Abstract
Background
Speaking depends on refined control of jaw opening and closing movements. The medial pterygoid muscle (MPT), involved in jaw closing, and the lateral pterygoid muscle (LPT), involved in jaw opening, are two key mandibular muscles in mastication and are likely to be recruited for controlled movements in speech.
Objectives
Three hypotheses were investigated, that during speech the MPT and LPT: (1) were both active, (2) but exhibited different patterns of activity, (3) which fluctuated with the vowels and consonants in speech.
Methods
Intramuscular EMG recordings were made from the right inferior head of the LPT and/or the right MPT in five participants during production of 40 target nonsense words (NWs) consisting of three syllables in the form /V1 C 1 V 2 C2ə/ (V = vowel; C = consonant; ə = unstressed, reduced vowel), spoken by each participant 10 times per NW; analysis focussed on the target syllable, C 1 V 2 .
Results
Both MPT and LPT exhibited robust increases in EMG activity during utterance of most NWs, relative to rest. Peak LPT activation was time‐locked to the final part of the target consonant (C1) interval when the jaw begins opening for the target vowel (V2), whereas peak MPT activation occurred around the temporal midpoint of V2, when the jaw begins closing for C2. EMG amplitude peaks differed in magnitude between “high” vowels, i.e., for which the tongue/jaw are high (e.g., in S EE K), and “low” vowels, i.e., for which the tongue/jaw are low (e.g., in S O CK).
Conclusions
These novel findings suggest a key role for the LPT and MPT in the fine control of speech production. They imply that speech may impose major synergistic demands on the activities of the MPT and the LPT, and thereby provide insights into the possible interactions between speech activities and orofacial activities (e.g. mastication) and conditions (e.g. Temporomandibular Disorders) that involve the masticatory muscles.
Keywords: computed tomography, electromyography, language, mandibular muscles, masticatory muscles, speech sounds
Intramuscular electromyographic (EMG) recordings were made from the inferior head of the lateral pterygoid muscle (LPT) and the medial pterygoid muscle (MPT) during production of target nonsense words (NWs) consisting of three syllables. Both MPT and LPT exhibited robust increases in EMG activity during utterance of most NWs, relative to rest. These novel findings suggest a key role for the pterygoid muscles in speech which may therefore impose major demands on the activities of the pterygoid muscles.

1. INTRODUCTION
Speech is a major orofacial motor activity which, for most individuals, is more frequently performed than mastication and is essential to human social interaction and quality of life including mental health. Patients frequently present to their dentists and dental specialists to improve masticatory ability as well as speech intelligibility, and routine prosthodontic, orthodontic, restorative, and surgical procedures can have significant impacts on speech. For example, producing a clear /s/ sound requires a closely approximated upper and lower anterior tooth position. 1 , 2 After many restorative and prosthodontic procedures, dentists will listen for the clear /s/ sounds in patients' production of words such as “six and seven” and “Mississippi” to assess not only an appropriate anterior tooth position but also an adequate “speaking space” at an acceptable vertical dimension of occlusion. 1 , 2 Given the importance of clear speech following dental procedures, it is remarkable that the dental and the speech production literatures have remained largely unconnected.
