Abstract
Calprotectin, a heterodimer of MRP8 and MRP14 with antimicrobial properties, is found in the cytosol of neutrophils, monocytes, and human gingival keratinocytes. During inflammation of the oral mucosa, the expression of immunoreactive calprotectin appears upregulated. Given the possible cell sources, we sought to learn if epithelial cells upregulate calprotectin in response to proinflammmatory agents. First, human gingival keratinocytes were maintained in primary culture until senescence. At each passage, cells were harvested and analyzed for quantitative expression of MRP8 and MRP14 subunit mRNA by RNase protection assays and calprotectin complex by enzyme-linked immunosorbent assay. Calprotectin expression was constitutive in the primary gingival keratinocytes, but calprotectin-specific mRNA and protein tended to increase as the cells neared senescence. To test whether calprotectin expression was inducible, immortalized gingival keratinocyte cultures were treated for 2 to 4 h with lipopolysaccharide (LPS) or interleukin-1β (IL-1β). As a positive control for inducible expression, immortalized keratinocytes were incubated with phorbol myristate acetate (PMA) (50 ng/ml) for 24 h. Incubation with PMA stimulated increased expression of MRP8 and MRP14 mRNA within 2 h, peaking within 5 h. MRP8- and MRP14-specific mRNA expression by immortalized keratinocytes appeared to be unaffected by LPS or IL-1β. In contrast, LPS, IL-1β, and PMA each upregulated IL-8. These data show that calprotectin mRNA is expressed constitutively in cultured keratinocytes, while expression by immortalized cells appears to be independent of the exogenous proinflammatory agents LPS and IL-1β.
Calprotectin appears to be constitutively expressed by squamous mucosal epithelia but is expressed by normal epidermis only in certain inflammatory skin diseases. During inflammation of the skin and mucosa, keratinocytes can actively contribute to immunological events that occur by expressing class II major histocompatibility complex (MHC) molecules (2), cell adhesion molecules (4, 14), and various cytokines (22, 40). Concomitantly, calprotectin is induced in skin and may be upregulated in mucosal epithelium as detected immunohistochemically. A major constituent in the cytoplasm of granulocytes and monocytes, calprotectin constitutes up to 45 to 60% of the total cytosolic protein in neutrophils (9, 13) but is absent in resident tissue macrophages. The biological functions of the calprotectin complex are poorly defined.
Calprotectin is a heterodimer of two noncovalently associated polypeptides, MRP8 and MRP14 (10.8 and 14 kDa, respectively). The calprotectin heterodimer is anionic and calcium binding. MRP8-MRP14 (31) synonyms include cystic fibrosis antigen (8), L1 antigen (1, 7), calgranulin A and B (42), and S100A8 and S100A9 (36, 43). MRP8 and MRP14 belong to the S100 protein family, whose members are involved in cell cycle progression, cell differentiation, and cytoskeletal membrane interactions with the plasma membrane (20). MRP14 phosphorylates in both monocytes and neutrophils (9). The phosphorylated amino acid (Thr-113) is within a domain with a sequence identical to neutrophil immobilizing factor (41; P. Freemont, N. Hogg, and J. Edgeworth, Letter, Nature 339:516, 1989), suggesting that calprotectin may be important in localization and adherence of myeloid cells during inflammation. Calprotectin complex, but not its subunits, has been shown to inhibit the activity of casein kinases I and II and protein kinase-mediated stimulation of RNA polymerase (25, 27), suggesting a role for the complex in gene regulation and cell differentiation.
Calprotectin also shows antimicrobial activity in vitro against the periodontopathic bacterium Capnocytophaga sputigena, as well as Candida strains, Escherichia coli, Staphylococcus aureus, and S. epidermidis (24, 28, 38, 39). Suprabasal oral gingiva shows constant expression of calprotectin detected by 27E10, a monoclonal antibody that detects the complex form of calprotectin (5, 37). In periodontitis, gingivitis, and other oral mucosal inflammatory diseases, the expression of immunoreactive calprotectin increases in the gingival epithelium (11, 37). With periodontitis, calprotectin in the gingival transudate (crevicular fluid) increases in concentration directly with the severity of the lesions (18). Yet the cellular source(s) of immunoreactive calprotectin in the gingiva during periodontitis and other mucosal inflammatory states is unclear. Increased tissue calprotectin may be produced by neutrophils during infiltration and transmigration through the mucosal epithelium. Similarly, gingival keratinocytes may increase expression in response to proinflammatory molecules. In this study, we tested the influence of the proinflammatory agents lipopolysaccharide (LPS) and interleukin-1β (IL-1β) on the expression of MRP8 and MRP14 mRNA in human gingival keratinocytes.
MATERIALS AND METHODS
Cell culture and RNA isolation.
Using an informed consent protocol that was reviewed and approved by the Human Subjects Institutional Review Board of the University of Minnesota, healthy human gingiva was excised from sites overlying impacted third molar teeth. Gingival keratinocytes were isolated and cultured by the method of Oda and Watson (30) and passaged in KGM (Clonetics; BioWhittaker, Inc., Walkersville, Md.). The cells were propagated through successive passages to senescence. Cells were plated at 5,000 cells/cm2 in T75 flasks and grown until the epithelial layer reached approximately 70% confluency. Cells were then harvested by trypsinization and seeded equally into two new T75 flasks. One flask was used for analysis of calprotectin mRNA and protein antigen expression, while the other flask was used for further propagation of the cell line.
