Abstract
Background
In adults and adolescents with asthma, use of ≥3 short‐acting β2‐agonist (SABA) canisters/year is associated with increased exacerbation risk. Whether this association is present in younger children remains unknown. In this SABA use IN Asthma (SABINA) Junior study, we assessed the association of SABA collection with exacerbation risk in the general Swedish pediatric asthma population.
Methods
This population‐based cohort study utilized linked data from the Swedish national healthcare registries involving patients with asthma (<18 years) treated in secondary care between 2006–2015. Exacerbation risk, by baseline SABA collection (0–2 vs. ≥3 canisters, further examined as ordinal/continuous variable) and stratified on comorbid atopic disease (allergic rhinitis, dermatitis and eczema, and food/other allergies), was assessed for 1‐year follow‐up using negative binomial regression.
Results
Of 219,561 patients assessed, 45.4%, 31.7%, and 26.5% of patients aged 0–5, 6–11, and 12–17 years, respectively, collected ≥3 SABA canisters during the baseline year (high use). Collection of ≥3 SABA canisters (vs. 0–2) was associated with increased exacerbation risk during follow‐up (incidence rate ratios [95% confidence interval]: 1.35 [1.29–1.42], 1.22 [1.15–1.29], and 1.26 [1.19–1.34] for 0–5‐, 6–11‐, and 12–17‐year‐olds, respectively); the association persisted with SABA as a continuous variable and was stronger among patients without atopic diseases (32%–44% increased risk versus. 14%–21% for those with atopic disease across groups).
Conclusions
High SABA use was associated with increased asthma exacerbation risk in children, particularly in those without comorbid atopic diseases, emphasizing the need for asthma medication reviews and reformative initiatives by caregivers and healthcare providers on SABA use.
Keywords: asthma, children, exacerbations, pediatric, SABINA junior study, short‐acting β2‐agonists, Sweden
Short abstract

Key message.
This population‐based cohort SABINA Junior study assessed the association between short‐acting β2‐agonist (SABA) collection and exacerbation risk in a general pediatric population with asthma in Sweden. Our findings revealed that of the 219,561 patients, a considerable proportion across all age groups (45.4%, 31.7%, and 26.5% of patients aged 0–5, 6–11, and 12–17 years, respectively) collected ≥3 SABA canisters during the baseline year (considered high SABA use), which was associated with increased exacerbation risk during follow‐up. This association was particularly strong among patients without comorbid atopic diseases.
1. INTRODUCTION
Asthma is the most common noncommunicable disease in children globally, 1 with a reported prevalence in Sweden of ~7% in children (7–8 years) 2 and 11% in adolescents (14–15 years). 3 Childhood asthma is characterized by multiple phenotypes, and unlike in adults, clinical manifestations of these phenotypes are more likely to change over time, thus hindering diagnosis and treatment, especially among those aged ≤5 years. 4 , 5 Birth cohorts from the United Kingdom (UK), the Netherlands, and Sweden have identified several phenotypes that optimally characterize the differences in childhood asthma over time. 5 , 6 Asthma phenotypes emerging in early childhood may be related to temporal patterns of wheeze, where persistent wheeze is strongly associated with atopy and increased airway responsiveness. 7 Among 6–17‐year‐olds, phenotypes differ and may be based on the presence of other allergic conditions, atopy, or eosinophilia; children with asthma and comorbid atopy and/or eosinophilia are at increased exacerbation risk. 8 , 9 , 10 , 11 Thus, similar to adults, children with asthma need to be carefully monitored and may require personalized treatment.
Childhood asthma exerts a persistent burden on patients, caregivers, and healthcare systems, 12 with a considerable proportion of children experiencing ≥1 exacerbation annually, 13 contributing to school absenteeism, reduced activity, disrupted sleep patterns, and associated psychological consequences. 14 , 15 , 16 The burden of childhood asthma may be further compounded by short‐acting β2‐agonist (SABA) over‐reliance for rapid symptom relief. The SABA use IN Asthma (SABINA) program in adults and adolescents with asthma reported that SABA overuse (≥3 canisters/year) is prevalent in Sweden 17 and associated with poor asthma‐related outcomes. 18 , 19 Few studies have evaluated SABA use in the pediatric population or its impact on clinical outcomes. 20 , 21 , 22 , 23 , 24 , 25 Examining the extent of SABA collection and associated clinical outcomes in children across age groups and asthma phenotypes may provide further insights into reducing asthma morbidity in children. The SABINA Junior study in Sweden describes the pediatric asthma population in secondary care, their treatment regimens, and the association between SABA collection and asthma‐related exacerbations.
2. METHODS
The study protocol was approved by the Stockholm Regional Ethics Committee (registration number: 2017/4:2) and conducted in compliance with the Declaration of Helsinki. In Sweden, patients need not provide consent for use of public register data. 26 , 27
2.1. Study design and population
This population‐based, nationwide retrospective cohort study included pediatric Swedish residents with physician‐diagnosed asthma (aged 0–17 years) in secondary care utilizing linked data from Swedish national healthcare registries (Appendix S1).
Asthma was identified based on the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD‐10) codes J45 or J46.9 in the Swedish National Patient Register (NPR). Patients were categorized by the number of SABA canisters collected from pharmacies at baseline, that is, the 12‐month period between initial asthma diagnosis and the index date, and followed up from index date until study end, death, emigration, or up to 3 years, whichever occurred first (Figure 1). Analytical outcomes were assessed for 1 and 3 years of follow‐up. As children can outgrow their asthma, the 1‐year follow‐up was considered for primary analysis.
FIGURE 1.
