Abstract
Atrial fibrillation (AF) is the most common arrhythmia in the general population. Systemic thromboembolism from left atrial appendage (LAA) thrombosis is a well‐known complication of AF, whereas thromboembolic complications from a right atrial (RA) thrombus are infrequent. Nevertheless, the prevalence of RA thrombosis is debated; despite having a low prevalence in echocardiographic studies, the higher prevalence found in autoptic studies rises the hypothesis of an under detection of RA clots, possibly related to the limited evaluation of right atrial appendage (RAA) with non‐invasive imaging. Here we present a review of the current literature about RA thrombosis, regarding its diagnosis, differentials, and best treatment options.
Keywords: atrial fibrillation, echocardiography, non‐invasive imaging, right atrial appendage, right atrial thrombus
Right atrial thrombosis is a rare complication of atrial fibrillation, which prevalence is still under debate. Right atrial screening with transesophageal echocardiography should be of routine before cardioversion and in case of pulmonary embolism without any detected deep vein thrombosis. Other non‐invasive investigation may be useful when echocardiography is inconclusive.

Abbreviations
- 3D
three‐dimensional
- AF
atrial fibrillation
- CMR
cardiac magnetic resonance
- CT
computed tomography
- DOACs
direct oral anticoagulants
- EGE
early gadolinium enhancement
- INR
international normalized ratio
- LA
left atrium
- LAA
left atrial appendage
- LGE
late gadolinium enhancement
- MDCT
multidetector computed tomography
- PFO
patent foramen ovale
- RA
right atrium
- RAA
right atrial appendage
- SEC
spontaneous echocontrast
- SSFP
steady state free precession
- STIR
short‐TI inversion recovery
- TEE
transesophageal echocardiography
- TTE
transthoracic echocardiography
1. INTRODUCTION
Atrial fibrillation (AF) is a common disease, with a prevalence of 2% to 4% in the entire population, which increases in the elderly, reaching a prevalence of 13% in the over 80 years old. 1
The most frightening complication of AF is systemic thromboembolism, causing 20%–30% of all ischemic strokes and 10% of cryptogenic strokes. 2
Left atrial appendage (LAA) thrombi are the main source of embolization, whereas pulmonary thromboembolism from a right atrial (RA) thrombus is considered as a rare complication of AF. However, their exact prevalence has not been established yet, as much as their potential risk of embolization in the pulmonary artery or in the systemic circulation through a patent foramen ovale (PFO). Moreover, while transesophageal echocardiography (TEE) is the gold‐standard for routine screening of LA and LAA, RA thrombotic mass detection may be more challenging, secondary to the limited visualization of the right atrial appendage (RAA). When facing a RA mass, differential diagnosis between a thrombus and other intracardiac masses, most frequently atrial myxomas, needs to be made. Multidetector computed tomography (MDCT) and tissue‐characterization by cardiac magnetic resonance (CMR) may be powerful tools to give additional information for reaching the diagnosis and for choosing the appropriate therapeutic strategy. Treatment options may include anticoagulation, thrombolysis, or surgical embolectomy: there is no evidence supporting the superiority of one above the others, so that therapeutic strategy should be decided case‐by‐case.
2. PREVALENCE OF RIGHT ATRIAL THROMBOSIS
Atrial thrombosis is the most common complication of AF, reaching up a prevalence of 13% of cases in the Assessment of Cardioversion Using Transesophageal Echocardiography (ACUTE) trial, with 88.2% of thrombi being localized in the LAA and 11.8% of thrombi belonging to the RA/RAA. 3
Thus, RA thrombus detection is considered as a rare finding, which may be responsible for severe thromboembolic complications, such as pulmonary thromboembolism and paradoxical systemic embolism trough a PFO. A RA/RAA thrombus in the context of pulmonary embolism was found in less than 4% of cases, 4 , 5 rising to 18% in intensive care setting, 6 while only case series describe paradoxical embolism from a RA clot. 7
The exact prevalence of RA thrombosis in the context of AF is still under debate, as it varies basing on the different series or on the different detection modalities. Concerning TEE studies, a review of the images of 508 patients, obtained from the ACUTE trial, found spontaneous echocontrast (SEC) or RA thrombi in 79 patients (14%), divided into 68 patients with SEC and 5 patients with RA thrombus. 8 , 9 A higher prevalence of right atrial SEC (57% and 51% respectively) and RA thrombi (7% for both) was found by de Divitiis et al. 10 and by Bilge et al. 8 in patients with AF.