Immortalized human gingival keratinocytes (a gift from R. Lamont and D. Oda, University of Washington) were passaged in Keratinocyte SFM (Gibco-BRL, Grand Island, N.Y.) containing 50 U of penicillin and 50 μg of streptomycin per ml. Immortalized keratinocytes were stimulated by incubation with lipopolysaccharide from E. coli O55:B5 (Sigma, St. Louis, Mo.), phorbol 12-myristate 13-acetate (PMA; Sigma) or recombinant human IL-1β (R&D Systems, Inc., St. Paul, Minn.). Total RNA was extracted from keratinocytes using TRIzol (Gibco-BRL).
Immunohistochemistry.
Human gingival keratinocytes and immortalized human gingival keratinocytes were grown overnight on coverslips, and then cells were washed once with Dulbecco phosphate-buffered saline and fixed with 4% paraformaldehyde for 10 min. The cells were then washed, permeabilized with 0.5% Triton X-100 for 2 min, washed again, and subsequently incubated with 27E10 monoclonal antibody or biotinylated 27E10 (Bachem Bioscience, King of Prussia, Pa.) which was diluted 1:50 in 3% bovine serum albumin in PBS. Cells were incubated overnight with antibody at 4°C, washed, and then incubated for 2 h with either goat anti-mouse Cy3 (Jackson Laboratories, Inc., West Grove, Pa.) or Alexa350-conjugated streptavidin (diluted 1:200; Molecular Probes, Eugene, Oreg.). Cells were mounted with Fluoromount G (Southern Biotechnology, Birmingham, Ala.), examined with a Nikon Eclipse epifluorescence microscope, and photographed using a Spot Camera and software (Diagnostic Instruments, Inc., Sterling Heights, Mich.).
Cytosol extraction and ELISA.
Cytosol was released from keratinocytes using digitonin according to the method of Bakouche et al. (3). In brief, keratinocytes were suspended in 500 μl of sucrose-KCl medium (125 mM sucrose, 60 mM KCl, 3 mM K-HEPES; pH 7.1) containing 0.02% digitonin (Sigma), incubated on ice for 5 min, and pelleted by centrifugation at 200 × g for 5 min. The cytosol-containing supernatant was assayed for protein concentration by the BCA Assay (Pierce, Rockford, Ill.). MRP8, MRP14, and MRP8-MRP14 were analyzed in triplicate using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Bachem Bioscience) which detects MRP antigens by a sandwich ELISA.
Labeled RNA probe preparation.
Templates for RNA probe synthesis were generated by PCR from cDNA fragments of MRP8, MRP14, IL-8, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cloned into pCR-Script Vector (Stratagene, La Jolla, Calif.). The radiolabeled probes were synthesized under the following reaction conditions: 500 μM concentrations each of rCTP, rGTP, and rATP, and 1 μM rUTP; 3 μM [α-32P]UTP (800 Ci/mmol, 10 mCi/ml) (DuPont NEN Research Products, Boston, Mass.); 0.5 μl of PCR template; 1 U of T7 or T3 RNA polymerase (Stratagene); 2 μl of transcription buffer (Stratagene); 40 U of RNase inhibitor (Promega, Madison, Wis.); and distilled H2O to a total volume of 10 μl. Nonradioactive UTP (1 μM; see above) was added to reduce premature termination of RNA transcripts. To lower the specific activity of the internal control GAPDH, 150 μM rUTP was added. The reaction mixture was incubated for 1 h at 40°C and then inactivated at 95°C for 2 min. After the mixture cooled to 37°C, 0.5 μl of RNase-free DNase (Promega) was added for an additional 15 min to digest the DNA template. The reaction mixture was diluted with an equal volume of gel loading buffer (Ambion, Austin, Tex.). Single-stranded RNA was then purified by gel electrophoresis in a 5% acrylamide–8 M urea gel. After exposure to autoradiographic film the full-length transcript was excised from the gel and eluted overnight in elution buffer (Ambion). Before gel electrophoresis and after elution of the labeled probe from the gel, 1-μl aliquots were sampled for scintillation counting, and the specific activity of the probe and yield of the reaction were determined.
RNase protection assay.
RNase protection assays were performed using the RPA II Kit (Ambion) according to the manufacturer's protocol. Briefly, each reaction contained 8 μg of total RNA, 8 × 104 cpm of the 32P-labeled probe (gene of interest), and 2.5 × 104 cpm of the housekeeping gene probe (GAPDH). Control reactions were performed simultaneously using 8 μg of yeast tRNA. After hybridization for 18 h at 42°C, single-stranded RNA was digested for 30 min at 37°C by using a mixture of RNase A and T1. Reaction products were separated on a 5% acrylamide–8 M urea gel and exposed to a storage phosphor screen (Molecular Dynamics, Sunnyvale, Calif.). The screen was scanned using a Storm 840 imager (Molecular Dynamics) in storage phosphor mode. Signal intensity was quantified using ImageQuant software.