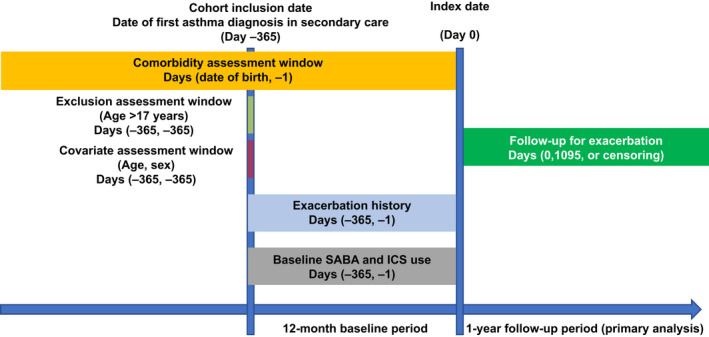
Study design ICS, inhaled corticosteroid; SABA, short‐acting β2‐agonist.
2.2. Variables and outcomes
SABA canisters were dichotomized as 0–2 versus. ≥3 based on evidence from studies in adults and adolescents. 18 , 19 Owing to potential differences in younger children, SABAs were further examined as ordinal (0–2, 3–5, 6–10, and ≥ 11 canister categories) and continuous variables.
Other baseline data comprised demographics; asthma medication, including inhaled corticosteroid (ICS); asthma severity or treatment severity step based on the Global Initiative for Asthma (GINA) 2018 recommendations 19 , 28 and comorbidities (Table S1) from birth until index date.
The analytical outcome was the incidence of asthma exacerbations during follow‐up, defined as hospitalization, emergency room (ER) visit due to asthma, or an oral corticosteroid (OCS) claim for asthma treatment.
2.3. Statistical analysis
All analyses were stratified by 0–5‐, 6–11‐, and 12–17‐year age groups. Analyses in 0–5‐year‐olds were exploratory. Baseline SABA canister collection was described by sex and treatment step of patients collecting SABA and the total number of ICS canisters collected by them. The Kruskal‐Wallis test assessed statistical significance between groups. The relation between baseline SABA use and individual exacerbation rates during first year of follow‐up was modeled using cubic B‐splines 29 using the R package ggplot2 (https://cran.r‐project.org/web/packages/ggplot2/index.html RRID:SCR_014601). 30
Negative binomial regression models assessed exacerbation risk (incidence rate ratios [IRRs] with 95% confidence intervals [CIs]) during the first follow‐up year and for 3 years of follow‐up, based on baseline SABA collection.
The risk of the first observed asthma exacerbation was assessed using Cox proportional hazard models. For this analysis, follow‐up for exacerbations started on the index date and ended at the first subsequent exacerbation, adulthood, death, emigration, study end, or 3 years after index, whichever occurred first. All multivariable analyses were adjusted for sex, treatment step, and baseline exacerbation history.
A post hoc analysis evaluated the interaction of SABA and atopic disease on exacerbation risk through model cross‐terms (SABA continuous times atopic disease 0/1). The interaction effect was tested through a likelihood‐ratio test. The multivariable analyses were stratified on atopic disease.
To visualize the change in exacerbation rate across SABA categories in the stratified groups, predicted exacerbation rate during the first follow‐up year for a patient profile stratified on atopic disease was calculated through estimated marginal means using the emmeans R package 31 applied to a negative binomial regression model (Appendix S1).
3. RESULTS
3.1. Study population
Of the 219,561 patients analyzed (Figure 2), 52.1%, 26.5%, and 21.4% were aged 0–5, 6–11, 12–17 years, respectively. Patients with longer‐term follow‐up and frequent hospital contacts could be included in more than one age‐specific cohort, that is, a patient with a visit at age 4, 8, and 14 years was included in all three cohorts (Figure S1). Overall, 40.2% of patients were female (Table 1) and 21.9%, 49.1%, and 58.5% of patients aged 0–5, 6–11, and 12–17 years, respectively, had comorbid atopic disease (Results and Table S2).
FIGURE 2.
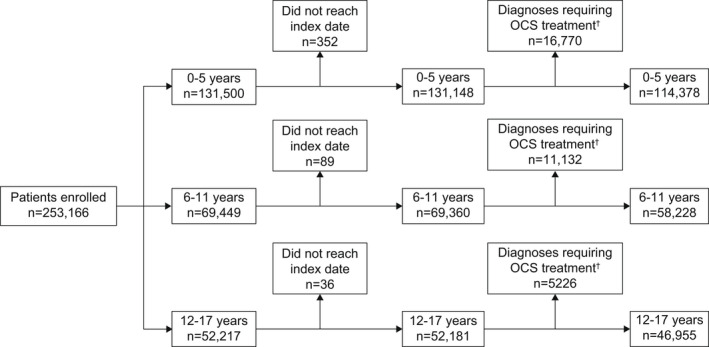
Patient disposition and study population stratified by age groups among pediatric patients with asthma. BPD, bronchopulmonary dysplasia; OCS, oral corticosteroid. †Diagnoses include cystic fibrosis, bronchiectasis, BPD originating in the perinatal period, and chronic lung disease following BPD/prematurity, croup, Crohn's disease, ulcerative colitis, juvenile arthritis, and anaphylaxis.
TABLE 1.