During speech, the mandible moves rapidly in the sagittal plane with frequent reversals in direction, and the trajectories can vary with the production of different consonants and vowels and with changes to the anterior teeth. 3 , 4 , 5 The medial pterygoid muscle (MPT), active in mandibular closing, and the lateral pterygoid muscle (LPT), active in mandibular opening, are critically important muscles in mastication, and are also likely to be two key contributors to the control of mandibular movements in speech. First, of the jaw muscles, the LPT is anatomically the best oriented to generate horizontal force vectors on the mandible in the sagittal plane, 6 , 7 , 8 and the superior, anterior, and medial orientation of each MPT 8 , 9 means that it can act synergistically with the LPT to provide the rapid reversals in mandibular direction in the sagittal plane during speech, and as well to help stabilise mandibular position to allow the lip and tongue muscles to operate during speech. Second, electromyographic (EMG) studies of MPT and LPT activity patterns during standardised mandibular tasks provide evidence for a prominent role for the MPT and LPT in the fine control of horizontal mandibular movements and forces particularly in the sagittal plane 10 , 11 , 12 , 13 , 14 and for the MPT in stabilising mandibular movements. 12 There are also some previous data from unverified EMG recordings that the MPT and LPT are active during speech. 15 , 16 Given the deep location of these muscles, however, it is important to verify, e.g., through computed tomography (CT), that the EMG recordings are in fact coming from these muscles. 17
If the pterygoid muscles, two of the classical masticatory muscles, are shown to be active in speech and to be intimately concerned with the fine details of speech production, then the effects of prosthodontic, orthodontic, restorative, and surgical procedures on mandibular muscle activity may not be limited to their effects on muscle activity during mastication 18 but also during speech. The data may also have broader implications because both muscles have been implicated for many years as playing a special role in the pathophysiology of Temporomandibular Disorders (TMD). 19 If the pterygoid muscles are shown to be frequently active during speech, then speech might impose a significant additional load on the muscles that may be an etiological factor in TMD as well as being an acknowledged activity that is affected by TMD. 20 Although speech would be likely only to impose low loads on the jaw muscles, such low loads could still contribute to TMD given the evidence that wake‐time non‐functional tooth contacts such as frequent low level mandibular clenching have associations with TMD. 21 , 22 , 23 Analogous observations have been made in the spinal musculoskeletal system where occupations characterised by static low muscle loads and cyclic repetitive actions exhibit a high prevalence of work‐related musculoskeletal disorders. 24 Speech is also affected in other disease conditions such as Parkinson's disease, stroke, and neoplasia, 20 , 25 as well as medication‐induced orofacial dyskinesias and dystonias. The present findings could point to mandibular muscle activity as a potential factor in how these diseases and medications affect speech. The aims of the present study were to test three hypotheses, that during speech the MPT and LPT: (1) were both active, (2) but exhibited different patterns of activity, (3) which fluctuated with the vowels and consonants in speech.
2. METHODS
Inclusion criteria for the five recruited participants (three males, two females; age range: 29–61 years) were voluntary written informed consent and an ability to fluently read and clearly pronounce English nonsense words. None had a history of orofacial pain or neuromuscular disorders and recent or anticipated computer tomography (CT) scans. Ethics approval was obtained from the Western Sydney Local Health District Human Research Ethics Committee (Reference number: HREC2012/4/4.3[3484]). Some of the EMG procedures have been previously described in detail, 10 , 12 and more details are in the Appendix S1.
2.1. EMG and speech acoustic recordings
Intramuscular EMG recordings were made from the right inferior head of the LPT and/or the right MPT by inserting bipolar teflon‐coated fine wires (Mediwire, USA; 110 μm × 25 mm) via a sterilised needle through the mucosa. The insertion point for the LPT was above the upper right second molar tooth to the lateral surface of the lateral pterygoid plate, and for the MPT, at the same insertion point as for an inferior alveolar nerve block but with the needle angled slightly lateral to the lower right posterior teeth, and advanced ~10–30 mm. After each insertion, the needle was removed, and the wires remained within the muscle and were passed loosely out at the corner of the mouth to reduce physical interference during speech. Electrodes were placed in the LPT only, in one participant, in the MPT only, in two participants, and in both muscles in two participants. The EMG activity from each electrode was amplified (Model DBA‐S, World Precision Instruments Ltd, Hertfordshire, UK; up to 20 000x), filtered (bandwidth: ~0.1–5 kHz), and digitised (micro1401; Cambridge Electronic Design, Cambridge, UK; ~20 000 samples/s) for offline analysis. Participants remained in an unrestrained seated position throughout the recordings.