On the other side, a Swedish population‐based study on 23 796 autopsies found that RA thrombosis was as common as LA one, with a prevalence of 3.1% for each chamber. 9 Moreover, RA thrombosis was doubled in patients with pulmonary embolism, highlighting its causal role in the genesis of thromboembolic events. The higher prevalence of RA intracardiac thrombi in the autoptic series rises the hypothesis of an under detection of RA clots in clinical practice, which may be related to the limited visualization of RAA with transthoracic echocardiography (TTE) and TEE.
Considering all the different studies, prevalence of RA thrombus varies from 0.4% to 7.5%. 8 , 11
3. ANATOMICAL DIFFERENCE BETWEEN LEFT AND RIGHT ATRIUM
It is still unknown why RA and RAA are usually spared from thrombosis in comparison to LA, even though both atria are fibrillating. It has been hypothesized an anatomical difference between the two atria as a cause of clot formation in the left chamber. A TEE study showed larger RAA neck widths and smaller RA areas in comparison to LAA and LA ones, which may avoid blood stasis and subsequent clot formation 12 ; moreover, in patients with AF, RAA did not show any structural remodeling. Since an increase in LAA size has already been associated to an increased risk of LAA thrombus in patients with AF, 13 the absence of RAA enlargement may explain the lower incidence of RA thrombi.
4. ECHOCARDIOGRAPHIC EVALUATION OF RIGHT ATRIAL THROMBOSIS
TTE may be a first line approach, allowing detection of large size thrombi, which may address clinical suspect (i.e., presence of a RA mass in a patient presenting with dyspnea may suggest a diagnosis of pulmonary embolism). An example of a large RA thrombus detected by TTE is shown in Figure 1. However, a complete examination of the whole RA is usually not possible with TTE, and RAA is not detectable in almost all patients. Moreover, because of the limited accuracy of the technique, information about size, composition, and mobility of the mass may be limited. Considering all cardiac chambers, TTE has a sensitivity of 55%–93% for detection of intracardiac masses, 14 which we may suppose to decrease when considering only the RA chamber.
FIGURE 1.
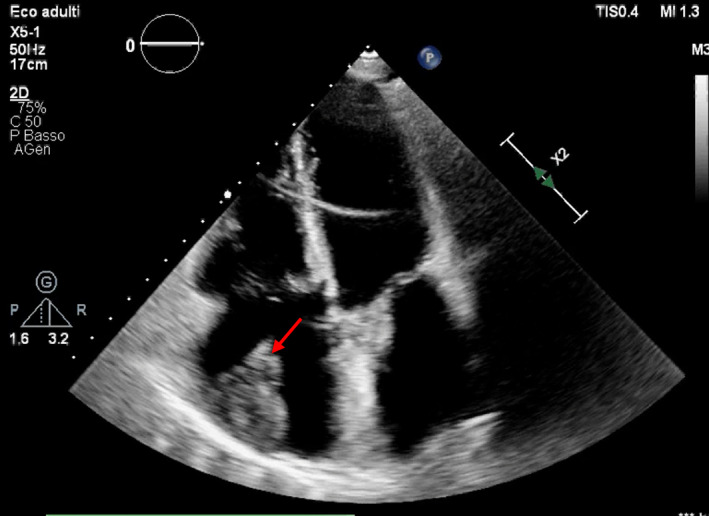
Transthoracic echocardiography (TTE) shows an echolucent structure attached to the right atrial (RA) walls, suggestive for a RA thrombus. The arrow indicates the mass. Courtesy of our center
TEE represents the gold standard modality to exclude LA thrombosis before cardioversion of AF. As it is consistent for left chamber evaluation, it may provide an evaluation of RA anatomy and it may detect RA/RAA clots. Three types of RA thrombi have been described. 15 The first type, referred as type A, is a thing and long mobile clot, defined as “worm‐like”, lodged in the RA chamber: they are usually associated with deep vein thrombosis, as they represent clot‐in‐transit from the venous circulation into the pulmonary arteries. The second type, referred as type B, is a more rounded clot, although morphology may be highly variable, attached to the atrial walls, with no or little mobility: it forms directly inside the RA, and it is associated to cardiac predisposing factors, such as AF, RA enlargement, correction of interatrial septal defects and others. Figure 2 shows an example of a type B thrombus, whom precise location and characterization was provided by TEE. The third type is a non‐A non‐B thrombus, identified as type C, typically resembling a myxoma. 15
FIGURE 2.

