Abstract
Cardiac tumors may be primary (either benign or malignant) or secondary (malignant) and are first detected by echocardiography in most cases. The cardiologist often challenges their identification, the differential diagnosis and the best therapeutic approach. Malignant tumors have usually a poor prognosis, which may be significantly improved by appropriate and timely therapies. The echocardiographic aspects of benign and malignant cardiac tumors described in this article, along with a clinical evaluation may orient the differential diagnosis and aid in choosing the further steps useful to define the nature of the mass,
Keywords: cardiac masses, cardiac tumors, diagnostic pathways, echocardiography, multimodality imaging
All the malignant and some benign cardiac have a dismal prognosis without a prompt treatment. Correctly recognizing cardiac tumors and assessing their potential clinical risks is of utmost importance. The clinical aspects and echocardiographic information should be integrated to plan the diagnostic and therapeutic pathways.
Abbreviations
- CDI
Color Doppler imaging
- CT
computed tomography
- HF
heterogeneity factor
- MRI
magnetic resonance imaging
- PET
psitron emission tomogrsaphy
- SDI
spectral Doppler imaging
- SUVmax
maximum standardized uptake
- TOE
transoesephageal echocardiography
- TTE
transthoracic echocardiography
- UEA
ultrasound enhancement agents
- 2D
two‐dimensional
- 3D
three‐dimensional
1. INTRODUCTION
Secondary cardiac tumors are quite rare, and primary tumors are even rarer; the prognosis of malignant tumors is often poor. 1 , 2 , 3 However, in the recent years the prognosis of several primary malignant tumors has improved, thanks to the progresses of both surgical and medical treatments. 4
The most frequent secondary tumors are represented by pericardial metastases of carcinomas (mostly lung or breast carcinomas) or melanoma, myocardial metastases originating by solid or hematological tumors, and by intracavitary metastases or extension of gynecologic, urological, or lung tumors. 5 , 6 The primary tumors are represented by benign and by malignant tumors; some tumors originating by a given histotype may grow as benign masses or differentiate in a malignant way (Table 1). 7 Some tumors (as the inflammatory myofibroblastic tumor, paragangliomas, and some germ cell tumors) may also show an intermediate or uncertain clinical behavior; the classification of cardiac tumors is further complicated by the possible presence of different histotypes (i.e., myxoid, fibrous, condroid and bone areas, and so on) in the same mass. 7
TABLE 1.
Simplified classification of the most common benign and malignant cardiac tumors. The arrows show the benign tumors which may have a malignant counterpart
| Benign | Malignant | ||
|---|---|---|---|
| Myxoma | >>>> | Myxosarcoma | |
| Rhabdomioma | >>>> | Rhabdomiosarcoma | |
| Angiomas | >>>> | Angiosarcomas | |
| Fibromas | >>>> | Fibrosarcoma | |
| Lipoma | >>>> | Liposarcoma | |
| Schwannoma | >>>> | Malignant peripheral nerve sheat tumor (MPNST) | |
| Cystic tumor of the atrio‐ventricular node | Other (myxofibro, leiomyo, undifferentiated pleomorphic, synovial, extrasketal osteo, etc.) sarcomas | ||
| Papillary fibroelastoma | Lymphomas | ||
| Metastatic tumors | |||
| Mesothelioma | |||
| Intermediate or uncertain behavior | |||
| Paraganglioma | |||
| Inflammatory myofibroblastic tumor | |||
| Germ cell tumors | Mature teratoma | >>>> | Immature teratoma |
| Yolk sac tumor | |||
The benign tumors may either be surgically removed or kept on follow‐up if they do not interfere with the hemodynamics and are not at risk of embolization. Some tumors‐like rhabdomyomas‐ may also show a spontaneous regression over time; thus a follow‐up strategy is usually adopted. 8 , 9 , 10 , 11 , 12 However, even benign tumors may cause clinical symptoms due to hemodynamic impairment (valve obstruction, compression of vessels and cardiac chambers), arrhythmias, and embolism requiring therapy, either medical or surgical. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Rhabdomyomas inducing atrial or ventricular arrhythmias have been reported in 16%–47% of cases; an interesting observation is that the tumor cells are structurally similar to Purkinje cells and can function like accessory pathways creating continuity at the atrioventricular junction inducing a pre‐excitation syndrome which disappears with the tumor regression. 21 , 22 The mainstay of treatment of hematological neoplasms (either primary, like some lymphomas, or metastatic) is chemotherapy, and a surgical approach is seldom required. 23 , 24 , 25 Also, for metastatic melanomas, the standard approach is target therapy and/or immunotherapy. 26 For primary cardiac sarcomas, on the contrary, the mainstay of treatment is surgery, which often must be associated with chemo‐ or radiotherapy. 27 To improve survival both radical surgery and a multi‐therapeutic approach are relevant. 28
When a cardiac mass is detected in a patient with a known systemic tumor, obtaining a differential diagnosis between a thrombus and metastasis is essential to orient the therapeutic approach and has a relevant prognostic role. 29 , 30
Thus, the differential diagnosis is of utmost importance to plan the most appropriate therapy, and a multimodality imaging approach is often required. 1 , 31 , 32 , 33 , 34 , 35
2. GENERAL DIAGNOSTIC APPROACH
For an appropriate diagnostic approach, the first step is differentiating between cardiac tumors and other cardiac masses such as thrombi or vegetations. Four steps should be followed to orient the diagnosis. 36
The clinical setting. In a patient with fever and positive blood cultures, or any other sign suggesting infective endocarditis, an aortic mass is more likely vegetation rather than a tumor. In a patient with coronary artery disease, with severe mitral stenosis or long‐lasting atrial fibrillation not on anticoagulant therapy, a mass on the akinetic left ventricular wall or –respectively‐ in an enlarged left atrium is probably a thrombus rather than a tumor. On the other hand, a mass extending from the inferior vena cava into the right atrium in a patient with a history of liver carcinoma is probably a metastasis or a tumor thrombus (Figure 1). A right atrial mass without any relationship with the inferior vena cava and with the same echogenicity of intrapleural metastasis of sarcoma is probably a metastasis (Figure 1). Tumor thrombus is a consequence of tumor invasion into the venous system, with activation of the coagulation and the simultaneous growth of both tumor and thrombus; it is rather frequent in the portal system in patients with hepatocarcinoma, but it can be observed also in the inferior vena cava invaded by a liver, gynecological or other abdominal cancers and requires a therapeutic approach not limited to anticoagulation (as is the case of simple thrombosis). 37 , 38 , 39 , 40 , 41 , 42 Tumor thrombi have been reported also in the pulmonary veins. 43
The histology‐based likelihood and the age of the patient at the time of presentation. (Table 2). The most frequent benign tumors in adult age are myxomas and papillary fibroelastomas. In fetal life and in early childhood the most frequent benign tumors are rhabdomyomas (which may be associated with tuberous sclerosis, neurofibromatosis, or other hereditary syndromes) and fibromas. The primary malignant tumors, both in children and in adults, are mostly represented by sarcomas (in adults, by angiosarcomas). 44 , 45 , 46 Amongst the tumors with uncertain behavior, the inflammatory myofibroblastic tumor, teratomas and yolk sac tumors are typical of fetuses and children; paragangliomas are more common in adults. 7
The tumor location. The intracardiac tumors can arise in every cardiac cavity or in every cardiac wall, even though each type of cardiac tumor prefers a well‐defined location. Myxomas are most commonly detected in the left atrium. The most common tumors on cardiac valves are papillary fibroelastomas (Figure 2; Video clip 1). Unlike vegetations in patients with native valve endocarditis, papillary fibroelastomas are usually attached downstream of the valve (on the aortic side of the aortic valve or on the left ventricular side of the mitral valve) (Video clip 2). The malignant tumors are usually sessile (with a broad implant) and often infiltrate the surrounding structures. Angiosarcomas, the most frequent primary malignant tumor, usually originate from the right atrial walls (near the inferior vena cava or involving the whole free wall, atrial roof, and interatrial septum) and often extend to the pericardium and to the right ventricle (Figures 2B and 3) but may be found also in the left chambers. 47 , 48 , 49 The tumors arising in the pulmonary artery or in the aorta are usually intimal sarcomas; in other cardiac cavities (atria, ventricles) the most frequent histotype are spindle cell sarcoma and leiomyosarcoma. 50 Left atrial tumors occupying also one or more pulmonary veins may be an extension of lung cancer, cardiac or other intrathoracic tumors. 51 , 52 , 53 , 54 , 55 , 56 , 57
FIGURE 1.
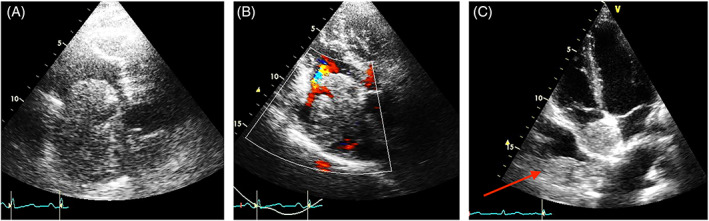
Tumor thrombus extending from the inferior vena cava to the right atrium in a patient with liver carcinoma. A. TTE‐2D Apical four‐chamber modified. B. The color Doppler helps to better define the irregular shape, typical of tumor thrombi. C. Right atrial mass without any relationship with the inferior vena cava, in a patient with metastatic sarcoma. TTE‐2D Apical four chamber. The mass has the same echogenicity of the intrapleural metastasis (red arrow). This mass should be considered a metastasis
TABLE 2.