Prior to recordings, participants briefly clenched and opened their mandibles against resistance to confirm muscle activity only from the MPT and LPT electrode leads, respectively. Electrode placement was verified at the end of 4/5 recording sessions, by 1‐3‐mm thick CT‐axial slices taken inferior to and parallel with the clinically approximated Frankfort Horizontal Plane. During these EMG recordings, data related to jaw and tongue motion were also acquired but were not analysed for this paper (see Appendix S1). Speech acoustics were recorded with a microphone capable of flat recording between 60 and 15 000 Hz and were amplified and digitised (micro1401; Cambridge Electronic Design) at a rate of 20 000 samples/s simultaneously with both EMG recordings.
2.2. Spoken nonsense words
Nonsense words (NWs) (Table 1) were used to capture the consonant‐vowel sequences and syllable structure needed for our analyses, which was not possible with real words. The NWs were displayed on a monitor in front of each participant. Each NW contained three syllables of the form /V1ˈC1V2.C2ə/ (V: vowel; C: consonant) where V1 and V2 are identical, C1 and C2 are identical, the second syllable is stressed, and the final /ə/ vowel is unstressed, as in the real word/name aurora (o‐RO‐rə). Appendix S1 describes and Table 1 lists the 40 NWs, which constitute combinations of five vowels /æ, a, i, e, o/ with eight consonants /p, f, t, s, ʃ, l, r, k/, where /ʃ/ is the “sh” sound. In each trial, a NW was repeated 10 times with a timed pause (~1 s) between the repetitions. Only a subset of the NWs was used for the current paper (see next section).
TABLE 1.
The 40 nonsense words (NWs)
| p | f | t | s | ʃ | l | r | k | |
|---|---|---|---|---|---|---|---|---|
| a | apapə | afafə | atatə | asasə | aʃaʃə | alalə | ararə | akakə |
| i | ipipə | ififə | ititə | isisə | iʃiʃə | ililə | irirə | ikikə |
| e | epepə | efefə | etetə | esesə | eʃeʃə | elelə | ererə | ekekə |
| o | opopə | opopə | ototə | ososə | oʃoʃə | ololə | ororə | okokə |
| æ | æpæpə | æfæfə | ætætə | æsæsə | æʃæʃə | ælælə | ærærə | ækækə |
Note: Nonsense words (NWs), which constitute combinations of five vowels /æ, A, i, e, o/ with eight consonants /p, F, t, s, ʃ, l, r, k/, where /ʃ/ is the “sh” sound. In each trial, a NW was repeated 10 times with a ~ 1 s pause between the repetitions.
2.3. Data analysis
To test the first hypothesis, a qualitative analysis assessed the presence or absence of EMG activity within LPT and MPT during speech in comparison to the rest periods between the NWs. To test the second hypothesis, the EMG data were first filtered using a proprietary algorithm, developed by author CC, for estimating the spectral density of the underlying system noise. An amplitude envelope of the filtered EMG data was subsequently created by computing the absolute values of the Hilbert transform (see Appendix S1) of the filtered data. Since the level of muscle activation can vary between the muscles, the final filtered and enveloped data was presented in percentages of the total ranges observed for each muscle in each participant's data. Scaling the data in this way emphasises the temporal coordination between the two muscles while retaining the variation in activity levels across different contexts. Each NW production was also time‐normalised by resampling the EMG data, before combining the data from the 10 repetitions of that NW. This time normalisation was applied to each separate C and V in the NW by either stretching or compressing its duration to be equal to the average duration of that C or V across the 10 repetitions.
