Abstract
The NLRP3 inflammasome is a multiprotein binding compound comprising NLRP3, connector protein ASC, and effector protein pro-caspase-1. When the NLRP3 inflammasome senses a danger signal from the host or pathogen, activated caspase-1 cleaves the precursors of interleukin (IL)-1β and IL-18 into mature proinflammatory cytokines, simultaneously causing lysis via the pore-forming protein gasdermin D. This induction of cell inflammatory pyroptosis suggests that it is a key process in the innate immune response to pathogens or cellular stress. Recent studies have shown that NLRP3 inflammasome also plays an important role in regulating autoimmune liver diseases, including autoimmune hepatitis, primary biliary cholangitis, and primary sclerosclerotic cholangitis. In this review, we summarize the structure, activation and modulation of the NLRP3 inflammasome, highlight the progress in research on the role of NLRP3 inflammasome in the occurrence and development of autoimmune liver diseases, and discuss potential strategies for targeting the NLRP3 inflammasome in the treatment of autoimmune liver diseases.
Keywords: NLRP3 inflammasome, autoimmune liver diseases, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis
Introduction
Innate pathogen pattern recognition receptor (PRR) systems play a key role in primary and adaptive immune responses. PRRs directly recognize common antigenic determinants in almost all types of pathogens (also called pathogen-associated molecular patterns or PAMPs) and initiate specific intracellular signaling pathways to produce specific immune responses. Additionally, when cells respond to stress, they recognize endogenous ligands known as damage-related molecular patterns (DAMPs) [ 1, 2] . There are five PRR systems: Toll-like receptors (TLRs), retinoic acid-induced gene I (RIG-I)-like receptors, C-type lectin receptors, nucleotide-binding and oligomeric domain (NOD)-like receptors, and absent-in-melanoma (AIM) receptors. The NOD-like receptors (NLRs) comprise a family of cytoplasmic pattern recognition receptors [3] encoded by 22 and 34 genes in humans and mice, respectively [ 4, 5] , and are considered ancient cell sentinels that mediate protective immune responses triggered by PAMPs or DAMPs [6]. NLR proteins, which contain nucleotide-binding domains (NBDs) and leucine-rich repeats (LRRs) and are distinguishable by their N-terminal effector domains, include the NLRA, NLRB, NLRC, NLRP and NLRX subfamilies. The largest of these is the N-terminal pyrin domain (PYD) subfamily, which comprises NLRP1–NLRP14 [7]. These NLRs have a tripartite domain structure consisting of an N-terminal PYD domain, a central nucleotide-binding and oligomerization (NACHT) domain, and a C-terminal LRR domain [ 8, 9] , with two exceptions: NLRP1 has an additional C-terminal caspase activation and recruitment domain (CARD) [10], and NLRP10 lacks an LRR domain [11]. Some receptors in the NLR family can form inflammasome complexes by linking connector protein ASC and effector protein pro-caspase-1, which then activates caspase-1, resulting in the transformation of precursors of interleukin (IL)-1β and IL-18 into their mature forms. This list reportedly includes NLRP1, NLRP2, NLRP3, NLRP6, NLRP7, NLRP12 and NLRC4 [ 12– 17] . In addition to the NLR family, AIM2 and pyrin can constitute inflammasome structures and exert similar effects [ 18, 19] . Most of the remaining NLR proteins have been tested and shown not to form inflammasome complexes. These noninflammasome NLRs show diverse functions, mainly involving the reproductive system, early embryonic arrest [ 20– 22] , and regulation of adaptive immunity [ 23, 24] . The NLRP3 inflammasome is widely researched by scholars worldwide, with a massive number of published reports in recent years. The NLRP3 inflammasome is a large molecular complex comprising three parts: (1) NLRP3, (2) apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), and (3) pro-caspase-1, whose assembly is dependent on the protein interaction domain known as the death domain superfamily which includes PYD and CARD [25]. Activation of inflammasomes results in the hydrolysis and cleavage of inactive pro-caspase-1 to activate caspase-1. Activated caspase-1 allows for the cleavage of pro-IL-1β and pro-IL-18 into the active cytokines IL-1β and IL-18, respectively, and mediates a unique version of cell death involving DNA fragmentation, cell lysis, and inflammatory cytokine release that is known as “pyroptosis” [ 26– 28] .
Research has shown that NLRP3 inflammasome is strongly associated with the pathogenesis of a variety of diseases, including autoinflammatory diseases, autoimmune diseases, diabetes, gout and atherosclerosis [ 29– 33] . The NLRP3 inflammasome is also related to the occurrence, development and pathogenesis of autoimmune liver disease, but the specific mechanism remains unclear.
In this review, the activation and regulation pathways of inflammasomes, as well as the mechanism underlying the relationship between NLRP3 inflammasome and autoimmune liver disease, are systematically summarized. By exploring the possibility of NLRP3 inflammasome as a cost-effective drug target, we hope to improve the cure rate and alleviate the suffering of patients with autoimmune liver diseases.
Activation of the NLRP3 Inflammasome
Activation of the NLRP3 inflammasome is an important host defense mechanism against pathogen invasion. Because overactivation of the NLRP3 inflammasome can lead to inflammation, promote disease development, and damage tissue and organ functions, it is crucial that its activation be balanced in vivo. NLRP3 inflammasome activation occurs through both canonical and noncanonical pathways, as described below.
Canonical pathway
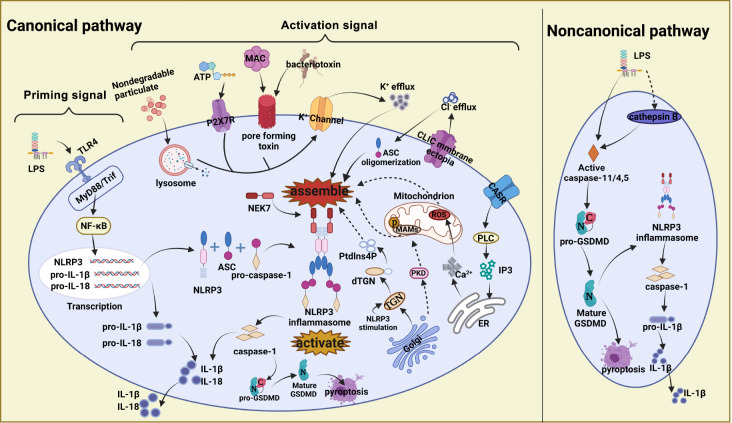
The canonical pathway of NLRP3 inflammasome activation includes a priming signal and an activation signal, as shown in Figure 1. Step one involves regulation of transcriptional and posttranscriptional expression levels under the priming signal. PRRs are critical in the priming process. TLR4 recognizes TLR ligands such as bacterial lipopolysaccharide (LPS) through extracellular domains, leading to activation of the transcription factor nuclear factor (NF)-κB pathway, which is dependent on the adaptor known as MyD88. In response to this initiation signal, transcription of the NLRP3, pro-IL-1β and pro-IL-18 genes begins [ 34, 35] .
Figure 1 .