Clinicopathologic features of primary heart tumors
| Histologic type | Age | Site in heart | Multiplicity | Syndromic association | |||
|---|---|---|---|---|---|---|---|
| Fetuses, infants | Children | Adults | Layer | Location | |||
| Benign congenital tumors | |||||||
| Rhabdomyoma | ++ | + | Myocardium a | Ventricles | Usual | Tuberous sclerosis | |
| Fibroma | + | ++ | + | Myocardium | Ventricle, ventricular septum | Rare | Gorlin syndrome |
| Histiocytoid cardiomyopathy | ++ | +/1 | Endocardium, myocardium | Ventricles, atrial, and AV SA nodes | Always | ||
| Benign acquired tumors | |||||||
| Myxoma | +/− | ++ | Endocardium | LA, atrial septum | Rare | Carney complex | |
| RA, atrial septum | |||||||
| Papillary fibroelastoma | ++ | Endocardium | Valves > atria > ventricles | Occasional | |||
| Hemangioma b | + | + | + | Myocardium Endocardium c | Atria > ventricles | Unusual | |
| Lipomatous hypertrophy | ++ | Myocardium of atrial septum | |||||
| Lipoma | ++ | Myocardium, epicardium, endocardium | All sites | Rare | |||
| Inflammatory myofibroblastic tumor d | ++ | + | +/− | Endocardium | Valves > atria | Occasional | |
| Germ cell tumors | |||||||
| Teratoma | ++ | + | +/− | Pericardial cavity | No | ||
| Ventricular septum (rare) | |||||||
| Yolk sac tumor | ++ | + | Pericardial cavity | No | |||
| Ventricular septum (rare) | |||||||
| Malignant tumors | |||||||
| Angiosarcoma | +/− | ++ | All layers | Right atrium, pericardium | Occasional | ||
| UPS/myxofibrosarcoma | +/− | +/− | ++ | Endocardium | Left atrium | Rare | |
| Other sites | |||||||
| Rhabdomyosarcoma | +/− | ++ | + | Myocardium | Ventricles | No | |
| Leiomyosarcoma | +/− | ++ | Endocardium | Left atrium | No | ||
| Lymphoma | +/− | ++ | Myocardium | Right atrium, others | Occasional | ||
Abbreviations: AV, atrioventricular; LA, left atrium; RA, right atrium; UPS, undifferentiated pleomorphic sarcoma; SA, sinoatrial; WHO, World Health Organization.
Boldface indicates an exclusive site.
Hemangioma is considered alternatively as a congenital tumor, especially in children.
Especially, the capillary type.
Inflammatory myofibroblastic tumors are sometimes considered low‐grade malignancies, although none in the heart has metastasized. Reproduced, with permission, from Ref. 7
FIGURE 2.
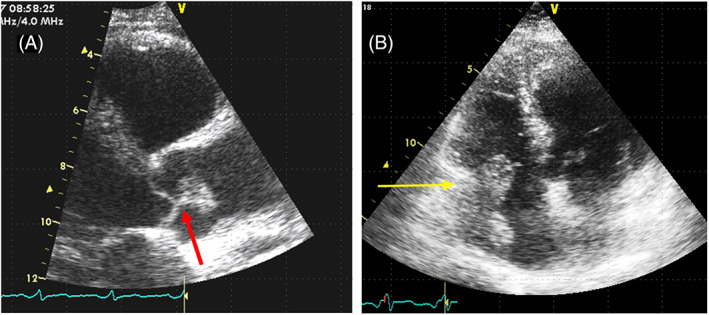
A. Papillary fibroelastoma of the aortic valve. TTE‐2D Long x is in diastole. Thin stalk at the aortic surface of the cusps (red arrow). B. Right atrium Angiosarcoma. TTE‐2D apical four chamber. Broad insertion on the lateral right atrial wall, with infiltration of the tricuspid annulus (yellow arrow). The mass has a lobulated surface
FIGURE 3.
Two cases of angiosarcoma. A: Apical four chamber view of a huge mass infiltrating the right atrium, right ventricle and the pericardium. B: TOE‐2D is focused on the right chambers. The mass infiltrates the interatrial septum, the roof and the lateral wall of the right atrium
Cardiac lymphomas may occupy any cardiac chamber or the pericardial space and show usually a very fast growth (Figure 4).
FIGURE 4.
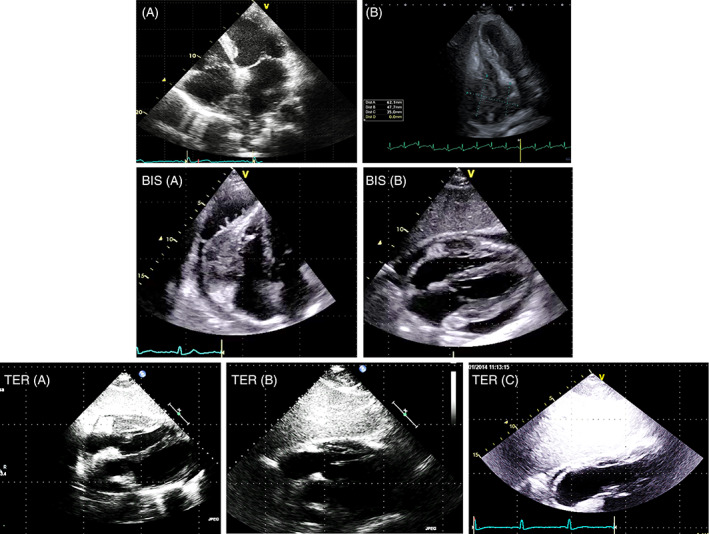
Two cases of lymphoma (TTE‐2D Apical four‐chamber). A. The mass infiltrates the interatrial septum, the roof of the right atrium and the superior vena cava. B: A large massoccupying most of the right atrIum infiltrating the lateral wall, the atrio‐ventricular groove and the right ventricle BIS. Pericardial lymphoma. A. TTE‐2D Apical four‐chamber. B. TTE‐2D subcostal four‐chamber The huge mass infiltrates the free wall of the right ventricle and is not homogeneous (haemorrhagic foci). The mass takes up pericardial effusion. Cardiac MRI, CT scan and PET were performed. Biopsy allowed the phenotypic characterization and the tailored chemiotherapy TER. The same patient of BIS. A‐B‐C TTE‐2D subcostal four‐chamber. Echocardiography was performed within 2 months of each other to assess the efficacy of chemiotherapy. In C the lymphoma is no longer recognizable
Tumors may also arise from atypical sites. Myxomas have been described as attached to the inferior part of the atrial septum, below the fossa ovalis region, or to the lateral wall of the left atrium, and even in the left or right ventricle. 58
-
4
The morphological and functional characteristics: size, shape, mobility, tissue characterization, vascular supply, and metabolic activity.
Points 3 and 4 may be investigated using different imaging techniques: echocardiography, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT). Each technique has advantages and disadvantages in terms of costs, availability, and diagnostic power: often they must be used in combination. 33 , 59
Transthoracic echocardiography (TTE) and transoesophageal echocardiography (TOE), thanks to their availability and low cost, are generally the initial diagnostic tests utilized for the evaluation of a suspected cardiac tumor.
In this article, we will analyze the echocardiographic aspects of cardiac tumors.
3. ECHOCARDIOGRAPHIC APPROACH
All modalities of TTE and TOE examinations are used to obtain the most accurate information about the tumor characteristics. Two‐dimensional (2D), three‐dimensional (3D), Color Doppler imaging (CDI), spectral Doppler imaging (SDI), and additional techniques such as the use of agitated saline as well as ultrasound enhancement agents (UEAs) are used to make the diagnosis. TOE‐2D, TOE‐3D and intracardiac ultrasounds are also used as a guide to the bioptic procedure necessary for obtaining a histology specimen. 60 , 61
The natural contrast between the mass and the blood of cardiac chambers or the intraluminal blood of the vessels facilitates the visualization of intracavitary tumors. The color Doppler may help to define the contours of some tumors with low intrinsic echogenicity (Figure 5).
FIGURE 5.
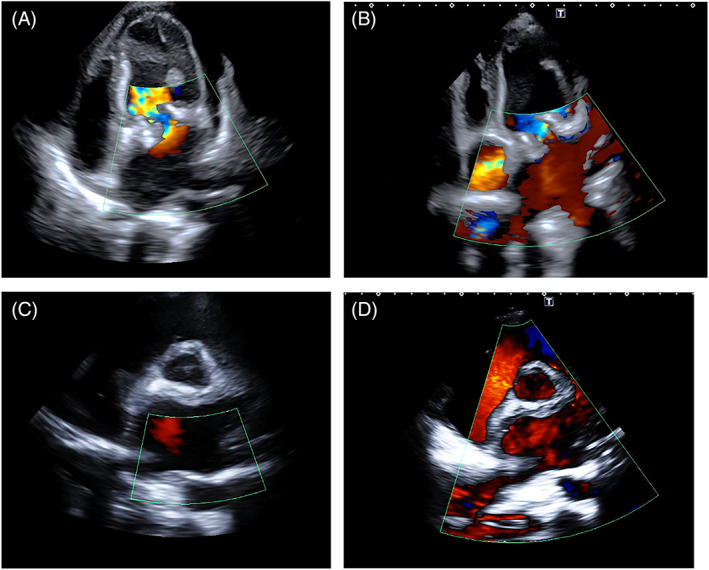
Patient with mitral bioprosthetic valve and diagnosis of left atrium sarcoma. A low‐echogenic mass occupies almost entirely the left atrium. The color Doppler allows the identification of the contour line of the mass. TTE‐2D 4 chamber (top) and short axis (bottom) pre (left) and after (right) chemotherapy. After chemotherapy, the mass is markedly reduced in size and the color fills most of the left atrial cavity and the pulmonary veins
Cardiac tumors, regardless of their nature, primary or metastatic and benign or malignant, are studied using a systematic approach with the goal of defining morphologic and dynamic appearance, evaluating hemodynamic consequences, and the possible therapeutic interventions.
4. SIZE OF THE TUMOR
Cardiac tumors can range in size from a few millimeters to several centimeters. In many cases, the size of the tumor may be correctly evaluated by TTE.
