physiologic dependence that can be misinterpreted as drug seeking or relapse behavior. 166 Moreover, comorbid psychiatric and medical illnesses can mask the effects of analgesics and complicate effective pain management. 167 When individuals with a substance use disorder of some type experience pain, they are less likely to receive adequate pain management than individuals in the general population. 168 Nevertheless, pain control can be achieved if practitioners follow basic principles such as those put forward by the World Health Organization, a stepladder approach to pain management. Acute pain, the most common presentation in dental settings, is treated in a similar fashion for all patients regardless of addiction history and non‐steroideal anti‐inflammatory drugs are usually the preferred choice for pain control. 169 However, follow‐up is important to prevent relapse and it is prudent for dental practitioners to consult with the patient's physician prior to prescribing any analgesics and caution must be exercised when administering nitrous oxide. 170 The ultimate goal of chronic pain management (consider temporomandibular disorders, orofacial pain) in addicted patients is the same as individuals without addictive disorders: to maximize functionality while providing pain relief. 169 The restriction to only one practitioner of the entire team of healthcare providers in providing all pain medication prescriptions for an individual patient is important, to minimize use potential. Prescribing around the clock, providing the minimum effective dose of opioids, being aware of tolerance potential, weaning periodically to reassess pain control, and using nonpsychotropic pain medications when possible, are strategies to manage substance use patients. Practitioners should be reminded that while relapse in a recovering individual may occur despite appropriate use of opioids and psychotropic medications required for effective pain management, inadequate pain relief is also a significant risk factor for relapse. 171 In addition, the risk of relapse is related to the quality of the patient's individualized substance use recovery and support system. 172 During the period of pain management, active involvement in a recovery support program should be initiated or intensified. In all circumstances, if methadone has been prescribed, it should be maintained and not ceased, and taken into consideration during treatment.
8. CONCLUSIONS
The dental treatment of subjects who use illegal substances is becoming more common in the daily clinical practice of periodontists and other dental clinicians. It is essential to manage their addiction properly in the comprehensive treatment when we encounter such patients.
Regarding the impact of illegal substances use on periodontal conditions, there is moderate evidence that regular long‐term use of cannabis is a risk factor for periodontal disease, manifesting as loss of periodontal attachment, deep pockets, gingival recessions, and gingival enlargements. Limited evidence also shows that the use of cocaine can cause a series of gingival conditions that mostly presents as chemical induced‐traumatic lesions (application of cocaine on the gingiva) or necrotizing ulcerative lesions. There is a scarcity of data regarding the impact of other drug use on periodontal health. Further studies and clinical observations are required to obtain sound and definitive information.
The overall dental management for substance use patients may be complex, and a systematic approach in health care entailing screening, interviews, counseling, and potential referral can prevent the practitioners' accidental negligence on important information in relevance to providing care for substance users. Such information is considered sensitive and difficult to be retrieved, hence caution in enquiring must be stressed. Attention to fine details would prevent malpractice, especially when medications are to be prescribed. The potential risks and complications related to the interaction between local esthetics and other dental products with illegal drugs have been discussed and precautions in administration cannot be further emphasized. When necessary, dental treatment must be postponed for the safety of both patients and clinicians. Careful assessment and multidisciplinary management of illicit drug users can prevent unnecessary tragedy and optimize treatment outcomes. Restoring patients' oral and general health plus their recovery in psychosocial well‐being should be the ultimate goals in treating this special group of patients.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest related to this manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Professor Jerry Boquot, Western Virginia, USA, for the provision of figures 4–7 and 10 and 11, which have been made available for use through the copyright‐free database, “BouquotToGofiles”. The authors would also like to acknowledge and thank Professor Manlio Quaranta, Rome, Italy, for his enduring support during these years and for being an extraordinary academic guide, mentor, and role model. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Quaranta A, D’Isidoro O, Piattelli A, Hui WL, Perrotti V. Illegal drugs and periodontal conditions. Periodontol 2000. 2022;90:62‐87. doi: 10.1111/prd.12450
Name of the Guest Editor: Professor Ivan Darby, University of Melbourne, Australia
REFERENCES
- 1. United Nations office on drugs and crime world drug report 2016 . www.unodc.org/wdr2016. Accessed May 26, 2022.
- 2. Sanz M, van Winkelhoff AJ, Working Group 1 of Seventh European Workshop on Periodontology . Periodontal infections: understanding the complexity–consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38:3‐6. [DOI] [PubMed] [Google Scholar]
- 3. Nunn ME. Understanding the etiology of periodontitis: an overview of periodontal risk factors. Periodontol. 2000;2003(32):11‐23. [DOI] [PubMed] [Google Scholar]
- 4. Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041‐1049. [DOI] [PubMed] [Google Scholar]
- 5. Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol. 2013;62(1):59‐94. [DOI] [PubMed] [Google Scholar]
- 6. Rees TD. Oral effects of drug use. Crit Rev Oral Biol Med. 1992;3(3):163‐184. [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Association , ed. Diagnostic and statistics manual of mental disorders. DSM‐5®. 5th ed. American Psychiatric Association Publishing; 2013. [Google Scholar]
- 8. American Society of Addiction Medicine . Public policy statement: definition of addiction short definition of addiction. https://www.asam.org/resources/definition‐of‐addiction. Accessed October 7, 2017.