RESULTS
Immunohistochemistry.
Human gingival keratinocytes and immortalized human gingival keratinocytes were grown overnight on coverslips. In both types of keratinocytes, calprotectin was detected with monoclonal antibody to 27E10, which specifically recognizes the complex of MRP8-MRP14 (5). Reactivity to the 27E10 epitope was seen in the cytoplasm with the strongest immunofluoresence detected in a perinuclear distribution (Fig. 1). A similar distribution of the 27E10 epitope was detected in both the immortalized and the human gingival keratinocytes.
FIG. 1.
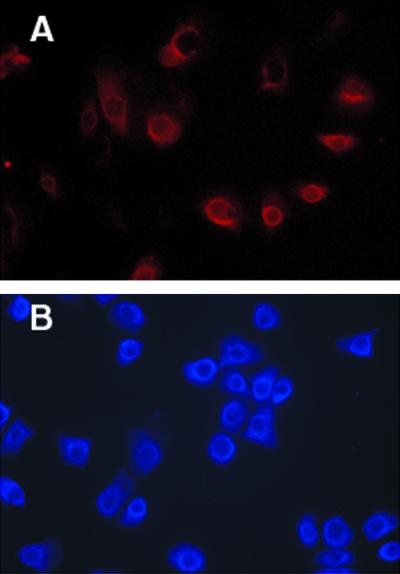
27E10 reactivity in cultured immortalized and human gingival keratinocytes. Gingival keratinocytes were fixed and permeabilized before reaction with the antibody 27E10 or biotinylated 27E10 against the MRP8-MRP14 complex. (A) Human gingival keratinocytes (27E10 secondary antibody Cy3). (B) Immortalized gingival keratinocytes (Alexa 350-conjugated streptavidin). Nonspecific immunoglobulin G controls showed no detectable fluoresence. Magnification, ×400.
Calprotectin protein antigen and mRNA expression by cultured gingival keratinocytes.
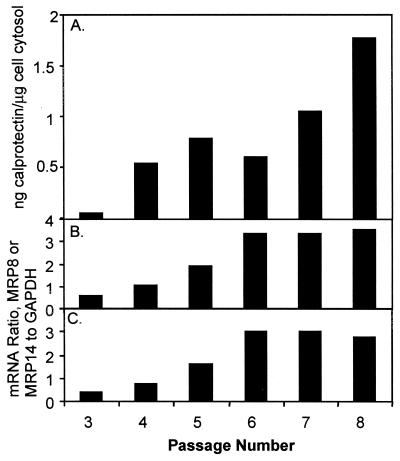
Healthy gingival keratinocytes were isolated from four individuals. Cells were cultured for successive passages through senescence. Cells survived an average of 9 passages (range, 6 to 16) before death. Keratinocytes harvested at each passage were analyzed for cytoplasmic MRP8 and MRP14 subunit antigens and MRP8-MRP14 complex by sandwich ELISA. The calprotectin complex was expressed at each passage and through cell death by each keratinocyte cell line (Fig. 2A). At later passages, expression of calprotectin tended to increase. At each passage, MRP8 and MRP14 were expressed below the detection limits of the ELISA. At each passage, mRNAs specific for MRP8 and MRP14 was detected by the RNase protection assay. Relative expression of MRP8- and MRP14-specific mRNA appeared to increase in successive passages to about threefold, after which expression appeared to plateau (Fig. 2B and C).
FIG. 2.
Expression of calprotectin in gingival keratinocytes. (A) Calprotectin complex (MRP8-MRP14) was detected in keratinocyte cytosol by sandwich ELISA. Results are representative of four experiments. (B and C) MRP8 (B) and MRP14 (C) mRNA in keratinocytes detected by RNase protection assay.
Expression of MRP8-, MRP14-, and IL-8-specific mRNAs by immortalized keratinocytes.
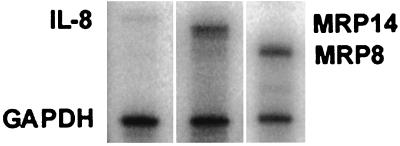
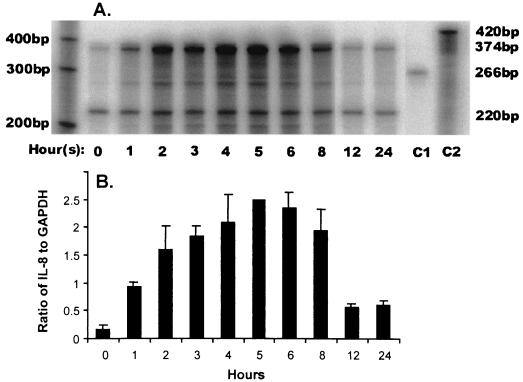
Immortalized keratinocytes expressed MRP8- and MRP14-specific mRNAs under normal culture conditions; IL-8 mRNA was expressed at relatively low levels (Fig. 3). Cells were incubated with 50 ng of PMA per ml for 24 h. Within 1 h, IL-8 mRNA expression was elevated, peaking with a 15-fold increase at 5 h (Fig. 4). After 5 h of incubation with PMA, IL-8 mRNA levels declined but remained significantly elevated from the baseline level through 12 to 24 h.