Goswami reported in his experience in 70 consecutive patients with 73 cardiac myxomas a range in size from 2.0 to 9.5 cm in maximum diameter assessed by 2D echocardiography. The echocardiographic dimension correlated very well with the anatomic measurement at surgery. 62 In our experience, cardiac myxomas show high variability of size (Figure 6).
FIGURE. 6.
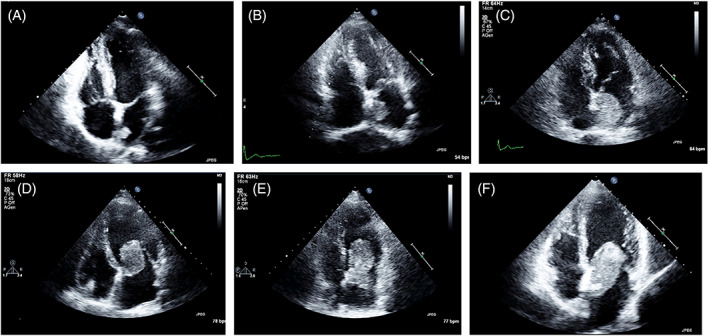
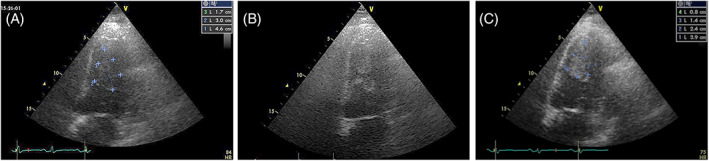
TTE‐2D. Four‐chamber apical view in six patients with left atrial myxomas of different sizes
However, for their distensibility and mobility, very soft tumors (myxoid or gelatinous type mass) when prolapsing deform and elongate, modifying their size. Also, in the case of irregularly shaped tumors, 2D echocardiography may lead to an erroneous assessment of the largest diameter. 3D Echocardiography in full volume modality images the entire volume of the tumor and facilitates linear measurements along orthogonal planes and allows volume measurements (Figure 7).
FIGURE 7.
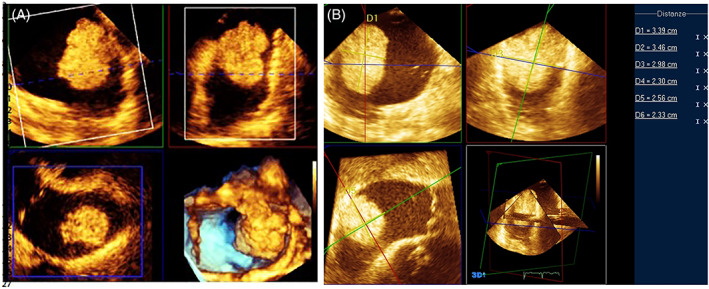
TOE‐3D. Multiplanar reconstruction of the mass (A) and linear measurements along orthogonal planes (B)
5. SITE AND MODALITY OF ATTACHMENT
The attachment site gives an important clue to defining the type of cardiac tumor. Left atrium myxomas are usually attached to the fossa ovalis region of the atrial septum (Figure 8). Right atrium angiosarcomas from any part of the right atrial wall (Figures 2B, 3 and 9). Tumors may also arise from atypical sites. Left atrium myxomas may be attached to the inferior part of the atrial septum, below the fossa ovalis region, or to the lateral wall of the left atrium (Figure 10).
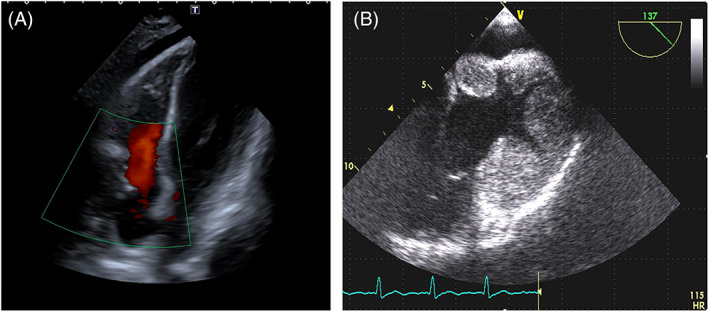
FIGURE 8.
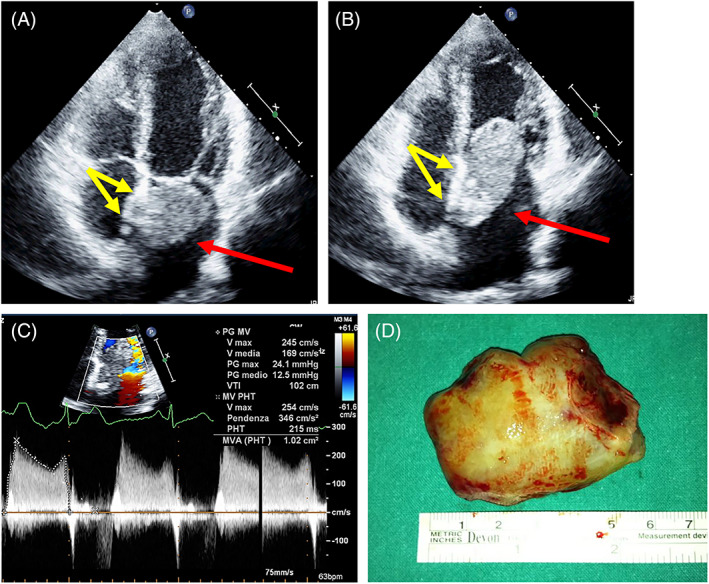
Left atrial myxoma attached to the fossa ovalis (usual site). TOP: TTE‐2D Apical four chamber. Systole (A), Diastole (B). The red arrow indicates left atrial mass. The yellow arrows indicate the site of attachment. Bottom: transmittal Doppler study assessing the obstruction (C), And the surgical specimen
FIGURE 9.
Right atrium angiosarcoma. TOE‐2D mid‐upper position. The two views are focused on right chambers in systole. An irregular and dishomogeneous giant mass (60 × 45 mm) infiltrates the free wall and the tricuspid annulus and protrudes into the right atrium
FIGURE 10.
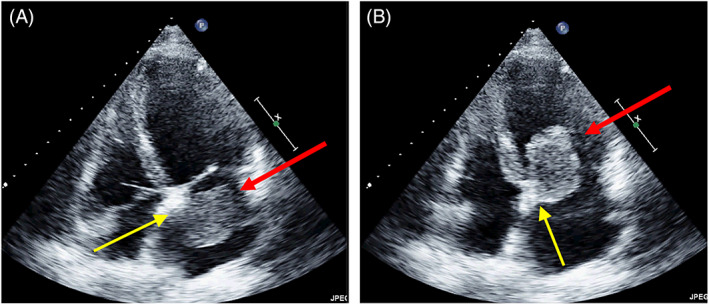
A left atrial myxoma attached on atrial septum below the fossa ovalis (unusual site). Tee‐2d apical four chamber. Systole (A), Diastole (B). The red arrow indicates left atrial mass. The yellow arrows indicate the stalk
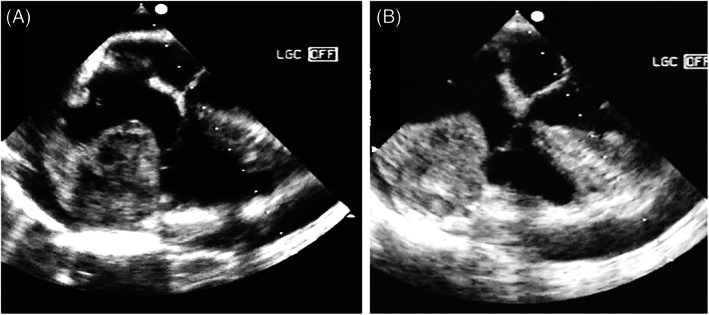
Tumors are attached via a stalk (pedunculated tumors) or directly to the cardiac structures (sessile tumors). The stalk is usually narrow in papillary fibroelastomas (Figure 11) but is of different sizes in myxomas (from narrow, the most common feature, to broad) (Figures 6, 7, 8 and 10) and in other tumors. Sometimes it is difficult to detect where the mass is attached because the stalk is not clearly seen with TTE. The stalk, depending on its position, can be easily detected with TOE. TOE‐3D allows the exact location of the attachment of the stalk and its relationship with surrounding structures (Figure 7; Video clips 2 and 3).
FIGURE. 11.
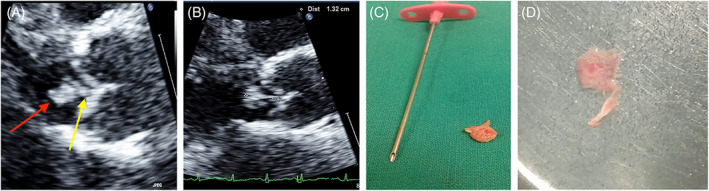
Top: TEE long axis view of an aortic papillary fibroelastoma. The mass is measured on zoomed still frames (1.32 mm). Red arrow indicates the head of the mass. The yellow arrow indicates the stalk. The attachment is on the ventricular side of aortic cusp at level of the mid portion. (An unusual site of attachment, Bottom: surgical specimen
Sessile cardiac tumors are broad‐based and, when malignant, may extend and infiltrate the surrounding structures (Figures 2B, 3, 4, 5, and 9).
6. SHAPE AND SURFACE
On 2D‐3D echocardiography, the shape of tumors depends on several factors: size, consistency, mobility, and size of the recipient chamber.
Primary benign tumors usually have a regular shape and are grossly rounded. Atrial myxomas show the most variability in shape and consistency. Capsulated myxomas are round/ovoid with regular borders and a smooth surface.
Papillary and gelatinous myxomas, not capsulated, have a more irregular shape and are soft with a multilobate surface. A high rate of embolization characterizes this irregular‐shaped myxoma because of the fragmentation of its surface. The ovoid shape becomes elongated when prolapsing in the ventricle.