Baseline demographics, disease characteristics, and medications among pediatric patients with asthma in Sweden
| 0–5 years (n = 114,378) | 6–11 years (n = 58,228) | 12–17 years (n = 46,955) | |
|---|---|---|---|
| Female, n (%) | 43,994 (38.5) | 22,988 (39.5) | 21,286 (45.3) |
| Index age, years | |||
| Mean (SD) | 1.7 (1.5) | 7.8 (1.8) | 13.7 (1.7) |
| Atopic disease, n (%) | 25,098 (21.9) | 28,601 (49.1) | 27,492 (58.5) |
| Treatment step, n (%) | |||
| 0 | 16,624 (14.5) | 9482 (16.3) | 8218 (17.5) |
| 1 | 6990 (6.1) | 4961 (8.5) | 4438 (9.5) |
| 2 | 44,407 (38.8) | 21,295 (36.6) | 11,104 (23.6) |
| 3 | 46,357 (40.5) | 12,566 (21.6) | 10,661 (22.7) |
| 4 | 0 (0.0) | 4354 (7.5) | 6611 (14.1) |
| 5 | 0 (0.0) | 5570 (9.6) | 5923 (12.6) |
| SABA canisters | |||
| Mean (SD) | 2.7 (2.5) | 2.1 (2.2) | 1.8 (2.3) |
| Number of SABA canisters, n (%) | |||
| 0 | 26,000 (22.7) | 18,264 (31.4) | 18,966 (40.4) |
| 1 | 786 (0.7) | 2772 (4.8) | 2951 (6.3) |
| 2 | 35,641 (31.2) | 18,751 (32.2) | 12,574 (26.8) |
| 3–5 | 36,694 (32.1) | 14,007 (24.1) | 9417 (20.1) |
| 6–10 | 13,471 (11.8) | 3911 (6.7) | 2590 (5.5) |
| ≥11 | 1786 (1.6) | 523 (0.9) | 457 (1.0) |
| Number of ICS canisters, n (%) | |||
| 0 | 26,658 (23.3) | 16,136 (27.7) | 14,520 (30.9) |
| 1 | 26,978 (23.6) | 15,828 (27.2) | 11,859 (25.3) |
| 2 | 21,531 (18.8) | 10,408 (17.9) | 8147 (17.4) |
| 3–5 | 28,063 (24.5) | 12,223 (21.0) | 9610 (20.5) |
| 6–10 | 9434 (8.2) | 3051 (5.2) | 2428 (5.2) |
| ≥11 | 1714 (1.5) | 582 (1.0) | 391 (0.8) |
| Daily dose of ICS, μg a | |||
| Mean (SD) | 117.1 (111.2) | 175.7 (147.5) | 221.6 (186.9) |
| Baseline exacerbation rate, per 100 person‐years (95% CI) | |||
| Hospitalization | 6.14 (6.00–6.29) | 0.69 (0.62–0.76) | 0.40 (0.34–0.45) |
| ER visit | 3.59 (3.48–3.70) | 0.06 (0.04–0.08) | 0.09 (0.06–0.11) |
| OCS | 7.15 (6.99–7.30) | 11.52 (11.24–11.79) | 15.73 (15.37–16.08) |
| Any | 16.52 (16.29–16.76) | 12.19 (11.91–12.48) | 16.09 (15.73–16.45) |
| Medications, n (%) | |||
| SABA | 86,184 (75.4) | 24,433 (42.0) | 12,389 (26.4) |
| Any ICS | 87,720 (76.7) | 42,092 (72.3) | 32,435 (69.1) |
| ICS monotherapy | 86,968 (76.0) | 35,598 (61.1) | 18,346 (39.1) |
| LABA | 185 (0.2) | 1638 (2.8) | 2705 (5.8) |
| ICS/LABA | 2031 (1.8) | 9104 (15.6) | 16,829 (35.8) |
| LTRA | 18,954 (16.6) | 7895 (13.6) | 7699 (16.4) |
| OCS | 7284 (6.4) | 5567 (9.6) | 5903 (12.6) |
| Antibiotics | 15,231 (13.3) | 1878 (3.2) | 2935 (6.3) |
| Cough and cold medications | 26,185 (22.9) | 7423 (12.7) | 4531 (9.6) |
| Antihistamines | 42,990 (37.6) | 43,282 (74.3) | 40,746 (86.8) |
| Nasal corticosteroids | 4232 (3.7) | 16,663 (28.6) | 23,113 (49.2) |
| Antidepressants | <5 b | 108 (0.2) | 966 (2.1) |
| Commonly used oral antibiotics | 46,557 (40.7) | 10,326 (17.7) | 5715 (12.2) |
| Amoxicillin | 15,171 (13.3) | 1459 (2.5) | 718 (1.5) |
| Phenoxymethylpenicillin | 39,721 (34.7) | 9370 (16.1) | 5198 (11.1) |
| Antibiotics for systemic use | 21,495 (18.8) | 21,641 (37.2) | 20,373 (43.4) |
Abbreviations: CI, confidence interval; ER, emergency room; ICS, inhaled corticosteroid;
LABA, long‐acting β2‐agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short‐acting β2‐agonist; SD, standard deviation.
Computed among patients using ICS.
The number of patients was too low to report.
Most patients collected 0–2 ICS‐containing canisters at baseline (65.7%, 72.8%, and 73.5% of 0–5‐, 6–11‐, and 12–17‐year‐olds, respectively), with 23.3%, 27.7%, and 30.9% collecting zero canisters (Table 1). Baseline exacerbation rate was highest in 0–5‐year‐olds (rate per 100 person‐years [95% CI]: 16.52 [16.29–16.76]), followed by 12–17‐year‐olds (16.09 [15.73–16.45]) and 6–11‐year‐olds (12.19 [11.91–12.48]; Table 1). Across age groups, exacerbations requiring OCS were most common (rates per 100 person‐years: 7.2–15.7).
3.2. SABA collection during the baseline year
Overall, 45.4%, 31.7%, and 26.5% of 0–5‐, 6–11‐, and 12–17‐year‐olds, respectively, claimed ≥3 SABA canisters in the baseline year (Table 1). SABA collection increased with the number of ICS canisters and treatment step at baseline across age groups (Table S3).
3.3. Exacerbations at follow‐up
The unadjusted exacerbation rate during 1‐year follow‐up increased with baseline SABA collection across all age groups and was highest for 12–17‐year‐olds and lowest for 0–5‐year‐olds (Figure 3). In an adjusted multivariable analysis, across age groups, ≥3 versus 0–2 SABA collection at baseline was associated with an increased exacerbation rate during 1‐year follow‐up (IRRs [95% CI]: 1.35 [1.29–1.42], 1.22 [1.15–1.29], and 1.26 [1.19–1.34] for 0–5‐, 6–11‐, and 12–17‐year‐olds, respectively; Table 2). A “dose–response” relation was noted when SABA was examined as an ordinal and continuous variable (Table 2). Similar results were observed during 3 years of follow‐up (Table S4).