The presence of differences in EMG amplitude peaks between “high” vowels (e.g. /i/ as in “l ea k”, and /e/ as in “l a ke”) and “low” vowels (e.g. /a/ as in “l o ck”, and /æ/ as in “l a ck”) was used to test the third hypothesis. High vowels were compared with low vowels because the amount of mandibular opening has been clearly demonstrated to be less with high vowels, during which the tongue body is closer to the palate [high], than low vowels during which the mandible is more open, i.e., the tongue body is lowered from the palate. 26 If there are differences in the characteristics of the EMG activity between high and low vowels, then this suggests that muscle activity fluctuates with the magnitude of jaw opening and closing in speech. In order to highlight these possible differences, we only include here consonants that are produced with the tip of the tongue pressed against the alveolar ridge (and, thus, the mandible in a more closed position than for vowels), i.e. the consonants /s/ and /t/; this helps ensure that the mandible traverses a maximum range of displacement between each consonant and vowel. Therefore, the NW subset used to test this third hypothesis includes the eight combinations of the four vowels /i, e, a, æ/ and the two consonants /s, t/. The analysis for this third hypothesis focussed on the second syllable, the target syllable, in which C1 is the target consonant and V2 is the target vowel.
3. RESULTS
An example from one participant of CT verification data is shown in Figure 1A for the MPT and 1B for the LPT.
FIGURE 1.

Computer tomography (CT) verification data from one participant and displaying two horizontal CT slices (anterior is uppermost) that show the location of an intramuscular EMG electrode (red circle) within the medial pterygoid muscle (A) and the lateral pterygoid muscle (B). The actual recording region of each electrode is 2–4 mm from the end of the wires illustrated in the centre of each red circle (see Appendix S1).
All recordings from the MPT (n = 4) and the LPT (n = 3) exhibited robust changes in EMG activity, in comparison with rest periods between the NWs, for all NWs. Figure 2 shows representative raw data from a section of a trial in one participant during the speaking of five NWs (apapǝ, atatǝ, ililǝ, alalǝ, ӕtӕtǝ). There are clear changes (mostly increases) in EMG activity in both the MPT and the LPT during each NW in comparison with the between‐NW rest periods, and the bursts of activity of the MPT appear to be mostly out of phase with those of the LPT.
FIGURE 2.

Representative data from a section of a trial in one participant for the nonsense words apapǝ, atatǝ, ililǝ, alalǝ, ӕtӕǝ. The lowermost panel is the speech audio output, and the two top panels show the unfiltered electromyographic signal from the lateral pterygoid muscle (LPT, uppermost panel) and the medial pterygoid muscle (MPT, middle panel).
Figure 3 displays representative data of speech audio (top panels), filtered EMG activity (middle panels), and Hilbert energy envelopes (bottom panels) for the NW /isisa/ (left column, high vowel) and the NW /æsæsa/ (right column, low vowel). The LPT and the MPT are denoted by different line colours, the beginning and end of the NWs are denoted by vertical dashed lines, and in the target syllable the consonant (C1) interval is denoted by the dark grey shaded area, and the vowel (V2) interval is denoted by the light grey shaded area. Note the marked difference in LPT and MPT EMG activity between the high vowel NW (left panel) compared with the low vowel NW (right panel). It is also apparent that the largest increases in MPT activity (implicated in mandibular closing) tended to occur during the target vowel, while the largest increases in LPT activity (implicated in mandibular opening) appeared mostly at the end of the target consonant.
FIGURE 3.

Examples in one participant of speech audio output (top panels), filtered electromyographic (EMG) activation signals (middle panels), and Hilbert energy envelopes (bottom panels) for the nonsense word containing a high vowel, “isisa” (left column), and the nonsense word containing a low vowel, “æsæsa” (right column). The lateral pterygoid and medial pterygoid muscle activities are denoted by different line colours, the beginning and end of the nonsense words are denoted by vertical dashed lines, the interval of the target consonant (C1) is denoted by the dark grey rectangle, and the interval of the target vowel (V2) is denoted by the light grey rectangle.
Muscle activity patterns from the LPT and the MPT are displayed for the NWs containing target syllables with low vowels (/a, æ/; see “Data analysis”) in Figure 4, and with high vowels (/i, e/) in Figure 5. For both low and high vowels, the LPT is most active during the target consonant and the MPT is most active during the target vowel. A comparison of the time‐normalised participant patterns (panel A) with the group patterns (panel B) in both figures shows that the LPT reaches peak activation during the latter portion of the target consonant interval (see also panel C), whereas the MPT reaches peak activation just after the temporal midpoint of the target vowel. During high vowels (Figure 5), the MPT reached an average of ~60% and LPT reached an average of ~40% of the maximum level observed during the low vowels (Figure 4).