Canonical and noncanonical pathways of NLRP3 inflammasome activation
The canonical pathway for activation of the NLRP3 inflammasome has been proposed as a two-signal model. TLR4-mediated priming signals activate the NF-κB pathway and induce transcription and posttranscriptional processing of the NLRP3, pro-IL-1β and pro-IL-18 genes. The activation signal is mainly driven by diverse PAMPs and DAMPs, such as extracytoplasmic ATP binding to P2X7R, bacteriotoxin and complement MAC, to induce the formation of cell membrane pores, as well as the phagocytosis of nondegradable particulate matters (silica, alum, MSU, etc.). Overall, this process leads to K + efflux through direct transmembrane transport, promoting the assembly and activation of the NLRP3 inflammasome. Membrane ectopia of CLICs induces chloride efflux and oligomerization of ASC. The ER releases Ca 2+ in response to mitochondrial production of ROS. Upon NLRP3 inflammasome stimulation, the TGN within the Golgi disperses to form the dTGN, generating Ptdlns4P, which also promotes NLRP3 inflammasome assembly and activation. NLRP3 inflammasome activation promotes IL-1β or IL-18 maturation and causes caspase-1-mediated, GSDMD-dependent cell pyroptosis. The noncanonical pathway mediated by LPS directly or indirectly (by cathepsin B) activates caspase-11/4/5 and leads to pro-GSDMD maturation, which then causes cell pyroptosis or activates NLRP3 inflammasome to release the proinflammatory cytokine IL-1β.
The priming signals provided by the NF-κB activator is a prerequisite for a second stimulus, called the activation signal, which regulates the assembly of NLRP3 inflammasome and a subsequent cascade of reactions [ 36, 37] . The activation signal is mainly driven by diverse PAMPs and DAMPs, such as viral RNA [38], monosodium urate (MSU), extracellular ATP, amyloid β and silicon dioxide [ 39– 41] , to facilitate the assembly of NLRP3 inflammasome. NLRP3 inflammasome takes shape via a central NACHT domain that connects the N-terminal PYD and C-terminal LRR domains; the PYD structure of NLRP3 inflammasome connects the homotype PYD structure of ASC and interacts with each other; and the CARD domain of ASC mediates connection with the homotypic CARD structure of pro-caspase-1 [ 25, 42] . The NLRP3 inflammasome causes pro-caspase-1 to decompose into active caspase-1, which results in the transformation of pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18, respectively [43]. Purified recombinant gasdermin D (GSDMD) was shown to be capable of being cleaved by the active tetramer form of caspase-1, thereby forming a 22-kDa C-terminal fragment and a 38-kDa N-terminal mature cleavage product. GSDMD can cause extensive cell death with apparent pyroptosis morphology mainly through its N-terminal product and controls the secretion of IL-1β without affecting its maturation [ 44, 45] .
With the deepening of research in recent years, there are several hypotheses for the mechanism that triggers the second-step activation signal. The most common of these are inflow and outflow of cytoplasmic ions, lysosomal rupture, mitochondrial dysfunction, and Golgi diffusion, as shown in Figure 1. Changes in intracellular ion concentrations, including K + outflow, Ca 2+ influx and Cl − outflow, are thought to be important for the activation of NLRP3 inflammasome [46]. The involvement of the K + efflux mechanism is generally recognized, but it is not specific for the activation of NLRP3 inflammasome and may represent a distant upstream signaling event. Different NLRP3 inflammasome activation signals lead to K + efflux through diverse mechanisms, for which there are two typical pathways. In the first pathway, extracytoplasmic ATP binds to the purinergic ligand-gated ion channel 7 receptor (P2X7R), causing the K + channel to open, which leads to K + efflux and a relative decrease in intracellular K + concentration [ 47, 48] . In the second pathway, the formation of cell membrane pores induced by bacteriotoxin and the complement membrane attack complex (MAC), as well as the phagocytosis of nondegradable particulate matter (such as silica, alum, and MSU), leads to K + efflux through direct transmembrane transport [ 49– 51] . Macrophages cultured without any stimulatory signal in medium free of K + were found to activate NLRP3 inflammasome in vitro, whereas a culture environment with a high K + concentration inhibits NLRP3 inflammasome activation and is accompanied by cytotoxicity [49]. These observations indicate that inhibition of NLRP3 inflammasome activation can be achieved by inhibiting K + outflow. Mitotic kinase NEK7 is a vital factor that bridges adjacent NLRP3 subunits through binary interactions to form a high-molecular-weight NLRP3-NEK7 complex, which plays a role downstream of K + and regulates NLRP3 oligomerization and activation [ 52, 53] . Studies have shown that the oligomerization of ASC into specks is dependent on Cl − channels, and the process is dynamic and reversible.
NLRP3-dependent ASC oligomerization and speck formation only occur under conditions free of Cl −. Furthermore, these specks remain inactive and do not recruit active caspase-1 until the absence of K +, and Cl − induces the formation of a high-molecular-weight NLRP3-NEK7 complex, which leads to speck activation [54]. Other scholars have reported on the activation mechanism of K +, mitochondrial reactive oxygen species (ROS) and Cl − on NLRP3 inflammasome. An NLRP3 inflammasome agonist induces K + efflux, causing mitochondrial damage and ROS production, which then induces membrane ectopia of chloride intracellular channel proteins (CLICs) and Cl − efflux, promoting NEK7-NLRP3-ASC interaction, inflammasome assembly, activation of caspase-1 and secretion of IL-1β [ 48, 55] . Calcium-sensing receptor (CASR), which increases the release of Ca 2+ stored in the endoplasmic reticulum (ER), promotes the assembly of inflammasome components and mediates the reduction of cellular cAMP, independently activating the NLRP3 inflammasome [56]. Therefore, the maintenance of ionic equilibrium is essential, and the continued identification and deepening of our understanding of ionic mechanisms may reveal possible therapeutic targets. However, because of the ubiquitous nature of ion signaling in all cell types, inhibition of ion channels is not an ideal therapeutic strategy and would inevitably lead to off-target effects.
Activation of the NLRP3 inflammasome is also associated with organelles, including the ER, mitochondria and Golgi apparatus. Studies have shown that NLRP3 inflammasome activity is negatively regulated by autophagy and positively regulated by ROS accumulation during mitochondrial damage and is also associated with mitochondrial DNA release [ 57, 58] . The trans-Golgi network (TGN) disintegrates and disperses upon NLRP3 inflammasome stimulation to form the dTGN, which acts as a polymeric scaffold for NLRP3 and ASC, inducing aggregation of both, thereby activating downstream responses [59]. Additionally, Golgi-mediated protein kinase D (PKD) signaling leads to phosphorylation of NLRP3 located in the mitochondria-associated ER membrane (MAM), leading to the assembly of the NLRP3 inflammasome in the cytoplasm and affecting downstream signaling [60], as shown in Figure 1.
Noncanonical pathway
Studies have shown that LPS not only acts as a starting signal to activate inflammasomes but also directly activates caspase-11 independently of TLR4 to cause pyroptosis, a process called atypical NLRP3 inflammasome activation ( Figure 1) [ 61, 62] . Human caspase-4/5 and mouse caspase-11, which have high homology, act directly on LPS and lipid A with high specificity and affinity to induce cytotoxicity [61]. Another study indicated that after LPS-induced changes in lysosomal membrane stability, the caspase-11 activator cathepsin B flows out to activate caspase-11 and plays a role in the activation of atypical inflammasomes [63]. In this case, activation of caspase-11 is mediated by cathepsin B rather than by direct binding to LPS. Mechanistically, caspase-4/5/11 specifically destroy the N-terminal and C-terminal domains in GSDMD, and the exposed N-terminal fragment induces pyroptosis in a certain way, confirming the key role of GSDMD in the pyroptosis mechanism mediated by caspase-11 [ 44, 62, 64] . This discovery of the activation of atypical inflammasomes adds a new chapter to the mapping of the complex network of inflammasomes, which is of great significance.