FIGURE 3.
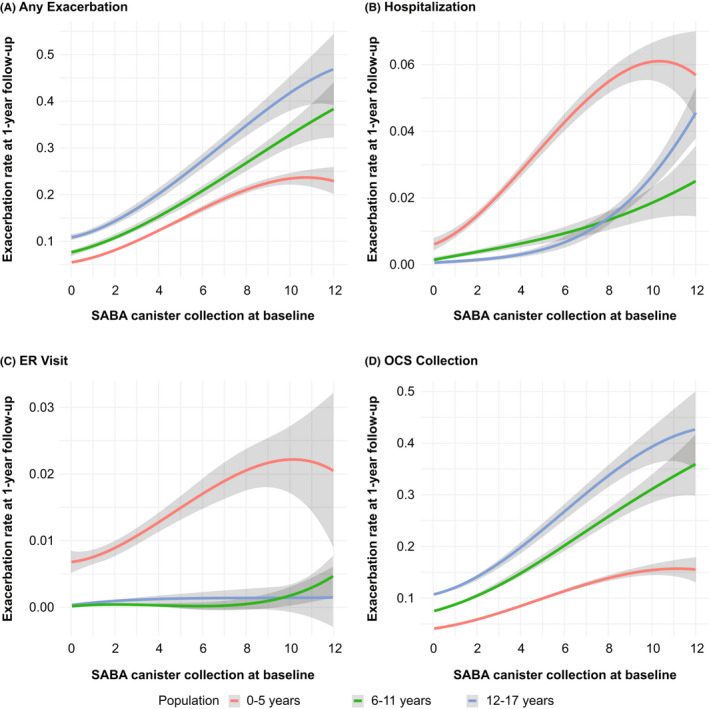
Unadjusted exacerbation rate during the first follow‐up year by baseline SABA use† among pediatric patients with asthma Smoothed curves derived using cubic B‐splines. The scale on the y‐axis is different in the four panels. The gray band denotes 95% confidence intervals. ER, emergency room; OCS, oral corticosteroid; SABA, short‐acting β2‐agonist. †Continuous variable.
TABLE 2.
Association of SABA a with exacerbations during the first follow‐up year among pediatric patients with asthma
| Baseline SABA canisters | 0–5 years (n = 114,378) | 6–11 years (n = 58,228) | 12–17 years (n = 46,955) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Number of events | IRR (95% CI) | Number of patients | Number of events | IRR (95% CI) | Number of patients | Number of events | IRR (95% CI) | |
| Overall | |||||||||
| 0–2 | 62,427 | 3750 | 1.00 (ref) | 39,787 | 3254 | 1.00 (ref) | 34,491 | 3852 | 1.00 (ref) |
| 3–5 | 36,694 | 3759 | 1.24 (1.18–1.31) | 14,007 | 1790 | 1.16 (1.08–1.23) | 9417 | 1657 | 1.16 (1.08–1.24) |
| 6–10 | 13,471 | 2172 | 1.57 (1.47–1.67) | 3911 | 742 | 1.31 (1.19–1.43) | 2590 | 707 | 1.44 (1.30–1.60) |
| ≥11 | 1786 | 504 | 1.86 (1.64–2.12) | 523 | 210 | 1.89 (1.57–2.26) | 457 | 264 | 1.98 (1.65–2.38) |
| 0–2 | 62,427 | 3750 | 1.00 (ref) | 39,787 | 3254 | 1.00 (ref) | 34,491 | 3852 | 1.00 (ref) |
| ≥3 | 51,951 | 6435 | 1.35 (1.29–1.42) | 18,441 | 2742 | 1.22 (1.15–1.29) | 12,464 | 2628 | 1.26 (1.19–1.34) |
| Continuous | 114,378 | 10,185 | 1.06 (1.05–1.07) | 58,228 | 5996 | 1.05 (1.04–1.06) | 46,955 | 6480 | 1.04 (1.03–1.06) |
| Atopic | |||||||||
| 0–2 | 12,728 | 1401 | 1.00 (ref) | 18,573 | 2414 | 1.00 (ref) | 19,707 | 2958 | 1.00 (ref) |
| 3–5 | 8526 | 1437 | 1.17 (1.07–1.27) | 7432 | 1336 | 1.09 (1.01–1.17) | 5780 | 1266 | 1.10 (1.02–1.19) |
| 6–10 | 3383 | 770 | 1.24 (1.12–1.38) | 2262 | 580 | 1.23 (1.11–1.36) | 1698 | 538 | 1.37 (1.22–1.53) |
| ≥11 | 461 | 193 | 1.72 (1.41–2.09) | 334 | 148 | 1.52 (1.25–1.85) | 307 | 193 | 1.83 (1.49–2.23) |
| 0–2 | 12,728 | 1401 | 1.00 (ref) | 18,573 | 2414 | 1.00 (ref) | 19,707 | 2958 | 1.00 (ref) |
| ≥3 | 12,370 | 2400 | 1.21 (1.12–1.31) | 10,028 | 2064 | 1.14 (1.07–1.22) | 7785 | 1997 | 1.20 (1.12–1.28) |
| Continuous | 25,098 | 3801 | 1.04 (1.02–1.05) | 28,601 | 4478 | 1.03 (1.02–1.05) | 27,492 | 4955 | 1.04 (1.03–1.05) |
| Nonatopic | |||||||||
| 0–2 | 49,699 | 2349 | 1.00 (ref) | 21,214 | 840 | 1.00 (ref) | 14,784 | 894 | 1.00 (ref) |
| 3–5 | 28,168 | 2322 | 1.29 (1.20–1.38) | 6575 | 454 | 1.24 (1.09–1.42) | 3637 | 391 | 1.33 (1.15–1.54) |
| 6–10 | 10,088 | 1402 | 1.78 (1.64–1.94) | 1649 | 162 | 1.39 (1.13–1.70) | 892 | 169 | 1.60 (1.28–2.00) |
| ≥11 | 1325 | 311 | 1.97 (1.67–2.32) | 189 | 62 | 2.88 (1.93–4.25) | 150 | 71 | 2.47 (1.65–3.67) |
| 0–2 | 49,699 | 2349 | 1.00 (ref) | 21,214 | 840 | 1.00 (ref) | 14,784 | 894 | 1.00 (ref) |
| ≥3 | 39,581 | 4035 | 1.44 (1.35–1.54) | 8413 | 678 | 1.32 (1.17–1.49) | 4679 | 631 | 1.44 (1.27–1.64) |
| Continuous | 89,280 | 6384 | 1.07 (1.06–1.08) | 29,627 | 1518 | 1.07 (1.05–1.09) | 19,463 | 1525 | 1.06 (1.04–1.08) |
Note: Negative binomial regression models were used; all analyses were adjusted for sex, treatment steps, and baseline exacerbation history (any type and categorized as 0, 1, and ≥2 exacerbations).Atopic diseases comprised vasomotor and allergic rhinitis, dermatitis and eczema, and allergies, including those of food and substances other than drugs and biological substances, as classified by respective ICD codes. Nonatopic disease was defined as the absence of atopic diseases.