FIGURE 4.
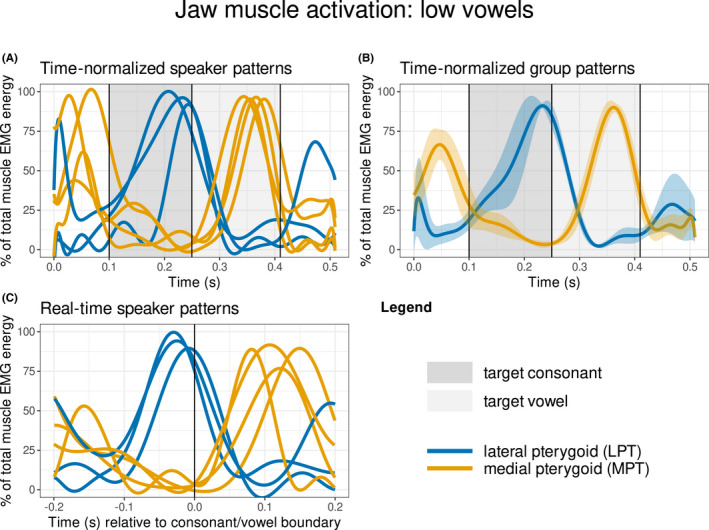
Muscle activation patterns for target syllables containing low vowels /a, æ/. The lateral pterygoid and medial pterygoid muscles are denoted by different line colours, the interval of the target consonant (C1) is denoted by the dark grey rectangle, and the interval of the target vowel (V2) is denoted by the light grey rectangle. Panel A shows the inter‐participant patterns of muscle activation with time normalisation of the target consonant and target vowel; a 100 ms window has been included before the target consonant and after the target vowel to provide a context for the activation patterns. Panel B displays the same information as panel A, but as average patterns across all participants along with 95% confidence interval bands. Panel C displays the same information as Panel A, but without time normalisation; the vertical line denotes the temporal boundary between the target consonant and target vowel.
FIGURE 5.
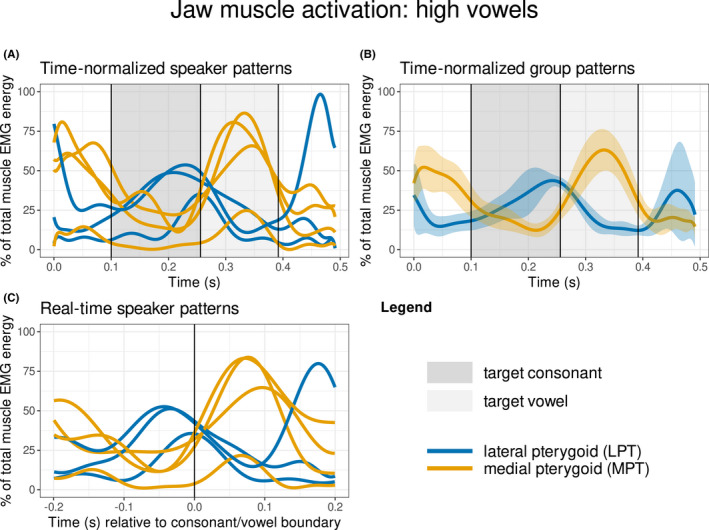
Muscle activation patterns for target syllables containing high vowels /i, e/. See Figure 4 legend for detailed description.