Over the years, the activation mechanism of NLRP3 inflammasome has attracted great attention and interest from an increasing number of scientists, but many questions remain unanswered. For example, what is the universal, definitive mechanism that coordinates the activation of the complex inflammasome pathway, including its multiple ion channels, organelles, and interacting molecular proteins? Furthermore, does changing one activation pathway cause other pathways to change, and if so, what are the intersections between the different pathways? Efforts have been made to identify the ultimate source of activation, in the expectation that the mechanism of this complex system can be traced to a common cause.
Regulation of the NLRP3 Inflammasome
Several mechanisms have been identified that regulate NLRP3 inflammasome activation, including transcriptional and posttranscriptional modifications of NLRP3, posttranslational modifications, and the involvement of interacting proteins or regulators. By positively or negatively regulating the activation of the NLRP3 inflammasome, homeostasis is maintained and the disease state is improved.
Transcription and posttranscriptional modification
Many stimulators of the NLRP3 inflammasome priming phase have been identified, including TLRs, FAS-associated protein with death domain (FADD) [65], and IL-1 receptor (IL-1R) ligands [ 65– 67] . The unique expression patterns of these mediators regulate the different phases of inflammasome activity, as follows: NLRP3 and IL-1β gene transcription occur at the priming step, thus targeting the MyD88 requirement in the early stage; the adapter-inducing IFN-β-containing Toll/IL-1R domain (TRIF) is required for subsequent intermediate stages [ 35, 68] ; and the kinase IL-1R-associated kinase (IRAK1) is essential for the rapid activation of NLRP3 by the MyD88 pathway [ 69, 70] .
MicroRNAs (miRNAs) are endogenous single-stranded noncoding RNAs that are 19–24 nucleotides in length. Each miRNA exerts its regulatory function by binding to the 3′ untranslated region (3′UTR) of a protein-coding mRNA [71], and miRNA has been shown to play an important role in the posttranscriptional regulation of the NLRP3 inflammasome [72]. NLRP3 mRNA can be regulated by multiple miRNAs, including miR-223 which binds to a highly conserved region of the 3′UTR of NLRP3 mRNA and subsequently interferes with protein translation [ 73, 74] , as well as miR-22 and miR-7 which target NLRP3 expression and regulate NLRP3 inflammasome activation [ 75– 77] .
Posttranslational modifications (PTMs)
NLRP3 inflammasome activity is modulated by dynamic posttranslational modification of proteins through phosphorylation and deubiquitination and by protein kinase or autophagy-mediated degradation. Exploration of the regulatory mechanisms of NLRP3 is expected to yield alternative methods for the treatment of inflammatory diseases.
Currently, research indicates that deubiquitination and phosphorylation modulate NLRP3 and ASC to facilitate the assembly of the NLRP3 inflammasome. BRCC3 isopeptidase is a multiprotein complex formed by the linkage of BRCC3 with ABRO1, NBA1 and BRE, and can specifically cleave the Lys63 ubiquitin chain [78]. Mechanistic studies have shown that BRCC3 and ABRO1 are key regulators of NLRP3 inflammasome activation. BRCC3 mediates the deubiquitination of the LRR domain of NLRP3, which in turn recruits ASC, activates caspase-1, and releases the proinflammatory cytokine IL-1β, for which ABRO1 binding to the NBD and NACHT domain of NLRP3 is essential [ 78, 79] . To deal with endotoxin, the polyubiquitination of the NLRP3-K63 linkage that is mediated by the deubiquitin enzyme STAMBP is a signal of nondegradation and activation of the NLRP3 inflammasome [80]. Apart from promoting assembly of the inflammasome complex, deubiquitination also enhances the transcription of NLRP3 and pro-IL-1β, thereby promoting the activation of the NLRP3 inflammasome. For example, UAF1/USP12 and UAF1/USP46 complexes promote NF-κB activation and enhance the transcription of NLRP3 and pro-IL-1β by inhibiting ubiquitin-mediated degradation of p65 [81]. Ubiquitin ligase plays a negative regulatory role in the activation. Previously, inhibition of the NLRP3 inflammasome by the neurotransmitter dopamine, mediated by the dopamine D1 receptor signal through binding of the second messenger cAMP to NLRP3, followed by the action of the E3 ubiquitin ligase MARCH7 to promote the ubiquitination and degradation of NLRP3, was reported to play a negative regulatory role [82]. Additionally, TRIM31 and FBXL2 have also been reported to promote the ubiquitination and degradation of NLRP3 [ 83, 84] . Evidence has shown that inflammasomes are regulated by protein kinases and phosphatases that target the phosphorylation of inflammasome components [85]. The possibility of targeting NLRP3 and ASC phosphorylation has been investigated. During the initiation event, TLRs activate mitogen-activated protein kinases, including JNK. JNK1-mediated phosphorylation of NLRP3 at S194, which is a key step leading to oligomerization of NLRP3, contributes to subsequent inflammasome assembly [86]. Using mass spectrometry, researchers identified three phosphorylated serines in NLRP3, one of which is located at the charge interaction interface within the PYD of NLRP3. Phosphorylation interferes with the charge interaction between PYD and PYD within the interface and inhibits assembly of the inflammasome, while protein phosphatase 2A (PP2A) plays a role in the dephosphorylation of NLRP3 and activates the inflammasome [87]. The protein kinase A (PKA) signaling pathway inhibits inflammasome activation through specific phosphorylation of Ser/Thr residues of NLRP3 [88]. Phosphorylation of ACS controls the formation of ASC speck aggregates. Syk kinase phosphorylates the ASC Y146 and Y187 residues, Syk and JNK phosphorylate Tyr144, and Pyk2 phosphorylates Tyr146, which are involved in the formation of ASC specks and the regulation of inflammasome activation [ 85, 89, 90] . In summary, regulated kinase and phosphatase activities effectively target the activation of the NLRP3 inflammasome, providing a new avenue for the selection of clinical drugs to inhibit inflammasome activation and target the treatment of diseases associated with NLRP3 inflammasome.
Apart from the ubiquitination and phosphorylation of NLRP3 inflammasome during PTM, studies have shown that S-nitrosylation blocks its activity [91], while sumoylation and ADP-ribosylation can promote activation of the NLRP3 inflammasome [ 92, 93] .