Abbreviations: CI, confidence interval; ICD, International Statistical Classification of Diseases and Related Health Problems; IRR, incidence rate ratio; ref, reference; SABA, short‐acting β2‐agonist.
≥3 canisters versus 0–2 canisters.
When stratified by presence of atopic disease, the association between baseline SABA collection and risk of asthma exacerbations was significant for both groups, but the association was stronger among patients without versus those with comorbid atopic disease (p < .001; Table S5). Collection of ≥3 versus 0–2 SABA canisters at baseline was associated with a greater risk of any asthma exacerbation during 1‐year follow‐up among patients with nonatopic versus atopic disease (IRRs [95% CI]: 1.44 [1.35–1.54] versus 1.21 [1.12–1.31], 1.32 [1.17–1.49] versus 1.14 [1.07–1.22], and 1.44 [1.27–1.64] versus 1.20 [1.12–1.28] for 0–5‐, 6–11‐, and 12–17‐year‐olds, respectively; Figure 4 and Table 2). This relation persisted during 3 years of follow‐up (Table S4). Similar results were observed for the risk of the first exacerbation, in the overall analysis and when stratified on atopic disease, regardless of the follow‐up period (Table S6 and Figures S2 and S3).
FIGURE 4.
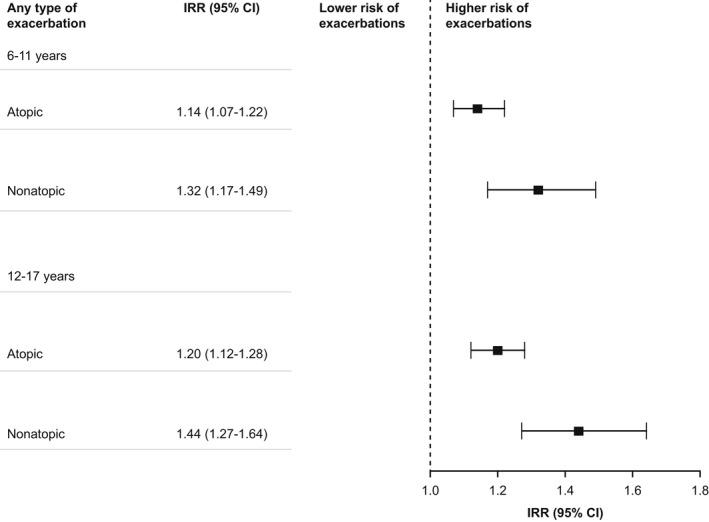
Multivariable associations of SABA† with exacerbation rates during the first follow‐up year stratified by atopic disease and age group. Negative binomial regression models were used; all analyses were adjusted for sex, treatment steps, and baseline exacerbation history (any type and categorized as 0, 1, and ≥2 exacerbations). Atopic diseases comprised allergic rhinitis, dermatitis and eczema, and allergies, including those of food and substances other than drugs and biological substances, as classified by respective ICD codes. Nonatopic disease was defined as the absence of atopic diseases. CI, confidence interval; ICD, International Statistical Classification of Diseases and Related Health Problems; IRR, incidence rate ratio; SABA short‐acting β2‐agonist. † ≥ 3 canisters versus 0–2 canisters.
When stratified by presence of atopic disease, the predicted rate of any exacerbation type during 1‐year follow‐up increased with higher baseline SABA canister collection (Figure S4). Despite a lower exacerbation rate with collection of zero SABA canisters in patients with nonatopic versus atopic disease, a steeper increase in the exacerbation rate with a high SABA canister collection was observed in the nonatopic group.
4. DISCUSSION
Overall, 45%, 32%, and 27% of patients with asthma aged 0–5, 6–11, and 12–17 years, respectively, collected ≥3 SABA canisters during the baseline year (considered overuse). SABA overuse was associated with an increased exacerbation risk by 35%, 22%, and 26% for 0–5‐, 6–11‐, and 12–17‐year‐olds, respectively. While this association was observed irrespective of atopic status at baseline, it was stronger among patients without comorbid atopic diseases.
SABAs have long been recommended for symptom relief in the management of childhood asthma. However, GINA no longer recommends as‐needed SABA monotherapy for patients ≥6 years, although monotherapy continues to be recommended for those under 5 years owing to lack of clinical data on an alternative reliever. 5 Instead, GINA recommends ICS‐formoterol as the preferred reliever for adolescents with mild asthma and for those with moderate‐to‐severe asthma prescribed ICS‐formoterol as maintenance and reliever therapy (MART). 4 In 6–11‐year‐olds, SABA is the only reliever option, except for those on MART, with a recommendation to use low‐dose ICS with as‐needed SABA at step 1. Adoption of updated GINA recommendations in clinical practice may reduce future SABA overuse. However, globally, discordance between local clinical recommendations and those of GINA may hinder progress.