- 9. Friedlander AH, Mills MJ. The dental management of the drug‐dependent patient. Oral Surg Oral Med Oral Pathol. 1985;60(5):489‐492. [DOI] [PubMed] [Google Scholar]
- 10. National Institute on Drug Abuse . The neurobiology of drug addiction. www.drugabuse.gov/publications/teaching‐packets/neurobiology‐drug‐addiction. Accessed May, 26 2022.
- 11. NCSL . State medical marijuana laws – national conference of state legislatures. 2014. www.ncsl.org/research/health/state‐medical‐marijuana‐laws.aspx. Accessed May 26, 2022.
- 12. Shipton EA, Shipton EE. Should doctors be allowed to prescribe cannabinoids for pain in Australia and New Zealand? Aust N Z J Psychiatry. 2014;48(4):310‐313. [DOI] [PubMed] [Google Scholar]
- 13. Maloney WJ. Significance of cannabis use to dental practice. NY State Dent J. 2011;77(3):36‐39. [PubMed] [Google Scholar]
- 14. Grafton SE, Huang PN, Vieira AR. Dental treatment planning considerations for patients using cannabis: a case report. J Am Dent Assoc. 2016;147(5):354‐361. [DOI] [PubMed] [Google Scholar]
- 15. Joshi S, Ashley M. Cannabis: a joint problem for patients and the dental profession. Br Dent J. 2016;220(11):597‐601. [DOI] [PubMed] [Google Scholar]
- 16. Ashton CH, Moore PB, Gallagher P, Young AH. Cannabinoids in bipolar affective disorder: a review and discussion of their therapeutic potential. J Psychopharmacol. 2005;19(3):293‐300. [DOI] [PubMed] [Google Scholar]
- 17. Iversen L. Cannabis and the brain. Brain. 2003;126(Pt 6):1252‐1270. [DOI] [PubMed] [Google Scholar]
- 18. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50(2):70‐74. [DOI] [PubMed] [Google Scholar]
- 19. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327‐360. [DOI] [PubMed] [Google Scholar]
- 20. Aryana A, Williams MA. Marijuana as a trigger of cardiovascular events: speculation or scientific certainty? Int J Cardiol. 2007;118(2):141‐144. [DOI] [PubMed] [Google Scholar]
- 21. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42(11 Suppl):58S‐63S. [DOI] [PubMed] [Google Scholar]
- 22. Clayton HB, Lowry R, Ashley C, Wolkin A, Grant AM. Health risk behaviors with synthetic cannabinoids versus marijuana. Pediatrics. 2017;139(4):e20162675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berry JH, Sullivan CR. Understanding the diseases of substance use disorders. In: O'Neil M, ed. The ADA practical guide to substance use disorders and safe prescribing. Wiley Blacwell; 2015:11‐30. [Google Scholar]
- 24. Hollister LE. Drug‐induced psychiatric disorders and their management. Med Toxicol. 1986;1(6):428‐448. [DOI] [PubMed] [Google Scholar]
- 25. Biehl JR, Burnham EL. Cannabis smoking in 2015: a concern for lung health? Chest. 2015;148(3):596‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tashkin DP, Baldwin GC, Sarafian T, Dubinett S, Roth MD. Respiratory and immunologic consequences of marijuana smoking. J Clin Pharmacol. 2002;42(11 Suppl):71S‐81S. [DOI] [PubMed] [Google Scholar]
- 27. Tennant FS, Prendergast TJ. Medical manifestations associated with hashish. JAMA. 1971;216(12):1965‐1969. [PubMed] [Google Scholar]
- 28. Donald PJ. Marijuana smoking‐possible cause of head and neck carcinoma in young patients. Otolaryngol Head Neck Surg. 1986;94(4):517‐521. [DOI] [PubMed] [Google Scholar]
- 29. Scully C. Scully's handbook of medical problems in dentistry. Elsevier Health Sciences; 2016. [Google Scholar]
- 30. Schrot RJ, Hubbard JR. Cannabinoids: medical implications. Ann Med. 2016;48(3):128‐141. [DOI] [PubMed] [Google Scholar]
- 31. NIDA research on the therapeutic benefits of cannabis and cannabinoids . U.S. National Institute on Drug Abuse Website, 2015. www.drugabuse.gov/drugs‐abuse/marijuana/nida‐research‐therapeutic‐benefits‐cannabis‐cannabinoids. Accessed November 5, 2017.