NLRP3 interacting partners
In addition to interacting with ASC and pro-IL-1β to form the inflammasome complex, NLRP3 also interacts with many other proteins, including NEK7, which regulates oligomerization and activation of NLRP3 [54], thioredoxin-interacting protein (TXNIP), the molecular chaperone heat shock protein 90 (HSP90) and its cochaperone SGT1, guanylate-binding protein 5 (GBP5), and others. TXNIP is associated with insulin resistance. After inflammasome activators such as MSU cause an increase in ROS, TXNIP is induced to dissociate from thioredoxin, which in turn binds to NLRP3 [94]; thus, it is possible to regulate the NLRP3 inflammasome by altering TXNIP. Functional HSP90 keeps NLRP3 stable and prevents degradation by the proteasome [95]. The SGT1-HSP90 complex binds to the NLRP3 LRR domain, which interacts with NLRP3 to maintain NLRP3 in an inactive but signaling-competent state; upon detection of an activation signal, SGT1-HSP90 dissociates from NLRP3, thereby inducing the NLRP3-associated inflammatory cascades.
The SGT1-HSP90 complex binds to the NLRP3 LRR domain [95]. GBP5 binds to the NLRP3 PYD domain, and tetrameric GBP5 promotes NLRP3-mediated ASC oligomerization [96], suggesting that the NLRP3 inflammasome could be regulated through GBP5.
NLRP3 Inflammasome in Autoimmune Liver Diseases
Autoimmune hepatitis
Autoimmune hepatitis (AIH) is a relatively rare and highly heterogeneous disease. The most common clinical phenotype is characterized by insidiousness, without obvious symptoms or with one or more nonspecific symptoms, which include fatigue, general malaise, upper right abdominal pain, lethargy, anorexia, weight loss, nausea, and itching. Typical biochemical characteristics include: (1) elevated bilirubin and transaminase; (2) γ-globulin or immunoglobulin G levels > 1.5 times the upper limit of normal; (3) titers of > 1:80 for anti-nuclear (ANA), anti-smooth muscle (SMA); and (4) anti-liver/kidney microsomal type 1 (LKM1) autoantibodies [ 97, 98] .
Regulatory T cells and effector T cells play an important role in the pathogenesis of AIH. T helper type 1 cells secrete IL-2 and IFN-γ to stimulate cytotoxic T lymphocytes on liver and activate macrophages to release IL-1 and tumor necrosis factor-α. Both IL-1β and IL-18 belong to the IL-1 family [99], and IL-1β is abundant in the liver microenvironment of AIH [98]. Concanavalin A (ConA)-induced hepatitis leads to activation of T lymphocytes, production of abundant proinflammatory cytokines, and damage or killing of hepatocytes, thus mimicking the AIH pattern. The expression levels of NLRP3, cleaved caspase-1, and IL-1β were shown to be upregulated in the livers of ConA model mice. To further evaluate the functional contribution of NLRP3 inflammasome in AIH, the authors investigated ConA-induced diseases in NLRP3 −/− mice. Compared with wild-type mice, NLRP3 −/− mice exhibited significantly reduced hepatocellular damage, reduced serum levels of alanine aminotransferase and aspartate transaminase, and downregulated levels of cleaved caspase-1 and IL-1β proteins in hepatocyte homogenates [100], suggesting that NLRP3 inflammasome plays a key role in the process of AIH. Trichloroethylene (TCE)-mediated AIH inflammation is similar to ConA-mediated hepatitis, and both are related to T cells. TCE stimulation leads to the activation of inflammasomes induced by oxidative stress, resulting in dysregulation of the liver immune response and inducing the formation of autoimmune diseases [101]. Studies have shown that the induction of AIH by dichloroacetyl chloride, a metabolite of TCE, is also related to the activation of inflammasomes [102]. These studies strongly suggest that NLRP3 inflammasome activation is involved in the inflammatory response of AIH and plays an important role in its pathogenesis.
The possibility that inhibiting the activation of the NLRP3 inflammasome can alleviate the inflammatory response in AIH is being further explored. The inflammasome activation could be inhibited through direct binding of NLRP3. For example, previous studies have demonstrated that miR-223 can target the binding site of the NLRP3 3′UTR, prevent the accumulation of inflammasomes and inhibit the production of IL-1β, thereby inhibiting liver inflammation and inflammatory cell death for a hepatoprotective effect [ 103, 104] . In other studies, the NLRP3 inflammasome is suppressed by inhibiting cytokines or key links related to the inflammasome activation pathway, primarily targeting IL-1β inhibitors and antioxidants, all in model mice. For example, studies in ConA-induced AIH have shown that the use of the IL-1β inhibitor rhIL-1Ra improves liver inflammation and damage in two ways: (1) blocking the role of IL-1β in the disease; and (2) eliminating ROS and restoring mitochondrial function to inhibit the activation of the NLRP3 inflammasome [100].
Activated nuclear factor erythroid 2-related factor 2 (NRF2), which promotes the expression of antioxidant genes, attenuates TCE-mediated autoimmunity. The antioxidants sulforaphane and tertiary butylhydroquinone have the same protective effect [105]. Could this effect be related to the inflammasome? It was reported that the ROS scavenger N-acetylcysteine (NAC) ameliorates immune disorders caused by TCE exposure, probably by inhibiting oxidative stress and activating the NLRP3 inflammasome [101]. Another study showed that curcumin ameliorates hepatic inflammatory injury by interfering with the NLRP3/caspase-1/GSDMD pyroptotic signaling pathway while scavenging malondialdehyde (the final product of ROS-induced lipid peroxidation) accumulation and regulating NRF2 signaling, thereby inhibiting hepatotoxicity and oxidative stress [106]. Additionally, the authors mentioned that curcumin may inhibit the activation of the NLRP3 inflammasome by inhibiting the ROS-TXNIP-NLRP3 pathway. Therefore, there is a close relationship between the antioxidant level and activation of the NLRP3 inflammasome, which may lead to new ideas for the treatment of AIH. In summary, the role of NLRP3 inflammasome in the pathogenesis and treatment of AIH has only been confirmed at the animal level, and systematic studies have been insufficient. The correlation between NLRP3 inflammasome and AIH needs further exploration and verification.
Primary biliary cholangitis
Primary biliary cholangitis (PBC) is an autoimmune cholestatic liver disease characterized by cholestasis, serological reaction of specific autoantibodies, including AMA and ANA, and intrahepatic cholangitis. Without timely treatment, PBC will eventually develop into advanced biliary cirrhosis [107].
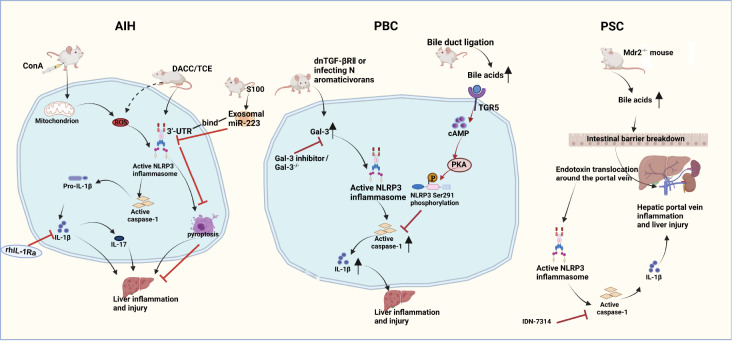
Bile acids (BAs) play an important role in the pathogenesis of PBC. When the concentration of BAs exceeds the normal physiological level, they exert a cytotoxic effect that causes cell necrosis [108]. In one report, LPS-primed bone marrow-derived macrophages (BMDMs) that were treated with BAs at 50 μM, which is within the physiological range of BAs in the portal vein (10–80 μM) and peripheral circulation (2–10 μM) of humans and mice, inhibited caspase-1 activation and IL-1β secretion, thereby specifically inhibiting NLRP3 inflammasome activation [109]. The detailed mechanism is shown in Figure 2. However, in another study, 50 μM BAs had the exact opposite effect: the NLRP3 inflammasome was activated in mouse BMDMs, and human peripheral blood monocytes differentiated into macrophages [110]. This discrepancy should be explored further in PBC. The inhibition of inflammasome activation at physiological concentrations of BAs raises the question: what is the effect of a pathological concentration? This area also needs more in-depth research.