4. DISCUSSION
This is the first evidence from verified EMG recordings that the MPT and the LPT, two important masticatory muscles, 6 , 7 also play an important role in speech. The data support the hypotheses by showing that, during speech, the MPT and LPT muscles are active during a variety of speech sounds, the activity of the MPT is different from that of the LPT, and that variation in MPT and LPT EMG activity correlate with aspects of speech, i.e., differences in vowels. These results have important implications for dentistry, suggesting that dental procedures that affect speech (e.g. prosthodontic, orthodontic, restorative, and surgical procedures) may also influence the activity of the mandibular muscles during speech. For example, modifying anterior tooth position will likely significantly affect the activity of both the MPT and the LPT during speech. The findings may also help explain why speech may be impaired in TMD patients. 20
4.1. Implications for TMD
As speech is performed without tooth‐to‐tooth contact, in contrast to mastication, dentists may have assumed that speech, and by implication any changes to the dentition resulting in alterations to speech, are unlikely to impose excessive demands on the activities of the mandibular muscles. The findings that both the MPT and the LPT are not only intensely active throughout production of many different speech sounds, but also appear to modulate their activity in close relation with high vowels vs. low vowels, suggest that these muscles play a prominent role in the fine details of speech production. Previous work has noted that verbal and emotional expression may be affected in TMD. 20 Given the evidence cited earlier that low‐level prolonged mandibular muscle activations may lead to tissue damage and pain in both the trigeminal and spinal musculoskeletal systems, 21 , 22 , 23 there is the potential for speech to be a source of prolonged mandibular muscle activations particularly in individuals with high speech requirements, for example, broadcasters, telephonists, singers, teachers, journalists and politicians. It should be mentioned however, that there is a substantial difference in muscle activity patterns between the rapidly changing muscle contractions during speech and the low‐level static muscle activation when holding the teeth in contact in low level clenching. Nonetheless, the presence of pterygoid muscle activity in speech provides the opportunity for high demand on these muscles. An earlier observation identified a co‐contraction of the masseter and suprahyoid muscles in TMD patients, and which was absent in pain‐free controls. 27 If the pterygoid muscles co‐contract in a similar fashion in TMD patients, then this may have implications for possible effects on speech. More detailed information about mandibular muscle activity during speech may provide insights into the possible bidirectional relationship between speech activities and orofacial pain conditions affecting the masticatory muscles. Speech could also be explored as a possible therapeutic exercise in TMD patients with myalgia to assist in jaw muscle re‐training.
4.2. Detailed activities in speech
For both low and high vowels, LPT activation is consistent across participants in a time‐invariant manner (i.e. the peak is time‐locked to the final part of the target consonant interval) whereas MPT activation is consistent across participants in a time‐relative manner (i.e. the peak occurs near or just after the temporal midpoint of the target vowel). Given that the LPT has been shown to be an ideal muscle to achieve a precise anterior–posterior positioning of the mandible, 10 , 11 , 13 , 14 we interpret these patterns as indicating that the LPT is involved in precise anterior–posterior mandibular positioning for the production of the consonant prior to the formation of the following vowel. In comparison, we extrapolate from the role of the MPT in closing the mandible and in stabilising vertical mandibular position throughout horizontal mandible movements with the teeth apart, 6 , 12 that the MPT is used in speech for closing the mandible near the middle of the vowel to begin forming the following consonant. In this way, LPT and MPT activation is coordinated asynchronously to help achieve complementary speech goals throughout the target syllable.
4.3. Future directions
Future studies could explore the temporal relationship between muscle activation and jaw kinematics. It is also unclear if the same motor units used during mastication are also used during speech. This could have implications for whether speech exercises might be useful in re‐training the jaw muscles in therapeutic interventions and/or whether speech may contribute to the development of TMD. This could be investigated with EMG recordings during both mastication and speech. The present findings also provide baseline data for developing algorithms driving actuators in the development of robotic faces simulating speech. Speech in current robotic faces (e.g., Sophia: Hanson Robotics) appears artificial, which may partly be due to the absence in these robots of a ‘mandible’ that is being moved in a similar way as in human speech.
5. CONCLUSIONS
The present findings show that the MPT and the LPT are active during speech, but in different ways, and that fluctuations in their activity are associated with differences in production of some consonants and vowels. These novel findings suggest that speech imposes substantial demands on the activities of the MPT and the LPT, both of which are also involved in mastication.