Bicaval view from a transesophageal echocardiography (TEE) shows presence of a multilobated mass attached to right atrial appendage (RAA). Courtesy of our center
In the context of AF, other than clots detection, standard TEE allows measurement of RA area and description of RAA morphology, with precise definition of the number of lobes, the neck width, the flow velocities, and the presence of spontaneous echocontrast (SEC). SEC is considered as the first step alteration before thrombus formation, 16 so that RAA thrombi are usually found in association with right atrial SEC, and isolated RA clot in absence of SEC is a rare finding. 17
Consequently, we would recommend to routinely perform a standard TEE examination before cardioversion with a comprehensive RA assessment, whereas in clinical practice focus is given on the LA/LAA, and RA it is usually overlooked.
Three‐dimensional (3D) TEE provides incremental information regarding location, size, and mobility of a RA mass: it allows visualization from multiple angles, giving a comprehensive idea of the anatomy of the clot. By cropping at different levels, it allows visualization of the internal part of a mass, providing additional information about its composition (i.e. an echolucent part inside the mass may indicate the presence of lysis which may suggest presence of a thrombus), which may be useful in the differential diagnosis with a tumoral mass. 18 An example of the utility of 3D TEE is given in Figure 3.
FIGURE 3.

Three‐dimensional transesophageal echocardiography allows reconstruction of a mass, in order to better define its location, size and mobility. By cutting at different planes, it may visualize the internal composition of the mass. Courtesy of our center
Nonetheless, RAA screening with TEE may be limited by the non‐visualization of RAA, with a prevalence ranging from 1.3% to 16% in different series. 17 , 19
Summarizing, TEE is the preferred methodic for RA/RAA thrombi detection, with a sensitivity varying from 0.92 to 1.00 and a specificity varying from 0.98 to 1.00. 20 In the authors'view it should be performed before cardioversion to rule out a RA/RAA thrombus. In the context of pulmonary embolism, a large RA thrombus or a thrombus straddling the PFO are usually seen routinely with TTE; however, we suggest considering TEE when the TTE window is poor and no deep vein thrombosis is detected, to look for a RAA thrombus secondary to AF or a clot‐in‐transit from a undetected vein thrombosis.
5. ALTERNATIVE NON‐INVASIVE INVESTIGATIONS
Other noninvasive modalities may be considered when TEE is not practicable or is unconclusive, such as multidetector computed tomography (MDCT) or cardiac magnetic resonance (CMR). These imaging modalities have only been studied for LAA evaluation: MDCT may permit successful identification of left atrial SEC and LAA thrombi, with a negative predictive value of 100% for LA thrombi exclusion, and a positive predictive value varying from 41% to 92%. 21 , 22 Figure 4 shows an RA thrombus with concomitant pulmonary embolism detected by MDCT.