High SABA use may also be ascribed to the reluctance of parents/caregivers of pediatric patients to use ICS, 16 likely due to safety concerns associated with ICS use in children. 32 Approximately three‐quarters of 6–11‐ and 12‐17‐year‐olds collected ICS‐containing medication at baseline. Although most patients were at treatment step 2 or 3, 66%–74% of patients across age groups collected only 0–2 canisters annually, indicating considerable ICS underuse. High SABA use may also be a consequence of poor response to ICS medications, particularly among patients with nonatopic disease. Children with eosinophilic airway inflammation, characteristic of atopic asthma, respond better to ICS treatment but not to leukotriene receptor antagonists (LTRAs), while nonatopic children demonstrate a varied response to ICS or LTRA treatment. 33 , 34
Our findings align with other studies that have shown an association between SABA overuse and exacerbations in children and adolescents. 23 The SABINA Junior UK study (manuscript submitted) also reported that prescriptions of ≥3 SABA canisters/year (vs. 0–2) were associated with higher exacerbation event rates across the three pediatric age groups. A study from the United States (US) demonstrated that children with commercial insurance and those with Medicaid using ≥3 SABA canisters/year (vs. 1–2) were at a greater risk of asthma‐related exacerbations requiring hospitalization, ER visits, or OCS treatment. 23 Although the study adopted the same SABA collection threshold, which is typically considered overuse in adults, 18 , 19 it is unclear what threshold is clinically most relevant for childhood asthma. Notably, stockpiling of SABA canisters at kindergarten/school and at home may be commonly practiced by caregivers of children with asthma. This practice is recommended in the US 35 and UK 36 and is likely common in Sweden. There was some variation in the overuse threshold based on the severity of asthma exacerbations, as well as across age groups, when SABA was examined as a continuous variable. Our findings indicate that for severe exacerbations necessitating hospitalization or ER visits, a higher SABA overuse threshold may be more clinically relevant.
No other studies besides ours and SABINA Junior UK, conducted in parallel, have evaluated the interaction of atopic disease and SABA use on exacerbation risk in children. We observed that although comorbid atopic disease per se puts patients at a greater initial risk of exacerbations in our prediction model, increased SABA canister collection was associated with a greater rate of risk increase in nonatopic (vs. atopic) patients with asthma. Pediatric cohorts have established a link between allergic comorbidity and asthma exacerbations, 8 , 37 with our findings suggesting that exacerbation risk by SABA collection varies based on atopic phenotype. We hypothesize that pediatric patients with asthma and atopic disease may be monitored carefully by healthcare providers due to a more persistent and overt phenotype. 8 , 37 Thus, after an asthma exacerbation, they may receive appropriate treatment and remain at lower risk of recurrent episodes. This may be coupled with a poorer response to ICS among children with nonatopic disease. 33 , 34 Indeed, certain nonatopic asthma phenotypes (e.g., asthma with childhood obesity 38 , 39 , 40 ) are often resistant to anti‐inflammatory therapies, such as ICS. 41 These patients may therefore receive less anti‐inflammatory protection against an exacerbation and may also be more likely to rely on their SABA inhalers, further compounding the problem.
Reducing SABA overuse in children requires the medical community to address the underlying factors contributing to such behavior and provide alternative reliever options to patients. 42 Proper communication, education, and training on correct inhaler technique, and medication adherence are essential to gain the most benefit from maintenance therapies. Studies on anti‐inflammatory reliever therapy in pediatric patients with mild asthma, such as the Children's Anti‐inflammatory REliever study, 43 are limited. Clinical trials, especially those that investigate the impact of asthma phenotypes such as presence of atopy on treatment and outcomes, are needed to bridge the evidence gap for asthma management in children across age groups and asthma severities.
Our study adds to the limited data on the extent of SABA use, its association with exacerbations, and influence of atopic comorbidities among children with asthma. Owing to the presence of transient phenotypes, results were presented for 1‐year follow‐up; however, long‐term results for 3 years of follow‐up demonstrated similar trends. The nationwide reach of this study reduces potential selection bias and ensures generalizability of results. Moreover, the validity of the Swedish NPR and Prescribed Drug Register is high for ICD‐10 and Anatomical Therapeutic Chemical (ATC) asthma codes. 44
This study has some limitations. The analysis for children aged 0–5 years was exploratory due to possible misclassification of asthma diagnoses in these children. Further, registry‐based data reporting pharmacy collections may not reflect actual medication use, and stockpiling of canisters is a possibility, contributing to potential overestimation of actual SABA use in children. As registers lack clinical data on objective asthma severity measures or lung function, influence from such factors cannot be discounted. Other potential confounders include inhaler technique, second‐hand smoke, obesity, and pollutants. The association analysis was not adjusted for ICS medication adherence, which may differ from SABA reliance. However, analyses were adjusted for disease severity using treatment step and prior exacerbations. Atopy was defined solely by the presence of comorbid atopic diseases without using any standardized measures of allergic sensitization to classify atopic and nonatopic asthma. Additionally, only patients in secondary care (with ≥1 hospital visit) were included, although prescriptions dispensed from primary care were included.
In conclusion, a large proportion of Swedish children with asthma aged 0–17 years collected ≥3 SABA canisters/year, which was associated with a significant risk of exacerbations and was stronger in children without atopic diseases. These results emphasize the need for educational initiatives targeted at pediatric patients, parents/caregivers, and clinicians, with a principal focus on aligning treatment of childhood asthma with updated global evidence‐based treatment recommendations.