FIG. 3.
Constitutive expression of IL-8, MRP8, and MRP14 mRNA in immortalized keratinocytes detected by RNase protection assay. IL-8-, MRP8-, and MRP14-specific mRNA are represented by single protected fragments of 374, 282, and 333 bp, respectively. The internal control GAPDH included in each assay tube is represented by a band at 220 bp.
FIG. 4.
Regulation of IL-8 mRNA expression in immortalized human gingival keratinocytes. (A) A representative storage phosphor scan demonstrates that, by using quantitative RNase protection assay, moderate IL-8 mRNA expression was detected in 8 μg of total RNA from immortalized keratinocytes under normal control conditions as seen at time zero. Following treatment of separate flasks of the same culture over 24 h with 50 ng of PMA per ml, IL-8 mRNA expression dramatically increases over a 6-h period, returning to nearly constitutive levels by 12 h. Test lanes, hours 0 to 24, show the protected probes after binding to IL-8 (374 bp) and GAPDH (220 bp) mRNA, respectively, within the sample, followed by RNase treatment. An increase in the band intensity of GAPDH at 2 to 6 h reflects an increase in the total amount of mRNA, as expected with PMA stimulation. This did not affect quantitation (see panel B, below), since the ratio of GAPDH to IL-8 was still proportionally constant. Control lanes (C1 and C2) show the two probes GAPDH (C1, 266 bp) and IL-8 (C2, 420 bp) to which no RNase has been added. The results are representative of three experiments. (B) Quantitation of IL-8 mRNA in immortalized keratinocytes following induction with PMA. From panel A the signal intensity determined for the IL-8 protected fragment was normalized to the abundance of the internal control GAPDH at each time point. The data shown are the mean ± the standard deviation (n = 3).
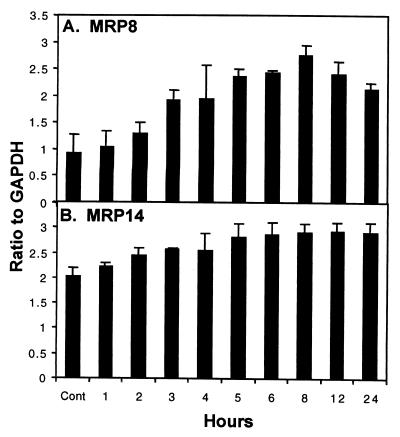
IL-8 mRNA expression was then measured in immortalized keratinocytes as a positive control for stimulation of cultured keratinocytes by PMA, LPS, and IL-1β. The same mRNA sample was then analyzed by quantitative RNase protection assay for the levels of MRP8- and MRP14-specific mRNAs. The expression of MRP8- and MRP14-specific mRNAs increased 3- and 1.5-fold above constitutive levels, respectively, upon treatment with 50 ng of PMA per ml (Fig. 5). Expression of both mRNAs appeared to maximize at about 8 h. Subsequently, MRP8 mRNA levels declined, while MRP14 mRNA remained elevated through 24 h.
FIG. 5.
Regulation of MRP8 and MRP14 mRNA expression in immortalized keratinocytes. Quantitation of MRP8-specific (A) and MRP14-specific (B) mRNA in immortalized keratinocytes following induction with 50 ng of PMA per ml for 24 h. The signal intensity determined for the MRP8 and MRP14 protected fragments was normalized to the abundance of the internal control GAPDH at each time point. The data shown are the mean ± the standard deviation (n = 3).
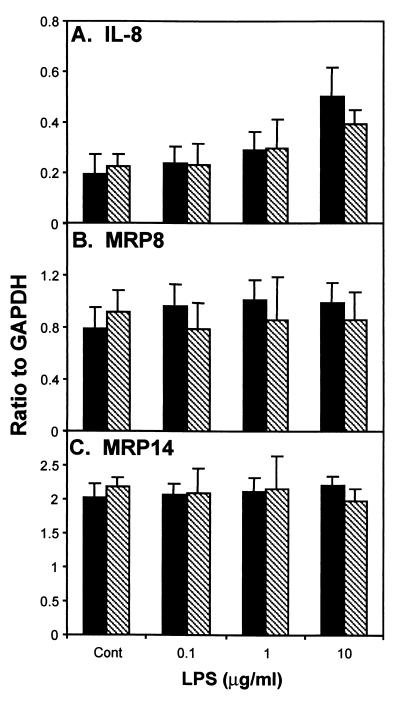
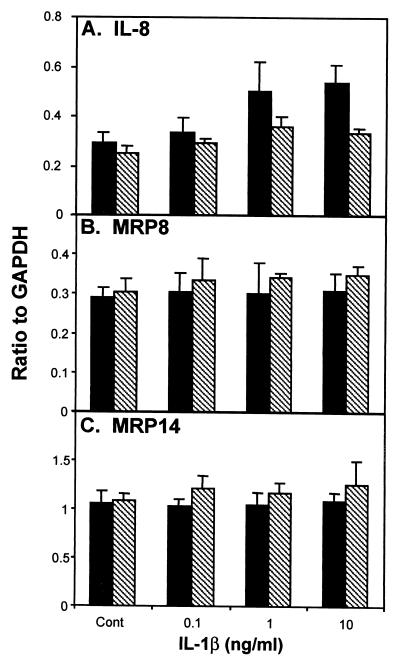
Treatment of the immortalized keratinocytes for 2 h with LPS (10 μg/ml) induced a 2.6-fold increase in the expression of IL-8 mRNA above that of the control (Fig. 6A). Incubation with IL-1β (10 ng/ml) for 2 h resulted in a 1.8-fold increase in IL-8 mRNA expression (Fig. 7A). After treatment with LPS (0.1, 1, and 10 μg/ml) or IL-1β (0.1, 1, and 10 ng/ml) for either 2 or 4 h, the expression levels of MRP8- and MRP14-specific mRNAs were similar to the constitutive levels obtained from untreated immortalized cultures (Fig. 6 and 7).