- 32. Darling MR, Arendorf TM. Review of the effects of cannabis smoking on oral health. Int Dent J. 1992;42(1):19‐22. [PubMed] [Google Scholar]
- 33. Yazdanian M, Armoon B, Noroozi A, et al. Dental caries and periodontal disease among people who use drugs: a systematic review and meta‐analysis. BMC Oral Health. 2020;20(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bellocchio L, Inchingolo AD, Inchingolo AM, et al. Cannabinoids drugs and oral health‐from recreational side‐effects to medicinal purposes: a systematic review. Int J Mol Sci. 2021;22(15):8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baddour HM, Audemorte TB, Layman FD. The occurrence of diffuse gingival hyperplasia in a patient using marijuana. J Tenn Dent Assoc. 1984;64(2):39‐43. [PubMed] [Google Scholar]
- 36. Silverstein SJ, Noel D, Heilbron D. Social drug use/abuse and dental disease. J Calif Dent Assoc. 1978;6(2):32‐37. [PubMed] [Google Scholar]
- 37. Firth NA. Marijuana use and oral cancer: a review. Oral Oncol. 1997;33(6):398‐401. [DOI] [PubMed] [Google Scholar]
- 38. Hashibe M, Ford DE, Zhang ZF. Marijuana smoking and head and neck cancer. J Clin Pharmacol. 2002;42(11 Suppl):103S‐107S. [DOI] [PubMed] [Google Scholar]
- 39. Mallat A, Roberson J, Brock‐Utne JG. Preoperative marijuana inhalation‐an airway concern. Can J Anaesth. 1996;43(7):691‐693. [DOI] [PubMed] [Google Scholar]
- 40. Rawal SY, Tatakis DN, Tipton DA. Periodontal and oral manifestations of marijuana use. J Tenn Dent Assoc. 2012;92(2):26‐31. [PubMed] [Google Scholar]
- 41. Versteeg PA, Slot DE, van der Velden U, van der Weijden GA. Effect of cannabis usage on the oral environment: a review. Int J Dent Hyg. 2008;6(4):315‐320. [DOI] [PubMed] [Google Scholar]
- 42. Mayol M, Andrade E, Rivoir SP, Rossy LAB, Rösing CK. Periodontal status in cannabis smokers. A systematic review. J Int Acad Periodontol. 2021;23(2):150‐166. [PubMed] [Google Scholar]
- 43. Schulz‐Katterbach MS, Imfeld T, Imfeld C. Cannabis and caries–does regular cannabis use increase the risk of caries in cigarette smokers? Schweiz Monatsschr Zahnmed. 2009;119(6):576‐583. [PubMed] [Google Scholar]
- 44. Lopez R, Baelum V. Cannabis use and destructive periodontal diseases among adolescents. J Clin Periodontol. 2009;36(3):185‐189. [DOI] [PubMed] [Google Scholar]
- 45. Melamede R. Cannabis and tobacco smoke are not equally carcinogenic. Harm Reduct J. 2005;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Melamede R. Harm reduction‐the cannabis paradox. Harm Reduct J. 2005;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnson GK, Slach NA. Impact of tobacco use on periodontal status. J Dent Educ. 2001;65(4):313‐321. [PubMed] [Google Scholar]
- 48. Baumeister SE, Alayash Z, Baurecht H, et al. Cannabis use and the risk of periodontitis: a two‐sample Mendelian randomization study. J Clin Periodontol. 2022. (in press);49:654‐661. [DOI] [PubMed] [Google Scholar]
- 49. Jamieson LM, Gunthorpe W, Cairney SJ, Sayers SM, Roberts‐Thomson KF, Slade GD. Substance use and periodontal disease among Australian Aboriginal young adults. Addiction. 2010;105(4):719‐726. [DOI] [PubMed] [Google Scholar]
- 50. Meier MH, Caspi A, Cerdá M, et al. Associations between cannabis use and physical health problems in early midlife: a longitudinal comparison of persistent cannabis vs tobacco users. JAMA Psychiat. 2016;73(7):731‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shariff JA, Ahluwalia KP, Papapanou PN. Relationship between frequent recreational cannabis (marijuana and hashish) use and periodontitis in adults in the United States: national health and nutrition examination survey 2011 to 2012. J Periodontol. 2017;88(3):273‐280. [DOI] [PubMed] [Google Scholar]
- 52. Thomson WM, Poulton R, Broadbent JM, et al. Cannabis smoking and periodontal disease among young adults. JAMA. 2008;299(5):525‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeng J, Williams SM, Fletcher DJ, et al. Reexamining the association between smoking and periodontitis in the Dunedin study with an enhanced analytical approach. J Periodontol. 2014;85(10):1390‐1397. [DOI] [PubMed] [Google Scholar]
- 54. Centers for Disease Control and Prevention (CDC) . National health and nutrition examination survey. http://www.cdc.gov/nchs/nhanes/participant.htm. Accessed May 26, 2022.