Papillary fibroelastomas are club‐shaped. The head and peduncle are well‐defined and rarely are strand‐like. The surface is usually smooth (Figure 11).
Intramural tumors that develop inside ventricular walls are grossly ovoid.
Primary malignant tumors are usually broad‐based and arise in a cardiac chamber with a multilobate or polyploidy shape. The shape of metastatic tumors depends on the mechanism and site of invasion (intrapericardial, intramyocardial, and intracavitary).
7. MOBILITY
The mobility of tumors depends on several factors: location and site of attachment, length and width of the stalk if present, size and tissue characteristics.
Intramyocardial or intrapericardial tumors move with the entire heart, but they do not have intrinsic mobility. They can, however, limit the cardiac kinetics (Figure 12, video clip 4).
FIGURE 12.
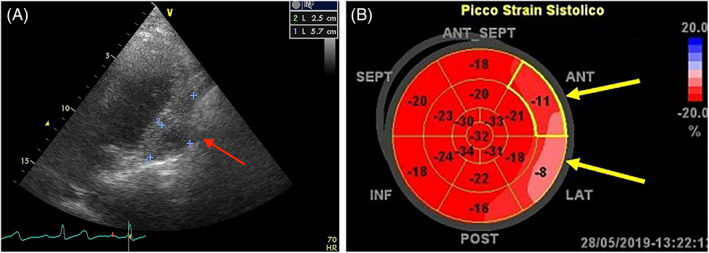
A. Sarcoma infiltrating the pericardium and the lateral wall of the left ventricle (Red arrow). B. Global Longitudinal strain shows low values of strain (−11% and – 8%) at level of infiltrated walls (Yellow arrow)
Intracavitary tumors show high mobility inside the cardiac chamber if pedunculated or when, even if broad‐based, they have some prolapsing extension (Video clip 5). Papillary fibroelastomas are usually highly mobile, with a fine fluttering when prolapsing into the cardiac chambers. Even not prolapsing papillary fibroelastomas may have any way high intrinsic mobility (Video clips 1 and 2).
More significant variability is shown by myxomas with a range from immobility to a higher degree of mobility. A broad‐based, capsulated, solid myxoma may appear as a no‐mobile mass. A huge, narrow‐stalked, gelatinous, and polypoid myxoma may be hypermobile when prolapsing (video clip 6).
Sometimes it is difficult to identify the origin of the atrial masses or masses attached to the cardiac valves at TTE. Both 2D and 3D‐TOE allow identifying the exact location of the attachment of the mass, the type of insertion (stalk vs. sessile), its relationship with surrounding structures, and a better definition of the characteristics of the whole mass and of its mobility, including the borders (regular or irregular) and, in the real‐time display, if there is any vibration of the surface. These aspects are relevant in the decisions about the therapeutic approach because tumors with thin stalks, irregular and/or vibrating surfaces have a higher risk of embolism. 63 , 64 , 65 , 66
8. TISSUE CHARACTERIZATION
Tumors, according to their 2D–3D echocardiographic intra‐mass appearance, may be either homogeneous or inhomogeneous as defined by echogenicity which provides some preliminary information regarding the tissue characteristics of the tumor.
Rhabdomyomas, rhabdomyosarcomas, and leiomyosarcomas usually show a homogenous echogenicity; hyperechogenic and hyperlucent central areas may represent foci of necrosis or of calcification. Lipomas may show different aspects, from hypo‐ to hyperechoic, but are usually homogenous. 67 , 68 , 69 Fibromas show usually an increased echogenicity compared to the normal myocardium. 70 , 71 , 72 Hemangiomas, angiosarcomas and lymphomas usually show an inhomogeneous echogenicity with scattered echolucent areas (Figure 13). 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81
FIGURE 13.
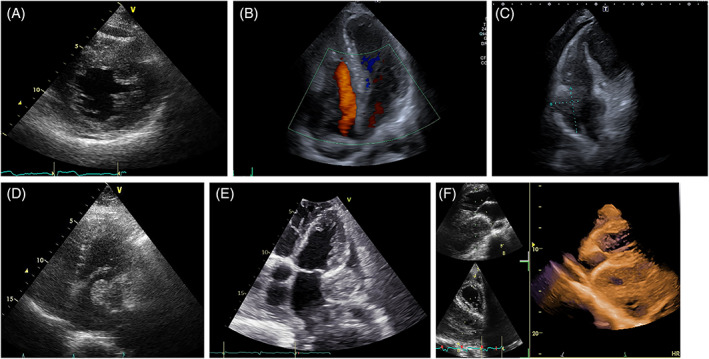
Different appearances depending on tissue characteristics. (A) Neuroendocrin tumor infiltrating the interventricular septum: granular echogenicity (B) non Hodgkin Lymphoma infiltrating the right chambers, with both hyper‐ and hypoechogenic areas. (C) angiosarcoma infiltrating the right chambers: irregular echogenicity. (D) Myxosarcoma with condroid areas, which appear hyperechogenic. (E) Extrascheletal osteosarcoma involving the pericardium, the left atrium and the right chambers: the hyperechogenic areas correspond to calcification. (F) Pericardial sarcoma with hypoechogenic areas due to necrosis: at 3D reconstruction, with cropping these areas appear as «holes» within the mass
The echogenicity of the tumors may change over time, following both chemotherapy and radiotherapy: chemotherapy may induce necrotic processes evident at echocardiography as anechoic areas; the necrosis induced by radiotherapy, on the contrary, may lead to inflammation and fibrosis which increases the echogenicity (Figures 14 and 15).
FIGURE 14.
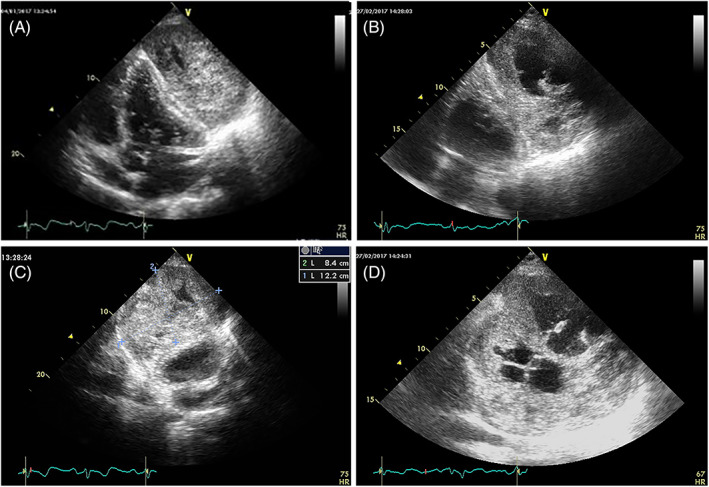
Changes in echogenicity in a case of pericardial leiomyosarcoma. Left: at diagnosis. Right: after chemotherapy. Large anechoic areas (necrosis, confirmed by magnetic resonance imaging) are evident
FIGURE 15.
Left ventricular leiomyosarcoma. Left: before RT, homogenous low echogenicity. Middle: during RT, anechoic and echoic areas. Right: at the end of RT the mass shows a uniform high echogenicity
Vascularity of cardiac angiomas and angiosarcomas can also be assessed with color flow Doppler imaging, setting the machine to optimize the vision of venous flows (Figure 16, Video clip 7) as also described for other angiosarcomas. 82
FIGURE 16.
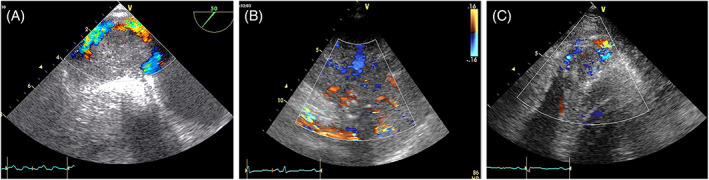
Three cases of angiosarcoma with Color Doppler showing their hypervascularization. A. TOE of a mass originating from the right atrium and inferior vena cava. B. long axis parasternal view of a mass extended from the right atrium to the right ventricle. C. Apical view of a mass infiltrating the right ventricular apex.
Ultrasound‐enhancing agents (UEAs) have been routinely used in the echographic detection of tumors, mostly in the liver and other abdominal tumors, but their use is extending to many other tumors and to lymph nodes and is not limited to diagnosis. 83 , 84 , 85 , 86 , 87
Echocardiographic perfusion imaging using UEAs with very low mechanical index and with intermittent flash (high‐Mechanical Index flashes) has been used mostly for the detection of cardiac ischemia, but it can also characterize the vascularization of cardiac tumors differentiating highly vascular tumors from hypo‐vascular benign tumors and a‐vascular thrombi. 88 Even without the use of intermittent flash, UEAs may improve both the visualization of the tumor and the CDI of the abnormal tumor vessels (Video clip 7).
The UEAs tissue characterization is obtained by analyzing the qualitative and quantitative differences between the levels of enhancement of the tumor compared with adjacent myocardium. 89 The hyperenhancement of the tumor is due to abnormal neovascularization necessary to provide nourishment for rapidly growing malignant tumor cells and is typical of malignant tumors; the higher the vascularization, the earlier the hyperenhancement (Figure 17, Video clip 8). 90 , 91 However, also benign, highly vascularized tumors, such as hemangiomas, are enhanced by contrast agents. 92 , 93 Stromal tumors such as myxomas in which blood supply is poor are partially enhanced. The avascular thrombi and fibroelastomas do not show any enhancement. 94 , 95 , 96 , 97 , 98 , 99 , 100
FIGURE 17.