FIGURE 4.
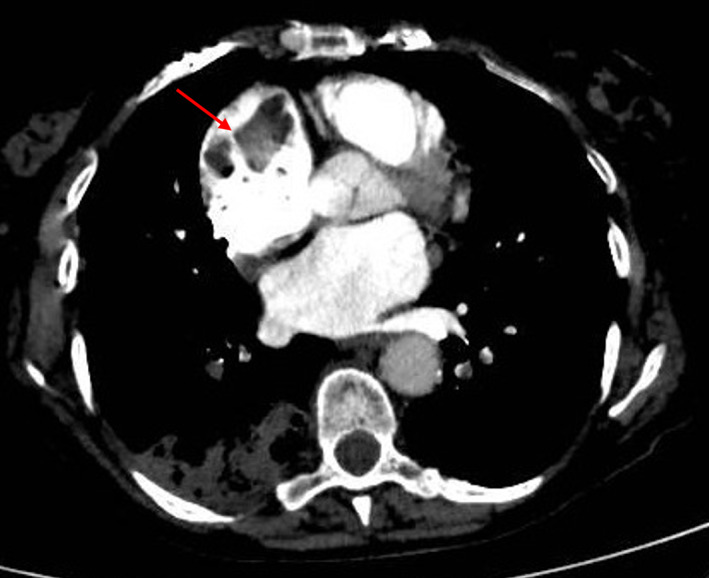
Chest computed tomography (CT) pulmonary angiography showing a dilated right atrium (RA) with a large hypointense mass inside. The patient was hospitalized with the diagnosis of acute pulmonary embolism from a RA thrombus in the context of atrial fibrillation (AF). The arrow indicates the mass. Courtesy of our center
CMR evaluates LAA size, anatomy, and function: it may detect thrombi as well as it may detect atrial wall fibrosis, which has been demonstrated to increase the risk or LAA thrombi formation. 23 An example of the CMR visualization of a RA thrombus is provide in Figure 5.
FIGURE 5.

Cardiac magnetic resonance (CMR) showing a dilated right atrium (RA) with inside an irregular and mobile formation, attached to the lateral wall and partially protruding into the right atrial appendage (RAA). The mass was hyperintense in T1‐ weighted and in short‐TI inversion recovery (STIR) sequences, hypointense inT2‐weighted and in early gadolinium enhancement (EGE); in late gadolinium enhancement (LGE) sequences it appeared hyperintense with a thin hypointense border. The arrow indicates the mass. Courtesy of our center
There are no formal recommendations regarding the use of these imaging modalities for RA/RAA thrombi detection. Basing on our experience, we summarized in Table 1 a list of advantages and disadvantages of each technique. We hypothesize that they may integrate TEE evaluation, especially when visualization of RAA is limited or when TEE is contraindicated or poorly tolerated. A flowchart of the authors' proposed evaluation for RA thrombi is provide in Figure 6.
TABLE 1.
Advantages and disadvantages of different imaging techniques for RA thrombi detection
| Imaging | Advantages | Disadvantages |
|---|---|---|
| TTE |
Non‐invasive High availability Low‐cost |
Limited sensitivity RAA not ordinarily seen |
| TEE |
Gold standard for RAA evaluation: describes anatomy and characteristics of the RAA Allows precise location of the mass Evaluation of RA flow and RAA emptying velocities |
Invasive RAA may not be seen in 1.3%–16% of patients May cause gastrointestinal bleeding Patient's discomfort Contraindicated in case of esophageal pathologies or patient intolerance |
| 3D echocardiography | Multiplane view of the mass and the anatomy of the RAA | Requires expertise |
| MDCT |
Non‐invasive May contemporarily detect pulmonary embolism* |
May cause contrast kidney damage Radiation exposure Limited evaluation in case of high heart rate |
| CMR |
Non‐invasive Tissue characterization with different sequences (T1W, T2W, LGE) may help in differential diagnosis |
High cost Limited availability Risk of nephrogenic systemic fibrosis Artifacts depending on slow flow in the RAA Lower temporal and spatial resolution compared to TEE |
Abbreviations: CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; MDCT, multidetector computed tomography; RA, right atrial; RAA, right atrial appendage; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Detection of pulmonary embolism with MDCT need confirmation with both angio and venous studies.