Figure 2 .
NLRP3 inflammasome activation in the disease processes of AIH, PBC and PSC
The NLRP3 inflammasome is activated and IL-1β is upregulated in ConA- or TCE-induced AIH model animals, which ultimately leads to the development of liver inflammation and injury that can be blocked by the IL-1 inhibitor rhIL-1Ra. Overexpressed miR-233 in exosomes from bone marrow-derived stem cells inhibits NLRP3 activation by binding to its 3′UTR, leading to NLRP3 mRNA degradation and thus suppression of liver inflammation and cell death, mediating a form of hepatoprotection. In a spontaneous PBC dnTGF-βRII mouse model and an induced PBC mouse model obtained by infection with Novosphingobium aromaticivorans, Gal3 promotes increased inflammation by enhancing the activation of the NLRP3 inflammasome and IL-1β production, inducing liver inflammation and injury that can be stopped by Gal3 inhibitors. Bile duct ligation triggers elevation in BAs, activating the TGR5-cAMP-PKA pathway and inducing NLRP3 Ser291 phosphorylation, inhibiting caspase-1 activation and relieving liver damage. In a mouse Mdr2-knockout ( Mdr2 −/−) model that duplicates the human PSC, BAs augment intestinal barrier disruption. Impaired barrier function leads to bacterial endotoxin migration to the hepatic periportal vein and activation of the NLRP3 inflammasome, ultimately leading to liver damage. Treatment with the pancaspase inhibitor IDN-7314 ameliorates liver injury in Mdr2 −/− mice.
PBC initially causes inflammation of the small bile ducts in the liver that can lead to the development of fibrosis and, in the absence of effective treatment, eventually liver cirrhosis [111]. For moderate-to-severe patients, it is very important to alleviate the process of liver fibrosis. There are no reports of a direct examination of the relationship between liver fibrosis and NLRP3 inflammasome in PBC, but recent studies of fibrosis caused by alcoholic and nonalcoholic fatty liver and nonalcoholic steatohepatitis have clarified the key role of NLRP3 inflammasome activation as a triggering factor for the development of various liver diseases [ 112, 113] . These results suggest that further exploration of the possible internal connection between liver fibrosis and the NLRP3 inflammasome in PBC could be fruitful.
As previously mentioned, the NLRP3 inflammasome can be activated by DAMPs, and the multifunctional glycoprotein Gal-3 is considered a DAMP molecule [114]. Studies in spontaneous PBC mouse models with dominant negative transforming growth factor β receptor II (dnTGF-βRII) have shown that the lectin Gal3 acts as the initiator of inflammatory signals to directly stimulate the activation of NLRP3 inflammasome and induce IL-17 proinflammatory cascades [115]. This activation of the NLRP3 inflammasome by Gal3 has also been confirmed in another PBC model. In C57BL/6 mice induced by infection with Novosphingobium aromaticivorans, Gal3 enhances the activation of the NLRP3 inflammasome, and IL-1β is produced to promote the increase in inflammation [116]. These results indicate that inhibition of Gal3 signal transduction may be a potential target for the treatment of PBC; however, what is the specific mechanism by which Gal3 plays a role in PBC remains to be further studied.
PBC is a cholestatic liver injury disease that is currently treated with ursodeoxycholic acid, an internationally recognized drug, but approximately 40% of patients have no biochemical reaction to it [117]. The search for drugs with better curative effects and tolerability and fewer adverse reactions has become urgent for PBC patients. Studies on the role of MCC950, an effective and selective small molecule inhibitor of NLRP3 inflammasome in liver injury [ 113, 117] , have prompted us to explore whether NLRP3 inflammasome small molecule inhibitors also play a similar role in the process of PBC. Such research on therapeutic drugs targeting NLRP3 inflammasome may provide more options for the clinical treatment of PBC.
Primary sclerosing cholangitis
Primary sclerosing cholangitis (PSC) is a chronic bile duct disease that is diagnosed based on cholangiographic (or histological) features shown by magnetic resonance cholangiopancreatography, dynamic liver magnetic resonance imaging and/or contrast computed tomography examination. There is little evidence that medications are useful to prevent disease progression [ 118, 119] .
Elevated markers of NRLP3 inflammasome activation have been detected in liver slices from PSC patients, suggesting a higher degree of inflammasome activity in these patients than in healthy individuals. Consistent results were observed in a mouse Mdr2-knockout ( Mdr2 −/−) model that is similar to human PSC [ 120, 121] , providing support for the need to further investigate the relationship between NLRP3 inflammasome and PSC pathogenesis. It has been pointed out that the microbiome is the cornerstone of PSC development, paving the way for translational diagnosis and treatment methods [122]. Given the important role of gut microbes in PSC disease, what role might the NLRP3 inflammasome play in the intestinal flora and PSC?
Despite an abundance of mechanistic theories, the pathogenesis of PSC remains unclear. BA toxicity and intestinal leakage are considered to be the two main hypothetical mechanisms [123]. PSC is an autoimmune cholestatic liver disease. Changes in the intestinal flora affect the BA pool and can exacerbate cholestasis, and excessive accumulation of BAs may, in turn, affects the composition of the intestinal microbiota [108]. Additionally, BAs are closely related to NLRP3 inflammasome. Therefore, it is possible that changes in the intestinal flora can lead to the accumulation of BAs, which affects the activation of inflammasomes through BAs and lead to PSC. It was reported that the intestinal barrier function of PSC model mice is impaired, leading to increased translocation of bacterial DNA and other bacterial products (such as LPS) into the portal system and that there is excessive activation of NLRP3 inflammasome in the liver and intestines of mice [121]. The detailed mechanism is shown in Figure 2.
As previously shown, the inflammasome may play a role in PSC in two ways. First, the intestinal permeability changes, allowing the intestinal flora to enter the liver as a PAMP, causing excessive activation of NLRP3 inflammasome in the liver and aggravating the disease process. Second, changes in the intestinal flora aggravate cholestasis, overactivate the inflammasome, and lead to aggravation of the disease.
Whether the increased activation of NLRP3 inflammasome is the result of changes in BA levels or in the gut microbiome is unknown. Transplant-free survival in PSC has not been shown to be prolonged by single drugs or therapy [124], so, research into the role of NLRP3 inflammasome in PSC is still in its infancy.