FIG. 6.
Treatment of immortalized keratinocytes with LPS (0.1, 1, and 10 μg/ml) for 2 or 4 h. Immortalized keratinocytes were incubated with various concentrations of LPS (0.1, 1, and 10 μg/ml) for 2 h (black bars) or 4 h (crosshatched bars). Expression of mRNA for IL-8-, MRP8-, and MRP14-specific mRNA was determined by RNase protection assay. The signal intensity for the protected fragments was normalized to the abundance of the internal control GAPDH. The data shown are the mean ± the standard deviation (n = 3).
FIG. 7.
Treatment of immortalized keratinocytes with IL-1β (0.1, 1, and 10 ng/ml) for 2 or 4 h. Immortalized keratinocytes were incubated with various concentrations of IL-1β (0.1, 1, and 10 ng/ml) for 2 h (black bars) or 4 h (crosshatched bars). Expression of mRNA for IL-8-, MRP8-, and MRP14-specific mRNA was determined by RNase protection assay. The signal intensity for the protected fragments was normalized to the abundance of the internal control GAPDH. The data shown are the mean ± the standard deviation (n = 3).
DISCUSSION
In cultured keratinocytes derived from oral epithelial mucosa, the data show that calprotectin is expressed constitutively. Normal human gingival keratinocytes in culture express mRNAs specific for the calprotectin subunits MRP8 (S100A8) and MRP14 (S100A9). When expressed constitutively, immunoreactive calprotectin localizes primarily in the cytoplasm, with a higher density in the perinuclear region. The calprotectin was recovered from keratinocyte cytoplasm exclusively as heterodimeric complexes of MRP8 and MRP14. Free or unassociated MRP8 and MRP14 proteins were virtually undetectable by ELISA.
During successive passages, MRP8- and MRP14-specific mRNAs appear to increase in association with a cytoplasmic elevation in immunoreactive calprotectin. As the passage number increases, keratinocytes from patients with cystic fibrosis show a higher turnover rate than the normal controls (17). Detection of immunoreactive MRP14 (cystic fibrosis antigen) increases as keratinocytes from both sources age in culture. Based on pulse-chase experiments, the increase in calprotectin reflects the rate of synthesis, and the rate of degradation and secretion does not change (17). Consistent with these observations, the abundance of calprotectin-specific mRNAs plateaus, and immunoreactive calprotectin levels continue to increase as gingival keratinocytes approach senescence.
In the gingiva and other squamous epithelia, calprotectin localizes to the spinous and subcorneal layers of the stratum spinosum, but it is not seen in the basal or cornified cell layers (10). Immature basal cells and mature cornified cells do not express calprotectin. Indeed, the cornified cells may actually release calprotectin without replenishment by the cell. Since monolayer keratinocyte cultures do not cornify, senescence may modulate expression of calprotectin-specific messages. When grown in serum-free media, gingival keratinocytes express keratins that are typically associated with basal cells (29), yet insufficient information is available to stage the cells in culture to model the maturation of certain epithelial layers. Exposure of epidermal keratinocytes to PMA promotes keratinocyte differentiation (15). Indeed, terminal differentiation of various epithelial cell lines was linked to the expression of calprotectin (35). Therefore, calprotectin expression by epidermal and mucosal keratinocytes may be controlled in association with cellular maturation.
During mucosal inflammation, the epithelial turnover rate increases (32). Higher levels of immunoreactive calprotectin are seen in affected epithelia and in transudative fluids that bathe these tissues (11, 18, 37). In addition to keratinocytes, inflamed mucosal epithelia contain several other cell sources of calprotectin. Calprotectin is expressed by transmigrating neutrophils and monocytes, which increase in number with inflammation. Hence, we tested the influence of the proinflammatory agents LPS and IL-1β on the expression of MRP8 and MRP14 mRNA in human gingival keratinocytes.
To test for the regulation of calprotectin expression by LPS and IL-1β, immortalized gingival epithelial cells were used as surrogates for normal cells in culture. Culture of normal gingival keratinocytes is labor-intensive, with low yields and donor-dependent heterogeneity. The immortalized gingival epithelial cells used in this study were developed from human gingival keratinocytes that had been transformed with human papillomavirus type 16 E6/E7 open reading frames (16). These cells express E- and P-cadherins and show contact-dependent growth inhibition (16). Like normal cells, immortalized keratinocytes were shown to express MRP8 and MRP14 mRNA and immunoreactive calprotectin complex, albeit at lower levels (data not shown). Similarly, normal and immortalized cells constitutively expressed IL-8. Collectively, these data showed that the immortalized keratinocytes were suitable surrogates for normal human gingival keratinocytes.