FIGURE 6.

Authors'proposed flowchart for the diagnosis and evaluation of right atrial thrombosis
6. DIFFERENTIAL DIAGNOSIS WITH OTHER INTRACARDIAC MASSES
A RA thrombus initially presents as a RA mass, which needs to be differentiated by other physiological findings or by malignant or benign intracardiac masses. A list of differential diagnosis to be considered when a RA mass is found is presented in Table 2. 24
TABLE 2.
Differential diagnosis of RA thrombosis
| Differential diagnosis of RA thrombus | |
|---|---|
| Findings | |
| Physiological | Crista terminalis |
| Chiari network | |
| Lipomatous hypertrophy of the interatrial septum | |
| Trabeculation of the RA appendage | |
| Iatrogenic | Atrial suture line after surgery |
| Pacemaker leads | |
| Swan‐Ganz catheter, central venous line | |
| Pathological | Myxoma |
| Melanoma | |
| Adrenocortical carcinoma | |
| Lymphoma | |
| Thyroid carcinoma* | |
| Inflammatory pseudo tumor* | |
| Rhabdomyoma** | |
| Hypernephroma** | |
| Hepatoma** | |
| Fibroelastoma*** | |
Abbreviation: RA, right atrial.
May be seen at the level of the right atrium/superior vena cava.
May be seen at the level of the right atrium/inferior vena cava.
May be seen at the level of the tricuspid valve.
A RA myxoma should be excluded. As it happens for thrombosis, RA myxomas are less frequent than their LA counterparts, accounting for 15–20% of all myxomas. 25 Preoperatively differentiating between an intracardiac myxoma and a thrombus may be challenging.
On echocardiography, thrombi are usually localized in the RAA, and they are accompanied by SEC. An acute thrombus is rounded and has smooth contours, while a chronic thrombus appear as a crescent mass along the endocardial borders with linear contours. RA myxomas usually localize at the level of the posterior wall, with either a sessile or pedunculated attachment, the latter being often mobile. Their contours are most commonly lobulated, and less frequently smooth or irregular. Their surface may be covered by thrombus, complicating its diagnosis. With perfusion echocardiography, a thrombus will appear as not enhanced, while a myxoma will be hypoenhanced; malignancies will be hyperhenanced masses. 26
At the CMR imaging, the appearance of a thrombus depends on its age. Acute thrombi will be hyperintense in T1 and T2 weighted images, while chronic thrombi will be hypointense in both T1 and T2. 27 An atrial myxoma usually present as isointense in T1, hyperintense in T2 and with a blackberry appearance at the steady state free precession (SSFP) images; they show post‐contrast enhancement weaker than the surrounding myocardium. However, it may also have a “thrombus‐like” presentation or a “pseudocystic” presentation. 28
At the MDCT, thrombi are low attenuation, non‐enhancing lesions, 29 while myxomas appear homogeneous and isodense, with absent or weak contrast perfusion. 28
The behavior of the mass may also provide incremental information about its nature, so that an increase in size may suggest a growing myxoma. However, myxomas'growth rate is highly variable among individuals, so that stability of the mass should not be used as a criterion to exclude the diagnosis of a cardiac myxoma. 30
7. TREATMENT OF RIGHT ATRIAL THROMBOSIS
Therapeutic options for RA thrombosis include anticoagulation, thrombolysis or surgery. Figure 7 shows an example of an atomical piece of a RA clot obtained from surgical thrombectomy. To authors'knowledge, no randomized clinical trial has been performed to evaluate the effect of the different strategies, and only retrospective studies and clinical cases are available.