- 55. Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7‐13. [DOI] [PubMed] [Google Scholar]
- 56. López R, Fernández O, Baelum V. Social gradients in periodontal diseases among adolescents. Community Dent Oral Epidemiol. 2006;34(3):184‐196. [DOI] [PubMed] [Google Scholar]
- 57. López R, Baelum V. Oral health impact of periodontal diseases in adolescents. J Dent Res. 2007;86(11):1105‐1110. [DOI] [PubMed] [Google Scholar]
- 58. Momen‐Heravi F, Kang P. Management of cannabis‐induced periodontitis via resective surgical therapy: a clinical report. J Am Dent Assoc. 2017;148(3):179‐184. [DOI] [PubMed] [Google Scholar]
- 59. Angelopoulos AP. A clinicopathological review. Diphenylhydantoin gingival hyperplasia: 2. Aetiology, pathogenesis, differential diagnosis and treatment. Dent J 1975:41(5):275–7, 83. [PubMed] [Google Scholar]
- 60. Seymour RA, Heasman PA. Drugs and the periodontium. J Clin Periodontol. 1988;15(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 61. Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42(11):11S‐19S. [DOI] [PubMed] [Google Scholar]
- 62. Arya R, Gulati S. Phenytoin‐induced gingival overgrowth. Acta Neurol Scand. 2012;125(3):149‐155. [DOI] [PubMed] [Google Scholar]
- 63. Fratto G, Manzon L. Use of psychotropic drugs and associated dental diseases. Int J Psychiatry Med. 2014;48(3):185‐197. [DOI] [PubMed] [Google Scholar]
- 64. Woyceichoski IE, de Arruda EP, Resende LG, et al. Cytomorphometric analysis of crack cocaïne effects on the oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(6):745‐749. [DOI] [PubMed] [Google Scholar]
- 65. National Institute on Drug Abuse . www.drugabuse.gov/sites/default/files/cocaine10.pdf. Accessed October, 28, 2017.
- 66. Blanksma CJ, Brand HS. Cocaine abuse: orofacial manifestations and implications for dental treatment. Int Dent J. 2005;55(6):365‐369. [DOI] [PubMed] [Google Scholar]
- 67. Lee CY, Mohammedi H, Dixons RA. Medical and dental implications of cocaine abuse. J Oral Maxillofac Surg. 1991;49(3):290‐293. [DOI] [PubMed] [Google Scholar]
- 68. Jatlow P. Cocaethylene: pharmacologic activity and clinical significance. Ther Drug Monit. 1993;15(6):533‐536. [PubMed] [Google Scholar]
- 69. Knuepfer MM. Cardiovascular disorders associated with cocaine use: myths and truths. Pharmacol Ther. 2003;97(3):181‐222. [DOI] [PubMed] [Google Scholar]
- 70. Shanti CM, Lucas CE. Cocaine and the critical care challenge. Crit Care Med. 2003;31(6):1851‐1859. [DOI] [PubMed] [Google Scholar]
- 71. Friedlander AH, Gorelick DA. Dental management of the cocaine addict. Oral Surg Oral Med Oral Pathol. 1988;65(1):45‐48. [DOI] [PubMed] [Google Scholar]
- 72. Gómez FM, Areso MP, Giralt MT, Sainz B, García‐Vallejo P. Effects of dopaminergic drugs, occlusal disharmonies, and chronic stress on non‐functional masticatory activity in the rat, assessed by incisal attrition. J Dent Res. 1998;77(6):1454‐1464. [DOI] [PubMed] [Google Scholar]
- 73. Gaio DC, Bastos FI, Moysés SJ, et al. Assessing oral health of crack users in Brazil: perceptions and associated factors, findings from a mixed methods study. Glob Public Health. 2021;16(4):502‐516. [DOI] [PubMed] [Google Scholar]
- 74. Mattson‐Gates G, Jabs AD, Hugo NE. Perforation of the hard palate associated with cocaine abuse. Ann Plast Surg. 1991;26(5):466‐468. [DOI] [PubMed] [Google Scholar]
- 75. Tsoukalas N, Johnson CD, Engelmeier RL, Delattre VF. The dental management of a patient with a cocaine‐induced maxillofacial defect: a case report. Spec Care Dentist. 2000;20(4):139‐142. [DOI] [PubMed] [Google Scholar]
- 76. Brand HS, Gonggrijp S, Blanksma CJ. Cocaine and oral health. Br Dent J. 2008;204(7):365‐369. [DOI] [PubMed] [Google Scholar]
- 77. Penarrocha M, Bagan JV, Penarrocha MJ, Silvestre FJ. Cluster headache and cocaine use. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(3):271‐274. [DOI] [PubMed] [Google Scholar]
- 78. Parry J, Porter S, Scully C, Flint S. Mucosal lesions due to oral cocaine use. Br Dent J. 1996;180(12):462‐464. [DOI] [PubMed] [Google Scholar]
- 79. Krutchkoff DJ, Eisenberg E, O'Brien JE, Ponzillo JJ. Cocaine‐induced dental erosions. N Engl J Med. 1990;322(6):408. [DOI] [PubMed] [Google Scholar]
- 80. Araujo NS, das Graças Alonso Oliveira M, AVB N, de Oliveira Lima Arsati YB, Dos Santos JN, Cury PR. Salivary flow rates and buffer capacity and its relationship with oral health status: a cross‐sectional study on crack‐cocaine‐addicted males. Environ Sci Pollut Res Int. 2020;27(33):41876‐41884. [DOI] [PubMed] [Google Scholar]