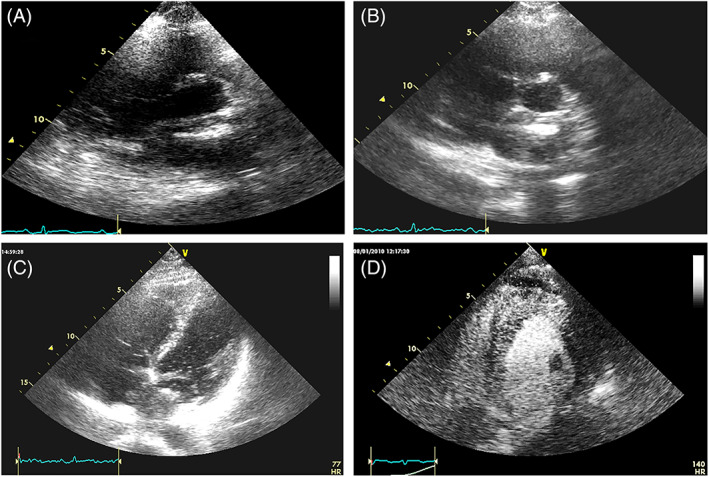
Left atrial sarcoma, with low echogenicity, hardly defined at standard TTE (A). 5 min after the injection of SonoVue, when the cardiac chambers are not anymore opacified, the echogenicity of the mass is enhanced (B and C). D: myocardial infiltration by non‐Hodgkin lymphoma. Shortly after the injection of SonoVue the myocardium shows a granular echogenicity
9. HEMODYNAMIC IMPACT
The atrial tumors are defined as obstructive if the mitral or tricuspid area is smaller than 2 cm2 calculated by pressure half‐time (Figure 8). The mechanism of obstruction is the reduction of the atrioventricular orifice caused by the prolapsing mass (Video clip 5). Sarcomas and lymphomas of both the left and right atrium and with rapid growth may also become obstructive by infiltrating the mitral or tricuspid annulus; Pulmonary artery sarcomas often induce significant stenosis (Figure 18). The venae cavae or the pulmonary veins may also be obstructed by sarcomas extending from the atria (Figures 5 and 16). Valve regurgitation is a rare complication of the valvular fibroelastomas: the mechanism is the traction of the cusp. The extracardiac tumors, mainly huge and solid tumors, may produce the compression of vessels or cardiac chambers.
FIGURE 18.
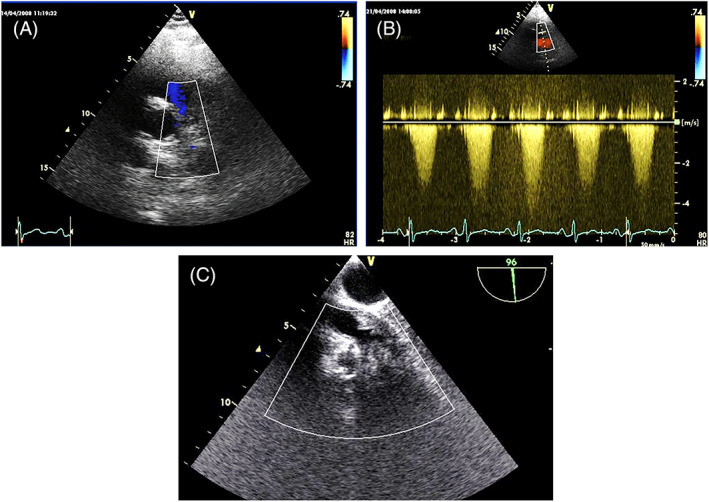
Pulmonary artery sarcoma. (A). poorly echogenic mass with severe pulmonary artery stenosis (B). (C) The mass is better defined from the TOE approach
10. INTEGRATING THE ECHOCARDIOGRAPHIC INFORMATION IN A CLINICAL FRAMEWORK
Most of the information necessary to define the mass (location and site of attachment, size, shape and surface, tissue characterization, mobility, spatial relationship to adjacent structures, the hemodynamic impact of the mass, pericardial effusion) can be assessed by echocardiography. For its good temporal resolution, Echocardiography is an optimal imaging modality to detect and image small and highly mobile masses, especially when arising from valves.
TTE is the first diagnostic tool for patients with a suspected cardiac mass because of its ubiquitous availability but other more advanced echocardiographic techniques are often required to better define the cardiac mass, mostly for intraatrial masses. In fact, several cases of malignant tumors diagnosed at surgery or after recurrence after a misdiagnosis of myxoma have been reported. 101 , 102 , 103
TOE‐2D, TOE‐3D, and UEAs are usually planned according to the clinical setting and to the further information required.
TOE‐2D is usually necessary to obtain a more precise assessment of the atrial masses and of their relationship with the pulmonary veins and with the superior vena cava. It is also useful in the study of pulmonary artery tumors, and of valvular masses.
TOE‐3D offers incremental value for the evaluation of intracardiac masses by providing more accurate assessment of the location and site of attachment, size and shape of the tumor as well as tissue characterization and the relationship between the mass and adjacent structures. 104
Table 3 summarizes the usefulness of various echocardiographic techniques in the analysis of different mass aspects.
TABLE 3.
Comparison of the five echocardiographic modalities for diagnostic features of cardiac tumors
| TTE‐2D | TTE‐3D | TOE‐2D | TOE‐3D | UEAs | |
|---|---|---|---|---|---|
| detection | +++ | + | ++++ | ++ | − |
| Location | +++ | ++ | ++++ | ++++ | + |
| Attachment | ++ | ++ | +++ | ++++ | − |
| Size | ++ | +++ | +++ | ++++ | − |
| Shape/surface | ++ | ++ | +++ | ++++ | − |
| Mobility | +++ | +++ | ++++ | ++++ | |
| Tissue characterization | ++ | ++ | +++ | ++ | ++++ |
| Hemodynamic impact | +++ | ++ | +++ | ++ | − |
Abbreviations: TTE, transthoracic echocardiography; TOE, transoesophageal echocardiography; UEAs, ultrasound‐enhancing agents.
Note: Utility: – no, + fair, ++ good, +++ very good, ++++ very very good.
Echocardiography may have some limits in assessing accurately the origin of the mass (especially if it arises from outside the standard echocardiographic views, or is very large), the extent and relationship with adjacent structures, and the tissue characterization; moreover, some benign and malignant tumors (see Table 1) may appear morphologically identical, mostly in the first stages, and the final diagnosis may be obtained only by pathology. These limits of echocardiography can be partially overcome by other imaging techniques. The best imaging technique in differentiating benign masses from their malignant counterpart (myxoma vs. myxosarcoma, fibroma vs. fibrosarcoma, angioma vs. angiosarcoma and so on) is PET/CT, which is based on the metabolic activity of the tissues: using 18‐Fluorodeoxiglucose (18FDG), a high maximum standardized uptake (SUVmax) and intratumoral glucose metabolic heterogeneity factor (HF) may discriminate well between malignant (sarcomas, lymphomas, or other malignant tumors) and benign masses, as well as in differentiating a tumor thrombus from a simple thrombus. 105 , 106 , 107 , 108 , 109 , 110 To improve the sensitivity and specificity of 18FDG in the particular setting of cardiac tumors, the physiologic glucose uptake by the myocardium must be minimized. This can be obtained with a low‐carbohydrate, high‐fat diet and with the administration of 50 Units/Kg of body weight of unfractioned heparin before the injection of18 FDG. 111 , 112 Other tumors, as neuroendocrine tumors and paragangliomas, require the use of different tracings as 68Ga‐DOTA‐peptide or others. 113 , 114
For ventricular tumors, mostly if they are in close relationship with a coronary artery, a coronary angiography or a CT scan with cardiac synchronization and 3D reconstruction may be considered to better define both the size of the mass and its relationship with the neighboring structures; CT and MRI may also aid in the tissue characterization.
In some cases, however, an extensive multimodality imaging workout may be avoided or delayed following the proposed diagnostic algorithm on histology‐based likelihood, age, location, and echocardiographic imaging characteristics. Thus, the second step should be planned according to the clinical setting (Tables 2 and 4).
In children with high probability of having a benign tumor with a tendency to spontaneous regression, a strict follow‐up of the mass dimensions may be sufficient, avoiding invasive procedures or exposition to radiations.
In a patient with clinically high suspicion of atrial thrombosis an attempt of anticoagulation with short term follow up may be considered. However, at least a TOE should be performed, to visualize the entire atrium: if the left atrial appendage is free and, on the contrary, one or more pulmonary veins are obstructed, the possibility of facing a sarcoma should be considered. (Figure 5). In this case a CT, MRI and/or a PET/CT scan are necessary to obtain the correct diagnosis and plan the therapy.
In adults with a highly mobile pedunculated mass attached to a cardiac valve, with clinical history and blood tests ruling out an infective endocarditis a presumptive diagnosis of papillary fibroelastoma can be done; the patient should be immediately referred to a cardiac surgeon, considering the high embolic risk. 115 (Video clips 1 and 2). Also other pedunculated masses may be considered for primary surgery if they appear at embolic risk and can be completely resected with a wide margin.
In the presence of a large mass invading the atria or the ventricles (with typical features suggesting its malignancy) and causing a hemodynamic impairment, a transvenous biopsy should be obtained first, to start timely chemotherapy, even if a CT scan, MRI and PET/CT will be relevant in the staging process. 116 , 117 , 118 , 119 If the mass is in continuity with a cava or pulmonary vein, suggesting an extension of an extracardiac tumor, the biopsy might more easily obtained from the primary mass, if identified (Figure 1, video clip 9).
Whenever a malignant nature of the mass is suspected, the diagnostic workout should be planned from the beginning by a multidisciplinary team with expertise in oncology, including cardio‐oncologists, cardiac surgeons, imaging specialists, and sarcoma oncologists. 120 This is essential for the therapeutic approach, which might be chemotherapy, surgery (either preceded or followed by chemotherapy), radiotherapy or a multimodal treatment. 1 , 2 , 3 , 4 , 10 , 27 , 28 , 31 , 32 , 33 , 34
TABLE 4.