FIGURE 7.

Anatomical piece from surgical thrombectomy of a right atrial appendage (RAA) thrombus resistant to anticoagulation. Courtesy of our center
Evidence from retrospective studies is conflicting. In the multicenter European Cooperative Study, the thrombus‐related short‐term mortality was higher for patients treated with anticoagulation (67%) in comparison to thrombolysis (40%) or surgical thrombectomy (27%) 15 ; however, these results refer to type A thrombi, probably clot‐in‐transit, while prognosis for type B thrombi, which are the ones usually forming in the case of AF, was benign irrespective of the type of treatment. The more recent retrospective study by Barrios et al. regarding RA thrombosis in the context of pulmonary embolism, showed no difference in mortality and in major bleeding complications between a reperfusion strategy plus anticoagulation versus anticoagulation alone, with a higher rate of venous thromboembolism recurrence for patients who received reperfusion plus anticoagulation (6.2% for reperfusion plus anticoagulation vs. 0% for anticoagulation alone). 31 The International Cooperative Pulmonary Embolism Registry (ICOPER) group showed similar mortality for pulmonary embolism associated with RA thrombus, regardless of the treatment provided (either heparin, thrombolysis or surgical embolectomy). 5
Because of the lack of evidence, the 2019 ESC (European Society of Cardiology) guidelines on pulmonary embolism do not recommend any specific strategy in the context of acute pulmonary embolism from a RA thrombosis, 32 so that treatment should be decided case‐by‐case basing on pulmonary embolism risk and hemodynamic conditions.
Concerning TEE studies before cardioversion for AF, if a RA clot is found, cardioversion should be delayed and performed after documented resolution of thrombosis. 2 In the authors' view, it may be reasonable to initiate anticoagulation with heparin or warfarin, with a therapeutic INR of 2 to 3. No data regarding the use of DOACs is available.
8. CONCLUSION
In conclusion, despite RA/RAA thrombi being considered a rare finding in AF, they may potentially lead to severe complications from thromboembolic events. However, evaluation with TEE in patients with AF is often focused to the LA, possibly causing an under detection of RA thrombi, which may result into the development of pulmonary embolism.
TEE is the gold‐standard technique to detect thrombotic atrial masses in the left chambers, whereas RA thrombotic mass detection could be more challenging. In our view, accurate screening for RA thrombi should be performed before cardioversion and when pulmonary embolism occurs during AF and without any detected deep vein thrombosis. Other cardiac masses are rarely present in the RA chamber, and they may be mistaken for a thrombus: differential diagnosis becomes crucial, as their treatments are completely different. Echocardiography may provide information about the nature and the mobility of the mass; MDCT and tissue‐characterization by CMR are powerful tools that give relevant additional information to correctly classify the mass. No large evidence regarding appropriate therapeutic strategy for RA thrombosis is available, and more data about when to choose anticoagulation instead of thrombolysis or surgery needs to be collected.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
This article does not contain any studies with human or animal subjects performed by any of the authors.
INFORMED CONSENT
Verbal informed consent was obtained from the patient(s) for their anonymized images to be published in this article.
ACKNOWLEDGMENTS
We thank our radiology department for providing CT and CMR images and our echocardiographic team for the realization of the echocardiographic images. We thank our cardiac surgeons for providing pictures of the resected thrombus. Open Access Funding provided by Universita degli Studi del Piemonte Orientale Amedeo Avogadro within the CRUI‐CARE Agreement.