AUTHOR CONTRIBUTIONS
GM: Contributed to conception, design, data acquisition and interpretation, drafted sections of the manuscript and critically revised the manuscript. CC: Contributed to data interpretation, performed most of the statistical analyses, drafted sections of the manuscript and critically revised the manuscript. TW: Contributed to conception, design, data acquisition and interpretation, performed some statistical analyses, drafted sections of the manuscript and critically revised the manuscript. JG: Contributed to data interpretation, performed some of the data analyses, and critically revised the manuscript. CB: Contributed to conception, design, data acquisition and interpretation, drafted sections of the manuscript and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
CONFLICT OF INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/joor.13377.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
We dedicate this paper to Eric Vatikiotis‐Bateson, who brought us together as the instigator of this project and formulator of its core hypothesis. Sadly, Eric passed away before the project was completed but his spirit has continued to energise and inspire us. The conception, design, data acquisition, and some of the interpretation for these studies were performed in the Jaw Function and Orofacial Pain Research Unit, Faculty of Dentistry, The University of Sydney. The Unit was closed in 2018. The assistance of Dr Christian Kroos and Dr Donald Derrick is acknowledged in some of the recording sessions. The project was not associated with any specific source of funding. Consumables and computer tomography (CT) scans were funded from discretionary funds from the Jaw Function and Orofacial Pain Research Unit. Costs associated with the electromagnetic articulometry aspects of the recording sessions were supported by funds from MARCS Institute, Western Sydney University. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Murray GM, Carignan C, Whittle T, Gal JA, Best C. Pterygoid muscle activity in speech: A preliminary investigation. J Oral Rehabil. 2022;49:1135‐1143. doi: 10.1111/joor.13377
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Murray CG. Re‐establishing natural tooth position in the edentulous environment. Aust Dent J. 1978;23(5):415‐421. [DOI] [PubMed] [Google Scholar]
- 2. Pound E. Recapturing esthetic tooth position in the edentulous patient. J Am Dent Assoc. 1957;55(2):181‐191. [DOI] [PubMed] [Google Scholar]
- 3. Nasir SM, Ostry DJ. Control of movement precision in speech production. In: Maassen B, van Lieshout PHHM, eds. Speech Motor Control: New Developments in Basic and Applied Research. Oxford University Press; 2010:1‐25. [Google Scholar]
- 4. Ostry DJ, Gribble PL, Gracco VL. Coarticulation of jaw movements in speech production: is context sensitivity in speech kinematics centrally planned? J Neurosci. 1996;16(4):1570‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Serrano P, Faot F, Cury A, Garcia R. Effect of dental wear, stabilization appliance and anterior tooth reconstruction on mandibular movements during speech. Braz Dent J. 2008;19(2):151‐158. [DOI] [PubMed] [Google Scholar]
- 6. Hannam AG, McMillan AS. Internal organization in the human jaw muscles. Crit Rev Oral Biol Med. 1994;5(1):55‐89. [DOI] [PubMed] [Google Scholar]
- 7. Miller AJ. Craniomandibular Muscles: their Role in Function and Form. CRC Press; 1991. [Google Scholar]
- 8. Schumacher GH. Funktionelle Morphologie der Kaumuskulatur. Veb Gustav Fischen Verlag; 1961. [Google Scholar]
- 9. El Haddioui A, Bravetti P, Gaudy JP. Anatomical study of the arrangement and attachments of the human medial pterygoid muscle. Surg Radiol Anat. 2007;29(2):115‐124. [DOI] [PubMed] [Google Scholar]