AUTHOR CONTRIBUTIONS
Erik Melén: Conceptualization (equal); Formal analysis (supporting); Investigation (lead); Methodology (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Bright I. Nwaru: Conceptualization (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Fredrik Wiklund: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (equal); Resources (equal); Software (lead); Supervision (equal); Validation (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Sofie de Fine Licht: Conceptualization (equal); Investigation (equal); Methodology (equal); Project Administration (supporting); Resources (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Gunilla Telg: Conceptualization (equal); Data curation (lead); Formal analysis (supporting); Funding acquisition (lead); Investigation (equal); Methodology (equal); Project Administration (lead); Resources (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Ekaterina Maslova: Conceptualization (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Ralf J. P. van der Valk: Conceptualization (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Trung N. Tran: Conceptualization (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Magnus Ekström: Conceptualization (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing. Christer Janson: Conceptualization (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing ‐ original draft (equal); Writing ‐ review and editing.
FUNDING INFORMATION
This study was funded by AstraZeneca. Dr. Melén is supported by grants from the Swedish Research Council, the Swedish Heart‐Lung Foundation, and Region Stockholm (ALF). AstraZeneca funded the SABINA studies and was involved in designing the program, developing the study protocol, conducting the studies, and performing the analyses. AstraZeneca was given the opportunity to review the manuscript before submission.
CONFLICT OF INTEREST
Dr. de Fine Licht, Mrs. Telg, Dr Maslova, Dr. van der Valk, and Dr. Tran are full‐time employees of AstraZeneca. Mrs. Telg and Dr. Maslova own shares in AstraZeneca. Dr. van der Valk owns shares in AstraZeneca and GlaxoSmithKline. Dr. Melén has received personal fees from AstraZeneca, Chiesi, Novartis, and Sanofi outside the submitted work. Drs. Nwaru and Ekström report no conflicts of interest relevant to this work. Dr. Janson has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline PLC, Novartis, and Teva outside the submitted work. Dr. Wiklund is an employee of Statisticon, of which AstraZeneca is a client. The authors have no other conflicts of interests to declare.
DATA AVALIBILITY STATEMENT
The dataset is still subject to further analyses but will continue to be held and managed by the Department of Public Health and Caring Sciences, Uppsala University, Uppsala, Sweden. Relevant anonymized patient‐level data are available on reasonable request from the authors.
ETHICAL APPROVAL
The study protocol was approved by the Stockholm Regional Ethics Committee (registration number: 2017/4:2) and conducted in compliance with the Declaration of Helsinki. In Sweden, patients need not provide consent for use of public register data.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13885.
Supporting information
AppendixS1
ACKNOWLEDGEMENTS
Medical writing and editorial support were provided by Michelle Rebello, PhD, and Tejaswini Subbannayya, PhD, of Cactus Life Sciences (part of Cactus Communications), Mumbai, India, in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and funded by AstraZeneca.
Melén E, Nwaru BI, Wiklund F, et al. Short‐acting β2‐agonist use and asthma exacerbations in Swedish children: A SABINA Junior study. Pediatr Allergy Immunol. 2022;33:e13885. doi: 10.1111/pai.13885
Associate Editor: Ömer Kalayci
REFERENCES
- 1. Asher MI, Rutter CE, Bissell K, et al. Worldwide trends in the burden of asthma symptoms in school‐aged children: global asthma network phase I cross‐sectional study. Lancet. 2021;398(10311):1569‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hicke‐Roberts A, Aberg N, Wennergren G, Hesselmar B. Allergic rhinoconjunctivitis continued to increase in Swedish children up to 2007, but asthma and eczema levelled off from 1991. Acta Paediatr. 2017;106(1):75‐80. [DOI] [PubMed] [Google Scholar]
- 3. Stridsman C, Backman H, Eklund BM, Ronmark E, Hedman L. Adolescent girls with asthma have worse asthma control and health‐related quality of life than boys‐a population based study. Pediatr Pulmonol. 2017;52(7):866‐872. [DOI] [PubMed] [Google Scholar]
- 4. Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention. 2022. https://ginasthma.org/wp‐content/uploads/2022/05/GINA‐Main‐Report‐2022‐FINAL‐22‐05‐03‐WMS.pdf Accessed September 29, 2022.
- 5. Ödling M, Wang G, Andersson N, et al. Characterization of asthma trajectories from infancy to young adulthood. J Allergy Clin Immunol Pract. 2021;9(6):2368‐2376.e3. [DOI] [PubMed] [Google Scholar]
- 6. Savenije OE, Granell R, Caudri D, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011;127(6):1505‐1512.e14. [DOI] [PubMed] [Google Scholar]
- 7. Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid‐childhood. Thorax. 2008;63(11):974‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arabkhazaeli A, Vijverberg SJ, van Erp FC, Raaijmakers JA, van der Ent CK, Maitland van der Zee AH. Characteristics and severity of asthma in children with and without atopic conditions: a cross‐sectional study. BMC Pediatr. 2015;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garden FL, Simpson JM, Mellis CM, Marks GB, Investigators C. Change in the manifestations of asthma and asthma‐related traits in childhood: a latent transition analysis. Eur Respir J. 2016;47(2):499‐509. [DOI] [PubMed] [Google Scholar]
- 10. Jackson DJ, Gern JE, Lemanske RF Jr. Lessons learned from birth cohort studies conducted in diverse environments. J Allergy Clin Immunol. 2017;139(2):379‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mallah N, Rodriguez‐Segade S, Gonzalez‐Barcala FJ, Takkouche B. Blood eosinophil count as predictor of asthma exacerbation.A meta‐Analysis. Pediatr Allergy Immunol. 2021;32(3):465‐478. [DOI] [PubMed] [Google Scholar]
