Abstract
Background
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has been leading to dramatic health, social and economic problems around the world. It was necessary to introduce worldwide vaccination program against SARS-CoV-2 virus. Vaccination of billions of people around the world leads to many questions about risk of vaccines and possible side effects. It is well known that acute disseminated encephalomyelitis (ADEM) is a rare, but possible complication of vaccines. Previously, cases of ADEM following various COVID-19 vaccines, including the vaccines from AstraZenica, Pfizer, Sputnik V, SinoVac, Moderna, Sinopharm, have been described. In this case report, we present the first documented case of ADEM following the COVID-19 vaccine Ad26.COV2.S from Johnson & Johnson.
Case presentation
We present the case of a 31-year-old female with gradually progression of right-sided weakness and numbness during a three-week period. Four weeks prior to symptom onset, the patient received the single-dose SARS-CoV-2 vaccine Ad26.COV2.S. Neuroimaging revealed five large juxtacortical T2 FLAIR hyperintense lesions with incomplete contrast enhancement on post-contrast T1 images located supratentorial: one in the right cerebral hemisphere and four in left cerebral hemisphere. The patient was followed up for four months. Symptom debut, clinical picture and MRI were typical for ADEM and the patient completely recovered after high dose intravenous methylprednisolone treatment.
Conclusions
This is, to the best of our knowledge, the first case report of ADEM following the COVID-19 vaccine Ad26.COV2.S. This case illustrates, although ADEM is a rare complication following SARS-CoV-2 vaccines, the necessity of maintaining a vaccine safety monitoring system to identify patients at high risk from developing severe complications from the vaccines.
Keywords: Acute disseminated encephalomyelitis, ADEM, COVID-19, SARS-CoV-2, Vaccine, Johnson & Johnson, Ad26.COV2.S
Background
A variety of vaccines have been granted emergency use authorization to fight the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic, also referred to as the COVID-19 pandemic. In the post-authorization phase, a broad range of neurological complications have been reported including neurovascular, neurometabolic and neuroinflammatory diseases (Garg and Paliwal 2022; Finsterer 2022). The reports of post-vaccination CNS inflammation included transverse myelitis, acute encephalitis, neuromyelitis optica and acute disseminated encephalomyelitis (ADEM).
ADEM is an autoimmune inflammatory disease of the CNS, characterized by a brief and widespread demyelination, predominantly involving the white matter, and often appears following a viral or bacterial infection.
We have been able to identify 17 case reports that have described ADEM, including one case report of ADEM-like presentation, following a COVID-19 vaccination: seven cases with mRNA vaccine (from Moderna and Pfizer), (Kania et al. 2021; Vogrig et al. 2021; Shimizu et al. 2021; Lee et al. 2022; Poli et al. 2022; Ahmad et al. 2022; Maramattom et al. 2022) seven cases with a viral vector vaccine (from AstraZenica and Sputnik V) (Rinaldi et al. 2022; Permezel et al. 2022; Mumoli et al. 2022; Nagaratnam et al. 2022; Al-Quliti et al. 2022; Lazaro et al. 2022; Garg et al. 2022) and three cases with vaccines based on inactivated virus (from SinoVac and Sinopharm) (Cao and Ren 2022; Ozgen Kenangil et al. 2021; Yazdanpanah et al. 2022). However, this case report is the first described case of ADEM following exposure to the Ad26.COV2.S vaccine from Johnson & Johnson.
Case presentation
A 31-year-old Caucasian female, with no previous neurological history (Table 1—Patient characteristics), was admitted to the hospital due to slow progression of right-sided weakness and numbness during the past three weeks. At symptom onset, the patient suffered from headache, nausea, mild neck pain, and balance impairment, but no dizziness. Additionally, the patient and some of her family members had noticed mild behavioral changes and cognitive impairments including memory and concentration issues. These symptoms remitted spontaneously after 5–7 days. The patient reported no diarrhea, respiratory infections, fever or chills.
Table 1.
Patient characteristics
| Age | 31 years |
| Sex | Female |
| BMI | 31.6 |
| Ethnic group | Caucasian |
| Medication | None |
| Medical history | No relevant history |
| Surgery history | Appendectomy—over 20 years ago |
| Family history | No relevant history |
Four weeks prior to symptom onset, the patient received the single-dose SARS-CoV-2 vaccine from Johnson & Johnson, Ad26.COV2.S, a recombinant, replication-incompetent Ad26 vector, encoding a stabilized variant of the SARS-CoV-2 spike protein.