Conclusions and Perspectives
Pathogen invasion causes the activation of the NLRP3 inflammasome, which comprises a host defense mechanism to clear the pathogen and maintain homeostasis. However, the excessive activation of NLRP3 inflammasome by foreign pathogens and self-danger signals can affect a variety of autoimmune liver diseases, including AIH, PBC and PSC. Indeed, previous studies have proven that substances acting as PAMPs or DAMPs activate a pivotal protein platform of the NLRP3 inflammasome and that the downstream signaling molecules caspase-1 and IL-1β are overactivated and upregulated in multiple autoimmune liver diseases. The activation mechanism of the NLRP3 inflammasome varies among autoimmune liver diseases. Further studies are needed to determine whether there is a common upstream mechanism that plays a decisive role in activation. Intestinal flora and BAs have indispensable functions in autoimmune liver disease, but which one plays a leading role in the pathogenesis of NLRP3 inflammasome remains to be determined.
According to the current literature on the characteristic role of the NLRP3 inflammasome in autoimmune liver disease, it is crucial to explore the therapeutic effects of targeted inhibition of the NLRP3 inflammasome or related signaling pathways. Small molecule inhibitors of NLRP3 inflammasome, such as MCC950, BA transport inhibitors, and BA receptor agonists, such as TGR5 and FXR, inhibit the activation pathway of the inflammasome and play a role in alleviating liver injury, which provides a new idea for the selection and application of clinical drugs. Most of the existing research drugs are based on reducing cholestasis, and the NLRP3 inflammasome may be a downstream signal of BAs, which causes the diseases. Therefore, the next step is to explore the targeted inhibition of the inflammasome itself or the upstream and downstream signals of inflammasome activation pathways to find new therapeutic targets.
In summary, the NLRP3 inflammasome plays an important role in the pathogenesis and development of autoimmune liver diseases and is a promising therapeutic target. However, because the existing research data are derived from animal models only, there is still a long way to go in this regard.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81502123 and 81973332), the Natural Science Foundation of Anhui Province (No. 1308085QH130), the Open Project Program of MOE Key Laboratory of Population Health Across Life Cycle (No. JKZD20212), the Open Fund of Key Laboratory of Anti Inflammatory and Immune Medicine, Ministry of Education, China (Anhui Medical University) (Nos. KFJJ-2020-12 and KFJJ-2021-9), and the Key Laboratory of Dermatology, Anhui Medical University, Ministry of Education, China (No. AYPYS2021-2).
References
- 1.Couto D, Zipfel C. Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol. 2016, 16: 537–552 . [DOI] [PubMed]
- 2.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. . 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Oh JE, Lee HK. Pattern recognition receptors and autophagy. Front Immunol. . 2014;5:300. doi: 10.3389/fimmu.2014.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harton JA, Linhoff MW, Zhang J, Ting JPY. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J Immunol. . 2002;169:4088–4093. doi: 10.4049/jimmunol.169.8.4088. [DOI] [PubMed] [Google Scholar]
- 5.Inohara N, Nuñez G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene. . 2001;20:6473–6481. doi: 10.1038/sj.onc.1204787. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstiel P, Jacobs G, Till A, Schreiber S. NOD-like receptors: ancient sentinels of the innate immune system. Cell Mol Life Sci. . 2008;65:1361–1377. doi: 10.1007/s00018-008-7502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev. . 2015;95:149–178. doi: 10.1152/physrev.00009.2014. [DOI] [PubMed] [Google Scholar]
- 8.Ting JPY, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, et al. The NLR gene family: a standard nomenclature. Immunity. . 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Zmasek CM, Godzik A. Domain architecture evolution of pattern-recognition receptors. Immunogenetics. . 2010;62:263–272. doi: 10.1007/s00251-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinon F, Burns K, Tschopp J. The inflammasome. Mol Cell. . 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 11.Su MY, Kuo CI, Chang CF, Chang CI. Three-dimensional structure of human NLRP10/PYNOD pyrin domain reveals a homotypic interaction site distinct from its mouse homologue. PLoS ONE. . 2013;8:e67843. doi: 10.1371/journal.pone.0067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng D, Kern L, Elinav E. The NLRP6 inflammasome. Immunology. . 2021;162:281–289. doi: 10.1111/imm.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song AQ, Gao B, Fan JJ, Zhu YJ, Zhou J, Wang YL, Xu LZ, et al. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J Neuroinflammation. . 2020;17:178. doi: 10.1186/s12974-020-01848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulon PG, Dhanushkodi N, Prakash S, Srivastava R, Roy S, Alomari NI, Nguyen AM, et al. NLRP3, NLRP12, and IFI16 inflammasomes induction and caspase-1 activation triggered by virulent HSV-1 strains are associated with severe corneal inflammatory herpetic disease. Front Immunol. . 2019;10:1631. doi: 10.3389/fimmu.2019.01631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minkiewicz J, de Rivero Vaccari JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia. . 2013;61:1113–1121. doi: 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- 16.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity. . 2012;36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. . 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. . 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heilig R, Broz P. Function and mechanism of the pyrin inflammasome. Eur J Immunol. . 2018;48:230–238. doi: 10.1002/eji.201746947. [DOI] [PubMed] [Google Scholar]
- 20.Chang B, Liu X, Liu J, Quan F, Guo Z, Zhang Y. Developmental expression and possible functional roles of mouse Nlrp4e in preimplantation embryos. In Vitro Cell Dev Biol Anim. . 2013;49:548–553. doi: 10.1007/s11626-013-9638-9. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Qian Y, Liu Y, Wang Q, Wang R, Zhou Y, Zhang C, et al. A novel homozygous variant in NLRP5 is associate with human early embryonic arrest in a consanguineous Chinese family . Clin Genet. . 2020;98:69–73. doi: 10.1111/cge.13744. [DOI] [PubMed] [Google Scholar]
- 22.Docherty LE, Rezwan FI, Poole RL, Turner CLS, Kivuva E, Maher ER, Smithson SF, et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat Commun. . 2015;6:8086. doi: 10.1038/ncomms9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenbarth SC, Williams A, Colegio OR, Meng H, Strowig T, Rongvaux A, Henao-Mejia J, et al. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature. . 2012;484:510–513. doi: 10.1038/nature11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C, Su Z, Lin M, Ou J, Zhao W, Cui J, Wang RF. NLRP11 attenuates Toll-like receptor signalling by targeting TRAF6 for degradation via the ubiquitin ligase RNF19A. Nat Commun. . 2017;8:1977. doi: 10.1038/s41467-017-02073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae JY, Park HH. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem. . 2011;286:39528–39536. doi: 10.1074/jbc.M111.278812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. . 2021;18:2114–2127. doi: 10.1038/s41423-021-00740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. . 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J Biol Chem. . 2002;277:29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Dong Y, Ye M, Jin S, Yang J, Joosse ME, Sun Y, et al. The pathogenic role of NLRP3 inflammasome activation in inflammatory bowel diseases of both mice and humans. J Crohns Colitis. . 2016;11:jjw219. doi: 10.1093/ecco-jcc/jjw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu XY, Li KT, Yang HX, Yang B, Lu X, Zhao LD, Fei YY, et al. Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J Autoimmun. . 2020;106:102336. doi: 10.1016/j.jaut.2019.102336. [DOI] [PubMed] [Google Scholar]
- 31.Guo C, Fu R, Zhou M, Wang S, Huang Y, Hu H, Zhao J, et al. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J Autoimmun. . 2019;103:102286. doi: 10.1016/j.jaut.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renaudin F, Orliaguet L, Castelli F, Fenaille F, Prignon A, Alzaid F, Combes C, et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Ann Rheum Dis. . 2020;79:1506–1514. doi: 10.1136/annrheumdis-2020-217342. [DOI] [PubMed] [Google Scholar]