- 12. Ferrante G, La Grutta S. The burden of pediatric asthma. Front Pediatr. 2018;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engelkes M, Baan EJ, de Ridder MAJ, et al. Incidence, risk factors and re‐exacerbation rate of severe asthma exacerbations in a multinational, multidatabase pediatric cohort study. Pediatr Allergy Immunol. 2020;31(5):496‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chipps BE, Haselkorn T, Rosén K, Mink DR, Trzaskoma BL, Luskin AT. Asthma exacerbations and triggers in children in TENOR: impact on quality of life. J Allergy Clin Immunol Pract. 2018;6(1):169‐176.e2. [DOI] [PubMed] [Google Scholar]
- 15. Moonie SA, Sterling DA, Figgs L, Castro M. Asthma status and severity affects missed school days. J Sch Health. 2006;76(1):18‐24. [DOI] [PubMed] [Google Scholar]
- 16. Szefler SJ, Chipps B. Challenges in the treatment of asthma in children and adolescents. Ann Allergy Asthma Immunol. 2018;120(4):382‐388. [DOI] [PubMed] [Google Scholar]
- 17. Janson C, Menzies‐Gow A, Nan C, et al. SABINA: an overview of short‐acting β2‐agonist use in asthma in European countries. Adv Ther. 2020;37(3):1124‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bloom CI, Cabrera C, Arnetorp S, et al. Asthma‐related health outcomes associated with short‐acting β2‐agonist inhaler use: an observational UK study as part of the SABINA global program. Adv Ther. 2020;37(10):4190‐4208. [DOI] [PubMed] [Google Scholar]
- 19. Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short‐acting β2‐agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butz AM, Ogborn J, Mudd S, et al. Factors associated with high short‐acting β2‐agonist use in urban children with asthma. Ann Allergy Asthma Immunol. 2015;114(5):385‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasselgren M, Gustafsson D, Stallberg B, Lisspers K, Johansson G. Management, asthma control and quality of life in Swedish adolescents with asthma. Acta Paediatr. 2005;94(6):682‐688. [DOI] [PubMed] [Google Scholar]
- 22. Kivistö JE, Karjalainen J, Huhtala H, Protudjer JLP. The use of short‐acting beta‐2 adrenergic receptor agonists for asthma increased among Finnish and Swedish children from 2006 to 2017. Acta Paediatr. 2020;109(8):1620‐1626. [DOI] [PubMed] [Google Scholar]
- 23. Stanford RH, Shah MB, D'Souza AO, Dhamane AD, Schatz M. Short‐acting β‐agonist use and its ability to predict future asthma‐related outcomes. Ann Allergy Asthma Immunol. 2012;109(6):403‐407. [DOI] [PubMed] [Google Scholar]
- 24. Van Ganse E, Texier N, Dima AL, et al. Effects of short‐ and long‐acting beta‐agonists on asthma exacerbations: a prospective cohort. Ann Allergy Asthma Immunol. 2020;124(3):254‐260. [DOI] [PubMed] [Google Scholar]
- 25. Wang CY, Lai CC, Wang YH, Wang HC. The prevalence and outcome of short‐acting β2‐agonists overuse in asthma patients in Taiwan. NPJ Prim Care Respir Med. 2021;31(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicholls SG, Quach P, von Elm E, et al. The reporting of studies conducted using observational routinely‐collected health data (RECORD) statement: methods for arriving at consensus and developing reporting guidelines. PLoS One. 2015;10(5):e0125620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85(11):867‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Global Initiative for Asthma (GINA) . 2018. Global strategy for asthma management and prevention. https://ginasthma.org/wp‐content/uploads/2019/01/2018‐GINA.pdf Accessed September 29, 2022.
- 29. Perperoglou A, Sauerbrei W, Abrahamowicz M, Schmid M. A review of spline function procedures in R. BMC Med Res Methodol. 2019;19(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer Cham; 2016. [Google Scholar]
- 31. Emmeans LR. Estimated Marginal Means, aka Least‐Square Means. 2020. R package version 1.4.4. https://CRAN.R‐project.org/package=emmeans Accessed September 29, 2022.
- 32. Ye Q, He XO, D'Urzo A. A review on the safety and efficacy of inhaled corticosteroids in the management of asthma. Pulm Ther. 2017;3:1‐18. [Google Scholar]
- 33. Rabinovitch N, Mauger DT, Reisdorph N, et al. Predictors of asthma control and lung function responsiveness to step 3 therapy in children with uncontrolled asthma. J Allergy Clin Immunol. 2014;133(2):350‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within‐subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115(2):233‐242. [DOI] [PubMed] [Google Scholar]
- 35. American Lung Association . Asthma in schools: The basics for parents. https://www.lung.org/lung‐health‐diseases/lung‐disease‐lookup/asthma/living‐with‐asthma/creating‐asthma‐friendly‐environments/asthma‐in‐schools#:~:text=Keep%20asthma%20well%20controlled%20by,including%20an%20annual%20flu%20shot. Accessed September 29, 2022.
- 36. Asthma and Lung UK . Your child's asthma action plan. https://www.asthma.org.uk/advice/child/manage/action‐plan/ Accessed September 29, 2022.
- 37. Friedlander JL, Sheehan WJ, Baxi SN, et al. Food allergy and increased asthma morbidity in a school‐based Inner‐City asthma study. J Allergy Clin Immunol Pract. 2013;1(5):479‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE, North west adelaide health study . Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol. 2006;118(6):1284‐1291. [DOI] [PubMed] [Google Scholar]
- 39. Musaad SM, Patterson T, Ericksen M, et al. Comparison of anthropometric measures of obesity in childhood allergic asthma: central obesity is most relevant. J Allergy Clin Immunol. 2009;123(6):1321‐1327 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and nutrition examination survey 1999‐2006. J Asthma. 2010;47(7):822‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santamaria F, Borrelli M, Baraldi E. GINA 2021: the missing pieces in the childhood asthma puzzle. Lancet Respir Med. 2021;9(10):e98. [DOI] [PubMed] [Google Scholar]
- 43. Hatter L, Bruce P, Holliday M, et al. The Children's anti‐inflammatory reliever (CARE) study: a protocol for a randomised controlled trial of budesonide‐formoterol as sole reliever therapy in children with mild asthma. ERJ Open Res. 2021;7(4):002712021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Örtqvist AK, Lundholm C, Wettermark B, Ludvigsson JF, Ye W, Almqvist C. Validation of asthma and eczema in population‐based Swedish drug and patient registers. Pharmacoepidemiol Drug Saf. 2013;22(8):850‐860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1