In immortalized gingival keratinocytes, the expression of MRP8-, MRP14-, and IL-8-specific mRNAs increased in the presence of PMA, a result consistent with the activation of protein kinase C. Expression appears to be tightly regulated since the level of calprotectin upregulation by PMA was significant but modest compared to IL-8. In contrast, incubation with LPS and IL-1β only increased the expression of IL-8. Cells were also incubated with 1 μg of LPS per ml over a 24-h period to learn if an increase might require longer exposure. No change in the expression of MRP8 or MRP14 was observed (data not shown). These data suggest that LPS and IL-1β do not exclusively regulate calprotectin expression. The presence of LPS and IL-1β in inflamed mucosal epithelia may be insufficient to explain the apparent increase in the expression of calprotectin in keratinocytes and the interstitial tissues.
Release of calprotectin into the interstitial tissues from keratinocytes is likely to involve a Golgi-independent process. Calprotectin lacks a signal peptide, membrane anchor, or N-linked glycosylation sequence, suggesting that the protein complex is neither targeted or packaged for export nor routed to the exterior as an integral membrane protein or glycoprotein to be proteolytically cleaved and released into the external environment. In monocytes, calprotectin is released after activation of protein kinase C by a tubulin-dependent mechanism that is Golgi independent (33). In neutrophils, calprotectin is released in complex with arachidonic acid (17, 19). Similar mechanisms could stimulate release from epithelial cells, since PMA, which activates protein kinase C, upregulated the expression of calprotectin in immortalized gingival keratinocytes.
Healthy human gingiva expresses calprotectin strictly in the suprabasal cells, as suggested by histopathological studies (10, 11, 37). If released by keratinocytes, immunoreactive calprotectin in the tissues would be expected to increase in proximity to the suprabasal layer of cells in the stratum spinosum. As inflammation increases, calprotectin does appear to be expressed by cells throughout the stratum spinosum, within and proximal to the keratinocytes. While IL-1β and LPS would explain the upregulation of IL-8 by epithelial cells in inflamed tissues, these signals and mediators appear insufficient to explain the upregulation of calprotectin.
Calprotectin expression in squamous mucosal epithelium differs from that in skin and intestinal epithelia. Normal skin and intestinal epithelia do not express calprotectin. During inflammation, calprotectin expression is upregulated in the epidermis and intestinal epithelia (21, 23). In inflammatory dermatoses of the skin, calprotectin expression requires de novo mRNA transcription. For example, MRP8 and MRP14 are expressed and protein kinase C is activated in psoriatic epithelial cells (34). In the inflammatory skin diseases lichen planus, lupus erthematosus, and psoriasis vulgaris, 27E10 epitope expression increases, but independently of the MHC class II molecules and the adhesion molecule, ICAM-1 (22). The MHC class II molecules and ICAM-1 are upregulated during epidermal inflammation but apparently in association with keratinocyte turnover. In contrast to gingival keratinocytes, cultured epidermis upregulates calprotectin in the absence of proinflammatory agents and without concomitant upregulation of MHC class II (6). Calprotectin expression is apparently tissue specific and appears to be regulated by a different mechanism than MHC class II molecules and ICAM-1.
Keratinocyte production of calprotectin may contribute to the antimicrobial barrier function and the innate immunity of mucosal epithelia. For example, calprotectin protein and mRNA are found in the upper and middle spinous cell layers in oral pseudomembranous candidiasis (12). Fungal hyphae penetrate the parakeratin layer but appear not to extend beyond the boundary of calprotectin expressed in the spinous layer. Epithelial cells transfected to express calprotectin become significantly resistant to invasion by key mucosal pathogens, including Listeria monocytogenes and salmonellae (K. Nisapakultorn, K. F. Ross, and M. C. Herzberg, unpublished results). In contrast, calprotectin-free superficial mucosal epithelial cells appear to harbor potentially pathogenic oral bacteria (34a). Therefore, constitutive expression of calprotectin may explain in part the relative resistance of the oral mucosa to infection and invasion by commensals, oral pathogens, and enteric pathogens in transit.
ACKNOWLEDGMENTS
We thank Kanakwan Nisapakultorn for the immunohistochemical staining of the gingival keratinocytes.
This work was supported by NIH grants P30DE09737 and RO1DE11831.