- 81. Brown RS, Johnson CD. Corrosion of dental gold restorations from inhalation of ‘crack’ cocaine. Gen Dent. 1994;42(3):242‐246. [PubMed] [Google Scholar]
- 82. Shibli JA, Marcantonio E, Spolidorio LC, Marcantorio E Jr. Cocaine associated with onlay bone graft failure: a clinical and histologic report. Implant Dent. 2005;14(3):248‐251. [DOI] [PubMed] [Google Scholar]
- 83. Vissink A, van Nieuw Amerongen A, Oremus ET. The effect of drugs on the orofacial area. Ned Tijdschr Tandheelkd. 1999;106(7):254‐263. [PubMed] [Google Scholar]
- 84. de M Thiele MC, Bohn JC, Chaiben CL, Grégio AM, Machado MÂ, de Lima AA. Nucleolar organizer regions of oral epithelial cells in crack cocaine users. Iran Biomed J. 2013;17(2):107‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Webber LP, Pellicioli AC, Magnusson AS, et al. Nuclear changes in oral mucosa of alcoholics and crack cocaïne users. Hum Exp Toxicol. 2016;35(2):184‐193. [DOI] [PubMed] [Google Scholar]
- 86. Antoniazzi RP, Zanatta FB, Rösing CK, Feldens CA. Association among periodontitis and the use of crack cocaine and other illicit drugs. J Periodontol. 2016;87(12):1396‐1405. [DOI] [PubMed] [Google Scholar]
- 87. Casarin M, Antoniazzi RP, Vaucher RA, Feldens CA, Zanatta FB. RT‐PCR quantification of periodontal pathogens in crack users and non‐users. Oral Dis. 2017;23(3):324‐330. [DOI] [PubMed] [Google Scholar]
- 88. Cury PR, Oliveira MG, Dos Santos JN. Periodontal status in crack and cocaine addicted men: a cross‐sectional study. Environ Sci Pollut Res Int. 2017;24(4):3423‐3429. [DOI] [PubMed] [Google Scholar]
- 89. Yukna RA. Cocaine periodontitis. Int J Periodontics Restorative Dent. 1991;11(1):72‐79. [PubMed] [Google Scholar]
- 90. Antoniazzi RP, Palmeira RV, Schöffer C, Dos Santos BZ, Zanatta FB, Feldens CA. Use of crack cocaine increases tooth loss. Am J Dent. 2021;34(6):317‐321. [PubMed] [Google Scholar]
- 91. Quart AM, Small CB, Klein RS. The cocaine connection. Users imperil their gingiva. J Am Dent Assoc. 1991;122(1):85‐87. [PubMed] [Google Scholar]
- 92. Sastry RC, Lee D, Har‐El G. Palate perforation. Otolaryngol Head Neck Surg. 1997;116(4):565‐566. [DOI] [PubMed] [Google Scholar]
- 93. Drugs.com . www.drugs.com/amphetamine.html. Accessed May 26, 2022.
- 94. U.S. Department of Health and Human Services, National Institute on Drug Abuse . Methamphetamine. 2007. NIDA infofacts, www.nida.nih.gov/infofacts/methamphetamine.html. Accessed May 2022
- 95. Hamamoto DT, Rhodus NL. Methamphetamine abuse and dentistry. Oral Dis. 2009;15(1):27‐37. [DOI] [PubMed] [Google Scholar]
- 96. Brand HS, Dun SN, Nieuw Amerongen AV. Ecstasy (MDMA) and oral health. Br Dent J. 2008;204(2):77‐81. [DOI] [PubMed] [Google Scholar]
- 97. Klasser GD, Epstein J. Methamphetamine and its impact on dental care. J Can Dent Assoc. 2005;71(10):759‐762. [PubMed] [Google Scholar]
- 98. Cole JC, Michailidou K, Jerome L, Sumnall HR. The effects of stereotype threat on cognitive function in ecstasy users. J Psychopharmacol. 2006;20(4):518‐525. [DOI] [PubMed] [Google Scholar]
- 99. de la Torre R, Farré M, Navarro M, Pacifici R, Zuccaro P, Pichini S. Clinical pharmacokinetics of amfetamine and related substances, monitoring in conventional and non conventional matrices. Clin Pharmacokinet. 2004;43(3):157‐185. [DOI] [PubMed] [Google Scholar]
- 100. Navarro M, Pichini S, Farré M, et al. Usefulness of saliva for measurement of 3,4‐methylenedioxymethamphetamine and its metabolites: correlation with plasma drug concentrations and effect of salivary pH. Clin Chem. 2001;47(10):1788‐1795. [PubMed] [Google Scholar]
- 101. Hall AP, Henry JA. Acute toxic effects of 'Ecstasy' (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96(6):678‐685. [DOI] [PubMed] [Google Scholar]
- 102. Ahmed M, Islam S, Hoffman GR. Widespread oral and oropharyngeal mucosal oedema induced by ecstasy (MDMA): a case for concern. Br J Oral Maxillofac Surg. 2007;45(6):496‐498. [DOI] [PubMed] [Google Scholar]
- 103. Lineberry TW, Bostwick JM. Methamphetamine abuse: a perfect storm of complications. Mayo Clin Proc. 2006;81(1):77‐84. [DOI] [PubMed] [Google Scholar]
- 104. National Institute on Drug Abuse . Research report series: methamphetamine abuse and addiction. www.nida.nih.gov/PDF/RRMetham.pdf. Accessed May 26, 2022.