- 10. Phanachet I, Whittle T, Wanigaratne K, Murray GM. Functional properties of single motor units in inferior head of human lateral pterygoid muscle: task relations and thresholds. J Neurophysiol. 2001;86(5):2204‐2218. [DOI] [PubMed] [Google Scholar]
- 11. Bhutada MK, Phanachet I, Whittle T, Wanigaratne K, Peck CC, Murray GM. Threshold properties of single motor units in superior head of human lateral pterygoid muscle. Arch Oral Biol. 2007;52(6):552‐561. [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Whittle T, Gal JA, Murray GM, Klineberg IJ. The medial pterygoid muscle: a stabiliser of horizontal jaw movement. J Oral Rehabil. 2017;44(10):779‐790. [DOI] [PubMed] [Google Scholar]
- 13. Schindler HJ, Rues S, Turp JC, Schweizerhof K, Lenz J. Jaw clenching: muscle and joint forces, optimization strategies. J Dent Res. 2007;86(9):843‐847. [DOI] [PubMed] [Google Scholar]
- 14. Ruangsri S, Whittle T, Wanigaratne K, Murray GM. Functional activity of superior head of human lateral pterygoid muscle and isometric force. J Dent Res. 2005;84(6):548‐553. [DOI] [PubMed] [Google Scholar]
- 15. Folkins JW. Muscle activity for jaw closing during speech. J Speech Hear Res. 1981;24(4):601‐615. [DOI] [PubMed] [Google Scholar]
- 16. Tuller B, Harris KS, Gross B. Electromyographic study of the jaw muscles during speech. J Phon. 1981;9(2):175‐188. [Google Scholar]
- 17. Orfanos T, Sarinnaphakorn L, Murray GM, Klineberg IJ. Placement and verification of recording electrodes in the superior head of the human lateral pterygoid muscle. Arch Oral Biol. 1996;41(5):493‐503. [DOI] [PubMed] [Google Scholar]
- 18. Bourdiol P, Hennequin M, Peyron M‐A, Woda A. Masticatory adaptation to occlusal changes. Front Physiol. 2020;11(263):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lund JP, Murray GM, Svensson P. Pain and motor reflexes. In: Sessle BJ, Lavigne G, Lund JP, Dubner R, eds. Orofacial Pain: from Basic Science to Clinical Management. Vol 2nd. Quintessence; 2008:109‐116. [Google Scholar]
- 20. Ohrbach R, Larsson P, List T. The jaw function limitation scale: development, reliability, and validity of 8‐item and 20‐item versions. J Orofac Pain. 2008;22(3):219‐230. [PubMed] [Google Scholar]
- 21. Palla S, Farella M. Masticatory muscle pain. In: Mense S, Gerwin RD, eds. Muscle Pain: Diagnosis and Treatment. Springer‐Verlag; 2010:193‐227. [Google Scholar]
- 22. Michelotti A, Cioffi I, Festa P, Scala G, Farella M. Oral parafunctions as risk factors for diagnostic TMD subgroups. J Oral Rehabil. 2010;37(3):157‐162. [DOI] [PubMed] [Google Scholar]
- 23. Ohrbach R, Bair E, Fillingim RB, et al. Clinical orofacial characteristics associated with risk of first‐onset TMD: the OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):T33‐T50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madeleine P. On functional motor adaptations: from the quantification of motor strategies to the prevention of musculoskeletal disorders in the neck‐shoulder region. Acta Physiol. 2010;199(s679):1‐46. [DOI] [PubMed] [Google Scholar]
- 25. Ribeiro GR, Campos CH, Rodrigues Garcia RCM. Parkinson's disease impairs masticatory function. Clin Oral Investig. 2017;21(4):1149‐1156. [DOI] [PubMed] [Google Scholar]
- 26. Lindblom BE, Sundberg JE. Acoustical consequences of lip, tongue, jaw, and larynx movement. J Acoust Soc Am. 1971;50(4):1166‐1179. [DOI] [PubMed] [Google Scholar]
- 27. Stohler C, Yamada Y, Ash MM Jr. Antagonistic muscle stiffness and associated reflex behaviour in the pain‐dysfunctional state. Schweiz Monatsschr Zahnmed. 1985;95(8):719‐726. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Research data are not shared.