Neurological examination revealed moderate right-sided hemiparesis and hypoesthesia. No impaired consciousness was found. Blood tests including complete blood count; glucose; electrolytes; vitamins; thyroid-, kidney and liver function were all within normal reference ranges. Anti-aquaporin-4, anti-myelin oligodendrocyte glycoprotein, and antinuclear antibodies were negative. Screening for HIV and HCV was negative. HBV antigen was negative and HBV antibody was positive (HBs IgM + IgG), due to previous HBV vaccination.
Pharyngeal swab was negative for SARS-CoV-2 on reverse-transcriptase polymerase chain reaction assay. The cerebrospinal fluid (CSF) white blood cell count was 1 × 106/L (reference range 0–5 × 106/L); protein levels were 0.31 g/L (reference range 0.15–0.5 g/L); IgG index was 0.47 (reference range 0.38–0.67); and oligoclonal bands in CSF and blood were negative.
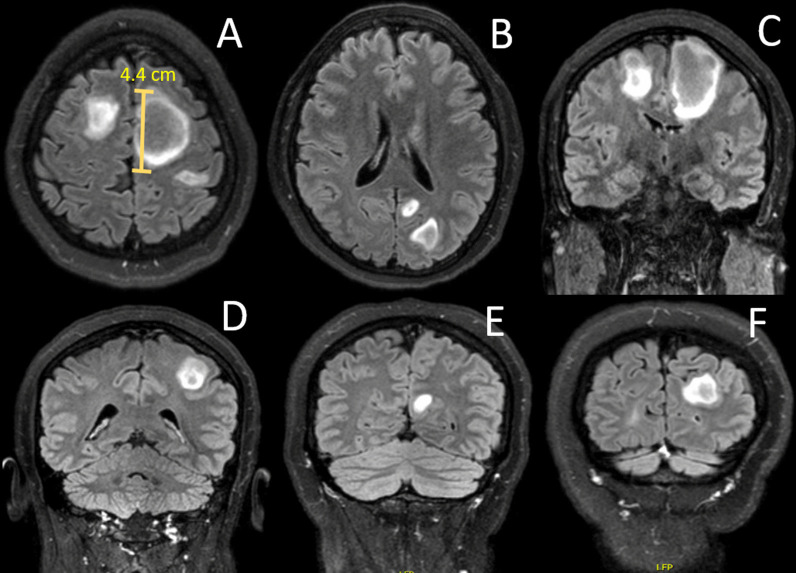
Brain and spinal cord magnetic resonance imaging (MRI) revealed five large juxtacortical T2 FLAIR hyperintense lesions with incomplete contrast enhancement on post-contrast T1 images located supratentorial: one in the right cerebral hemisphere and four in left cerebral hemisphere. The largest lesion was in the right frontal lope with a diameter of approximately 4.4 cm (Fig. 1). No infratentorial or spinal cord lesions were found. The clinical and radiological findings raised the suspicion of ADEM.
Fig. 1.
Axial T2 fluid-attenuated inversion recovery (T2 FLAIR) MRIs at admission showing five large juxtacortical lesions supratentorially. One lesion in the right cerebral hemisphere and four in the left cerebral hemisphere. The largest lesion was located in the left frontal lope and measured approximately 4.4 cm in diameter (A). A + B Axial sections. C–F Coronal sections
On the day at admission, the patient was treated with high-dose IV methylprednisolone, 1000 mg for three consecutive days followed by oral administration of 500 mg prednisolone for four days with significant clinical improvements. She was discharged from the hospital six days after admission where physical examination only revealed mild right-sided hemiparesis.
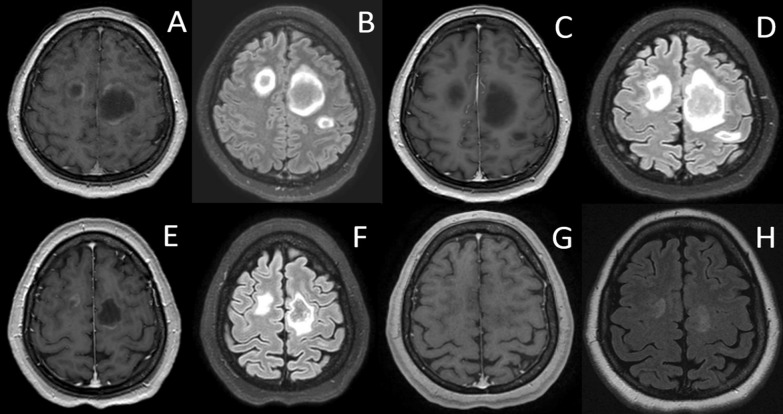
A five-week follow-up MRI showed volume reduction in all the lesions, and no new lesions were found. Two lesions showed incomplete ring enhancement. The last MRI follow-up at four months showed regression in all five lesions with extensive lesion volume reduction and almost complete resolution (Fig. 2). Additionally, the patient presented with complete clinical recovery at the four-month follow-up.