- 105. Solowij N, Hall W, Lee N. Recreational MDMA use in Sydney: a profile of ‘ecstacy’ users and their experiences with the drug. Br J Addict. 1992;87(8):1161‐1172. [DOI] [PubMed] [Google Scholar]
- 106. Vollenweider FX, Gamma A, Liechti M, Huber T. Psychological and cardiovascular effects and short‐term sequelae of MDMA (‘ecstasy’) in MDMA‐naive healthy volunteers. Neuropsychopharmacology. 1998;19(4):241‐250. [DOI] [PubMed] [Google Scholar]
- 107. Cole JC, Sumnall HR. Altered states: the clinical effects of Ecstasy. Pharmacol Ther. 2003;98(1):35‐38. [DOI] [PubMed] [Google Scholar]
- 108. McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352(9138):1433‐1437. [DOI] [PubMed] [Google Scholar]
- 109. Gantos MA, Manzotti A, Yuan JC, et al. Prosthodontics treatment considerations for methamphetamine‐dependent patients. J Prosthodont. 2015;24(1):64‐70. [DOI] [PubMed] [Google Scholar]
- 110. Panenka WJ, Procyshyn RM, Lecomte T, et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129(3):167‐179. [DOI] [PubMed] [Google Scholar]
- 111. Screaton GR, Singer M, Cairns HS, Thrasher A, Sarner M, Cohen SL. Hyperpyrexia and rabdomyolysis after MDMA (‘ecstasy’) abuse. Lancet. 1992;339(8794):677‐678. [DOI] [PubMed] [Google Scholar]
- 112. Sharma A, Singh S, Mathur A, et al. Route of drug abuse and its impact on oral health‐related quality of life among drug addicts. Addict Health. 2018;10(3):148‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine‐induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81(1):198‐204. [DOI] [PubMed] [Google Scholar]
- 114. Morgan MJ. Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology. 2000;152(3):230‐248. [DOI] [PubMed] [Google Scholar]
- 115. Parrott AC. Recreational Ecstasy/MDMA, the serotonin syndrome, and serotonergic neurotoxicity. Pharmacol Biochem Behav. 2002;71(4):837‐844. [DOI] [PubMed] [Google Scholar]
- 116. Parrott AC, Rodgers J, Buchanan T, Ling J, Heffernan T, Scholey AB. Dancing hot on Ecstasy: physical activity and thermal comfort ratings are associated with the memory and other psychobiological problems reported by recreational MDMA users. Hum Psychopharmacol. 2006;21(5):285‐298. [DOI] [PubMed] [Google Scholar]
- 117. Marshall BD, Werb D. Health outcomes associated with methamphetamine use among young people: a systematic review. Addiction. 2010;105(6):991‐1002. [DOI] [PubMed] [Google Scholar]
- 118. Cretzmeyer M, Walker J, Hall JA, Arndt S. Methamphetamine use and dental disease: results of a pilot study. J Dent Child (Chic). 2007;74(2):85‐92. [PubMed] [Google Scholar]
- 119. Morio KA, Marshall TA, Qian F, Morgan TA. Comparing diet, oral hygiene and caries status of adult methamphetamine users and nonusers: a pilot study. J Am Dent Assoc. 2008;139(2):171‐176. [DOI] [PubMed] [Google Scholar]
- 120. Breivik T, Bogen IL, Haug KH, et al. Effects of long‐term exposure of 3,4‐methylenedioxymethamphetamine (MDMA; "ecstasy") on neuronal transmitter transport, brain immuno‐regulatory systems and progression of experimental periodontitis in rats. Neurochem Int. 2014;72:30‐36. [DOI] [PubMed] [Google Scholar]
- 121. Donaldson M, Goodchild JH. Oral health of the methamphetamine abuser. Am J Health Syst Pharm. 2006;63(21):2078‐2082. [DOI] [PubMed] [Google Scholar]
- 122. Colado MI, Williams JL, Green AR. The hyperthermic and neurotoxic effects of 'Ecstasy' (MDMA) and 3,4 methylenedioxyamphetamine (MDA) in the Dark Agouti (DA) rat, a model of the CYP2D6 poor metabolizer phenotype. Br J Pharmacol. 1995;115(7):1281‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kalant H. The pharmacology and toxicology of "ecstasy" (MDMA) and related drugs. CMAJ. 2001;165(7):917‐928. [PMC free article] [PubMed] [Google Scholar]
- 124. Zervogiannis FH, Wiechers E, Bester G. The ‘E' in rave: a profile of young ecstasy (MDMA) users. S Afr J Psychol. 2003;33(3):162‐169. [Google Scholar]
- 125. Shetty V, Mooney LJ, Zigler CM, Belin TR, Murphy D, Rawson R. The relationship between methamphetamine use and increased dental disease. J Am Dent Assoc. 2010;141(3):307‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dinis‐Oliveira RJ, Caldas I, Carvalho F, Magalhães T. Bruxism after 3,4‐methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila). 2010;48(8):863‐864. [DOI] [PubMed] [Google Scholar]
- 127. Saini T, Edwards PC, Kimmes NS, Carroll LR, Shaner JW, Dowd FJ. Etiology of xerostomia and dental caries among methamphetamine abusers. Oral Health Prev Dent. 2005;3(3):189‐195. [PubMed] [Google Scholar]
- 128. Nixon PJ, Youngson CC, Beese A. Tooth surface loss: does recreational drug use contribute? Clin Oral Investig. 2002;6(2):128‐130. [DOI] [PubMed] [Google Scholar]