REFERENCES
- 1.Andersson K B, Sletten K, Berntzen H B, Dale I, Brandtzaeg P, Jellum E, Fagerhol M K. The leucocyte L1 protein: Identity with the cystic fibrosis antigen and the calcium-binding MRP-8 and MRP-14 macrophage components. Scand J Immunol. 1988;28:241–245. doi: 10.1111/j.1365-3083.1988.tb02437.x. [DOI] [PubMed] [Google Scholar]
- 2.Aubock J, Romani N, Grubauer G, Fritsch P. HLA-DR expression on keratinocytes is a common feature of diseased skin. Br J Dermatol. 1986;114:465–472. doi: 10.1111/j.1365-2133.1986.tb02851.x. [DOI] [PubMed] [Google Scholar]
- 3.Bakouche O, Brown D C, Lachman L B. Subcellular localization of human monocyte interleukin 1: evidence for an inactive precursor molecule and a possible mechanism for IL 1 release. J Immunol. 1987;138:4249–4255. [PubMed] [Google Scholar]
- 4.Barker J N, Mitra R S, Griffiths C E, Dixit V M, Nickoloff B J. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 5.Bhardwaj R S, Zotz C, Zwadlo-Klarwasser G, Roth J, Goebeler M, Mahnke K, Falk M, Meinardus-Hager G, Sorg C. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol. 1992;22:1891–1897. doi: 10.1002/eji.1830220732. [DOI] [PubMed] [Google Scholar]
- 6.Clark B R, Kelly S E, Fleming S. Calgranulin expression and association with the keratinocyte cytoskeleton. J Pathol. 1990;160:25–30. doi: 10.1002/path.1711600107. [DOI] [PubMed] [Google Scholar]
- 7.Dale I, Fagerhol M K, Naesgaard I. Purification and partial characterization of a highly immunogenic human leukocyte protein, the L1 antigen. Eur J Biochem. 1983;134:1–6. doi: 10.1111/j.1432-1033.1983.tb07522.x. [DOI] [PubMed] [Google Scholar]
- 8.Dorin J R, Novak M, Hill R E, Brock D J, Secher D S, van Heyningen V. A clue to the basic defect in cystic fibrosis from cloning the CF antigen gene. Nature. 1987;326:614–616. doi: 10.1038/326614a0. [DOI] [PubMed] [Google Scholar]
- 9.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;12:7706–7713. [PubMed] [Google Scholar]
- 10.Eversole L R, Miyasaki K T, Christensen R E. The distribution of the antimicrobial protein, calprotectin, in normal oral keratinocytes. Arch Oral Biol. 1992;37:963–968. doi: 10.1016/0003-9969(92)90068-j. [DOI] [PubMed] [Google Scholar]
- 11.Eversole L R, Miyasaki K T, Christensen R E. Keratinocyte expression of calprotectin in oral inflammatory mucosal diseases. J Oral Pathol Med. 1993;22:303–307. doi: 10.1111/j.1600-0714.1993.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 12.Eversole L R, Reichart P A, Ficarra G, Schmidt-Westhausen A, Romagnoli P, Pimpinelli N. Oral keratinocyte immune responses in HIV-associated candidiasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:372–380. doi: 10.1016/s1079-2104(97)90035-4. [DOI] [PubMed] [Google Scholar]
- 13.Fagerhol M K, Andersson K B, Naess-Andresen C-F, Brandtzaeg P, Dale I. Calprotectin (the L1 leukocyte protein) In: Smith V L, Dedman J R, editors. Stimulus response coupling: the role of intracellular calcium-binding proteins. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 187–210. [Google Scholar]
- 14.Griffiths C E, Voorhees J J, Nickoloff B J. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989;20:617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- 15.Hawley-Nelson P, Stanley J R, Schmidt J, Gullino M, Yuspa S H. The tumor promoter, 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp Cell Res. 1982;137:155–167. doi: 10.1016/0014-4827(82)90017-9. [DOI] [PubMed] [Google Scholar]
- 16.Kandikonda S, Oda D, Niederman R, Sorkin B C. Cadherin-mediated adhesion is required for normal growth regulation of human gingival epithelial cells. Cell Adhes Commun. 1996;4:13–24. doi: 10.3109/15419069609010760. [DOI] [PubMed] [Google Scholar]
- 17.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274:32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 18.Kido J, Nakamura T, Kido R, Ohishi K, Yamauchi N, Kataoka M, Nagata T. Calprotectin, a leukocyte protein related to inflammation, in gingival crevicular fluid. J Periodontal Res. 1998;33:434–437. doi: 10.1111/j.1600-0765.1998.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 19.Klempt M, Melkonyan H, Nacken W, Wiesmann D, Holtkemper U, Sorg C. The heterodimer of the Ca2+-binding proteins MRP8 and MRP14 binds to arachidonic acid. FEBS Lett. 1997;408:81–84. doi: 10.1016/s0014-5793(97)00394-3. [DOI] [PubMed] [Google Scholar]
- 20.Kligman D, Hilt D C. The S100 protein family. Trends Biochem Sci. 1988;13:437–443. doi: 10.1016/0968-0004(88)90218-6. [DOI] [PubMed] [Google Scholar]
- 21.Kunz M, Roth J, Sorg C, Kolde G. Epidermal expression of the calcium binding surface antigen 27E10 in inflammatory skin diseases. Arch Dermatol Res. 1992;284:386–390. doi: 10.1007/BF00372067. [DOI] [PubMed] [Google Scholar]