Fig. 2.
Post-contrast T1 weighted MRI (A, C, E and G) and axial T2 fluid-attenuated inversion recovery (T2 FLAIR) (B, D, F and H) during hospitalization and follow-up. First day at hospitalization (A + B), six days follow-up (C + D), five weeks follow-up (E + F) and four months follow-up (G + H)
Conclusions
Many of the previous mentioned SARS-CoV-2 vaccines have been approved by federal agencies or national authorities, e.g., the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) as it has been assessed that the overall benefits of the vaccines outweigh their risks. However, due to the extensive global use of SARS-Cov-2 vaccines. it is important to ensure a fast-acting safety monitor system to discover the rare complications to the vaccines, e.g., ADEM. This would improve the likelihood of identifying the right patients who are at high risk from developing severe complications from the vaccines.
The manifestation of the clinical and paraclinical findings in this case did meet the criteria for ADEM (Krupp et al. 2007). Our patient presented with multifocal neurological abnormalities including mild encephalopathy not explained by fever, postictal symptoms, or systemic illness. MRI of the brain showed diffuse, large (the largest at approximately 4.4 cm) lesions in the cerebral white matter and normal findings of the CSF including negative oligoclonal bands and normal IgG index. We saw a rapid clinical remission with high-dose corticosteroid treatment. It is well known that ADEM is associated with vaccines (i.e., measles, rubella or mumps). A large epidemiological study, which evaluated epidemiological features of post-vaccine ADEM, found that the time interval from vaccination to ADEM onset was 2–30 days in 61% of the cases (Pellegrino et al. 2013). In our case, the patient had symptom onset four weeks after vaccination, in line with the previously reported time-line from vaccine to onset of ADEM.
During the four-month follow-up, the clinical and paraclinical features followed a monophasic disease course; however, a prolonged observation is required to confirm this.
Overall, we have identified 17 previous case reports of patients with ADEM following SARS-CoV-2 vaccination, including vaccines based on mRNA, viral vector and inactivated virus (Kania et al. 2021; Vogrig et al. 2021; Shimizu et al. 2021; Lee et al. 2022; Poli et al. 2022; Ahmad et al. 2022; Maramattom et al. 2022; Rinaldi et al. 2022; Permezel et al. 2022; Mumoli et al. 2022; Nagaratnam et al. 2022; Al-Quliti et al. 2022; Lazaro et al. 2022; Garg et al. 2022; Cao and Ren 2022; Ozgen Kenangil et al. 2021; Yazdanpanah et al. 2022); however, this case report is the first described case of ADEM following exposure to the Ad26.COV2.S vaccine.
In previous cases, the patients age ranged from 19 to 88 years but were predominantly in the ages between 30 and 50, and the majority was females, which is in line with our case. In contrast, the previous cases primarily had time from vaccination to symptoms of 10–14 days, which is half the time as the patient in our case. Additionally, most patients from the previous cases, similar to the patient in our case, had no previous significant medical history, and none of the patients were treated with immune-modifying drugs. This illustrates, although ADEM is a rare complication following SARS-CoV-2 vaccines, the necessity of maintaining a vaccine safety monitoring system to identify patients at high risk from developing severe complications from the vaccines.
Acknowledgements
Not applicable.