- 129. Shaner JW, Kimmes N, Saini T, Edwards P. “Meth mouth”: rampant caries in methamphetamine abusers. AIDS Patient Care STDs. 2006;20(3):146‐150. [DOI] [PubMed] [Google Scholar]
- 130. Ye T, Sun D, Dong G, et al. The effect of methamphetamine abuse on dental caries and periodontal diseases in an Eastern China city. BMC Oral Health. 2018;18(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hasan AA, Ciancio S. Relationship between amphetamine ingestion and gingival enlargement. Pediatr Dent. 2004;26(5):396‐400. [PubMed] [Google Scholar]
- 132. Brazier WJ, Dhariwal DK, Patton DW, Bishop K. Ecstasy related periodontitis and mucosal ulceration – a case report. Br Dent J. 2003;194(4):197‐199. [DOI] [PubMed] [Google Scholar]
- 133. McGrath C, Chan B. Oral health sensations associated with illicit drug abuse. Br Dent J. 2005;198(3):159‐162. [DOI] [PubMed] [Google Scholar]
- 134. Nugent G, Basyuni S, McAnerney D, Cameron M. Oral surgery: mutilation following MDMA. Br Dent J. 2017;222(2):68. [DOI] [PubMed] [Google Scholar]
- 135. Maloney WJ, Raymond G. The significance of ecstasy use to dental practice. NY State Dent J. 2014;80(6):24‐27. [PubMed] [Google Scholar]
- 136. American Dental Association . Methamphetamine use (Meth Mouth) 2009. https://www.mouthhealthy.org/en/az‐topics/m/meth‐mouth. Accessed May 26, 2022.
- 137. Ravenel MC, Salinas CF, Marlow NM, Slate EH, Evans ZP, Miller PM. Methamphetamine abuse and oral health: a pilot study of “meth mouth”. Quintessence Int. 2012;43(3):229‐237. [PubMed] [Google Scholar]
- 138. Shaner JW. Caries associated with methamphetamine abuse. J Mich Dent Assoc. 2002;84(9):42‐47. [PubMed] [Google Scholar]
- 139. Goodchild JH, Donaldson M. Methamphetamine abuse and dentistry: a review of the literature and presentation of a clinical case. Quintessence Int. 2007;38(7):583‐590. [PubMed] [Google Scholar]
- 140. Henry JA. Amphetamines. In: Ford MD, Delaney KA, Ling LJ, Erickson T, eds. Clinical toxicology. 1st ed. W.B. Saunders Co; 2001:620‐627. [Google Scholar]
- 141. Spolsky VW, Clague J, Murphy DA, et al. Periodontal status of current methamphetamine users. J Am Dent Assoc. 2018;149(3):174‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tipton DA, Legan ZT, Dabbous MK. Methamphetamine cytotoxicity and effect on LPS‐stimulated IL‐1beta production by human monocytes. Toxicol In Vitro. 2010;24(3):921‐927. [DOI] [PubMed] [Google Scholar]
- 143. Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121‐135. [DOI] [PubMed] [Google Scholar]
- 144. Henkel K, Altenburger MJ, Auwärter V, Neukamm MA. Full validation of a method for the determination of drugs of abuse in non‐mineralized dental biofilm using liquid chromatography‐tandem mass spectrometry and application to postmortem samples. Talanta. 2018;176:360‐366. [DOI] [PubMed] [Google Scholar]
- 145. Antoniazzi RP, Del'Agnese CC, Bento LW, Santos BZ, Skupien JA, Feldens CA. Association between crack cocaine use and dental caries experience: a cross‐sectional study in southern Brazil. Environ Sci Pollut Res Int. 2021;28(48):68417‐68425. [DOI] [PubMed] [Google Scholar]
- 146. Verheijden SL, Henry JA, Curran HV. Acute, sub‐acute and long‐term subjective consequences of ‘ecstasy’ (MDMA) consumption in 430 regular users. Hum Psychopharmacol. 2003;18(7):507‐517. [DOI] [PubMed] [Google Scholar]
- 147. Peroutka SJ, Newman H, Harris H. Subjective effects of 3,4 methylenedioxymethamphetamine in recreational users. Neuropsychopharmacology. 1988;1(4):273‐277. [PubMed] [Google Scholar]
- 148. Hegazi F, Alhazmi H, Abdullah A, et al. Prevalence of oral conditions among methamphetamine users: NHANES 2009–2014. J Public Health Dent. 2021;81(1):21–28. [DOI] [PubMed] [Google Scholar]
- 149. Strobbe S. Prevention and screening, brief intervention, and referral to treatment for substance use in primary care. Prim Care. 2014;41(2):185‐213. [DOI] [PubMed] [Google Scholar]
- 150. Maloney WJ, Raymond GF. Common Substances and Medications of Abuse. In: O'Neil M, ed. The ADA practical guide to substance use disorders and safe prescribing. Wiley Blacwell; 2015:83‐118. [Google Scholar]
- 151. Melton ST, Orr RA. Detection and deterrence of substance use disorders and drug diversion in dental practice. In: O'Neil M, ed. The ADA practical guide to substance use disorders and safe prescribing. Wiley Blacwell; 2015:148‐158. [Google Scholar]
- 152. 139. 151. SAMSHA‐HRSA Center for Integrated Health Solutions . SBIRT: screening, brief intervention, and referral to treatment. http://www.integration.samhsa.gov/clinical‐practice/SBIRT. Accessed May 26, 2022.