- 33.Sharma A, Choi JSY, Stefanovic N, Al-Sharea A, Simpson DS, Mukhamedova N, Jandeleit-Dahm K, et al. Specific NLRP3 inhibition protects against diabetes-associated atherosclerosis. Diabetes. . 2021;70:772–787. doi: 10.2337/db20-0357. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Wise L, Fukuchi KI. TLR4 cross-talk with NLRP3 inflammasome and complement signaling pathways in alzheimer′s disease. Front Immunol. . 2020;11:724. doi: 10.3389/fimmu.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. . 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 36.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. . 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Mao L, Meng G. The NLRP3 inflammasome activation in human or mouse cells, sensitivity causes puzzle. Protein Cell. . 2013;4:565–568. doi: 10.1007/s13238-013-3905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhi X, Zhang Y, Sun S, Zhang Z, Dong H, Luo X, Wei Y, et al. NLRP3 inflammasome activation by Foot-and-mouth disease virus infection mainly induced by viral RNA and non-structural protein 2B. RNA Biol. . 2020;17:335–349. doi: 10.1080/15476286.2019.1700058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. . 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 40.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol. . 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. . 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oroz J, Barrera-Vilarmau S, Alfonso C, Rivas G, de Alba E. ASC pyrin domain self-associates and binds NLRP3 protein using equivalent binding interfaces. J Biol Chem. . 2016;291:19487–19501. doi: 10.1074/jbc.M116.741082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. . 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 44.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. . 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 45.He W, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. . 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong T, Yang Y, Jin T, Jiang W, Zhou R. Orchestration of NLRP3 inflammasome activation by ion fluxes. Trends Immunol. . 2018;39:393–406. doi: 10.1016/j.it.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Franceschini A, Capece M, Chiozzi P, Falzoni S, Sanz JM, Sarti AC, Bonora M, et al. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. . 2015;29:2450–2461. doi: 10.1096/fj.14-268714. [DOI] [PubMed] [Google Scholar]
- 48.Domingo-Fernández R, Coll RC, Kearney J, Breit S, O′Neill LAJ. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1β transcription and activate the NLRP3 inflammasome. J Biol Chem. . 2017;292:12077–12087. doi: 10.1074/jbc.M117.797126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. . 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamon MA, Cossart P. K + efflux is required for histone H3 dephosphorylation by listeria monocytogenes listeriolysin O and other pore-forming toxins . Infect Immun. . 2011;79:2839–2846. doi: 10.1128/IAI.01243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suresh R, Chandrasekaran P, Sutterwala FS, Mosser DM. Complement-mediated “bystander” damage initiates host NLRP3 inflammasome activation. J Cell Sci. . 2016;129:1928. doi: 10.1242/jcs.179291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, Hauenstein AV, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. . 2019;570:338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. . 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green JP, Yu S, Martín-Sánchez F, Pelegrin P, Lopez-Castejon G, Lawrence CB, Brough D. Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Proc Natl Acad Sci USA. . 2018;115:e9371. doi: 10.1073/pnas.1812744115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, Cui J, et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. . 2017;8:202. doi: 10.1038/s41467-017-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. . 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. . 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 58.Nakahira K, Haspel JA, Rathinam VAK, Lee SJ, Dolinay T, Lam HC, Englert JA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. . 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. . 2018;564:71–76. doi: 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z, Meszaros G, He WT, Xu Y, de Fatima Magliarelli H, Mailly L, Mihlan M, et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J Exp Med. . 2017;214:2671–2693. doi: 10.1084/jem.20162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. . 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 62.Kayagaki N, Stowe IB, Lee BL, O′Rourke K, Anderson K, Warming S, Cuellar T, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. . 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 63.Chen N, Ou Z, Zhang W, Zhu X, Li P, Gong J. Cathepsin B regulates non-canonical NLRP3 inflammasome pathway by modulating activation of caspase-11 in Kupffer cells. Cell Prolif. . 2018;51:e12487. doi: 10.1111/cpr.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, et al. Non-canonical inflammasome activation targets caspase-11. Nature. . 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 65.Gurung P, Anand PK, Malireddi RKS, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. . 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allam R, Lawlor KE, Yu ECW, Mildenhall AL, Moujalled DM, Lewis RS, Ke F, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non‐apoptotic caspase‐8 is required for inflammasome priming. EMBO Rep. . 2014;15:982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. . 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lei Q, Yi T, Chen C. NF-κB-Gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. . 2018;24:6044–6052. doi: 10.12659/MSM.908529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin KM, Hu W, Troutman TD, Jennings M, Brewer T, Li X, Nanda S, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci USA. . 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, Alnemri ES. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. . 2013;191:3995–3999. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. . 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 72.Tezcan G, Martynova EV, Gilazieva ZE, McIntyre A, Rizvanov AA, Khaiboullina SF. MicroRNA post-transcriptional regulation of the NLRP3 inflammasome in immunopathologies. Front Pharmacol. . 2019;10:451. doi: 10.3389/fphar.2019.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neudecker V, Haneklaus M, Jensen O, Khailova L, Masterson JC, Tye H, Biette K, et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. . 2017;214:1737–1752. doi: 10.1084/jem.20160462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. . 2012;189:4175–4181. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 75.Li S, Liang X, Ma L, Shen L, Li T, Zheng L, Sun A, et al. MiR-22 sustains NLRP3 expression and attenuates H. pylori-induced gastric carcinogenesis. Oncogene. . 2018;37:884–896. doi: 10.1038/onc.2017.381. [DOI] [PubMed] [Google Scholar]
- 76.Huang WQ, Wei P, Lin RQ, Huang F. Protective effects of microrna-22 against endothelial cell injury by targeting NLRP3 through suppression of the inflammasome signaling pathway in a rat model of coronary heart disease. Cell Physiol Biochem. . 2017;43:1346–1358. doi: 10.1159/000481846. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegeneration. . 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren G, Zhang X, Xiao Y, Zhang W, Wang Y, Ma W, Wang X, et al. ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. EMBO J. . 2019;38:e100376. doi: 10.15252/embj.2018100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. . 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Bednash JS, Johns F, Patel N, Smail TR, Londino JD, Mallampalli RK. The deubiquitinase STAMBP modulates cytokine secretion through the NLRP3 inflammasome. Cell Signal. . 2021;79:109859. doi: 10.1016/j.cellsig.2020.109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song H, Zhao C, Yu Z, Li Q, Yan R, Qin Y, Jia M, et al. UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat Commun. . 2020;11:6042. doi: 10.1038/s41467-020-19939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. . 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 83.Song H, Liu B, Huai W, Yu Z, Wang W, Zhao J, Han L, et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat Commun. . 