Degiovanni A, Carassia C, De Vecchi S, Erbetta R, Patti G. Atrial thrombosis: Not only left, think also about right!. J Clin Ultrasound. 2022;50(8):1194‐1201. doi: 10.1002/jcu.23311
DATA AVAILABILITY STATEMENT
Due to the nature of this article, no data have been used by any of the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke Statistics‐2019 update: a report from the American Heart Association [published correction appears in circulation. 2020 Jan 14;141(2):e33]. Circulation. 2019;139(10):e56‐e528. [DOI] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio‐thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373‐498. [DOI] [PubMed] [Google Scholar]
- 3. Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344(19):1411‐1420. [DOI] [PubMed] [Google Scholar]
- 4. Casazza F, Becattini C, Guglielmelli E, Floriani I, Morrone V, Caponi C, Pizzorno L, Masotti L, Bongarzoni A, Pignataro L Prognostic significance of free‐floating right heart thromboemboli in acute pulmonary embolism: results from the Italian pulmonary embolism registry. Thromb Haemost 2014;111(1):53–57. [DOI] [PubMed] [Google Scholar]
- 5. Torbicki A, Galié N, Covezzoli A, Rossi E, de Rosa M, Goldhaber SZ. Right heart thrombi in pulmonary embolism: results from the international cooperative pulmonary embolism registry. J Am Coll Cardiol. 2003;41(12):2245‐2251. [DOI] [PubMed] [Google Scholar]
- 6. Casazza F, Bongarzoni A, Centonze F, Morpurgo M. Prevalence and prognostic significance of right‐sided cardiac mobile thrombi in acute massive pulmonary embolism. Am J Cardiol. 1997;79(10):1433‐1435. [DOI] [PubMed] [Google Scholar]
- 7. van Laecke S, Dhondt A, de Sutter J, Vanholder R. Right atrial thrombus in an asymptomatic hemodialysis patient with malfunctioning catheter and patent foramen ovale. Hemodial Int. 2005;9(3):236‐240. [DOI] [PubMed] [Google Scholar]
- 8. Bilge M, Eryonucu B, Güler N, Erkoç R. Right atrial appendage function in patients with chronic nonvalvular atrial fibrillation. Jpn Heart J. 2000;41(4):451‐462. [DOI] [PubMed] [Google Scholar]
- 9. Ogren M, Bergqvist D, Eriksson H, Lindblad B, Sternby NH. Prevalence and risk of pulmonary embolism in patients with intracardiac thrombosis: a population‐based study of 23 796 consecutive autopsies. Eur Heart J. 2005;26(11):1108‐1114. [DOI] [PubMed] [Google Scholar]
- 10. de Divitiis M, Omran H, Rabahieh R, et al. Right atrial appendage thrombosis in atrial fibrillation: its frequency and its clinical predictors. Am J Cardiol. 1999;84(9):1023‐1028. [DOI] [PubMed] [Google Scholar]
- 11. Rozenberg V, Boccara F, Benhalima B, Lamisse N, Buyukoglu B, Cohen A. Comparison of echocardiographic markers of embolism in atrial flutter and fibrillation: frequency of protruding atherosclerotic plaques in the thoracic aorta. Echocardiography. 2000;17(6 Pt 1):555‐562. [DOI] [PubMed] [Google Scholar]
- 12. Subramaniam B, Riley MF, Panzica PJ, Manning WJ. Transesophageal echocardiographic assessment of right atrial appendage anatomy and function: comparison with the left atrial appendage and implications for local thrombus formation. J Am Soc Echocardiogr. 2006;19(4):429‐433. [DOI] [PubMed] [Google Scholar]
- 13. Rubin DN, Katz SE, Riley MF, Douglas PS, Manning WJ. Evaluation of left atrial appendage anatomy and function in recent‐onset atrial fibrillation by transesophageal echocardiography. Am J Cardiol. 1996;78(7):774‐778. [DOI] [PubMed] [Google Scholar]
- 14. Mügge A, Daniel WG, Haverich A, Lichtlen PR. Diagnosis of noninfective cardiac mass lesions by two‐dimensional echocardiography. Comparison of the transthoracic and transesophageal approaches. Circulation. 1991;83(1):70‐78. [DOI] [PubMed] [Google Scholar]