- 153. Raymond GF, Maloney WJ. Interviewing and counseling patients with known or suspected substance use disorders: dealing with drug‐seeking patients. In: O'Neil M, ed. The ADA practical guide to substance use disorders and safe prescribing. Wiley Blacwell; 2015:159‐168. [Google Scholar]
- 154. The substance abuse and mental health services administration (SAMHSA) : https://www.samhsa.gov/. Accessed May 26, 2022.
- 155. Resnicow K, DiIorio C, Soet JE, Ernst D, Borrelli B, Hecht J. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol. 2002;21(5):444‐451. [PubMed] [Google Scholar]
- 156. Miller WR, Rollnick S. Motivational interviewing: preparing people for change. 2nd ed. Guildford Press; 2002. [Google Scholar]
- 157. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta‐analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843‐861. [DOI] [PubMed] [Google Scholar]
- 158. Bolla KI, Cade JL. Exogenous acquired metabolic disorders of the nervous system: toxins and illicit drugs. In: Goetz CG, ed. Textbook of clinical neurology. 3rd ed. Saunders Elsevier; 2007:865‐896. [Google Scholar]
- 159. Little JW, Falace DA, Miller CS, Rhodus NL. Neurological, behavioral and psychiatric disorders. Dental management of the medically compromised patient. 7th ed. Mosby Elsevier; 2008:493‐574. [Google Scholar]
- 160. McGee SM, McGee DN, McGee MB. Spontaneous intracerebral hemorrhage related to methamphetamine abuse: autopsy findings and clinical correlation. Am J Forensic Med Pathol. 2004;25(4):334‐337. [DOI] [PubMed] [Google Scholar]
- 161. Wang P, Chen X, Zheng L, Guo L, Li X, Shen S. Comprehensive dental treatment for “meth mouth”: a case report and literature review. J Formos Med Assoc. 2014;113(11):867‐871148. [DOI] [PubMed] [Google Scholar]
- 162. Morales S. Aesthetic reconstruction of “meth mouth”. Dent Today. 2006;25(86):8‐91. [PubMed] [Google Scholar]
- 163. Padilla R, Av R. Meth mouth: methamphetamine and oral health. J Esthet Restor Dent. 2008;20(2):148‐149. [DOI] [PubMed] [Google Scholar]
- 164. Charnock S, Owen S, Brookes V, Williams M. A community based programme to improve access to dental services for drug users. Br Dent J. 2004;196(7):385‐388. [DOI] [PubMed] [Google Scholar]
- 165. Deutsch HL, Millard DR. A new cocaine abuse complex. Involvement of nose, septum, palate, and pharynx. Arch Otolaryngol Head Neck Surg. 1989;115(2):235‐237. [DOI] [PubMed] [Google Scholar]
- 166. Newman RG. The need to redefine addiction. N Engl J Med. 1983;308(18):1096‐1098. [DOI] [PubMed] [Google Scholar]
- 167. Koenig TW, Clark MR. Advances in comprehensive pain management. Psychiatr Clin North Am. 1996;19(3):589‐611. [DOI] [PubMed] [Google Scholar]
- 168. Portenoy RK, Dole V, Joseph H, et al. Pain management and chemical dependency. Evol Persp JAMA. 1997;278(7):592‐593. [PubMed] [Google Scholar]
- 169. Prater CD, Zylstra RG, Miller KE. Successful pain management for the recovering addicted patient. Prim Care Companion J Clin Psychiatry. 2002;4(4):125‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Maloney W. The significance of illicit drug use to dental practice. Webmed Central Dent Drug Abuse. 2010;1(7):WMC00455. [Google Scholar]
- 171. Savage S. Principles of pain treatment in the addicted patient. In: Graham AW, Schultz TK, eds. Principles of Addiction Medicine. 2nd ed. American Society of Addiction Medicine; 1998:919‐946. [Google Scholar]
- 172. Dunbar SA, Katz NP. Chronic opioid therapy for nonmalignant pain in patients with a history of substance abuse: report of 20 cases. J Pain Symptom Manag. 1996;11(3):163‐171. [DOI] [PubMed] [Google Scholar]