Abbreviations
- ADEM
Acute disseminated encephalomyelitis
- CSF
Cerebrospinal fluid
- HBV
Hepatitis B-virus
- HCV
Hepatitis C-virus
- HIV
Human immunodeficiency virus
- MRI
Magnetic resonance imaging
- SARS-CoV-2
The severe acute respiratory syndrome coronavirus-2
- T2 FLAIR
T2 fluid-attenuated inversion recovery
Author contributions
SG wrote the original draft and was reviewed and edited by AW and MMN. SG and AW carried out the clinical work and diagnostics. MMN described MRI images. All authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The data that support the findings of this case report are available from the corresponding author, [SG], upon reasonable request.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad HR, Timmermans VM, Dakakni T. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Am J Case Rep. 2022;23:e936574. doi: 10.12659/AJCR.936574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Quliti K, Qureshi A, Quadri M, Abdulhameed B, Alanazi A, Alhujeily R. acute demyelinating encephalomyelitis post-COVID-19 vaccination: a case report and literature review. Diseases. 2022;10(1):13. doi: 10.3390/diseases10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2022;122(3):793–795. doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol Scand. 2022;145(1):5–9. doi: 10.1111/ane.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3–40. doi: 10.1007/s10072-021-05662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Batra PK, Gupta P. Post COVID-19 vaccination acute disseminated encephalomyelitis: a case report. Curr Med Imaging. 2022 doi: 10.2174/1573405618666220509205457. [DOI] [PubMed] [Google Scholar]
- Kania K, Ambrosius W, TokarzKupczyk E, Kozubski W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS-CoV-2. Ann Clin Transl Neurol. 2021;8(10):2000–2003. doi: 10.1002/acn3.51447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E, International Pediatric Multiple Sclerosis Study Group International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261–7. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- Lazaro LG, PereaCossio JE, Luis MB, Tamagnini F, Paguay Mejia DA, Solarz H, Fernandez Liguori NA, Alonso RN. Acute disseminated encephalomyelitis following vaccination against SARS-CoV-2: a case report. Brain Behav Immun Health. 2022;20:100439. doi: 10.1016/j.bbih.2022.100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hor JY, Koh KL, Chia YK. Central nervous system demyelination following COVID-19 mRNA-based vaccination: two case reports and literature review. J Cent Nerv Syst Dis. 2022;24(14):11795735221102747. doi: 10.1177/11795735221102747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maramattom BV, Lotlikar RS, Sukumaran S. Central nervous system adverse events after ChAdOx1 vaccination. Neurol Sci. 2022;43(6):3503–3507. doi: 10.1007/s10072-022-06000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumoli L, Vescio V, Pirritano D, Russo E, Bosco D. ADEM anti-MOG antibody-positive after SARS-CoV2 vaccination. Neurol Sci. 2022;43(2):763–766. doi: 10.1007/s10072-021-05761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaratnam SA, Ferdi AC, Leaney J, Lee RLK, Hwang YT, Heard R. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol. 2022;22(1):54. doi: 10.1186/s12883-022-02575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OzgenKenangil G, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021;121(4):1089–1091. doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino P, Carnovale C, Perrone V, Pozzi M, Antoniazzi S, Clementi E, Radice S (2013) Acute disseminated encephalomyelitis onset: evaluation based on vaccine adverse events reporting systems. PLoS ONE 8(10):e77766. 10.1371/journal.pone.0077766. Erratum in: PLoS ONE. 2013;8(12). 10.1371/annotation/1d544202-04f5-4848-83f1-696c2de4221e [DOI] [PMC free article] [PubMed]
- Permezel F, Borojevic B, Lau S, de Boer HH. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci Med Pathol. 2022;18(1):74–79. doi: 10.1007/s12024-021-00440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli K, Poli S, Ziemann U. Multiple autoimmune syndromes including acute disseminated encephalomyelitis, myasthenia gravis, and thyroiditis following messenger ribonucleic acid-based COVID-19 vaccination: a case report. Front Neurol. 2022;13:913515. doi: 10.3389/fneur.2022.913515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi V, Bellucci G, Romano A, Bozzao A, Salvetti M. ADEM after ChAdOx1 nCoV-19 vaccine: a case report. Mult Scler J. 2022;28(7):1151–1154. doi: 10.1177/13524585211040222. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Ogaki K, Nakamura R, Kado E, Nakajima S, Kurita N, Watanabe M, Yamashiro K, Hattori N, Urabe T. An 88-year-old woman with acute disseminated encephalomyelitis following messenger ribonucleic acid-based COVID-19 vaccination. eNeurologicalSci. 2021;25:100381. doi: 10.1016/j.ensci.2021.100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogrig A, Janes F, Gigli GL, Curcio F, Negro ID, D'Agostini S, Fabris M, Valente M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah F, Iranpour P, Haseli S, Poursadeghfard M, Yarmahmoodi F. Acute disseminated encephalomyelitis (ADEM) after SARS- CoV-2 vaccination: a case report. Radiol Case Rep. 2022;17(5):1789–1793. doi: 10.1016/j.radcr.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this case report are available from the corresponding author, [SG], upon reasonable request.