- 22.Kupper T S. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Investig Dermatol. 1990;94:146S–150S. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- 23.Lugering N, Stoll R, Kucharzik T, Schmid K W, Rohlmann G, Burmeister G, Sorg C, Domschke W. Immunohistochemical distribution and serum levels of the Ca2+-binding proteins MRP8, MRP14 and their heterodimeric form MRP8/14 in Crohn's disease. Digestion. 1995;56:406–414. doi: 10.1159/000201267. [DOI] [PubMed] [Google Scholar]
- 24.Miyasaki K T, Bodeau A L, Murthy A R K, Lehrer R I. In vitro antimicrobial activity of the human neutrophil cytosolic S-100 protein complex, calprotectin, against Capnocytophaga sputigena. J Dent Res. 1993;72:517–523. doi: 10.1177/00220345930720020801. [DOI] [PubMed] [Google Scholar]
- 25.Murao S. Review: two calcium-binding proteins, MRP8 and MRP14: a protein complex associated with neutrophil and monocyte activation. Acta Histochem Cytochem. 1994;27:107–116. [Google Scholar]
- 26.Murao S, Collart F, Huberman E. A protein complex expressed during terminal differentiation of monomyelocytic cells is an inhibitor of cell growth. Cell Growth Differ. 1990;1:447–454. [PubMed] [Google Scholar]
- 27.Murao S, Collart F R, Huberman E. A protein containing the cystic fibrosis antigen is an inhibitor of protein kinases. J Biol Chem. 1989;264:8356–8360. [PubMed] [Google Scholar]
- 28.Murthy A, Lerher R, Harwig S, Miyasaki K. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151:6291–6301. [PubMed] [Google Scholar]
- 29.Oda D, Dale B A, Bourekis G. Human oral epithelial cell culture. II. Keratin expression in fetal and adult gingival cells. In vitro Cell Dev Biol. 1990;26:596–603. doi: 10.1007/BF02624209. [DOI] [PubMed] [Google Scholar]
- 30.Oda D, Watson E. Human oral epithelial cell culture. I. Improved conditions for reproducible culture in serum-free medium. In Vitro Cell Dev Biol. 1990;26:589–595. doi: 10.1007/BF02624208. [DOI] [PubMed] [Google Scholar]
- 31.Odink K, Cerletti N, Brüggen J, Clerc R G, Tarcsay L, Zwaldo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 32.Page R C, Schroeder H E. Structure and pathogenesis. In: Schluger S, Yuodelis R A, Page R C, editors. Periodontal disease. Philadelphia, Pa: Lea & Febiger; 1977. pp. 168–195. [Google Scholar]
- 33.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen H H, Celis J E. Evidence for an altered protein kinase C (PKC) signaling pathway in psoriasis. J Investig Dermatol. 1993;101:560–566. doi: 10.1111/1523-1747.ep12365986. [DOI] [PubMed] [Google Scholar]
- 34a.Rudney J D, Chen R, Sedgewick G J. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect Immun. 2001;69:2700–2707. doi: 10.1128/IAI.69.4.2700-2707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saintigny G, Schmidt R, Shroot B, Juhlin L, Reichert U, Michel S. Differential expression of calgranulin A and B in various epithelial cell lines and reconstructed epidermis. J Investig Dermatol. 1992;99:639–644. doi: 10.1111/1523-1747.ep12668098. [DOI] [PubMed] [Google Scholar]
- 36.Schäfer B W, Wicki R, Engelkamp D, Mattei M-G, Heizmann C W. Isolation of a YAC clone covering a cluster of nine S100 genes on human chromosome 1q21: rationale for a new nomenclature of the S100 calcium-binding protein family. Genomics. 1995;25:638–643. doi: 10.1016/0888-7543(95)80005-7. [DOI] [PubMed] [Google Scholar]
- 37.Schlegel Gómez R, Langer P, Pelka M, Von den Driesch P, Johannessen A C, Simon M., Jr Variational expression of functionally different macrophage markers (27E10, 25F9, RM3/1) in normal gingiva and inflammatory periodontal disease. J Clin Periodontol. 1995;22:341–346. doi: 10.1111/j.1600-051x.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 38.Sohnle P G, Collins-Lech C, Wiessner J H. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis. 1991;163:187–192. doi: 10.1093/infdis/163.1.187. [DOI] [PubMed] [Google Scholar]
- 39.Steinbakk M, Naess-Andresen C-F, Lingaas E, Dale I, Brandtzaeg P, Fagerhol M K. Antimicrobial actions of calcium-binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 40.Stoof T J, Boorsma D M, Nickoloff B J. Keratinocytes and immunological cytokines. In: Leigh I, Lane B, Watt F, editors. The keratinocyte handbook. Cambridge, England: Cambridge University Press; 1994. pp. 365–399. [Google Scholar]
- 41.Watt K W, Brightman I L, Goetzl E J. Isolation of two polypeptides comprising the neutrophil-immobilizing factor of human leucocytes. Immunology. 1983;48:79–86. [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson M M, Busuttil A, Hayward C, Brock D J H, Dorin J R, van Heyningen V. Expression pattern of two related cystic fibrosis-associated calcium-binding proteins in normal and abnormal tissues. J Cell Sci. 1988;91:221–230. doi: 10.1242/jcs.91.2.221. [DOI] [PubMed] [Google Scholar]
- 43.Zimmer D B, Cornwall E H, Landar A, Song W. The S100 protein family: history, function, and expression. Brain Res Bull. 1995;37:417–429. doi: 10.1016/0361-9230(95)00040-2. [DOI] [PubMed] [Google Scholar]