Preliminary diagnostic hypothesis according to the clinical presentation and echocardiographic aspects of the mass
| Intracavitary |
| (a) left atrium: myxoma, sarcoma, and lung cancer metastasis |
|
|
|
|
| (b) right atrium, right ventricle: angiosarcoma, liver or ovarian metastasis, lymphoma |
|
|
|
|
| (c) Left ventricle: leiomyosarcoma, myxoma |
| (d) Valve: papillary fibroelastoma (differential diagnosis: endocarditis) |
| (e) Pulmonary artery: intimal sarcoma |
| Intramural |
|
|
|
| Intrapericardial |
|
|
|
11. CONCLUSIONS
Echocardiography is the most used imaging tool in the study of cardiac tumors. When integrated in the clinical setting, it can orient the diagnosis and recognize the patients who should be referred urgently to the surgeon, treated for nonneoplastic pathologies, or kept on watchful follow‐up. However, other imaging techniques are often necessary to differentiate tumors from other masses or to plan the therapeutic approach: namely CT scan, MRI, and PET/CT. A detailed analysis of possibilities, advantages, limits and costs of these techniques is beyond the scope of this article. The selection of the most appropriate imaging techniques should be done on the basis of the clinical presentation and—in case of suspect malignant tumor—by a multidisciplinary team including cardiologists, oncologists, radiologists, nuclear medicine experts, and possibly a cardio‐oncologist.
Supporting information
Video S1 Videoclips
Pino PG, Moreo A, Lestuzzi C. Differential diagnosis of cardiac tumors: General consideration and echocardiographic approach. J Clin Ultrasound. 2022;50(8):1177‐1193. doi: 10.1002/jcu.23309
DATA AVAILABILITY STATEMENT
This is a review, without experimental data to share
REFERENCES
- 1. Elbardissi AW, Dearani JA, Daly RC, et al. Survival after resection of primary cardiac tumors: a 48‐year experience. Circulation. 2008;118(14 Suppl):S7‐S15. [DOI] [PubMed] [Google Scholar]
- 2. Sultan I, Bianco V, Habertheuer A, et al. Long‐term outcomes of primary cardiac malignancies: multi‐institutional results from the National Cancer Database. J Am Coll Cardiol. 2020;75:2338‐2347. [DOI] [PubMed] [Google Scholar]
- 3. Yin K, Luo R, Wei Y, et al. Survival outcomes in patients with primary cardiac sarcoma in the United States. J Thorac Cardiovasc Surg. 2021;162:107‐115. [DOI] [PubMed] [Google Scholar]
- 4. Sultan I, Aranda‐Michel E, Habertheuer A, et al. Long‐term outcomes of primary cardiac lymphoma. Circulation. 2020;142:2194‐2195. [DOI] [PubMed] [Google Scholar]
- 5. Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol. 1990;3:195‐198. [PubMed] [Google Scholar]
- 6. Butany J, Leong SW, Carmichael K, Komeda M. A 30‐year analysis of cardiac neoplasms at autopsy. Can J Cardiol. 2005;21:675‐680. [PubMed] [Google Scholar]
- 7. Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. 2016;11:441‐452. [DOI] [PubMed] [Google Scholar]
- 8. Satge D, De Geeter B. Rhabdomyomes cardiaques et apoptose: les régressions sont‐elles contrôlées par l'organisme? [Cardiac rhabdomyoma and apoptosis: are regression controlled by the body?]. Arch mal Coeur Vaiss. 1992;85:603‐608. [PubMed] [Google Scholar]
- 9. Yuan SM. Fetal cardiac tumors: clinical features, management and prognosis. J Perinat Med. 2018;46:115‐121. [DOI] [PubMed] [Google Scholar]
- 10. Stiller B, Hetzer R, Meyer R, et al. Primary cardiac tumours: when is surgery necessary? Eur J Cardiothorac Surg. 2001;20:1002‐1006. [DOI] [PubMed] [Google Scholar]
- 11. Padalino MA, Reffo E, Cerutti A, et al. Medical and surgical management of primary cardiac tumours in infants and children. Cardiol Young. 2014;24:268‐274. [DOI] [PubMed] [Google Scholar]
- 12. Svobodov AA, Glushko LA, Ergashov AY. Surgical treatment of primary cardiac tumors in children systematic review and meta‐analysis. Pediatr Cardiol. 2022;43:251‐266. [DOI] [PubMed] [Google Scholar]
- 13. Shi L, Wu L, Fang H, et al. Identification and clinical course of 166 pediatric cardiac tumors. Eur J Pediatr. 2017;176:253‐260. [DOI] [PubMed] [Google Scholar]
- 14. Sugalska M, Tomik A, Jóźwiak S, Werner B. Treatment of cardiac Rhabdomyomas with mTOR inhibitors in children with tuberous sclerosis complex‐a systematic review. Int J Environ Res Public Health. 2021;18:4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao AS, Chao A, Wang TH, et al. Outcome of antenatally diagnosed cardiac rhabdomyoma: case series and a meta‐analysis. Ultrasound Obstet Gynecol. 2008;31:289‐295. [DOI] [PubMed] [Google Scholar]
- 16. Nathan M, Fabozzo A, Geva T, Walsh E, del Nido PJ. Successful surgical management of ventricular fibromas in children. J Thorac Cardiovasc Surg. 2014;148:2602‐2608. [DOI] [PubMed] [Google Scholar]
- 17. Jones JP, Ramcharan T, Chaudhari M, et al. Ventricular fibromas in children, arrhythmia risk, and outcomes: a multicenter study. Heart Rhythm. 2018;15:1507‐1512. [DOI] [PubMed] [Google Scholar]
- 18. Qian T, Wu Z, Yang Y, et al. Surgery for primary cardiac tumors in children: successful Management of Large Fibromas. Front Cardiovasc Med. 2022;9:808394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griborio‐Guzman AG, Aseyev OI, Shah H, Sadreddini M. Cardiac myxomas: clinical presentation, diagnosis and management. Heart. 2022;108:827‐833. [DOI] [PubMed] [Google Scholar]
- 20. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146:404‐410. [DOI] [PubMed] [Google Scholar]
- 21. Mas C, Penny DJ, Menahem S. Pre‐excitation syndrome secondary to cardiac rhabdomyomas in tuberous sclerosis. J Paediatr Child Health. 2000;36:84‐86. [DOI] [PubMed] [Google Scholar]
- 22. O'Callaghan FJ, Clarke AC, Joffe H, et al. Tuberous sclerosis complex and Wolff‐Parkinson‐white syndrome. Arch Dis Child. 1998;78:159‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nascimento AF, Winters GL, Pinkus GS. Primary cardiac lymphoma: clinical, histologic, immunophenotypic, and genotypic features of 5 cases of a rare disorder. Am J Surg Pathol. 2007;31:1344‐1350. [DOI] [PubMed] [Google Scholar]
- 24. Lestuzzi C, Spina M, Martellotta F, Carbone A. Massive myocardial infiltration by HIV‐related non‐Hodgkin lymphoma: echocardiographic aspects at diagnosis and at follow‐up. J Cardiovasc Med (Hagerstown). 2012;13:836‐838. [DOI] [PubMed] [Google Scholar]
- 25. Matsunaga K, Kobayashi T, Takahashi M, Gohra H. Huge primary cardiac malignant lymphoma in the left ventricle. Ann Thorac Surg. 2020;110:e115‐e118. [DOI] [PubMed] [Google Scholar]
- 26. Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, et al. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2:CD011123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torabi S, Arjomandi Rad A, Vardanyan R, et al. Surgical and multimodality treatment of cardiac sarcomas: a systematic review and meta‐analysis. J Card Surg. 2021;36:2476‐2485. [DOI] [PubMed] [Google Scholar]
- 28. Isambert N, Ray‐Coquard I, Italiano A, et al. Primary cardiac sarcomas: a retrospective study of the French sarcoma group. Eur J Cancer. 2014;50:128‐136. [DOI] [PubMed] [Google Scholar]
- 29. Chan AT, Plodkowski AJ, Pun SC, et al. Prognostic utility of differential tissue characterization of cardiac neoplasm and thrombus via late gadolinium enhancement cardiovascular magnetic resonance among patients with advanced systemic cancer. J Cardiovasc Magn Reson. 2017;19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mousavi N, Cheezum MK, Aghayev A, et al. Assessment of cardiac masses by cardiac magnetic resonance imaging: histological correlation and clinical outcomes. J Am Heart Assoc. 2019;8:e007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lestuzzi C, De Paoli A, Baresic T, et al. Malignant cardiac tumors: diagnosis and treatment. Future Cardiol. 2015;11:485‐500. [DOI] [PubMed] [Google Scholar]
- 32. Yanagawa B, Chan EY, Cusimano RJ, Reardon MJ. Approach to surgery for cardiac tumors: primary simple, primary complex, and secondary. Cardiol Clin. 2019;37:525‐531. [DOI] [PubMed] [Google Scholar]
- 33. Tyebally S, Chen D, Bhattacharyya S, et al. Cardiac tumors: JACC CardioOncology state‐of‐the‐art review. JACC CardioOncol. 2020;2:293‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rezaeian N, Hosseini L, Toloueitabar Y, Hemmati Komasi MM . The role of cardiac CT and MRI in the diagnosis and management of primary cardiac lymphoma: a comprehensive review. Trends Cardiovasc Med. 2022;32:408‐420. [DOI] [PubMed] [Google Scholar]
- 35. Lemasle M, Lavie Badie Y, Cariou E, et al. Contribution and performance of multimodal imaging in the diagnosis and management of cardiac masses. Int J Cardiovasc Imaging. 2020;36:971‐981. [DOI] [PubMed] [Google Scholar]