2016;7:13727. doi: 10.1038/ncomms13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han SH, Lear TB, Jerome JA, Rajbhandari S, Snavely CA, Gulick DL, Gibson KF, et al. Lipopolysaccharide primes the NALP3 inflammasome by inhibiting its ubiquitination and degradation mediated by the SCFFBXL2 E3 ligase. J Biol Chem. . 2015;290:18124–18133. doi: 10.1074/jbc.M115.645549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, et al. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol. . 2013;14:1247–1255. doi: 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. . 2017;68:185–197.e6. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 87.Stutz A, Kolbe CC, Stahl R, Horvath GL, Franklin BS, van Ray O, Brinkschulte R, et al. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J Exp Med. . 2017;214:1725–1736. doi: 10.1084/jem.20160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan H, Lin Y, Dou J, Fu Z, Yao Y, Ye S, Zhang S, et al. Wedelolactone facilitates Ser/Thr phosphorylation of NLRP3 dependent on PKA signalling to block inflammasome activation and pyroptosis. Cell Prolif. . 2020;53:e12868. doi: 10.1111/cpr.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin YC, Huang DY, Wang JS, Lin YL, Hsieh SL, Huang KC, Lin WW. Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J Leukoc Biol. . 2015;97:825–835. doi: 10.1189/jlb.3HI0814-371RR. [DOI] [PubMed] [Google Scholar]
- 90.Chung IC, OuYang CN, Yuan SN, Li HP, Chen JT, Shieh HR, Chen YJ, et al. Pyk2 activates the NLRP3 inflammasome by directly phosphorylating ASC and contributes to inflammasome-dependent peritonitis. Sci Rep. . 2016;6:36214. doi: 10.1038/srep36214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mishra BB, Rathinam VAK, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, Sassetti CM. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome–dependent processing of IL-1β. Nat Immunol. . 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barry R, John SW, Liccardi G, Tenev T, Jaco I, Chen CH, Choi J, et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat Commun. . 2018;9:3001. doi: 10.1038/s41467-018-05321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bose S, Segovia JA, Somarajan SR, Chang TH, Kannan TR, Baseman JB. ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. mBio. . 2014;5:e02186. doi: 10.1128/mBio.02186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. . 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 95.Mayor A, Martinon F, De Smedt T, Pétrilli V, Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. . 2007;8:497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- 96.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. . 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 97.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol. 2015, 63: 971–1004 . [DOI] [PubMed]
- 98.Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis—update 2015. J Hepatol. . 2015;62:S100–S111. doi: 10.1016/j.jhep.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 99.Muratori L, Longhi MS. The interplay between regulatory and effector T cells in autoimmune hepatitis: implications for innovative treatment strategies. J Autoimmun. . 2013;46:74–80. doi: 10.1016/j.jaut.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 100.Luan J, Zhang X, Wang S, Li Y, Fan J, Chen W, Zai W, et al. NOD-Like receptor protein 3 inflammasome-dependent IL-1β accelerated cona-induced hepatitis. Front Immunol. . 2018;9:758. doi: 10.3389/fimmu.2018.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang H, Wang G, Liang Y, Du X, Boor PJ, Sun J, Khan MF. Redox regulation of hepatic NLRP3 inflammasome activation and immune dysregulation in trichloroethene-mediated autoimmunity. Free Radical Biol Med. . 2019;143:223–231. doi: 10.1016/j.freeradbiomed.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang H, Wang G, Ansari GAS, Khan MF. Trichloroethene metabolite dichloroacetyl chloride induces apoptosis and compromises phagocytosis in Kupffer cells: activation of inflammasome and MAPKs. PLoS ONE. . 2018;13:e0210200. doi: 10.1371/journal.pone.0210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J Immunol. . 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 104.Chen L, Lu FB, Chen DZ, Wu JL, Hu E, Xu LM, Zheng MH, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol. . 2018;93:38–46. doi: 10.1016/j.molimm.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 105.Banerjee N, Wang H, Wang G, Khan MF. Enhancing the Nrf2 antioxidant signaling provides protection against trichloroethene-mediated inflammation and autoimmune response. Toxicol Sci. . 2020;175:64–74. doi: 10.1093/toxsci/kfaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y, Liu F, Liu M, Zhou X, Wang M, Cao K, Jin S, et al. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem Toxicol. . 2022;161:112823. doi: 10.1016/j.fct.2022.112823. [DOI] [PubMed] [Google Scholar]
- 107.Hirschfield GM, Beuers U, Corpechot C, Invernizzi P, Jones D, Marzioni M, Schramm C. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. . 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. . 2017;16:885–896. doi: 10.1016/j.autrev.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. . 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 110.Hao H, Cao L, Jiang C, Che Y, Zhang S, Takahashi S, Wang G, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. . 2017;25:856–867.e5. doi: 10.1016/j.cmet.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. . 2015;386:1565–1575. doi: 10.1016/S0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 112.Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med. . 2014;92:1069–1082. doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qu J, Yuan Z, Wang G, Wang X, Li K. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int Immunopharmacol. . 2019;70:147–155. doi: 10.1016/j.intimp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 114.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble β-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. . 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 115.Tian J, Yang G, Chen HY, Hsu DK, Tomilov A, Olson KA, Dehnad A, et al. Galectin‐3 regulates inflammasome activation in cholestatic liver injury. FASEB J. . 2016;30:4202–4213. doi: 10.1096/fj.201600392RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arsenijevic A, Milovanovic J, Stojanovic B, Djordjevic D, Stanojevic I, Jankovic N, Vojvodic D, et al. Gal-3 deficiency suppresses Novosphyngobium aromaticivorans inflammasome activation and IL-17 driven autoimmune cholangitis in mice . Front Immunol. . 2019;10:1309. doi: 10.3389/fimmu.2019.01309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. . 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aabakken L, Karlsen TH, Albert J, Arvanitakis M, Chazouilleres O, Dumonceau JM, Färkkilä M, et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy. . 2017;49:588–608. doi: 10.1055/s-0043-107029. [DOI] [PubMed] [Google Scholar]
- 119.Chapman MH, Thorburn D, Hirschfield GM, Webster GGJ, Rushbrook SM, Alexander G, Collier J, et al. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut. . 2019;68:1356–1378. doi: 10.1136/gutjnl-2018-317993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maroni L, Agostinelli L, Saccomanno S, Pinto C, Giordano DM, Rychlicki C, De Minicis S, et al. Nlrp3 activation induces Il-18 synthesis and affects the epithelial barrier function in reactive cholangiocytes. Am J Pathol. . 2017;187:366–376. doi: 10.1016/j.ajpath.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 121.Liao L, Schneider KM, Galvez EJC, Frissen M, Marschall HU, Su H, Hatting M, et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut. . 2019;68:1477–1492. doi: 10.1136/gutjnl-2018-316670. [DOI] [PubMed] [Google Scholar]
- 122.Mooser C, Ganal-Vonarburg SC. Microbiota as a cornerstone in the development of primary sclerosing cholangitis: paving the path for translational diagnostic and therapeutic approaches. Gut. . 2019;68:1353–1355. doi: 10.1136/gutjnl-2019-318487. [DOI] [PubMed] [Google Scholar]
- 123.Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis—a comprehensive review. J Hepatol. . 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 124.Dyson JK, Beuers U, Jones DEJ, Lohse AW, Hudson M. Primary sclerosing cholangitis. Lancet. . 2018;391:2547–2559. doi: 10.1016/S0140-6736(18)30300-3. [DOI] [PubMed] [Google Scholar]