- 15. The European cooperative study on the clinical significance of right heart thrombi. European working group on echocardiography. Eur Heart J. 1989;10(12):1046‐1059. [DOI] [PubMed] [Google Scholar]
- 16. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373(9658):155‐166. [DOI] [PubMed] [Google Scholar]
- 17. Bashir M, Asher CR, Garcia MJ, et al. Right atrial spontaneous echo contrast, and thrombi in atrial fibrillation: a transesophageal echocardiography study. J Am Soc Echocardiogr. 2001;14(2):122‐127. [DOI] [PubMed] [Google Scholar]
- 18. Papadopoulos CH, Michalakeas CA, Paraskevaidis I, Ikonomidis I, Anastasiou‐Nana M. Differential diagnosis of a left atrial mass: role of three‐dimensional transoesophageal echocardiography. Hellenic J Cardiol. 2010;51(6):546‐548. [PubMed] [Google Scholar]
- 19. Ozer O, Sari I, Davutoglu V. Right atrial appendage: forgotten part of the heart in atrial fibrillation. Clin Appl Thromb Hemost. 2010;16(2):218‐220. [DOI] [PubMed] [Google Scholar]
- 20. Anfinogenova ND, Vasiltseva OY, Vrublevsky AV, et al. Right atrial thrombosis and pulmonary embolism: a narrative review. Semin Thromb Hemost. 2020;46(8):895‐907. [DOI] [PubMed] [Google Scholar]
- 21. Martinez MW, Kirsch J, Williamson EE, et al. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging. 2009;2(1):69‐76. [DOI] [PubMed] [Google Scholar]
- 22. Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta‐analysis. Circ Cardiovasc Imaging. 2013;6(2):185‐194. [DOI] [PubMed] [Google Scholar]
- 23. Akoum N, Fernandez G, Wilson B, Mcgann C, Kholmovski E, Marrouche N. Association of atrial fibrosis quantified using LGE‐MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24(10):1104‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alizadehasl A, Sadeghpour A. Cardiac masses and tumors. In: Sadeghpour A, Alizadehasl A, eds. Case‐Based Textbook of Echocardiography. Springer; 2018. [Google Scholar]
- 25. Reynen K. Cardiac myxomas. N Engl J Med. 1995;333(24):1610‐1617. [DOI] [PubMed] [Google Scholar]
- 26. Wu CM, Bergquist PJ, Srichai MB. Multimodality imaging in the evaluation of Intracardiac masses. Curr Treat Options Cardiovasc Med. 2019;21(10):55. [DOI] [PubMed] [Google Scholar]
- 27. Barkhausen J, Hunold P, Eggebrecht H, et al. Detection and characterization of intracardiac thrombi on MR imaging. AJR Am J Roentgenol. 2002;179(6):1539‐1544. [DOI] [PubMed] [Google Scholar]
- 28. Colin GC, Gerber BL, Amzulescu M, Bogaert J. Cardiac myxoma: a contemporary multimodality imaging review. Int J Cardiovasc Imaging. 2018;34(11):1789‐1808. [DOI] [PubMed] [Google Scholar]
- 29. Hur J, Pak HN, Kim YJ, et al. Dual‐enhancement cardiac computed tomography for assessing left atrial thrombus and pulmonary veins before radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol. 2013;112(2):238‐244. [DOI] [PubMed] [Google Scholar]
- 30. Karlof E, Salzberg SP, Anyanwu AC, Steinbock B, Filsoufi F. How fast does an atrial myxoma grow? Ann Thorac Surg. 2006;82(4):1510‐1512. [DOI] [PubMed] [Google Scholar]
- 31. Barrios D, Chavant J, Jiménez D, et al. Treatment of right heart thrombi associated with acute pulmonary embolism [published correction appears in am J med. 2017 Jul;130(7):874]. Am J Med. 2017;130(5):588‐595. [DOI] [PubMed] [Google Scholar]
- 32. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543‐603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of this article, no data have been used by any of the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.