- 36. Bruce CJ. Cardiac Tumors in Otto CM. The practice of clinical echocardiography. 5th ed. Elsevier; 2017. [Google Scholar]
- 37. Kraft C, Schuettfort G, Weil Y, et al. Thrombosis of the inferior vena cava and malignant disease. Thromb Res. 2014;134:668‐673. [DOI] [PubMed] [Google Scholar]
- 38. Kojiro M, Nakahara H, Sugihara S, Murakami T, Nakashima T, Kawasaki H. Hepatocellular carcinoma with intra‐atrial tumor growth. A clinicopathologic study of 18 autopsy cases. Arch Pathol Lab Med. 1984;108:989‐992. [PubMed] [Google Scholar]
- 39. Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol. 2013;20:914‐922. [DOI] [PubMed] [Google Scholar]
- 40. Wassef J, Xu S. Hepatocellular carcinoma with tumor thrombus to the hepatic veins and the right atrium: a case report and review exploring various presentations and treatment options. Cureus. 2020;12:e8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee JM, Lee KW, Hong SK, et al. Unusual techniques for preserving surgical and oncologic safety in hepatectomy of advanced adrenal malignancy with vena cava and liver invasion. Ann Surg Oncol. 2018;25:3324‐3325. [DOI] [PubMed] [Google Scholar]
- 42. Sun N, Zhang J, Li B, Li A, Lv M, Zhang C. Favorable response to multimodal treatment in hepatocellular carcinoma with inferior vena cava and right atrial tumor thrombus and left adrenal gland metastasis: a case report and literature review. Medicine (Baltimore). 2021;100:e27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cassar MP, Stirrup J. Left atrial tumor thrombus in metastatic thyroid cancer. J Cardiovasc Comput Tomogr. 2020;14:e155‐e156. [DOI] [PubMed] [Google Scholar]
- 44. Lee E, Mahani MG, Lu JC, Dorfman AL, Srinivasan A, Agarwal PP. Primary cardiac tumors associated with genetic syndromes: a comprehensive review. Pediatr Radiol. 2018;48:156‐164. [DOI] [PubMed] [Google Scholar]
- 45. Isaacs H Jr. Fetal and neonatal cardiac tumors. Pediatr Cardiol. 2004;25:252‐273. [DOI] [PubMed] [Google Scholar]
- 46. Chen J, Wang J, Sun H, et al. Fetal cardiac tumor: echocardiography, clinical outcome and genetic analysis in 53 cases. Ultrasound Obstet Gynecol. 2019;5:103‐109. [DOI] [PubMed] [Google Scholar]
- 47. Kupsky DF, Newman DB, Kumar G, Maleszewski JJ, Edwards WD, Klarich KW. Echocardiographic features of cardiac Angiosarcomas: the Mayo Clinic experience (1976–2013). Echocardiography. 2016;33:186‐192. [DOI] [PubMed] [Google Scholar]
- 48. Maleszewski JJ, Anavekar NS, Moynihan TJ, et al. Pathology, imaging, and treatment of cardiac tumours. Nat Rev Cardiol. 2017;14:536‐549. [DOI] [PubMed] [Google Scholar]
- 49. Masroor S, Marla R. Left atrial angiosarcoma: an unusual presentation and location. J Heart Valve Dis. 2011;20:229‐231. [PubMed] [Google Scholar]
- 50. Bussani R, Castrichini M, Restivo L, et al. Cardiac tumors: diagnosis, prognosis, and treatment. Curr Cardiol Rep. 2020;22:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lynch M, Balk MA, Lee RB, Martin RP. Role of transesophageal echocardiography in the management of patients with bronchogenic carcinoma invading the left atrium. Am J Cardiol. 1995;76:1101‐1102. [DOI] [PubMed] [Google Scholar]
- 52. Desai MY, Mankad S. Extension of bronchogenic carcinoma through pulmonary vein into the left atrium detected by echocardiography. Echocardiography. 2004;21:189‐191. [DOI] [PubMed] [Google Scholar]
- 53. Lestuzzi C, Viel E, Mimo R, Meneguzzo N. Left atrial invasion by lung carcinoma through a pulmonary vein. Int J Cardiovasc Imaging. 2001;17:107‐110. [DOI] [PubMed] [Google Scholar]
- 54. Koh TW. Invasion of lung mesenchymal chondrosarcoma into the left atrium via the pulmonary vein detected on transoesophageal echocardiography. Eur J Echocardiogr. 2011;12:556. [DOI] [PubMed] [Google Scholar]
- 55. Nosrati A, Nabati M, Vahedi L, Shokri M. Invasion of poorly differentiated large‐cell neuroendocrine tumor of the lung through right pulmonary veins into the left atrium: a very rare case report. J Clin Ultrasound. 2020;48:560‐564. [DOI] [PubMed] [Google Scholar]
- 56. Reynoso‐Hermosillo M, Sandoval‐García J, López‐Rosales B, et al. Malignant primary tumor in left atrium with mitral valve invasion, pulmonary veins and cerebral metastasis. A case report. Tumor primario maligno en aurícula izquierda con invasión a válvula mitral, venas pulmonares y metástasis cerebral. Informe de un Caso. Cir Cir. 2020;88:91‐94. [DOI] [PubMed] [Google Scholar]
- 57. Pietras CM, Spittell PC, Nuttall GA, Eleid MF, Said SM. Pulmonary venous obstruction from a large left atrial sarcoma: resection combined with mitral valve bypass. Ann Thorac Surg. 2016;102:e237‐e239. [DOI] [PubMed] [Google Scholar]
- 58. Samanidis G, Khoury M, Balanika M, Perrea DN. Current challenges in the diagnosis and treatment of cardiac myxoma. Kardiol pol. 2020;78:269‐277. [DOI] [PubMed] [Google Scholar]
- 59. Lestuzzi C. Primary tumors of the heart. Curr Opin Cardiol. 2016;31:593‐598. [DOI] [PubMed] [Google Scholar]
- 60. Fazzari F, Mantovani R, Curzi M, Raspante D, Bragato RM. Real‐time three‐dimensional trans‐oesophageal echocardiography: a guidance in challenging endomyocardial biopsy‐cardiac angiosarcoma involving upper and lower vena cava. Eur Heart J Cardiovasc Imaging. 2016;17:891. [DOI] [PubMed] [Google Scholar]
- 61. Ambrus N, Havasi K, Kalapos A, et al. Primary cardiac angiosarcoma: a case report. Echocardiography. 2018;35:267‐271. [DOI] [PubMed] [Google Scholar]
- 62. Goswami KC, Shrivastava S, Bahl VK, Saxena A, Manchanda SC, Wasir HS. Cardiac myxomas: clinical and echocardiographic profile. Int J Cardiol. 1998;63:251‐259. [DOI] [PubMed] [Google Scholar]
- 63. Engberding R, Daniel WG, Erbel R, et al. Diagnosis of heart tumours by transoesophageal echocardiography: a multicentre study in 154 patients. European cooperative study group. Eur Heart J. 1993;14:1223‐1228. [DOI] [PubMed] [Google Scholar]
- 64. Cianciulli TF, Cozzarin A, Soumoulou JB, et al. Twenty years of clinical experience with cardiac Myxomas: diagnosis, treatment, and follow up. J Cardiovasc Imaging. 2019;27:37‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kalçık M, Bayam E, Güner A, et al. Evaluation of the potential predictors of embolism in patients with left atrial myxoma. Echocardiography. 2019;36:837‐843. [DOI] [PubMed] [Google Scholar]
- 66. Rosário M, Fonseca AC, Sotero FD, Ferro JM. Neurological complications of cardiac tumors. Curr Neurol Neurosci Rep. 2019;19:15. [DOI] [PubMed] [Google Scholar]
- 67. Bai R, Zhang Y, Wang H, Yang J, Sun D. Invasive cardiac lipoma diagnosis based on echocardiography: case report and literature review. J Clin Ultrasound. 2021;49:408‐412. [DOI] [PubMed] [Google Scholar]
- 68. Shu S, Yuan H, Kong X, Wang J, Wang J, Zheng C. The value of multimodality imaging in diagnosis and treatment of cardiac lipoma. BMC Med Imaging. 2021;21:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu L, Zuo Y, Huang Y, Cao L. Echocardiographical findings of giant cardiac lipoma: a case report. Medicine (Baltimore). 2019;98:e14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kusajima K, Hata H, Fujita T, et al. Successful surgical treatment for recurrent cardiac fibroma 21 years after resection. Surg Case Rep. 2015;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gasparovic H, Coric V, Milicic D, et al. Left ventricular fibroma mimicking an acute coronary syndrome. Ann Thorac Surg. 2006;82:1891‐1892. [DOI] [PubMed] [Google Scholar]
- 72. Chu ZG, Zhu ZY, Liu MQ, Lv FJ. Cardiac fibromas in the adult. J Card Surg. 2014;29:159‐162. [DOI] [PubMed] [Google Scholar]
- 73. Miao H, Yang W, Zhou M, et al. Atrial hemangioma: A case report and review of the literature. Ann Thorac Cardiovasc Surg. 2019;25:71‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nakamura E, Nakamura K, Furukawa K, et al. A Giant cardiac cavernous hemangioma involving the left atrial roof in an elderly woman. Ann Thorac Cardiovasc Surg. 2019;25:60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brandt RR, Arnold R, Bohle RM, Dill T, Hamm CW. Cardiac angiosarcoma: case report and review of the literature. Z Kardiol. 2005;94:824‐828. [DOI] [PubMed] [Google Scholar]
- 76. Duyuler S, Demirkan B, Guray Y, Demir C, Guray U. Echocardiography in differential diagnosis of chest pain and elevated cardiac biomarkers: a case of cardiac angiosarcoma. Eur J Echocardiogr. 2011;12:406‐407. [DOI] [PubMed] [Google Scholar]
- 77. Uemura K, Sano H, Takaoka H, Okita Y. Cardiac angiosarcoma in the right ventricle treated by surgical resection. BMJ Case Rep. 2021;14:e238736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rao JN, Gowda GD, Anand R, et al. Angiosarcoma of the right atrium and right ventricle. J Card Surg. 2017;32:807‐808. [DOI] [PubMed] [Google Scholar]
- 79. De Filippo M, Chernyschova N, Maffei E, et al. Primary cardiac Burkett's type lymphoma: transthoracic echocardiography, multidetector computed tomography and magnetic resonance findings. Acta Radiol. 2006;47:167‐171. [DOI] [PubMed] [Google Scholar]
- 80. Khalid K, Faza N, Lakkis NM, Tabbaa R. Cardiac involvement by Burkitt lymphoma in a 49‐year‐old man. Tex Heart Inst J. 2020;47:210‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li Y, Zhou Z, Xin F, et al. Primary cardiac lymphoma in both atria: a case report. J Clin Ultrasound. 2019;47:561‐563. [DOI] [PubMed] [Google Scholar]
- 82. Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dietrich CF, Nolsøe CP, Barr RG, et al. Guidelines and good clinical practice recommendations for contrast‐enhanced ultrasound (CEUS) in the liver‐update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579‐2604. [DOI] [PubMed] [Google Scholar]
- 84. Yin T, Zheng B, Lian Y, et al. Contrast‐enhanced ultrasound improves the potency of fine‐needle aspiration in thyroid nodules with high inadequate risk. BMC Med Imaging. 2022;22:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sogani J, Mango VL, Keating D, Sung JS, Jochelson MS. Contrast‐enhanced mammography: past, present, and future. Clin Imaging. 2021;69:269‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jung EM, Weber MA, Wiesinger I. Contrast‐enhanced ultrasound perfusion imaging of organs. Kontrastmittelverstärkter Ultraschall zur Perfusionsdiagnostik in Organen. Radiologe. 2021;61(Suppl 1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lacerda Q, Tantawi M, Leeper DB, Wheatley MA, Eisenbrey JR. Emerging applications of ultrasound‐contrast agents in radiation therapy. Ultrasound Med Biol. 2021;47:1465‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aggeli C, Dimitroglou Y, Raftopoulos L, et al. Cardiac masses: the role of cardiovascular imaging in the differential diagnosis. Diagnostics (Basel). 2020;10:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Strachinaru M, Damry N, Duttmann R, et al. Ultrasound contrast quantification for the diagnosis of Intracardiac masses. JACC Cardiovasc Imaging. 2016;9:747‐750. [DOI] [PubMed] [Google Scholar]
- 90. Lepper W, Shivalkar B, Rinkevich D, Belcik T, Wei K. Assessment of the vascularity of a left ventricular mass using myocardial contrast echocardiography. J Am Soc Echocardiogr. 2002;15:1419‐1422. [DOI] [PubMed] [Google Scholar]
- 91. Yang HS, Sengupta S, Umland MM, et al. Primary cardiac angiosarcoma evaluated with contrast two‐dimensional and real‐time three‐dimensional echocardiography. Eur J Echocardiogr. 2008;9:733‐738. [DOI] [PubMed] [Google Scholar]
- 92. Mongal LS, Salat R, Anis A, et al. Enormous right atrial hemangioma in an asymptomatic patient: a case report and literature review. Echocardiography. 2009;26:973‐976. [DOI] [PubMed] [Google Scholar]
- 93. Gao Y, Wu W, Zhang L, et al. Multimodality imaging in preparation for resection of a right atrial cavernous hemangioma. Echocardiography. 2020;37:465‐466. [DOI] [PubMed] [Google Scholar]
- 94. Hari P, Mohamad T, Kondur A, Jahania SM, Afonso L. Incremental value of contrast echocardiography in the diagnosis of atrial myxoma. Echocardiography. 2010;27:E46‐E49. [DOI] [PubMed] [Google Scholar]
- 95. Yelamanchili P, Wanat FE, Knezevic D, Nanda NC, Patel V. Two‐dimensional transthoracic contrast echocardiographic assessment of metastatic left ventricular tumors. Echocardiography. 2006;23:248‐250. [DOI] [PubMed] [Google Scholar]
- 96. Kirkpatrick JN, Wong T, Bednarz JE, et al. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412‐1419. [DOI] [PubMed] [Google Scholar]
- 97. Duke J, Greaves K, Dettrick A. Use of microbubble contrast in the diagnosis of a left ventricular papillary fibroelastoma. Echo Res Pract. 2015;2:K43‐K45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bhattacharyya S, Khattar R, Senior R. Characterisation of intra‐cardiac masses by myocardial contrast echocardiography. Int J Cardiol. 2013;163:e11‐e13. [DOI] [PubMed] [Google Scholar]
- 99. Uenishi EK, Caldas MA, Tsutsui JM, et al. Evaluation of cardiac masses by real‐time perfusion imaging echocardiography. Cardiovasc Ultrasound. 2015;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Porter TR, Mulvagh SL, Abdelmoneim SS, et al. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography guidelines update. J Am Soc Echocardiogr. 2018;31:241‐274. [DOI] [PubMed] [Google Scholar]
- 101. Hasegawa T, Nakagawa S, Chino M, Kunihiro T, Ui S, Kimura M. Primary cardiac sarcoma mimicking benign myxoma: a case report. J Cardiol. 2002;39:321‐325. [PubMed] [Google Scholar]
- 102. Kim JT, Baek WK, Kim KH, Yoon YH, Kim DH, Lim HK. A primary cardiac sarcoma preoperatively presented as a benign left atrial myxoma. Yonsei Med J. 2003;44:530‐533. [DOI] [PubMed] [Google Scholar]
- 103. Kakaty D, Grapow M, Huber B, Lardinois D. Long‐term survival after extended resection of primary atrial myxosarcoma. J Surg Case Rep. 2015;2015:rju146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zaragosa‐Macias E, Chen MA, Gill EA. Real‐time three‐dimensional echocardiography evaluation of intracardiac masses. Echocardiography. 2012;29:207‐219. [DOI] [PubMed] [Google Scholar]
- 105. Lim HJ, Johnny Ong CA, et al. Utility of positron emission tomography/computed tomography (PET/CT) imaging in the evaluation of sarcomas: A systematic review. Crit Rev Oncol Hematol. 2019;143:1‐13. [DOI] [PubMed] [Google Scholar]
- 106. Cheson BD. PET/CT in lymphoma: current overview and future directions. Semin Nucl Med. 2018;48:76‐81. [DOI] [PubMed] [Google Scholar]
- 107. Chen B, Feng H, Xie J, et al. Differentiation of soft tissue and bone sarcomas from benign lesions utilizing 18F‐FDG PET/CT‐derived parameters. BMC Med Imaging. 2020;20:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hughes NM, Jacene HA. PET imaging for hematologic malignancies. Radiol Clin North Am. 2021;59:705‐723. [DOI] [PubMed] [Google Scholar]
- 109. Friedman SN, Itani M, Dehdashti F. PET imaging for gynecologic malignancies. Radiol Clin North Am. 2021;59:813‐833. [DOI] [PubMed] [Google Scholar]
- 110. Sonin AH, Mazer MJ, Powers TA. Obstruction of the inferior vena cava: a multiple‐modality demonstration of causes, manifestations, and collateral pathways. Radiographics. 1992;12:309‐322. [DOI] [PubMed] [Google Scholar]
- 111. Kobayashi Y, Kumita S, Fukushima Y, Ishihara K, Suda M, Sakurai M. Significant suppression of myocardial (18)F‐fluorodeoxyglucose uptake using 24‐h carbohydrate restriction and a low‐carbohydrate, high‐fat diet. J Cardiol. 2013;6:314‐319. [DOI] [PubMed] [Google Scholar]
- 112. Masuda A, Naya M, Manabe O, et al. Administration of unfractionated heparin with prolonged fasting could reduce physiological 18F‐fluorodeoxyglucose uptake in the heart. Acta Radiol. 2016;57:661‐668. [DOI] [PubMed] [Google Scholar]
- 113. Wong RKS, Metser U, Veit‐Haibach P. Neuroendocrine tumors: imaging perspective. PET Clin. 2021;16:353‐364. [DOI] [PubMed] [Google Scholar]
- 114. Carrasquillo JA, Chen CC, Jha A, et al. Imaging of Pheochromocytoma and Paraganglioma. J Nucl Med. 2021;62:1033‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sousa‐Uva M, Cardim N. Cardiac papillary fibroelastoma: so small and yet so dangerous. Rev Port Cardiol (Engl Ed). 2018;37:987‐989. [DOI] [PubMed] [Google Scholar]
- 116. Saito M, Saraya T, Oda M, et al. Rapidly progressive respiratory failure with multiple halo signs on computed tomography in a patient with primary cardiac angiosarcoma derived from the right atrium: a case report. BMC Pulm Med. 2020;20:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Keller DI, Hunziker P, Buser P. Biopsy of right atrial angiosarcoma guided by transesophageal echocardiography. J Am Soc Echocardiogr. 2002;15:475‐477. [DOI] [PubMed] [Google Scholar]
- 118. Chang L, Gong C, Lu H, et al. Percutaneous intravenous catheter forceps biopsy in right atrial mass: two case reports and literature review. BMC Cardiovasc Disord. 2022;22:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kaiafa G, Bobos M, Savopoulos C, et al. Heart and lymphoma: an unusual case of secondary cardiac lymphoma manifested through presyncope and syncope episodes and atrial flutter. Hellenic J Cardiol. 2018;59:182‐185. [DOI] [PubMed] [Google Scholar]
- 120. Lestuzzi C, Reardon MJ. Primary cardiac malignancies: the need for a multidisciplinary approach and the role of the cardio‐oncologist. [editorial]. J Am Coll Cardiol. 2020;12(75):2348‐2351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Videoclips
Data Availability Statement
This is a review, without experimental data to share


